Abstract
Background
Synthetic magnetic resonance (MR) is a method allowing reduction of examination time and access to quantitative imaging.
Purpose
This study sought to assess the image quality and diagnostic accuracy of synthetic magnetic resonance imaging (MRI) compared to standard MRI in patients with knee pain.
Material and Methods
In total, 22 patients underwent standard 1.5 knee MRI with an added synthetic sequence. Quantitative T1, T2, and proton density (PD) images were generated synthetically; T1, PD, and short tau inversion recovery (STIR) weighted images were created with chosen echo time (TE), repetition time (TR), and inversion time (TI). Two blinded musculoskeletal radiologists evaluated the overall sequence quality, visualization of anatomic structures, and presence of artifacts using a 3-point score.
Results
The synthetic sequence was acquired in 39% less time than the conventional MRI. Synthetic PD, T1, and STIR images were rated fair (2%, 5%, and 2%, respectively) or good quality (98%, 95%, and 98%, respectively), despite the presence of popliteal artery artifacts. Cartilage and meniscus were well visualized in all cases. Anterior cruciate ligament visualization was rated poor in 7%, 14%, and 30% of PD, STIR, and T1 images, respectively.
Conclusion
Our pilot study confirmed the feasibility of synthetic MRI in knee examinations, proving faster and achieving appropriate quality and good diagnostic confidence.
Keywords: Knee, magnetic resonance imaging, synthetic, quantitative imaging, artifact, soft-tissue injuries, cartilage
Introduction
Magnetic resonance imaging (MRI) is a widely available modality for investigating knee trauma. It is a crucial tool in clinical applications for detecting bone, cartilage, and soft-tissue injuries, offering excellent anatomical details (1,2). MRI has been proven to shorten time to diagnosis and change knee trauma management (3). Numerous studies have demonstrated the high accuracy of MRI in the detection of meniscal and anterior cruciate ligament injury, reaching 95% in some (1,4–7). Assessing cartilage lesions is still difficult, even when using cartilage-specific sequences (8,9) and MRI offers good specificity with poor sensitivity (10). The standard protocol is usually composed of an intermediate two-dimensional (2D) fat-suppressed spin echo MR (proton density [PD]-weighted sequence) in all three planes and a T1-weighted (T1W) sequence in one plane (mostly the coronal plane), both sensitive for ligament and meniscal injuries (11). The protocol takes 13 min (12). Sequences such as short tau inversion recovery (STIR), which are more specific for the detection of fluid and bone marrow edema, are added in some protocols, as in our institution (4).
Currently, as demonstrated in several publications (13–15), three-dimensional (3D) isotropic fat-suppressed PD-weighted MRI can replace the 2D sequence in knee imaging with the same efficacy for evaluating the meniscus and ligaments, with the added advantage of providing multiplanar reconstructions. More recently, isotropic 3D balanced fast field-echo imaging has been developed to assess cartilage defects (14). The duration of sequences differs between protocols, reaching 10 min in some studies (12). Furthermore, all studies were carried out using high fields (3-T).
However, advanced-imaging techniques, such as quantitative imaging, are not possible without a special sequence able to perform T2 mapping which increases acquisition time. Furthermore, time is one of the most important factors when working with MRI, as a significant number of patients must be scanned in a typical day and each must be allocated a well-defined time slot.
Recently, a new sequence called synthetic MRI has become available, which enables both a significant reduction in examination time and access to quantitative imaging. This technique has already been used in some cerebral applications, notably in multiple sclerosis. There are only a few publications concerning this technique in the literature, most focused on brain imaging and especially on white-matter diseases (16,17). As concerns the applications of synthetic MRI in musculoskeletal imaging, there are no publications at all to our knowledge. Nevertheless, it seems promising to put standard imaging protocols in place, such as those established for knee imaging, with the advantage of not only reducing acquisition time but also generating mapping of the cartilage, for example.
This study sought to assess the overall image quality and diagnostic accuracy of synthetic MRI compared to conventional MRI in patients with post-traumatic or degenerative knees.
Material and Methods
Patients
This study received approval from the institutional review ethics board and informed consent was waived.
