Abstract
Sinomenine, a bioactive alkaloid isolated from the traditional Chinese herb Sinomenium acutum, possesses antiinflammatory, antinociceptive, antifibrotic, and antitumorigenic properties. In this work, we sought to explore the biological effects of sinomenine on glioma cells. It was found that sinomenine caused a concentration-dependent inhibition of viability in both U87 and U251 glioma cells. Sinomenine at 16 μmol/L caused 55% to 60% reduction in the proliferation of U87 and U251 cells. Moreover, sinomenine treatment induced a G0/G1 cell cycle arrest and apoptosis. Mechanistically, sinomenine promoted p53 expression and acetylation and reduced the expression of sirtuin 1. Ectopic expression of sirtuin 1 significantly prevented sinomenine-induced p53 acetylation and growth suppression in glioma cells. Moreover, sinomenine inhibited the growth of U87 xenograft tumors in vivo and raised the p53 protein expression. Collectively, sinomenine shows antiproliferative effects against glioma cells which is mediated through downregulation of sirtuin 1 and induction of p53 activity.
Keywords: acetylation, cell cycle arrest, glioma, natural compounds, p53, SIRT1
Introduction
Malignant gliomas are highly aggressive and show resistance to radiotherapy and chemotherapy.1,2 Although many efforts have been made to improve therapeutic outcomes, the survival time for patients with malignant gliomas is still low.3 Therefore, identifying novel therapeutic agents for malignant glioma is of great importance.
Sirtuin 1 (SIRT1) is a member of the family of nicotinamide adenine dinucleotide–dependent deacetylases and plays an important role in a broad range of biological processes, such as oxidative stress,4 inflammation,5 glucose metabolism,6 and tumor progression.7 Sirtuin 1 has the capacity to induce posttranslational modification of many proteins including oncogenic or tumor suppressor proteins.8,9 p53 is a well-defined substrate of SIRT1. It has been reported that downregulation of SIRT1 leads to an increase in p53 acetylation, consequently causing p53-dependent apoptosis in chronic lymphocytic leukemia cells.10 Likewise, inhibition of SIRT1 exerts antiproliferative effects against human melanoma cells by enhancing p53 activity.11 In glioma cells, SIRT1 also regulates cell proliferation and apoptosis,12 underscoring its potential as a therapeutic target.
Sinomenine (Figure 1A) is a natural bioactive alkaloid that is extracted from the traditional Chinese herb Sinomenium acutum.13 Clinically, sinomenine has a long history to be used to treat rheumatoid arthritis.14 Apart from anti-inflammatory activity, sinomenine also possesses antinociceptive,15 antifibrotic,16 and antitumorigenic17 properties. It has been reported that sinomenine can inhibit the growth and metastasis of osteosarcoma.17 Similarly, this compound elicits antiproliferative effects against lung cancer cells.18 Despite these studies, the anticancer potential of sinomenine in glioma has not been investigated.
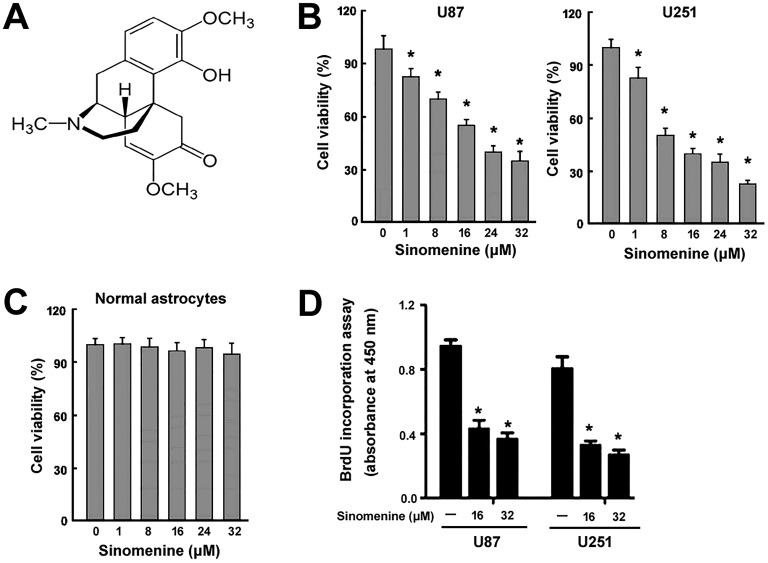
Figure 1.
