Abstract
Background
Adult-onset growth hormone deficiency (AO-GHD) is associated with an increased prevalence of the metabolic syndrome (MetS).
Aim
To determine the effect of GH replacement on the prevalence of MetS in AO-GHD and to study the impact of MetS on the incidence of cardiovascular events during GH replacement.
Patients and methods
1449 AO-GHD patients (males 48.9%; mean age 48.9 ± 12.8 year) were retrieved from KIMS (Pfizer International Metabolic Database). The prevalence of MetS (using International Diabetes Federation criteria) and its components were calculated at baseline and after one year of GH replacement. The relative risk to develop cardiovascular events according to the presence of MetS at baseline was assessed in another group of 3282 patients after prolonged GH replacement.
Results
The prevalence of MetS was 46.9% at baseline and 48.2% after one year of GH replacement (P = NS). The percentage of patients with abnormal waist circumference decreased significantly (80.3 vs 77.4%; P < 0.001), but impaired glucose metabolism (17.1 vs 23.3%; P < 0.001) increased and HDL cholesterol (48.2 vs 50.9%; P = 0.011) decreased. Switch from MetS to NoMS (18.5%) and from NoMS to MetS (18.8%) occurred. All patients showed a significant and comparable amelioration of quality of life. During seven years of GH replacement patients with MetS had a 66% higher risk (P = 0.0016) to develop a new coronary disease compared to NoMS.
Conclusion
MetS prevalence remains unchanged in AO-GHD during one year of GH replacement whereas its components are differentially affected. Besides GH replacement, consequent pharmacotherapy of all risk factors and endorsement of lifestyle intervention appears to be of uttermost importance together with early GHD diagnosis to prevent cardiovascular disease during prolonged treatment.
Keywords: metabolic syndrome, hypopituitarism, growth hormone deficiency, GH replacement, cardiovascular disease
Introduction
It is now well-recognized that patients with adult-onset growth hormone deficiency (AO-GHD), apart from experiencing a poor quality of life (QoL), present with an increased risk of developing a metabolic syndrome (MetS) (1, 2, 3, 4). MetS describes the clustering of five cardiovascular risk factors: increased waist circumference due to visceral fat accumulation, elevated blood pressure, lowered HDL cholesterol, increased triglycerides, and impaired glucose tolerance and is considered an important predictor of cardiovascular disease (5, 6). There are different MetS definitions, of which the revised National Cholesterol Education Program (NCEP) and the International Diabetes Federation (IDF) are most commonly used (7, 8). It is estimated that now about one third of the European adult population has MetS according to the IDF criteria and that both males and females with MetS are about 1.5 times more likely to die from cardiovascular complications (9).
Patients with AO-GHD have a prevalence of MetS between 1.3- and 2-fold that of the general population (1, 4) and this varies depending on the studies between 31.0 and 56.6% (1, 2, 3, 4). The elevated MetS prevalence in GHD is probably of particular relevance as an increased cardiovascular mortality has been found in patients with hypopituitarism receiving conventional hormonal replacement without GH (10, 11, 12). A recent KIMS study assessing GHD patients with MetS before GH replacement also reported a higher prevalence ratio (PR) of coronary and cerebrovascular morbidity compared to patients without MetS (3). It has clearly been documented that GH replacement had a favourable effect on QoL and on the different MetS components, apart from a possible deterioration of the glycaemic control, particularly in patients with elevated BMI (13, 14, 15, 16). However, no study assessing the occurrence of MetS during GH replacement has so far indicated any improvement in its prevalence (1, 2, 4). Furthermore, the impact of GH replacement on the increased cardiovascular mortality is unsettled (4, 17).
The first aim of the present study was to use the KIMS (Pfizer International Metabolic Database) in order to investigate the effect of one year of GH replacement on the prevalence of MetS and of its components in the largest available cohort of AO-GHD patients. The subsequent aim was to characterize and compare the two groups of patients who changed their MetS status during this period. The final objective was to assess the incidence of cardiovascular and cerebrovascular events occurring during the whole follow-up period of GH replacement.
Patients and methods
Patients
In the prevalence analysis were included: patients of European origin with severe AO-GHD confirmed by an accepted GH stimulatory test, naïve or semi-naïve (without GH replacement for at least 6 months prior to study entry) to GH replacement, with a complete set of data on the five MetS components at baseline (BL) and after 1 year of GH replacement (Y1). A total of 1449 patients fulfilled the inclusion criteria for this study. Females numbered 740 (51.1%; mean age, 47.7 ± 12.8 year), divided into age categories <40 year (n = 216), 40–60 year (n = 386), >60 year (n = 138) and males numbered 709 (48.9%; mean age, 50.2 ± 12.7 year), divided into age categories <40 year (n = 158), 40–60 year (n = 380), >60 year (n = 171). The group with MetS consisted of 679 patients (46.9%; mean age, 51.1 ± 12.1 year), while the group without MetS (NoMS) consisted of 770 patients (53.1%; mean age, 47.0 ± 13.1 year).
The cardiovascular complications analysis consisted of patients recruited according to the same inclusion criteria, except for the addition of non-naïve patients (already on GH replacement at baseline). A total number of 3282 patients (50.2% females) fulfilled the inclusion criteria for this study. The MetS group consisted of 1516 patients (46.2%; mean age, 51.1 ± 12.0 year; mean duration of follow-up, 6.8 ± 4.7 year; number of patient-years, 10373), while the NoMS group consisted of 1766 patients (53.8%; mean age, 46.6 ± 13.1 year; mean duration of follow-up, 7.8 ± 5.1 year; number of patient-years, 13713).
