Abstract
Purpose of Review
Epidemiological and animal studies suggest that air pollution may negatively affect the central nervous system (CNS) and contribute to CNS diseases. Traffic-related air pollution is a major contributor to global air pollution, and diesel exhaust (DE) is its most important component.
Recent findings
Several studies suggest that young individuals may be particularly susceptible to air pollution-induced neurotoxicity, and that perinatal exposure may cause or contribute to developmental disabilities and behavioral abnormalities. In particular, a number of recent studies have found associations between exposures to traffic-related air pollution and autism spectrum disorders (ASD), which are characterized by impairment in socialization and in communication, and by the presence of repetitive and unusual behaviors. The cause(s) of ASD are unknown, and while it may have a hereditary component, environmental factors are increasingly suspected as playing a pivotal role in its etiology, particularly in genetically susceptible individuals.
Summary
Autistic children present higher levels of neuroinflammation and systemic inflammation, which are also hallmarks of exposure to traffic-related air pollution. Gene-environment interactions may play a relevant role in determining individual susceptibility to air pollution developmental neurotoxicity. Given the worldwide presence of elevated air pollution, studies on its effects and mechanisms on the developing brain, genetic susceptibility, role in neurodevelopmental disorders, and possible therapeutic interventions, are certainly warranted.
Keywords: Traffic-related air pollution, diesel exhaust, neuro-inflammation, autism spectrum disorders, reelin, gene-environment interactions
Introduction
Air pollution is a mixture of several components, including gases, organic compounds, metals, and ambient particulate matter (PM); the latter is believed to be the most widespread threat, and has been heavily implicated in disease [1, 2]. The populations of many countries, particularly in South and East Asia, are often exposed to relatively high levels of PM (≥ 100 μg/m3) [3, 4]. PM is broadly characterized by aerodynamic diameter (e.g. PM10, equivalent to <10 μm in diameter). Traffic-related air pollution is a major contributor to global air pollution, and diesel exhaust (DE) is its most important component [5]. DE contains more than 40 toxic air pollutants, and is a major constituent of ambient PM, particularly of fine (PM2.5) and ultrafine (UFPM; <100 nm) PM [6]. DE exposure is often utilized as a measure of traffic-related air pollution. The association between air pollution and morbidity and mortality caused by respiratory and cardiovascular diseases is well established [7, 8], and oxidative stress and inflammation are believed to be the most relevant contributors to such effects [9].
In recent years evidence has been accumulating from human epidemiological and animal studies which indicates that air pollution may negatively affect the central nervous system (CNS) and contribute to CNS diseases [10–15]. PM2.5 and UFPM are of much concern, as these particles can enter the circulation and distribute to various organs, including the brain [12, 16], in addition to gaining direct access to the brain through the nasal olfactory mucosa [17–20]. Decreased cognitive function, olfactory dysfunction, auditory deficits, depressive symptoms and other adverse neuropsychological effects have also been reported [21–26]. Post-mortem investigations in highly exposed individuals have revealed increased markers of neuroinflammation and of neurodegenerative pathologies [24, 27–29]. Animal studies corroborate the human observations [2, 30]. For example, dogs exposed to heavy air pollution presented evidence of chronic inflammation and neurodegeneration in various brain regions [10, 31], and mice exposed to traffic in a highway tunnel had higher levels of pro-inflammatory cytokines in brain [32]. Controlled exposure to DE has been reported to alter motor activity, spatial learning and memory, and novel object recognition ability in mice, and to alter emotional behavior and learning capability in rats [33, 34]. Prominent effects of DE exposure in the CNS are oxidative stress and neuro-inflammation [35–40].
Developmental neurotoxicity of air pollution
Epidemiological and animal studies suggest that young individuals may be particularly susceptible to air pollution-induced neurotoxicity [22, 24, 26–28, 41–45]. Human studies have revealed a series of biochemical and behavioral alterations in children exposed pre- and/or post-natally to elevated air pollution. In addition, developmental exposure to air pollution, particularly traffic related air pollution, has been suggested to play a role as an etiological factor in autism spectrum disorders (see following sections).
A series of studies in Mexico City have revealed elevated levels of neuroinflammatory markers in brain of children exposed to high air pollution, as well as cognitive deficits [24, 27, 42, 46]. Saenen et al. [47] found a decreased expression of genes associated with the brain-derived growth factor signaling pathway in placenta upon exposure to PM2.5. Newman et al. [48] reported hyperactivity in 7-year old children associated with early life exposure to traffic-related air pollution. In six European cohorts, exposure to air pollution during pregnancy was found to be associated with delayed psychomotor development [43]. Similar results were found in a study in Japan, in which air pollution exposure during gestation was associated with delays in developmental milestones in children at both 2.5 and 5.5 years of age [49]. Additional studies reported that exposure to traffic-related air pollution was inversely associated with sustained attention in adolescents [50], and to lower cognitive development in primary school children [51]. The latter was confirmed in another study in Spain, in which developmental exposure to PM2.5 was associated with a 11–30% reduction in cognitive development [52]. In a population of children in Eastern Massachusetts, mid-childhood exposure to air pollution, particularly to black carbon, was reported to be associated with diminished executive functions at 6–10 years of age [53]. Chiu et al. [53] reported that pre-natal exposure to air pollution was associated with a number of behavioral alterations in children, mostly in boys. In particular, exposure to PM2.5 in gestational weeks 31–36 was associated with lower IQ, while earlier exposures (weeks 20–26) were associated with lower attention. Deficits in reaction time and memory were also found [54]. In a recent review Xu et al. [55] identified a total of 41 human studies which examined the potential effects of ambient or traffic-related air pollution on children (including those specifically investigating autism). They concluded that “evidence suggests that prenatal exposure to air pollutants may have impacts of child neurodevelopment regardless of different study designs, study populations, air pollution exposure assessments, and outcome measurements” [55].
Experimental studies also indicate that developmental exposure to DE may cause neurotoxicity [56]. In utero exposure to high levels of DE (1.0 mg/m3) caused alterations in motor activity, motor coordination and impulsive behavior, as well as changes in neurotransmitters, in male offspring [34, 57, 58]. Depression-like responses were found in mice exposed prenatally to urban air nanoparticles at somewhat lower concentrations (350 μg/m3) [59]. Additional studies have shown that developmental DE exposure of mice alters motor activity, spatial learning and memory, and novel object recognition ability, and causes changes in gene expression, neuro-inflammation, and oxidative damage [33, 60–64]. Prenatal exposure of mice to a low level of DE (90 μg/m3) has been found to enhance territorial aggression induced by social isolation in male mice [65]. Early postnatal exposure of mice to concentrated ambient PM was reported to cause various behavioral changes, including long-term impairment of short term memory, and impulsivity-like behavior [66, 67]. Additional human and animal studies have focused on the potential effects of developmental air pollutant exposure on autism-like behaviors and of their potential etiological role in autism, and are discussed in a following section.
