Abstract
Background:
Autism spectrum disorder is a neurodevelopmental disorder. It is believed that the cause of autism is multifactorial, where genetic predispositions interact with environmental factors. In this context, micronutrients play a crucial role.
Objective:
To present evidence on current micronutrient status in Palestine and highlight its possible role in increasing problems of neurodevelopment disorders in general and autism in particular.
Method:
Analytical review of results.
Results And Conclusions:
The Palestinian Micronutrient Survey was conducted to assess micronutrient status in most vulnerable groups and also micronutrient deficiencies. The data from Palestinian population showed severe anaemia due to iron, Zn, B12, and folic acid deficiencies. One in every 3 Palestinian pregnant women is anaemic. Moreover, 78.2% and 87.1% of lactating mothers from the West Bank and Gaza Strip are Zn deficient. For children from 6 to 59 months old, 1 in every 4 boys and 1 in every 5 girls are considered anaemic. Similar trend was found with respect to vitamins E, D, A, and folic acid. We reviewed the literature that linked micronutrient deficiencies to neurodevelopmental disorders and expected the number of neurodevelopmental disorder cases, including autism, to increase.
Keywords: Palestinian Micronutrient Survey (PMS), neurodevelopmental disorders, anaemia, autism
Introduction
The frequency of autism spectrum disorder (ASD) diagnoses has been increasing for decades. Approximately 1 in 68 children aged 8 years living in sites participating in the ADDM Network surveillance areas (USA) met the ASD case criteria for the 2012 surveillance year.1 Some hypotheses were suggested to explain this increase, such as an increased awareness, improved detection, expanding definition, or an actual increase in incidence or a combination of these factors.2 Although both genetic and multiple environmental risk factors have been studied extensively, many potentially modifiable risk factors including nutritional and immune function–related risk factors such as vitamin D, folic acid, and metabolic syndrome have not received sufficient attention.3 Several recent studies have put forward hypotheses to explain the mechanism of association between both folic acid and vitamin D and autism. A continuous rise in the prevalence of autism in many countries around the world has coincided with a significant enhancement of maternal folate status. There is also a growing body of research that suggests that vitamin D status either in utero or early in life may be a risk for autism.
The aim of this study is to highlight the low nutritional status, presented by the latest Palestinian survey, of the Palestinian mothers and children as a risk factor of neurodevelopmental disorders and/or autism prevalence.
Palestinian Micronutrient Survey
Nutrition is one programme in primary health care and public health programmes in the Ministry of Health in Palestine. Its services expand to cover both Clinical Nutrition and Public Nutrition. Micronutrient deficiency prevention in Palestine is achieved through micronutrient supplementation, flour fortification, salt iodisation, and promotion of diet diversification.
Micronutrient supplementation includes folic acid tablets 3 months before pregnancy and 3 months postnatal, vitamin A and D drops for infant starting from birth until 12 months, and iron and folic acid tablets for pregnant women 3 months after conception until 3 months postnatal. These actions are expected to reduce the risk of micronutrient deficiency and anaemia.
Flour fortification is a public nutrition programme since 2006 that is supposed to reach most communities. For this purpose, 10 micronutrients are added to the flour, including B1, B2, B6, B12, folic acid, niacin, iron, zinc, vitamin A, and vitamin D.4
Despite all the above-mentioned actions, anaemia prevalence is still high among pregnant women, lactating mothers, and children less than 5 years of age. The Ministry of Health decided to conduct a Palestinian Micronutrient Survey (PMS) to assess the Palestinian micronutrient profile, to explore the causes of anaemia, and to provide an adequate and valid nutritional information allowing to evaluate the current intervention strategies and supporting the decision making process in preventing micronutrient deficiencies and improving and optimising infant and young child feeding practices.4
Methodology
The PMS is an ambitious project that included 664 clinics and schools, 6000 questionnaires, 10 635 samples, 1 172 640 data entry figures, 550 staff, 50 training sessions, and 147 345 tests, using more than 50 nutrition and health indicators.4 Targeted groups and distribution of groups, number of questionnaires, number of samples, and number of tests are shown in Table 1.
Table 1.
Targeted groups and distribution of groups, number of questionnaires, number of samples, and number of tests including total cost of the project.
| Target group | Schools or clinics | Questionnaires | Samples | Data entry figures | Team members | Training sessions | Tests | Cost (US$) million |
|---|---|---|---|---|---|---|---|---|
| Primary school | 88 | / | 1200 | 8400 | 550 | 50 | 3600 | |
| Adolescents | 160 | 2400 | 2400 | 391 200 | 55 200 | |||
| Pregnant | 80 | 1200 | 1200 | 229 200 | 27 600 | |||
| Lactating | 80 | 1200 | 1200 | 246 000 | 27 600 | 1.6 | ||
| Children 6-59 mo | 80 | 1200 | 1200 | 238 800 | 27 600 | |||
| Table salt | 88 | / | 1200 | 3600 | 2400 | |||
| Flour | / | / | 555 | 50 400 | 1665 | |||
| Bread | 88 | / | 1680 | 5040 | 1680 | |||
| Total | 664 | 6000 | 10 635 | 117 2640 | 550 | 50 | 147 345 | 1.6 |
Samples of blood serum, blood plasma, and 24-hour urine were taken, refrigerated, and kept at −20 to −80°C until analysis. Bread, table salt, and flour from homes and bakeries were transferred at ambient temperature and then frozen until further analysis. Four types of questionnaires were used, one for each targeted group. The nature of data collected was identification (name, gender, residency), personal, social, socioeconomic, micronutrient supplements, health and nutrition education and knowledge, health service, smoking, physical activity, eating habits, food frequency, and anthropometric (weight and height). A software was developed by the Nutrition Department (Ministry of Health) for data entry and data cleaning.
Further analysis was conducted using SPSS 22 (International Business Machines Corp, USA) WHO Anthro 3.2.2 and WHO Anthro Plus 1.0.4 for statistical, anthropometric calculator, individual assessment, and nutritional survey.
