Abstract
Purpose:
Intravenous (IV) compounding safety has garnered recent attention as a result of high-profile incidents, awareness efforts from the safety community, and increasingly stringent practice standards. New research with more-sensitive error detection techniques continues to reinforce that error rates with manual IV compounding are unacceptably high. In 2014, our team published an observational study that described three types of previously unrecognized and potentially catastrophic latent chemotherapy preparation errors in Canadian oncology pharmacies that would otherwise be undetectable. We expand on this research and explore whether additional potential human failures are yet to be addressed by practice standards.
Methods:
Field observations were conducted in four cancer center pharmacies in four Canadian provinces from January 2013 to February 2015. Human factors specialists observed and interviewed pharmacy managers, oncology pharmacists, pharmacy technicians, and pharmacy assistants as they carried out their work. Emphasis was on latent errors (potential human failures) that could lead to outcomes such as wrong drug, dose, or diluent.
Results:
Given the relatively short observational period, no active failures or actual errors were observed. However, 11 latent errors in chemotherapy compounding were identified. In terms of severity, all 11 errors create the potential for a patient to receive the wrong drug or dose, which in the context of cancer care, could lead to death or permanent loss of function. Three of the 11 practices were observed in our previous study, but eight were new. Applicable Canadian and international standards and guidelines do not explicitly address many of the potentially error-prone practices observed.
Conclusion:
We observed a significant degree of risk for error in manual mixing practice. These latent errors may exist in other regions where manual compounding of IV chemotherapy takes place. Continued efforts to advance standards, guidelines, technological innovation, and chemical quality testing are needed.
INTRODUCTION
The past few decades have brought a growing awareness of the effect of human error on health care, including in oncology practice. Although several studies have established rates of error, or active failures, with chemotherapy,1-4 theorists such as Reason5 have highlighted the importance of studying working conditions so that the underlying potential human failures, or latent errors, can be remedied. For example, whereas an active failure would be the selection and use of the wrong drug during chemotherapy mixing, a latent error would be the storage of look-alike drugs beside each other.
In 2014, members of our team published an observational study that described three types of previously unrecognized latent chemotherapy preparation errors in Canadian oncology pharmacies.6 The following potentially catastrophic compounding errors would not have been detectable if they occurred:
For drugs that require reconstitution, the incorrect volume or type of diluent could be injected into a chemotherapy vial because quality checks of reconstitution were not in routine use and may not always be effective when they are used.
The incorrect medication could be selected and drawn into a syringe but the correct vial shown to the pharmacist during their check because multiple drugs often were stored in proximity in the biologic safety cabinet (BSC).
The wrong patient-specific drug label could be applied to a correctly mixed bag because labels often were not physically affixed to the bag in which the drugs were to be injected until after mixing was complete.
Since, intravenous (IV) compounding safety has garnered increased attention as a result of some high-profile incidents,7,8 awareness efforts from the safety community,9-11 and increasingly stringent practice standards.12-16 Meanwhile, new research with more-sensitive error detection techniques than in past studies has continued to reinforce that error rates with manual IV compounding are unacceptably high.17-19
As did our original work, this study used human factors–informed observation and analysis to identify and describe latent errors. Whereas our previous study explored latent errors across the entire chemotherapy delivery pathway from ordering to administration, the goal of this study was to gain a deeper understanding of latent errors specific to manual chemotherapy compounding practices.
METHODS
Field observations were conducted in four cancer center pharmacies in four Canadian provinces from January 2013 to February 2015. Sites were chosen from among the organizations that had provided support for the study. Production ranged from 15 to 300 chemotherapy mixes per day.
At each site for approximately 12 hours over a period of 2 to 3 days, human factors specialists observed and interviewed pharmacy managers, oncology pharmacists, pharmacy technicians, and pharmacy assistants as they carried out their work. The scope of observation was from the point the pharmacy received a chemotherapy order to when a completed product was ready to be sent for administration. Emphasis was on potential errors that could lead to outcomes of wrong drug, wrong dose, or wrong diluent. Sterility of the product and safe handling of hazardous drugs, although important, were not the focus.
