Abstract
This study aims to validate our hypothesis that acid-sensing ion channels (ASICs) may contribute to the symptom of pain in patients with chronic prostatitis (CP). We first established a CP rat model, then isolated the L5-S2 spinal dorsal horn neurons for further studies. ASIC1a was knocked down and its effects on the expression of neurogenic inflammation-related factors in the dorsal horn neurons of rat spinal cord were evaluated. The effect of ASIC1a on the Ca2+ ion concentration in the dorsal horn neurons of rat spinal cord was measured by the intracellular calcium ([Ca2+]i) intensity. The effect of ASIC1a on the p38/mitogen-activated protein kinase (MAPK) signaling pathway was also determined. ASIC1a was significantly upregulated in the CP rat model as compared with control rats. Acid-induced ASIC1a expression increased [Ca2+]i intensity in the dorsal horn neurons of rat spinal cord. ASIC1a also increased the levels of neurogenic inflammation-related factors and p-p38 expression in the acid-treated dorsal horn neurons. Notably, ASIC1a knockdown significantly decreased the expression of pro-inflammatory cytokines. Furthermore, the levels of p-p38 and pro-inflammatory cytokines in acid-treated dorsal horn neurons were significantly decreased in the presence of PcTx-1, BAPTA-AM, or SB203580. Our results showed that ASIC1a may contribute to the symptom of pain in patients with CP, at least partially, by regulating the p38/MAPK signaling pathway.
Keywords: ASIC1a, chronic prostatitis, pain symptom
INTRODUCTION
Prostatitis is the most frequently diagnosed illness in men under 50 years, accounting for approximately 8% of all consultations with urologists.1 The clinical characteristics include genital symptoms (pain or discomfort in the penis or in the genital area), nonspecific urinary symptoms (frequency and/or nocturia), or other symptoms (an ache in the inside thigh).2 According to the National Institutes of Health (NIH), prostatitis is classified into four categories (category I, II, III, and IV). Notably, category III, also known as chronic prostatitis (CP) or chronic pelvic pain syndrome (CPPS), is well known as the most common type of prostatitis.3 It is difficult to design treatment strategies for improving symptoms of CP/CPPS and is often considered as a challenge for both the patient and urologist.4 The symptom of pain appears to be the chief complaint in 61% of patients with CP/CPPS and the secondary complaint in the remainder.4 Pain localized in the suprapubic perineal, pelvic, scrotal, and lower back areas is characteristic of CP.5 Moreover, pain is the only symptom that significantly decreases functional status and quality of life in individuals with CP.4
A previous study confirmed that the peripheral processes of dorsal root ganglion (DRG) cells dichotomize to the sphincter, prostate, and somatic parties simultaneously. Some of these cells contain calcitonin gene-related peptide and substance P, indicating that referred pain (a characteristic of visceral pain and pain that can be explained by neurologic mechanisms) in the perineum and pelvic floor of CP/CPPS patients may be caused by an axon reflex in the peripheral processes of DRG neurons.5 Acid-sensing ion channels (ASICs), belonging to the degenerin/epithelial Na+ channel superfamily, are ligand-gated cation channels activated by extracellular protons and contribute to sensory transmission, including nociception, and pain, particularly.6 At least, six subunits of ASICs (1a, 1b, 2a, 2b, 3, and 4) have been identified.7 Some researchers suggested that neurogenic inflammation plays an important role in the pathophysiological mechanisms of CPPS.8,9 Additionally, increased expression of ASIC1a, ASIC2b, and ASIC3 in the DRG was found after peripheral inflammation. Thus, we speculated that ASICs may contribute to the symptom of pain in patients with CP.
To validate our hypothesis, we first established a CP rat model. The L5-S2 spinal dorsal horn neurons were isolated since the L6 and S1 segments received the major nociceptive input from the prostate.10 The expression levels of ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC3 in spinal dorsal horn neurons from the rat model were detected by quantitative reverse transcription-polymerase chain reaction (qRT-PCR), Western blotting, and immunohistochemical analysis. ASIC1a was identified as an upregulated gene and the effects of ASIC1a on the Ca2+ ion concentration in the dorsal horn neurons of rat spinal cord were measured by the intracellular calcium ([Ca2+]i) intensity. ASIC1a was knocked down and its effects on neurogenic inflammation-related factors in the dorsal horn neurons of rat spinal cord were detected. The effect of ASIC1a on the p38/mitogen-activated protein kinase (MAPK) signaling pathway was also determined. Our results showed that ASIC1a may contribute to the symptom of pain in patients with CP, at least partially, by regulating the p38/MAPK signaling pathway.
