Abstract
BACKGROUND
Dry eye disease is a common chronic condition that is characterized by ocular discomfort and visual disturbances that decrease quality of life. Many clinicians recommend the use of supplements of n–3 fatty acids (often called omega-3 fatty acids) to relieve symptoms.
METHODS
In a multicenter, double-blind clinical trial, we randomly assigned patients with moderate-to-severe dry eye disease to receive a daily oral dose of 3000 mg of fish-derived n–3 eicosapentaenoic and docosahexaenoic acids (active supplement group) or an olive oil placebo (placebo group). The primary outcome was the mean change from baseline in the score on the Ocular Surface Disease Index (OSDI; scores range from 0 to 100, with higher scores indicating greater symptom severity), which was based on the mean of scores obtained at 6 and 12 months. Secondary outcomes included mean changes per eye in the conjunctival staining score (ranging from 0 to 6) and the corneal staining score (ranging from 0 to 15), with higher scores indicating more severe damage to the ocular surface, as well as mean changes in the tear break-up time (seconds between a blink and gaps in the tear film) and the result on Schirmer’s test (length of wetting of paper strips placed on the lower eyelid), with lower values indicating more severe signs.
RESULTS
A total of 349 patients were assigned to the active supplement group and 186 to the placebo group; the primary analysis included 329 and 170 patients, respectively. The mean change in the OSDI score was not significantly different between the active supplement group and the placebo group (−13.9 points and −12.5 points, respectively; mean difference in change after imputation of missing data, −1.9 points; 95% confidence interval [CI], −5.0 to 1.1; P=0.21). This result was consistent across prespecified subgroups. There were no significant differences between the active supplement group and the placebo group in mean changes from baseline in the conjunctival staining score (mean difference in change, 0.0 points; 95% CI, −0.2 to 0.1), corneal staining score (0.1 point; 95% CI, −0.2 to 0.4), tear break-up time (0.2 seconds; 95% CI, −0.1 to 0.5), and result on Schirmer’s test (0.0 mm; 95% CI, −0.8 to 0.9). At 12 months, the rate of adherence to treatment in the active supplement group was 85.2%, according to the level of n–3 fatty acids in red cells. Rates of adverse events were similar in the two trial groups.
CONCLUSIONS
Among patients with dry eye disease, those who were randomly assigned to receive supplements containing 3000 mg of n–3 fatty acids for 12 months did not have significantly better outcomes than those who were assigned to receive placebo. (Funded by the National Eye Institute, National Institutes of Health; DREAM ClinicalTrials.gov number, NCT02128763.)
Dry eye disease (also known as kera-toconjunctivitis sicca) is a common chronic, inflammatory, age-related condition that causes ocular discomfort, fatigue, and visual disturbances that interfere with reading, computer use, driving, and other aspects of quality of life.1–3 The prevalence of symptomatic dry eye disease among adults in the United States is approximately 14%; rates are higher among women and increase with age.4 Dry eye disease is one of the most common reasons for seeking eye care.2 When the costs of medical care and productivity loss are combined, the annual cost to the U.S. economy is more than $55 billion.5 Patients with dry eye disease use a variety of approaches for symptom relief, including artificial tears, lid scrubs, punctal plugs, and prescription antiinflammatory eyedrops. Many clinicians recommend and many patients take dietary supplements of n–3 fatty acids (often called omega-3 fatty acids), because they have antiinflammatory activity and are not associated with substantial side effects.6
There is no definitive evidence of the efficacy of n–3 fatty acid supplements in the relief of symptoms or in the resolution of signs of dry eye disease. The American Academy of Ophthalmology Preferred Practice Pattern guidelines state that n–3 fatty acid products may be beneficial, “though the evidence is insufficient to establish the effectiveness.”7 The Dry Eye Assessment and Management (DREAM) trial was designed to provide comprehensive information on the effects of n–3 fatty acid supplementation on dry eye disease.
METHODS
TRIAL POPULATION
From October 2014 through July 2016, a total of 923 patients completed a screening visit at 27 clinical centers in the United States (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). At the screening visit, 678 patients (73.5%) met none of the exclusion criteria; these patients were provided with run-in supplements (placebo capsules) and were scheduled to attend an eligibility-confirmation visit approximately 2 weeks later. Of the 615 patients who returned for the eligibility-confirmation visit, 535 (87.0%) were eligible for inclusion in the trial.
The trial was designed to include a broad spectrum of patients with symptomatic moderate-to-severe dry eye disease. Eligibility criteria were an age of 18 years or older, the presence of ocular symptoms related to dry eye disease for at least 6 months, the use of or a desire to use artificial tears an average of at least two times per day during the 2 weeks before the screening visit, and a score on the Ocular Surface Disease Index (OSDI) of 25 to 80 at the screening visit and of 21 to 80 at the eligibility-confirmation visit. Scores on the 12-item OSDI range from 0 to 100, with a score of 0 indicating no ocular discomfort and higher scores indicating greater symptom severity. The minimal clinically meaningful change in score is 10 points.8,9 Scores on three subscales of the OSDI (ocular symptoms, vision-related function, and environmental triggers) also range from 0 to 100, with higher scores indicating greater symptom severity.
