Abstract
The epidemiological pattern of acute kidney injury (AKI) in tropical countries during monsoon reflects infectious disease as the most important cause. AKI is a confounding factor and may be overlooked by primary health-care providers and underreported in health statistics. The present study prospectively helps estimate the burden of disease and analyze etiology, clinical profile, and outcome in a tertiary care hospital of a metropolitan city in a tropical country. The study period included monsoon season of 2012 and 2013, a total of 8 months. AKI staging was done as per the AKI Network (AKIN) criteria. Patients were treated for primary disease. Renal replacement therapy (RRT) was given as required. Patients were followed up during hospitalization till recovery/death. Out of a total of 9930 admissions during this period, 1740 (17.52%) were for infections and 230 (2.31%) had AKI secondary to infectious diseases during monsoon. The incidence of AKI (230/1740) in infectious diseases during monsoon was 13.21%. The study population (n = 230) comprised 79.5% of males and the mean age was 40.95 ± 16.55 years. Severe AKI: AKIN Stage III was seen in 48.26% of patients and AKIN Stage I in 41.74%. The most common etiology of AKI was malaria (28.3%) followed by acute gastroenteritis (23%), dengue (16.5%), leptospirosis (13%), undifferentiated fever (10.4%), more than one etiology (5.4%), and enteric fever (3.5%). RRT was required in 44.78% of patients. Requirement for RRT was maximum in patients with more than one etiology followed by leptospirosis, malaria, dengue, and least in typhoid. The overall mortality was 12.17%. In multivariate analysis, vasopressor support and assisted ventilation were risk factors for mortality.
Keywords: Acute kidney injury, AKIN criteria, infections, monsoon
Introduction
In tropical countries, the epidemiological pattern of acute kidney injury (AKI) during monsoon reflects infectious disease as the most prominent cause. These infections present mostly with fever as cardinal sign. Clinical profile of AKI in tropics is modified by a number of factors such as severe systemic infection, severe toxemia, shock, dehydration, disseminated intravascular coagulation, hepatocellular injury, pulmonary injury, or central nervous system involvement. The catabolic effect of infection with uremic state results in considerable negative nitrogen balance, which has a major impact on outcome. In addition, there is an effect of delayed referral to tertiary care units. Malaria, leptospirosis, dengue, acute gastroenteritis (AGE), cholera, hepatitis A, and hepatitis E are prime causes of morbidity and mortality during monsoon period. Climatic influences, poor sanitation, overcrowding, rapid urbanization and migration, and poverty lead to aggravation of these infections.[1]
AKI, in these infections, is a result of direct invasion of renal parenchyma by microbial agents, tubular necrosis due to hemodynamic disturbances, renal inflammation due to immune response, or iatrogenic renal injury associated with treatment.[1] AKI is a significant complication in these illnesses, but since it is a confounding factor in associated systemic illness, it may be overlooked by primary health-care providers and underreported in health statistics. It is assumed that these illnesses contribute to more than two-thirds of AKI in tropics. The incidence of AKI in tropics is unknown owing to the lack of reliable registries and unified definitions being adapted only in the last decade. However, considering the reported incidence of AKI in the prevailing spectrum of primary causes and referring it to the known prevalence of these conditions in tropical environment suggest extremely high figures, at least 10 folds higher than those reported.[2] At least 80% of AKI in tropics is community acquired.[3]
Basu et al. reported 41.1% incidence of AKI among the tropical acute febrile illnesses (AFIs).[4]
Most studies till date have highlighted the causes of AKI in tropics or individual disease with AKI due to malaria or leptospirosis. In a study by Mehta et al., in Mumbai, AKI necessitating dialysis was seen in 92% of patients with AKI in malaria.[5] Thanachartwet et al. from Thailand reported 115 patients of Falciparum malaria with severe AKI over 4 years.[6] Daher et al. from Brazil retrospectively analyzed 287 patients of leptospirosis with AKI between 1985 and 2008.[7]
Studies which have addressed AKI due to infectious etiology in epidemic proportions during monsoon are sparse. Bhagat et al. in their analysis of AKI in AFI in rainy season enrolled 100 patients over 2 months. Among these 100 patients, 30 patients required renal replacement therapy (RRT) in the form of hemodialysis.[8]
Materials and Methods
This is an observational, prospective study to assess incidence, etiology, clinical profile, and outcome of AKI of infectious origin during monsoon seasons (June to September) of 2012 and 2013 in a tertiary care hospital in a metro city.
Inclusion criteria
All patients, with age ≥18 years and hospitalized with fever, in medicine or nephrology wards including intensive care units (ICUs), with AFI, suspected of infectious etiology and AKI, were included in the study. AKI was defined as an absolute increase in serum creatinine concentration of 0.3 mg/dl or greater as per the AKI Network (AKIN) consensus definition. Written informed consent was obtained from all cases.
Exclusion criteria
All known chronic kidney disease patients confirmed by ultrasound studies or blood or urine abnormalities of renal function of 3 months and pregnant females were excluded from the study.
Detailed history taking and clinical examination of patients were carried out and noted. Biochemical investigations were carried out on Olympus 400 Full Autoanalyzer. All were evaluated with complete blood count, renal function tests, electrolytes, liver function tests, urine examination, blood culture, urine culture, stool culture, X-ray chest, ultrasonography of kidney, ureter, and bladder, peripheral smear for malarial parasite, rapid diagnostic test for malaria (for the detection of histidine-rich protein II, specific to Plasmodium falciparum and Plasmodium lactate dehydrogenase specific to Plasmodium vivax in human blood sample), serum IgM antibody for leptospirosis, and serum NS1 antigen for dengue. Widal test was performed in all to identify infection. Anti-hepatitis A virus and hepatitis E virus IgM antibodies, anti-HIV, and hepatitis B virus and hepatitis C virus antibodies were done as per indication.