By using the method of contextual inquiry20 observers asked questions; took detailed notes; collected artifacts (eg, policies, worksheets, de-identified labels); and captured photographs of equipment, materials, and the environment. A spreadsheet was used to document each site’s production practices consistently and followed the format of a task analysis.21 In other words, generalized steps and substeps in chemotherapy production were listed in rows, and practice details for each site were listed in columns. Latent errors were identified by reviewing each practice and asking, “Given human limitations such as memory, attention, and cognitive biases, is an error possible?”
RESULTS
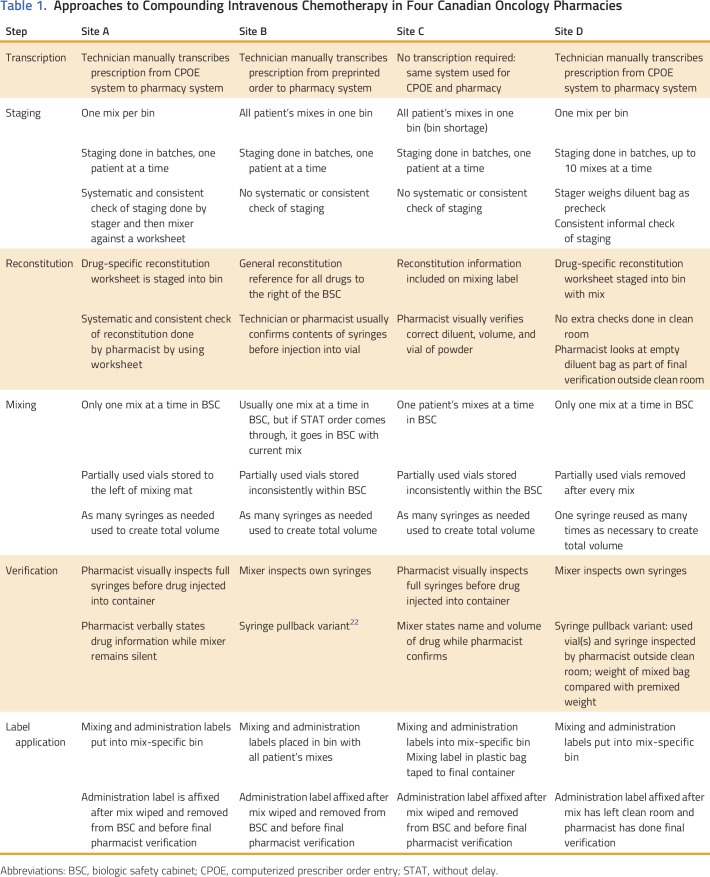
Various approaches to six preparation-related steps were observed among the four sites (Table 1).
Table 1.
Approaches to Compounding Intravenous Chemotherapy in Four Canadian Oncology Pharmacies
Latent Errors
As expected given the relatively short observational period, no active failures or actual errors were observed. However, 11 latent errors were identified and are grouped herein by process category. All three of the latent errors described in our first study6 were observed again (identified with an asterisk), and eight new latent errors were identified. In terms of severity, all 11 latent errors created the potential for a patient to receive the wrong drug or dose, which in the context of cancer care, could lead to death or permanent loss of function. In risk management frameworks, such as Healthcare Failure Mode and Effect Analysis,23 this level of severity would be classified as catastrophic.
Transcription
Transcription comprises the entering of details from the physician’s order into the pharmacy computer system. Pharmacy systems are used downstream from the ordering process, including for the critical step of generating labels, which often act as compounding instructions.
1. Manual transcription of prescription into pharmacy system: In three of the four sites, someone (usually a technician) manually transcribed the chemotherapy prescription details into the pharmacy system, even when the systems were both electronic (two of four sites) because these systems did not interface with each other. Compared with electronic transcription, human transcription is error prone.24 If an error in transcription were to occur, it has the potential to propagate downstream through the system, meaning that a patient could receive a mix that contains any type of error, including wrong drug or wrong dose. Although these sites had transcription checks in place, human error-checking processes are not fail-safe25,26 and were labor intensive.
Staging
Staging encompasses the selection and grouping of materials for compounding before their entry into the BSC. All sites used bins/baskets for staging. During staging, the wrong materials could be chosen or grouped, which can introduce new errors, such as wrong drug, wrong diluent, or wrong dose (eg, if wrong-sized syringes are chosen, the mixer may misinterpret the mixing volume).