We included 22 patients referred to our department between March and October 2016 for MRI due to knee pain. The mean age was 42 ± 19 years and the gender ratio was 0.29 (5 women, 17 men). Indications for MRI were suspected meniscal, ligament, or cartilage injury due to trauma or arthritis. Patients with one of the following criteria were excluded: massive traumatic injuries; postoperative knee; advanced chondropathy; and tumoral or inflammatory diseases.
MRI
Before synthetic MRI, all patients underwent a conventional MRI examination according to our center’s protocol, using an Ingenia 1.5-T MRI Philips (Best, Netherland) with a 16-channel-knee phased-array coil. The protocol included coronal fast spin-echo T1W, coronal STIR, and sagittal fast spin-echo PD-weighted scanning. We usually also include 3D isotropic fat-suppression PD-weighted sequences. This sequence was not, however, included in the study, as the synthetic sequence is 2D without the possibility of multiplanar reconstructions. All sequence parameters are provided in Table 1.
Table 1.
Summary of sequence parameters.
| Sequence | FOV X (mm) | FOV Y (mm) | Res X (mm) | Res Y (mm) | Rec res X (mm) | Rec res Y (mm) | TE (ms) | TR (ms) | TI (ms) | Slice thickness (mm) | Gap (mm) | Acquisition time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 cor | 160 | 160 | 0.35 | 0.49 | 0.25 | 0.25 | 10 | 524 | NA | 3 | 0.3 | 00:03:13 |
| STIR cor | 160 | 160 | 0.61 | 0.73 | 0.37 | 0.37 | 60 | 3700 | 140 | 3 | 0.3 | 00:04:04 |
| PD sag | 160 | 160 | 0.3 | 0.46 | 0.25 | 0.25 | 30 | 2000 | NA | 3 | 0.3 | 00:04:23 |
| SyntAc sag | 200 | 150 | 0.45 | 0.59 | 0.45 | 0.45 | 13/100 | 3100 | NA | 5 | 1 | 00:07:07 |
Cor, coronal; sag, sagittal; PD, proton density; STIR, short tau inversion recovery; res, resolution; FOV, field of view; rec, reconstruction; TE, echo time; TR, repetition time; TI, inversion time.
Sagittal SyntAc (multiple-dynamic multiple-echo [MDME] sequence, provided by Synthetic MRI AB, Linköping, Sweden) was added to the protocol. We opted for the sagittal plane primarily to assess the cruciate ligaments for feasibility. Adding the coronal and axial planes would significantly lengthen the examination time and not conform to the daily MRI workflow. The SyntAc sequence is based on a TSE sequence with a saturation pulse (120°), with four different inversion times (TI) and two echo times (TE), providing eight images with different contrasts. These images are used by the SyMRI v8 software (SyntheticMR AB, Linköping, Sweden) to generate quantitative T1, T2, and PD images, then it synthetically creates T1, PD, and STIR-weighted images with user-chosen TE, repetition time (TR) and TI. The sequence parameters are summarized in Table 1. In this study, we chose TE/TR to match the conventional sequences and adequate TI to suppress the fat signal in the image (190 ms).
Image analysis
Two blinded, experienced, musculoskeletal radiologists (with five and two years of experience) separately evaluated the conventional MRI and synthetic images, anonymized by a third person. The acquisition time was compared between the two techniques. Overall sequence quality and visualization of anatomical structures (bone, femoro-tibial cartilage, meniscus, anterior and posterior cruciate ligaments, and extensor tendons) were evaluated using a 3-point score (1 = poor, 2 = fair, 3 = good). The presence of artifacts was also assessed using a 3-point score (1 = severe, 2 = moderate, 3 = none). Conventional MRI images (henceforth referred to with “c” prefix) were coronal cT1, cSTIR, and sagittal cPD-weighted images. Synthetic MRI (henceforth referred to with “s” prefix) were sagittal sT1, sSTIR, and sPD-weighted images. In addition, each final diagnosis (cruciate ligament tear, meniscus tear, bone edema or fracture, and chondropathy) was compared between conventional and synthetic MRI. Collateral ligaments were not evaluated, since the SyntAc sequence was only acquired in the sagittal plane. T1 and T2 mapping were not evaluated in this preliminary study of technique feasibility.
Statistical analysis
Evaluation of score data was performed with median values. Comparison of scores between conventional and synthetic images was performed based on the results given by Reader 1 (the most experienced), using a signed-rank Wilcoxon paired test. Comparison between the two readers was also performed with a signed-rank Wilcoxon paired test. Kappa was not used to test inter-rater reliability, due to the high similarity of scores between the two readers (18).