Developmental exposure to air pollution and autism spectrum disorders
Autism is a neurodevelopmental disorder characterized by marked reduction of social and communicative skills, and by the presence of stereotyped behaviors [68]. The term autism spectrum disorders (ASD) is usually utilized to include autism and a range of similar disorders, such as Asperger’s syndrome. The symptoms of ASD are typically present before the age of three, and are often accompanied by abnormalities of cognitive functioning, learning, attention, and sensory processing [68]. The incidence of ASD appears to have increased in the past few decades, and it is now estimated at about 7–9/1000, though certain studies have identified up to 27/1000 children affected by ASD [69, 70]. ASD is more common in males than in females [71], and represents an important societal problem, as the economic burden of caring for an individual with ASD and intellectual disability during his/her lifespan has been estimated at $2.4 million [72]. Children with ASD present a number of morphological abnormalities in the brain [68, 73, 74], and alterations in certain neurotransmitter systems [75]. They also have higher levels of oxidative stress [76–78], as well as neuroinflammation and increased systemic inflammation [79–83].
The etiological basis of ASD is unknown, and susceptibility is attributable to both genetic and environmental factors [68, 84–88]. Several candidate susceptibility genes for ASD have been identified, but no single anomaly appears to predominate, and the total fraction of ASD attributable to genetic inheritance may be only about 30–50% [85, 89]. DNA methylation is also altered in the autistic brain, suggesting that epigenetic dysregulation may also contribute to ASD [90, 91]. It is thus apparent that ASD likely results from the complex interactions between genes conferring vulnerability and diverse environmental factors. In addition to air pollution (particularly traffic-related), which is discussed in the next section, chemicals studied in this regard include metals (e.g. mercury, lead), pesticides (e.g. organophosphates) and other industrial chemicals (e.g. polybrominated diphenyl ethers, organochlorine compounds) [87, 88, 92, 93]. Perhaps the strongest association between an environmental factor and ASD has been found with maternal infection [94]. Studies in humans and in various animal species have indeed evidenced that maternal immune activation (MIA), due to viral or bacterial infection, increases neuroinflammation in the placenta and in the fetal brain, leading to offspring that display ASD-like behaviors [95–98]. As discussed in a further section, several effects seen in MIA are also found upon developmental exposure to air pollution.
Traffic-related air pollution and ASD
Several studies have found associations between exposures to traffic-related air pollution and ASD [2, 99]. Two studies in California by Volk et al. [100, 101] found that residential proximity to freeways and gestational and early-life exposure to traffic-related air pollution were associated with autism (OR=1.86; 95% CI=1.04–3.45). Similar results were obtained in another epidemiological study in California [102], and in another one, part of the Nurses’ Health Study II, in which perinatal DE exposure was significantly associated with ASD, particularly in boys [103]. Two further studies in Taiwan [104] and in Pennsylvania [105] also reported of an increased risk of ASD associated with PM and air pollution exposure, while a study by Guxens et al. [106] in four European cohorts found no associations. An additional study in two cohorts in North Carolina and California reported an association between PM exposure and ASD, particularly when exposure occurred in the third trimester of pregnancy [107]. The higher susceptibility of third trimester exposure was also evidenced by a study of Raz et al. [108] in the Nurses’ Health Study II cohort.
The few available animal studies are in agreement with the human observations [2, 30]. Prenatal exposure to DE has been shown to disrupt DNA methylation in the brain [109]. Prenatal and early life exposure of mice to DE is associated with a number of behaviors similar to those present in humans with ASD, including higher levels of motor activity, elevated levels of self-grooming, and increased rearing [110]. Postnatal exposure, on postnatal days (PND) 4–7 and 10–13, to concentrated ambient ultrafine particles caused persistent glial cell (astrocytes and microglia) activation, and ventriculomegaly (lateral ventricular dilation), which occurred preferentially in male mice [67, 111]. Brain region- and sex-dependent alterations in cytokines and neurotransmitters were also found in exposed male and female mice [111]. Using the same exposure protocol these investigators also reported a decreased corpus callosum in both male and female mice, and an increase of glutamate levels, with an excitatory/inhibitory imbalance [112]. Chang et al. (in preparation) found that perinatal exposure of mice to DE at environmentally relevant concentrations [250–300 μg/m3, from gestational day (GD) 0 to PND 21] caused significant behavioral alterations relevant to ASD, in the domains of persistent/repetitive behaviors, communication, and social interactions. Interestingly, the effects of developmental DE exposure were more robust if exposure occurred in both the pre-natal (GD 0 - birth) and post-natal (PND 1–21) periods. Human studies indicated that the association between air pollution and ASD is stronger when exposure occurs in the third trimester of pregnancy [107, 108, 113, 114]. Due to different rates of brain development, the third trimester of pregnancy in humans is equivalent to the first few postnatal weeks in mice and rats [115, 116]. Animal studies, which report robust effects when exposure occurred or continued postnatally (66, 67, 110; Chang et al. in preparation], are thus in agreement with human observations.
Possible mechanisms of developmental neurotoxicity of traffic related air pollution
Currently, the most prominent reported effects of air pollution on the CNS are related to microglia activation with ensuing oxidative stress and neuroinflammation. Such effects have been found in vivo [35–39], and have been reproduced in vitro [117, 118]. For example, in the latter study [118] it was found that diesel exhaust particles activate microglia; microglia-generated oxidant species and pro-inflammatory cytokines such as IL-6 cause neuronal toxicity, which can be prevented by inhibiting microglia activation.
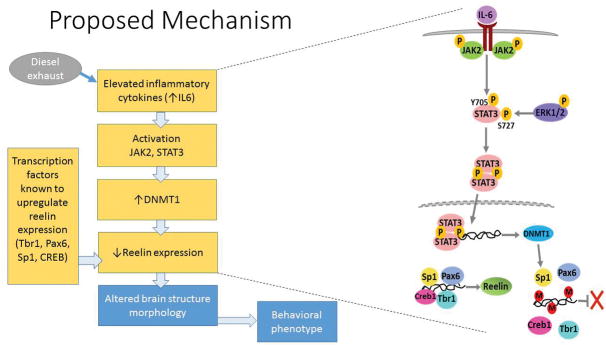
This chain of events may explain many of the observed effects seen in brain of rodents following developmental air pollution exposure. For example, microglia-generated pro-inflammatory cytokines could lead to the observed hypomyelination and ventriculomegaly via toxicity to oligodendrocytes [112]. Closely related to ASD is also the hypothesis of a possible impairment by DE of the reelin signaling system (se Fig. 1). Reelin is a signaling glycoprotein, secreted in the marginal zone of the developing cerebral cortex by Cajal-Retzius cells [119], which plays a most relevant role in neuronal migration and establishment of neuronal polarity [120–123]. In the adult nervous system, reelin is expressed in GABAergic interneurons in the cortex and the hippocampus, where it modulates learning and memory processes, and its reduction may contribute to Alzheimer’s disease [124]. The canonical reelin signaling pathway is activated upon binding of reelin to VLDL receptor and APoE receptor 2, which triggers tyrosine phosphorylation of the intracellular adaptor protein disabled-1 (Dab1). Phosphorylated Dab1 then activates a kinase cascade involving PI-3 kinase, LIM kinase-1, and several others [123]. Such complex networks of signaling pathways mediate the ultimate effects of reelin on neuronal migration and polarity in the developing brain. Strong evidence exists for an involvement of reelin in ASD. First, reelin expression is significantly decreased in brain from ASD subjects [121, 125]. Second, the reelin gene, which maps at chromosome 7q22, is affected in several autistic pedigrees [126–129]. Third, the methylation pattern at the reelin gene promoter is different is ASD and control post-mortem brains [130]. Fourth, mice lacking the C-terminal region of reelin exhibit behavioral abnormalities related to ASD [131]; Fifth, the reeler (rl−/−) mouse, a spontaneously arising mutant mouse, displays several ASD-like morphological and behavioral traits [132, 133]. Sixth, cortical disorganization has been reported in reelin-deficient mice and in ASD patients [74, 134]. Seventh, dysregulation of reelin-driven cortical neuron migration is present in ASD [133]. In addition to all this, MIA which leads to offspring that display neuro-inflammation and ASD-like behaviors [95, 96], has been shown to decrease levels of reelin protein and mRNA in brain of offspring [135–138]. The notion that oxidative stress and neuroinflammation may play an important role in modulating reelin expression is also supported by studies showing that N-acetylcysteine completely prevents lipopolysaccharide (LPS)-induced decreases of reelin [137]. In our laboratory we have found that developmental DE exposure (250–300 μg/m3 from GD0 to PND21) causes neuroinflammation (as evidenced by an increase in IL-6 mRNA) and a decrease of reelin expression (Chang et al. unpublished results).