Sinomenine inhibits the viability and proliferation of glioma cells in vitro. (A) Chemical structure of sinomenine. (B) Glioma cells and (C) normal human astrocytes were treated with indicated concentrations of sinomenine for 48 hours and measured for viability using the CCK-8 assay. (D), Cell proliferation analysis. Glioma cells were treated with 16 or 32 μmol/L of sinomenine for 48 hours and subjected to BrdU incorporation assay. *P < .05 relative to vehicle-treated control cells. CCK-8 indicates cell counting kit 8; BrdU, bromodeoxyuridine.
Therefore, in this work, we aimed to explore the biological effects of sinomenine on the proliferation, cell cycle progression, apoptosis, and tumorigenesis of glioma cells. Additionally, we examined whether sinomenine modulated the expression and activity of SIRT1.
Materials and Methods
Cell Culture
Human glioma cell lines U87 and U251 were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, California) in an atmosphere of 5% CO2 at 37°C. Normal human astrocytes were obtained from ScienCell Research Laboratories (Carlsbad, California; catalog no 1800) and cultured in astrocyte medium (ScienCell Research Laboratories) containing 10% FBS.
Cell Viability Assay
Cells were plated at 8 × 103 cells/well in 96-well plates and treated with different concentrations of sinomenine (1-32 μmol/L; Sigma-Aldrich, St Louis, Missouri) or 0.1% dimethyl sulfoxide (DMSO; Sigma-Aldrich) for 48 hours. Cell viability was measured using the cell counting kit 8 (CCK-8) assay according to the manufacturer’s protocols (Dojindo, Kumamoto, Japan). In brief, cells were incubated with CCK-8 solution for 4 hours. The absorbance was measured at 450 nm.
Bromodeoxyuridine Cell Proliferation Assay
Cells were plated in 96-well plates (1 × 104 cells/well) and treated with 16 and 32 μmol/L sinomenine or DMSO for 48 hours. Cell proliferation was assessed using the bromodeoxyuridine (BrdU) cell proliferation enzyme-linked immunosorbent assay kit (Abcam, Cambridge, United Kingdom) per the manufacturer’s instructions.
Cell Cycle and Apoptosis Analysis by Flow Cytometry
For analysis of cell cycle distribution, cells were incubated with the staining solution (Sigma-Aldrich) containing propidium iodide (PI; 50 μg/mL) and RNase A (20 μg/mL) for 1 hour in the dark. For apoptosis detection, cells were incubated with annexin-V–fluorescein isothiocyanate and PI (BD Biosciences, Franklin Lakes, New Jersey) according to the manufacturer’s protocol. Stained cells were analyzed by a FACSCalibur flow cytometer (BD Biosciences).
Western Blot Analysis
Tissue and cellular lysates were prepared using ice-cold radioimmunoprecipitation assay buffer (Abcam) supplemented with complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Equal amounts of protein (40 μg per lane) were resolved with 10% to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were incubated overnight at 4°C with primary antibodies (1:500) against SIRT1 (#2310), total p53 (#2524), acetylated p53 (Lys382; #2525), and β-actin (#4970; all from Cell Signaling Technology, Beverly, Massachusetts). Afterward, the membranes were incubated with horseradish peroxidase–conjugated secondary antibody (Sigma-Aldrich; 1:5000 dilution). Protein bands were visualized by the enhanced chemiluminescence system according to the manufacturer’s instructions (Cell Signaling Technology). Signals were quantitated by densitometry using Quantity One software (Bio-Rad Laboratories, Hercules, California).
Plasmids and Transfections
Human Sirt1-expressing plasmids were obtained from Origene (Rockville, Maryland). Cells were transfected with the Sirt1-expressing plasmid or empty vector using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. Twenty-four hours later, cells were exposed to 32 μmol/L of sinomenine for additional 48 hours. The cells were then examined for gene expression, cell cycle progression, and apoptosis.