In addition, patients had to have at least two of the following four signs in at least one eye: a conjunctival lissamine-green staining score of 1 or more (on a scale ranging from 0 to 6, with higher scores indicating greater abnormality), a corneal fluorescein staining score of 4 or more (on a scale ranging from 0 to 15, with higher scores indicating greater abnormality), a tear break-up time (the time from a blink to the appearance of gaps in the tear film, with shorter times indicating greater abnormality) of 7 seconds or less, and a result on Schirmer’s test with anesthesia (the length of wetting of paper strips placed in the inferior cul de sac of the lower eyelid, with shorter lengths indicating greater abnormality) of 1 to 7 mm in 5 minutes. The same qualifying signs had to be present in the same eye at both the screening visit and the eligibility-confirmation visit.
Patients were excluded from the trial if they did not take at least 90% of the run-in supplements (five per day) or if they had worn contact lenses during the 30 days before the screening visit, had undergone laser-assisted in situ keratomileusis or recent ocular surgery (within the past 6 months), or had a history of ocular infection or contraindications to treatment with high-dose n–3 fatty acid supplementation (see the Supplementary Appendix and the protocol, available at NEJM.org). Patients who were regularly using treatments for dry eye disease (including n–3 fatty acid supplements: eicosapentaenoic acid [EPA] plus docosahexaenoic acid [DHA] at a dose of <1200 mg daily), systemic medications that are known to cause ocular dryness, systemic glucocorticoids, or other immunosuppressive agents were allowed to continue those treatments if they committed to using them for the next 12 months. Patients with a history of thyroid disease, Sjögren’s syndrome, rheumatoid arthritis, or inflammatory diseases could be included in the trial if they were otherwise eligible.
The DREAM Study Research Group had control of the design and conduct of the trial and the interpretation of the data. The protocol was approved by the institutional review board at each center, and the trial was carried out under a Food and Drug Administration Investigational New Drug application. The first four members of the writing committee vouch for the accuracy and completeness of the data and the fidelity of the trial to the protocol. All the patients provided written informed consent.
TRIAL GROUPS
Patients were randomly assigned, in a 2:1 ratio, to receive active or placebo supplements for 12 months. Randomization was performed with the use of a Web-based module and was stratified according to clinical center with a permuted-block method with randomly chosen block sizes. Personnel at the Investigational Drug Service, University of Pennsylvania, mailed the supplements directly to the patients.
In both trial groups, the regimen was five soft-gelatin capsules per day. Each active capsule contained 400 mg of EPA and 200 mg of DHA, for a total daily dose of 2000 mg of EPA and 1000 mg of DHA. Each placebo capsule contained 1000 mg of refined olive oil; each capsule was 68% oleic acid, 13% palmitic acid, and 11% linoleic acid. The active and placebo capsules contained 3 mg of vitamin E (alpha-tocopherol), as an antioxidant, as well as masking flavor and lemon flavor. The Access Business Group manufactured and donated the capsules. The fish oil concentrate in triglyceride form that was included in the active supplements was donated by Epax. The contents of the active and placebo capsules were verified by an independent laboratory (Nutra-source Diagnostics). The regimen was reduced or suspended when the patient reported gastrointestinal symptoms or when a contraindication to treatment with the full dose of active supplements developed. With resolution of symptoms or contraindications, the patient could restart or increase the regimen.
OUTCOME MEASURES
The primary outcome was the mean change from baseline in the OSDI score. The following measures were prespecified secondary outcomes: the proportion of patients with a decrease from baseline in the OSDI score of 10 points or more, changes in the percentages of EPA and DHA in total fatty acids in red cells (by weight), changes in signs of dry eye disease (as assessed by conjunctival staining score, corneal staining score, tear break-up time, and the result on Schirmer’s test), changes in the scores on the physical health and mental health subscales of the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36; scores range from 0 to 100, with higher scores indicating better health-related quality of life), changes in the scores on the discomfort and pain interference subscales of the Brief Ocular Discomfort Index (BODI; scores range from 0 to 100, with higher scores indicating greater discomfort), changes in treatments used for dry eye disease, changes in visual acuity and intraocular pressure (safety outcomes), and the incidence of adverse events. Coordinators asked patients about adverse events during each visit (at 3, 6, and 12 months) and by telephone (at 9 months) and coded these events according to the Medical Dictionary for Regulatory Activities (MedDRA) system; a medical monitor reviewed serious adverse events and their codes. All patients, clinical staff, and laboratory personnel were unaware of the trial-group assignments.
RPS Diagnostics provided their InflammaDry Detector test kits, TearLab provided materials for their TearLab Osmolarity System, and Tear-Science provided their Meibomian Gland Evaluators at a discounted cost. Results of these tests were used to define subgroups for analyses shown in the Supplementary Appendix.
STATISTICAL ANALYSIS
For the primary and secondary outcomes, baseline values were the means of values obtained during the screening and eligibility-confirmation visits, except for values obtained only during the eligibility-confirmation visit (e.g., visual acuity and results of impression cytology). The values used for assessing change were the means of values obtained during the 6-month and 12-month visits; if a value from only one of these visits was available, that value was used. The 97.5 percentile for the mean change from baseline in the EPA level at 6 months in the placebo group (0.32 percentage points) was used as a threshold for adherence to treatment in the active supplement group. Analyses were performed according to the intention-to-treat principle.