2. More than one mix staged in each bin. Two of the sites staged materials for one patient in a single bin, which often meant multiple mixes in one bin. Staging more than one mix per bin creates the potential for the wrong drugs, syringes, final containers, mixing instructions, and/or labels to be loaded into the BSC, which would lead to mixing errors.6
3. No systematic check of staging. Two of the sites did not consistently use systematic staging double-checks. A third site used checks, but they were not systematic. Although double-checks are not fail-safe, they can reduce the potential for error, especially when done independently and with well-designed supportive tools, such as a checklist.25,26 Without an effective staging check, a staging error will likely propagate downstream into mixing and beyond.
Reconstitution
Some chemotherapy drugs must be reconstituted with a precise type and quantity of diluent before being used for compounding. Some manufacturers package the drug with the exact diluent to be added, whereas others require the manual selection and measurement of diluent from the pharmacy’s stock. Each pharmacy had both types of drugs for reconstitution.
If an error in reconstitution occurs, no way of detecting it downstream may exist and could result in a significant dose error. Drug-specific reconstitution protocols and independent reconstitution checks, therefore, are critical.
4. No drug-specific reconstitution instructions staged with mix. One site had a single document outside and to the right of the BSC that listed all the drugs’ reconstitution instructions. Therefore, a technician would need to lean outward and scan the whole document to find a specific drug’s instructions and then interpret them correctly. However, the document was not always referenced, and even when it was, the visual scanning process meant that it would be easy to read the wrong instructions, which would lead to a reconstitution error.
5. No live verification of reconstitution.* Another site had no live inspection of reconstitution: The pharmacist verified reconstitution only after the completed admixture had left the clean room and used proxy methods, such as examination of the diluent bag to estimate correctness (see Verification). Proxy methods of verification could fail, which would lead to a reconstitution error.
Mixing
Mixing entails the combination of precise volumes of drug(s) and a base solution into a final container, such as a diluent bag, bottle, or syringe. Having more than one mix in the BSC at once could result in errors: The technician could withdraw from the incorrect vial of chemotherapy drug but show the correct vial during verification (or have the check fail), and labels could be misapplied.
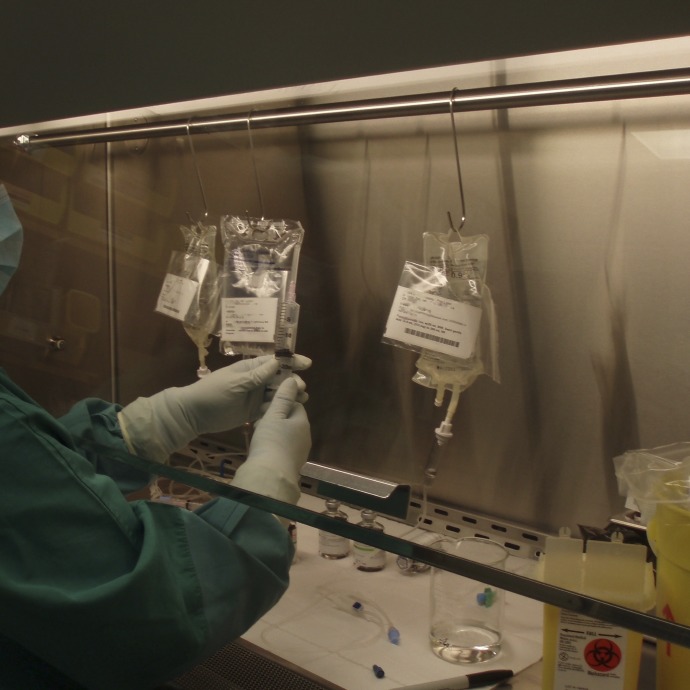
6. More than one mix at a time in BSC.* The two sites that staged patients’ mixes in the same bin also sometimes had one or more mixes in the BSC at once (Fig 1). A mix-up of drug, dose, and/or label, therefore, was possible.
Fig 1.
Several mixes are in the biologic safety cabinet. Labels are paired with associated final containers by using tape and zip-top bags.
One of the challenges with mixing is the management of partially used vials of chemotherapy drugs throughout the day. Two options exist: Keep the vials in the BSC until they are empty or remove partially used vials as soon as the mix is complete then return them to the BSC the next time they are needed. Keeping vials in the BSC means that they have less exposure to environmental contaminants and less chance of a spill; however, a resulting risk of error exists.