Informed consent was obtained from patients in accordance with local regulations. The studies were performed in accordance with the Declaration of Helsinki (18). The data collection into KIMS was approved by the ZNA/OCMW Antwerp Institutional Review Boards/Ethical Committees as required by local regulations in each participating country. Written informed consent was obtained from all the patients before any data were entered into KIMS.
Methods
Background and baseline data consisted of gender, age, medical history (hypertension, hyperlipidaemia, diabetes mellitus), BMI, blood pressure and waist circumference. Plasma glucose was measured at the site of the participating centres, while serum lipids (total cholesterol, HDL cholesterol, triglycerides) were performed in a central laboratory according to well-defined methods (3). Serum IGF-I was measured centrally using methods detailed in previous KIMS publications (3). Quality of life was assessed by the QoL-AGHDA questionnaire, which scores ranges from 0 to 25, with the higher score corresponding to a poorer quality of life (19). All these parameters have been determined at BL and Y1. The GH dose used at one year of replacement was also retrieved from the database.
Definition of MetS
The diagnosis of MetS was defined according to the IDF criteria (8). Central obesity (defined by a waist circumference ≥94 cm for males and ≥80 cm for females) plus any two of the following four factors: a blood pressure ≥130/85 mmHg or the use of anti-hypertensive drugs, a serum high-density cholesterol ≤40 mg/dL (1.0 mmol/L) for males and ≤50 mg/dL (1.3 mmol/L) for females or the use of lipid-lowering drugs, serum triglycerides ≥150 mg/dL (1.7 mmol/L) or the use of lipid-lowering drugs, raised fasting glucose >100 mg/dL (5.6 mmol/L) or type 2 diabetes (defined by a random or 2 h OGTT glucose ≥200 mg/dL (11.1 mmol/L); a HbA1c ≥6.4% or the use of anti-diabetic drugs (20). The MetS score, ranging between 0 and 5, corresponds to the sum of the abnormal MetS components, with the higher score denoting the most adverse MetS profile.
Study design
The MetS PR, comparing MetS prevalence after 1 year of GH replacement with the MetS prevalence at KIMS entry before GH replacement, was first calculated for the whole group of patients, and then separated by gender and age. Similarly, the prevalence of the five separate MetS components was determined before and after 1 year of GH replacement, crude and by age and gender categories. The relative impact of the separate MetS components upon the global MetS score was calculated. Additionally, the absolute changes in the five components between MetS patients and NoMS patients at baseline were compared.
Four groups of patients, corresponding to retention or change of their MetS status during GH replacement, were characterized by the MetS score and its components, and by related factors, such as changes in BMI, IGF-I and QoL-AGHDA. Significant differences between the two groups with MetS status reversal were calculated.
All patients, irrespective of GH replacement naïvety and with complete data on MetS components, were followed up to the occurrence of a new specific cardiovascular or cerebrovascular event, or to the reported date of last visit or date of death. The incidence rates of these complications occurring in the group of MetS patients at baseline were compared to the group of NoMS patients.
Statistics
Changes in the prevalence of MetS after 1 year of GH replacement were assessed. The change was expressed as percentage change or as the ratio of the prevalence at the 1-year visit and the prevalence at KIMS entry (start of GH replacement). Analyses were also performed based on the 4 possible transition groups (i.e. 2 groups where no change in MetS status was observed and 2 groups where MetS status changed between the 2 time points).
Results are expressed as counts and percentage for categorical variables and as mean ± s.d. for numerical variables. The McNemar’s test for paired samples was used in the crude analysis to analyse the changes during GH replacement in the prevalence of MetS, as well as on the different MetS components (waist, blood pressure, HDL cholesterol, triglycerides and glucose). In the multiple regression analyses of PR at the 1-year visit compared to BL visit, Robust Poisson regression methods with repeated measurements were applied, with adjustment for age and gender. In the quantitative analyses of change between visits in MetS components, multiple linear regression was performed with adjustment for gender and age. In special analyses, end-point specific drug therapy at baseline was incorporated as a potential confounding indicator variable. Estimates are presented with 95% confidence intervals (CIs) and statistical significance was set to 5%. SAS v9.2 Proc Genmod (SAS Inc., Cary, NC, USA) was used.
Results
Prevalence of MetS and of its components during one year of GH replacement
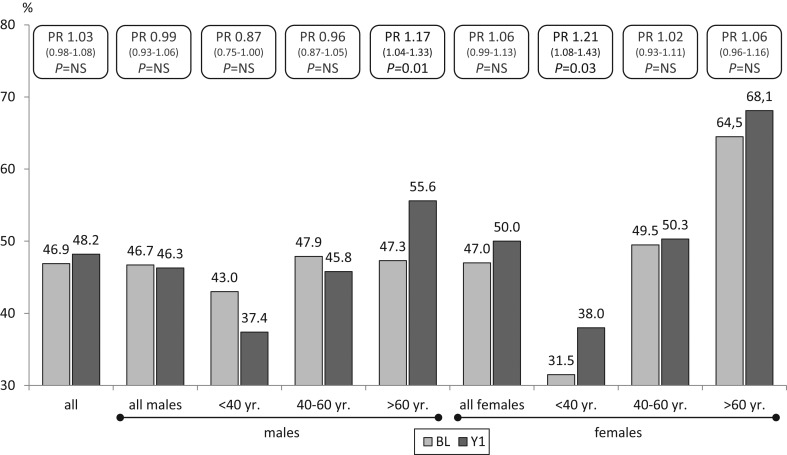
The number of MetS patients increased at Y1 from 679 to 698 (46.9% vs 48.2%; P = NS), corresponding to an increase of 3% (95% CI, −2% to 8%) or a PR increase of 1.03 (95% CI, 0.98–1.08). When separated by age and gender, a significant increase was noticed in MetS for males >60 year and females <40 year, while a decrease was only noted in males <40 year and between 40 and 60 year, although the difference was not significant (Fig. 1).