Fig. 1.
Scheme of a proposed mechanism of developmental effects of DE involving disruption of the reelin pathway (see text for details).
Additional mechanistic hypotheses may and should be formulated with regard to possible effects of developmental air pollution exposure on the observed excitatory/inhibitory imbalance, which is believed to be relevant in ASD [139]. While such imbalance may be due to a reduced GABAergic action or to an increased glutamatergic one, recent evidence suggests that in individuals with ASD the deficit lies in a reduced GABAergic action [140]. The “reelin hypothesis” discussed above may provide at least a partial mechanistic explanation even in this case. By inducing neuroinflammation, and specifically by increasing levels of IL-6, air pollution would also increase expression of DNA methyltransferase-1 (DNMT1) via the JAK/STAT pathway [141]. DNMT1, which in turn modulates the expression of reelin [142, 143], has been found to be increased upon developmental DE exposure (Chang et al. unpublished results). Since DNMT1 also decreases the expression of GAD 67 (glutamic acid decarboxylase 67), a marker of inhibitory GABAergic interneurons [143], this decrease would diminish inhibitory GABAergic neurotransmission, thereby disrupting the balance of excitation/inhibition, as found in ASD and in mouse models of ASD [144]. Of interest is that also maternal immune activation causes a decrease of GAD67 [145].
Possible gene-environment interactions in the developmental neurotoxicity of traffic related air pollution
Animal models that resemble core human autistic symptoms may be useful for studying the etiology and molecular pathogenesis of autism, and for discovering gene-environment interactions [146]. Various strains of mice have been identified that display at least some behavioral traits relevant to ASD, either carrying specific genetic mutations [144, 147], or others whose genetic traits have not been fully characterized, such as the BTBR mouse [148, 149]. However, the marked alterations already present in these mice may represent a “ceiling” effect, and these strains may not be amenable to investigate gene-environment interactions. Nevertheless, De Felice et al. [150, 151] investigated the effects of the organophosphorus insecticide chlorpyrifos in BTBR mice exposed in utero. They found that the effects of chlorpyrifos on oxidative stress and on behavioral maturation were enhanced in BTBR mice compared to C57 mice. These findings suggest that these mice may also be amenable for studying the developmental neurotoxicity of air pollution.
Another interesting transgenic model to investigate potential gene-environment interactions related to developmental DE exposure and ASD, may be the heterozygote reeler mouse (rl+/−). In contrast to the reeler mouse (rl−/−) in which the absence of reelin causes severe disorganization of brain development and severe behavioral effects [132], the rl+/− mouse displays only moderate behavioral abnormalities [152, 153]. The applicability of this model has been shown by the finding that developmental exposure of rl+/− mice to 6 ppm methylmercury increases ASD-like behaviors, particularly in male animals, compared to rl+/+ mice [154]. The hypothesis discussed in the previous section involving a primary role for reelin in the developmental neurotoxicity of air pollution, would be in tune with a potential gene-environment interaction in rl+/− mice.
As oxidative stress and neuroinflammation are preponderant responses to DE exposure (35, 36, 38, 39], another potential interesting model is represented by the Gclm mouse, which lacks the modifier subunit of glutamate-cysteine ligase, the first and rate-limiting enzyme in the synthesis of glutathione (GSH), a main player in cellular defense against oxidative stress. Gclm−/− mice have very low levels of GSH in all tissues including the brain [155], though they may up-regulate other antioxidant pathways; in contrast, Gclm+/− mice have only moderate reductions in GSH, but may not up-regulate alternate defense pathways. In addition, Gclm+/− mice may more closely resemble a very common human polymorphism of Gclm [156]. Gclm+/− mice have been shown to be most sensitive to oxidative stress and neuro-inflammation induced by acute DE exposure (250–300 μg/m3 for 6 h) [30, 39]. This finding confirms a previous observation of enhanced lung inflammation in Gclm+/− mice upon exposure to DE compared to wild type mice [157]. Of great relevance is also the finding that in brain of subjects with ASD, there is a 37% decrease of GCLM protein level, and a 38% decrease in GCL activity [158], which is in agreement with the reported reduced levels of GSH [76]. Thus, the proposed transgenic model (Gclm+/− mice) would be highly relevant to study gene-environment interactions related to developmental exposure to air pollution and ASD.
Conclusion and research needs
While several chemicals present in the environment or in the diet have been considered and studied for potential developmental neurotoxicity, little had been done until recently in this regard for chemicals present in the air. Yet, the air we breathe seems a logical potential source of exposure for chemicals which may exert neurotoxicity or developmental neurotoxicity. Though attention has been limited for several decades only to effects on the respiratory system, and more recently on the cardiovascular system, evidence has been accumulating during the past several years providing strong support to the notion that exposure to high levels of air pollution, very common in many cities all around the world, is associated with damage to the CNS. Human and animal studies have evidenced a series of common adverse effects of air pollution (particularly traffic-related), with oxidative stress and neuro-inflammation emerging as the hallmark biochemical effects, and clinical manifestations which included a variety of behavioral alterations.
As the developing nervous system is particularly sensitive to toxic insult [159], the issue of developmental neurotoxicity of air pollution is especially relevant. Particularly troublesome is the suggestion that air pollution may contribute to the etiopathology of neurodevelopmental diseases whose incidence seems to be increasing in the global populations. This review has focused on ASD, which have been the most studied in this regard, but other disorders such as early onset schizophrenia, or attention deficit hyperactivity disorder, also need to be considered.
Measures to decrease emissions leading to poor air quality are the obvious first choice to pursue in order to protect human health. However, further studies aimed at better characterizing the effects of air pollution on the CNS, its underlying mechanisms, and its role in the etiology of neurodevelopmental diseases are certainly warranted. In particular, the possibility that sexes may be differentially affected by air pollution, with males being more susceptible, needs to be further investigated, in light of the higher incidence of neurodevelopmental disorders (e.g. ASD) in males [71]. In addition, gene-environment interactions still need to be investigated in the context of exposure to high air pollution and effects on the CNS, as developmental abnormalities are likely to be manifest only or especially in susceptible individuals. In this respect, there is the need for experimental studies utilizing transgenic animal models of certain neurodevelopmental disorders (e.g. the reelin heterozygote mouse for ASD) or other transgenic animals addressing specific mechanistic hypotheses (e.g. the Gclm+/− mouse). Markers of genetic susceptibility should also be incorporated in human epidemiological studies, something that has been missing so far. Last but not least, these studies should provide important novel information for therapeutic interventions involving for examples, anti-inflammatory and/or anti-oxidant compounds, drugs that inhibit microglia activation, or others that facilitate GABAergic neurotransmission.