Tumor Xenografts in Nude Mice
The experimental procedures involving animals were approved by the Animal Care and Use Committee of Xinjiang Uygur Autonomous Region People’s Hospital (Urumqi, China). Male Balb/c nude mice (4 weeks of age) were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). U87 cells were injected subcutaneously into the right flank of nude mice (4 × 106 cells per mouse; 4 mice per group), and tumor formation was monitored. When tumors reached the size of 150 mm3, tumor-bearing mice were randomly assigned to the control and sinomenine treatment groups. In the sinomenine treatment group, sinomenine (100 mg/kg body weight)19 was administered intraperitoneally every 3 days for 3 weeks. Control animals underwent the same procedure, except that physical saline was given. Tumor volume was measured weekly for 4 weeks. Tumor growth curves were plotted using the tumor volumes at different time points. The mice were killed after the last measurement of tumor volume. Tumors were resected and weighed. For Ki-67 immunohistochemistry, tumor samples were processed according to standard procedures and stained with anti-Ki-67 antibody (ab15580; 1:300 dilution; Abcam). The percentage of Ki-67-positive nuclei relative to total nuclei was calculated by counting 500 cells from 5 random microscopic fields (400×).
Statistical Analysis
Data are expressed as mean (standard deviation). Statistical differences were determined using the Student t test or 1-way analysis of variance followed by the Tukey multiple comparison test. A P value < .05 was considered to indicate a statistically significant difference.
Results
Sinomenine Inhibits the Viability and Proliferation of Glioma Cells In Vitro
U87 and U251 glioma cells were treated with different concentrations of sinomenine for 48 hours and measured for viability using the CCK-8 assay. Sinomenine significantly inhibited the viability of U87 and U251 cells in a concentration-dependent manner (Figure 1B). In contrast, sinomenine up to 32 μmol/L exerted no significant cytotoxicity to normal human astrocytes as determined by CCK-8 assays (Figure 1C). To determine whether sinomenine also inhibits glioma cell proliferation, BrdU incorporation assay was conducted. Sinomenine at 16 μmol/L significantly suppressed the proliferation of U87 and U251 cells by 55% to 60%, and mildly greater inhibition of cell proliferation was observed when 32 μmol/L sinomenine was used (Figure 1D). Therefore, in the following in vitro experiments, 16 μmol/L sinomenine was employed.
Sinomenine Promotes G0/G1-Phase Cell Cycle Arrest and Apoptosis
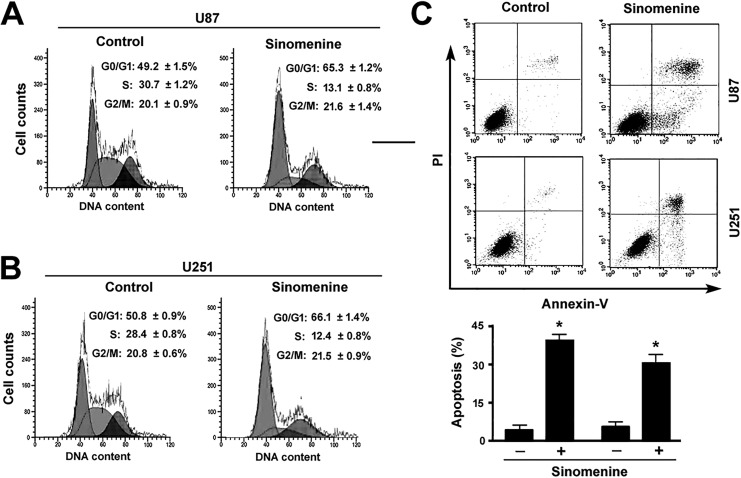
Analysis of cell cycle distribution after PI staining revealed that sinomenine (16 μmol/L) for 48 hours resulted in an accumulation of cells at the G0/G1 phase and a concomitant reduction of cells at the S phase (Figure 2A and B). Moreover, sinomenine treatment triggered significant apoptosis in both U87 and U251 cells as determined by flow cytometry after annexin-V/PI staining (Figure 2C).
