Abstract
Chemotherapy in prostate cancer (PCa) has undergone dramatic landscape changes. While earlier studies utilized varying chemotherapy regimens which were found to be largely palliative in nature and hardly resulted in durable or meaningful responses, docetaxel resulted in the first chemotherapy agent that showed improvement in overall survival in metastatic castration-resistant prostate cancer (mCRPC). However, combination chemotherapy or any agents added to docetaxel have failed to yield incremental benefits. The improvement in overall survival as well as secondary endpoints of prostate-specific antigen (PSA) and time to recurrence when using docetaxel in the metastatic hormone-sensitive state has changed the standard of care for treatment of newly diagnosed de novo metastatic PCa. There are also promising results in locally advanced PCa and high-risk PCa in both the neoadjuvant and adjuvant settings. This review summarizes the historical as well as the more contemporary use of chemotherapeutic agents in PCa in varying states and phases of disease.
Keywords: docetaxel, metastatic castration-resistant prostate cancer, prostate cancer
INTRODUCTION
The first case of prostate cancer (PCa) was described as a very rare disease by J Adams at the London Hospital in 1853.1,2 Today, PCa has emerged as the most common noncutaneous malignancy in men with 161 360 cases projected to occur in 2017 alone in the United States.3 For localized PCa, apart from active surveillance that is emerging as a viable form of management, radical prostatectomy or radiotherapy represents the two main curative forms of therapy.4 Androgen deprivation therapy has been considered the backbone of treatment for advanced and metastatic cancer.5 The aim of this review article is to explore the role of chemotherapy in the treatment of varying stages and phases of PCa, from high-risk locally advanced PCa to hormone-sensitive metastatic disease and to metastatic castration-resistant prostate cancer (mCRPC).
HISTORY OF CHEMOTHERAPY USE IN PCA – FROM PALLIATIVE TO SURVIVAL ADVANTAGE
In the 1940s, Dr. Charles Huggins and Dr. Clarence Hodges were the first to demonstrate that bilateral orchiectomy or estrogen therapy resulted in shrinkage of prostate tumors by decreasing testosterone levels and inducing castration.6 Since then, androgen ablation therapy has been the mainstay of treatment of PCa. Initially, most patients responded to androgen-ablative therapy or androgen deprivation therapy (ADT), but became resistant with time and developed fatal disease.2 At that time, there were no clear methods to measure tumor burden. Most patients would have soft tissue diseases involving the prostate and lymph nodes where they could not be easily measured, along with extensive bone metastases. They mostly suffered from bone pain which when localized usually transiently responded to radiation therapy. Obstructive symptoms were treated with transurethral resection. It was the patients with generalized bone pain in whom effective palliation was difficult, and so came the need for clinical trials of chemotherapy in these selected patients.7
Between the 1950s and 1970s, several small trials took place using alkylating agents but these investigations along with results were poorly documented. A large number of single agents have been studied. However, most of these trials were broad, unfocused Phase II clinical trials without specific criteria for response and with a small number of patients.8 PCa was largely considered a chemotherapy unresponsive disease.9 This is why for a certain drug, results varied widely among different investigators and showed a lack of reproducibility. For this reason, several trials using two or more drugs in combination were performed. None of the combinations showed any major impact on response or survival. In 1972, the National Prostatic Cancer Project (NPCP) began a series of randomized Phase II and III studies on single agents and combinations in what was then termed "hormonally-resistant" but later coined "castration-resistant" PCa patients. They used response as their main endpoint and compared chemotherapy drugs to the standard treatment which consisted of palliative therapies (radiation, hormonal therapies, or analgesic use). The NPCP criteria were complete response (CR), partial response (PR), and objective stabilization as endpoints of therapeutic efficacy. These trials showed higher rates of objective responses on the chemotherapy arms, but there was no survival advantage, and most of the response was stable disease with CR and PR occurring rarely. Whether the stability of the disease was due to the chemotherapy or to the slow progression of PCa in general could not be determined. The NPCP also tested the efficacy of chemotherapy in combination with hormonal therapy but also showed no difference between the study arms. At that time, all existing data did not show any evidence that the addition of chemotherapy would prolong survival and there was no solid evidence to support the use of any chemotherapeutic agent as a standard of care.10 Reports of NPCP and other trials suggested that patients may have improvement of pain and other symptoms after treatment with any of several drugs, but all drugs added toxicity.7 The different chemotherapy regimens that have been studied were vast and some of the earlier trial results during that period are summarized.
Cyclophosphamide is an alkylating agent that affects cell division by cross-linking deoxyribonucleic acid (DNA) strands and thus decreasing DNA synthesis. In early conducted trials, these drugs were collectively felt to deliver suboptimal responses in urologic tumors.11 Carter and Wasserman showed a response in 8 out of 57 patients, although response criteria used were unclear.12 In the NPCP trials, CR was reported in 0% of patients and PR in 7% of patients, and 26%–46% of patients had stable disease.9 Cyclophosphamide was also used orally with modest responses,13 though later repurposing with interest in cyclophosphamide's role in angiogenesis inhibition through metronomic cycling,14 brought a resurgence of interest in the use of this drug for docetaxel failures.15
Cisplatin is a platinum-containing compound that inhibits DNA synthesis by cross-linking and denaturing DNA strands. Cisplatin was studied using a weekly schedule for 6 weeks then every 3 weeks maintenance,16 and was found to have a complete and partial remission in 17 patients (31%) out of 54. Another study reported four CRs and PRs in 21 patients (19%).17 Yagoda et al.18 observed only three CRs and PRs (12%), while an Eastern Cooperative Oncology Group (ECOG) study observed no response in 17 evaluable patients.19 The NCPC 1100 study showed no CR in patients treated with cisplatin,20 but 4% PR and 32% stable disease, making up 36% objective response rates, and protocol 1200 showed a CR and PR in none of patients, and stable disease in 21%.21 In 209 cases reviewed, cisplatin showed a modest antitumor activity with a PR in 12% (95% confidential interval [CI]: 4%–20%) and thus was continued to be investigated as a single agent and in combination.9
Carboplatin, a cisplatin derivative that results in intra- and inter-strand cross-linkage DNA damage, had been studied in earlier trials as a single agent with minimal responses.22,23 However, when combined with other chemotherapy drugs such as paclitaxel and estramustine, declines in serum prostate-specific antigen (PSA) levels of 50%, 80%, and 90% were seen in 67% (95% CI: 55%–79%), 48% (95% CI: 35%–61%), and 39% (95% CI: 26%–52%), respectively. There were two patients (6%) of 33 patients who had a CR and 13 (39%) had a PR.24
Satraplatin, the first oral 4th generation platinum analog found to be effective against cisplatin- and carboplatin-resistant cell lines,25 held a lot of promise in castration-resistant PCa,26 and while it showed improvement in time to pain progression, it failed to improve overall survival (OS) in the Phase III SPARC registration trial.27
5-fluorouracil (5-FU) is a pyrimidine analog that inhibits DNA synthesis during the synthesis (S) phase by inhibiting thymidylate synthetase. Studies in the aggregate comprising of 124 patients receiving 5-FU in various doses and schedules showed modest antineoplastic activity with a response rate of around 9% (95% CI: 4%–14%).28,29 In one of the larger trials that included 147 patients comparing doxorubicin to 5-FU, doxorubicin yielded 25% (15 out of 61 patients) response compared to 8% (3 out of 42 patients) treated with 5-FU alone.30
Capecitabine, an oral fluoropyrimidine carbamate that delivers 5-FU to tumor cells, was studied in castrate-resistant PCa with PSA response rates in the 12%.31
Methotrexate, a dihydrofolic acid reductase inhibitor which inhibits purine and thymidylic acid synthesis, serves to interfere with DNA synthesis. It has been investigated in several earlier Phase II trials and by the NPCP. In the NPCP Phase III randomized trial (protocol 1100) using methotrexate at varying doses,32 only one out of 63 patients had a PR (2%, 95% CI: 0–6%), and stable disease occurred in 20%.