Figure 1.
Prevalence ratio of MetS by gender and age category at BL and Y1.
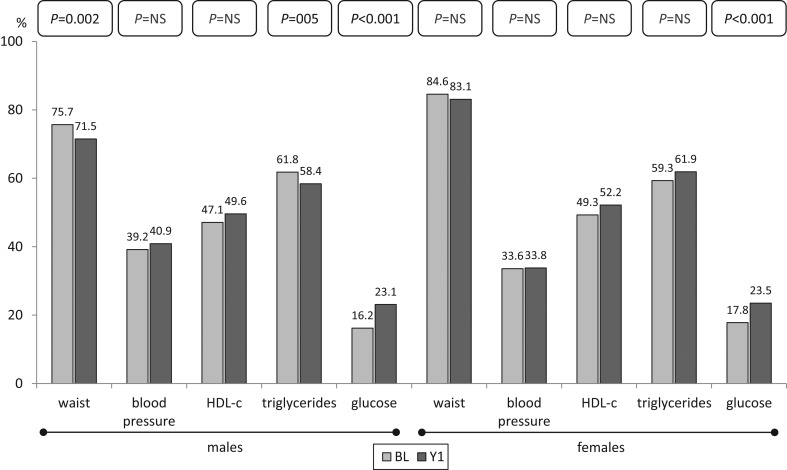
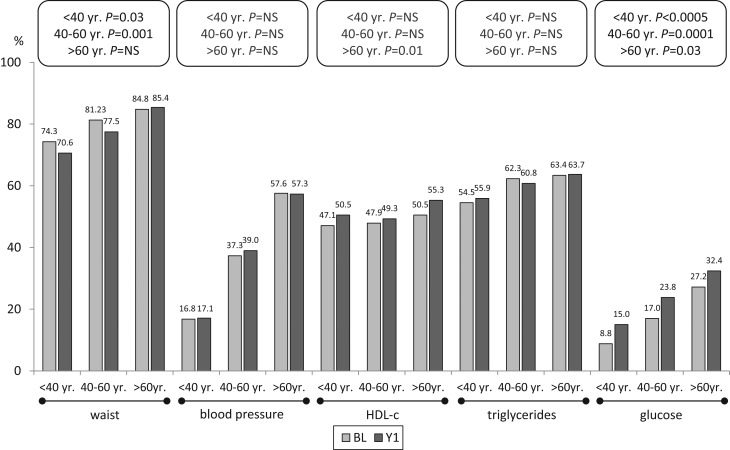
The percentage of patients with abnormal waist decreased significantly at Y1 (80.3 vs 77.4%; P < 0.001), whereas impaired glucose metabolism increased (17.1 vs 23.3%; P < 0.001) as well as unfavourable HDL (48.2 vs 50.9%; P = 0.011). No change was observed for blood pressure and triglycerides. Separate analysis by gender of the prevalence of the components at Y1 showed a significant decrease in abnormal waist and triglycerides in males, but also a significant increase of impaired glucose metabolism in both males and females (Fig. 2). Similar analysis by age category indicated a significant decrease in abnormal waist in all patients <60 year. On the other hand, abnormal HDL increased in patients >60 year, while the increase in impaired glucose metabolism was significant in each age category (Fig. 3).
Figure 2.
Prevalence of components determining MetS by gender at BL and Y1.
Figure 3.
Prevalence of components determining MetS by age category at BL and Y1.
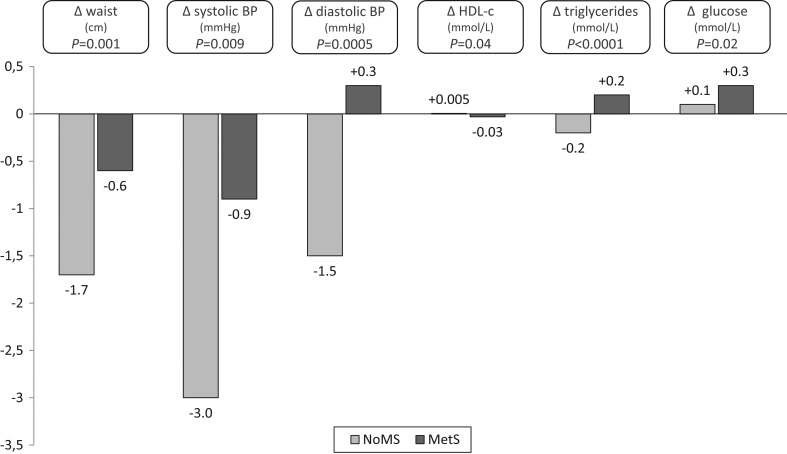
GH replacement induced in NoMS patients a positive effect on all components except for the glucose component, while this effect is only apparent for waist and systolic blood pressure in MetS patients (Fig. 4). A comparison, after adjustment for gender, age, the component value at baseline and the drug therapy, showed a significant more favourable change for all components in the NoMS group.