Figure 2.
Sinomenine promotes G1-phase cell cycle arrest and apoptosis. (A B) U87 and U251 cells were treated with or without 16 μmol/L of sinomenine for 48 hours and examined for cell cycle distribution by flow cytometry after PI staining. The sub-G1 population was gated out, and the percentages of cells at the G0/G1, S, and G2/M phases were calculated. The statistic data in this figure represent the results from 3 independent experiments. (C), Apoptosis analysis by flow cytometry after annexin-V/PI staining. Top: representative dot plots of cells stained with annexin-V and PI. *P < .05 relative to vehicle-treated control cells. PI indicates propidium iodide.
Sinomenine Increases p53 Activity Via Reduction in SIRT1 Expression
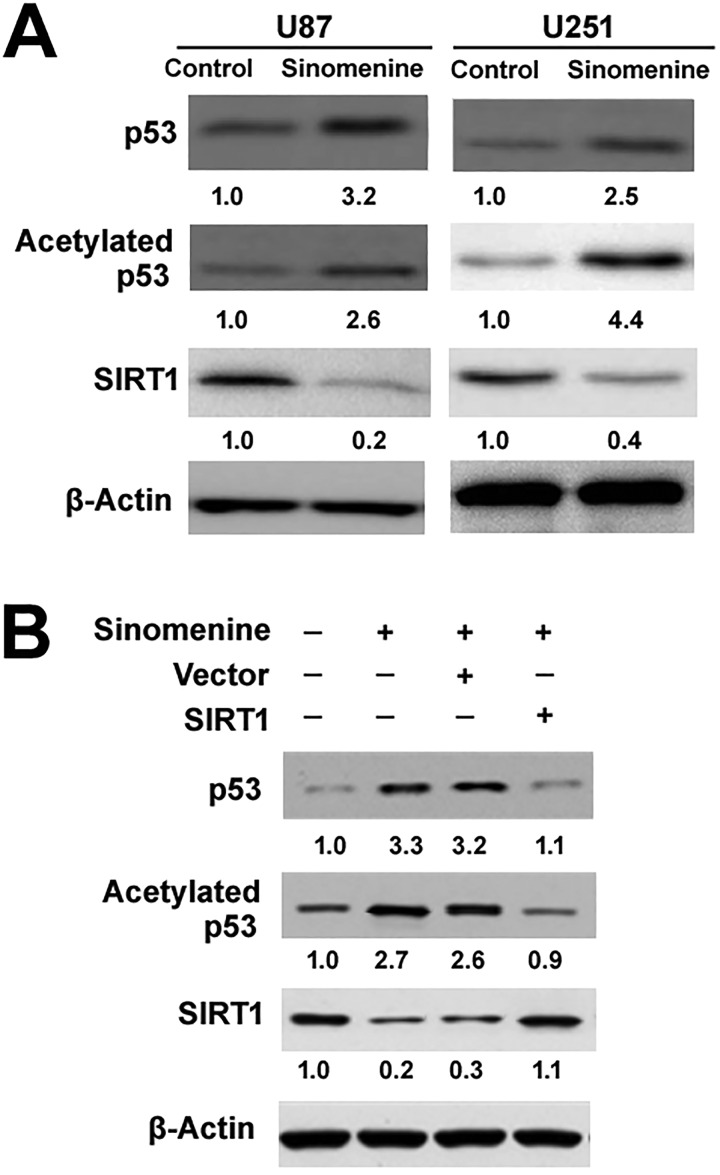
Western blot analysis revealed that sinomenine treatment raised the protein levels of p53 compared to vehicle-treated cells (Figure 3A). We further examined the level of p53 acetylation, an important posttranslational modification leading to enhancement of p53 activity.20 The results confirmed that sinomenine treatment led to a marked increase in the amount of acetylated p53 (Figure 3A). In contrast, sinomenine reduced the expression of SIRT1 in glioma cells (Figure 3A). To test whether sinomenine-induced p53 acetylation is associated with downregulation of SIRT1, we overexpressed SIRT1 (Figure 3B) in glioma cells prior to sinomenine exposure. Of note, enforced expression of SIRT1 significantly counteracted p53 expression and acetylation evoked by 32 μmol/L of sinomenine in U87 cells (Figure 3B).