Results
MRI
The SyntAc sequence was acquired in 39% less time than the conventional sequences (11 min 40 s for conventional vs. 7 min 7 s for SyntAc).
Image quality
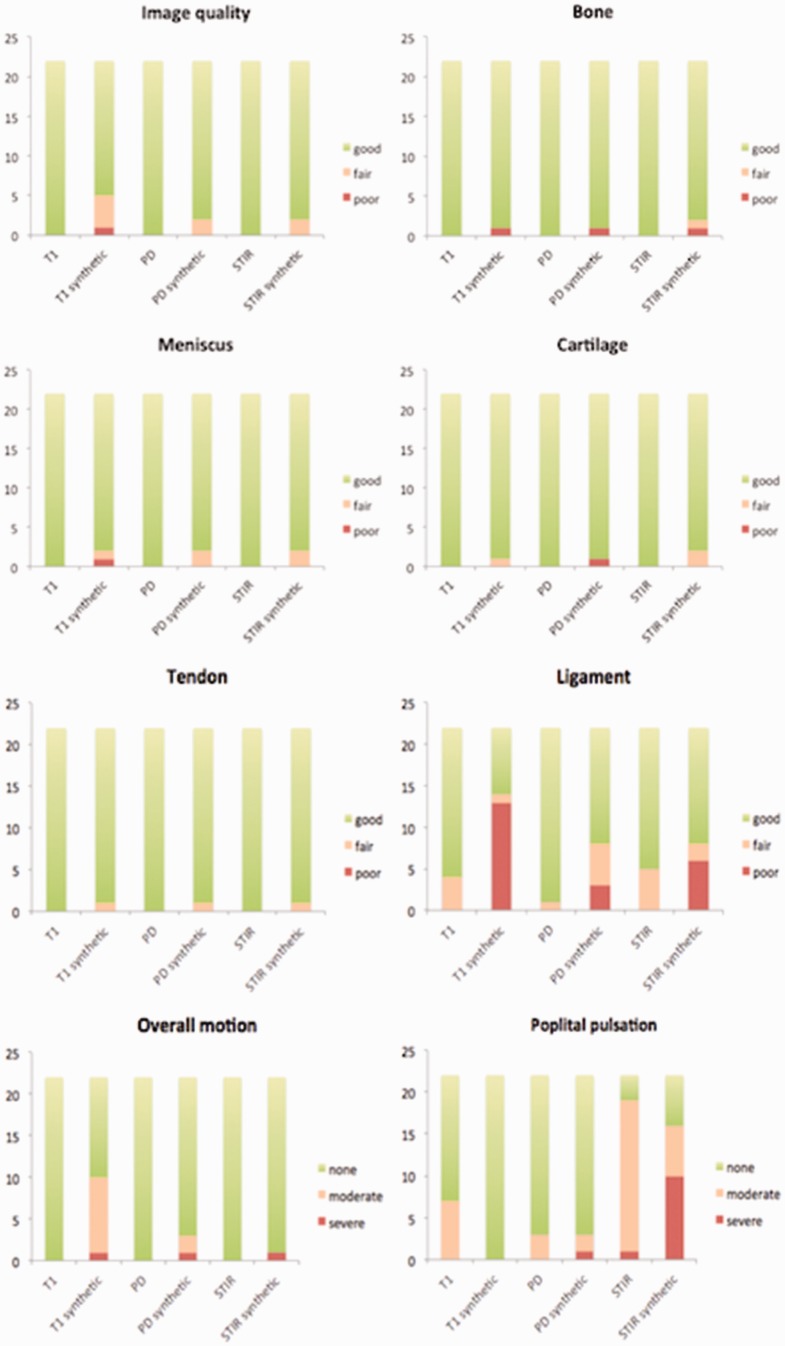
Fig. 1 shows the distribution of scores given for each parameter evaluated by Reader 1.
Fig. 1.
Distribution of scores obtained for each evaluated parameter by Reader 1 .