7. Accumulation of partially used vials in BSC. Three of the four sites chose to avoid removing partially used vials of chemotherapy drugs from the BSC until the end of the day. If there was a partially used vial of the target drug available in the BSC, it would be used first, followed by a new vial. Of these three sites, two had inconsistent approaches to storage of the vials within the BSC, which makes mix-ups more likely. The risk of having additional high-risk vials in the BSC along with the target vials is the same as having more than one mix at a time: The mixer could mistakenly choose one of these vials instead of the target vial for the mix but still show the correct vial during the check (and/or have the check fail).
Another challenge with mixing is the management of syringes.
8. Reuse of same syringe to create total dose. To reduce environmental waste, one site reused a syringe as many times as necessary to achieve a target volume. Thus, for example, to achieve a total volume of 24 mL, it might use a 10-mL syringe to withdraw 8 mL, inject it into the bag, and repeat twice more. The risk with this approach is the possibility of losing track and injecting too little or too much drug into the final container. The person who checks the mix also could lose track or make a mistake in mental math. Without being able to see all the syringes at once, reliable confirmation of the total volume is difficult.
Verification
Verification of the mixing steps is a widely required and performed practice, but the way in which a verification is performed is critical to safety. Small differences in practice can have a large effect on error detection.
9. Proxy methods of mixing verification. Two of the four sites used a variant of the denounced syringe pullback method.22 At both sites, the mixer would withdraw the drug into the syringe and inject it into the final container without direct observation by a second individual. With the drug still in the syringe, the technician would write the short form of the drug name and mark the line of the drug volume on the barrel of the syringe. The syringes and empty vials would be inspected later. At one site, a mixing assistant would check empty syringes and vials before the mix left the clean room, and at another site, a pharmacist would check empty syringes and vials outside the clean room as part of the final verification. This same site also used a weight-based check where the premixing weight of the bags was documented during staging and the postmixing weight checked against an estimated target weight during the final verification. Although this weight-based check could catch potential mixing errors, some errors could easily be missed. For example, an error in reconstitution could be made but the correct volume of drug withdrawn, or two errors could cancel each other out to create the correct weight.
10. Verification biased by drug and dose being spoken aloud. At two sites, the mixer spoke aloud the name and volume of drug just withdrawn, for example, “five of cyclophosphamide.” Telling the checker what to expect can create confirmation bias: The checker may accept what has been stated aloud rather than see what is being shown. For example, if the mixer believed that he or she had withdrawn (and, therefore, spoke aloud) cyclophosphamide but had withdrawn cyclosporine, the checker might not notice the error. All downstream visual checks would subsequently fail because the final container and the label would be correct.
Labeling
Application of the final label is a critical activity to preventing errors. If a mix has been prepared according to instructions but the wrong label applied, a patient could get the entirely wrong mix and receive his or her own drug out of sequence and at the wrong rate.
11. Label applied after mix has left BSC.* At two of the four sites, administration labels were affixed after the mix had left the BSC, which created the potential for the labels to become lost and/or mixed up and, ultimately, applied to the wrong final container.
DISCUSSION
In this study, 11 latent errors in chemotherapy compounding that could have catastrophic consequences to patients were observed. Three of the 11 problematic compounding practices had been observed in our previous study (indicated with an asterisk), but eight were new:
Manual transcription of prescription into pharmacy system
More than one mix staged in each bin
No systematic check of staging
No drug-specific reconstitution instructions staged with mix
No live verification of reconstitution*
More than one mix at a time in BSC*
Accumulation of partially used vials in BSC
Reuse of same syringe to create total dose
Proxy methods of mixing verification
Verification biased by drug and dose being spoken aloud
Label applied after mix has left BSC*
Relevant regulatory standards do not address these issues. Canadian standards published by Accreditation Canada and the National Association of Pharmacy Regulatory Authorities make no reference to the avoidance of these practices.15,27,28 The equivalent American standards of USP79712 and USP80029 are similar in this respect. Although intended to address safety, most of the content is heavily focused on protecting the final sterile product from contamination and/or on protecting the environment and staff from hazardous exposure.