Comparisons of the mean change in continuous measures between trial groups and associated 95% confidence intervals were based on linear regression with a robust variance estimator. Generalized estimating equations were used for ocular measures to accommodate the correlation between eyes in the same person.10 Propensity scores and the regression method of multiple imputation were used for missing OSDI scores at month 6 or 12.11 In accordance with the protocol, an analysis of the mean change in the OSDI score with adjustment for the baseline EPA level was performed because of an imbalance between trial groups in the EPA level (P<0.10). Comparisons of categorical outcomes were based on chi-square tests, and 95% confidence intervals for the difference in proportions were calculated with the Wilson method.12 Differences between trial groups in the cumulative proportion of patients with an adverse event were evaluated with the log-rank test; Fisher’s exact test was used when the number of patients in a group with a given adverse event was 5 or fewer. The significance of differences between trial groups for 18 secondary outcomes, measures of adherence to the trial regimen, and safety measures were evaluated with post hoc application of the Benjamini–Hochberg adjustment.
Prespecified subgroups were defined according to baseline severity of symptoms (OSDI score ≥40 vs. <40), severity of signs (severe [conjunctival staining score, ≥2; corneal staining score, ≥4; tear break-up time, <5 seconds; and the result on Schirmer’s test, ≤7 mm in 5 minutes] vs. not severe), EPA and DHA levels in red cells (both levels above vs. one or both levels equal to or below the mean levels in the reference population of the central laboratory [DHA, 3.7%; EPA, 0.6%]), and ocular inflammation (0 vs. 1 vs. 2 eyes with a percentage of HLA-DR+ epithelial cells on impression cytology that was greater than the median percentage among all patients in the trial [5%]). Tests of interaction were used to evaluate whether the effect of supplementation with n–3 fatty acids differed among subgroups.
We determined that a sample of 505 patients would provide the trial with 90% statistical power to detect a 6-point mean difference between trial groups in the mean change in the OSDI score, assuming a standard deviation of 18 points and missing data for 15% of patients. This report includes data that were available by October 24, 2017. Statistical computations were performed with SAS software, version 9.4 (SAS Institute).
RESULTS
PATIENTS AND ADHERENCE
A total of 349 patients were assigned to the active supplement group and 186 to the placebo group. There were no significant imbalances between trial groups in baseline characteristics, except for the higher mean EPA level in the active supplement group than in the placebo group (0.63% vs. 0.56%; P = 0.047) (Table 1, and Table S1 in the Supplementary Appendix). A total of 974 of the 1044 scheduled follow-up visits (93.3%) were completed in the active supplement group, and 498 of the 558 visits (89.2%) were completed in the placebo group. The change in the mean EPA level was 2.2 percentage points in the active supplement group as compared with 0.0 percentage points in the placebo group (P<0.001); the change in the mean DHA level was 1.6 percentage points as compared with −0.1 percentage points (P<0.001), and the change in the mean oleic acid level was −0.1 percentage points and 0.1 percentage points, respectively (P = 0.40) (Table 2). In the active supplement group, for 264 of the 290 patients who were assessed at 6 months (91.0%) and for 247 of the 290 patients who were assessed at 12 months (85.2%), the EPA level exceeded the threshold for adherence to treatment. At 12 months, 269 of 324 patients in the active supplement group (83.0%) and 136 of 164 patients in the placebo group (82.9%) reported taking five capsules daily.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Active Supplement (N = 349) | Placebo (N = 186) |
|---|---|---|
| Age — yr | 58.3±13.5 | 57.5±12.6 |
|
| ||
| Sex — no. (%) | ||
| Female | 284 (81.4) | 150 (80.6) |
| Male | 65 (18.6) | 36 (19.4) |
|
| ||
| Race — no. (%)† | ||
| White | 265 (75.9) | 133 (71.5) |
| Black | 39 (11.2) | 25 (13.4) |
| Other | 45 (12.9) | 28 (15.1) |
|
| ||
| Ethnic group — no. (%)† | ||
| Hispanic or Latino | 44 (12.6) | 24 (12.9) |
| Other | 305 (87.4) | 162 (87.1) |
|
| ||
| OSDI score‡ | ||
| Total | 44.6±14.0 | 44.1±14.6 |
| Vision-related function subscale | 37.2±17.8 | 38.8±17.6 |
| Ocular symptoms subscale | 47.4±16.5 | 45.0±18.2 |
| Environmental triggers subscale | 56.5±23.9 | 53.8±25.4 |
|
| ||
| Treatments used for dry eye disease — no. (%) | ||
| Artificial tears, either drops or gel | 277 (79.4) | 147 (79.0) |
| Cyclosporine drops | 134 (38.4) | 71 (38.2) |
| Warm lid soaks | 80 (22.9) | 34 (18.3) |
| Lid scrubs or baby shampoo | 56 (16.0) | 27 (14.5) |
| Other | 170 (48.7) | 99 (53.2) |
|
| ||
| Fatty acid levels in red cells — %§ | ||
| Eicosapentaenoic acid¶ | 0.63±0.43 | 0.56±0.35 |
| Docosahexaenoic acid | 3.91±1.17 | 3.85±1.11 |
| Oleic acid | 11.11±1.24 | 11.10±1.38 |
|
| ||
| Signs of dry eye disease|| | ||
| Conjunctival staining score | 3.1±1.4 | 2.9±1.4 |
| Corneal staining score | 4.0±2.9 | 3.7±2.4 |
| Tear break-up time — sec | 3.1±1.4 | 3.1±1.6 |
| Result on Schirmer’s test — mm in 5 minutes | 9.3±6.2 | 10.2±7.0 |
Plus–minus values are means ±SD. Baseline values were the means of values obtained during the screening and eligibility-confirmation visits, except for values for fatty acid levels and treatments used for dry eye disease, which were obtained only at the eligibility-confirmation visit.
