Abstract
Despite impressive survival benefits with immunotherapy in patients with various solid tumors, the full potential of these agents in prostate cancer has yet to be realized. Sipuleucel-T demonstrated a survival benefit in this population, indicating that prostate cancer is an immunoresponsive disease; however, these results have not been matched by other agents. A large trial with ipilimumab in prostate cancer failed to meet its primary objective, and small trials with PD-1/PD-L1 inhibitors did not yield a significant improvement in overall response. However, several late-stage clinical trials are underway with other vaccines in prostate cancer. Reports of clinical benefit with immunotherapies, particularly when used in combination or a select population, have provided the framework to develop sound clinical trials. Understanding immunogenic modulation, antigen spread, biomarkers, and DNA-repair defects will also help mold future strategies. Through rational patient selection and evidence-based combination approaches, patients with prostate cancer may soon derive durable survival benefits with immunotherapies.
Keywords: checkpoint inhibitor, immunotherapy, prostate cancer, therapeutic vaccine
INTRODUCTION
Since 2010, the treatment repertoire for metastatic castration-resistant prostate cancer (mCRPC) has significantly expanded and now offers multiple agents proven to extend overall survival (OS) in this population. In addition to docetaxel, the chemotherapy backbone of mCRPC, cabazitaxel provides an additional conventional approach. Through androgen inhibition, enzalutamide and abiraterone offer an improved toxicity profile. Radium-223 is a radiopharmaceutical that offers a unique option for patients with symptomatic bone metastasis. The approval of sipuleucel-T for asymptomatic or minimally symptomatic mCRPC began the modern era of cancer immunotherapy.
Soon thereafter, durable improvements in OS were experienced with checkpoint inhibitors by patients with a variety of solid tumors resulting in practice-changing treatment approaches. However, response rates and survival benefits of checkpoint inhibitors in prostate cancer have been underwhelming thus far, though hints of clinical benefit suggest that these agents should not be abandoned. Strategic patient selection and tactical combination approaches may be the key to unlocking the potential of immunotherapy in this disease.
CHECKPOINT INHIBITORS
CTLA-4 inhibitors
The first immune checkpoint inhibitor approved by the FDA was ipilimumab in 2011. Ipilimumab is a cytotoxic T-lymphocyte antigen 4 (CTLA-4)-blocking antibody that has demonstrated a noteworthy improvement in OS in advanced melanoma.1,2 This fully human IgG monoclonal antibody inhibits the binding of CTLA-4 with B-7 on antigen-presenting cells (APCs). The inhibition of the CTLA-4/B-7 interaction unleashes T-cell activation and proliferation. Early ipilimumab clinical trial data in mCRPC captured a glimpse of clinical activity and provided the rationale for additional research in this population. Small and colleagues3 conducted the first trial to evaluate prostate-specific antigen (PSA) modulation and safety with ipilimumab in mCRPC. In this monotherapy pilot trial, 14 patients received one or two infusions of intravenous ipilimumab at a dose of 3 mg kg−1. Two patients demonstrated a PSA decline of ≥50% lasting 135 days and 60 days, respectively and an additional eight patients experienced a <50% PSA decline. Although PSA response is not a true surrogate for radiographic response and clinical benefit, these improvements suggest that ipilimumab warranted further evaluation. Another trial in mCRPC evaluated escalating doses of ipilimumab alone or in combination with radiotherapy.4 Among the 50 patients receiving ipilimumab 10 mg kg−1 in this Phase I/II trial, 8 patients had PSA declines of ≥50%, 1 had a complete response (CR), and 6 were reported to have stable disease. Immune-related adverse events (irAEs) were considered manageable and consisted of diarrhea (54%), colitis (22%), rash (32%), and pruritis (20%). Grade 3/4 irAEs were colitis (16%) and hepatitis (10%).
Based on a noteworthy, but manageable toxicity profile (primarily consisting of autoimmune toxicity) and suggested clinical benefit, two large Phase III trials were conducted to evaluate survival with ipilimumab in mCRPC. The first was a randomized, double-blind study that evaluated ipilimumab versus placebo after radiotherapy in mCRPC.5 In this trial, 799 men with docetaxel-refractory prostate cancer and at least 1 bone metastasis received bone-directed radiotherapy followed by either ipilimumab 10 mg kg−1 or placebo every 3 weeks for up to 4 doses. Patients without progression could continue maintenance ipilimumab or placebo every 3 months thereafter. The primary objective was OS. Median OS was 11.2 months in the ipilimumab arm compared to 10.0 months in the placebo arm (hazard ratio [HR]: 0.85, 95% CI: 0.72–1.00; P = 0.053). Although this study did not meet its primary objective, a post hoc subgroup analysis revealed an OS disparity in patients exhibiting poor prognostic factors including at least one of the following: presence of visceral metastasis, elevated alkaline phosphatase, or decreased hemoglobin. Patients with good prognostic features experienced an OS benefit (P = 0.0038) whereas patients with poor prognostic features did not experience the same outcomes (P = 0.8756).