Other practice guidance documents cover a few of the observed latent errors, although most medication error prevention statements are too high level to address the specific issues that we observed. Safety standards from ASCO,14 error prevention guidelines from the American Society of Hospital Pharmacists,30 and standards of practice from the Canadian Association of Pharmacy in Oncology31 do not have statements that directly address the issues. The Canadian Society for Hospital Pharmacists compounding guidelines32 address issues 3, 6, and 11. The issue of storage of partiality used vials (7) also is addressed somewhat in these guidelines with the statement “used syringes, bottles, vials, and other supplies shall be removed from the critical area in the primary clean air device.” The International Society for Oncology Pharmacy Practice standards of practice33 refers to issue 3 (checking staged materials before they enter the BSC). Its safe compounding guidelines13 exclusively focus on error prevention in compounding and directly address four of the 11 the latent errors, specifically 2, 6, 9, and 11. Thus, no one guideline addresses all issues, a combination of guidelines address some of the issues, and nothing specifically addresses the issues of manual transcription of prescription to pharmacy system, no drug-specific reconstitution instructions with mix, no live verification of reconstitution, reuse of same syringe to create total dose, and verification biased by drug and dose being spoken aloud.
Practice standards must be updated to include statements that address the specific mechanisms of latent error observed in the current study. Although the balancing of requirements for sterility and hazardous protection with those of error prevention may be challenging, the former issues are being given a disproportionate amount of attention in these documents.
Although standards and guidelines are critical elements of a safe system, they are not sufficient. Automated compounding processes from robotics to bar coding and gravimetric weighing, with built-in error prevention functions, have shown promise in error reduction18,19,34-36 and may address or eliminate many of the latent errors observed in the current study. Meanwhile, unlike other industries with high-risk production processes, chemotherapy compounding has not adopted live quality control mechanisms that confirm the actual contents of the prepared compounds (with the exception of a few French sites).37,38 Use of techniques such as spectrometry, high-performance liquid chromatography, and sensors should be implemented to catch errors that do slip through.
In addition, it is technologically feasible for all order-based information technology systems, such as computerized prescriber order entry, and pharmacies to interface, yet often they do not, which can lead to transcription errors. This is unacceptable.
Although these changes are essential, they take time and significant resource investments. Therefore, ongoing observational research and local quality improvement initiatives that identify and address additional latent errors are critical to the overall reduction of preventable errors with chemotherapy. In addition, interim low-technology strategies, such as drug-specific compounding worksheets,39 should be considered.
This observational study included a small number of sites in only four Canadian provinces. As a result, the opportunity to observe many other latent errors likely was missed. However, in this small sample, light has been shed on practice issues that are not addressed by most current safety mechanisms. Another limitation is that no actual errors were observed, which means that it was not possible to know how likely they are to occur. However, for such high-risk activity as chemotherapy compounding, the anticipation and addressing of issues before they result in patient harm are essential.
In conclusion, in this observational, human factors–based study of four Canadian oncology pharmacies, a significant degree of risk for serious error in manual mixing practice was observed. That these latent errors exist in other regions where manual compounding of IV chemotherapy takes place is reasonable to assume. Much room for improvement in IV compounding exists. Continued efforts to advance standards, guidelines, technological innovation, and chemical quality testing are needed.
ACKNOWLEDGMENT
Supported by the Canadian Association of Provincial Cancer Agencies, the BC Cancer Agency, CancerCare Manitoba, Alberta Health Services, and the Princess Margaret Hospital. An unrestricted educational grant from CareFusion Canada was provided to support the manuscript preparation process.