The severity of acute kidney injury was defined by the Acute Kidney Injury Network staging criteria as follows[9]
Stage I, serum creatinine increase to 1.5–2 fold of baseline
Stage II, serum creatinine increase to >2–3 fold of baseline
Stage III, serum creatinine increase to >3 fold of baseline or serum creatinine of >4 mg/dl
All patients requiring dialysis were categorized in Stage III.
Patients were followed up regularly during the period of hospitalization. Follow-up investigations, mainly renal function tests, were noted. Recovery or progression of illness with involvement of other organ systems, mainly pulmonary, cardiovascular, hematology, and central nervous system, was noted. Patients were assessed for the requirement of RRT and were treated accordingly as per indications.
Treatment
Conservative treatment
Optimizing hemodynamic stability by maintaining hydration and blood pressure
Vasopressor support to maintain mean arterial pressure ≥65 mmHg or systolic blood pressure more than 100 mmHg if conservative management failed to maintain blood pressure
Treatment of the underlying cause of AKI when the etiology was known. Patients of undifferentiated etiology were treated empirically as per hospital policy with antimalarials and broad-spectrum antibiotics covering leptospirosis, considering the common causes of treatable infections (intravenous artesunate with ceftriaxone)
Transfusion of blood and blood products required as per clinical assessment and patient requirements
Assisted ventilation whenever indicated.
RRT as intermittent hemodialysis/sustained low-efficiency dialysis was initiated for either single or a combination of appropriate indications which included metabolic acidosis, pulmonary edema, fluid overload, hyperkalemia, azotemia, and oliguria/anuria.
Data were analyzed for demographic and clinical profile, incidence and etiology of AKI, requirement for RRT, and outcome (recovery vs. mortality).
Statistical data analysis methods
Categorical variables were summarized as frequency and percentage. Continuous variables were represented using mean, standard deviation, and median. Association between categorical independent and dependent variables was determined using Chi-square test and Fisher's exact test for all 2 × 2 tables where P value of Chi-square test was not valid due to small counts (e.g., sex of the patient vs. mortality). Predictiveness of factors for mortality (outcome of AKI) was assessed using binary logistic regression analysis. Results were graphically represented where deemed necessary. All tests of significance were two sided, with P < 0.05 indicating statistical significance. The data were analyzed using the Statistical Package for the Social Sciences software version 17.0 [SPSS Inc, Chicago IL].
Results
There were a total of 9930 admissions, during the study period in the medicine wards. Of these, 1740 patients (17.52%) were admitted with AFI and 230 (2.31%) developed AKI. AKI incidence in monsoon-related infectious disease was 13.21% (230/1740) [Figure 1].
Figure 1.
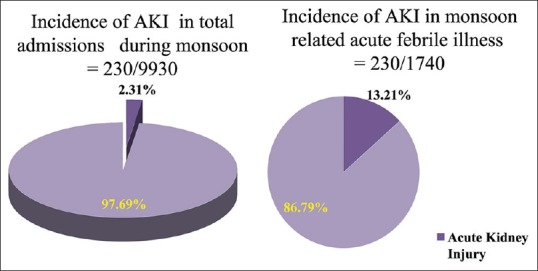
Incidence of acute kidney injury of infectious etiology during monsoon 2.31% of total admissions developed acute kidney injury while 13.21% of acute febrile illness during monsoon developed acute kidney injury
The study population comprised 79.6% of males and 20.4% of females. Mean age of the study population was 40.95 ± 16.55 years: 56.5% were in the age group of 18–40 years, 26.5% were in the age group of 41–60 years, and 17% were more than 60 years old. Predominant presenting features included fever (89.6%), vomiting (84.3%), oliguria (70.4%), high-colored urine (62.2%), and breathlessness (40.4%). Forty-nine patients (21.3%) were in altered sensorium, five of them had convulsions. Twenty patients (8.7%) presented with bleeding manifestations as hemoptysis, hematemesis, melena, gum bleed, or bleed per rectum [Table 1]. Examination revealed pallor in 120 patients, icterus in 93 patients while 64 had pedal edema. Tachypnea (116 patients), hypotension (93), and tachycardia (85) were significant findings on admission. Petechiae, purpura, and erythema were noted in 54 patients. Nineteen patients had subconjunctival hemorrhage on admission [Figure 2].
Table 1.
Clinical demography of acute kidney injury in 230 patients
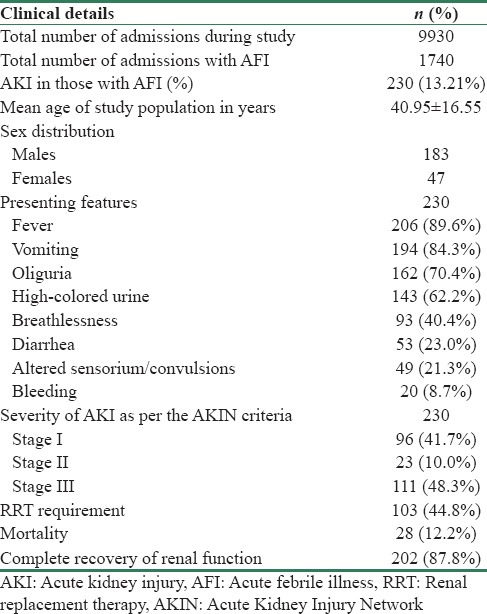
Figure 2.