Doxorubicin is an anthracycline that intercalates between DNA base pairs and impairs topoisomerase II function inhibiting replication and transcription. Doxorubicin has also shown only marginal responses against PCa. In the earliest trials, responses varied from a 29% response rate as reported by O’Bryan et al.33 to a 5% response rate as reported by Scher et al.34 Each study had a different response criteria which explain the variability in results. The NPCP results showed clinical benefit with a response rate that included stable disease reaching 84%.10 Subsequent trials utilizing additional ketoconazole with doxorubicin alternating with vinblastine and etoposide chemotherapy showed no additional benefit to hormonal therapy alone.35
Etoposide alters DNA replication, induces G2 phase arrest, and kills cells in G2 and late S phases. Studies showed poor response rates with an overall response rate of 3% (95% CI: 0–7%) in the aggregate.36,37,38 Vinblastine, a vinca alkaloid that inhibits microtubule formation, had been shown in older studies to induce a 21% remission rate in a small number of 39 patients.29
Estramustine, an estradiol and nornitrogen mustard carbamate-linked combination which has an antiandrogen effect and antimicrotubule effect, was extensively studied by the NPCP and had been reported to have a palliative effect in castration resistant PCa patients, but objective response was rare in NPCP trials. Estramustine as a single agent was evaluated in varying NPCP protocols that included 163 patients, where CR plus PR rates varied between 0-4%, but with SD included, rates increased to 18%-34%.9 Similarly, when estramustine was studied in combination with prednimustine, vincristine, and cisplatin, no remarkable added benefit was shown.29 Given notable side effects of estramustine such as nausea and diarrhea, its use was not widely adopted,7 though it did garner the first the United States Food and Drug Administration (FDA) approval for a cytotoxic therapy in 1981 for its clinical response given lack of other active agents.39,40 While later studies combining it with docetaxel showed promising results,41,42 efficacy was felt to be more due to docetaxel, omitting use of estramustine altogether due to side effect profile.
Mitoxantrone is an anthracenedione, a Type II topoisomerase inhibitor that serves to interfere with DNA intercalation and damage. Prednisone, on the other hand, was believed to produce a negative feedback on the pituitary gland that would inhibit the secretion of adrenocorticotropic hormone leading to decreased dehydroepiandrosterone (DHEA) and dehydroepiandrosterone-sulfate (DHEAS) which can be metabolized to small amounts of testosterone. In patients no longer responding to primary androgen ablation, up to about 30% may have improvement of symptoms, mainly bone pain, with low-dose prednisone and mitoxantrone.43 In 1996, a randomized controlled trial was published that compared mitoxantrone plus prednisone versus prednisone alone in symptomatic CRPC patients with a total of 161 fairly symptomatic men with pain.44 The primary endpoint of the study was palliation with pain relief as its primary indicator. In the patients who received mitoxantrone with prednisone, a 29% palliative response over a duration of 48 weeks was observed versus 12% over 18 weeks in patients who received prednisone alone. Progression-free survival (PFS) was also shown to be better in the mitoxantrone with prednisone arm although OS was similar in both arms. Mitoxantrone was well tolerated except for possible cardiac toxicity in 5 patients.
Another Phase III trial, the Cancer and Leukemia Group B (CALGB) 9182, randomized 244 CRPC patients to mitoxantrone plus hydrocortisone versus hydrocortisone alone.45 It also showed improvement in pain control but no effect on survival (12.3 vs 12.6 months, P = 0.3298). Once these studies demonstrated mitoxantrone's palliative benefits, mitoxantrone became the next cytotoxic drug to be approved by the United States FDA for use in mCRPC for quality of life results.40 Other trials were done to evaluate mitoxantrone's role in OS, but failed to demonstrate any benefit. Today, mitoxantrone is used with the goal of improving quality of life and pain control as second- or third-line or beyond chemotherapy.
PCA WORKING GROUP CRITERIA FOR RESPONSE
Given the challenges and lack of standardization in defining PSA responses as well as progression, the PSA working group initially convened a consensus conference and published the guidelines in 1999 in order to guide selection of candidate agents for further testing and choosing which agents that can proceed to Phase III trials especially if they are based on different gauge of PSA changes.46 They also proposed that response duration and time to PSA progression may be important clinical endpoints. The working group criteria were further revised in 2009 with an emphasis on using different parameters, not just PSA progression alone, and patients with early changes in PSA and/or pain are not encouraged to be acted upon without other evidence of objective disease progression such as radiographic technetium scan or computed tomography scans using the Response Evaluation Criteria in Solid Tumors (RECIST) criterion and pain scales.47 In addition, given drugs that were felt to be more cytostatic than cytotoxic, treatment was encouraged to be continued for at least 3 months so that drug exposure was ensured to be adequate. The Prostate Cancer Clinical Trials Working Group 3 (PCWG3) reconvened and published updated guidelines in 2016. The emphasis was to be able to distinguish between first progression and the clinical need to switch treatment, with the provision for using blood-based diagnostics, novel imaging and biologic profiling wherever applicable.48
DOCETAXEL: ROLE IN MCRPC
Up until 2004, there was still no standard front-line or second-line chemotherapy for mCRPC that improved OS. Treatment options for mCRPC at the time often included second-line hormonal therapy, radiation therapy, cytotoxic chemotherapy, investigational therapy, or supportive care. Chemotherapy was clearly shown to provide palliative benefit but no survival benefit, and the regimens available at the time, as aforementioned, were mitoxantrone, estramustine, or docetaxel.49
Docetaxel is a taxane derivative that works by binding to microtubules and preventing androgen receptor nuclear translocation and causing apoptosis through B-cell lymphoma (Bcl-2) phosphorylation.50 Studies using docetaxel as a single agent or in combination with estramustine showed objective response rates in up to 38% of patients, PSA declines of more than 50% in 69% of patients.51,52 These findings encouraged subsequent two trials: the TAX 327 trial and the Southwest Oncology Group (SWOG) 99-16 trial.