Figure 4.
Changes of absolute values of MetS components at Y1 related to baseline MetS status.
There was no difference between NoMS and MetS patients in the presence of TSH deficiency (77.2 vs 77.0%), ACTH deficiency (74.1 vs 72.5%) or LH-FSH-deficiency in females (77.3 vs 76.6%). Compared to NoMS, more MS patients had ADH deficiency (23.3 vs 26.3%, P = 0.05) and LH-FSH deficiency in males (85.4 vs 89.8%). The number of patients with isolated GH was equal in both groups (8.67 vs 7.58%, P = 0.33). An equal percentage of patients with NoMS and MetS were substituted for TSH deficiency (both 99.4%), ACTH deficiency (99.0 vs 98.8%) and LH-FSH deficiency in males (98.6 vs 98.5%). Compared to NoMS, less MetS patients were substituted for ADH (99.2 vs 97.1, P = 0.03) and LH-FSH deficiency in females (88.2 vs 76.4, P < 0.001).
Reversal of MetS status during one year of GH replacement
While 625 patients retained their NoMS status and 553 their MetS status, a cross-over of patients was apparent in both directions: a total of 126 patients (18.5%) switched from MetS to NoMS and 145 patients (18.8%) switched from NoMS to MetS. Details on changes in BMI, IGF1 SDS, QoL-AGHDA, MetS score and MetS components are reported in Table 1. Noteworthy is the finding that the dissimilarity in response was not related to a different baseline IGF1 SDS, GH replacement dose or IGF1 SDS at Y1, while a significant change in BMI was observed between the cross-over groups. Also of interest is the fact that the overall comparable poor QoL improved importantly and similarly irrespective of the MetS status during GH replacement.
Table 1.
Clinical and biochemical characteristics of GHD patients at baseline (BL) and after one year of GH replacement (Y1).
| NoMS | MetS | ||||
|---|---|---|---|---|---|
| N (% female) | |||||
| At BL→Y1 | 770 (50.1) → 751 (49.2) | 679 (51.3) → 698 (53.0) | |||
| NoMS → NoMS | MetS → NoMS | P MetS → NoMS vs NoMS → MetS | NoMS → MetS | MetS → MetS | |
| N (% female) | |||||
| At Y1 | 625 (49.8) | 126 (46.8) | 145 (55.9) | 553 (52.3) | |
| Age | |||||
| At BL | 46.4 ± 13.1 | 47.4 ± 11.1 | 0.21 | 49.2 ± 13.3 | 52.0 ± 12.2 |
| BMI (kg/m²) | |||||
| At BL | 26.2 ± 4.5 | 29.8 ± 4.4 | NS | 29.6 ± 5.7 | 31.4 ± 5.1 |
| ∆ BL→Y1 | 0.0 ± 1.4 | –0.4 ± 1.6 | <0.0001 | 0.5 ± 2.1 | 0.0 ± 1.7 |
| IGF-I SDS | |||||
| At BL | −1.9 ± 1.8 | −1.7 ± 1.6 | NS | −1.8 ± 1.6 | −1.6 ± 1.5 |
| ∆ BL→Y1 | 2.2 ± 1.7 | 2.0 ± 1.5 | NS | 2.0 ± 1.6 | 2.1 ± 1.5 |
| GH dose (mg/day) | |||||
| At Y1 | 0.4 ± 0.2 | 0.4 ± 0.2 | NS | 0.4 ± 0.2 | 0.3 ± 0.2 |
| QoL-AGHDA (score) | |||||
| At BL | 9.6 ± 7.3 | 10.9 ± 7.7 | NS | 10.4 ± 7.0 | 11.1 ± 6.8 |
| ∆ BL→Y1 | −3.7 ± 5.3 | −3.4 ± 4.9 | NS | −3.3 ± 5.5 | −3.5 ± 6.0 |
| MetS (score) | |||||
| At BL | 1.33 ± 0.80 | 3.15 ± 0.36 | <0.0001 | 1.81 ± 0.57 | 3.66 ± 0.70 |
| ∆ BL→Y1 | −0.01 ± 0.72 | −1.28 ± 0.61 | <0.0001 | +1.48 ± 0.62 | 0.09 ± 0.63 |
| Waist circumference | |||||
| ∆ BL→Y1 (cm) | −1.7 ± 5.5 | −3.1 ± 6.1 | <0.0001 | +1.4 ± 5.9 | −0.9 ± 5.5 |
| ∆ BL→Y1 (score) | 0.59 → 0.53 | 1.00 → 0.00 | N/A | 0.78 → 1.00 | 1.00 → 1.00 |