General examination findings of study group
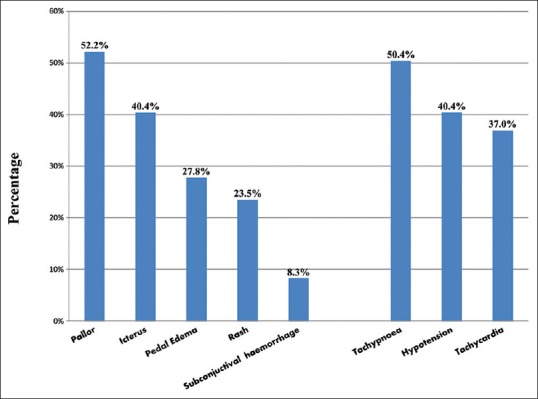
Multisystem involvement was noted in 64.74% of patients [Figure 3]. Seventy-seven patients showed respiratory system involvement (acute respiratory distress syndrome [ARDS] in 36, pulmonary edema in 22). Hematologic involvement was the most common feature with thrombocytopenia in 170 and anemia in 163 patients. The mean hemoglobin and total leukocyte counts on admission were 11.63 g% and 11946/cmm, respectively [Table 2]. Mean values of platelet counts and serum creatinine were 110,211/cmm and 3.42 mg%, respectively. Total bilirubin, serum glutamic oxaloacetic transaminase, and serum glutamic pyruvic transaminase revealed significant elevation on admission (mean value at 6.21 mg/dl, 186.32 IU/L, and 109.4 IU/L, respectively).
Figure 3.
Systemic examination findings of the study group
Table 2.
Laboratory investigations on admission
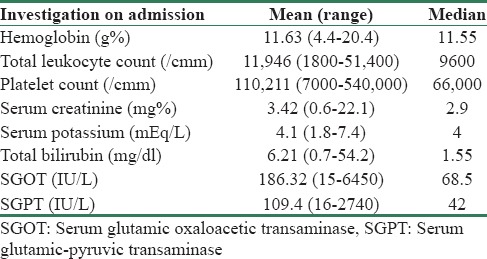
Severity of acute kidney injury as per the Acute Kidney Injury Network criteria and etiology
About 48.26% of patients were classified in AKIN Stage III, 10% in AKIN Stage II, and 41.74% in AKIN Stage I [Figure 4]. The most common etiology was malaria affecting 28.3% of patients followed by AGE (23%), dengue (16.5%), leptospirosis (13%), undifferentiated fever (10.4%), mixed infection (5.4%), and typhoid (3.5%). None had hepatitis A- or E-induced AKI [Table 3].
Figure 4.
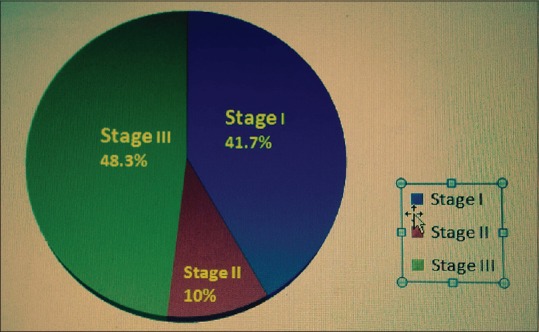
Severity of acute kidney injury as per the Acute Kidney Injury Network criteria
Table 3.
Distribution of study group as per infectious disease etiology
Association between infectious disease etiology and severity of acute kidney injury (Acute Kidney Injury Network Stages)
Majority of patients with leptospirosis (20 of 30), malaria (35/65), and multiple etiology (7/12) presented in AKIN Stage III. Most patients with AGE (24/53), dengue (19/38), and typhoid (6/8) presented with AKIN Stage I. Those with undifferentiated etiology had 11 cases each in AKIN Stages I and III [Figure 5]. Distribution of AKI as per etiology and AKIN stages was statistically significant (P = 0.001).
Figure 5.
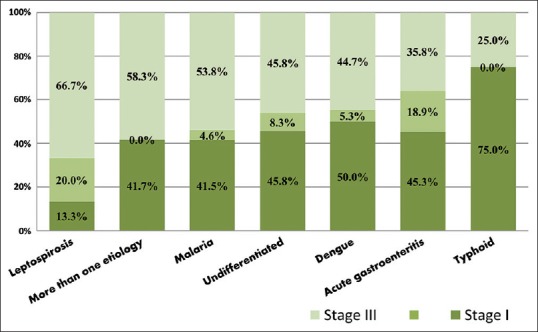
Association among study group between etiology and Acute Kidney Injury Network stages
Factors associated with severe acute kidney injury – Acute Kidney Injury Network Stage III
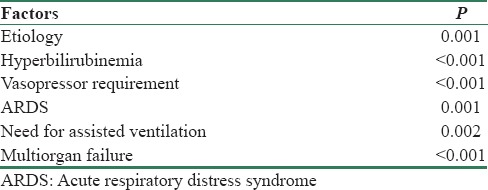
In addition to etiology, hyperbilirubinemia, vasopressor requirement, ARDS, need for assisted ventilation, and multiorgan failure were associated with severe AKI – AKIN III [Table 4].
Table 4.
Factors associated with severe acute kidney injury - Acute Kidney Injury Network Stage III
Association between renal replacement therapy and infectious disease etiology
Out of 230 patients, 103 (44.78%) required dialysis [Table 1]. Requirement of dialytic support was highest; 58.3% in those diagnosed with mixed etiology [Seven of 12 with mixed etiology needed RRT] [Figure 6]. This was followed by leptospirosis 56.7% [17/30], malaria 52.3% [34/65], dengue 44.7% [17/38], undifferentiated 41.7% [10/24] & AGE 32.1% [17/53]. Requirement of dialysis was least with typhoid: 1 patient (12.5%). Most patients diagnosed with more than one etiology had concomitant malaria [Table 3]. The distribution of etiology and requirement of RRT was statistically significant (P = 0.041).