Main Findings of PMS
Anaemia-related micronutrients
Pregnant women
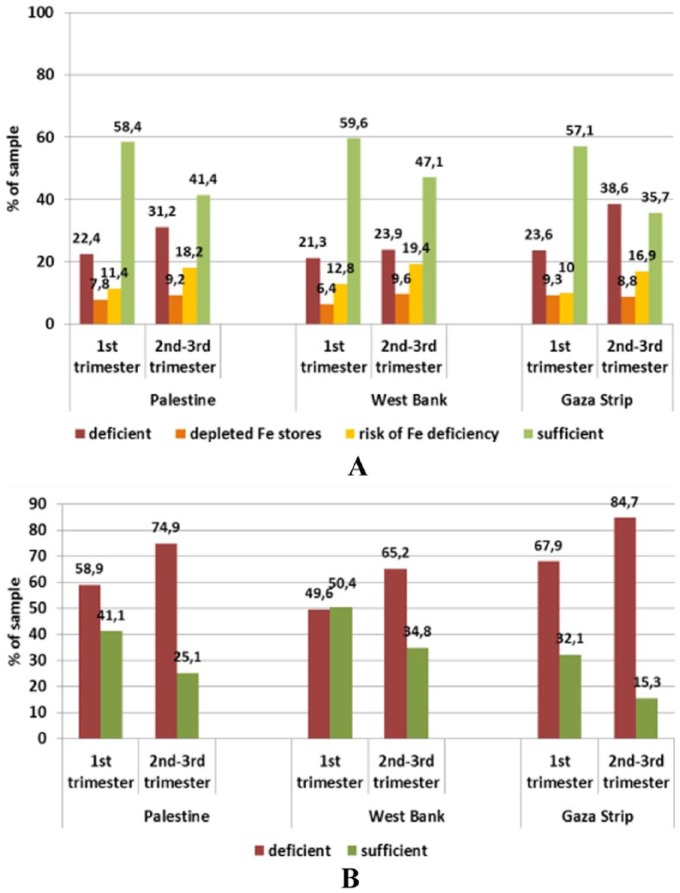
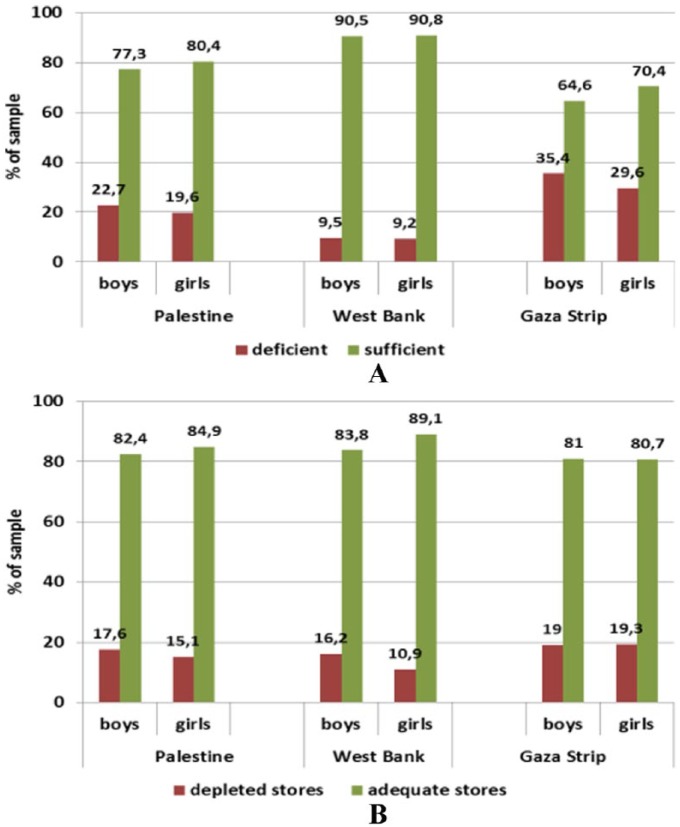
The results showed that anaemia in Palestine hit 1 in every 3 pregnant women. Iron-, zinc-, folate-, and B12-deficient women accounted to 17.1%, 71.1%, 7.4%, and 62%, respectively (Figures 1 and 2). Although all these micronutrient deficiencies interact to cause anaemia, it appears that zinc and B12 are the major limiting factors that promote anaemia. Biomarkers of iron status were on average within reference ranges in pregnant women in the first as well as the second and third trimesters in both provinces. However, there was a trend towards a lower status in later compared with earlier pregnancy, particularly in the Gaza Strip. Accordingly, ferritin levels suggested iron deficiency (ID) in 21.3% and 23.9% of women in the first and the second-third trimester, respectively, from the West Bank and in 23.6% of women in the first trimester from the Gaza Strip compared to 38.6% of women in the second-third trimester from the Gaza Strip. This prevalence was significantly higher than in the other tested population groups (P < .001).
Figure 1.
Status of (A) iron and (B) zinc in pregnant women (18-43 years) by trimester and province assessed through serum ferritin and zinc levels, respectively.
Reference levels – ferritin: deficient <12 ng/mL, depleted Fe stores <15 ng/mL, sufficient >20 ng/mL; zinc deficiency: first trimester <8.6 μmol/L, second-third trimester <7.6 μmol/L.
Figure 2.
Status of (A) folic acid and (B) vitamin B12 in pregnant women (18-43 years) by trimester and province assessed through serum levels.
Reference levels – folic acid: deficient <6.8 nmol/L, low <13.4 nmol/L, sufficient >13.4 nmol/L; vitamin B12: deficient <110 pmol/L, low <147 pmol/L, sufficient >147 pmol/L.