Race and ethnic group were reported by the patient.
Ocular Surface Disease Index (OSDI) scores range from 0 to 100, with a score of 0 indicating no ocular discomfort and higher scores indicating greater symptom severity. The minimal clinically meaningful change in score is 10 points. Scores on three subscales of the OSDI (ocular symptoms, vision-related function, and environmental triggers) also range from 0 to 100, with higher scores indicating greater symptom severity.
Data are missing for 11 patients in the active supplement group and 4 patients in the placebo group.
P = 0.047 for the mean difference between trial groups.
Data are from assessments of 665 eyes in the active supplement group and 357 eyes in the placebo group. The following results constitute signs of dry eye disease in an eye: a conjunctival lissamine-green staining score of 1 or more (on a scale ranging from 0 to 6, with higher scores indicating greater abnormality), a corneal fluorescein staining score of 4 or more (on a scale ranging from 0 to 15, with higher scores indicating greater abnormality), a tear break-up time (the time from a blink to the appearance of gaps in the tear film, with shorter times indicating greater abnormality) of 7 seconds or less, and a result on Schirmer’s test with anesthesia (the length of wetting of paper strips placed in the inferior cul de sac of the lower eyelid, with shorter lengths indicating greater abnormality) of 1 to 7 mm in 5 minutes.
Table 2.
Primary and Secondary Outcomes.*
| Outcome | Active Supplement (N = 329) | Placebo (N = 170) | Mean Difference (95% CI) | P Value† | ||
|---|---|---|---|---|---|---|
| no. of patients | no. of patients | |||||
| Change in OSDI score | ||||||
| Total | 329 | −13.9±15.6 | 170 | −12.5±18.2 | −1.4 (−4.6 to 1.8) | 0.40 |
| Vision-related function subscale | 329 | −12.1±18.1 | 170 | −12.5±20.3 | −0.4 (−3.2 to 4.0) | 0.83 |
| Ocular symptoms subscale | 329 | −13.9±17.3 | 170 | −12.2±18.8 | −1.7 (−5.1 to 1.7) | 0.32 |
| Environmental triggers subscale | 327 | −17.3±23.5 | 167 | −13.1±27.7 | −4.1 (−9.0 to 0.8) | 0.10 |
|
| ||||||
| Patients with decrease in OSDI score of ≥10 points — no. (%)‡ | 329 | 202 (61) | 170 | 91 (54) | 8 (−1 to 17) | 0.09 |
|
| ||||||
| Change in BODI score§ | 329 | 170 | ||||
| Discomfort subscale | −11.3±18.1 | −9.8±16.3 | −1.5 (−4.6 to 1.6) | 0.34 | ||
| Pain interference subscale | −9.4±15.0 | −8.9±16.2 | −0.4 (−3.4 to 2.5) | 0.77 | ||
|
| ||||||
| Change in SF-36 score¶ | 329 | 170 | ||||
| Physical health subscale | 0.1±6.9 | 0.1±6.3 | 0.0 (−1.2 to 1.2) | 0.95 | ||
| Mental health subscale | −0.9±6.3 | 0.4±7.0 | −1.2 (−2.5 to 0.0) | 0.051 | ||
|
| ||||||
| Patients with change in treatments used for dry eye disease at 12 months — no. (%)‡ | 324 | 164 | 0.60 | |||
| Fewer treatments used, with no additions | 171 (53) | 93 (57) | −4 | |||
| No change | 80 (25) | 36 (22) | 3 | |||
| Same number of treatments used or fewer, with ≥1 treatment switched | 40 (12) | 23 (14) | −2 | |||
| More treatments used | 33 (10) | 12 (7) | 3 | |||
|
| ||||||
| Change in fatty acid levels in red cells — percentage points | 309 | 155 | ||||
| Eicosapentaenoic acid | 2.2±1.2 | 0.0±0.2 | 2.2 (2.0 to 2.3) | <0.001 | ||
| Docosahexaenoic acid | 1.6±1.2 | −0.1±0.7 | 1.8 (1.6 to 1.9) | <0.001 | ||
| Oleic acid | −0.1±1.0 | −0.1±1.0 | −0.1 (−0.3 to 0.1) | 0.40 | ||
|
| ||||||
| Change in signs of dry eye disease | 629|| | 327|| | ||||
| Conjunctival staining score | −0.4±1.1 | −0.4±1.0 | 0.0 (−0.2 to 0.1) | 0.77 | ||
| Corneal staining score | −0.6±1.9 | −0.7±1.7 | 0.1 (−0.2 to 0.4) | 0.61 | ||
| Tear break-up time — sec | 0.7±2.1 | 0.6±1.6 | 0.2 (−0.1 to 0.5) | 0.25 | ||
| Result on Schirmer’s test — mm in 5 minutes | 0.4±5.3 | 0.3±5.0 | 0.0 (−0.8 to 0.9) | 0.95 | ||
|
| ||||||
| Change in visual-acuity score** | 656|| | −0.5±5.0 | 340|| | −0.2±4.8 | −0.3 (−1.0 to 0.4) | 0.42 |
|
| ||||||
| Change in intraocular pressure — mm Hg | 658|| | 0.0±2.3 | 340|| | 0.3±2.4 | −0.3 (−0.7 to 0.1) | 0.17 |
Plus–minus values are means ±SD. Baseline values were the means of values obtained during the screening and eligibility-confirmation visits, except for values for fatty acid levels and treatments used for dry eye disease, which were obtained only at the eligibility-confirmation visit. The values used for assessing change from baseline were the means of values obtained during the 6-month and 12-month visits.