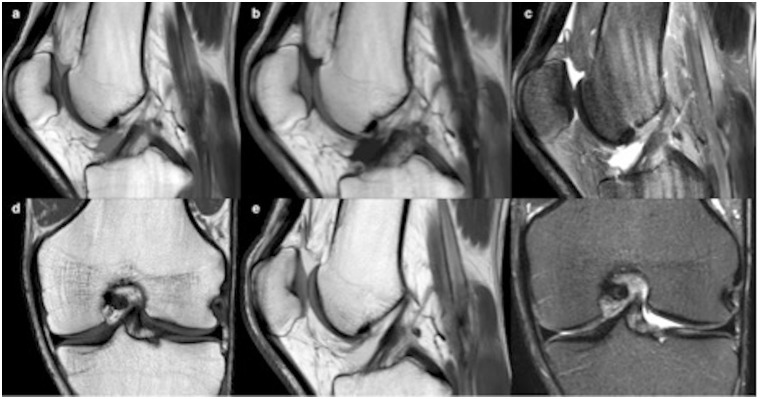
The synthetic image quality was rated “good” for all contrasts. All structures were well visualized (median score = 3) with all three contrasts, except for the cruciate ligaments in sT1 (13 = “poor;” 1 = “fair;” 8 = “good”). Femoro-tibial cartilage, meniscus, and extensor tendons were well visualized in all cases in sT1, sPD, and sSTIR, with a median score of 3 (all “good,” except 1 “poor” on sPD; 1 “fair” on sT1; and 2 “fair” on sSTIR), as illustrated in Fig. 2.
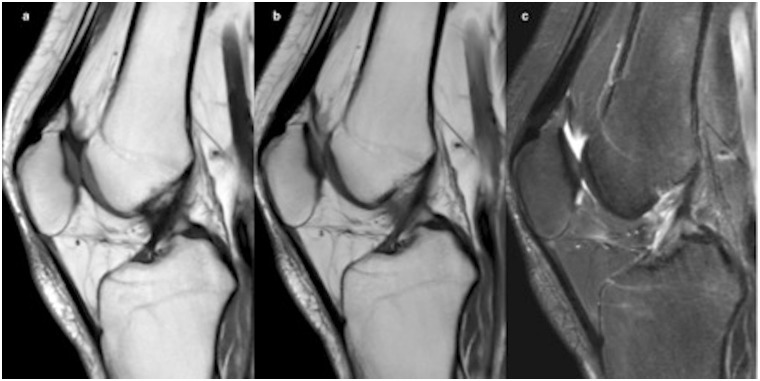
Fig. 2.
Post-traumatic left knee MRI in a 32-year-old patient: synthetic MRI with sagittal T1 (a), PD (b), and STIR (c) weighting compared to conventional images for coronal T1 (d), sagittal PD (e), and coronal STIR (f) weighting.
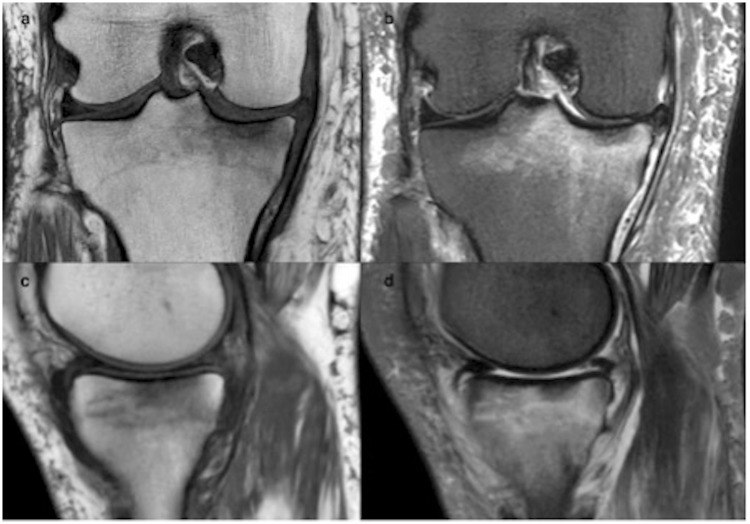
We particularly observed good sensitivity for the assessment of bone edema, rated “good” (only 1 “poor” and 1 “fair”) in sSTIR when present, as illustrated in Fig. 3.
Fig. 3.