In 2004, the pivotal TAX 327, a randomized nonblinded Phase III trial, was published.53 It involved 1006 men with PCa refractory to hormonal treatment who were assigned to three arms: 12 mg m−2 of mitoxantrone every 3 weeks compared to 75 mg m−2 every 3 weeks, or 30 mg m−2 of docetaxel weekly for 5 out of every 6 weeks and all these patients were followed for about 20 months. The primary endpoint was OS and secondary endpoints were pain, PSA levels, and quality of life. The results showed that the survival rate in the group of docetaxel every 3 weeks was significantly higher than the mitoxantrone group, 18.9 months versus 16.5 months, respectively (P = 0.009). The group of weekly docetaxel did yield an OS of 17.4 months, not significantly higher than the mitoxantrone group (P = 0.36), and the every 3 weeks of docetaxel therefore became the default standard of care thereafter. Pain reduction was more frequently observed among patients receiving docetaxel every 3 weeks than those receiving mitoxantrone (35% vs 22%, P = 0.01), while weekly docetaxel did not significantly differ than mitoxantrone alone (31% vs 22%, P = 0.08). PSA response, defined as >50% reduction in PSA levels in this study, was also significantly higher in the patients receiving docetaxel every 3 weeks (45%) as compared to mitoxantrone (32%), as well as in the patients receiving docetaxel weekly (48%). This study concluded that cytotoxic chemotherapy, more specifically docetaxel with prednisone, can significantly prolong OS in men with mCRPC and was the basis of the United States FDA approval of docetaxel in 2004.
Another Phase III trial, which was published at the same time with the TAX 327 trial, was the SWOG 99-16 which enrolled 770 men with a similar eligibility criteria with mCRPC patients randomized to two treatment arms each given in 21-day cycles: 280 mg of estramustine three times daily on days 1 to 5, 60 mg m−2 of docetaxel on day 2 and 60 mg of dexamethasone before docetaxel, versus 12 mg m−2 of mitoxantrone on day 1 plus 5 mg of prednisone twice daily.41 The primary endpoint of OS was shown to be significantly longer in the docetaxel plus estramustine arm compared to mitoxantrone arm (17.5 vs 15.6 months, P = 0.02). The secondary endpoints such as the median time to progression were significantly better in the docetaxel and estramustine arm (6.3 vs 3.2 months, P < 0.001), although pain improvement was not shown to be different in either group. Adverse events and Grade 3/4 toxicities, such as gastrointestinal, cardiovascular, thromboembolic events, infections, and neuropathy, were all shown to be higher in the arm that contained docetaxel and estramustine (54% of patients) compared to the mitoxantrone arm (34% patients). However, this difference was not associated with an increased rate of treatment-related deaths or discontinuation of treatment. This study concluded that treatment with estramustine and docetaxel moderately not only increases the survival but also increases the rate of adverse events. Despite demonstrated survival advantage, the combination of docetaxel plus estramustine is rarely used now due to significant toxicity of the regimen.
These two studies, primarily the TAX 327 and secondarily the SWOG 9916, have set the standard of care for men with mCRPC. Numerous subsequent combination trials have been performed in an attempt to improve upon the efficacy of docetaxel, but most of these have been largely negative trials.
CABAZITAXEL: ROLE AS SECOND-LINE SALVAGE CHEMOTHERAPY
After failure of docetaxel as first-line chemotherapy, second-line treatment options included mitoxantrone, retreatment with docetaxel, or clinical trials.8 It was not until 2010 that another chemotherapeutic drug, cabazitaxel, was FDA approved for the treatment of PCa.
Cabazitaxel is a third-generation, semisynthetic tubulin-binding taxane drug that was developed after resistance was seen with the other taxanes.54 It was found to be as potent as docetaxel in cell lines and has antitumor activity in models resistant to paclitaxel and docetaxel. A randomized Phase III open-label clinical trial termed the TROPIC trial (XRP6258 Plus Prednisone Compared to Mitoxantrone Plus Prednisone in Hormone Refractory Metastatic Prostate Cancer), was reported in 2010, in which the aim was to assess the role of cabazitaxel plus prednisone in patients with mCRPC who progressed after docetaxel.55 Seven hundred and fifty-five patients were randomized to receive either cabazitaxel (25 mg m−2) or mitoxantrone (12 mg m−2) on day 1 of each 21-day cycle, and all patients received prednisone 10 mg daily. The primary endpoint of the trial was median OS and was superior in the cabazitaxel arm at 5.1 (95% CI: 14.1–16.3) months compared to mitoxantrone group at 12.7 (95% CI: 11.6–13.7) months translating to a 30% reduction in relative risk of death (HR: 0.70, 95% CI: 0.59–0.83, P < 0.0001). On the other hand, cabazitaxel showed higher adverse events, the most frequent was hematological mostly neutropenia, leukopenia, and anemia, thereby adding on to the label of cabazitaxel upon FDA approval in 2010 with use of growth factors for prophylaxis for patients older than 65 years of age or those with significant comorbidities.
The PROSELICA trial (Cabazitaxel at 20 mg m−2 compared to 25 mg m−2 with Prednisone for the Treatment of Metastatic Castration Resistant Prostate Cancer) was a Phase III study that sought to compare two different dosing of cabazitaxel at 20 mg m−2 versus cabazitaxel at the standard dose of 25 mg m−2 in 1200 patients with mCRPC who progressed after docetaxel. The study results showed the noninferiority of the 20 mg m−2 as compared to 25 mg m−2 dose of cabazitaxel every 3 weeks in combination with prednisone (median OS: 13.4 vs 14.5 months, respectively) and it showed that the 20 mg m−2 dose had less adverse events (39.7% vs 54.5%). Particularly, the rate of Grade 4 neutropenia was 21.3% in the lower dose and 48.6% in the higher dose groups.56 The FIRSTANA trial (Cabazitaxel vs Docetaxel Both With Prednisone in Patients With Metastatic Castration Resistant Prostate Cancer) was a Phase III study that compared cabazitaxel to docetaxel in chemotherapy-naïve patients with mCRPC and recent results did not reveal superiority in OS (median: 24.5 months, 25.2 months, and 24.3 months in the cabazitaxel 20 mg m−2 group, cabazitaxel 25 mg m−2 group, and docetaxel 75 mg m−2 group, respectively; HR: 0.97, 95% CI: 0.819–1.16, P = 0.7574),57 therefore showing that docetaxel remains the most appropriate first-line chemotherapy regimen for patients with mCRPC.
Cabazitaxel therefore remains an option for patients with mCRPC who have failed docetaxel and there are no data to support greater efficacy of cabazitaxel over docetaxel in the chemotherapy-naïve patients.