Acknowledgments
Research by the authors is supported by grants from NIEHS (R01ES22949, P30ES07033, P42ES04696, T32ES07032) and NICHD (U54HD083091) and by funds from the Department of Environmental and Occupational Health Sciences, University of Washington).
Footnotes
Compliance with ethics guidelines
Conflict of interest
Lucio G. Costa, Yu-Chi Chang, and Toby B. Cole declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This review article does not contain any studies with human or animal subjects performed by any of the authors. Studies with animals by the authors and reported elsewhere are referred to. These studies were approved by the Institutional Animal Review Board.
References
- 1.Møller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, Vesterdal LK, Forchhammer L, Wallin H, Loft S. Role of oxidative damage in toxicity of particulates. Free Rad Res. 2010;44:1–46. doi: 10.3109/10715760903300691. [DOI] [PubMed] [Google Scholar]
- 2.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicants are in the air: convergence of human and in vitro studies on the effects of air pollution on the brain. BioMed Res Int. 2014:8. doi: 10.1155/2014/736385. ID 736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook RD, Rajagopalan S, Pope A, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitesel L, Kaufman JD on behalf of the American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease. An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 4*.Van Donkelaar A, Martin RV, Brauer M, Boys BL. Use of satellite observations for long-term exposure of global concentrations of fine particulate matter. Environ Health Perspect. 2015;123:135–143. doi: 10.1289/ehp.1408646. This study provides useful information on the global burden of PM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghio AJ, Smith CB, Madden MC. Diesel exhaust particles and airway inflammation. Curr Op Pulm Med. 2012;18:144–150. doi: 10.1097/MCP.0b013e32834f0e2a. [DOI] [PubMed] [Google Scholar]
- 6.USEPA (United States Environmental Protection Agency) Health Assessment Document for Diesel Engine Exhaust. National Center for Environmental Assessment, USEPA; Washington, DC: 2002. p. 669. [Google Scholar]
- 7.Brook RD, Rajagopalan S. Air pollution and cardiovascular events. New Engl J Med. 2007;356:2104–2105. doi: 10.1056/NEJMc070556. [DOI] [PubMed] [Google Scholar]
- 8.Gill EA, Curl CL, Adar SD, Allen RW, Auchincloss AH, O’Neill MS, Park SK, Ven Hee VC, Diez Roux AV, Kaufman JD. Air pollution and cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Progr Cardiovasc Res. 2011;53:353–360. doi: 10.1016/j.pcad.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Møller P, Danielsen PH, Karottki DG, Jantzen K, Rousgaard M, Klingberg H, Jensen DM, Christophersen DV, Hemmingsen JG, Cao Y, Loft S. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat Res. 2014;762:133–166. doi: 10.1016/j.mrrev.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Calderon-Garciduenas L, Azzarelli B, Acuna H, Garcia R, Gambling TM, Osnaya N, Monroy S, Del Rosario Tizapantzi M, Carson JL, Villareal-Calderon A, Rewcastle B. Air pollution and brain damage. Toxicol Pathol. 2002;30:373–389. doi: 10.1080/01926230252929954. [DOI] [PubMed] [Google Scholar]
- 11.Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. J Toxicol. 2012:23. doi: 10.1155/2012/782462. ID 782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, Cory-Slechta DA, Costa D, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33:972–984. doi: 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JC, Wang X, Wellenius GA, Serre ML, Driscoll I, Casanova R, McArdle JJ, Manson JE, Chui HC, Espeland MA. Ambient air pollution and neurotoxicity on brain structure: evidence from Women’s Health Initiative Memory study. Ann Neurol. 2015;78:466–476. doi: 10.1002/ana.24460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, Melly SJ, Wang Y, Dominici F, Zanobetti A. Long-term PM2.5 exposure and neurological hospital admissions in the Northeastern United States. Environ Helath Perspect. 2016;124:23–29. doi: 10.1289/ehp.1408973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberdoerster G, Utell MJ. Ultrafine particles in the urban air: to the respiratory tract –and beyond? Environ Health Perspect. 2002;110:A440–A441. doi: 10.1289/ehp.110-1240959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberdoerster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 18.Peters A, Veronesi B, Calderon-Garciduenas L, Gehr P, Chen LC, Geiser M, Reed W, Rothen-Rutishauser B, Schurch S, Schulz H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Particle Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucchini RG, Dorman DC, Elder A, Veronesi B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology. 2012;33:838–841. doi: 10.1016/j.neuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia GJM, Schroeter JD, Kimbell JS. Olfactory deposition of inhaled nanoparticles in humans. Inhal Toxicol. 2015;27:394–403. doi: 10.3109/08958378.2015.1066904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranft U, Schikowski T, Sugiri D, Krutmann J, Kramer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109:1004–1011. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Freire C, Ramos R, Puertas R, Lopez-Espinosa MJ, Julvez J, Aguilera I, Cruz F, Fernandez MF, Sunyer J, Olea N. Association of traffic-related air pollution with cognitive development in children. J Epidemiol Comm Health. 2010;64:223–228. doi: 10.1136/jech.2008.084574. [DOI] [PubMed] [Google Scholar]
- 23.Calderon-Garciduenas L, Franco-Lira M, Henriquez-Roldan C, Osnaya N, Gomzalez-Maciel A, Reynoso-Robles R, Villareal-Calderon R, Herritt L, Brooks D, Keefe S, Palacios-Moreno J, Villareal-Calderon R, Torres-Jardon R, Medina-Cortina H, Delgado-Chavez R, Aiello-Mora M, Maronpot RR, Doty RL. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol. 2010;62:91–102. doi: 10.1016/j.etp.2009.02.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderon-Garciduenas L, Engle R, Mora-Tiscareno A, Styner M, Gomez-Garza G, Zhu H, Jewells V, Torres-Jardon R, Romero L, Monroy-Acosta ME, Bryant C, Gonzalez-Gonzalez LO, Median-Cortina H, D’Angiulli A. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cognition. 2011;77:345–355. doi: 10.1016/j.bandc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, Nelson RJ. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psych. 2011;16:987–995. doi: 10.1038/mp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guxens M, Sunyer J. A review of epidemiological studies on neuropsychological effects of air pollution. Swiss Med Wkly. 2012;141:w13322. doi: 10.57187/smw.2012.13322. [DOI] [PubMed] [Google Scholar]
- 27.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villareal-Calderon R, Osnaya N, Stone I, Garcia R, Brooks DM, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 28.Calderon-Garciduenas L, Kavanaugh M, Block M, D’Angiulli A, Delgado-Chavez R, Torres-Jardon R, Gonzales-Maciel A, et al. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheim Dis. 2012;28:93–107. doi: 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- 29.Levesque S, Surace MJ, McDonald J, Block ML. Air pollution and the brain: subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammat. 2011a;8:105. doi: 10.1186/1742-2094-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2016 doi: 10.1016/j.neuro.2015.11.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calderon-Garciduenas L, Maronpot RR, Torres Jardon R, Henriquez-Roldan C, Schoonhoven R, Acuna-Ayala H, Villareal-Calderon A, Nakamura J, Fernando R, Reed W, Azzarelli B, Swenberg JA. DNA Damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003;31:524–538. doi: 10.1080/01926230390226645. [DOI] [PubMed] [Google Scholar]