Comparison of conventional MRI on coronal T1 and STIR (a, b) vs. synthetic STIR on sagittal T1 and STIR (c, d) for a 45-year-old patient consulting for internal knee pain. For this particular example, the conventional STIR was acquired also in the sagittal orientation (usually in coronal). The edema is clearly visible on the tibial endplate on synthetic imaging.
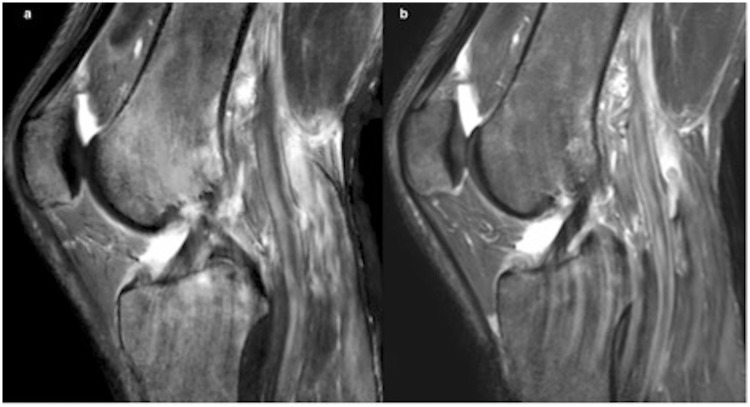
The median score for artifacts differed for overall motion in T1 (P = 0.003) and popliteal pulsation in STIR (P = 0.02) (Fig. 4) between conventional and synthetic sequences. However, we obtained good reliability with no significant difference between the two methods for the rest of the sequences.
Fig. 4.
Illustration of popliteal pulsations in STIR sequences in a 37-year-old man, in synthetic (a) and conventional (b) MRI. When STIR was acquired in the sagittal plane, popliteal pulsations were more evident, with no significant difference with synthetic acquisition.
Table 2 presents the results of the different evaluated parameters.
Table 2.
Median scores* obtained for all evaluated parameters.
| Median | T1 | T1 synthetic | P (Wilcoxon test) between methods | PD | PD synthetic | P (Wilcoxon test) between methods | STIR | STIR synthetic | P (Wilcoxon test) between methods |
|---|---|---|---|---|---|---|---|---|---|
| Image quality | 3 | 3 (2) | 0.05 | 3 | 3 | 0.3 | 3 | 3 (2)* | 0.3 |
| Bone | 3 | 3 | 1 | 3 | 3 | 1 | 3 | 3† | 0.4 |
| Cartilage | 3 | 3 | 1 | 3 | 3 | 1 | 3 | 3 | 0.3 |
| Meniscus | 3 | 3 (2.5)† | 0.4 | 3 | 3 | 0.3 | 3 | 3 | 0.3 |
| Ligaments | 3 | 1 (2) | 0.001 | 3 | 3† | 0.02 | 3 | 3 | 0.04 |
| Tendons | 3 | 3 | 1 | 3 | 3 | 1 | 3 | 3 | 1 |
| Overall motion | 3 | 3† | 0.003 | 3 | 3 | 0.2 | 3† | 3 (2)* | 1 |
| Popliteal pulsation | 3† | 3† | 0.02 | 3 | 3 | 0.8 | 2† | 2 | 0.2 |
For image quality and visualization of the different structures, 3 = good, 2 = fair, 1 = poor.
For overall motion and popliteal pulsation, 3 = none, 2 = moderate, 1 = severe.
Boldface values = p = 0.05. All Reader 1 results provided with Reader 2 results given in brackets when different. When the difference is significant between readers, it is indicated as *P < 0.01 or †P < 0.05).
Despite a median score of 3 (good) for ligament visualization on sPD and sSTIR contrast (all “good” except 3 “poor,” 5 “fair,” and 6 “poor;” 2 “fair,” respectively), we obtained a significantly lower rating with the synthetic sequence compared to the conventional sequence, whereas the visualization on the sT1 sequence was clearly rated “poor” (13 “poor,” 1 “fair,” 8 “good”). This may be explained by the low contrast and artifacts in intercondylar notch obscuring the ligament enthesis (Fig. 5).
Fig. 5.
Sagittal synthetic MRI for anterior cruciate ligament in T1 (a), PD (b), and STIR (c) sequences in a 24-year-old patient complaining of persistent pain following ski trauma. Ligament hyposignal is clear, the intensity at the enthesis is less apparent, explaining the poor evaluation in T1 for a majority of cases by both readers (a).