DOCETAXEL – ROLE IN EARLY HORMONE-SENSITIVE PCA
For metastatic hormone-sensitive PCa, the cornerstone of treatment has been aimed toward addressing the androgen pathway, but most of these patients will progress to castration resistant PCa in 1–2 years. The mechanism of action of docetaxel raised the question of its possible benefit in hormone-sensitive PCa, which led to further investigations.58
A randomized study from the Genito-Urinary Group and the French Association of Urology (GETUG-AFU) 15 was a Phase III trial that evaluated the role of docetaxel in 385 men with metastatic hormone-sensitive PCa. Patients were randomized to receive either androgen deprivation therapy alone or with docetaxel (75 mg m−2 every 3 weeks) and were followed up for 50 months. Results for the primary endpoint, OS, were not statistically significant (58.9 months in ADT plus docetaxel vs 54.2 months in the group given ADT alone, HR: 1.01, 95% CI: 0.75–1.36), but the ADT plus docetaxel reported more adverse events (72 more serious adverse events). The authors concluded that the results of the trial did not support the use of docetaxel as part of first-line treatment for patients with noncastrate metastatic PCa.59 In 2015, long-term follow-up results were published that also showed no statistically significant benefit (62.1 vs 48.6 months; HR: 0.88, 95% CI: 0.68–1.14, P = 0.3) but did show an absolute difference in median OS of 14 months.60
Another larger study, the ECOG-run ChemoHormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED) study, enrolled 790 metastatic hormone-sensitive PCa patients and randomized them to receive either ADT alone or ADT with docetaxel (75 mg m−2 every 3 weeks).61 OS was 16.6 months longer, 57.6 months with the addition of early docetaxel to ADT versus 44 months with ADT alone (HR: 0.61, 95% CI: 0.47–0.80, P < 0.001). This study showed that combining docetaxel with ADT early only for patients diagnosed with de novo castration-sensitive PCa resulted in longer OS, longer time to develop castration resistance, and better cancer control especially for the high volume disease group.
The Systemic Therapy in Advanced or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE) trial was the largest of the trials that aimed at investigating the efficacy of using various treatments including docetaxel and zoledronic acid as front-line with hormonal therapy in men with newly diagnosed locally advanced or metastatic PCa and men who have relapsed after local therapy.62 It enrolled 2862 eligible patients with PCa who were newly diagnosed as metastatic, node positive, or high-risk locally advanced (with at least two of disease features pT3/4, Gleason score of 8–10, and PSA ≥40 ng ml−1) or previously treated with radical surgery, radiotherapy, or both and relapsing with high-risk features. They were randomized in a 2:1:1:1 ratio to standard of care only, standard of care plus zoledronic acid, standard of care plus docetaxel, and standard of care plus zoledronic acid and docetaxel. Patients who received docetaxel along with standard of therapy have improved OS with a 10-month difference (77 vs 67 months; HR: 0.76, 95% CI: 0.63–0.91, P = 0.003), in addition to improvements in failure-free survival. However, subgroup analyses showed that patients with nonmetastatic (M0) disease did not benefit (HR: 1.01, 95% CI: 0.65–1.56). They concluded that standard of care for patients with metastatic castration-sensitive disease should include docetaxel chemotherapy.
A subsequent meta-analysis published in 2015 reviewed all relevant trials on docetaxel with standard of care in castration-sensitive PCa to study the effects of this therapy.63 It showed that the addition of docetaxel improves survival in men with M1 disease, but not M0 disease. Results of the 3 trials (CHAARTED, GETUG-15, and STAMPEDE) all showed that docetaxel as an addition to standard care would improve 4-year survival by 9% (HR: 0.77, 95% CI: 0.68–0.87, P < 0.0001), and reduces 4-year failure rates by 16% (HR: 0.64, 95% CI: 0.58–0.70, P < 0.0001). However, based on the results of STAMPEDE as well as two other trials (GETUG-12, RTOG 0521) in men with locally advanced but M0 disease, unanimous benefit was not shown from the addition of docetaxel (HR: 0.87, 95% CI: 0.69–1.09, P = 0.218). While strong evidence was found to support the addition of docetaxel to standard care with ADT for men with metastatic castration-sensitive PCa, more evidence is still needed to use early chemotherapy for men with M0 disease.
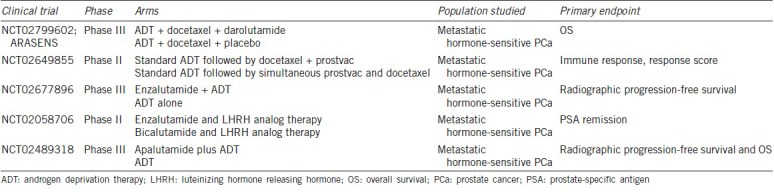
Table 1 lists ongoing trials that utilize chemotherapy in castration-sensitive or hormone-sensitive metastatic PCa. The promising results of early docetaxel chemotherapy have paved the way for dramatic changes in the current standard of care to include docetaxel chemotherapy as part of the cornerstone of treatment in men with newly diagnosed metastatic hormone-sensitive PCa.
Table 1.
Selected ongoing clinical trials in metastatic hormone-sensitive prostate cancer
NEOADJUVANT CHEMOTHERAPY PRIOR TO RADICAL PROSTATECTOMY
The majority of patients with localized PCa who undergo radical prostatectomy are cured but as many as one-third experience recurrence.64 Recently, there has been a growing interest in neoadjuvant treatment in an attempt to eradicate micrometastases and improve surgical outcomes in patients with varying cancers. Given the lack of mature Phase III trials evaluating the role of neoadjuvant chemotherapy in PCa and the availability of a multitude though limited number of patients in Phase II trials that utilizes different chemotherapy agents65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81 (Table 2), there remains to be a limited role of neoadjuvant chemotherapy with or without ADT in the management of localized PCa prior to radical prostatectomy.
Table 2.
Neoadjuvant chemotherapy trials

A Phase II study enrolled 19 patients with high-risk PCa defined as biopsy Gleason scores of 8–10, serum PSA levels >20 ng ml−1, and/or clinical stage T3 disease.82 The recruited patients received weekly docetaxel (36 mg m−2) for 6 months, followed by radical prostatectomy. PSA declines of >50% were seen in 11 of 19 patients and endorectal magnetic resonance imaging (MRI) showed maximum tumor volume reduction of at least 25% in 13 of 19 patients and at least 50% in 4 patients. Sixteen patients completed chemotherapy and had radical prostatectomy and none of them achieved pathologic complete response.
Another Phase II trial also evaluated the role of weekly docetaxel for 6 weeks followed by radical prostatectomy.83 The study showed a statistically significant reduction in PSA (P < 0.03) with 79% of patients experiencing any reduction and 24% of patients experiencing >50% reduction in PSA. There was no complete pathologic response and the positive surgical margin rate was 3.5%.
Neoadjuvant chemotherapy in combination with ADT has also been evaluated.84 In a Phase II study that included 22 patients with high-risk PCa, neoadjuvant docetaxel and estramustine was given and followed for a PSA nadir with a gonadotropin-releasing hormone (GnRH) agonist. One patient achieved a pathologic CR and six patients achieved low residual tumor confined to ≤10% of prostate volume. The mean 5-year disease-free survival at 53 months was 80% for patients with ≤10% residual cancer and 20% for those with >10%.
The Canadian Urologic Oncology Group (CUOG) conducted another larger Phase II trial of combined neoadjuvant chemotherapy with ADT.85 A total of 72 men with high-risk PCa were treated with docetaxel (weekly for 6 of 8 weeks for 3 cycles) and ADT (buserelin acetate every 8 weeks for 3 doses and an antiandrogen for 4 weeks) followed by radical prostatectomy. Median PSA before surgery was 0.14 μg l−1 representing a median decrease of 98.4%. Of the 64 patients completing the protocol, two (3%) had a pathologic CR and 16 patients (25%) had ≤5% tumor in the RP specimen. There were 34 patients (53%) with pathologic T2 disease, 17 patients (27%) with positive margins, and 4 patients (6%) with regional lymph node involvement. After a median follow-up of 42.7 months, a total of 19 patients (30%) had PSA recurrence.