MATERIALS AND METHODS
Animals
A total of fifty male Sprague-Dawley (SD) rats, 4-month-old, weighing 401–465 g, were obtained from the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Animals were bred and maintained under specific pathogen-free conditions. The experimental protocols were approved by the Ethics Committee of Anhui Medical University (IRB approval number: LLSC20140068).
Establishment of the chronic prostatitis rat model
The establishment of CP model was based on a previous study.11 In brief, SD rats were anesthetized with 10% chloral hydrate and then underwent castration surgery. They were then injected intramuscularly with 15 000 IU kg−1 penicillin for 3 days. After 7 days, the rats were administered 17β-estradiol diluted in saline at a dose of 0.25 mg kg−1 via subcutaneous injection for one month to induce CP. Rats in the control group did not undergo castration surgery. Subcutaneous injection of an equal volume of saline was performed for one month.
Isolation of dorsal horn neurons
Dorsal horn neurons were dissociated from rat spinal cord according to a previous study.6 Briefly, rats were killed by decapitation and a segment of lumbosacral (L5-S2) spinal cord was dissected out. 400 μm transverse slices were then sectioned with a vibratome tissue slicer (VT1000S; Leica, Wetzlar, Germany), and a vibration-isolation system was used to mechanically dissociate neurons from the spinal slices.
Spinal neuron culture and si-RNA transfection
The neurons were grown in Dulbecco's Modified Eagle's Medium (DMEM; Invitrogen, Carlsbad, CA, USA) with L-glutamine plus 10% fetal bovine serum (Invitrogen) and 10% F12 nutrient mixture (Invitrogen). Neurobasal medium (Invitrogen) with 2% B27 serum-free supplement (Invitrogen) was replaced one day after cell plating. The cultures were maintained at 37°C in 5% CO2 humidified atmosphere. The si-ASIC1a sequence was synthesized and transfected as previously described.12 10 μmol l−1 Ca2+ chelator BAPTA-AM, 10 μmol l−1 p38-MAPK inhibitor SB203580, and 100 ng ml−1 ASIC1a antagonist PcTx-1 were used to explore the underlying mechanism.
Measurement of cytokine levels
We measured the levels of six cytokines (interleukin [IL]-2, IL-4, IL-6, IL-10, tumor necrosis factor-α [TNF-α], and interferon-γ [IFN-γ]) in the supernatant, using Bio-Plex (Bio-Rad Laboratories Inc., Hercules, CA, USA) with cytokine multiplex kits (Bio-Plex Pro™ Rat Cytokine Th1/Th2 Assay;Bio-Rad Laboratories Inc.).
Immunohistochemical studies
Rats were anesthetized and the dorsal horn of spinal cord tissues was collected for immunohistochemical studies. Tissues were perfused with 4% paraformaldehyde in phosphate buffer (0.1 mol l−1, pH 7.4). The spinal cord was removed and cryoprotected overnight in 30% sucrose solution at 4°C. Transverse frozen sections (30 μm thick) were cut from the lumbar cord. Coimmunostaining was performed on parallel sections with antibodies against ASIC1a (1:200; Millipore, Temecula, CA, USA). Secondary antibodies were then used. The percentage of positive staining area was evaluated by a computer imaging analysis system with Aperio Image Scope Software Version 9.0 (AperioTechnologies Inc., Vista, CA, USA).
Measurement of intracellular calcium concentration ([Ca2+]i)
DRG neurons grown on coverslips were first washed twice with Hanks solution, and then incubated in 100 μl of 10 μmol l−1 Fluo-3/AM and 0.02% F-127 solution at 37°C for 3 min. The coverslips were then equilibrated in Hanks solution and [Ca2+]i was measured within 10 min. For the detection of [Ca2+]i in the absence of calcium, the Hanks solution was replaced with D-Hanks, and 5 μmol l−1 nimodipine solution was added to the culture dish and the [Ca2+]i was scanned for 20 s, the extracellular fluid (pH 6.0) was added using a micro-sampler and the dynamic changes of fluorescence intensity were detected. In the other treatment group, 100 μmol l−1 amiloride was added to the culture dish, extracellular fluid (pH 6.0) was added using a micro-sampler, and the dynamic changes in fluorescence intensity were detected. The [Ca2+]i was scanned per 6 s for 10 min, with each scan covering 7 cells. The wavelength of the excitation light was 488 nm and the wavelength of the emitted light was 525 nm.