Some parameters were also rated differently by the two readers, particularly for T1 and STIR contrasts, even though the median scores were still “good” overall. The PD contrast, which is one of the most commonly used sequences in knee examination, was rated “good” for all evaluated parameters by both readers, with no artifacts. Only the ligament structures generated different ratings between readers. Cruciate-ligament visualization was rated “poor” by Reader 1 in 7%, 14%, and 30% of cases on sPD, sSTIR, and sT1 contrasts, respectively, due to inadequate visualization of the anterior cruciate ligament, despite good visualization of the posterior cruciate ligament. This was attributed to the slice thickness (5 mm in synthetic MRI, 3 mm in conventional PD sagittal) and had no impact on diagnosis.
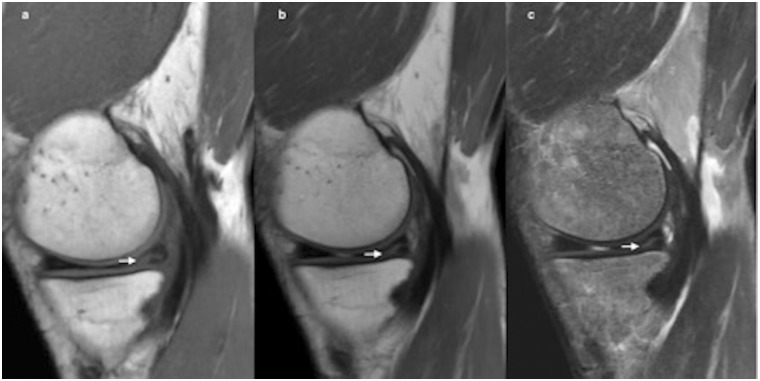
Finally, diagnostic accuracy was identical in conventional and synthetic MRI in all cases, i.e. all findings observed on the conventional sequences were also found on the synthetic sequences for visualized structures in the sagittal plane, mainly for meniscus tear (Fig. 6).
Fig. 6.
Tear in the internal meniscus extends to tibial surface (arrow), showed in synthetic MRI in sagittal T1 (a), PD (b), and STIR (c) sequences in a 46-year-old patient.
Discussion
In this preliminary study, we demonstrated the feasibility of using synthetic MRI in musculoskeletal imaging, taking the knee as an example. The knee joint is among the most frequently investigated structures in trauma centers, and in the majority of cases conventional MRI is needed to exclude soft-tissue injuries, including meniscus and ligament trauma and bone or cartilage bruising. The accuracy of MRI to assess traumatic lesions is close to 95% (4), and as high for meniscus and cruciate ligaments too in some studies. Synthetic MRI is proposed by our pilot study as a new technique enabling the acquisition of T1, PD, and STIR contrasts with a single acquisition. Synthetic MRI is an emerging tool in neuroradiology, up until now used in the analysis of white-matter diseases like multiple sclerosis (17,19). Studies performed in the brain have demonstrated that the contrast in synthetic MRI is higher, yet so is the level of noise, in T1 and T2, and that despite an inferior image quality, synthetic MRI achieved similar diagnostic accuracy as conventional sequences (20). Visual assessment of the image did not suggest any loss of signal-to-noise ratio (SNR) in our study, no doubt due to the greater slice thickness used in synthetic MRI than in conventional images. Another study in children’s brains produced similar conclusions about the potential use of synthetic sequences for diagnosis, yet outlined some limitations regarding the FLAIR contrast, which proved lower quality than conventional sequence (21). All in all, both studies reported that the synthetic sequence’s shorter acquisition time was a great advantage compared to conventional sequences.
One of the most significant benefits of synthetic MRI is its reduction of examination time by generating multiple image contrasts based on a single scan. In our study, we focused on the diagnostic quality of synthetic MRI with a scan time of 7 min in comparison to conventional protocol taking 11.5 min. This is a clear advantage for clinical practice, and it should also not be forgotten that other contrasts can also still be generated with the same single acquisition. Another advantage of synthetic MRI that was not analyzed in this first feasibility study is its tissue characterization capacities with the quantification of T1, T2, and PD values. This can provide additional information for the diagnosis and should be investigated in more detail in the future. Tissue characterization offers the added advantage of enabling volume estimation, facilitating follow-up of diseases, such as in bone marrow infection or malignant disease (22,23).