The results of this post hoc analysis contribute to the growing evidence that patients with better baseline prognostic factors may derive greater benefit from immunotherapy.6,7,8 A concurrent Phase III trial also evaluated ipilimumab in what may be considered an optimal mCRPC population. In this double-blind, placebo controlled trial, chemotherapy-naive patients with asymptomatic or minimally symptomatic mCRPC without visceral metastasis were randomized (2:1) to receive ipilimumab 10 mg kg−1 (n = 399) or placebo (n = 199).9 Infusions were administered every 3 weeks for 4 doses followed by every 3 months in patients without progression. The primary objective of this study, OS, was not found to be statistically significant between the two arms. Median OS was 28.7 months in the ipilimumab arm versus 29.7 months in the placebo arm (HR: 1.11; 95.87% CI: 0.88–1.39; P = 0.3667). Modest improvements in secondary and exploratory endpoints were noted. Median progression-free survival (PFS) was 5.6 months in the ipilimumab arm versus 3.8 months in the placebo arm (HR: 0.67; 95.87% CI: 0.55–0.81), and PSA response rate was 23% with ipilimumab compared to 8% with placebo. Toxicity was again noteworthy, but similar to previous trials. The most common treatment-related adverse events were diarrhea, rash, pruritus, fatigue, nausea/vomiting, and decreased appetite. Diarrhea was the only grade 3/4 adverse event reported in >10% of patients. Nine treatment-related deaths occurred in the ipilimumab arm whereas no deaths occurred in the placebo arm: a finding requiring further investigation.
Another anti-CTLA-4 agent in clinical trials, tremelimumab, has been studied in patients with various solid tumors. One study evaluated safety and PSA kinetics following tremelimumab plus short-term androgen deprivation therapy (ADT) in 11 patients with PSA-recurrent prostate cancer.10 No PSA changes were observed in this small population; however, 3 patients experienced a prolonged PSA doubling time immediately after the 2 doses of tremelimumab which continued for months following treatment. Although PSA responses with CTLA-4 inhibitors are intriguing, further analysis is needed especially in light of the recent disappointing outcomes with ipilimumab monotherapy in prostate cancer and the accompanying toxicity.
PD-1/PD-L1 inhibitors
Data with FDA-approved programmed death-1 (PD-1)/ligand-1 (PD-L1) including nivolumab, pembrolizumab, durvalumab, atezolizumab, and avelumab in prostate cancer has been lackluster thus far when compared to impressive results in other solid tumors. The results of select trials evaluating checkpoint inhibitors in prostate cancer are presented in Table 1. One of the first trials evaluating nivolumab in solid tumors included 17 patients with prostate cancer; no objective responses were reported.11 A Phase Ib study evaluated pembrolizumab 10 mg kg−1 every 2 weeks in 23 patients with mCRPC and ≥1% PD-L1 expression by immunohistochemistry.12 Despite a population selected for PD-L1 expression, only 3 patients had a confirmed partial response (PR) resulting in an overall response rate (ORR) of 13% (95% CI: 3%–34%) with a median duration of response of 59 weeks (range, 28–62 weeks). Although the response rate was modest, the duration of response is encouraging. The PD-L1 inhibitor, avelumab, was evaluated in a cohort of 18 men with mCRPC at a dose of 10 mg kg−1 administered every 2 weeks.13 No objective responses were noted. However, in the small subgroup of 5 patients that enrolled with a rising PSA while on enzalutamide, 3 patients experienced stable disease for >24 months.
Table 1.