The CALGB 90203 trial (Preoperative Use of Neoadjuvant ChemoHormonal Therapy [PUNCH trial]) is a Phase III neoadjuvant trial that randomized patients with clinically localized high-risk PCa and having opted for radical prostatectomy to receive neoadjuvant ADT and docetaxel prior to surgery versus surgery alone. The trial has finished accrual and results are eagerly awaited. The use of neoadjuvant chemotherapy prior to radical prostatectomy remains investigational and is currently not part of the standard of care of patients with PCa.
THE ROLE OF ADJUVANT CHEMOTHERAPY
The role of adjuvant chemotherapy after radiation therapy in PCa was recently evaluated in a large Phase III trial, the RTOG 0521 that randomized a total of 563 high-risk PCa patients to either ADT and radiotherapy or ADT and radiotherapy followed by sequential docetaxel and prednisone.86 Androgen suppression was given for 24 months; external-beam radiation therapy was given for 8 weeks; and docetaxel was given at 75 mg m−2 on day 1 for 6 cycles, starting 4 weeks after the completion of radiotherapy along with prednisone 10 mg. The enrolled patients had Gleason scores between 8 and 10, PSA ≥20 ng ml−1 (but <150 ng ml−1 ), or ≥T2 stage. At a median follow-up of 5.5 years, 4-year OS was 89% in ADT/radiation arm and 93% with the addition of docetaxel, for an absolute benefit of 4% (one-sided P = 0.04) resulting in a 30% reduction in risk of death favoring adjuvant docetaxel. In addition, there was an absolute 10% reduction in the rate of disease-free survival at 6 years (65% vs 55%, P = 0.04) and the risk of biochemical failure was reduced by 20% in the docetaxel-containing arm. As expected, there was more Grade 3 or 4 hematologic toxicity in the chemotherapy arm. This was one of the promising trials that evaluated adjuvant chemotherapy after radiation which was included in a provisionary statement in the National Comprehensive Cancer Network (NCCN) Guidelines for PCa treatment in men with high-risk disease as a consideration for selected patients who are fit to receive chemotherapy.87
On the other hand, adjuvant chemotherapy postprostatectomy has not uniformly shown to be equally as promising (see Table 3). One large trial conducted and reported from the Genito-Urinary Group and the French Association of Urology (GETUG) 12, randomized 207 patients with high-risk disease to ADT in addition to docetaxel and estramustine while 206 patients to ADT alone.88 High-risk features were considered one of the followings: stage T3–T4 disease, Gleason score of ≥8, PSA levels of >20 ng ml−1, or pathological node-positive disease as evidenced by patients undergoing lymph node dissection. Results showed that while the 8-year relapse-free survival was superior in the combination arm with ADT plus docetaxel and estramustine at 62% (95% CI: 55-69) versus 50% (95% CI: 44–57) in the ADT only group (adjusted HR: 0.71, 95% CI: 0.54–0.94, P = 0.017), results on OS and metastasis-free survival were not yet mature.
Table 3.
Adjuvant chemotherapy studies
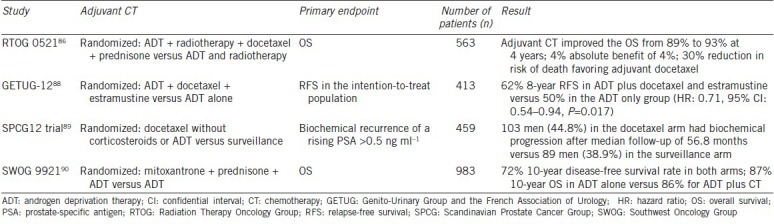
Another adjuvant chemotherapy after radical prostatectomy trial was reported in the Scandinavian Prostate Cancer Group (SPCG) 12 trial, a Phase III study that randomized 459 patients who underwent radical prostatectomy to receive either docetaxel 75 mg m−2 every 3 weeks for 6 cycles (without corticosteroids or ADT) or to undergo surveillance.89 The primary endpoint of the study was biochemical recurrence of a rising PSA >0.5 ng ml−1. The patients were enrolled if they had high-risk disease postprostatectomy defined as having either a pT2 tumor with Gleason score 4 + 3 or 8−10 and positive margins or any pT3a tumor with Gleason score of at least 4 + 3 or any Gleason Grade 4 tumor with pT3b, or any node-positive tumor if Gleason Grade 4 or higher. During a median follow-up of 56.8 months, the rate of biochemical progression was higher in the docetaxel arm (103 men, 44.8%) than the surveillance arm (89 men, 38.9%) in the intention to treat analysis (P = 0.78 for comparison of Kaplan–Meier curves). The authors concluded that there was no benefit and potential harm from the addition of docetaxel in high-risk PCa patients after prostatectomy.
Another adjuvant trial postprostatectomy was recently reported; results of SWOG 9921 trial were recently presented.90 This trial was designed in 1999 to evaluate the role of the addition of chemotherapy (6 cycles of mitoxantrone/prednisone) to 2 years of adjuvant ADT in high-risk PCa after radical prostatectomy. A total of 983 patients were randomized in a 1:1 ratio to either ADT alone or ADT and 6 cycles of mitoxantrone/prednisone. At a median follow-up of 11.2 years, there was no evidence that mitoxantrone improved PCa-specific survival when added to 2 years of ADT and increased the risk of leukemia. The 10-year disease-free survival rate was 72% in both arms, and 10-year OS was 87% for ADT alone compared with 86% for ADT plus chemotherapy. Given the noncontemporary use of an older drug (mitoxantrone), it is unlikely that the findings would result in any meaningful change in the standard practice of adjuvant therapy postprostatectomy.
CONCLUSIONS
Chemotherapy in PCa has evolved from that of palliation to improvement in OS. Docetaxel has been the mainstay of chemotherapy that has been used for PCa with cabazitaxel as second-line therapy. Varying combinations with docetaxel have been attempted but not found to be successful. Changes in the treatment landscape with institution of docetaxel earlier in the disease course have made it the default standard of care for metastatic hormone-sensitive or castration-sensitive PCa along with ADT. Further evaluation as neoadjuvant and adjuvant therapy especially for men with locally advanced high-risk disease is currently underway. Mechanisms of resistance to chemotherapy is discussed extensively elsewhere91 but certainly an area of active investigation as well. The promising results of decades of investigation have finally changed the treatment paradigm in PCa.