The first limitation of our study was the small number of patients due to our exclusion of non-traumatic cases and time constraints. The second limitation was the acquisition in sagittal plane for synthetic MRI, rendering analysis of collateral ligaments difficult, and ideally an additional coronal plane would have been beneficial. We chose this orientation due to our focus on the cruciate ligaments in this preliminary study. For the future, the acquisition will be evaluated in the coronal orientation instead of sagittal. Another limitation for this preliminary study was the slice thickness of 5 mm offered by this first-generation synthetic sequence, compared to the 3 mm of conventional sequences. This thickness was obligatory due to the use of a 1.5-T field in this study, as well as the compromise between acquisition time and image quality for synthetic MRI. There is now the possibility of implementing this sequence in a 3-T field enabling thinner slices and better spatial resolution, which should be considered. The third limitation was the absence of quantitative evaluation. Synthetic MRI offers access to T1, T2, and PD quantification, which is certainly of high potential for the characterization of various pathologies, such as the distinction between benign and malignant diseases like bone marrow diseases. T2 mapping is already of great interest for cartilage assessment and can thus be obtained without additional acquisition time. Moreover, the combination of quantitative T1 and T2 also reveals new information, completely independent of the MRI technique, and is expected to be more accurate and relevant than traditional MRI results according to a recent publication from the European Society of Radiology (3). Furthermore, the effect of contrast agents has not been studied. Finally, we must clarify that synthetic MRI is intrinsically incapable of producing spectral fat saturation in its current design. Synthetic MRI is based on T1, T2, and PD measurements in each voxel, which enables calculation of signal intensity using Bloch equations for a spin echo sequence. This can produce any signal intensity for a given TE, TR, or TI. In this workflow, therefore, the addition of a spectrally selective fat saturation was not included. The only way to perform suppression of lipid signal is to use inversion recovery, as we did using the STIR sequence. The advantage here is that the synthetic STIR is produced with the same spatial resolution as the other sequences, which is rarely the case in practice since this sequence has a lower SNR than conventional sequences (without fat saturation) due to the inversion of the spins.
For the future, the application of this technique at higher field strength could benefit from higher SNR and enable the acquisition of thinner slices. Also, one major improvement in the MRI technique was the development of 3D isotropic sequences, which improved cartilage investigation, thus rendering multiplanar reconstructions available. It is clear that the development of a 3D sequence for synthetic acquisition would be of great interest in the future and would probably benefit knee imaging.
Synthetic MRI alone provided good diagnostic confidence, with overall good image quality. This promising technique can be used in musculoskeletal medicine when several contrasts are needed, such as T1, T2, PD, or fat saturation weighted. The image quality is still slightly lower, however, particularly in T1 or STIR contrasts, with flow artifacts in the popliteal fossa.
In conclusion, our preliminary study showed that synthetic MRI is a method with potential use for evaluating the knee and provides as good quality as conventional sequences in a shorter time, despite the presence of some artifacts and its limitations in terms of interpreting ligament structures. We believe that these limitations can be overcome with the use of another acquisition orientation and thinner slices, and that the additional benefit of having a true quantitative acquisition is an important step for MRI standardization for lesion characterization. Further study with large numbers of cases is needed to validate this technique.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Ahn JM, El-Khoury GY. Role of magnetic resonance imaging in musculoskeletal trauma. Top Magn Reson Imaging 2007; 18: 155–168. [DOI] [PubMed] [Google Scholar]