After application of the Benjamini–Hochberg adjustment for multiple comparisons, only the differences in eicosapentaenoic acid and doc-osahexaenoic acid levels were significant, with a false discovery rate of 0.05.
The mean differences are in percentage points.
Brief Ocular Discomfort Index (BODI) scores range from 0 to 100, with higher scores indicating greater discomfort.
Medical Outcomes Study 36-Item Short Form Health Survey (SF-36) scores range from 0 to 100, with higher scores indicating better health-related quality of life.
This is the number of eyes assessed, rather than the number of patients.
Visual-acuity scores of 0 to 100 correspond to Snellen visual-acuity levels of worse than 20/800 to 20/10, respectively.
OUTCOMES
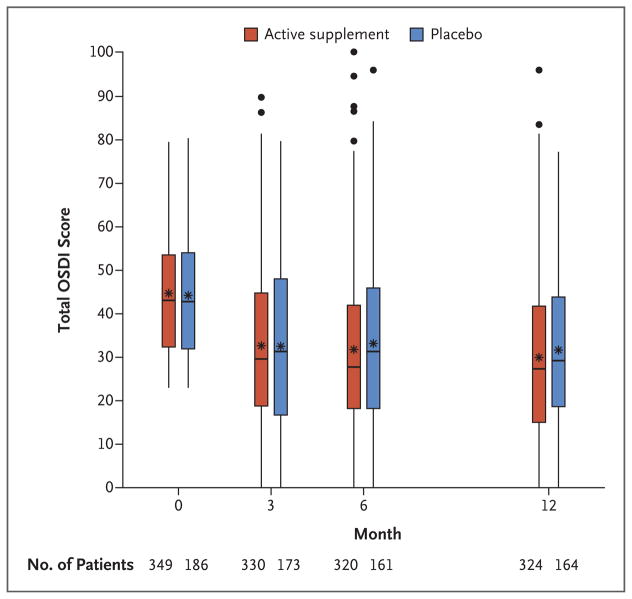
OSDI scores decreased between baseline and 12 months in the active supplement group and in the placebo group (P<0.001 for change in each group); most of the decrease was during the first 3 months (Fig. 1). The mean (±SD) change in the total OSDI score was −13.9±15.6 points in the active supplement group and −12.5±18.2 points in the placebo group, resulting in a mean difference in change of −1.9 points (95% confidence interval [CI], −5.0 to 1.1; P = 0.21) in an analysis performed with the regression method of multiple imputation for missing data (Table S2 in the Supplementary Appendix). The mean difference in change was −1.4 points (95% CI, −4.6 to 1.8; P = 0.40) in an analysis performed without multiple imputation (which included 499 patients) and was −1.6 points (95% CI, −4.8 to 1.6; P = 0.32) in an analysis performed with adjustment for the baseline EPA level (which included 486 patients) (Table 2). The mean changes in scores on the three OSDI subscales were not significantly different in the two trial groups (P≥0.10 for all comparisons).
Figure 1. Distribution of Scores on the Ocular Surface Disease Index.
Shown are box-and-whisker plots of the scores on the Ocular Surface Disease Index (OSDI) in the active supplement group (who received a daily capsule of 3000 mg of n–3 fatty acids) and the placebo group between baseline and 12 months. The upper and lower edges of the boxes correspond to the 75th and 25th percentiles, respectively. Within the boxes, the asterisks correspond to the mean and the lines correspond to the 50th percentile (median). The upper and lower ends of the whiskers correspond to the highest score within 1.5× the interquartile range of the 75th percentile and the lowest score within 1.5× the interquartile range of the 25th percentile, respectively. Each dot outside the whiskers corresponds to one score.