Lactating women
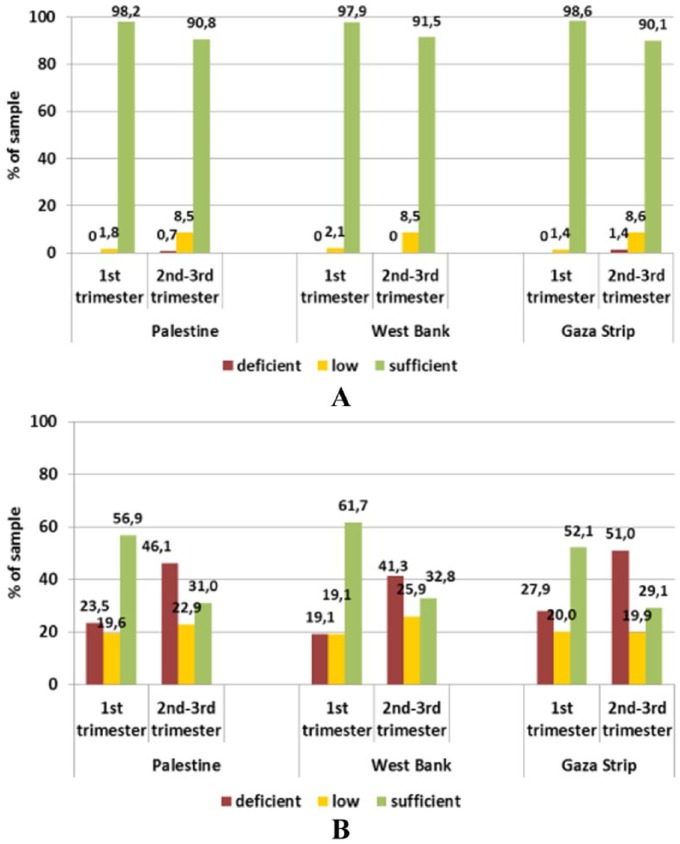
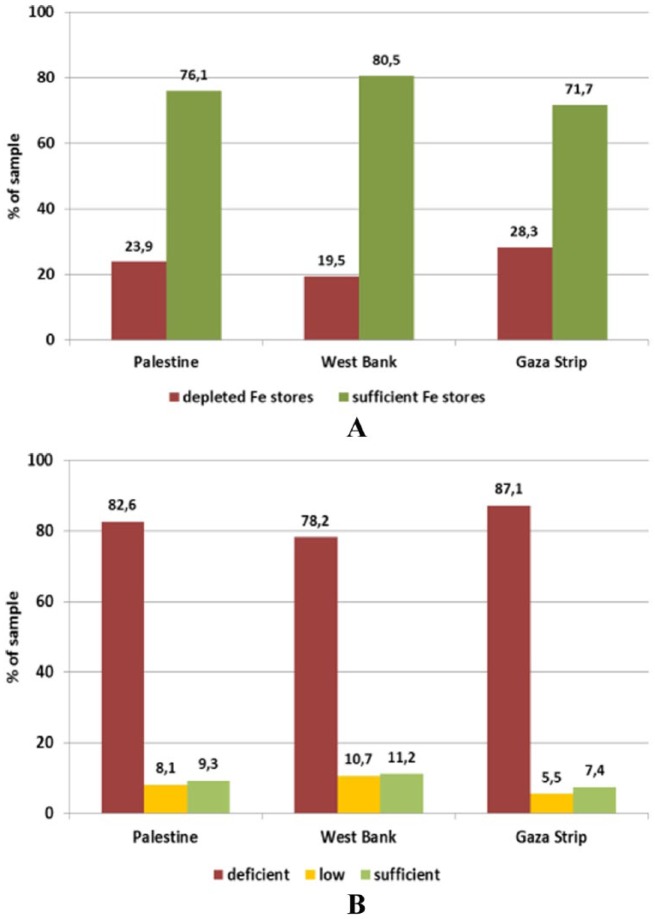
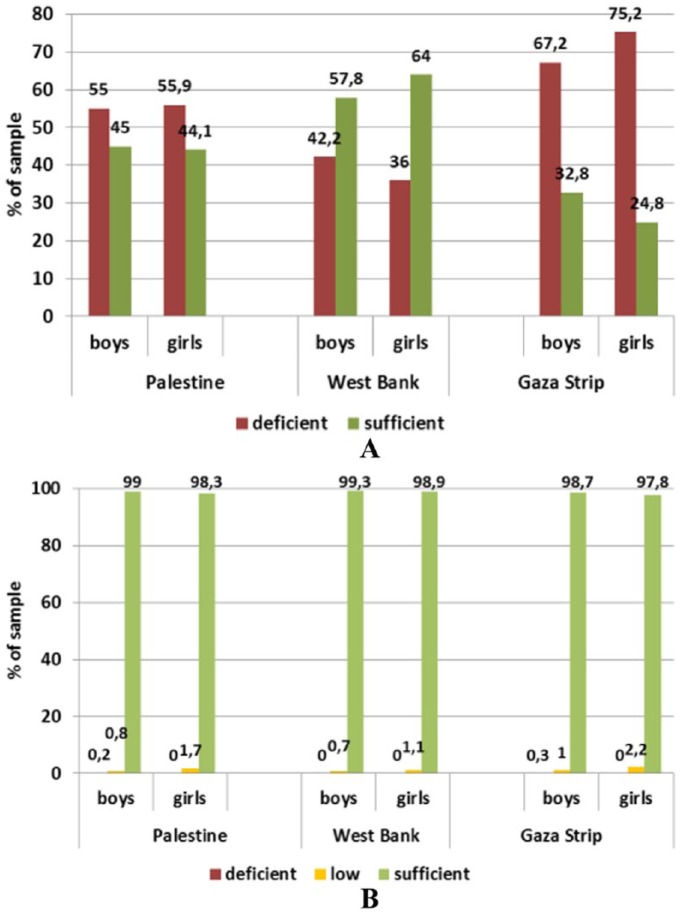
The results showed similar trends (Figures 3 and 4) in the number of anaemic women as an anaemic pregnant woman is more likely to be anaemic at the time of lactation. However, the major influential micronutrients during lactation were zinc and folate. Markers of iron status were on average within the respective reference ranges. Based on ferritin concentrations, iron stores can be considered sufficient in 80.5% and 71.7% of women from the West Bank and Gaza Strip, leaving 20% and 28%, respectively, with depleted stores (χ2(1) = 12.406; P = .001 between provinces). We expect that depletion of these micronutrients in such a period has neither been compensated by dietary intake nor by supplementation, especially for Zn. Although human milk is a poor source of iron and B12, maternal supplementation with vitamin B12 during lactation may be too late to restore adequate milk concentrations. Zinc status was shown to be critical with mean serum concentrations below the reference level in both provinces. Accordingly, 78.2% and 87.1% of lactating mothers from the West Bank and Gaza Strip had a deficient status, whereas only 11.2% and 7.4%, respectively, had a sufficient status – the differences between the provinces being statistically significant (χ2(2) = 17.097; P < .001).
Figure 3.
Status of (A) iron and (B) zinc in lactating women (18-48 years) by province assessed through serum ferritin and zinc levels, respectively.
Reference levels – ferritin: depleted Fe stores <15 ng/mL (WHO, 2001); zinc: deficient <11.5 μmol/L, low 11.5-13 μmol/L, sufficient 13-23 μmol/L, elevated >23 μmol/L.