AUTHOR CONTRIBUTIONS
RN, JEA, and JBAC designed the study, wrote the manuscript, had access to all data, and reviewed the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Adams J. The case of scirrhous of the prostate gland with corresponding affliction of the lymphatic glands in the lumbar region and in the pelvis. Lancet. 1853;1:393. [Google Scholar]
- 2.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–96. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Hamdy FC, Donovan JL, Neal DE. 10-year outcomes in localized prostate cancer. N Engl J Med. 2017;376:180. doi: 10.1056/NEJMc1614342. [DOI] [PubMed] [Google Scholar]
- 5.Sriprasad S, Feneley MR, Thompson PM. History of prostate cancer treatment. Surg Oncol. 2009;18:185–91. doi: 10.1016/j.suronc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Huggins C, Hodges CV. Studies on prostatic cancer I The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 7.Tannock IF. Is there evidence that chemotherapy is of benefit to patients with carcinoma of the prostate? J Clin Oncol. 1985;3:1013–21. doi: 10.1200/JCO.1985.3.7.1013. [DOI] [PubMed] [Google Scholar]
- 8.Aragon-Ching JB, Dahut WL. Chemotherapy in Androgen-Independent Prostate Cancer (AIPC): what's next after taxane progression? Cancer Ther. 2007;5A:151–60. [PMC free article] [PubMed] [Google Scholar]
- 9.Yagoda A, Petrylak D. Cytotoxic chemotherapy for advanced hormone-resistant prostate cancer. Cancer. 1993;71:1098–109. doi: 10.1002/1097-0142(19930201)71:3+<1098::aid-cncr2820711432>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberger MA, Simon R, O’Dwyer PJ, Wittes RE, Friedman MA. A reevaluation of nonhormonal cytotoxic chemotherapy in the treatment of prostatic carcinoma. J Clin Oncol. 1985;3:827–41. doi: 10.1200/JCO.1985.3.6.827. [DOI] [PubMed] [Google Scholar]
- 11.Yagoda A. Proceedings: non-hormonal cytotoxic agents in the treatment of prostatic adenocarcinoma. Cancer. 1973;32:1131–40. doi: 10.1002/1097-0142(197311)32:5<1131::aid-cncr2820320519>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Carter SK, Wasserman TH. The chemotherapy of urologic cancer. Cancer. 1975;36:729–47. doi: 10.1002/1097-0142(197508)36:2+<729::aid-cncr2820360818>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Nicolini A, Mancini P, Ferrari P, Anselmi L, Tartarelli G, et al. Oral low-dose cyclophosphamide in metastatic hormone refractory prostate cancer (MHRPC) Biomed Pharmacother. 2004;58:447–50. doi: 10.1016/j.biopha.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 15.Ladoire S, Eymard JC, Zanetta S, Mignot G, Martin E, et al. Metronomic oral cyclophosphamide prednisolone chemotherapy is an effective treatment for metastatic hormone-refractory prostate cancer after docetaxel failure. Anticancer Res. 2010;30:4317–23. [PubMed] [Google Scholar]
- 16.Merrin CE. Treatment of genitourinary tumours with cis-dichlorodiammineplatinum (II): experience in 250 patients. Cancer Treat Rep. 1979;63:1579–84. [PubMed] [Google Scholar]
- 17.Rossof AH, Coltman CA, Jr, Jones SE, Talley RW. Phase II evaluation of cis-dichlorodiammineplatinum (II) in lymphomas: a Southwest Oncology Group Study. Cancer Treat Rep. 1979;63:1605–8. [PubMed] [Google Scholar]
- 18.Yagoda A, Watson RC, Natale RB, Barzell W, Sogani P, et al. A critical analysis of response criteria in patients with prostatic cancer treated with cis-diamminedichloride platinum II. Cancer. 1979;44:1553–62. doi: 10.1002/1097-0142(197911)44:5<1553::aid-cncr2820440502>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Qazi R, Khandekar J. Phase II study of cisplatin for metastatic prostatic carcinoma. An Eastern Cooperative Oncology Group study. Am J Clin Oncol. 1983;6:203–5. doi: 10.1097/00000421-198304000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Loening SA, Beckley S, Brady MF, Chu TM, deKernion JB, et al. Comparison of estramustine phosphate, methotrexate and cis-platinum in patients with advanced, hormone refractory prostate cancer. J Urol. 1983;129:1001–6. doi: 10.1016/s0022-5347(17)52509-4. [DOI] [PubMed] [Google Scholar]
- 21.Soloway MS, Beckley S, Brady MF, Chu TM, deKernion JB, et al. A comparison of estramustine phosphate versus cis-platinum alone versus estramustine phosphate plus cis-platinum in patients with advanced hormone refractory prostate cancer who had had extensive irradiation to the pelvis or lumbosacral area. J Urol. 1983;129:56–61. doi: 10.1016/s0022-5347(17)51917-5. [DOI] [PubMed] [Google Scholar]
- 22.Trump DL, Marsh JC, Kvols LK, Citrin D, Davis TE, et al. A phase II trial of carboplatin (NSC 241240) in advanced prostate cancer, refractory to hormonal therapy. An Eastern Cooperative Oncology Group pilot study. Invest New Drugs. 1990;8(Suppl 1):S91–4. doi: 10.1007/BF00171992. [DOI] [PubMed] [Google Scholar]
- 23.Miglietta L, Cannobbio L, Boccardo F. Assessment of response to carboplatin in patients with hormone-refractory prostate cancer: a critical analysis of drug activity. Anticancer Res. 1995;15:2825–8. [PubMed] [Google Scholar]
- 24.Kelly WK, Curley T, Slovin S, Heller G, McCaffrey J, et al. Paclitaxel, estramustine phosphate, and carboplatin in patients with advanced prostate cancer. J Clin Oncol. 2001;19:44–53. doi: 10.1200/JCO.2001.19.1.44. [DOI] [PubMed] [Google Scholar]
- 25.Bhargava A, Vaishampayan UN. Expert Opin Investig Drugs. Vol. 18. Satraplatin: leading the new generation of oral platinum agents; 2009. pp. 1787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figg WD, Chau CH, Madan RA, Gulley JL, Gao R, et al. Phase II study of satraplatin and prednisone in patients with metastatic castration-resistant prostate cancer: a pharmacogenetic assessment of outcome and toxicity. Clin Genitourin Cancer. 2013;11:229–37. doi: 10.1016/j.clgc.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. 2009;27:5431–8. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 28.Yagoda A. Cytotoxic agents in prostate cancer: an enigma. Semin Urol. 1983;1:311–21. [PubMed] [Google Scholar]
- 29.Eisenberger MA, Abrams JS. Chemotherapy for prostatic carcinoma. Semin Urol. 1988;6:303–10. [PubMed] [Google Scholar]
- 30.DeWys WD, Begg CB, Brodovsky H, Creech R, Khandekar J. A comparative clinical trial of adriamycin and 5-fluorouracil in advanced prostatic cancer: prognostic factors and response. Prostate. 1983;4:1–11. doi: 10.1002/pros.2990040102. [DOI] [PubMed] [Google Scholar]