Figure 6.
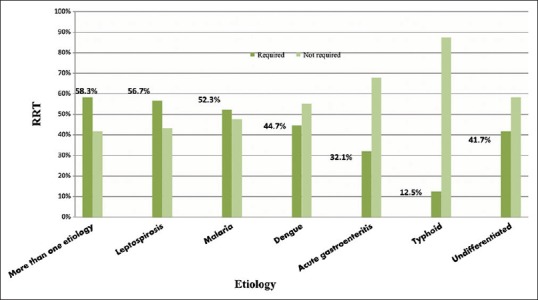
Association among study group between etiology and renal replacement therapy
All patients who required hemodialysis were classified in AKIN Stage III. Out of 230 patients, 103 patients (44.8%) required hemodialysis. The distribution of patients requiring RRT as per the AKIN stages was statistically significant (P < 0.001).
Association between mortality and infectious disease etiology
Mortality rate was 12.17% (28/230). The remaining 202 patients showed complete recovery of AKI. The highest mortality was seen with dengue (23.7%), leptospirosis (16.7%), multiple etiology (16.7%) followed by those with undifferentiated etiology (12.5%) and malaria (10.8%) and AGE (3.8%). No deaths were noted due to typhoid [Figure 7].
Figure 7.
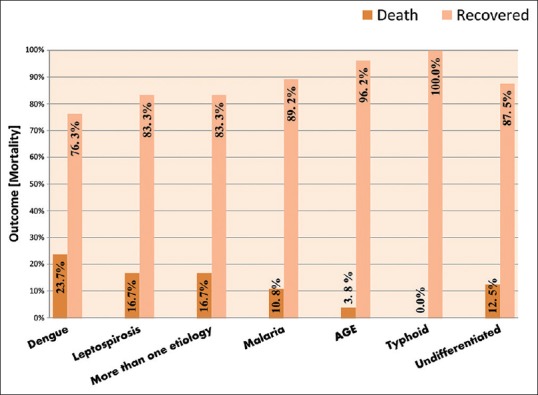
Distribution of study group as per etiology and mortality
Other factors significantly associated with mortality
Hyperbilirubinemia, severity of AKI, encephalopathy, hypotension, ARDS, sepsis, multiorgan failure, vasopressor requirement, blood product transfusion, assisted ventilation, and delay in treatment initiation were significantly associated with poor outcome [Table 5].
Table 5.
Prognostic factors significantly associated with mortality
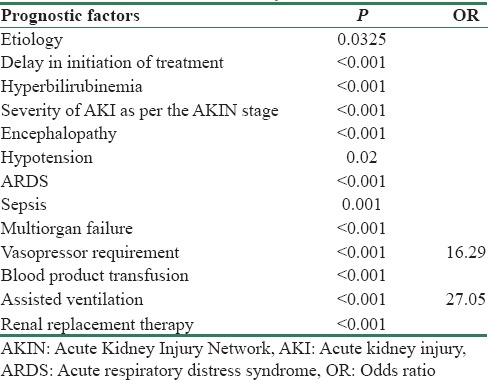
For the purpose of identifying the predictors and assessing the relationships of selected variables on outcome of AKI, a logistic regression was performed. Outcome of AKI was influenced by need for vasopressor and assisted ventilation. No statistical variations were found in the outcome of AKI by hypotension, encephalopathy, hyperbilirubinemia, ARDS, sepsis, requirement of blood and blood product transfusion, and dialysis. The odds of death among patients who required vasopressor were about 16.29 higher than those who did not require vasopressor. The odds of death among patients who were on assisted ventilation were 27.05 times higher than those who were not on ventilator.
Discussion
The cultural, economic, and geographical differences determine the epidemiology of AKI in different places. Tropical AFIs such as malaria, diarrhea, leptospirosis, dengue, typhoid,[10,11] and others are a major cause of AKI in tropics and have been occurring in epidemic proportions in Mumbai. In addition to its high incidence, AKI with other multiorgan failures, fatal pulmonary hemorrhage with leptospirosis, increasing incidence of dengue, undiagnosed viral etiologies, and severe stress on health machinery with higher need for dialysis facilities are very taxing for hospitals. The predisposing factors are rapid urbanization, massive construction activities, conducive environment for vector breeding, people with poor socioeconomic status in urban slums predisposed to infections, and rising costs of health care due to which people are opting either traditional medicine or taking inadequate treatment, thus predisposing to drug resistance. There is a renewal of interest with the emergence of such diseases in the developed nations and nontropical regions[12,13] due to global warming[14] and travel to tropics.[15]
Most of the studies in India have focused on AKI secondary to either malaria,[5,16,17] leptospirosis,[18,19] dengue,[20,21] or diarrhea.[22] Others have highlighted tropical infections leading to AKI in all seasons.[4] No single study has focused on AKI only during monsoon season. Only three studies, including the present study, have considered AKI in different infections together in one study.[4,23]
Incidence and clinical demography of acute kidney injury
It is generally believed that tropical infections contribute to more than 2/3rd of AKI cases in tropics which may account up to 800 per million population according to certain calculations.[24] More than twenty tropical infections may be associated with AKI; the most widely known are malaria, leptospirosis, cholera, shigellosis, dengue, and HIV.[24]
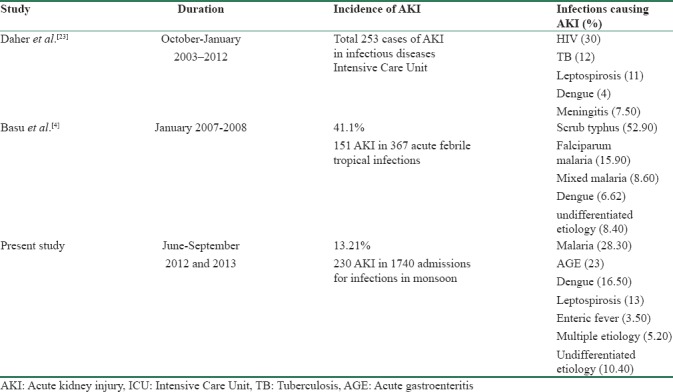
In the present study, the incidence of AKI in monsoon due to infectious etiology was 13.21% [Table 1]. Incidence of AKI of infectious etiology by Daher et al.[25] was 17.7% [Table 6]. The main causes of ICU admission were AIDS-related diseases, pneumonia, leptospirosis, meningitis, and tetanus. Basu et al.[4] from CMC Vellore in South India in 2007–2008 reported an incidence of 41.1% of AKI in infectious diseases, one of the highest incidences noted among tropical infections. Most of the patients in the study done by Basu et al. were in risk class and had milder degree of renal dysfunction. Our study included patients of AKI due to infections in monsoon season, in prominent metropolitan city of a tropical country. The enormous burden of infectious diseases is reflected in the number of patients being admitted in the present study in 8 months which is more than four times the number of patients admitted in 1 year in the study done by Basu et al.[4]
Table 6.