- 32.Bos I, DeBoever P, Emmerechts J, Buekers J, Vanoirbeek J, Meeusen R, Van Poppel M, Nemry B, Nawrot T, Panis LI. Changed gene expression in brains of mice exposed to traffic in a highway tunnel. Inhal Toxicol. 2012;24:676–686. doi: 10.3109/08958378.2012.714004. [DOI] [PubMed] [Google Scholar]
- 33.Win-Shwe TT, Yamamoto S, Fujitani Y, Hirano S, Fujimaki H. Spatial learning and memory function-related gene expression in the hippocampus of mouse exposed to nanoparticles-rich diesel exhaust. Neurotoxicology. 2008;29:940–947. doi: 10.1016/j.neuro.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Yokota S, Moriya N, Iwata M, Umezawa M, Oshio S, Takeda K. Exposure to diesel exhaust during fetal period affects behavior and neurotransmitters in male offspring mice. J Toxicol Sci. 2013;38:13–23. doi: 10.2131/jts.38.13. [DOI] [PubMed] [Google Scholar]
- 35.MohanKumar SMJ, Campbell A, Block M, Veronesi B. Particulate matter, oxidative stress and neurotoxicity. Neurotoxicology. 2008;29:479–488. doi: 10.1016/j.neuro.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RPF, Wang K, Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Particle Fibre Toxicol. 2010;7:12. doi: 10.1186/1743-8977-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Win-Shwe TT, Fujimaki H. Nanoparticles and neurotoxicity. Int J Mol Sci. 2011;12:6267–6280. doi: 10.3390/ijms12096267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, Johnson JA, Duke L, Kodavanti P, Surace MJ, Block ML. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect. 2011b;119:1149–1155. doi: 10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole TB, Coburn J, Dao K, Roqué P, Kalia V, Guilarte T, Diedzic J, Costa LG. Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology. 2016;374:1–9. doi: 10.1016/j.tox.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durga M, Devasena T, Rajasekar A. Determination of LC50 and sub-chronic neurotoxicity of diesel exhaust nanoparticles. Environ Toxicol Pharmacol. 2015;40:615–625. doi: 10.1016/j.etap.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Calderon-Garciduenas L, Torres-Jardon R, Kulesza RJ, Park SB, D’Angiulli A. Air pollution and detrimental effects on children’s brain. The need for a multidisciplinary approach to the issue complexity and challenges. Front Human Neurosci. 2014;8:Art. 613. doi: 10.3389/fnhum.2014.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calderon-Garciduenas L, Kulesza RJ, Doty RL, D’Angiulli A, Torres-Jardon R. Megacities air pollution problems: Mexico City Metropolitan Area critical issues on the central nervous system pediatric impact. Environ Res. 2015;137:157–169. doi: 10.1016/j.envres.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Guxens M, Garcia-Esteban R, Giorgis-Allemand L, Forns J, Badaloni C, Ballester F, Cesaroni G, Chatzi L, et al. Air pollution during pregnancy and childhood cognitive and psychomotor development. Epidemiology. 2014;25:636–647. doi: 10.1097/EDE.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 44.Suades-Gonzalez E, Gascon M, Guxens M, Sunyer J. Air pollution and neuropsychological development: a review of the latest evidence. Endocrinology. 2015;156:3473–3482. doi: 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health-A review of recent concerns. Int J Hyg Environ Health. 2016 doi: 10.1016/j.ijheh.2016.05.001. (in press) [DOI] [PubMed]
- 46.Calderon-Garciduenas L, Cross JV, Franco-Lira M, Aragon-flores M, Kavanaugh M, Torres-Jardon R, et al. Brain immune interactions and air pollution: macrophage inhibitory factor (MIF), prion cellular protein (PrPC), interleukin-6 (IL-6), interleukin 1 receptor antagonist (IL-1Ra), and serum interleukin-2 (IL-2) in cerebrospinal fluid and MIF in serum differentiate urban children exposed to severe vs. low air pollution. Front Neurosci. 2013;7:183. doi: 10.3389/fnins.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saenen ND, Plusquin M, Bijnens E, Jansen BG, Gyselaers W, Cox B, Fierens F, Molenberghs G, Penders J, Vrijens K, De Boever P, Nawrot TS. In utero fine particle air pollution and placental expression of genes in the brain-derived neurotrophic factor signaling pathway; an ENVIRONAGE birth cohort study. Environ Health Perspect. 2015;123:834–840. doi: 10.1289/ehp.1408549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newman NC, Ryan P, LeMasters G, Levin L, Bernstein D, Khurana Hershey GK, Lockey JE, et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ Health Perspect. 2013;121:731–736. doi: 10.1289/ehp.1205555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yorifuji T, Kashima S, Higa Diez M, Kado Y, Sanada S, Doi H. Prenatal exposure to traffic-related air pollution and child behavioral development milestone delays in Japan. Epidemiology. 2016;27:57–65. doi: 10.1097/EDE.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 50.Kicinski M, Vermeir G, Van Larebeke N, Den Hond E, Schoeters G, Bruckers L, Sioen I, et al. Neurobehavioral performance in adolescents is inversely associated with traffic exposure. Environ Int. 2015;75:136–143. doi: 10.1016/j.envint.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 51.Sunyer J, Esnaola M, Alvarez-Pedrerol M, Forns J, Rivas I, Lopez-Vicente M, Suades-Gonzales E, Foraster M, Garcia-Esteban R, et al. Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med. 2015;12:e1001792. doi: 10.1371/journal.pmed.1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Basagaña X, Esnaola M, Rivas I, Amato F, Alvarez-Pedrerol M, Forns J, Lopez-Vicente M, Pujol J, Nieuwenhuijsen M, Querol X, Sunyer J. Neurodevelopmental deceleration by urban fine particles from different emission sources: a longitudinal observational study. Environ Health Perspect. 2016 doi: 10.1289/EHP209. (in press). This is an example of recent studies investigating the behavioral effects of air pollution in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, Schwartz JD, Gryparis A, Kloog I, Koutrakis P, Bellinger DC, Belfort MB, Webster TF, White RF, Sagiv SK, Oken E. Prenatal and childhood traffic-related air pollution exposure and childhood executive function and behavior. Neurotoxicol Teratol. 2016 doi: 10.1016/j.ntt.2016.06.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiu YHM, Hsu HHL, Coull BA, Bellinger DC, Kloog I, Schwartz J, Wright RO, Wright RJ. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ Int. 2016;87:56–65. doi: 10.1016/j.envint.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu X, Uyen Ha S, Basnet R. A review of epidemiological research on adverse neurological effects of exposure to ambient air pollution. Front Public Health. 2016;4:31. doi: 10.3389/fpubh.2016.00157. Art. 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ema M, Naya M, Horimoto M, Kato H. Developmental toxicity of diesel exhaust: a review of studies in experimental animals. Reprod Toxicol. 2013;42:1–17. doi: 10.1016/j.reprotox.2013.06.074. [DOI] [PubMed] [Google Scholar]