Figure 3.
Sinomenine increases p53 activity via reduction in SIRT1 expression. (A) Western blot analysis of indicated proteins in glioma cells treated with or without 16 μM of sinomenine for 48 hours. (B) Western blot analysis of indicated proteins in U87 cells transfected with SIRT1-expressing plasmids or empty vector before treatment with 16 μmol/L of sinomenine. The relative protein levels were determined by densitometry after normalization to the values of β-actin. The results are expressed as fold change. Numbers below blots represent fold change in normalized protein expression relative to vehicle-treated control cells. SIRT1 indicates sirtuin 1.
Restoration of SIRT1 Rescues Glioma Cells From Sinomenine-Induced Growth Suppression
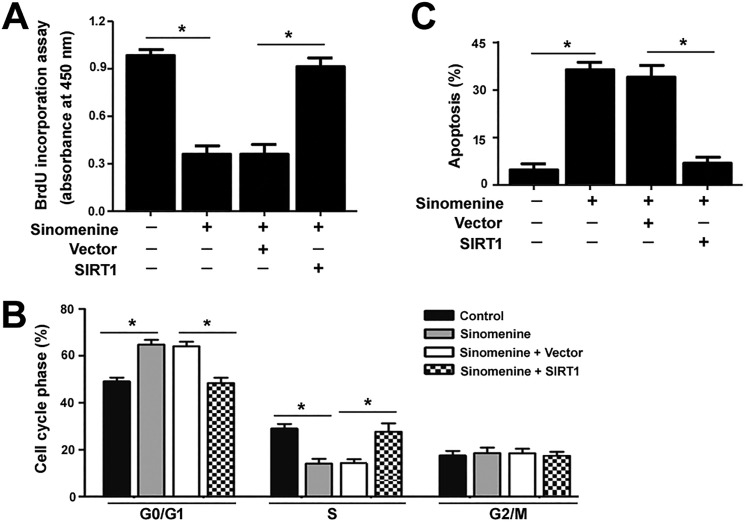
Next, we checked whether restoration of SIRT1 could rescue sinomenine-mediated toxic effects on glioma cells. As shown in Figure 4A, ectopic expression of SIRT1 rendered glioma cells more resistant to the antiproliferative effect of sinomenine (16 μmol/L). Moreover, sinomenine-induced cell cycle arrest (Figure 4B) and apoptosis (Figure 4C) were significantly reversed by overexpression of SIRT1. However, enforced expression of SIRT3 did not alter the cytotoxicity of sinomenine to glioma cells (data not shown), confirming the involvement of SIRT1 in the action of sinomenine.
Figure 4.
Restoration of SIRT1 rescues glioma cells from sinomenine-induced growth suppression. U87 cells were transfected with SIRT1-expressing plasmids or empty vector before treatment with 16 μM of sinomenine, and tested for proliferation (A), cell cycle distribution (B), and apoptosis (C). (A) BrdU incorporation assay. (B) Flow cytometric analysis of DNA contents after PI staining. (C) Apoptosis analysis after annexin-V/PI staining. *P < .05. SIRT1 indicates sirtuin 1; BrdU, bromodeoxyuridine; PI, propidium iodide.