| Blood pressure | |||||
| Systolic ∆ BL→Y1 (mmHg) | −1.5 ± 14.8 | −2.3 ± 17.0 | 0.03 | +2.1 ± 18.0 | −1.6 ± 17.8 |
| Diastolic ∆ BL→Y1 (mmHg) | −1.0 ± 10.0 | −1.2 ± 11.1 | 0.007 | +1.7 ± 11.8 | −0.5 ± 11.2 |
| ∆ BL→Y1 (score) | 0.18 → 0.17 | 0.41 → 0.27 | <0.0001 | 0.27 → 0.50 | 0.59 → 0.60 |
| HDL cholesterol | |||||
| ∆ BL→Y1 (mmol/L) | 0.0 ± 0.3 | +0.1 ± 0.2 | 0.01 | −0.2 ± 0.3 | 0.0 ± 0.2 |
| ∆ BL→Y1 (score) | 0.18 → 0.21 | 0.65 → 0.34 | <0.0001 | 0.26 → 0.64 | 0.84 → 0.85 |
| Triglycerides | |||||
| ∆ BL→Y1 (mmol/L) | 0.0 ± 0.8 | −0.4 ± 3.2 | <0.0001 | +0.4 ± 1.0 | −0.1 ± 1.5 |
| ∆ BL→Y1 (score) | 0.31 → 0.32 | 0.89 → 0.43 | <0.0001 | 0.41 → 0.80 | 0.93 → 0.91 |
| Glucose | |||||
| ∆ BL→Y1 (mmol/L) | +0.1 ± 1.0 | −0.1 ± 1.2 | 0.03 | +0.3 ± 0.7 | +0.2 ± 1.3 |
| ∆ BL→Y1 (score) | 0.06 → 0.10 | 0.20 → 0.08 | <0.0001 | 0.10 → 0.36 | 0.31 → 0.39 |
MetS at baseline and cardiovascular and cerebrovascular events during prolonged GH replacement
The relative risk to develop cardiovascular events according to the presence of MetS at baseline was assessed in 3282 patients: MetS group 6.8 ± 4.7 year of follow-up and NoMS group 7.8 ± 5.1 year of follow-up. Analysis of the crude incidence rate of new cardiovascular events during GH replacement showed a 66% higher risk in patients with MetS at baseline compared to NoMS patients (8.62 vs 5.19 per 1000 patient-years; relative risk (RR) 1.66; 95% CI, 1.21–2.27). By contrast, the incidence of cerebrovascular events was, although higher, not significantly different between MetS patients and NoMS patients (3.81 vs 3.17 per 1000 patient-years; RR 1.20; 95% CI, 0.78–1.86).
Analysis of the crude incidence rate of cardiovascular events showed a significant adverse impact of waist, blood pressure and glucose, which was confirmed for blood pressure and glucose by regression analysis (Table 2).
Table 2.
Crude incidence rate (CIR, estimate and 95% confidence limits) of cardiovascular and cerebrovascular events per 1000 patient-years during GH replacement in patients with MetS and without MetS (NoMS) at baseline and related to the presence of a specific MetS component with assessment of relative risk (RR).
| CIR estimate (95% CI) | MetS vs NoMS | |||
|---|---|---|---|---|
| NoMS at baseline | MetS at baseline | RR (95% CI) | P | |
| Cardiovascular event | ||||
| MetS | 5.19 (4.11–6.56) | 8.62 (6.98–10.63) | 1.66 (1.21–2.27) | 0.002 |
| Waist | 4.47 (3.02–6.61) | 7.34 (6.19–8.70) | 1.64 (1.07–2.52) | 0.02 |
| Blood pressure | 4.95 (3.97–6.17) | 10.23 (8.19–12.77) | 2.07 (1.51–2.83) | <0.0001 |
| HDL cholesterol | 6.26 (5.04–7.78) | 7.15 (5.71–8.97) | 1.14 (0.84–1.56) | 0.4 |
| Triglycerides | 5.47 (4.18–7.16) | 7.49 (6.18–9.07) | 1.37 (0.98–1.91) | 0.06 |
| Glucose | 6.01 (5.03–7.18) | 10.44 (7.53–14.47) | 1.74 (1.20–2.52) | 0.004 |
| Cerebrovascular event | ||||
| MetS | 3.17 (2.35–4.27) | 3.81 (2.78–5.22) | 1.20 (0.78–1.86) | 0.4 |
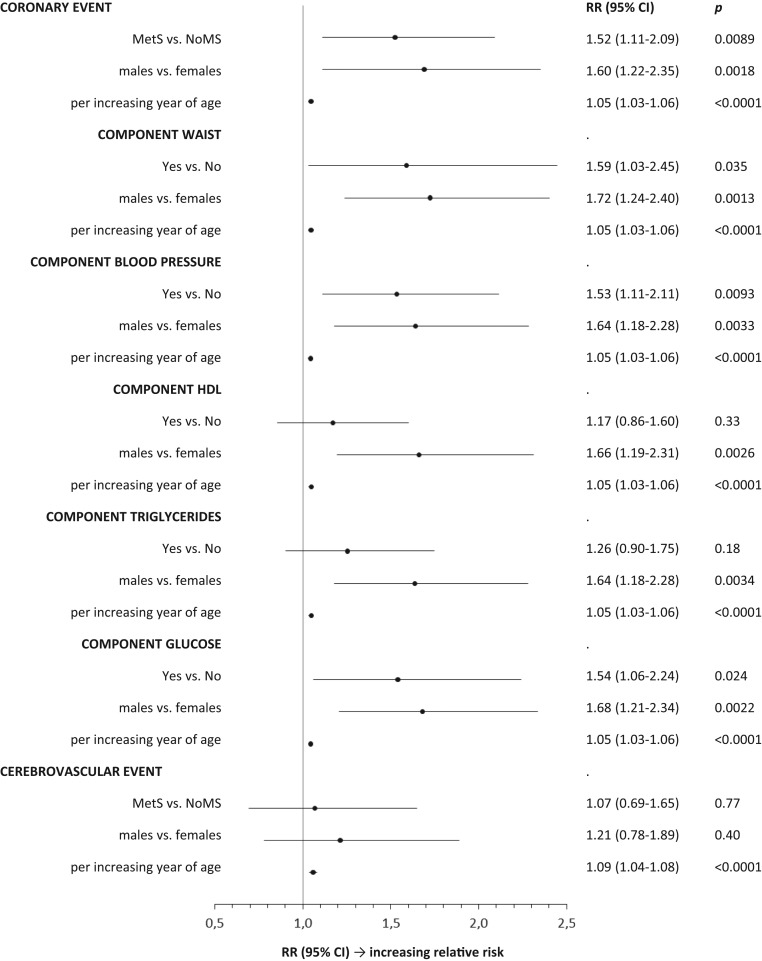
Assessment of the RR to develop a cardiovascular or cerebrovascular event was also determined after adjustment for gender and age (Fig. 5).