Figure 4.
Status of (A) folic acid and (B) vitamin B12 in lactating women (18-48 years) by province assessed through serum levels.
Reference levels – folic acid: deficient <6.8 nmol/L, low <13.4 nmol/L, sufficient >13.4 nmol/L; vitamin B12: deficient <110 pmol/L, low <147 pmol/L, sufficient >147 pmol/L.
Children aged 6 to 59 months
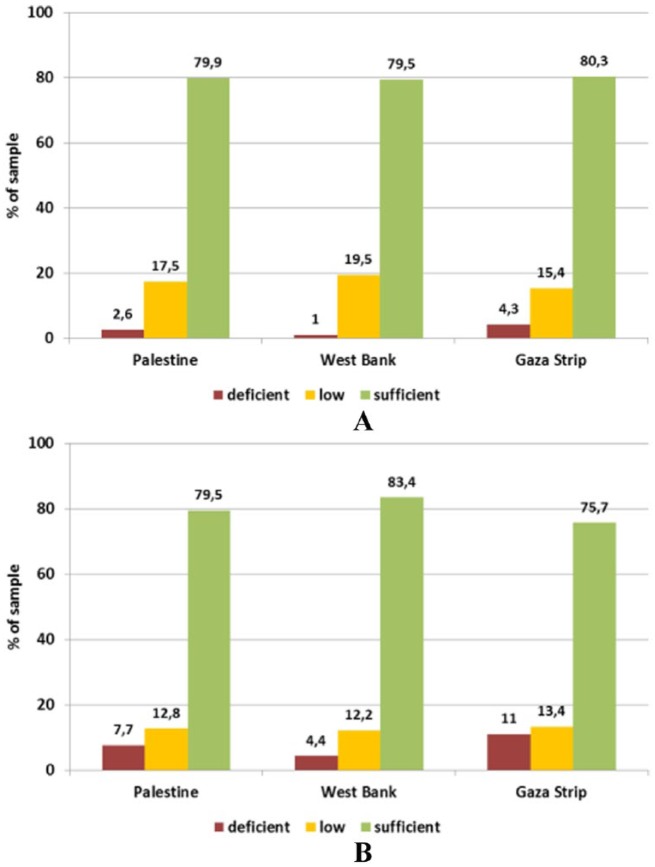
Generally speaking, anaemia prevalence among this age group was a reflection of their mothers’ status of micronutrients. One in every 4 boys and 1 in every 5 girls are considered anaemic. Iron and Zn deficiency played a major role in this type of anaemia, whereas the effect of other micronutrients has diminished; this could be due to the type of food taken by the children and/or the high demand on the iron requirement in this age. It is worthy to note that biomarkers of iron status showed a satisfactory picture. However, ferritin concentrations were below the reference level of 12 ng/mL in 16.4% of the children, with a higher prevalence in the Gaza Strip where 19% of children had depleted stores compared with about 13.7% in the West Bank. However, the difference between the provinces was statistically significant only in girls (χ2(1) = 7.392, P = .007). In the West Bank, boys were more affected than girls, whereas no sex difference was observed in the Gaza Strip (Figure 5). More than half of the children in this group are Zn deficient. Zinc status assessed through the serum level was on average above the reference level (9.9 μmol/L) only in children from the West Bank. In the Gaza Strip, 67.2% of boys and 75.2% of girls had serum zinc levels below the sufficiency threshold compared with 42.2% and 36.0%, respectively, in the West Bank (Figure 6).
Figure 5.
Iron status in children under 5 years (6-59 months) assessed through (A) serum iron levels and (B) serum ferritin levels by sex and province.
Reference levels: serum iron: <2 years ≥30 μg/dL (5.37 μmol/L); 2-5 years ≥ 40 μg/dL (7.16 μmol/L); serum ferritin: >12 ng/mL.
Figure 6.
Status of (A) zinc and (B) folic acid in children under 5 years (6-59 months) by sex and province assessed through serum levels.
Reference levels: zinc: >9.9 μmol/L; folic acid: deficient <6.8 nmol/L, low <13.4 nmol/L, sufficient >13.4 nmol/L.
As mentioned in the ‘Discussion’ section, late foetal/early neonatal period and late infancy/toddler period (6-36 months) are the times when children are more likely to be iron deficient. Moreover, children born to iron- and Zn-deficient women are struggling to normalise their levels during the first year of life.
Immunity-related micronutrients
Vitamins E and D
Pregnant women
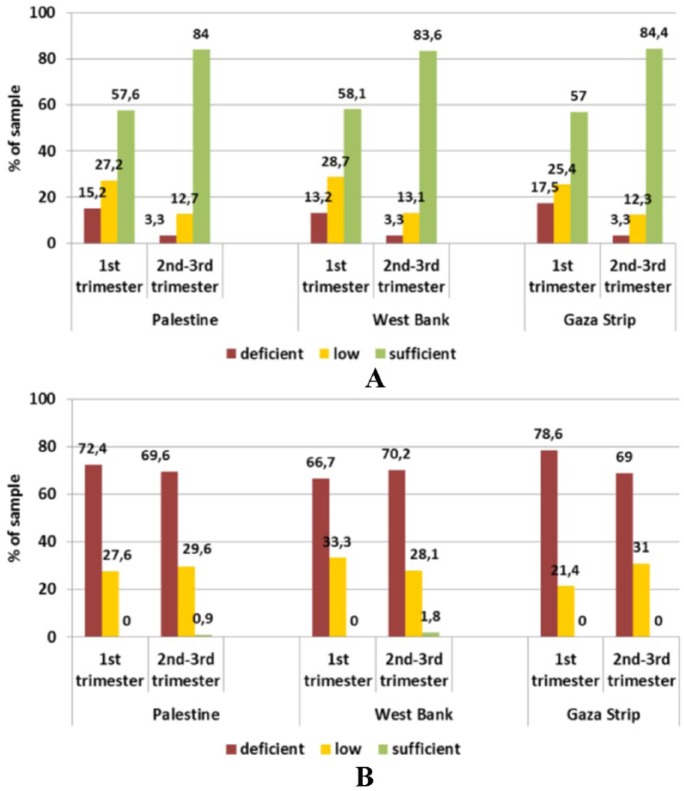
Almost 1 in every 5 women had vitamin E deficiency. Vitamin E is an important antioxidant and plays a major role in the protection and development of the embryo nervous system. Vitamin E status was sufficient in 58% and 57% of women in the first trimester in the West Bank and the Gaza Strip, and this percentage increased to 83% and 84%, respectively, for the second and third trimesters. In this latter group, deficient levels were found in only about 3% of women from both provinces. Thus, vitamin E status can be considered as quite satisfactory. An unsatisfying status was also observed for vitamin D whose mean serum level did not meet the reference value in any of the sub-groups, with more than two-thirds of the women being deficient. Women in their first trimester from the Gaza Strip were less well supplied, with 78.6% having deficient status compared with 66.7% in the West Bank. In turn, this difference between the provinces was not seen in women in their second and third trimesters (70.2% vs 69.0%). Moreover, no significant differences for any of the fat-soluble vitamin status were found between provinces (Figure 7).