Select clinical trials evaluating immunotherapy in prostate cancer
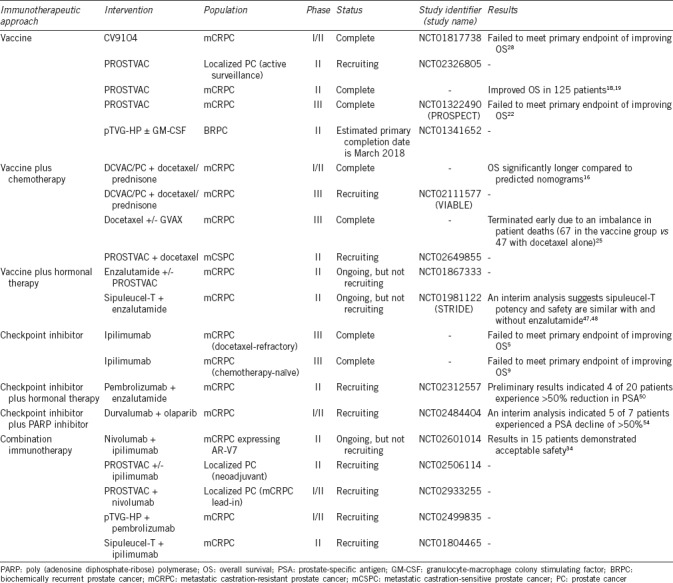
Clinical trials evaluating the use of checkpoint inhibitors in prostate cancer have suggested that using these agents alone would result in less than optimal improvements in OS. However, these trials provide a glimpse of efficacy, indicating that checkpoint inhibitors should not be altogether abandoned in this population. Through combination strategies with vaccines, hormonal agents, or other modalities, further studies should strive to understand the optimal approach to harness the antitumor effect of checkpoint inhibitors.
THERAPEUTIC CANCER VACCINES
Sipuleucel-T demonstrated an improvement in OS in patients with asymptomatic or minimally symptomatic mCRPC14,15 and ultimately led to the designation as the first FDA-approved therapeutic cancer vaccine. These practice-changing trials demonstrated that prostate cancer was responsive to immunotherapy and that vaccine therapy was a safe and effective treatment approach. Various clinical trials are underway to evaluate therapeutic cancer vaccines in prostate cancer as outlined in Table 1.
DCVAC/PCa
DCVAC/PCa is an autologous vaccine that combines activated dendritic cells pulsed with killed PSA-positive LNCaP cells. A Phase I/II open-label, single-arm clinical trial evaluated DCVAC/PCa in combination with standard dose docetaxel with prednisone in 25 men with mCRPC.16 The primary and secondary endpoints of the trial included safety and immune responses. The most common adverse events attributed to DCVAC/PCa were fatigue, back pain, and paresthesias (all grade 1 or 2). As part of the safety evaluation, OS was compared to predicted values with established nomograms. The OS observed with the DCVAC/PCa regimen was 19 months which was significantly longer than the Halabi and MSKCC nomograms predicted OS of 11.8 and 13 months, respectively (HR: 0.26, 95% CI: 0.13–0.51). A Phase III trial is currently underway to further explore the potential of this promising therapy (VIABLE; NCT02111577). VIABLE is a randomized, double-blind, placebo-controlled, parallel-group study to evaluate the safety and efficacy of docetaxel plus DCVAC/PCa versus docetaxel plus placebo in approximately 1200 patients. The primary objective is OS and the estimated study completion date is June 2018.17
PROSTVAC
Both sipuleucel-T and DCVAC/PCa present feasibility challenges in clinical practice as they are personalized vaccines requiring ex vivo processing. PROSTVAC is a poxviral-based vaccine encoding PSA as the target antigen in addition to three costimulatory molecules (B7.1, ICAM-1, and LFA-3). The PROSTVAC regimen consists of an off-the-shelf prime–boost approach. The primer vaccine (modified vaccinia vector) is administered subcutaneously for one dose followed by six booster vaccines (modified fowlpox vector). A Phase II randomized, double-blind trial in 125 patients with mCRPC demonstrated a significant improvement in OS with the PROSTVAC prime–boost regimen.18,19 Median OS was 25.1 months in the PROSTVAC arm compared to 16.6 months in the control arm, an improvement of 8.5 months (HR: 0.56, 95% CI: 0.37–0.85; P = 0.0061). Another study evaluated the immune impact induced by PROSTVAC administration in 104 patients.20 T-cell responses prevaccination and 4 weeks postvaccination were compared. Overall, 59 (57%) of 104 patients demonstrated an increase in PSA-specific T-cells and 19 of 28 patients (68%) mounted an immune response to tumor-associated antigens not present in the vaccine; a concept known as antigen spreading. These promising results led to a Phase III trial (PROSPECT; NCT01322490).21 PROSPECT is a double-blind trial in which 1297 men with asymptomatic or minimally symptomatic mCRPC were randomized to receive PROSTVAC, PROSTVAC plus granulocyte-macrophage colony stimulating factor (GM-CSF), or placebo. In September 2017, a preplanned interim analysis revealed that continuing the trial was futile as the primary outcome of OS could not be reached.22 Although the results are disappointing, the promise of immunotherapy in prostate cancer may lie in combination strategies. Clinical trials administering PROSTVAC in combination with other immunotherapeutic agents or earlier in the disease course are currently underway (NCT02933255, NCT02506114, NCT02649439, NCT02326805).