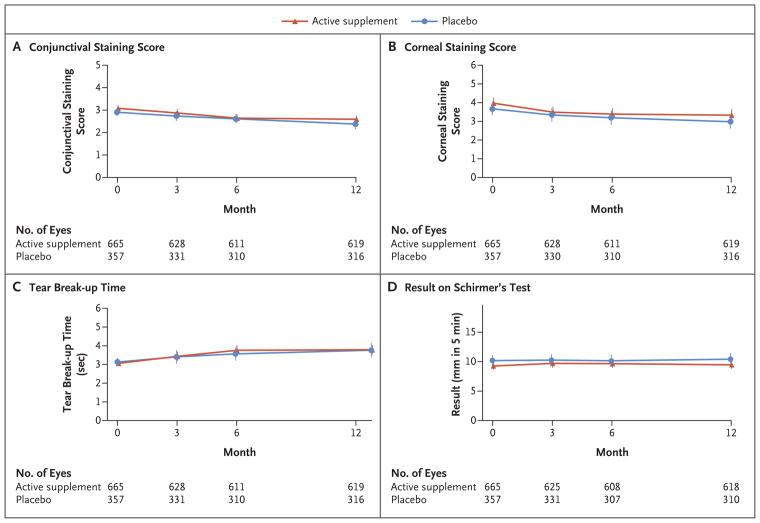
The 4 prespecified subgroup analyses did not reveal any significant interactions (P≥0.29 for all comparisons) (Table 3). Within subgroups, the estimated mean differences in change in the OSDI score between trial groups did not exceed 3.3 points. In addition, 18 exploratory analyses of subgroups defined according to baseline demographic characteristics, use of medications, systemic diseases, signs of dry eye disease, candidate biomarkers for dry eye disease, use of n–3 fatty acid supplements, and self-reported adherence did not reveal any significant interactions (Table S3 in the Supplementary Appendix). The changes in secondary outcome measures were similar in the two trial groups (Table 2). In each trial group, there were improvements between baseline and 12 months in the conjunctival staining score, the corneal staining score, and the tear break-up time (P<0.001 for change for each measure in each group) but not in the result on Schirmer’s test (P = 0.79 for change in the active supplement group; P = 0.46 for change in the placebo group) (Fig. 2). Changes in these signs were not significantly different in the two trial groups (P≥0.25 for all comparisons) (Table 2), as were the changes in mean visual acuity and mean intraocular pressure.
Table 3.
Primary Outcome in Prespecified Subgroups.*
| Subgroup | Active Supplement (N = 329) | Placebo (N = 170) | Mean Difference (95% CI) | P Value for Interaction | ||
|---|---|---|---|---|---|---|
| no. of patients | change in OSDI score | no. of patients | change in OSDI score | |||
| Severity of symptoms | 0.29 | |||||
| OSDI score <40 | 147 | −9.2±11.7 | 73 | −5.9±14.1 | −3.3 (−7.1 to 0.4) | |
| OSDI score ≥40 | 182 | −17.6±17.3 | 97 | −17.5±19.3 | −0.1 (−4.7 to 4.4) | |
|
| ||||||
| EPA and DHA levels† | 0.81 | |||||
| Both levels above the mean level | 99 | −12.4±15.5 | 47 | −10.3±17.1 | −2.1 (−7.8 to 3.6) | |
| One or both levels equal to or below the mean level | 220 | −14.6±15.7 | 120 | −13.3±18.0 | −1.3 (−5.1 to 2.6) | |
|
| ||||||
| Severity of signs‡ | 0.84 | |||||
| Not severe | 228 | −14.4±15.7 | 120 | −12.8±17.4 | −1.6 (−5.3 to 2.1) | |
| Severe | 101 | −12.7±15.4 | 50 | −11.8±20.0 | −0.8 (−7.1 to 5.4) | |
|
| ||||||
| No. of eyes with ocular inflammation§ | 0.67 | |||||
| 0 | 127 | −12.9±14.7 | 70 | −13.3±20.3 | 0.4 (−4.9 to 5.8) | |
| 1 | 65 | −16.9±12.5 | 30 | −15.3±16.2 | −1.7 (−8.1 to 4.7) | |
| 2 | 130 | −12.8±17.2 | 67 | −10.0±16.7 | −2.9 (−7.8 to 2.1) | |
Plus–minus values are means ±SD. Baseline values were the means of values obtained during the screening and eligibility-confirmation visits. The values used for assessing change from baseline were the means of values obtained during the 6-month and 12-month visits.
The mean levels in the reference population of the central laboratory were 3.7% for docosahexaenoic acid (DHA) and 0.6% for eicosapentaenoic acid (EPA). Data are missing for 10 patients in the active supplement group and 3 patients in the placebo group because no test results were available.
Severe signs were defined as a conjunctival staining score of 2 or more, a corneal staining score 4 or more, a tear break-up time of less than 5 seconds, and a result on Schirmer’s test of 7 mm or less in 5 minutes.
Ocular inflammation was defined as a percentage of HLA-DR+ epithelial cells on impression cytology that was greater than the median percentage among all patients in the trial (5%). Data are missing for 7 patients in the active supplement group and 3 patients in the placebo group because no sample was analyzed, less than 10,000 cells were available, or only one eye had results.