- 31.Morant R, Bernhard J, Dietrich D, Gillessen S, Bonomo M, et al. Capecitabine in hormone-resistant metastatic prostatic carcinoma – A phase II trial. Br J Cancer. 2004;90:1312–7. doi: 10.1038/sj.bjc.6601673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy GP, Priore RL, Scardino PT. Hormone-refractory metastatic prostatic cancer treated with methotrexate, cyclophosphamide plus adriamycin, cis-platinum plus 5-fluorouracil plus cyclophosphamide. National Prostatic Cancer Project randomized trial. Urology. 1988;32:33–40. doi: 10.1016/0090-4295(88)90450-5. [DOI] [PubMed] [Google Scholar]
- 33.O’Bryan RM, Baker LH, Gottlieb JE, Rivkin SE, Balcerzak SP, et al. Dose response evaluation of adriamycin in human neoplasia. Cancer. 1977;39:1940–8. doi: 10.1002/1097-0142(197705)39:5<1940::aid-cncr2820390505>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Scher H, Yagoda A, Watson RC, Serber M, Whitmore W. Phase II trial of doxorubicin in bidimensionally measurable prostatic adenocarcinoma. J Urol. 1984;131:1099–102. doi: 10.1016/s0022-5347(17)50829-0. [DOI] [PubMed] [Google Scholar]
- 35.Millikan RE, Wen S, Pagliaro LC, Brown MA, Moomey B, et al. Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. J Clin Oncol. 2008;26:5936–42. doi: 10.1200/JCO.2007.15.9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scher HI, Sternberg C, Heston WD, Watson RC, Niedzwiecki D, et al. Etoposide in prostatic cancer: experimental studies and phase II trial in patients with bidimensionally measurable disease. Cancer Chemother Pharmacol. 1986;18:24–6. doi: 10.1007/BF00253058. [DOI] [PubMed] [Google Scholar]
- 37.Trump DL, Loprinzi CL. Phase II trial of etoposide in advanced prostate cancer. Cancer Treat Rep. 1984;68:1195–6. [PubMed] [Google Scholar]
- 38.Walther PJ, Williams SD, Troner M, Greco FA, Birch R, et al. Phase II study of etoposide for carcinoma of the bladder: the Southeastern Cancer Study Group experience. Cancer Treat Rep. 1986;70:1337–8. [PubMed] [Google Scholar]
- 39.Hwang C. Overcoming docetaxel resistance in prostate cancer: a perspective review. Ther Adv Med Oncol. 2012;4:329–40. doi: 10.1177/1758834012449685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Amico AV. US Food and Drug Administration approval of drugs for the treatment of prostate cancer: a new era has begun. J Clin Oncol. 2014;32:362–4. doi: 10.1200/JCO.2013.53.9528. [DOI] [PubMed] [Google Scholar]
- 41.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 42.Figg WD, Li H, Sissung T, Retter A, Wu S, et al. Pre-clinical and clinical evaluation of estramustine, docetaxel and thalidomide combination in androgen-independent prostate cancer. BJU Int. 2007;99:1047–55. doi: 10.1111/j.1464-410X.2007.06763.x. [DOI] [PubMed] [Google Scholar]
- 43.Tannock I, Gospodarowicz M, Meakin W, Panzarella T, Stewart L, et al. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol. 1989;7:590–7. doi: 10.1200/JCO.1989.7.5.590. [DOI] [PubMed] [Google Scholar]
- 44.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–64. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 45.Kantoff PW, Halabi S, Conaway M, Picus J, Kirshner J, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17:2506–13. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 46.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 47.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martel CL, Gumerlock PH, Meyers FJ, Lara PN. Current strategies in the management of hormone refractory prostate cancer. Cancer Treat Rev. 2003;29:171–87. doi: 10.1016/s0305-7372(02)00090-7. [DOI] [PubMed] [Google Scholar]
- 50.Pienta KJ. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin Oncol. 2001;28:3–7. doi: 10.1016/s0093-7754(01)90148-4. [DOI] [PubMed] [Google Scholar]
- 51.Picus J, Schultz M. Docetaxel (Taxotere) as monotherapy in the treatment of hormone-refractory prostate cancer: preliminary results. Semin Oncol. 1999;26:14–8. [PubMed] [Google Scholar]
- 52.Berry W, Dakhil S, Gregurich MA, Asmar L. Phase II trial of single-agent weekly docetaxel in hormone-refractory, symptomatic, metastatic carcinoma of the prostate. Semin Oncol. 2001;28:8–15. doi: 10.1016/s0093-7754(01)90149-6. [DOI] [PubMed] [Google Scholar]
- 53.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 54.Mita AC, Denis LJ, Rowinsky EK, Debono JS, Goetz AD, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723–30. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 55.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 56.De Bono JS, Hardy-Bessard A, Kim C, Geczi L, Ford D, et al. Phase III non-inferiority study of cabazitaxel (C) 20 mg/m2 (C20) versus 25 mg/m2 (C25) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel (D) J Clin Oncol. 2016;34 abstr 5008. [Google Scholar]
- 57.Sartor AO, Oudard S, Sengelov L, Daugaard G, Mainwaring PN, et al. Cabazitaxel vs. docetaxel in chemotherapy-naive (CN) patients with metastatic castration-resistant prostate cancer (mCRPC): a three-arm phase III study (FIRSTANA) J Clin Oncol. 2016;34 abstr 5006. [Google Scholar]
- 58.Shenoy N, Kohli M. Role of systemic chemotherapy in metastatic hormone-sensitive prostate cancer. Indian J Urol. 2016;32:257–61. doi: 10.4103/0970-1591.191234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gravis G, Fizazi K, Joly F, Oudard S, Priou F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–58. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 60.Gravis G, Boher JM, Joly F, Soulie M, Albiges L, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 Trial. Eur Urol. 2016;70:256–62. doi: 10.1016/j.eururo.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vale CL, Burdett S, Rydzewska LH, Albiges L, Clarke NW, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17:243–56. doi: 10.1016/S1470-2045(15)00489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. doi: 10.1016/s0094-0143(05)70386-4. [DOI] [PubMed] [Google Scholar]
- 65.Clark PE, Peereboom DM, Dreicer R, Levin HS, Clark SB, et al. Phase II trial of neoadjuvant estramustine and etoposide plus radical prostatectomy for locally advanced prostate cancer. Urology. 2001;57:281–5. doi: 10.1016/s0090-4295(00)00914-6. [DOI] [PubMed] [Google Scholar]
- 66.Hussain M, Smith DC, El-Rayes BF, Du W, Vaishampayan U, et al. Neoadjuvant docetaxel and estramustine chemotherapy in high-risk/locallyadvanced prostate cancer. Urology. 2003;61:774–80. doi: 10.1016/s0090-4295(02)02519-0. [DOI] [PubMed] [Google Scholar]
- 67.Konety BR, Eastham JA, Reuter VE, Scardino PT, Donat SM, et al. Feasibility of radical prostatectomy after neoadjuvant chemohormonal therapy for patients with high risk or locally advanced prostate cancer: results of a phase I/II study. J Urol. 2004;171:709–13. doi: 10.1097/01.ju.0000108122.36893.5a. [DOI] [PubMed] [Google Scholar]
- 68.Magi-Galluzzi C, Zhou M, Reuther AM, Dreicer R, Klein EA. Neoadjuvant docetaxel treatment for locally advanced prostate cancer: a clinicopathologic study. Cancer. 2007;110:1248–54. doi: 10.1002/cncr.22897. [DOI] [PubMed] [Google Scholar]