- 57.Yokota S, Mizuo K, Moriya N, Oshio S, Sugawara I, Takeda K. Effect of prenatal exposure to diesel exhaust on dopaminergic system in mice. Neurosci Lett. 2009;449:38–41. doi: 10.1016/j.neulet.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki T, Oshio S, Iwata M, Saburi H, Odagiri T, Udagawa T, Sugawara I, Umezawa M, Takeda K. In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Particle Fibre Toxicol. 2010;7:7. doi: 10.1186/1743-8977-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis DA, Bortolato M, Godar SC, Sander TK, Iwata N, Pakbin P, Shih JC, Berhane K, McConnell R, et al. Prenatal exposure to urban air nanoparticles in mice causes altered neuronal differentiation and depression like responses. PLoS ONE. 2013;8(5):e64128. doi: 10.1371/journal.pone.0064128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hougard KS, Jensen KA, Nordly P, Taxvig C, Vogel U, Saber AT, Wallin H. Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Particle Fibre Toxicol. 2008;5:3. doi: 10.1186/1743-8977-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hougaard KS, Saber AT, Jensen KA, Vogel U, Wallin H. Diesel exhaust particles: effects on neurofunction in female mice. Basic Clin Pharmacol Toxicol. 2009;105:139–143. doi: 10.1111/j.1742-7843.2009.00407.x. [DOI] [PubMed] [Google Scholar]
- 62.Tsukue N, Watanabe M, Kumamoto T, Takano H, Takeda K. Perinatal exposure to diesel exhaust affects gene expression in mouse cerebrum. Arch Toxicol. 2009;83:985–1000. doi: 10.1007/s00204-009-0459-2. [DOI] [PubMed] [Google Scholar]
- 63.Win-Shwe TT, Fujitani Y, Kyi-Tha-Thu C, Furuyama A, Michikawa T, Tsukahara S, Nitta H, Hirano S. Effects of diesel engine exhaust origin secondary organic aerosols on novel object recognition ability and maternal behavior in BALB/C mice. Int J Environ Res Public Health. 2014;11:11286–11307. doi: 10.3390/ijerph111111286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yokota S, Sato A, Umezawa M, Oshio S, Takeda K. In utero exposure of mice to diesel exhaust particles affects spatial learning and memory with reduced N-methyl-D-aspartate receptor expression in the hippocampus of male offspring. NeuroToxicology. 2015;50:108–115. doi: 10.1016/j.neuro.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Yokota S, Oshio S, Moriya N, Takeda K. Social isolation-induced territorial aggression in male offspring is enhanced by exposure to diesel exhaust during pregnancy. PLoS ONE. 2016;11(2):e0149737. doi: 10.1371/journal.pone.0149737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allen JL, Conrad K, Oberdorster G, Johnston CJ, Sleezer B, Cory-Slechta DA. Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environ Health Perspect. 2013;121:32–38. doi: 10.1289/ehp.1205505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen JL, Liu X, Weston D, Prince L, Oberdörster G, Finkelstein JN, Johnston CJ, Cory-Slechta DA. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex dependent behavioral neurotoxicity and glial activation. Toxicol Sci. 2014a;140:160–178. doi: 10.1093/toxsci/kfu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, Visser S, Kogan MD. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011a;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 70.Wingate M, Mulvihill B, Kirby RS, Pettygrove S, Cunniff C, Meaney F, et al. Prevalence of autism spectrum disorders-Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- 71.Schaafsma SM, Pfaff DW. Etiologies underlying sex differences in autism spectrum disorders. Front Neuroendocrinol. 2014;35:255–271. doi: 10.1016/j.yfrne.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168:721–728. doi: 10.1001/jamapediatrics.2014.210. [DOI] [PubMed] [Google Scholar]
- 73.Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E, Ma SY, Chauhan A, Chauhan V, Wierzba Bobrowicz T, de Leaon M, Saint Louis LA, Cohen IL, London E, Brown WT, Wisniewski T. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuroptahol. 2010;119:755–770. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Wynshaw-Boris A, Colamarino SA, Lein ES, Courchesne E. Patches of disorganization in the neocortex of children with autism. New Engl J Med. 2014;370:1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lam KS, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder. A review of the literature. Rev Dev Disab. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, James SJ. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. 2012;2:e134. doi: 10.1038/tp.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Della Bernardina B, Bonassi S. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Rad Biol Med. 2012;52:2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 78.Napoli E, Wong S, Giulivi C. Evidence of reactive oxygen species-mediated damage to mitochondrial DNA damage in children with typical autism. Mol Autism. 2013;4:2. doi: 10.1186/2040-2392-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol. 2007;36:361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 80.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Ansary A, Al-Ayadhi L. Neuroinflammation in autism spectrum disorders. J Neuroinflammat. 2012;9:265. doi: 10.1186/1742-2094-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Theoharides TC, Asadi S, Patel AB. Focal brain inflammation and autism. J Neuroinflammat. 2013;10:46. doi: 10.1186/1742-2094-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Depino AM. Peripheral and central inflammation in autism spectrum disorders. Mol Cell Neurosci. 2013;53:69–76. doi: 10.1016/j.mcn.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miler J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiat. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, Mahajan M, Manaa D, Pawitan Y, Reichert J, Ripke S, Sandin S, Sklar P, Svantesson O, Reichenberg A, Hultman CM, Devlin B, Roeder K, Buxbaum JD. Most genetic risk for autism resides in common variation. Nature Genet. 2014;46:881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Op Pediatr. 2010;22:219–225. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- 87.Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: a review of epidemiological evidence. Curr Probl Pediatr Adolesc Health Care. 2014;44:277–318. doi: 10.1016/j.cppeds.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiat. 2014;4:e360. doi: 10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nardone S, Sams D, Reuveni E, Getselter D, Oron O, Karpuj, Elliott E. DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Trans Psychiat. 2014;4:e433. doi: 10.1038/tp.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berko ER, Greally JM. How might epigenetic dysregulation in early embryonic life contribute to autism spectrum disorder? Epigenomics. 2015;7:1–4. doi: 10.2217/epi.14.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. 2014;43:443–464. doi: 10.1093/ije/dyt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lyall K, Croen LA, Sjodin A, Yoshida CK, Zerbo O, Kharrazi M, Windham GC. Polychlorinated biphenyl and organochlorine pesticide concentrations in mid-pregnancy serum analysis: association with autism spectrum disorder and intellectual disability. Environ Health Perspect. 2016 doi: 10.1289/EHP277. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bauman MD, Iosif AM, Smith SEP, Bregere C, Amaral DG, Patterson PH. Activation of the maternal immune system during pregnancy alters behavioral development of Rhesus monkey offspring. Biol Psychiat. 2014;75:332–341. doi: 10.1016/j.biopsych.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97*.Estes ML, McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353:772–777. doi: 10.1126/science.aag3194. This review highlights the importance of maternal immune activation in numerous CNS disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones KL, Croen LA, Yoshida CK, Heuer L, Hansen R, Zerbo O, DeLorenze GN, Kharrazi M, Yolken R, Ashwood P, Van de Water J. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psych. 2016 doi: 10.1038/mp.2016.77. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wong CT, Wais J, Crawford DA. Prenatal exposure to common environmental factors affects brain lipids and increases risk of developing autism spectrum disorders. Eur J Neurosci. 2015;42:2742–2760. doi: 10.1111/ejn.13028. [DOI] [PubMed] [Google Scholar]