Figure 7.
Status of (A) vitamin E and (B) vitamin D in pregnant women (18-43 years) by trimester and province assessed through serum levels.
Reference levels – vitamin D: deficient <25 nmol/L, low <50 nmol/L, sufficient >50 nmol/L; vitamin E: deficient <11.6 μmol/L, low <16.2 μmol/L, sufficient >16.2 μmol/L.
Lactating women
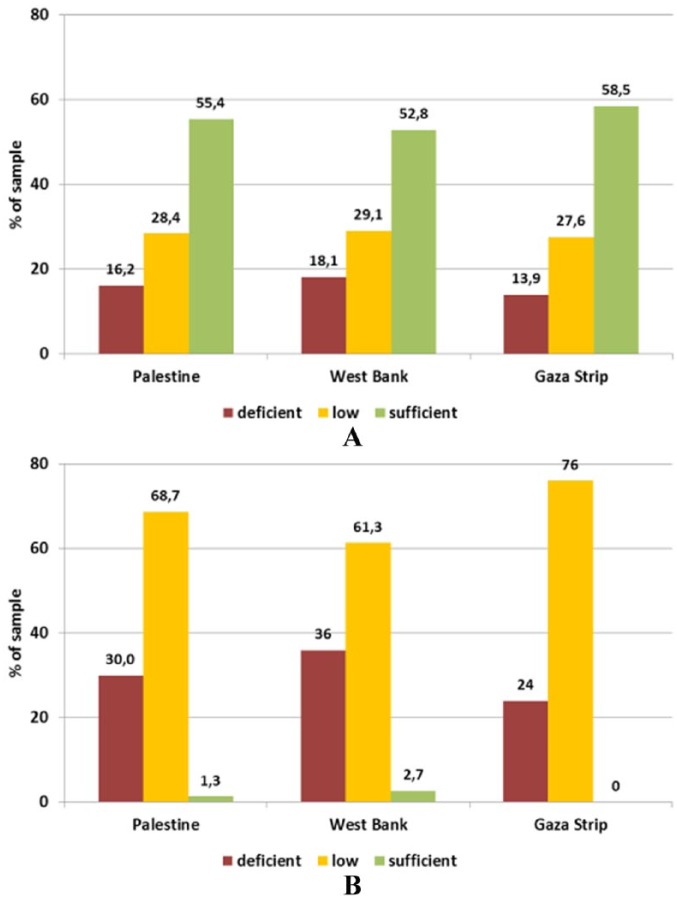
The average level of vitamin E was adequate. However, half of the subjects are still within low to deficient level. The status of vitamin E was better in lactating women from the Gaza Strip, although not significant. Vitamin D was on average above the threshold for deficiency (25 nmol/L) but still below the desirable level of 50 nmol/L. This level was achieved only by about 2.7% of the lactating women from the West Bank and none in the Gaza Strip, whereas 36% and 24% of lactating women from the respective provinces were vitamin D deficient. No significant differences were found between the provinces (Figure 8).
Figure 8.
Status of (A) vitamin E and (B) vitamin D in lactating women (18-48 years) by province assessed through serum levels.
Reference levels – vitamin D: deficient <25 nmol/L, low <50 nmol/L, sufficient >50 nmol/L; vitamin E: deficient <11.6 μmol/L, low <16.2 μmol/L, sufficient >16.2 μmol/L.
Children aged 6 to 59 months
As a result of the low quality of food the mothers of the same household were taking, there was an accumulating deficiency of vitamin E. It is more likely that children of vitamin-deficient women due to nutritional reasons are also well deficient in the same vitamins, although the severity is higher. More than 40% of girls from the West Bank and boys from both provinces had sufficient serum levels of vitamin D (>50 nmol/L 25-OH-D3), but this was only true for 21.9% of girls from Gaza Strip. In the total sample, low levels (<50 nmol/L) were encountered in 60.5% of children, though. Deficient status (<25 nmol/L) was measured in 6.1% of children of both sexes from Palestine, with the highest level in boys from the West Bank (11.1%; Figure 9).
Figure 9.
Status of (A) vitamin E and (B) vitamin D status in children (6-59 months) by sex and province assessed through plasma levels.
Reference levels – vitamin D: deficient <25 nmol/L, low <50 nmol/L, sufficient >50 nmol/L; vitamin E: deficient <11.6 μmol/L, low <16.2 μmol/L, sufficient >16.2 μmol/L.
Discussion
In this section, we will review the literature linking micronutrients under discussion to neurodevelopmental disorders in general and autism in particular.