Figure 2. Change in Signs of Dry Eye Disease.
The following results constitute signs of dry eye disease in an eye: a conjunctival lissamine-green staining score of 1 or more (on a scale ranging from 0 to 6, with higher scores indicating greater abnormality), a corneal fluorescein staining score of 4 or more (on a scale ranging from 0 to 15, with higher scores indicating greater abnormality), a tear break-up time (the time from a blink to the appearance of gaps in the tear film, with shorter times indicating greater abnormality) of 7 seconds or less, and a result on Schirmer’s test with anesthesia (the length of wetting of paper strips placed in the inferior cul de sac of the lower eyelid, with shorter lengths indicating greater abnormality) of 1 to 7 mm in 5 minutes. In each trial group, there was a significant change between baseline and 12 months (with time as a continuous variable) in the conjunctival staining score, the corneal staining score, and the tear break-up time (P<0.001 for change for each measure in each group) but not in the result on Schirmer’s test (P = 0.79 for change in the active supplement group; P = 0.46 for change in the placebo group).
ADVERSE EVENTS
The percentage of patients with at least one serious adverse event was 6.0% in the active supplement group and 8.1% in the placebo group (P = 0.31). The percentage of patients with at least one nonserious adverse event was similar in the active supplement group and the placebo group (61.9% and 60.8%, respectively; P = 0.87), as was the percentage of patients with an episode of bleeding (2.0% and 1.6%, respectively; P = 1.00). A higher percentage of patients reported diarrhea in the active supplement group than in the placebo group (4.9% and 1.6%, respectively; P = 0.09) (Tables S4 and S5 in the Supplementary Appendix).
DISCUSSION
In this randomized, multicenter clinical trial of 12 months of daily oral supplementation with 3000 mg of n–3 fatty acids for the treatment of dry eye disease, symptoms and signs improved both among patients who received the active supplement and among those who received placebo, and there was no significant difference in improvement between the two groups. The mean OSDI score decreased (improved) significantly, by approximately 13 points, in each group during follow-up, with greater improvement by 1.9 points (95% CI, −5.0 to 1.1; P = 0.21) in the active supplement group than in the placebo group. There was virtually no difference between the two groups in the improvement in four key signs of dry eye disease (P≥0.25 for all comparisons) (Table 2).
Several fundamental aspects of the design and conduct of the DREAM trial contribute to the validity and generalizability of the results. This trial was a “real world” clinical trial, including patients with typical dry eye disease who sought relief of symptoms despite the use of other interventions. Patients were recruited from private and academic optometry and ophthalmology practices throughout the United States and had symptoms and signs of moderate-to-severe dry eye disease on two consecutive examinations that were performed 2 weeks apart. Patients were allowed to continue their current treatments for dry eye disease, which is not the case in most industry-sponsored trials of treatments for this disease. Eye examinations were conducted according to a standard protocol by trial-certified staff to reduce variation between visits and examiners. The 1-year follow-up period minimized the effect of seasonal factors. The dose of n–3 fatty acids (3000 mg daily) was the highest dose used in previous clinical trials of fish-derived n–3 fatty acids. The daily placebo was approximately 1 teaspoon of olive oil, which primarily delivers n–9 oleic acid, a substance that is considered to be neutral with respect to changes in symptoms and signs of dry eye disease. An independent laboratory verified the fatty acid composition of the supplements. The change in the n–3 fatty acid level, as measured in red-cell membranes, indicated a high level of adherence to treatment among patients in the active supplement group; the mean EPA level increased by 400% in the active supplement group, whereas the mean oleic acid level changed by less than 1% in either trial group. The percentage of completed follow-up visits was high (93.3% in the active supplement group and 89.2% in the placebo group). As in other clinical trials of treatments for dry eye disease, the placebo effect was substantial.13,14 Results from the analysis of the primary outcome (mean change in the OSDI score), prespecified subgroup analyses, and analyses of all the secondary outcomes consistently showed a difference between the active supplement group and the placebo group that was small and not significant.
Direct comparison of the results of the DREAM trial with the results of previous randomized, placebo-controlled clinical trials of n–3 fatty acids for dry eye disease is complicated by differences in eligibility criteria, n–3 fatty acid dose, placebo content, duration of supplementation, criteria regarding the use of other treatments for dry eye disease, dietary practices of the participants (e.g., diet in India), and outcome measures.15–27 Aside from several large trials that were conducted in India by Bhargava et al.,15–18 the previous clinical trials involved fewer than 125 total patients. Most of these trials showed significantly greater improvement in symptoms or in at least one of several signs of dry eye disease in the n–3 fatty acid group than in the placebo group.15,18–22,24,26 Some studies had highly restrictive eligibility criteria. For example, of the 375 patients in the DREAM trial who had tear osmolarity measurements, only 24 (6.4%) would have met the following eligibility criteria in a recent study: an osmolarity of at least 312 mOsm per milliliter in at least one eye and mild meibomian-gland dysfunction in both eyes.20 Other studies included only patients with rosacea17 or with results on Schirmer’s test of less than 5 mm in 5 minutes.22 In the DREAM trial, analyses of subgroups that were defined according to these and other criteria did not yield evidence of a benefit from treatment as compared with placebo.