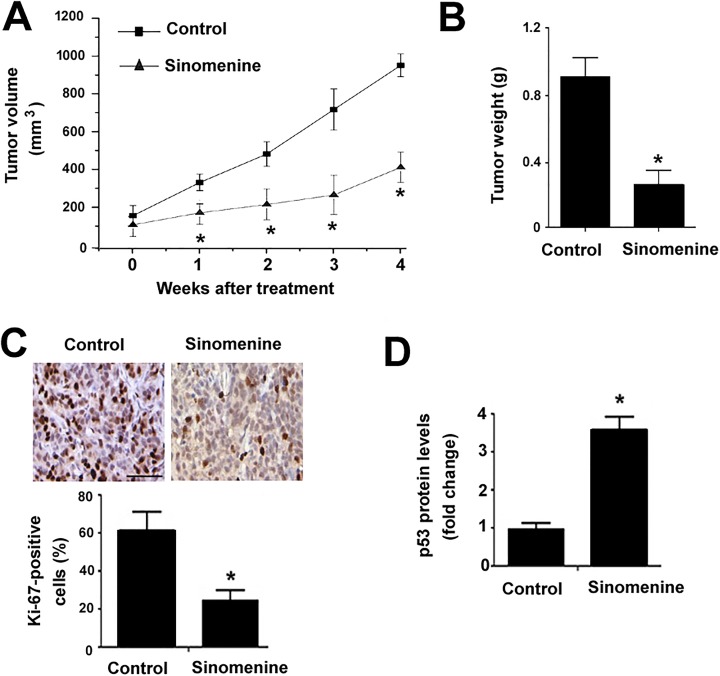
Sinomenine Retards the Growth of Glioma Xenografts In Vivo
To examine the antitumor effect of sinomenine in vivo, we established a subcutaneous xenograft model of U87 cells. Administration of sinomenine significantly inhibited the growth of xenograft tumors compared to vehicle-treated animals (Figure 5A). The final weight of sinomenine-treated U87 xenograft tumors was 67% lower than corresponding controls, respectively (Figure 5B). Tumor tissues were then processed for Ki-67 (a proliferation marker) staining. The results showed that the percentage of Ki-67-positive proliferating cells was significantly reduced in sinomenine-treated tumors (P < .05 vs control tumors; Figure 5C). Additionally, p53 protein levels were markedly elevated in sinomenine-treated tumors as determined by Western blot analysis (Figure 5D).
Figure 5.
Sinomenine retards the growth of glioma xenografts in vivo. U87 cells were injected subcutaneously into nude mice to form xenograft tumors. When tumors reached the size of 150 mm3, tumor-bearing mice were administered intraperitoneally with sinomenine or vehicle. (A) Tumor growth curves for each group. (B) Tumors were removed and weighed at 4 weeks after sinomenine treatment. (C) Ki-67 immunohistochemistry in sections of xenograft tumors. Top, representative images of Ki-67 immunostaining. Bottom, the percentage of Ki-67-positive cells in the tumors. Scale bar = 80 μm. (D) Western blot analysis of p53 protein levels in the U87 xenograft tumors. *P < .05 relative to the vehicle-treated control group.
Discussion
Natural compounds have gained increasing attention in the treatment of gliomas.21,22 For example, β-elemene, a bioactive agent isolated from a Chinese herb, exerts the anticancer effects against glioma stem-like cells.21 The natural polyphenol compound curcumin has exhibited the activity to suppress the growth, migration, and invasion of glioma cells.22 Our data demonstrated that sinomenine treatment led to a concentration-dependent inhibition of the viability of glioma cells. However, sinomenine at low-to-moderate concentrations (up to 32 μmol/L) had no significant cytotoxicity to normal human astrocytes. A preclinical study reported that sinomenine significantly inhibited the growth of colon carcinoma xenograft tumors in nude mice without causing obvious side effects.23 These results suggest that sinomenine can be used to selectively kill tumor cells.