Acute kidney injury in tropical infections
Out of 230 patients of AKI in our study, 183 (79.59%) were males and 47 (20.43%) were females. Reason for gender difference is unclear but it is likely related to work activities. Mean age of study population was 40.95 ± 16.55 years. It implies the occurrence of community-acquired AKI in younger age group in tropics as compared with developed countries. Relatively young patients aged 37–47 years develop AKI of tropical diseases as a result of factors encountered in their environment.[10]
No combination of symptoms, signs, or demographic data [Table 1 and Figures 2, 3] can reliably distinguish between the different causes of fever. The common presentation with multiorgan involvement also highlights the widespread inflammation with endothelial dysfunction in common tropical infections such as malaria, leptospirosis, and dengue.
Etiology of acute kidney injury
In our study, malaria was the most common etiology of AKI affecting 65 patients (28.30%) [Table 3]. Malaria is estimated to account for 60% of outpatient visits, 30%–50% of hospital admissions, and 40% of public health expenditure in countries with a high burden of malarial disease.[26] India contributes 80% of all the cases of malaria in Southeast Asia.[16,17] Basu et al.[4] reported malaria as the second most common tropical illness of their study accounting for 25.8% of cases of AKI of infectious etiology while Kaul et al.[27] noted that malaria was responsible for 18.8% of medical causes of community-acquired AKI. The changes in epidemiology of infections causing community-acquired AKI were highlighted by Prakash et al.,[28] with a significant increase in the number of AKI cases due to malaria from 4.7% in 1983–1995 to 17.09% in 1996–2008. Naqvi et al.[16] reported that 5.9% of community-acquired AKI was related to malaria.
AKI due to diarrheal diseases was the second most common cause of AKI accounting for 23% of patients in our cohort. RRT was necessary in 32.1% of these patients.
The incidence of AKI due to dengue was 16.50% in the present study. In Mumbai, dengue is a fast emerging important cause of AKI. As per the data obtained from the Satellite Disease Surveillance Unit, there is serial increase in the occurrence of number of cases of dengue in Mumbai along with consequent increase in the incidence of AKI (unpublished data). In our study, 19 patients (50%) with dengue presented in AKIN Class I, but 44.70% of the total patients with dengue required dialysis. Mortality was maximum with dengue in our cohort, at 23.7%. AKI is a complication of severe dengue infection and is typically associated with hypotension, rhabdomyolysis, or hemolysis.[29] Incidence of 16.5% of AKI in dengue in our group is in sharp contrast to 4% and 7.6% as reported by Dahr et al. and Basu et al., respectively. The prevalence of AKI was 1.6% among 617 children with dengue hemorrhagic fever (DHF) in Colombia, 3.3% in hospitalized adults with DHF, 4.9% in 81 Chinese patients with DHF/dengue shock syndrome, and 5% in DHF patients in Qatar.[30]
Leptospirosis is another emerging cause of AKI in Mumbai. There is a sharp increase in its incidence during rainy season or after floods. AKI occurs in 20–85% of cases of leptospirosis.[31] In the present study, the incidence of AKI due to leptospirosis was 13%. Major factors involved in pathogenesis are direct nephrotoxic action of leptospires, toxin-induced immune response, hyperbilirubinemia, rhabdomyolysis, and hemodynamic alterations due to decrease in peripheral vascular resistance, diarrhea, vomiting, and fever. Basu et al.[4] in their study had 3.3% incidence of AKI due to leptospirosis. Kaul et al.[27] in their study had 8% incidence of AKI due to leptospirosis. Prakash et al.[28] did not note any case of AKI due to leptospirosis during 26 years. Leptospirosis is the leading cause of AKI in Thailand and Singapore. Daher et al.[23,25] in their study of AKI in infectious disease in ICU noted a 11% incidence of leptospiral AKI. Thus, the incidence of AKI due to leptospirosis varies as per the region.