GVAX-PCa
Granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells (GVAX-PCa) is a vaccine comprised of cells from the LNCaP and PC3 cell lines and is genetically modified to secrete GM-CSF. A Phase I/II dose-escalation trial evaluated the safety and immunogenicity of GVAX in 80 patients with mCRPC.23 Overall, the vaccine was well tolerated with the most common adverse event being injection-site erythema. A significant proportion of patients, 89% in the high-dose group (P = 0.002), were able to develop an antibody response to one or both cell lines. Two Phase III studies were subsequently completed to evaluate OS and further establish safety. One trial24 evaluated GVAX versus standard docetaxel plus prednisone in men with chemotherapy-naive mCRPC. The trial was terminated early based on a futility analysis, indicating that the study was unlikely to meet the primary endpoint of OS. In the 626 patients included in the analysis, the median survival was 20.7 months for the GVAX arm versus 21.7 months for the control arm (HR: 1.03, 95% CI: 0.83–1.28; P = 0.78). Grade ≥3 adverse events were reported in 8.8% in the GVAX arm versus 43% in the docetaxel arm, and the investigators concluded that GVAX had a favorable toxicity profile. On the contrary, a Phase III trial of GVAX plus docetaxel versus docetaxel alone in 408 men with mCRPC was terminated early due to an imbalance in patient deaths (67 in the vaccine group versus 47 deaths with docetaxel alone).25 The imbalance was reflected in OS: 12.2 months with the vaccine combination versus 14.1 with chemotherapy alone (P = 0.0076). Further analysis is required.
CV9104
A Phase I/IIa trial evaluated the safety and immunogenicity of CV9103, an mRNA vaccine encoding the antigens PSA, prostate stem cell antigen (PSCA), prostate-specific membrane antigen (PSMA), and six-transmembrane epithelial antigen of the prostate 1 (STEAP1).26 In this study, 26 of 33 evaluable patients at the recommended Phase II dose (1280 mcg by intradermal injection) developed an immune response. Patients who demonstrated an immunological response to multiple antigens had statistically longer OS compared to nonimmunological responders or patients who responded to only 1 antigen (HR: 0.41, 95% CI: 0.17–0.95, P = 0.017). The second-generation CV9103 formulation, CV9104, encodes prostatic acid phosphatase (PAP) and MUC1 in addition to the 4 antigens in the first-generation formula.27 Despite the promising immunogenicity data reported in the Phase I/IIa trial, a Phase IIb trial with CV9104 failed to meet its primary endpoint of improving OS in patients with asymptomatic or minimally symptomatic mCRPC.28 Complete trial results are awaited.
COMBINATION STRATEGIES
As discussed earlier, efficacy with checkpoint inhibition alone for the treatment of mCRPC has been underwhelming thus far. It is therefore imperative to determine the mechanism behind this resistance. Recent analysis suggests that tumor mutational burden predicts a favorable response to PD-1/PD-L129,30,31 and CTLA-4 inhibitors.32 Prostate cancer is known to have a low mutational burden,33 thus providing further evidence that appears to support this hypothesis. Interestingly, one study concluded that outcomes following dual blockade with a CTLA-4 inhibitor plus a PD-1/PD-L1 inhibitor appeared to be independent of mutational burden.29
In addition, numerous trials have generated signs of clinical activity, indicating the need for additional research in specific patient subsets through evidence-based sequencing and combination therapy approaches. One strategy has evaluated ipilimumab with nivolumab in advanced prostate cancer patients with mutated androgen receptors.34 This combination has yielded benefit in melanoma, but with a large proportion of patients experiencing toxicity.35 As expected, this study was accompanied by toxicity with 1 of 15 patients having a 50% decline in PSA and 3 of 15 patients having durable PFS.34
Vaccines and checkpoint inhibitors
Understanding the effects of immunotherapies on the tumor microenvironment may help provide the framework for combination therapies. It is postulated that tumors with greater PD-L1 expression in the tumor microenvironment have a greater tendency to respond to PD-1/PD-L1 inhibition.36 Rekoske and colleagues37 evaluated clinical samples from patients with prostate cancer who previously received a DNA vaccine encoding PAP. The following changes were analyzed: checkpoint receptor expression on antigen-specific CD8+ T-cells, the effect of PD-1 blockade on elicited immune responses, and changes in checkpoint ligand expression on circulating tumor cells (CTCs). The results of this study demonstrated that following vaccination, PD-L1 expression was increased on CTCs and a link was suggested between this PD-L1 upregulation and extended PFS. The investigators also found a trend with the sipuleucel-T vaccine, which similarly targets PAP.