- 100.Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119:873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiat. 2013;70:71–77. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles County, California. Environ Health Perspect. 2013;121:380–386. doi: 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roberts AL, Lyall K, Hart JE, Laden F, just AC, Bobb JF, Koenen KC, Ascherio A, Weisskopf MG. Perinatal air pollutant exposures and autism spectrum disorder in the Children of Nurses’s Health Study II participants. Environ Health Perspect. 2013;121:978–984. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jung CR, Lin YT, Hwang BF. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. Plos ONE. 2013;8:e75510. doi: 10.1371/journal.pone.0075510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Talbott EO, Arena VC, Rager JR, Clougherty JE, Michanowicz DR, Sharma RK, Stacy SL. Fine particulate matter and the risk of autism spectrum disorders. Environ Res. 2015;140:414–420. doi: 10.1016/j.envres.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 106.Guxens M, Ghassabian A, Gong T, Garcia-Esteban R, Porta D, Giorgis-Allemand L, Almqvist C, et al. Air pollution exposure during pregnancy and childhood autistic traits in four European population-based cohort studies: the ESCAPE project. Environ Health Perspect. 2016;124:133–140. doi: 10.1289/ehp.1408483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107*.Kalkbrenner AE, Windham GC, Serre ML, Akita Y, Wang X, Hoffman K, Thayer BP, Daniels JL. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology. 2015;26:30–42. doi: 10.1097/EDE.0000000000000173. This study reports how third-trimester exposure has the strongest association between air pollution and ASD. [DOI] [PubMed] [Google Scholar]
- 108.Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, Weisskopf MG. Autism spectrum disorder and particulate matter air pollution before, during and after pregnancy: a nested case-control analysis within the nurses’ health study II cohort. Environ Health Perspect. 2015;123:264–270. doi: 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tachibana K, Takayanagi K, Akimoto A, Ueda K, Shinkai Y, Umezawa M, Takeda K. Prenatal diesel exhaust exposure disrupts the DNA methylation profile in the brain of mouse offspring. J Toxicol Sci. 2015;40:1–11. doi: 10.2131/jts.40.1. [DOI] [PubMed] [Google Scholar]
- 110.Thirtamara Rajamani K, Doherty-Lyons S, Bolden C, Willis D, Hoffman C, Zelikoff J, Chen LC, Gu H. Prenatal and early life exposure to high level diesel exhaust particles leads to increased locomotor activity and repetitive behaviors in mice. Autism Res. 2013;6:248–257. doi: 10.1002/aur.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111*.Allen JL, Liu X, Pelkowski S, Palmer B, Conrad K, Oberdörster G, Weston D, Mayer-Proschel M, Cory-Slechta D. Early postnatal exposure to ultrafine particulate matter air pollution: persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environ Health Perspect. 2014b;122:939–945. doi: 10.1289/ehp.1307984. This study provides important evidence in an animal model of CNS alterations induced by air pollution which are similar to ASD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allen JL, Oberdoerster G, Morris-Schaffer K, Wong C, Klocke C, Sobolewski M, Conrad K, Mayer-Proschel M, Cory-Slechta DA. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2016 doi: 10.1016/j.neuro.2015.12.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rich DQ, Liu K, Zhang J, Thurstin SW, Stevens TP, Pan Y, Kane C, Weinberger B, Ohman-Strickland P, Woodruff TJ, Duan X, Assibey-Mensah V, Zhang J. Differences in birth weight associated with the 2008 Bejing Olympics air pollution reduction: results from a natural experiment. Environ Health Perspect. 2015;123:880–887. doi: 10.1289/ehp.1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schembari A, de Hoogh K, Pedersen M, Dadvand P, Martinez D, Hoelk G, Petherick ES, Wright J, Nieuwenhujisen MJ. Ambient air pollution and newborn size and adiposity at birth: difference by maternal ethnicity (The Born in Bradford Study Cohort) Environ Health Perspect. 2015;123:1208–1215. doi: 10.1289/ehp.1408675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 116.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined pattern in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 117.Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong JS, Veronesi B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- 118.Roqué PJ, Dao K, Costa LG. Microglia mediate diesel exhaust particle-induced cerebellar neuronal toxicity through neuroinflammatory mechanisms. NeuroToxicology. 2016;56:2014–214. doi: 10.1016/j.neuro.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martinez-Cerdeño V, Noctor SC. Cajal, Retzius, and Cajal-Retzius cells. Front Neuroanat. 2014;8:48. doi: 10.3389/fnana.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jossin Y. Neuronal migration and the role of reelin during early development of the cerebral cortex. Mol Neurobiol. 2004;30:225–251. doi: 10.1385/MN:30:3:225. [DOI] [PubMed] [Google Scholar]
- 121.Folsom TD, Fatemi SH. The involvement of reelin in neurodevelopmental disorders. Neuropharmacology. 2013;68:122–135. doi: 10.1016/j.neuropharm.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sekine K, Kubo K, Nakajima K. How does reelin control neuronal migration and layer formation in the developing mammalian cortex? Neurosci Res. 2014;86:50–58. doi: 10.1016/j.neures.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 123.Förster E. Reelin, neuronal polarity and process orientation of cortical neurons. Neuroscience. 2014;269:102–111. doi: 10.1016/j.neuroscience.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 124.Yu NN, Tan MS, Yu JT, Xie AM, Tan L. The role of reelin signaling in Alzheimer’s disease. Mol Neurobiol. 2016;53:5692–5700. doi: 10.1007/s12035-015-9459-9. [DOI] [PubMed] [Google Scholar]
- 125.Fatemi SH, Snow AV, Stary JM, Araghi-Niknam M, Brooks AI, Pearce DA, Reutiman TJ, Lee S. Reelin signaling is impaired in autism. Biol Psych. 2005;57:777–787. doi: 10.1016/j.biopsych.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 126.Persico AM, D’Agruma L, Maiorano N, Totaro A, Militerni R, Bravaccio C, et al. Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol Psychiatry. 2001;6:150–159. doi: 10.1038/sj.mp.4000850. [DOI] [PubMed] [Google Scholar]
- 127.Serajee FJ, Zhong H, Huq AHMM. Association of reelin polymorphysms with autism. Genomics. 2006;87:75–83. doi: 10.1016/j.ygeno.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 128.Wang Z, Hong Y, Zou L, Zhong R, Zhu B, Shen N, Chen W, Lou J, Ke J, Zhang T, Wang W, Miao X. Reelin gene variants and risk of autism spectrum disorders: an integrated meta-analysis. Am J Med Genet Pt B. 2014;165B:192–200. doi: 10.1002/ajmg.b.32222. [DOI] [PubMed] [Google Scholar]