In 10.4% of patients with AKI, the etiology could not be determined and were labeled as cases of AKI due to undifferentiated etiology. AKI in most of these patients was suspected to be secondary to viral infection. The diagnosis is difficult requiring expensive antibody-based tests which are not available in a tertiary care charitable government hospital. These are diseases caused by RNA viruses belonging to Flaviviridae, Arenaviridae, Bunyaviridae, and Filoviridae groups.[32] In the present study, patients were not evaluated for scrub typhus, hantavirus, and melioidosis which are other emerging causes of tropical illness causing AKI. In addition, the rapid antibody-based tests for malaria, leptospirosis, and dengue are done at admission. These tests may report negative if done before antibodies are developed against the causative infection. These tests should ideally be repeated postadmission since they are not both 100% sensitive and specific. However, due to enormous workload during monsoon along with limited resources, these are usually not repeated if patient shows response to therapy. In the study done by Basu et al.,[4] there were 8.4% cases, of undifferentiated AFI with AKI.
In the present study, 12 patients were affected with mixed infections [Table 3]. About 58.3% of patients with AKI due to mixed etiology presented in more severe stages of AKI requiring dialytic support [Figure 6].
In our cohort, eight patients (3.5%) of typhoid were diagnosed with AKI. Basu et al.[4] reported 8.7% cases of AKI due to typhoid whereas Daher et al.[23] reported none.
Distribution of study group as per the Acute Kidney Injury Network stages and requirement of renal replacement therapy
In our cohort, maximum number of patients were seen in Class III: 111 patients (48.26%) [Table 1]. In the study done by Yang et al.[33] in China, 271 patients were classified as per the AKIN criteria with maximum number of patients seen in Class III: 141 patients (52%), followed by Class I: 86 patients (33.7%), and Class II: 44 patients (16.2%). Basu et al. classified AKI according to RIFLE criteria with maximum number of patients of AKI, 64 (42.4%) in risk class followed by 53 patients (35.1%) in failure class, and 34 patients (22.5%) in injury class. Thus, a significant number of patients are seen in more severe class of AKI irrespective of criteria used for classification in cases of community-acquired AKI.
Leptospirosis, mixed etiology, malaria, undifferentiated, etiology and dengue were associated with severe AKI in our study group [Figure 5], with more than 40% of each etiology needed RRT. Widespread inflammation with endothelial dysfunction in common tropical infections such as malaria, leptospirosis, and dengue along with multiorgan involvement is the culprit for severe AKI. Leptospirosis in the early 1980s was seen as benign infection with nonoliguric AKI, not requiring RRT. Over the past 30 years, it has become a more severe disease with AKI necessitating RRT and significant mortality. Basu et al.[4] in the year 2011 in South India reported an incidence of 12% for leptospirosis among which 6% developed AKI, but none of them required RRT and there was no mortality. The present scenario with higher mortality and morbidity may be related to severe strain development or drug resistance.
What is worrisome is dengue emerging as a cause of severe AKI. In the present study, 44.70% of total patients with dengue presented with Stage III AKIN and required dialysis. In the study by Basu et al.,[4] out of ten patients with dengue-related AKI, two required dialysis but mortality was maximum at 25% in patients with dengue. In the study done by Daher et al.,[23] 4% of total admissions of AKI in ICU were due to dengue. Acute renal failure is a potential complication of severe dengue infection and is typically associated with hypotension, rhabdomyolysis, or hemolysis.[29] Development of AKI in patients with dengue infection is associated with increased mortality. In Thailand, the prevalence of AKI in fatal DHF was 33.3%, compared with 0.3% in all DHF cases.[34]
Patients with AGE or typhoid presented with less severe class of AKI and lesser need for hemodialysis. Out of the 230 patients involved in the study, 103 patients (44.78%) required dialysis. In AKIN Stage III (92.80%), patients needed dialysis. In the present study, correlation of etiology with both, AKIN class depicting the severity of AKI and need for RRT, was statistically significant (P = 0.001).
The number of patients requiring dialysis varies in different studies of infection-related AKI carried out in India and other tropical countries. In the study by Basu et al.,[4] 19.2% of patients received dialysis in South India and 27.6% and 35% of patients received dialysis in two separate studies done by Daher et al.[23,25] in Brazil. Eighty-three percentage of patients received dialysis in the study done by Kaul et al.[27] in North India. Ninety-two percentage of patients received dialysis in the study done by Mehta et al.[5] in Mumbai. Requirement of dialysis in tropical infections varies from 19% to 92% depending on the underlying etiology and severity of illness. Studies by Daher et al.[23,25] enrolled ICU patients comprising mainly HIV/AIDS-related cases, pneumonia, meningitis, and tuberculosis. Infections such as leptospirosis and dengue comprise small proportion of total patient base which differs from patient base of the present study which involves infections such as malaria, leptospirosis, dengue, and more than one etiology comprising a combination of the same infections.
Predictors of severity of acute kidney injury
Association between severity of AKI with etiology, multiorgan failure, vasopressor support, hyperbilirubinemia, ARDS, and need for assisted ventilation was statistically significant in this study [Table 4].
Mortality and prognostic factors significantly associated with mortality
In the present study, maximum mortality [Figure 7] was seen in patients with dengue: 9 patients (23.7%) followed by leptospirosis: 5 patients (16.7%), and those with mixed etiology: 2 patients (16.7%) and undifferentiated etiology 3 patients (12.5%). Seven patients (10.8%) died due to malaria. Although malaria was the causative agent for the highest number of AKIs in our study, dengue and leptospirosis and those with multiple etiologies were responsible for higher mortality. Etiology as a risk factor for mortality was statistically significant in the present study [Table 5]. The development of AKI in patients with dengue infection is associated with increased mortality.[30] The mortality of AKI associated with a specific disease such as severe malaria, that is often complicated with multiple organ failure, is very high.[35] Leptospirosis presenting with AKI is a severe disease, frequently leading to multiorgan failure and death in one-third of the patients.[36] Significant morbidity and mortality has been reported by Yang et al.[37] in patients with AKI due to leptospirosis. Mortality with diarrheal illness is 3.8% which has decreased due to practices of early, rapid, and careful hydration with definitive treatment. No mortality was seen with typhoid. In two separate studies in Brazil, by Daher et al.[23,25] in AKI in infectious diseases ICU, the mortality rate was 62.8% and 66%, respectively, reflecting the more critical illness of patients admitted in ICU.