The safety and tolerability of ipilimumab and PROSTVAC was evaluated in a Phase I dose-escalation trial in 30 patients with mCRPC.38 PROSTVAC-V was administered subcutaneously on day 1 followed by monthly injections with PROSTVAC-F starting on day 15 in combination with ipilimumab monthly starting on day 15 at doses of 1, 3, 5, or 10 mg kg−1. The most commonly reported adverse events were vaccine injection-site reactions and irAEs such as colitis, rash, elevated aminotransferases, and endocrine. The median OS for all patients was 34.4 months and the 2-year OS was 73%, comparing favorably to previous studies of vaccine alone in this population, including the Phase III results of sipuleucel-T. Notably, this trial provided evidence of antigen spreading whereby an immune response was generated to tumor-associated antigens not present in the vaccine. Antigen spread may allow for a more durable and adaptable immune response potentially leading to improved long-term clinical outcomes.39
Further evidence of therapeutic vaccines and ipilimumab is provided in a small study of 9 men with mCRPC who received 3 doses of sipuleucel-T followed by ipilimumab 1 mg kg−1 at the following intervals: 1 week; 1 and 4 weeks; or 1, 4, and 7 weeks.40 Serum immunoglobulins directed at GM-CSF/PAP fusion protein (PA2024) and PAP were measured prior to sipuleucel-T, postsipuleucel-T, every other months for 5 months then every 3 months for 12 additional months. Sipuleucel-T plus ipilimumab was well tolerated. A significant increase from baseline to postsipuleucel-T was reported in IgG and IgG-IgM for PAP (P < 0.001 and P < 0.0001, respectively) and for PA2024 (P = 0.0001 and P < 0.0001, respectively). Furthermore, postsipuleucel-T to postipilimumab IgG and IgG-IgM significantly increased for PAP (P < 0.001 and P = 0.002, respectively) and PA2024 (P < 0.0001 and P = 0.001, respectively). A link between OS with sipuleucel-T and PA2024 and PAP-specific immune responses has previously been established,41 suggesting potential for the checkpoint vaccine regimen to yield clinical benefit.
Immunotherapy and enzalutamide
Enzalutamide competitively inhibits androgen binding and androgen receptor nuclear translocation and interaction with DNA. This second-generation antiandrogen has demonstrated the ability to extend survival in mCRPC,42,43 and preclinical studies have characterized its immunologic properties. Ardiani and colleagues44 exposed transgenic adenocarcinoma of the mouse prostate (TRAMP) mice to enzalutamide with or without a therapeutic vaccine. This study demonstrated an enhanced thymic production of naive T-cells and improved OS in mice treated with the combination compared to no treatment, vaccine alone, or enzalutamide alone (P ≤ 0.0001, P = 0.0003, and P = 0.0009, respectively). Another study demonstrated that LNCaP cells were more susceptible to T-cell killing by enzalutamide through immunogenic modulation.45 Furthermore, preliminary analysis from a Phase II trial in biochemically recurrent prostate cancer suggested increased naive T-cells and natural killer cells following administration with short-course enzalutamide alone (n = 12).46 Results from the enzalutamide plus vaccine arm of this ongoing trial have yet to be reported and may provide a further understanding of this antiandrogen's immunogenic response.
An ongoing Phase II trial, STRIDE (NCT01981122), will also provide a deeper understanding of the T-cell response with enzalutamide and vaccine. In this open-label study, 52 patients with mCRPC will be randomized to receive sipuleucel-T with enzalutamide administered concurrently (enzalutamide started 2 weeks prior to sipuleucel-T) or sequentially (enzalutamide initiated 10 weeks after the first sipuleucel-T infusion).47 An interim immune analysis indicated that PA2024-specific T-cell responses were elevated at all time points (P < 0.001).48 Cytokines including interferon (IFN)-γ, interleukin-2, and tumor necrosis factor (TNF)-α were also elevated in both arms. No difference was noted in toxicities between concurrent versus sequential administration.
Bishop and colleagues49 evaluated the upregulation of clinically relevant immunotherapy targets in enzalutamide resistant tumors, both in patients and preclinical models. PD-L1/2 and PD-1 were assessed on dendritic cells (DCs) and T-cells in patients who were enzalutamide naive or progressing or responding to enzalutamide. When compared to patients who responded or were naive, patients who progressed on enzalutamide had a significantly increased frequency of PD-L1/2-positive DCs (P = 0.0060 and P = 0.0037, respectively). Further evidence to suggest activity of anti-PD-1 agents in patients with enzalutamide-resistant mCRPC is provided by Graff and colleagues.50 In this ongoing Phase II trial (NCT02312557), patients with evidence of progression while on enzalutamide received pembrolizumab 200 mg intravenously every 3 weeks for 4 doses. Standard dose enzalutamide was continued. Preliminary results indicated that 3 of the first 10 patients experienced a rapid PSA decline to ≤0.2 ng ml−1, including 2 patients with measurable disease at the time of enrollment who achieved a PR. A more recent update of these data indicated that 4 of 20 patients had a >50% reduction in PSA.50 In addition, as described earlier, 3 patients with mCRPC and a rising PSA while on enzalutamide experienced extended stable disease following treatment with avelumab.13 Although the number of patients is small, the results of these trials indicate that further exploration is warranted.