Anaemia and intellectual abilities
Iron, folate, vitamin B12, vitamin A, zinc, and other micronutrient deficiencies can contribute to anaemia.5 Iron-deficiency anaemia is a major public health nutritional problem affecting people of all ages. Across the globe, 1.62 billion people are anaemic (24.8%), with the highest prevalence among preschool-age children (47.4%) and pregnant women (41.8%). Maternal anaemia is one of the important factors which determines the pregnancy outcome and is responsible for increased incidence of premature births, low birth weight, and high perinatal mortality.6 Moreover, it was reported as early as the thirties of last century that babies born for iron-deficient women, although exhibiting normal iron profile, had failed to maintain normal haemoglobin level during the first year of life.7 This may indicate that physiological abnormalities have occurred and became irreversible. An organ of interest in this context is the brain. The developing brain is a highly metabolic organ, consuming 50% of the energy used by the baby. While all nutrients are important for brain development, certain nutrients (eg, protein, long-chain polyunsaturated fatty acids, iron, copper, zinc, iodine, folate, choline, and vitamins A, B6, and B12) have particularly large effects early in life and exhibit critical or sensitive periods for neurodevelopment.8 All highly metabolic, rapidly developing organs have high iron requirements because iron supports fundamental metabolic processes.9 Early-life ID is particularly detrimental as this is a period of rapid neurodevelopment. Timing is crucial in this scenario. As embryo development is genetically timed, deficiency of a certain nutrient at the time of an important process will cause a detrimental effect even if this nutrient becomes available afterward. As a result, it is likely that the greatest effects of deficiency will occur in brain regions that are rapidly developing. As the brain regions work in harmony, an insult in one region will definitely affect other regions which work together to optimise behavioural functioning. Thus, insults to one brain region may negatively affect development in other later-developing regions.9
The late foetal/early neonatal period and late infancy/toddler period (6-36 months) are the times when children are more likely to be iron deficient. The neurobehavioral effects of deficiencies in those time periods are a function of which iron-dependent processes are most active. Three fundamental iron-dependent systems are rapidly developing from mid-late gestation through the first 3 postnatal years: monoamine (eg, dopamine) neurotransmission, myelination, and the hippocampus. Thus, it is not surprising that behavioural studies of children who are iron deficient10,11 during that time period show that they have abnormalities in mood/affect, speed of processing, learning, and memory, respectively.10 Moreover, the long-term effect of early-life ID was estimated to cause a loss of 10 IQ points.9 The brain’s potential for recovery from early damage has been widely studied.12 In the case of brain alterations caused by nutrient deficiency, recovery is plausible if nutrients become available during the time that the affected growth process is still occurring. In addition to nutrient repletion, enhanced sensory, linguistic, and social interactions may also facilitate recovery.12 Surkan et al13 reported that after 1 year, neither zinc nor iron-folic acid supplementation (10 mg zinc, 12.5 mg iron, and 50 µg folic acid, or a combination of 10 mg zinc, 12.5 mg iron, and 50 µg folic acid) in Nepali children improved attainment of motor or language milestones.
Association between maternal intake of iron and autism
Poor iron status is more prevalent in children with ASD and does not necessarily correlate with low iron intake, suggesting that these children could absorb and/or metabolise iron less efficiently.14 This was explained by low level of maternal iron. Consequences of low level of maternal iron have been associated with high risk of autism. Schmidt et al14 found that the highest iron intake during the index period (pregnancy and breastfeeding) was associated with reduced ASD risk compared with the lowest. Furthermore, they estimated that low iron intake significantly interacted with advanced maternal age and metabolic conditions have increased the risk by 5-fold.15
In animal model, effect of ID of mother rats accompanied with prenatal immune activation was more detrimental on offspring ability of learning and changes in metabolic biomarkers.16 It was reported that problems with immune system (brain-reactive antibodies) are more prevalent in mothers of autistic children compared with mothers of typically developed children.17
In Palestine, the only one study that addresses nutritional status of autistic children was conducted by Al-Ali et al.18 Their hypothesis was based on association between ASD and ID in children diagnosed with ASD in the northern west bank. Iron deficiency was detected in 20% (N = 6 of 30) of autistic children based on serum ferritin (SF) level (SF < 10 μ/L), compared with 0% for the 2 control groups (P = .0001).18 The study did not link ID with autism severity, nor showed the level of iron in the blood of corresponding mothers, as these may link ID as a autism risk factor.In the same context, researchers from Taiwan and the United Kingdom19 analysed 25 articles to see whether ID is a characteristic of ASD children. They found that results were inconsistent; moreover, they found that peripheral iron levels were not significantly different between the ASD and non-ASD groups, including SF or hair iron. Also, no significant difference in the quantity of iron in food content between both groups was found.19
Zinc association with autism
Zinc deficiency during pregnancy and during lactation has been shown to impair cognitive function and motor activity in offspring in the animal model.20 Zinc deficiency may affect cognitive development by alterations in attention, activity, neuropsychological behaviour, and motor development. The exact mechanisms are not clear. Evidence from human studies is limited. Low maternal intakes of zinc during pregnancy and lactation were found to be associated with less focused attention in neonates and decreased motor functions at 6 months of age.21 Healthy infants are usually able to get an adequate amount of zinc from breastfeeding during the first 6 months of life, as long as the mother’s milk supply is adequate and breast milk is not displaced by complementary foods.22 During the second 6 months of life when complementary foods are introduced, the risk for zinc deficiency increases because most traditional complementary foods are low in bioavailable zinc.23 Several studies have suggested a disturbance in the copper (Cu) and zinc (Zn) metabolism in ASDs.24 Zinc deficiency, excess Cu levels, and low Zn/Cu ratio are common in children diagnosed with ASD.
Association between folic acid and autism
Folic acid is essential for cell replication during periods of growth such as pregnancy, particularly during early prenatal periods. Supplementation with folic acid during the time of conception (perinatal period) reduces the risk of neural tube defects in offspring.25 This protective effect led to mandatory fortification of flour and cereal products in several countries,26 including Palestine.
In a large study sample of 85 176 derived from the population-based, prospective Norwegian Mother and Child Cohort, Surén et al27 found that of the children whose mothers took folic acid, 0.10% had autistic disorder, compared with 0.21% in children whose mothers did not take folic acid. Moreover, a case-control study of ASD from California showed that maternal intake of folic acid and prenatal vitamins during the 3 months prior to pregnancy and the first month of pregnancy was associated with a lower risk of ASD in the offspring.15
One of the core characteristics of autism is lack of social awareness and undesired ways of emotional expressions (self-hurting, continuous screaming, and unexplained laughter). In this context, de Graaf et al28 indicated in a population-based cohort study in Rotterdam, The Netherlands, that children of mothers with prenatal folate deficiency were at higher risk of emotional problems. Studies have found that children born to women taking prenatal vitamins or folic acid supplements preconceptionally or during the first 2 months of pregnancy had a 40% reduction in the risk of ASD.29 However, excess intake of folate did subsequently decrease the overall percent of autism incidence in these populations, suggesting that mothers with some gene polymorphism are at higher risk when folate is deficient,3 with one report hypothesising that children who develop autism may have received a massive dose of folic acid in utero, as well as after birth.30 It is imperative to emphasise that some researchers could not find such an association between folic acid and autism; this could be due to the fact that other micronutrients should be presented for folic acid effect to take place. In a systematic review of studies involving relationships between folic acid and ASD, Castro et al31 reported contradictory results of folic acid effect on ASD risk.