Figure 5.
Assessment of the relative risk to develop a cardiovascular or cerebrovascular event after adjustment for gender and age.
Discussion
The current findings analysing the effect of GH replacement on the MetS prevalence in AO-GHD patients do not permit a strict comparison with previous studies in view of different approaches (1, 2, 4). A first premise in the present study was the use of the IDF criteria instead of the NCEP criteria, as it accords a primordial importance to the waist component known to be favourably affected by GH replacement. The baseline data may be compared with an European IDF reference population showing a MetS prevalence 1.30 times higher in males (46.7% vs 35.9%) and 1.37 times higher in females (47.0% vs 34.1%) (8). Another premise was to repeat the analysis after one year since the GH effect on the different components can be considered completed at that time as indicated by the normalization of IFG1 SDS. This choice allowed to include a larger number of patients, also allowing additional analyses. Nonetheless, despite the different methodology, the crude analysis of the present study confirms the fact that GH replacement does not induce an improvement in the prevalence of MetS and is thus in line with previously analysed data using the NCEP criteria over a longer period of follow-up. An overview of the different studies is given in Table 3 which also highlights the dissimilarities between the components.
Table 3.
Overview of literature regarding the effect of GH replacement on the prevalence of MetS in AO-GHD patients and on MetS components.
| Study (reference) | van der Klaauw et al. (1)* | Attanasio et al. (2) | Claessen et al. (4) | Present study |
|---|---|---|---|---|
| MetS definition | NCEP | NCEP | NCEP | IDF |
| Country | Netherlands | Europe and United States | Netherlands | Europe |
| n | 50 | 346 | 160 | 1449 |
| Males (%) | 48.0 | 53.5 | 55.3 | 48.9 |
| Age (years) | 45.2 | 52.7 | 54.7 | 48.9 |
| Follow-up period (years) | 2 | 3 | 5 | 1 |
| BMI (kg/m²) | 26.7 | 30.2 | 27.5 | 29.0 |
| MetS at baseline (%) | 38.0 | 42.5 | 41.0 | 46.9 |
| MetS at end of study (%) | 42.0 | 45.7 | 53.4 | 48.2 |
| MetS end study vs baseline (P-value) | NS | NS | 0.007 | NS |
| Waist circumference (change) | = | ↓ | = | ↓ |
| Blood pressure (change) | = | ↑ | = | = |
| HDL cholesterol (change) | = | = | ↓ in males | ↓ |
| Triglycerides (change) | = | = | = | = |
| Glucose (change) | = | ↑ | ↑ | ↑ |
*Results of 5-year follow-up not included because of overlap with the data of Claessen et al. (4).
Analysis of the separate components, shown in Figs 2 and 3, suggests an explanation for the failure of GH replacement to decrease the MetS prevalence. MetS is mostly determined by the presence of an abnormal waist, which is favourably impacted by GH replacement in both genders and in patients younger than 60 years. However, this effect is abolished by the impaired glucose metabolism in both genders irrespective of age. This adverse effect, which mostly occurs in predisposed patients, has previously been documented and may represent an obstacle for a uniform and safe metabolic control (16, 21). Another factor that prevents a correction in the calculation of the MetS prevalence is the use at baseline of anti-hypertensive, lipid-lowering and anti-diabetic drugs, which probably are not discontinued during GH replacement. These findings question the suitability and the usefulness of establishing the diagnosis of MetS in order to express the outcome of GH replacement.
Another aspect that may influence the evaluation of GH replacement on the MetS prevalence is indicated by the finding, shown in Fig. 4, that patients without MetS are in a sense better GH responders than MetS patients, resulting in significantly more favourable changes for all five components. This behaviour reminds of a comparable observation in which overweight diabetic subjects lose less weight than their overweight nondiabetic spouses on a same weight-control program, a finding which has been confirmed afterwards (22, 23). This dissimilarity can be explained neither by gender, age, baseline component value and drug therapy as statistical analysis had been performed with adjustment for these variables, nor by a difference in GH dose and IGF1 change. Possibly, the prolonged metabolic changes induced by GHD in combination with MetS and a persisting propensity towards obesity are responsible for the relative unresponsiveness.