In conclusion, among patients who had moderate-to-severe dry eye disease despite the use of other treatments and were randomly assigned to receive either n–3 fatty acid or placebo supplements, symptoms and signs had improved. We found no evidence of a beneficial effect of n–3 fatty acid supplements as compared with placebo supplements among patients with dry eye disease.
Supplementary Material
Acknowledgments
Supported by cooperative agreements (U10EY022879 and U10EY022881) from the National Eye Institute, National Institutes of Health (NIH), and by grant supplements from the NIH.
Footnotes
Disclosure forms provided by the authors are available at NEJM.org.
References
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–65. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Wei Y, Asbell PA. The core mechanism of dry eye disease is inflammation. Eye Contact Lens. 2014;40:248–56. doi: 10.1097/ICL.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring Study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799–806. doi: 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30:379–87. doi: 10.1097/ICO.0b013e3181f7f363. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Ophthalmology Cornea/External Disease Panel. Preferred practice pattern guidelines: dry eye syndrome. San Francisco: Academy of Ophthalmology; 2013. ( https://www.aao.org/preferred-practice-pattern/dry-eye-syndrome-ppp--2013) [Google Scholar]
- 8.12-Item Ocular Surface Disease Index (OSDI) administration and scoring manual. Irvine, CA: Allergan; Nov, 2004. [Google Scholar]
- 9.Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the Ocular Surface Disease Index. Arch Ophthalmol. 2010;128:94–101. doi: 10.1001/archophthalmol.2009.356. [DOI] [PubMed] [Google Scholar]
- 10.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 11.Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- 12.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–90. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Foulks GN. Challenges and pitfalls in clinical trials of treatments for dry eye. Ocul Surf. 2003;1:20–30. doi: 10.1016/s1542-0124(12)70004-6. [DOI] [PubMed] [Google Scholar]
- 14.Holland EJ, Luchs J, Karpecki PM, et al. Lifetegrast for the treatment of dry eye disease: results of a phase III, randomized, double-masked, placebo-controlled trial (OPUS-3) Ophthalmology. 2017;124:53–60. doi: 10.1016/j.ophtha.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Bhargava R, Kumar P, Kumar M, Mehra N, Mishra A. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int J Ophthalmol. 2013;6:811–6. doi: 10.3980/j.issn.2222-3959.2013.06.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhargava R, Kumar P, Phogat H, Kaur A, Kumar M. Oral omega-3 fatty acids treatment in computer vision syndrome related dry eye. Cont Lens Anterior Eye. 2015;38:206–10. doi: 10.1016/j.clae.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Bhargava R, Chandra M, Bansal U, Singh D, Ranjan S, Sharma S. A randomized controlled trial of omega 3 fatty acids in rosacea patients with dry eye symptoms. Curr Eye Res. 2016;41:1274–80. doi: 10.3109/02713683.2015.1122810. [DOI] [PubMed] [Google Scholar]
- 18.Bhargava R, Kumar P. Oral omega-3 fatty acid treatment for dry eye in contact lens wearers. Cornea. 2015;34:413–20. doi: 10.1097/ICO.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 19.Deinema LA, Vingrys AJ, Wong CY, Jackson DC, Chinnery HR, Downie LE. A randomized, double-masked, placebo-controlled clinical trial of two forms of omega-3 supplements for treating dry eye disease. Ophthalmology. 2017;124:43–52. doi: 10.1016/j.ophtha.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35:1185–91. doi: 10.1097/ICO.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kangari H, Eftekhari MH, Sardari S, et al. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology. 2013;120:2191–6. doi: 10.1016/j.ophtha.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Kawakita T, Kawabata F, Tsuji T, Kawashima M, Shimmura S, Tsubota K. Effects of dietary supplementation with fish oil on dry eye syndrome subjects: randomized controlled trial. Biomed Res. 2013;34:215–20. doi: 10.2220/biomedres.34.215. [DOI] [PubMed] [Google Scholar]
- 23.Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:336–56. [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra C, Singh S, Chakma P, Jain AK. Effect of oral omega-3 fatty acid supplementation on contrast sensitivity in patients with moderate meibomian gland dysfunction: a prospective placebo-controlled study. Cornea. 2015;34:637–43. doi: 10.1097/ICO.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 25.Oleñik A, Jiménez-Alfaro I, Alejandre-Alba N, Mahillo-Fernández I. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin Interv Aging. 2013;8:1133–8. doi: 10.2147/CIA.S48955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheppard JD, Jr, Singh R, McClellan AJ, et al. Long-term supplementation with n-6 and n-3 PUFAs improves moderate-to-severe keratoconjunctivitis sicca: a randomized double-blind clinical trial. Cornea. 2013;32:1297–304. doi: 10.1097/ICO.0b013e318299549c. [DOI] [PubMed] [Google Scholar]
- 27.Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30:308–14. doi: 10.1097/ICO.0b013e3181f22e03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.