The maximum number of deaths involving 25 patients (22.5%) was seen in AKIN Stage III which correlates with the severity of AKI. Association of mortality with increasing severity of AKIN stages is statistically significant. Risk for mortality increased with increasing delay in seeking medical attention (P = 0.001).
More deaths were observed in patients requiring vasopressors (P = 0), thus implying the role of hemodynamic instability, hypoperfusion-related cellular injury, and multiorgan involvement increasing the risk for mortality.[38] In multivariate analysis [Table 5], need for vasopressor support (odds ratio [OR] = 16.29) and assisted ventilation (OR = 27.05) was the significant risk factor for mortality.
It would have been ideal to analyze the following parameters: time of diagnosis of AKI after admission, time of nephrology consult after admission, use of diuretics, RRT requirement, and type of RRT with respect to incidence of CKD after an episode of AKI. However, these patients once discharged after recovery are not on regular follow-up.
Conclusions
Tropical AKI presents in severe stage and in significant proportion during monsoon. Malaria and AGE are still the predominant etiologies while leptospirosis and dengue are emerging etiologies causing AKI during monsoon. Severity of AKI and requirement of dialysis were more in patients with mixed etiology, leptospirosis, malaria, and least with enteric fever. Hyperbilirubinemia, ARDS, need for vasopressor requirement, assisted ventilation, and multiorgan failure were predictors of severe AKI. Mortality was more in patients with dengue, leptospirosis, and least with enteric fever. Severity of AKI, delay in initiation of treatment, sepsis, multiorgan involvement, and need for supportive lifesaving therapies were significant risk factors for mortality. Awareness of potential renal complications and their prevention and treatment when indicated is the single most cost-effective lifesaving measure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sitprija V. Altered fluid, electrolyte and mineral status in tropical disease, with an emphasis on malaria and leptospirosis. Nat Clin Pract Nephrol. 2008;4:91–101. doi: 10.1038/ncpneph0695. [DOI] [PubMed] [Google Scholar]
- 2.Cerdá J, Bagga A, Kher V, Chakravarthi RM. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4:138–53. doi: 10.1038/ncpneph0722. [DOI] [PubMed] [Google Scholar]
- 3.Kideny Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2:1–138. [Google Scholar]
- 4.Basu G, Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JA, et al. Acute kidney injury in tropical acute febrile illness in a tertiary care centre – RIFLE criteria validation. Nephrol Dial Transplant. 2011;26:524–31. doi: 10.1093/ndt/gfq477. [DOI] [PubMed] [Google Scholar]
- 5.Mehta KS, Halankar AR, Makwana PD, Torane PP, Satija PS, Shah VB. Severe acute renal failure in malaria. J Postgrad Med. 2001;47:24–6. [PubMed] [Google Scholar]
- 6.Thanachartwet V, Desakorn V, Sahassananda D, Kyaw Win KK, Supaporn T. Acute renal failure in patients with severe falciparum malaria: Using the WHO 2006 and RIFLE criteria. Int J Nephrol. 2013;2013:841518. doi: 10.1155/2013/841518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daher EF, Silva GB, Jr, de Abreu KL, Mota RM, Batista DV, Rocha NA, et al. Leptospirosis-associated acute kidney injury: Penicillin at the late stage is still controversial. J Clin Pharm Ther. 2012;37:420–5. doi: 10.1111/j.1365-2710.2011.01312.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhagat A, Bhurke SP, Shetty PP, Mehta KS. ISN West Zone, India, Souvenir: (Abstract); 2012. Study of Acute Kidney Injury in Monsoon Related Acute Febrile Illness in Mumbai; p. 202. [Google Scholar]
- 9.Burdmann EA, Jha V, Sitprija V. Comprehensive Clinical Nephrology. 4th ed. Ch 67. Missouri: Elsevier Saunders; 2010. Acute kidney injury in tropics; pp. 813–20. [Google Scholar]
- 10.Jha V, Chugh KS. Community-acquired acute kidney injury in Asia. Semin Nephrol. 2008;28:330–47. doi: 10.1016/j.semnephrol.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Naicker S, Aboud O, Gharbi MB. Epidemiology of acute kidney injury in Africa. Semin Nephrol. 2008;28:348–53. doi: 10.1016/j.semnephrol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Desai S, van Treeck U, Lierz M, Espelage W, Zota L, Sarbu A, et al. Resurgence of field fever in a temperate country: An epidemic of leptospirosis among seasonal strawberry harvesters in Germany in 2007. Clin Infect Dis. 2009;48:691–7. doi: 10.1086/597036. [DOI] [PubMed] [Google Scholar]
- 13.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with Chikungunya virus in Italy: An outbreak in a temperate region. Lancet. 2007;370:1840–6. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 14.Semenza JC, Menne B. Climate change and infectious diseases in Europe. Lancet Infect Dis. 2009;9:365–75. doi: 10.1016/S1473-3099(09)70104-5. [DOI] [PubMed] [Google Scholar]