Immunotherapy and abiraterone
Similar to enzalutamide, preclinical evidence suggests that abiraterone is associated with immunogenic modulation.45 A Phase II open-label study in 69 patients with mCRPC evaluated the effect of sequential or concurrent abiraterone administration with sipuleucel-T on immune responses.51 Patients were randomized to receive either abiraterone plus prednisone starting day 1 (concurrent) or abiraterone plus prednisone starting 10 weeks after the first sipuleucel-T infusion (sequential). The primary objective was cumulative antigen-presenting cell activation. In both arms, ex vivo APC activation was significantly greater at the second and third infusions compared to baseline (P < 0.05), and peripheral immune responses were consistent with previous sipuleucel trials. This trial also provided evidence to suggest that low-dose prednisone (5 mg twice daily) may not affect the immunogenicity of sipuleucel-T.
Abiraterone plus prednisone was also evaluated in combination with ipilimumab in a Phase I/II trial in treatment-naive mCRPC.52 The primary objective was safety. In the first cohort, patients received abiraterone 1000 mg orally daily, prednisone 5 mg orally twice daily, and ipilimumab 3 mg kg−1 every 3 weeks for 4 cycles. Two patients experienced Grade 3 transaminases and 1 patient experienced Grade 3 fatigue and colitis leading to a regimen adjustment allowing for a 2-month abiraterone lead-in. However, extensive toxicities were still reported consisting of hypokalemia, dehydration, and elevated transaminases (all Grade 3). The study was stopped early as the regimen resulted in toxicities that surpassed the maximum tolerated dose.
Immunotherapy and PARP inhibition
Olaparib, a poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor, has demonstrated clinical activity in patients with mCRPC and DNA-repair defects.53 Deletions and/or deleterious mutations in DNA-repair genes, including BRCA1/2, ataxia telangiectasia mutated (ATM), Fanconi's anemia genes, and checkpoint kinase 2 (CHEK2), have been reported to occur in approximately one-third of this patient population. An ongoing single-arm pilot study (NCT02484404) evaluated the effect of durvalumab 1500 mg intravenous every 4 weeks with olaparib 300 mg orally twice daily in treatment-refractory mCRPC.54 A DNA repair pathway mutation was not required for enrollment. The interim analysis of 10 patients suggested that the combination has an acceptable toxicity profile and reported the following Grade 3/4 adverse events: anemia, thrombocytopenia, lymphopenia, neutropenia, nausea, fatigue, UTI, and lung infection. Of the patients on study >2 months, 5 (71%) of 7 experienced a PSA decline >50%. Although a small population thus far, the results of this trial are intriguing, especially given the unselected population.
PERSPECTIVES ON THE FIELD
With the exception of sipuleucel-T, single-agent immunotherapies in patients with mCRPC have not demonstrated a clear PFS or OS improvement in large clinical trials. Multiple Phase 3 studies, including ipilimumab5,9 and PROSTVAC,50 have been disappointing and provide evidence that prostate cancer is not as immunoresponsive as other genitourinary malignancies such as kidney and bladder cancer. However, since smaller trials have suggested benefit, complete abandonment of these agents may be premature. It is therefore imperative to determine tumor and patient characteristics that may impact response to immunotherapy. Furthermore, combination strategies may overcome tumor evasion of the immune response. Recent studies supplement the growing body of literature to suggest the use of single-agent immunotherapies in mCRPC should wane. Instead, resources should concentrate on strategies to optimize patient selection and maximize immune responses through sound combinations. Given the relatively unremarkable side effect profile of therapeutic vaccines, investigating these agents in the setting of localized prostate cancer may be worthwhile. Biochemical recurrent prostate cancer may prove to be an optimal target population for immunotherapy regimens with favorable toxicity profiles. Although checkpoint inhibitors are relatively well-tolerated when compared to cytotoxic chemotherapy,55 these agents have been associated with serious immune-related adverse events.56 PD-1/PD-L1 inhibitors have demonstrated improved tolerability over CTLA-4 inhibitors,56 but caution must be used particularly in a patient population with extended anticipated survival.