- 129.Lammert DB, Howell BW. RELN Mutations in autism spectrum disorder. Front Cell Neurosci. 2016;10 doi: 10.3389/fncel.2016.00084. art 84. doi:103389/fncel.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lintas C, Sacco R, Persico AM. Differential methylation at the RELN gene promoter in temporal cortex from autistic and typically developing post-puberal subjects. J Neurodev Dis. 2016;8:18. doi: 10.1186/s11689-016-9151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sakai K, Shoji H, Kohno T, Miyakawa T, Hattori M. Mice that lack the C-terminal region of reelin exhibit behavioral abnormalities related to neuropsychiatric disorders. Sc Rep. 2016;6:28636. doi: 10.1038/srep28636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Katsuyama Y, Terashima T. Developmental anatomy of reeler mutant mouse. Develop Growth Differ. 2009;51:271–286. doi: 10.1111/j.1440-169X.2009.01102.x. [DOI] [PubMed] [Google Scholar]
- 133.Reiner O, Karzburn E, Kshirsagar A, Kaibuchi K. Regulation of neuronal migration, an emerging topic in autism spectrum disorders (ASD) J Neurochem. 2016;136:440–456. doi: 10.1111/jnc.13403. [DOI] [PubMed] [Google Scholar]
- 134.Boyle MP, Bernard A, Thompson CL, Ng L, Boe A, Mortrud M, Hawrylcz MJ, Jones AR, Hevner RF, Lein ES. Cell-type-specific consequences of reelin deficiency in the mouse neocortex, hippocampus, and amygdala. J Comp Neurol. 2011b;519:2061–2089. doi: 10.1002/cne.22655. [DOI] [PubMed] [Google Scholar]
- 135.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ghiani CA, Mattan NS, Nobuta H, Malvar JS, Boles J, Ross MG, Waschek JA, Carpenter EM, Fisher RS, de Vellis J. Early effects of lipopolysaccharide-induced inflammation of foetal brain development in rat. ASN Neuro. 2011;3(4) doi: 10.1042/AN20110027. art:e00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Novais ARB, Guiramand J, Cohen-Sola C, Crouzin N, de Jesus Ferreira MC, Vignes M, Barbanel G, Cambonie G. N-acetyl-cysteine prevents pyramidal cell disarray and reelin-immunorecative neuron deficiency in CA3 after prenatal immune challenge in rats. Pediatr Res. 2013;73:750–755. doi: 10.1038/pr.2013.40. [DOI] [PubMed] [Google Scholar]
- 138.Depino AM. Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience. 2015;299:56–65. doi: 10.1016/j.neuroscience.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 139.Uzunova G, Pallanti S, Hollander E. Excitatory/inhibitory imbalance in autism spectrum disorders: implications for interventions and therapeutics. World J Biol Psych. 2016;17:174–186. doi: 10.3109/15622975.2015.1085597. [DOI] [PubMed] [Google Scholar]
- 140.Robertson CE, Ratai EM, Kanwisher N. Reduced GABAergic action in the autistic brain. Curr Biol. 2016;26:80–85. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 141.Shaun N, Thomas B. The STAT3-DMNT1 connection. JAK-STAT. 2012;1(4):257–260. doi: 10.4161/jkst.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Noh JS, Sharma RP, Veldic M, Salvacion AA, Jia X, Chen Y, Costa E, Guidotti A, Grayson DR. DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures. Proc Natl Acad Sci. 2005;102:1749–1754. doi: 10.1073/pnas.0409648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kundakovic M, Chen Y, Costa E, Grayson DR. DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes. Mol Pharmacol. 2007;71:644–653. doi: 10.1124/mol.106.030635. [DOI] [PubMed] [Google Scholar]
- 144.Han S, Tai C, Westenbroek RE, YUFH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. Autistic-like behavior in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nouel D, Burt M, Zhang Y, Harvey L, Boksa P. Prenatal exposure to bacterial endotoxin reduces the number of GAD67- and reelin-immunoreactive neurons in the hippocampus of rat offspring. Eur Neuropsychopharmacol. 2012;22:300–3017. doi: 10.1016/j.euroneuro.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 146.Schwartzer JJ, Koenig CM, Berman RF. Using mouse models of autism spectrum disorders to study the neurotoxicology of gene-environment interactions. Neurotoxicol Teratol. 2013;36:17–35. doi: 10.1016/j.ntt.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly S, Genestine M, Millonig JH, DiCicco-Bloom E, Crawley JN. Autism-relevant social abnormalities and cognitive deficits in Engrailed-2 knockout mice. PLoS ONE. 2012;7(7):e40914. doi: 10.1371/journal.pone.0040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 149.Silverman JL, Yang M, Lord C, Crawley JN. Behavioral phenotyping assays for mouse models of autism. Nature Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.De Felice A, Scattoni ML, Ricceri L, Calamandrei G. Prenatal exposure to a common organophosphate insecticide delays motor development in a mouse model of idiopathic autism. PLoS ONE. 2015;10(3):e0121663. doi: 10.1371/journal.pone.0121663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.De Felice A, Greco A, Calamandrei G, Minghetti L. Prenatal exposure to the insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J Neuroinflammat. 2016;13:149. doi: 10.1186/s12974-016-0617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, Pesold C. The phenotypic characteristics of heterozygous reeeler mouse. NeuroReport. 1999;10:1329–1334. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- 153.Podhorna J, Didriksen M. The heterozygous reeler mouse: behavioral phenotype. Behav Brain Res. 2004;153:43–54. doi: 10.1016/j.bbr.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 154.Biamonte F, Latini L, Giorgi FS, Zingariello M, Marino R, De Luca R, D’Ilio S, Majorani C, Petrucci F, Violante N, Senofonte O, Molinari M, Keller F. Associations among exposure to methylmercury, reduced reelin expression, and gender in the cerebellum of developing mice. Neurotoxicology. 2014;45:67–80. doi: 10.1016/j.neuro.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 155.Giordano G, White CC, McConnachie LA, Fernandez C, Kavanagh TJ, Costa LG. Neurotoxicity of domoic acid in cerebellar granule neurons in a genetic model of glutathione deficiency. Mol Pharmacol. 2006;70:2116–2126. doi: 10.1124/mol.106.027748. [DOI] [PubMed] [Google Scholar]
- 156.Nakamura S, Kugiyama K, Sugiyama S, Miyamoto S, Koide S, Fukushima H, Honda O, Yoshimura M, Ogawa H. Polymorphism in the 5’-flanking region of human glutamate-cysteine ligase modifier subunit gene is associated with myocardial infarction. Circulation. 2002;105:2968–2973. doi: 10.1161/01.cir.0000019739.66514.1e. [DOI] [PubMed] [Google Scholar]
- 157.Weldy CS, White CC, Wilkerson HW, Larson TV, Stewart JA, Gill SE, Parks WC, Kavanagh TJ. Heterozygosity in the glutathione synthesis gene Gclm increases sensitivity to diesel exhaust particulate induced lung inflammation in mice. Inhal Toxicol. 2012;23:724–735. doi: 10.3109/08958378.2011.608095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gu F, Chauhan V, Chauhan A. Impaired synthesis and antioxidant defense of glutathione in the cerebellum of autistic subjects: alterations in the activities and protein expression of glutathione-related enzymes. Free Rad Biol Med. 2013;65:488–496. doi: 10.1016/j.freeradbiomed.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 159.Costa LG, Aschner M, Vitalone A, Syversen T, Porat-Soldin O. Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol. 2004;44:87–110. doi: 10.1146/annurev.pharmtox.44.101802.121424. [DOI] [PMC free article] [PubMed] [Google Scholar]