Another factor that is directly affected by folic acid level is glutathione which is a product of methionine cycle that depends on folic acid and B12. Glutathione is involved in neuroprotection against oxidative stress and neuroinflammation in the brain.32 Glutathione was reported by many researchers to be deficient in children with autism compared with typically developed children.33 The methionine cycle involves the regeneration of methionine via the vitamin B12–dependent transfer of a methyl group from 5-methyltetrahydrofolate to homocysteine in the methionine synthase reaction. Methionine may then be activated by methionine adenosyltransferase to form S-adenosylmethionine (SAM), the primary methyl donor for most cellular methyltransferase reactions, including the methylation of DNA, RNA, proteins, phospholipids, and neurotransmitters.34
Vitamin D status as a risk factor for autism
The main source of vitamin D is the solar UV-B radiation–related conversion of 7-dehydrocholesterol to previtamin D3 in the skin and a lesser amount of vitamin D from food.35 There is a growing body of research that suggests that vitamin D status either in utero or early in life may be a risk of autism.36 Moreover, epidemiologic evidence supports the role of maternal vitamin D deficiency as a risk factor for the development of infantile autism.37 Maternal status of vitamin D is considered insufficient not only in countries where solar radiation is low but also in very sunny places such Saudi Arabia, UAE, Iran, and Jordan.38
Vitamin D functions in the body have been extensively studied by researchers. Its functions affect regulating cells, tissues, organs, and systems in the body. In fact, it is the most potent steroid hormone in the human body interacting with 2.5% of the human genome or some 1000 genes (a large number of which affect brain development and function) and working like a master on-off switch.35
The link between vitamin D and autism are based on 4 observational data:
The low concentrations of serotonin in the brain and its elevated concentrations in tissues outside the blood-brain barrier;
Low plasma concentrations of the vitamin D hormone precursor 25-hydroxyvitamin D (25(OH) D3);
High male incidence;
Presence of maternal antibodies to foetal brain tissue.35,36
The level of serotonin in the brain depends on the blood levels of tryptophan, which, unlike serotonin, crosses the blood-brain barrier. Serotonin in the brain promotes prosocial behaviour and correct assessment of emotional social cues. Brains of individuals with ASD display significantly lower concentrations of serotonin compared with the brains of non-autistic individuals.35 Patrick and Ames36 proposed that vitamin D (calcitriol) activates the transcription of serotonin-synthesizing gene tryptophan hydroxylase 2 (TPH2) in the brain and represses the transcription of TPH1 in tissues outside the blood-brain barrier.
It is important to mention in this context that the prevalence of autism in Somali children born in Sweden was found to be 3 to 4 times higher than in the non-Somali group (0.7% vs 0.19%).39 As Somalis have high levels of melanin, during migration to northern latitudes, such as Sweden, they would require 5 to 10 times more UV-B exposure than light-skinned individuals or an alternative vitamin D source, such as from the diet or supplementation.40 Moreover, Saad et al41 reported that 57% vitamin D deficient and 30% insufficient patients who received vitamin D3 (300 IU/kg/day not to exceed 5000 IU/day) for 3 months showed significant improved outcomes (80.72 %), which were mainly in the sections of the Childhood Autism Rating Scale (CARS) and aberrant behaviour checklist subscales that measure behaviour, stereotypy, eye contact, and attention span. Experiment on animal models showed that 1,25 dihydroxyvitamin D3 prenatal supplementations were protective for offsprings against induced maternal immune activation (MIA).42 Maternal immune activation in experimental animals reproduces ASD-like symptoms via significant increase of proinflammatory cytokines; however, administration of vitamin D has blocked the emergence of ASD-relevant deficits in social interaction, stereotyped behaviour, and emotional learning and memory.42 Recently, Sotodehasl et al43 have reviewed 80 articles linking autism and vitamin D. The authors found no significant differences between children with ASD and neurotypical developed children.43 This was also supported by a Canadian prospective cohort study that included 3852 children.44 Forty-one children were diagnosed with ASD, who have no difference in the level of vitamin D in their serum compared with non-ASD children.
Conclusions
Maternal status of micronutrients before pregnancy, during pregnancy, and during lactation is crucial not only to have a healthy child but also to establish a good state of neurodevelopment. The early 2 years are also important to decide the ability of the child to build healthy immune and nervous systems.
It was noticed that a micronutrient-deficient mother is more likely to bear a micronutrient-deficient child and higher risk of being neuro undeveloped.
On the community level, it was noticed that diet plays a major role in the manifestation of anaemia and vitamin E and D deficiency; however, other factors may interfere to make such a deficiency multifactorial. It is still not clear whether nutritional interventions, and in particular micronutrient supplements (to mother or baby), could reduce the risk of neurodevelopmental disorders by enhancing the early development of the immune system. More future studies are needed to elucidate this relationship, which may contribute to a better understanding of preventative mechanisms.
Acknowledgments
I would like to thank the Department of Nutrition in Ministry of Health represented by Eng. Alaa Abur Rub for their valuable work in producing PMS.
Footnotes
Funding:The PMS was conducted by the Nutrition Department, Ministry of Health, State of Palestine in collaboration with UNICEF (UN).
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MA wrote the article.