CONCLUSIONS
Although most do not consider prostate cancer an immune responsive tumor, there is an approved immunotherapy (sipuleucel-T) that has demonstrated a survival advantage in Phase 3 testing. However, survival benefits of checkpoint inhibitors in patients with prostate cancer have yet to be maximized. Multiple treatment strategies, such as those involving therapeutic vaccines, are being investigated in combination with these agents to augment their potential. Immunogenic modulation and antigen spread may provide the rationale for combination approaches which will be developed in future trials. In addition, optimal patient selection through biomarker identification or mutational analysis is currently under investigation. When administered alone, many of these agents do not produce remarkable clinical improvements, but in combination, they may yield more meaningful clinical benefits. As more immune-oncology studies are conducted in prostate cancer, knowledge will accumulate allowing for optimal combinations and patients selection and potentially enhance clinical outcomes for men with prostate cancer.
AUTHOR CONTRIBUTIONS
LMC, JLG, and RAM contributed to the writing of this manuscript. All authors had read and approved the final version of the manuscript and agreed with the order of presentation of the authors.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
The authors acknowledge the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, for their support of this manuscript.
REFERENCES
- 1.Eggermont AM, Shiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–55. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowry I, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–5. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 4.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–21. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, et al. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the immunotherapy for prostate adenocarcinoma treatment (IMPACT) trial. Urology. 2013;81:1297–302. doi: 10.1016/j.urology.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 8.Gulley JL, Madan RA, Schlom J. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol. 2011;18:e150–7. doi: 10.3747/co.v18i3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–7. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 10.McNeel DG, Smith HA, Eickhoff JC, Lang JM, Staab MJ, et al. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012;61:1137–47. doi: 10.1007/s00262-011-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen A, Massard C, Ott PA, Haas N, Lopez J, et al. Pembrolizumab for patients with advanced prostate adenocarcinoma: preliminary results from the KEYNOTE-028 study. Ann Oncol. 2016;27(Suppl 6) doi: 10.1093/annonc/mdy232. abstract 725PD. [DOI] [PubMed] [Google Scholar]
- 13.Fakhrejahani F, Madan RA, Dahut WL, Karzai K, Cordes LM, et al. Avelumab in metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2017;35(6 Suppl):159. [Google Scholar]
- 14.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–94. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 15.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 16.Podrazil M, Horvath R, Becht E, Rozkova D, Bilkova P, et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2015;6:18192–205. doi: 10.18632/oncotarget.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beer TM, Vogelzang N, Bartunkova J, Miller K, Oh W, et al. Autologous dendritic cell immunotherapy (DCVAC/PCa) added to docetaxel chemotherapy in a Phase III trial (viable) in men with advanced (mCRPC) prostate cancer. J Immunother Cancer. 2015;3(Suppl 2):P164. [Google Scholar]
- 18.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantoff PW, Gulley JL, Pico-Navarro C. Revised overall survival analysis of a phase II, randomized, double-blind, controlled study of PROSTVAC in men with metastatic castration-resistant prostate cancer. J Clin Oncol. 2017;35:124–5. doi: 10.1200/JCO.2016.69.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulley JL, Madan RA, Tsang KY, Jochems C, Marte JL, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. 2014;2:133–41. doi: 10.1158/2326-6066.CIR-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulley JL, Giacchino JL, Breitmeyer JB, Franzusoff AJ, Panicali D, et al. Prospect: a randomized double-blind phase 3 efficacy study of PROSTVAC-VF immunotherapy in men with asymptomatic/minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol. 2015;5:1509–12. [Google Scholar]
- 22.Bavarian Nordic [Press Release]. Independent Data Monitoring Committee Recommends Discontinuation of Bavarian Nordic's Phase 3 Study of Prostvac in Metastatic Prostate Cancer. [Last accessed on 2017 Sep 14]. Available from: http://www.bavarian-nordic.com/investor/news/news.aspx?news=5308 .