Our data showed that sinomenine at 16 μmol/L suppressed the proliferation of glioma cells as determined by BrdU incorporation assay. To gain more insight into the antiproliferative activity of sinomenine, we analyzed cell cycle progression and apoptosis after sinomenine treatment. It was found that sinomenine-treated glioma cells displayed an accumulation of cells at the G0/G1 phase, indicating a cell cycle arrest. Consistently, sinomenine arrested colon carcinoma cells at the G0/G1 phase, which was accompanied by increased expression of p21 and decreased expression of cyclin D1 and cyclin E.24 However, a previous study reported that sinomenine treatment caused a cell cycle arrest at the S phase in human osteosarcoma cells.17 Therefore, the antiproliferative activity of sinomenine is not restricted to the G0/G1 phase of the cell cycle. In addition to induction of cell cycle arrest, we found that sinomenine treatment triggered significant apoptotic death in glioma cells. The proapoptotic activity of sinomenine is also detected in other malignant cells, such as lung cancer cells18 and esophageal carcinoma cells.25
p53 is well known as a key tumor suppressor, inducing cell cycle arrest and apoptosis.26 In this study, we showed that sinomenine treatment significantly increased the levels of p53 protein in glioma cells. Moreover, sinomenine treatment promoted the acetylation of p53 protein, which contributes to the enhancement of p53 activity.20 These findings suggest the involvement of p53 in the anticancer activity of sinomenine. In contrast to enhanced p53 acetylation, the expression of SIRT1 was suppressed by sinomenine in glioma cells. The SIRT1 has been shown to modulate the activities of many proteins by inducing deacetylation.8,9 A previous study has demonstrated that SIRT1 downregulation enhances p53 acetylation and causes p53-dependent apoptosis in chronic lymphocytic leukemia cells.10 Our data showed that ectopic expression of SIRT1 abolished sinomenine-induced p53 acetylation in glioma cells. Moreover, the antiproliferative effects of sinomenine on glioma cells were reversed by overexpression of SIRT1. These results collectively indicate that sinomenine exerts its anticancer activity in glioma cells via downregulation of SIRT1 and induction of p53 acetylation. In support of this view, in vivo xenograft studies demonstrated that administration of sinomenine inhibited the growth of glioma xenografts and increased the levels of p53 protein.
In conclusion, we provide evidence that sinomenine exerts growth-suppressive effects on glioma cells, causing a G0/G1 cell cycle arrest and apoptosis. However, sinomenine has mild cytotoxicity to normal human astrocytes. The anticancer activity of sinomenine is causally linked to downregulation of SIRT1 and induction of p53 acetylation. These observations suggest that sinomenine may represent a promising natural anticancer agent for glioma.
Abbreviations
- BrdU
bromodeoxyuridine
- CCK-8
cell counting kit 8
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- PI
propidium iodide
- SIRT1
sirtuin 1.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Science Foundation of The Xinjiang Uygur Autonomous Region of China (grant no 2015211C210).
References
- 1. Miller JJ, Wen PY. Emerging targeted therapies for glioma. Expert Opin Emerg Drugs. 2016;21(4):441–452. [DOI] [PubMed] [Google Scholar]
- 2. Gallego-Perez D, Chang L, Shi J, et al. On-chip clonal analysis of glioma-stem-cell motility and therapy resistance. Nano Lett. 2016;16(9):5326–5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mittal S, Pradhan S, Srivastava T. Recent advances in targeted therapy for glioblastoma. Expert Rev Neurother. 2015;15(8):935–946. [DOI] [PubMed] [Google Scholar]
- 4. Xie Y, Tu W, Zhang J, et al. SirT1 knockdown potentiates radiation-induced bystander effect through promoting c-Myc activity and thus facilitating ROS accumulation. Mutat Res. 2015;772:23–29. [DOI] [PubMed] [Google Scholar]