The comparative analysis of the patients crossing from one MetS status to another yields another remarkable and significant finding. As shown in Table 1, while the therapeutic conditions for the two groups were comparable regarding GH dose and IGF1 response, a marked deterioration in the group developing MetS was observed for each component. Conversely, an amelioration of each component, including impaired glucose metabolism, was observed in the group losing the adverse MetS status. This divergent course can be related to an identical baseline BMI, evolving significantly into opposite direction by gaining or losing about 0.5 kg/m². Also of importance, as shown in Table 1, is that GH replacement succeeded to significantly and similarly improve QoL in the four groups irrespective of an eventual change in MetS status and in parallel with the GH dose and IGF1 increase. The QoL-AGHDA score thus obviously evolves independently of a clinical and biochemical assessment.
The increased incidence of cardiovascular complications in GHD is well documented (10, 11, 12). It was recently reported that GH-unreplaced patients with MetS after adjustment for age, gender and BMI had a significantly higher PR for coronary morbidity (1.91; 95% CI, 1.33–2.75) and for cerebrovascular morbidity (1.77; 95% CI, 1.09–2.87) than patients without MetS (3). The present study indicated that despite a prolonged GH replacement, the presence of MetS at baseline remained associated with a 66% higher incidence rate to develop new coronary events, whereas no increased incidence was found for cerebrovascular events. This finding possibly clarifies the perception that GH replacement on its own is not sufficient to normalize the enhanced cardiovascular risk induced by a longstanding GHD and that other therapeutic measures are required (3).
In conclusion, in the absence of prospective randomized trials, data from KIMS and a recent epidemiological study suggest that GH replacement is able to correct the increased mortality associated with GHD (17, 24). On the other hand, the present analysis using a surveillance database with its known limitations describes the absence of effect on the MetS status after one year of GH replacement and an elevated cardiovascular morbidity after seven years of follow-up (25). Possible explanations to be mentioned for this discrepancy are inadequate MetS criteria for monitoring and irredeemable metabolic damage after a too longstanding GHD. However, the present data also suggest that the model of GHD may consist of two independently evolving factors. The first one, QoL, is restored almost to normality independently of any condition. The other one, metabolic disruption, could be prevented by timely diagnosis of GHD and treated by adequate GH replacement, but it will also require a lifestyle intervention, physical activity and especially weight reduction as indicated by the cross-over patients in this study.
Declaration of interest
Johan Verhelst has received honoraria for lectures and/or advisory boards from Pfizer, Ipsen, Novartis. Anders Mattsson and Cecilia Camacho-Hübner are employed by Pfizer, Inc. All statistical analyses were performed by a statistician (Anders Mattsson). Anton Luger has received honoraria for lectures and/or advisory boards from Pfizer, Ipsen, Novo Nordisk and Eli Lilly and unrestricted grants to the Division of Endocrinology and Metabolism, Medical University of Vienna, Austria from Pfizer, Novo Nordisk and Ipsen. Roger Abs is a member of the KIMS Steering Committee. He has received grants from Pfizer, Novartis, Ipsen.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. The KIMS database is sponsored by Pfizer Inc.
References
- 1.van der Klaauw AA, Biermasz NR, Feskens EJ, Bos MB, Smit JW, Roelfsema F, Corssmit EP, Pijl H, Romijn JA, Pereira AM. The prevalence of the metabolic syndrome is increased in patients with GH deficiency, irrespective of long-term substitution with recombinant human GH. European Journal of Endocrinology 2007. 156 455–462. ( 10.1530/EJE-06-0699) [DOI] [PubMed] [Google Scholar]
- 2.Attanasio AF, Mo D, Erfurth EM, Tan M, Ho KY, Kleinberg D, Zimmermann AG, Chanson P. Prevalence of metabolic syndrome in adult hypopituitary growth hormone (GH)-deficient patients before and after GH replacement. Journal of Clinical Endocrinology and Metabolism 2010. 95 74–81. ( 10.1210/jc.2009-1326) [DOI] [PubMed] [Google Scholar]
- 3.Verhelst J, Mattsson AF, Luger A, Thunander M, Goth MI, Kołtowska-Häggström M, Abs R. Prevalence and characteristics of the metabolic syndrome in 2479 hypopituitary patients with adult-onset GH deficiency before GH replacement: a KIMS analysis. European Journal of Endocrinology 2011. 165 881–889. ( 10.1530/EJE-11-0599) [DOI] [PubMed] [Google Scholar]
- 4.Claessen KM, Appelman-Dijkstra NM, Adoptie DM, Roelfsema F, Smit JW, Biermasz NR, Pereira AM. Metabolic profile in growth hormone-deficient (GHD) adults after long-term recombinant human growth hormone (rhGH) therapy. Journal of Clinical Endocrinology and Metabolism 2013. 98 352–361. ( 10.1210/jc.2012-2940) [DOI] [PubMed] [Google Scholar]
- 5.Gallagher EJ, Leroith D, Karnieli E. The metabolic syndrome – from insulin resistance to obesity and diabetes. Medical Clinics of North America 2011. 95 855–873. ( 10.1016/j.mcna.2011.06.001) [DOI] [PubMed] [Google Scholar]