AUTHOR CONTRIBUTIONS
Conception and design: Rachel E. Gilbert, Melissa C. Kozak, Roxanne B. Dobish, Vishal Kukreti, Anthony C. Easty, Patricia L. Trbovich
Administrative support: Rachel E. Gilbert, Heather A. Logan
Provision of study materials or patients: Roxanne B. Dobish, Vishal Kukreti, Venetia C. Bourrier, Paul M. Koke
Collection and assembly of data: Rachel E. Gilbert, Melissa C. Kozak, Roxanne B. Dobish, Venetia C. Bourrier, Paul M. Koke
Data analysis and interpretation: Rachel E. Gilbert, Melissa C. Kozak, Roxanne B. Dobish, Paul M. Koke, Vishal Kukreti, Heather A. Logan, Anthony C. Easty, Patricia L. Trbovich
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Intravenous Chemotherapy Compounding Errors in a Follow-Up Pan-Canadian Observational Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Rachel E. Gilbert
Honoraria: BD
Consulting or Advisory Role: BD Canada
Research Funding: CareFusion Canada
Travel, Accommodations, Expenses: BD
Melissa C. Kozak
No relationship to disclose
Roxanne B. Dobish
No relationship to disclose
Venetia C. Bourrier
No relationship to disclose
Paul M. Koke
No relationship to disclose
Vishal Kukreti
Honoraria: Celgene, Takeda Pharmaceuticals
Heather A. Logan
No relationship to disclose
Anthony C. Easty
Stock and Other Ownership Interests: Bristol-Myers Squibb (I)
Patricia L. Trbovich
Honoraria: BD
Research Funding: BD (Inst)
REFERENCES
- 1.Taylor JA, Winter L, Geyer LJ, et al. : Oral outpatient chemotherapy medication errors in children with acute lymphoblastic leukemia. Cancer 107:1400-1406, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Walsh KE, Dodd KS, Seetharaman K, et al. : Medication errors among adults and children with cancer in the outpatient setting. J Clin Oncol 27:891-896, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Gandhi TK, Bartel SB, Shulman LN, et al. : Medication safety in the ambulatory chemotherapy setting. Cancer 104:2477-2483, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Schwappach DLB, Wernli M: Medication errors in chemotherapy: Incidence, types and involvement of patients in prevention. A review of the literature. Eur J Cancer Care (Engl) 19:285-292, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Reason J: Human error: Models and management. West J Med 172:393-396, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White R, Cassano-Piché A, Fields A, et al. : Intravenous chemotherapy preparation errors: Patient safety risks identified in a pan-Canadian exploratory study. J Oncol Pharm Pract 20:40-46, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention : Multistate outbreak of fungal meningitis and other infections, 2015. https://www.cdc.gov/hai/outbreaks/meningitis.html
- 8.Thiessen JJ. A review of the oncology under-dosing incident, 2013. http://www.health.gov.on.ca/en/public/programs/cancer/drugsupply/docs/report_thiessen_oncology_under-dosing.pdf
- 9.Institute for Safe Medication Practices : Technology and error-prevention strategies: Why are we still overlooking the IV room? 2013.
- 10.Institute for Safe Medication Practices : Safe practices in pharmacy sterile compounding areas, 2011.
- 11.American Society of Health-System Pharmacies : Pharmacy Sterile Compounding Summit: Summary of a Stakeholder Meeting Pharmacy Sterile Compounding Summit, 2013. http://www.pewtrusts.org/~/media/assets/2013/04/15/pharmacysterilecompoundingsummit_report.pdf?la=en
- 12.US Pharmacopeial Convention : General Chapter <797> Pharmaceutical Compounding—Sterile Preparations, 2007. http://www.usp.org/compounding/general-chapter-797
- 13.Institute for Safe Medication Practices : ISMP Guidelines for Safe Preparation of Compounded Sterile Preparations, 2016. https://www.ismp.org/Tools/guidelines/IVSummit/IVCGuidelines.pdf.