- 2.Kumaravel M, Weathers WM. Emergency magnetic resonance imaging of musculoskeletal trauma. Magn Reson Imaging Clin N Am 2016; 24: 391–402. [DOI] [PubMed] [Google Scholar]
- 3.Tuite MJ, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria acute trauma to the knee. J Am Coll Radiol 2015; 12: 1164–1172. [DOI] [PubMed] [Google Scholar]
- 4.Bohndorf K, Kilcoyne RF. Traumatic injuries: imaging of peripheral musculoskeletal injuries. Eur Radiol 2002; 12: 1605–1616. [DOI] [PubMed] [Google Scholar]
- 5.Sanders TG, Miller MD. A systematic approach to magnetic resonance imaging interpretation of sports medicine injuries of the knee. Am J Sports Med 2005; 33: 131–148. [DOI] [PubMed] [Google Scholar]
- 6.Magee T, Williams D. 3.0-T MRI of meniscal tears. Am J Roentgenol 2006; 187: 371–375. [DOI] [PubMed] [Google Scholar]
- 7.Oei EH, Nikken JJ, Verstijnen AC, et al. MR imaging of the menisci and cruciate ligaments: a systematic review. Radiology 2003; 226: 837–848. [DOI] [PubMed] [Google Scholar]
- 8.Kohl S, Meier S, Ahmad SS, et al. Accuracy of cartilage-specific 3-Tesla 3D-DESS magnetic resonance imaging in the diagnosis of chondral lesions: comparison with knee arthroscopy. J Orthop Surg 2015; 29: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Dyck P, Vanhevel F, Vanhoenacker FM, et al. Morphological MR imaging of the articular cartilage of the knee at 3 T-comparison of standard and novel 3D sequences. Insights Imaging 2015; 6: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith TO, Drew BT, Toms AP, et al. Accuracy of magnetic resonance imaging, magnetic resonance arthrography and computed tomography for the detection of chondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc 2012; 20: 2367–2379. [DOI] [PubMed] [Google Scholar]
- 11.De Smet AA, Tuite MJ. Use of the “two-slice-touch” rule for the MRI diagnosis of meniscal tears. Am J Roentgenol 2006; 187: 911–914. [DOI] [PubMed] [Google Scholar]
- 12.Jung JY, Yoon YC, Kwon JW, et al. Diagnosis of internal derangement of the knee at 3.0-T MR imaging: 3D isotropic intermediate-weighted versus 2D sequences. Radiology 2009; 253: 780–787. [DOI] [PubMed] [Google Scholar]
- 13.Homsi R, Gieseke J, Luetkens JA, et al. Three-dimensional isotropic fat-suppressed proton density-weighted MRI at 3 Tesla using a T/R-coil can replace multiple plane two-dimensional sequences in knee imaging. Rofo 2016; 188: 949–956. [DOI] [PubMed] [Google Scholar]
- 14.Jung JY, Yoon YC, Kim HR, et al. Knee derangements: comparison of isotropic 3D fast spin-echo, isotropic 3D balanced fast field-echo, and conventional 2D fast spin-echo MR imaging. Radiology 2013; 268: 802–813. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, Lee SY, Park NH, et al. Three-dimensional isotropic T2-weighted fast spin-echo (VISTA) knee MRI at 3.0 T in the evaluation of the anterior cruciate ligament injury with additional views: comparison with two-dimensional fast spin-echo T2-weighted sequences. Acta Radiol 2016; 57: 1372–1379. [DOI] [PubMed] [Google Scholar]
- 16.Andica C, Hagiwara A, Nakazawa M, et al. The advantage of synthetic MRI for the visualization of early white matter change in an infant with Sturge-Weber syndrome. Magn Reson Med Sci 2016; 15: 347–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granberg T, Uppman M, Hashim F, et al. Clinical feasibility of synthetic MRI in multiple sclerosis: a diagnostic and volumetric validation study. Am J Neuroradiol 2016; 37: 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012; 22: 276–282. [PMC free article] [PubMed] [Google Scholar]
- 19.Hagiwara A, Hori M, Yokoyama K, et al. Synthetic MRI in the detection of multiple sclerosis plaques. Am J Neuroradiol 2017; 38: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blystad I, Warntjes JB, Smedby O, et al. Synthetic MRI of the brain in a clinical setting. Acta Radiol 2012; 53: 1158–1163. [DOI] [PubMed] [Google Scholar]
- 21.Betts AM, Leach JL, Jones BV, et al. Brain imaging with synthetic MR in children: clinical quality assessment. Neuroradiology 2016; 58: 1017–1026. [DOI] [PubMed] [Google Scholar]
- 22.Mamere AE, Saraiva LA, Matos AL, et al. Evaluation of delayed neuronal and axonal damage secondary to moderate and severe traumatic brain injury using quantitative MR imaging techniques. Am J Neuroradiol 2009; 30: 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldman AD. Magnetic resonance imaging of brain tumors- time to quantify. Discov Med 2010; 9: 7–12. [PubMed] [Google Scholar]