- 69.Sella A, Zisman A, Kovel S, Yarom N, Leibovici D, et al. Neoadjuvant chemohormonal therapy in poor-prognosis localized prostate cancer. Urology. 2008;71:323–7. doi: 10.1016/j.urology.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 70.Friedman J, Dunn RL, Wood D, Vaishampayan U, Wu A, et al. Neoadjuvant docetaxel and capecitabine in patients with high risk prostate cancer. J Urol. 2008;179:911–5. doi: 10.1016/j.juro.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mellado B, Font A, Alcaraz A, Aparicio LA, Veiga FJ, et al. Phase II trial of short-term neoadjuvant docetaxel and complete androgen blockade in high-risk prostate cancer. Br J Cancer. 2009;101:1248–52. doi: 10.1038/sj.bjc.6605320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garzotto M, Higano CS, O’Brien C, Rademacher BL, Janeba N, et al. Phase 1/2 study of preoperative docetaxel and mitoxantrone for high-risk prostate cancer. Cancer. 2010;116:1699–708. doi: 10.1002/cncr.24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shepard DR, Dreicer R, Garcia J, Elson P, Magi-Galluzzi C, et al. Phase II trial of neoadjuvant nab-paclitaxel in high risk patients with prostate cancer undergoing radical prostatectomy. J Urol. 2009;181:1672–7. doi: 10.1016/j.juro.2008.11.121. [DOI] [PubMed] [Google Scholar]
- 74.Kim WY, Whang YE, Pruthi RS, Baggstrom MQ, Rathmell WK, et al. Neoadjuvant docetaxel/estramustine prior to radical prostatectomy or external beam radiotherapy in high risk localized prostate cancer: a phase II trial. Urol Oncol. 2011;29:608–13. doi: 10.1016/j.urolonc.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 75.Narita S, Tsuchiya N, Kumazawa T, Maita S, Numakura K, et al. Short-term clinicopathological outcome of neoadjuvant chemohormonal therapy comprising complete androgen blockade, followed by treatment with docetaxel and estramustine phosphate before radical prostatectomy in Japanese patients with high-risk localized prostate cancer. World J Surg Oncol. 2012;10:1. doi: 10.1186/1477-7819-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross RW, Galsky MD, Febbo P, Barry M, Richie JP, et al. Phase 2 study of neoadjuvant docetaxel plus bevacizumab in patients with high-risk localized prostate cancer: a Prostate Cancer Clinical Trials Consortium trial. Cancer. 2012;118:4777–84. doi: 10.1002/cncr.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thalgott M, Horn T, Heck MM, Maurer T, Eiber M, et al. Long-term results of a phase II study with neoadjuvant docetaxel chemotherapy and complete androgen blockade in locally advanced and high-risk prostate cancer. J Hematol Oncol. 2014;7:20. doi: 10.1186/1756-8722-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silberstein JL, Poon SA, Sjoberg DD, Maschino AC, Vickers AJ, et al. Long-term oncological outcomes of a phase II trial of neoadjuvant chemohormonal therapy followed by radical prostatectomy for patients with clinically localised, high-risk prostate cancer. BJU Int. 2015;116:50–6. doi: 10.1111/bju.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nosov A, Reva S, Petrov S, Mamijev E, Novikov R, et al. Neoadjuvant chemotherapy using reduced-dose docetaxel followed by radical prostatectomy for patients with intermediate and high-risk prostate cancer: a single-center study. Prostate. 2016;76:1345–52. doi: 10.1002/pros.23165. [DOI] [PubMed] [Google Scholar]
- 80.Bergstrom CP, Ruffell B, Ho CM, Higano CS, Ellis WJ, et al. Docetaxel and mitoxantrone before radical prostatectomy in men with high-risk prostate cancer: 10-year follow-up and immune correlates. Anticancer Drugs. 2017;28:120–6. doi: 10.1097/CAD.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Womble PR, VanVeldhuizen PJ, Nisbet AA, Reed GA, Thrasher JB, et al. A phase II clinical trial of neoadjuvant ketoconazole and docetaxel chemotherapy before radical prostatectomy in high risk patients. J Urol. 2011;186:882–7. doi: 10.1016/j.juro.2011.04.087. [DOI] [PubMed] [Google Scholar]
- 82.Febbo PG, Richie JP, George DJ, Loda M, Manola J, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–40. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 83.Dreicer R, Magi-Galluzzi C, Zhou M, Rothaermel J, Reuther A, et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology. 2004;63:1138–42. doi: 10.1016/j.urology.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 84.Prayer-Galetti T, Sacco E, Pagano F, Gardiman M, Cisternino A, et al. Long-term follow-up of a neoadjuvant chemohormonal taxane-based phase II trial before radical prostatectomy in patients with non-metastatic high-risk prostate cancer. BJU Int. 2007;100:274–80. doi: 10.1111/j.1464-410X.2007.06760.x. [DOI] [PubMed] [Google Scholar]
- 85.Chi KN, Chin JL, Winquist E, Klotz L, Saad F, et al. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol. 2008;180:565–70. doi: 10.1016/j.juro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 86.Sandler HM, Hu C, Rosenthal S, Sartor AO, Gomella LG, et al. A phase III protocol of androgen suppression (AS) and 3DCRT/IMRT versus AS and 3DCRT/IMRT followed by chemotherapy (CT) with docetaxel and prednisone for localized, high-risk prostate cancer (RTOG 0521) J Clin Oncol. 2015;33:LBA5002. [Google Scholar]
- 87.National Comprehensive Cancer Network (NCCN) Panel Guidelines. Prostate Cancer. Ver. 2.2017. 2017. [Last accessed on 2017 May 14]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf .
- 88.Fizazi K, Faivre L, Lesaunier F, Delva R, Gravis G, et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 2015;16:787–94. doi: 10.1016/S1470-2045(15)00011-X. [DOI] [PubMed] [Google Scholar]
- 89.Ahlgren G, Flodgren P, Tammela TL, Kellokumpu-Lehtinen P, Borre M, et al. A randomized phase III trial between adjuvant docetaxel and surveillance after radical prostatectomy for high risk prostate cancer: results of SPCG12. J Clin Oncol. 2016;34 doi: 10.1016/j.eururo.2018.01.012. abstr 5001. [DOI] [PubMed] [Google Scholar]
- 90.Glode ML, Tangen CM, Hussain M, Swanson G, Wood D, et al. Adjuvant androgen deprivation (ADT) versus mitoxantrone plus prednisone (MP) plus ADT in high-risk prostate cancer (PCa) patients following radical prostatectomy: a phase III intergroup trial (SWOG S9921) NCT00004124. J Clin Oncol. 2017;35 doi: 10.1200/JCO.2017.76.4126. abstr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lohiya V, Aragon-Ching JB, Sonpavde G. Role of chemotherapy and mechanisms of resistance to chemotherapy in metastatic castration-resistant prostate cancer. Clin Med Insights Oncol. 2016;10:57–66. doi: 10.4137/CMO.S34535. [DOI] [PMC free article] [PubMed] [Google Scholar]