References
- 1. Christensen DL, Baio J, Braun KVN, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neggers YH. Increasing prevalence, changes in diagnostic criteria, and nutritional risk factors for autism spectrum disorders. ISRN Nutr. 2014;2014:514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neggers Y. The relationship between folic acid and risk of autism spectrum disorders. Healthcare (Basel). 2014;2:429–444. doi: 10.3390/healthcare2040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elmadfa I, Abu Rub A, Ben-Abdullah K, et al. Palestinian Micronutrient Survey (PMS). Final report. State of Palestine, Ministry of Health, Primary Health Care & Public Health, General Directorate, Nutrition Department; Published 2014. [Google Scholar]
- 5. Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–520. [DOI] [PubMed] [Google Scholar]
- 6. Kaur M, Chauhan A, Manzar D, Rajput MM. Maternal anaemia and neonatal outcome: a prospective study on urban pregnant women. J Clin Diagn Res. 2015;9:QC04–QC08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strauss MB. Anemia of infancy from maternal iron deficiency in pregnancy. J Clin Invest. 1933;12:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wachs TD, Georgieff M, Cusick S, McEwen B. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci. 2014;1308:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doom JR, Michael K, Georgieff MD. Striking while the iron is hot: understanding the biological and neurodevelopmental effects of iron deficiency to optimize intervention in early childhood. Curr Pediatr Rep. 2014;2:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res. 2003;53:217–223. [DOI] [PubMed] [Google Scholar]
- 11. Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron-deficiency anemia. Child Dev. 1998;69:24–36. [PubMed] [Google Scholar]
- 12. Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72:267–284. [DOI] [PubMed] [Google Scholar]
- 13. Surkan PJ, Siegel EH, Patel S, et al. Zinc and iron supplementation on motor and language milestone scores of infants and toddlers. Nutrition. 2013;29:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, Ozonoff S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol. 2014;180:890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harvey L, Boksa P. Additive effects of maternal iron deficiency and prenatal immune activation on adult behaviors in rat offspring. Brain Behav Immun. 2014;40:27–37. [DOI] [PubMed] [Google Scholar]
- 17. Brimberg L, Sadiq A, Gregersen PK, Diamond B. Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatry. 2013;18:1171–1177. [DOI] [PubMed] [Google Scholar]
- 18. Al-Ali SFS, Russo Alkaissi A. Association between autism spectrum disorder and iron deficiency in children diagnosed autism spectrum disorder in the Northern West Bank. J Health Med Nurs. 2015;16:2–10. [Google Scholar]
- 19. Tseng PT, Cheng YS, Chen YW, et al. Peripheral iron levels in children with autism spectrum disorders vs controls: a systematic review and meta-analysis. Nutr Res. 2018;50:44–52. [DOI] [PubMed] [Google Scholar]
- 20. Tahmasebi BS, Naghdi N, Shahbazi M, et al. The effect of severe zinc deficiency and zinc supplement on spatial learning and memory. Biol Trace Elem Res. 2009;130:48–61. [DOI] [PubMed] [Google Scholar]
- 21. Bhatnagar S, Taneja S. Zinc and cognitive development. Br J Nutr. 2001;85:S139–145. [DOI] [PubMed] [Google Scholar]
- 22. Black MM. The evidence linking zinc deficiency with children’s cognitive and motor functioning. J Nutr. 2003;133:1473S-1476S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krebs NF, Westcott J. Zinc and breastfed infants: if and when is there a risk of deficiency? Adv Exp Med Biol. 2002;503:69–75. [DOI] [PubMed] [Google Scholar]
- 24. Sayehmiri F, Babaknejad N, Bahrami S, Sayehmiri K, Darabi M, Rezaei-Tavirani M. Zn/Cu levels in the field of autism disorders: a systematic review and meta-analysis. Iran J Child Neurol. 2015;9:1–9. [PMC free article] [PubMed] [Google Scholar]
- 25. Roth C, Magnus P, Schjølberg S, et al. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306:1566–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Center for Disease Control Prevention. CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep. 2010;59:980–984. [PubMed] [Google Scholar]
- 27. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Graaff JS, Sabine JR, Steegers APE, et al. Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am J Clin Nutr. 2012;95:1413–1421. [DOI] [PubMed] [Google Scholar]
- 29. Braun JM, Froehlich T, Kalkbrenner A, et al. Are autistic-behaviors in children related to prenatal vitamin use and maternal whole blood folate concentrations? J Autism Dev Disord. 2014;44:2602–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beard CM, Panser LA, Katusic SK. Is excess folic acid supplementation a risk factor for autism? Med Hypotheses. 2011;77:15–17. [DOI] [PubMed] [Google Scholar]
- 31. Castro KL, Klein D, Baronio D, Gottfried C, Riesgo R, Perry IS. Folic acid and autism: what do we know? Nutr Neurosci. 2016;19:310–317. [DOI] [PubMed] [Google Scholar]
- 32. Ghanizadeh A, Akhondzadeh S, Hormozi M, Makarem A, Abotorabi-Zarchi M, Firoozabadi A. Glutathione-related factors and oxidative stress in autism, a review. Curr Med Chem. 2012;19:4000–4005. [DOI] [PubMed] [Google Scholar]
- 33. Hodgson NW, Waly MI, Al-Farsi YM, et al. Decreased glutathione and elevated hair mercury levels are associated with nutritional deficiency-based autism in Oman. Exp Biol Med (Maywood). 2014;239:697–706. [DOI] [PubMed] [Google Scholar]
- 34. James SJ, Cutler P, Melnyk S, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–1617. [DOI] [PubMed] [Google Scholar]
- 35. Richer SP, Pizzimenti JJ. The importance of vitamin D in systemic and ocular wellness. J Optom. 2013;6:124–133. [Google Scholar]
- 36. Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. FASEB J. 2014;28:2398–2413. [DOI] [PubMed] [Google Scholar]
- 37. Grant WB, Soles CM. Epidemiologic evidence supporting the role of maternal vitamin D deficiency as a risk factor for the development of infantile autism. Dermatoendocrinol. 2009;1:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haq A, Svobodová J, Imran S, Stanford C, Razzaque MS. Vitamin D deficiency: a single centre analysis of patients from 136 countries. J Steroid Biochem Mol Biol. 2016;164:209–213. [DOI] [PubMed] [Google Scholar]
- 39. Barnevik-Olsson M, Gillberg C, Fernell E. Prevalence of autism in children born to Somali parents living in Sweden: a brief report. Dev Med Child Neurol. 2008;50:598–601. [DOI] [PubMed] [Google Scholar]
- 40. Barnevik-Olsson M, Gillberg C, Fernell E. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2008;81:353–373. [DOI] [PubMed] [Google Scholar]
- 41. Saad K, Abdel-Rahman AA, Elserogy YM, et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr Neurosci. 2016;19:346–351. [DOI] [PubMed] [Google Scholar]
- 42. Vuillermot S, Luan W, Meyer U, Eyles D. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol Autism. 2017;8:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sotodehasl N, Tamadon MR, Malek F. Vitamin D deficiency and autism; a review on recent findings. J Parathyr Dis. 2018;6:7–12. [Google Scholar]
- 44. Ali Y, Anderson LN, Smile S, et al. Prospective cohort study of vitamin D and autism spectrum disorder diagnoses in early childhood [published online ahead of print March 8, 2018]. Autism. doi: 10.1177/1362361318756787. [DOI] [PubMed] [Google Scholar]