- 6.Leroith D. Pathophysiology of the metabolic syndrome: implications for the cardiometabolic risks associated with type 2 diabetes. American Journal of the Medical Sciences 2012. 343 13–16. ( 10.1097/MAJ.0b013e31823ea214) [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Detection, Evaluation, and Treatment of High Blood Clolesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001. 285 2486–2497. ( 10.1001/jama.285.19.2486) [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabetic Medicine 2006. 23 469–480. ( 10.1111/j.1464-5491.2006.01858.x) [DOI] [PubMed] [Google Scholar]
- 9.Qiao Q. & DECODE Study Group. Comparison of different definitions of the metabolic syndrome in relation to cardiovascular mortality in European men and women. Diabetologia 2006. 49 2837–2846. ( 10.1007/s00125-006-0438-6) [DOI] [PubMed] [Google Scholar]
- 10.Rosen T, Bengtsson BÅ. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet 1990. 336 285–288. ( 10.1016/0140-6736(90)91812-O) [DOI] [PubMed] [Google Scholar]
- 11.Bülow B, Hagmar L, Mikoczy Z, Nordström CH, Erfurth EM. Increased cerebrovascular mortality in patients with hypopituitarism. Clinical Endocrinology 1997. 46 75–81. ( 10.1046/j.1365-2265.1997) [DOI] [PubMed] [Google Scholar]
- 12.Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, Sheppard MC, Stewart PM. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet 2001. 357 425–431. ( 10.1016/S0140-6736(00)04006-X) [DOI] [PubMed] [Google Scholar]
- 13.Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a meta-analysis of blinded, randomized, placebo-controlled trials. Journal of Clinical Endocrinology and Metabolism 2004. 89 2192–2199. ( 10.1210/jc.2003-030840) [DOI] [PubMed] [Google Scholar]
- 14.Götherström G, Bengtsson BA, Bosaeus I, Johannsson G, Svensson J. A 10-year, prospective study of the metabolic effects of growth hormone replacement in adults. Journal of Clinical Endocrinology and Metabolism 2007. 92 1442–1445. ( 10.1210/jc.2006-1487) [DOI] [PubMed] [Google Scholar]
- 15.Abs R, Feldt-Rasmussen U, Mattsson AF, Monson JP, Bengtsson BÅ, Góth MI, Wilton P, Kołtowska-Häggström M. Determinants of cardiovascular risk in 2589 hypopituitary GH-deficient adults – a KIMS database analysis. European Journal of Endocrinology 2006. 155 79–90. ( 10.1530/eje.1.02179) [DOI] [PubMed] [Google Scholar]
- 16.Luger A, Mattsson AF, Kołtowska-Häggström M, Thunander M, Góth M, Verhelst J, Abs R. Incidence of diabetes mellitus and evolution of glucose parameters in growth hormone-deficient subjects during growth hormone replacement therapy: a long-term observational study. Diabetes Care 2012. 35 57–62. ( 10.2337/dc11-0449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaillard RC, Mattsson AF, Åkerblad AC, Bengtsson BÅ, Cara J, Feldt-Rasmussen U, Kołtowska-Häggström M, Monson JP, Saller B, Wilton P, et al Overall and cause-specific mortality in GH-deficient adults on GH replacement. European Journal of Endocrinology 2012. 166 1069–1077. ( 10.1530/EJE-11-1028) [DOI] [PubMed] [Google Scholar]
- 18.Riis P. Thirty years of bioethics: the Helsinki declaration 1964–2003. New Review of Bioethics 2003. 1 15–25. ( 10.1080/1740028032000131396) [DOI] [PubMed] [Google Scholar]
- 19.McKenna SP, Doward LC, Alonso J, Kohlmann T, Niero M, Prieto L, Wirén L. The QoL-AGHDA: an instrument for the assessment of quality of life in adults with growth hormone deficiency. Quality of Life Research 1999. 8 373–383. ( 10.1023/A:1008987922774) [DOI] [PubMed] [Google Scholar]
- 20.International Diabetes Federation, Clinical Guidelines Task Force. Screening and diagnosis. In Global Guideline for Type 2 Diabetes, pp 9–14. Brussels, Belgium: IDF, 2012.. (available at: https://www.iapb.org/wp-content/uploads/Global-Guideline-for-Type-2-Diabetes-IDF-2012.pdf) [Google Scholar]
- 21.Abs R, Mattsson AF, Thunander M, Verhelst J, Góth MI, Wilton P, Kołtowska-Häggström M, Luger A. Prevalence of diabetes mellitus in 6050 hypopituitary patients with adult-onset GH deficiency before GH replacement: a KIMS analysis. European Journal of Endocrinology 2013. 168 297–305. ( 10.1530/EJE-12-0807) [DOI] [PubMed] [Google Scholar]
- 22.Wing RR, Marcus MD, Epstein LH, Salata R. Type II diabetic subjects lose less weight than their overweight nondiabetic spouses. Diabetes Care 1987. 10 563–566. ( 10.2337/diacare.10.5.563) [DOI] [PubMed] [Google Scholar]
- 23.Guare JC, Wing RR, Grant A. Comparison of obese NIDDM and nondiabetic women: short- and long-term weight loss. Obesity Research 1995. 3 329–335. ( 10.1002/j.1550-8528.1995.tb00158.x) [DOI] [PubMed] [Google Scholar]
- 24.Olsson DS, Trimpou P, Hallén T, Bryngelsson IL, Andersson E, Skoglund T, Bengtsson BÅ, Johannsson G, Nilsson AG. Life expectancy in patients with pituitary adenoma receiving growth hormone replacement. European Journal of Endocrinology 2017. 176 67–75. ( 10.1530/EJE-16-0450) [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez LP, Kołtowska-Häggström M, Jönsson PJ, Mattsson AF, Svensson D, Westberg B, Luger A. Registries as a tool in evidence-based medicine: example of KIMS (Pfizer International Metabolic Database). Pharmacoepidemiology and Drug Safety 2008. 17 90–102. ( 10.1002/pds.1510) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a