- 15.Wilson ME, Freedman DO. Etiology of travel-related fever. Curr Opin Infect Dis. 2007;20:449–53. doi: 10.1097/QCO.0b013e3282a95e27. [DOI] [PubMed] [Google Scholar]
- 16.Naqvi R, Ahmad E, Akhtar F, Naqvi A, Rizvi A. Outcome in severe acute renal failure associated with malaria. Nephrol Dial Transplant. 2003;18:1820–3. doi: 10.1093/ndt/gfg260. [DOI] [PubMed] [Google Scholar]
- 17.Prakash J, Singh AK, Kumar NS, Saxena RK. Acute renal failure in Plasmodium vivax malaria. J Assoc Physicians India. 2003;51:265–7. [PubMed] [Google Scholar]
- 18.Pilane KM, Jagasia B, Halankar AR. Renal involvement in leptospirosis. (Abstract) Indian J Nephrol (New Ser) 1993;4:23. [Google Scholar]
- 19.Mudur G. Mumbai braces itself for leptospirosis and waterborne infections. J Assoc Physicians India. 2004;52:623–5. doi: 10.1136/bmj.331.7512.307-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra N, Patel A, Abraham G, Reddy YN, Reddy YN. Acute kidney injury in dengue fever using Acute Kidney Injury Network criteria: Incidence and risk factors. Trop Doct. 2012;42:160–2. doi: 10.1258/td.2012.120023. [DOI] [PubMed] [Google Scholar]
- 21.Neeraja M, Iakshmi V, Teja VD, Lavanya V, Priyanka EN, Subhada K, et al. Unusual and rare manifestations of dengue during a dengue outbreak in a tertiary care hospital in South India. Arch Virol. 2014;159:1567–73. doi: 10.1007/s00705-014-2010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar SS, Paramananthan R, Muthusethupathi MA. Acute renal failure due to acute diarrhoeal diseases. J Assoc Physicians India. 1990;38:164–6. [PubMed] [Google Scholar]
- 23.Daher Ede F, Junior Silva GB, Vieira AP, Souza JB, Falcão Fdos S, Costa CR, et al. Acute kidney injury in a tropical country: A cohort study of 253 patients in an infectious diseases Intensive Care Unit. Rev Soc Bras Med Trop. 2014;47:86–9. doi: 10.1590/0037-8682-0223-2013. [DOI] [PubMed] [Google Scholar]
- 24.Barsoum RS. Critical Care Nephrology. 2nd ed. Ch 167. Philadelphia: Elsevier Saunders; 2009. Tropical infections causing acute kidney injury; pp. 867–71. [Google Scholar]
- 25.Daher EF, Marques CN, Lima RS, Silva Júnior GB, Barbosa AS, Barbosa ES, et al. Acute kidney injury in an infectious disease Intensive Care Unit – An assessment of prognostic factors. Swiss Med Wkly. 2008;138:128–33. doi: 10.4414/smw.2008.12062. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization (WHO) Malaria Fact Sheet No 94. 2010. [Last assessed on 2011 Jan 23]. Available from: http://www.who.int/mediacentre/factsheets/fs094/en/
- 27.Kaul A, Sharma RK, Tripathi R, Suresh KJ, Bhatt S, Prasad N. Spectrum of community-acquired acute kidney injury in India: A retrospective study. Saudi J Kidney Dis Transpl. 2012;23:619–28. [PubMed] [Google Scholar]
- 28.Prakash J, Singh TB, Ghosh B, Malhotra V, Rathore SS, Vohra R, et al. Changing epidemiology of community-acquired acute kidney injury in developing countries: Analysis of 2405 cases in 26 years from Eastern India. Clin Kidney J. 2013;6:150–5. doi: 10.1093/ckj/sfs178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima EQ, Nogueira ML. Viral hemorrhagic fever-induced acute kidney injury. Semin Nephrol. 2008;28:409–15. doi: 10.1016/j.semnephrol.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Lizarraga KJ, Nayer A. Dengue-associated kidney disease. J Nephropathol. 2014;3:57–62. doi: 10.12860/jnp.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthusethpathi MA, Shivakumr S, Rajendran S, Vijayakumar R, Jayakumar M. Leptospiral renal failure in Madras city. Indian J Nephrol. 1991;1:15–7. [Google Scholar]
- 32.Mathew AJ, George J. Acute kidney injury in the tropics. Ann Saudi Med. 2011;31:451–6. doi: 10.4103/0256-4947.84620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang F, Zhang L, Wu H, Zou H, Du Y. Clinical analysis of cause, treatment and prognosis in acute kidney injury patients. PLoS One. 2014;9:e85214. doi: 10.1371/journal.pone.0085214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee IK, Liu JW, Yang KD. Clinical characteristics, risk factors, and outcomes in adults experiencing dengue hemorrhagic fever complicated with acute renal failure. Am J Trop Med Hyg. 2009;80:651–5. [PubMed] [Google Scholar]
- 35.Blumberg L, Lee RP, Lipman J, Beards S. Predictors of mortality in severe malaria: A two year experience in a non-endemic area. Anaesth Intensive Care. 1996;24:217–23. doi: 10.1177/0310057X9602400213. [DOI] [PubMed] [Google Scholar]
- 36.Covic A, Goldsmith DJ, Gusbeth-Tatomir P, Seica A, Covic M. A retrospective 5-year study in Moldova of acute renal failure due to leptospirosis: 58 cases and a review of the literature. Nephrol Dial Transplant. 2003;18:1128–34. doi: 10.1093/ndt/gfg095. [DOI] [PubMed] [Google Scholar]
- 37.Yang CW, Wu MS, Pan MJ. Leptospirosis renal disease. Nephrol Dial Transplant. 2001;16(Suppl 5):73–7. doi: 10.1093/ndt/16.suppl_5.73. [DOI] [PubMed] [Google Scholar]
- 38.Maier RV. Harrison's Principles of Internal Medicine. 18th ed. Ch 279. United States of America: McGraw-Hill Medical; 2012. Approach to the patient with shock; pp. 2215–22. [Google Scholar]