- 14.Neuss MN, Gilmore TR, Belderson KM, et al. : 2016 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards, including standards for pediatric oncology. J Oncol Pract 12:1262-1271, 2016 [DOI] [PubMed] [Google Scholar]
- 15.National Association of Pharmacy Regulatory Authorities : Model standards for pharmacy compounding of hazardous sterile preparations, 2016. http://napra.ca/general-practice-resources/model-standards-pharmacy-compounding-hazardous-sterile-preparations
- 16.American Society of Health-System Pharmacists : ASHP guidelines on preventing medication errors in hospitals. Am J Hosp Pharm 50:305-314, 1993 [PubMed] [Google Scholar]
- 17.Poppe LB, Savage SW, Eckel SF: Assessment of final product dosing accuracy when using volumetric technique in the preparation of chemotherapy. J Oncol Pharm Pract 22:3-9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terkola R, Czejka M, Bérubé J: Evaluation of real-time data obtained from gravimetric preparation of antineoplastic agents shows medication errors with possible critical therapeutic impact: Results of a large-scale, multicentre, multinational, retrospective study. J Clin Pharm Ther 42:446-453, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Reece KM, Lozano MA, Roux R, et al. : Implementation and evaluation of a gravimetric i.v. workflow software system in an oncology ambulatory care pharmacy. Am J Health Syst Pharm 73:165-173, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Beyer H, Holtzblatt K: Contextual Design: Defining Customer-Centered Systems. San Francisco, CA, Morgan Kauffman Publishers, 1997 [Google Scholar]
- 21.Hackos JT, Redish JC. User and Task Analysis for Interface Design. New York, NY, Wiley, 1998 [Google Scholar]
- 22.Cohen M, Smetzer J: ISMP medication error report analysis. Hosp Pharm 48:803-806, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Veteran Affairs : The Basics of Healthcare Failure Mode and Effect Analysis. Ann Arbor, MI, Veteran Affairs National Center for Patient Safety, 2001 [Google Scholar]
- 24.Wahi MM, Parks DV, Skeate RC, et al. : Reducing errors from the electronic transcription of data collected on paper forms: A research data case study. J Am Med Inform Assoc 15:386-389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute for Safe Medication Practices : Independent double checks: Undervalued and misused: Selective use of this strategy can play an important role in medication safety, 2013. https://www.ismp.org/newsletters/acutecare/showarticle.aspx?id=51
- 26.White RE, Trbovich PL, Easty AC, et al. : Checking it twice: An evaluation of checklists for detecting medication errors at the bedside using a chemotherapy model. Qual Saf Health Care 19:562-567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Accreditation Canada : Qmentum Program Standards: Ambulatory Systemic Cancer Therapy Services. Ottawa, Ontario, Canada, Accreditation Canada, 2012 [Google Scholar]
- 28.Accreditation Canada : Required Organizational Practices Handbook 2016. Ottawa, Ontario, Canada, Accreditation Canada, 2015 [Google Scholar]
- 29.US Pharmacopeial Convention : USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings, 2018. http://www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare
- 30.ASHP Council on Professional Affairs : ASHP guidelines on preventing medication errors with antineoplastic agents. Am J Heal Syst Pharm 59:1648-1668, 2002. http://www.ajhp.org/content/59/17/1648.long?sso-checked=true [DOI] [PubMed] [Google Scholar]
- 31.Canadian Association of Pharmacy in Oncology : Standards of practice for oncology pharmacy in Canada, 2009. http://www.capho.org/standards-practice
- 32.Canadian Society for Hospital Pharmacists : Compounding: Guidelines for Pharmacies. Ottawa, Ontario, Canada, Canadian Society for Hospital Pharmacists, 2014. p. 150 [Google Scholar]
- 33.International Society of Oncology Pharmacy Practitioners Standards Committee : ISOPP standards of practice. Safe handling of cytotoxics. J Oncol Pharm Pract 13:1-81, 2007. (suppl) https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17933809&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 34.Carrez L, Bouchoud L, Fleury-Souverain S, et al. : Reliability of chemotherapy preparation processes: Evaluating independent double-checking and computer-assisted gravimetric control. J Oncol Pharm Pract 23:83-92, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Moniz TT, Chu S, Tom C, et al. : Sterile product compounding using an i.v. compounding workflow management system at a pediatric hospital. Am J Health Syst Pharm 71:1311-1317, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Nurgat Z, Faris D, Mominah M, et al. : A three-year study of a first-generation chemotherapy-compounding robot. Am J Health Syst Pharm 72:1036-1045, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Limat S, Drouhin JP, Demesmay K, et al. : Incidence and risk factors of preparation errors in a centralized cytotoxic preparation unit. Pharm World Sci 23:102-106, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Nardella F, Beck M, Collart-Dutilleul P, et al. : A UV-Raman spectrometry method for quality control of anticancer preparations: Results after 18 months of implementation in hospital pharmacy. Int J Pharm 499:343-350, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Dobish R, Shultz J, Neilson S, et al. : Worksheets with embedded checklists support IV chemotherapy safer practice. J Oncol Pharm Pract 22:142-150, 2016 [DOI] [PubMed] [Google Scholar]