- 5. Debelec-Butuner B, Ertunc N, Korkmaz KS. Inflammation contributes to NKX3.1 loss and augments DNA damage but does not alter the DNA damage response via increased SIRT1 expression. J Inflamm (Lond). 2015;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boutant M, Joffraud M, Kulkarni SS, et al. SIRT1 enhances glucose tolerance by potentiating brown adipose tissue function. Mol Metab. 2014;4(2):118–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim SY, Ko YS, Park J, et al. Forkhead transcription factor FOXO1 inhibits angiogenesis in gastric cancer in relation to SIRT1. Cancer Res Treat. 2016;48(1):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stünkel W, Peh BK, Tan YC, et al. Function of the SIRT1 protein deacetylase in cancer. Biotechnol J. 2007;2(11):1360–1368. [DOI] [PubMed] [Google Scholar]
- 9. Gomes AR, Yong JS, Kiew KC, et al. Sirtuin1 (SIRT1) in the acetylation of downstream target proteins. Methods Mol Biol. 2016;1436:169–188. [DOI] [PubMed] [Google Scholar]
- 10. Dal Bo M, D’Agaro T, Gobessi S, et al. The SIRT1/TP53 axis is activated upon B-cell receptor triggering via miR-132 up-regulation in chronic lymphocytic leukemia cells. Oncotarget. 2015;6(22):19102–19117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilking MJ, Singh C, Nihal M, Zhong W, Ahmad N. SIRT1 deacetylase is overexpressed in human melanoma and its small molecule inhibition imparts anti-proliferative response via p53 activation. Arch Biochem Biophys. 2014;563:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qu Y, Zhang J, Wu S, Li B, Liu S, Cheng J. SIRT1 promotes proliferation and inhibits apoptosis of human malignant glioma cell lines. Neurosci Lett. 2012;525(2):168–172. [DOI] [PubMed] [Google Scholar]
- 13. Zhang MF, Zhao Y, Jiang KY, et al. Comparative pharmacokinetics study of sinomenine in rats after oral administration of sinomenine monomer and Sinomenium acutum extract. Molecules. 2014;19(8):12065–12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu M, Liu L, Qi C, Deng B, Cai X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med. 2008;74(12):1423–1429. [DOI] [PubMed] [Google Scholar]
- 15. Zhu Q, Sun Y, Mao L, et al. Antinociceptive effects of sinomenine in a rat model of postoperative pain. Br J Pharmacol. 2016;173(10):1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin T, Yin S, Yang J, et al. Sinomenine attenuates renal fibrosis through Nrf2-mediated inhibition of oxidative stress and TGFβ signaling. Toxicol Appl Pharmacol. 2016;304:1–8. [DOI] [PubMed] [Google Scholar]
- 17. Xie T, Ren HY, Lin HQ, et al. Sinomenine prevents metastasis of human osteosarcoma cells via S phase arrest and suppression of tumor-related neovascularization and osteolysis through the CXCR4-STAT3 pathway. Int J Oncol. 2016;48(5):2098–2112. [DOI] [PubMed] [Google Scholar]
- 18. Jiang T, Zhou L, Zhang W, et al. Effects of sinomenine on proliferation and apoptosis in human lung cancer cell line NCI-H460 in vitro. Mol Med Rep. 2010;3(1):51–56. [DOI] [PubMed] [Google Scholar]
- 19. Zhang H, Ren Y, Tang X, et al. Vascular normalization induced by sinomenine hydrochloride results in suppressed mammary tumor growth and metastasis. Sci Rep. 2015;5:8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mellert H, Sykes SM, Murphy ME, McMahon SB. The ARF/oncogene pathway activates p53 acetylation within the DNA binding domain. Cell Cycle. 2007;6(11):1304–1306. [DOI] [PubMed] [Google Scholar]
- 21. Feng HB, Wang J, Jiang HR, et al. β-Elemene selectively inhibits the proliferation of glioma stem-like cells through the downregulation of Notch1. Stem Cells Transl Med. 2017;6(3):830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fei Y, Xiong Y, Zhao Y, et al. Cathepsin L knockdown enhances curcumin-mediated inhibition of growth, migration, and invasion of glioma cells. Brain Res. 2016;1646:580–588. [DOI] [PubMed] [Google Scholar]
- 23. Zhang JX, Yang ZR, Wu DD, et al. Suppressive effect of sinomenine combined with 5-fluorouracil on colon carcinoma cell growth. Asian Pac J Cancer Prev. 2014;15(16):6737–6743. [DOI] [PubMed] [Google Scholar]
- 24. Yang H, Yin P, Shi Z, et al. Sinomenine, a COX-2 inhibitor, induces cell cycle arrest and inhibits growth of human colon carcinoma cells in vitro and in vivo. Oncol Lett. 2016;11(1):411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang J, Yang ZR, Dong WG, et al. Cooperative inhibitory effect of sinomenine combined with 5-fluorouracil on esophageal carcinoma. World J Gastroenterol. 2013;19(45):8292–8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perri F, Pisconti S, Della Vittoria Scarpati G. P53 mutations and cancer: a tight linkage. Ann Transl Med. 2016;4(24):522. [DOI] [PMC free article] [PubMed] [Google Scholar]