- 23.Higano CS, Corman JM, Smith DC, Centeno AS, Steidle CP, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–84. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 24.Higano C, Saad F, Somer B, Curti B, Petrylak DP, et al. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic castration-resistant prostate cancer (CRPC) Proc Am Soc Clin Oncol (Genitourinary Cancer Symposium) 2009;27 abstract LBA150. [Google Scholar]
- 25.Small E, Demkow T, Gerritsen WR. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prosate cancer (CRPC) Genitourinary Cancers Symposium. 2009 abstract 7. [Google Scholar]
- 26.Kubler H, Scheel B, Gnad-Vogt U, Miller K, Schultze-Seemann W, et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J Immunother Cancer. 2015;3:26. doi: 10.1186/s40425-015-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rausch S, Schwentner C, Stenzl A, Bedke J. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum Vaccin Immunother. 2014;10:3146–52. doi: 10.4161/hv.29553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CureVac: Topline Results of Phase IIB Clinical Trial with CV9104, and RNAactive Prostate Cancer Vaccine. In. Presented at the 35th Annual J.P. Morgan Healthcare Conference San Francisco. 2017 [Google Scholar]
- 29.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boudadi K, Suzman DL, Luber B, Wang H, Silberstein J, et al. Phase 2 biomarker-driven study of ipilimumab plus nivolumab (Ipi/Nivo) for ARV7-positive metastatic castrate-resistant prostate cancer (mCRPC) J Clin Oncol. 2017;35 abstract 5035. [Google Scholar]
- 35.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–4. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rekoske BT, Olson BM, McNeel DG. Antitumor vaccination of prostate cancer patient elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunology. 2016;5:e1165377. doi: 10.1080/2162402X.2016.1165377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castriation-resistant prostate cancer: a phase I dose-escalation trial. Lancet Oncol. 2012;13:501–8. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulley JL, Madan RA, Pachynski R, Mulders P, Sheikh NA, et al. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J Natl Cancer Inst. 2017:109. doi: 10.1093/jnci/djw261. [doi: 101093/jnci/djw261] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholz M, Yep S, Chancey M, Kelly C, Chau K, et al. Phase I clinical trial of sipuleucel-T combined with escalating doses of ipilimumab in progressive metastatic castrate-resistant prostate cancer. Immunotargets Ther. 2017;6:11–6. doi: 10.2147/ITT.S122497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheikh NA, Petrylak D, Kantoff PW, Rosa CD, Stewart FP, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 cinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62:137–47. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, et al. Increased surivial with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 43.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ardiani A, Farsaci B, Rogers CJ, Protter A, Guo Z, et al. Combination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin Cancer Res. 2013;19:6205–18. doi: 10.1158/1078-0432.CCR-13-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ardiani A, Gameiro SR, Kwilas AR, Donahue RN, Hodge JW. Androgen deprivation therapy sensitizes prostate cancer cells to T-cell killing through androgen recepotr dependent modulation of the apoptotic pathway. Oncotarget. 2014;5:9335–48. doi: 10.18632/oncotarget.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madan RA, Donahue RN, Singh H, Karzai F, Theoret MR, et al. Clinical and immunologic impact of short course enzalutamide without androgen deprivation therapy for biochemically recurrent prostate cancer. J Clin Oncol. 2016;34(2 Suppl) abstract 214. [Google Scholar]
- 47.Quinn DI, Petrylak DP, Pieczonka CM, Sandler A, DeVries T, et al. A randomized phase II, open-label study of sipuleucel-T with concurrent or sequential enzalutamide in metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2014;32(Suppl 15) abstract e16071. [Google Scholar]
- 48.Quinn DI, Drake CG, Dreicer R, Antonarakis ES, Shore ND, et al. Immune response from STRIDE, a randomized, phase 2, open label study of sipuleucel-T (sip-T) with concurrent vs. sequential enzalutamide (enz) administration in metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2015;33(Suppl 15) abstract 5040. [Google Scholar]
- 49.Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, et al. PD-L1 is highly expressed in enzalutamide resistant prostate cancer. Oncotarget. 2015;6:234–42. doi: 10.18632/oncotarget.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, et al. First evidence of significant clinical activity of PD-1 inhibitors in metastatic, castration resistant prostate cancer (mCRPC) Ann Oncol. 2016;27(Suppl 6):7190. [Google Scholar]
- 51.Small EJ, Lance RS, Gardner TA, Karsh LI, Fong L, et al. A randomized phase II trial of sipuleucel-T with concurrent versus sequential abiraterone acetate pluse prednisone in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2015;21:3862–9. doi: 10.1158/1078-0432.CCR-15-0079. [DOI] [PubMed] [Google Scholar]
- 52.Danila DC, Kuzel T, Cetnar JP, Rathkopf DE, Morris MJ, et al. A phase 1/2 study combining ipilimumab with abiraterone acetate plus prednisone in chemotherapy-and immunotherapy-naive patients with progressive metastatic castration resistant prostate cancer (mCRPC) J Clin Oncol. 2016;34(Suppl 15) abstract e16507. [Google Scholar]
- 53.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karzai F, Madan RA, Owens H, Hankin A, Couvillon A, et al. Combination of PDL-1 and PARP inhibition in an unselected population with metastatic castrate-resistant prostate cancer (mCRPC) J Clin Oncol. 2017;35(Suppl 15) abstract 5026. [Google Scholar]
- 55.Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and tolerability fo PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta-analyssi. Oncologist. 2017;22:470–9. doi: 10.1634/theoncologist.2016-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jbag H, Carbonnel F, Robert C, Kerr KM, Peters S, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv119–42. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]