Reverse transcription-quantitative polymerase chain reaction
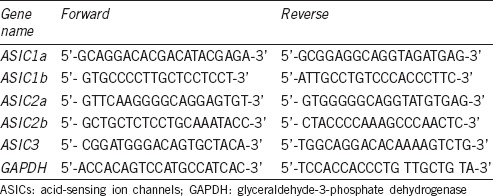
Total RNA was isolated from proximal tibias using the TRIzol method (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). cDNA was prepared by reverse transcription of single-stranded RNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. Briefly, 1 μg or 2 μg of mRNA, 2 μl of RT buffer, 0.8 μl of dNTP mixture, 2 μl of RT random primers, 1 μl of MultiScribe® reverse transcriptase, and 4.2 μl of nuclease-free water were used for each cDNA synthesis. After the reverse transcription, cDNA was stored at –20°C. Reactions were incubated in a PCR thermocycler at 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min, and then cooled to 4°C. RT-qPCR was carried out using the SYBR® Premix Ex Taq™kit (Takara Bio, Inc., Otsu, Japan), according to the manufacturer's instructions. The 20 μl reaction mix consisted of 2 μl 30-fold diluted 1st-strand cDNA, 10 μl 2X SYBR® Premix Ex Taq™, 0.4 μl 10 μmol l−1 forward and reverse primers, 0.4 μl 50X ROX Reference Dye, and 6.8 μl diethy pyrocarbonate (DEPC)-treated water. The primer pairs used for ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were listed in Table 1. Reactions were performed in an ABI7300 Real-Time quantitative instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: initial denaturation at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 31 s. The expression level of the internal control, GAPDH, was used as a housekeeping gene, and the comparative 2−ΔΔCq method was used to quantify gene expression levels.
Table 1.
Primers used for quantitative reverse transcription-polymerase chain reaction
Western blotting analysis
Protein was collected and lysed in radioimmunoprecipitation buffer (RIPA) containing protease inhibitors at 4°C for 30 min. Cell lysates were prepared with a RIPA lysis buffer kit (Santa Cruz Biotechnology, Inc., Calif., USA), and the protein concentrations were quantified using a Bio-Rad protein assay (Bio-Rad Laboratories, Inc.). Proteins (30 μg) were separated on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Amersham; GE Healthcare, Chicago, IL, USA). The membranes were blocked in 5% nonfat milk (Merck KGaA, Darmstadt, Germany) overnight at 4°C. Transferred membranes were then stained with the primary antibodies overnight at 4°C. Subsequently, protein bands were detected by incubation with a horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. The immune reactive proteins were visualized using SuperSignal ECL (Pierce, Rockford, IL, USA) and the densities of the bands were analyzed with Image J (NIH, Bethesda, MD, USA).
Statistical analyses
All values are expressed as the mean ± standard deviation, and analysis of variance was calculated using SPSS 11.5 statistical analysis software (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant. The unpaired Student's t-test was used to analyze differences between two groups. One-way ANOVA was used to analyze differences among multiple groups.
RESULTS
ASIC1a was significantly upregulated in the CP rat model as compared with the control rats
We constructed a CP rat model, and the expression levels of subunits of ASICs (1a, 1b, 2a, 2b, and 3) were detected. As shown in (Figure 1a), the mRNA level of ASIC1a was significantly upregulated in the CP rat model as compared with the control rats. No significant differences were found in the mRNA levels of ASIC2a, ASIC2b, and ASIC3 between CP and normal rats. We did not find any expression of ASIC1b in either the CP or normal rats. The Western blotting results were similar to the qRT-PCR results (Figure 1b). The immunohistochemical analysis also validated that the protein expression level of ASIC1a was significantly upregulated in the CP rat model (Figure 1c).
Figure 1.
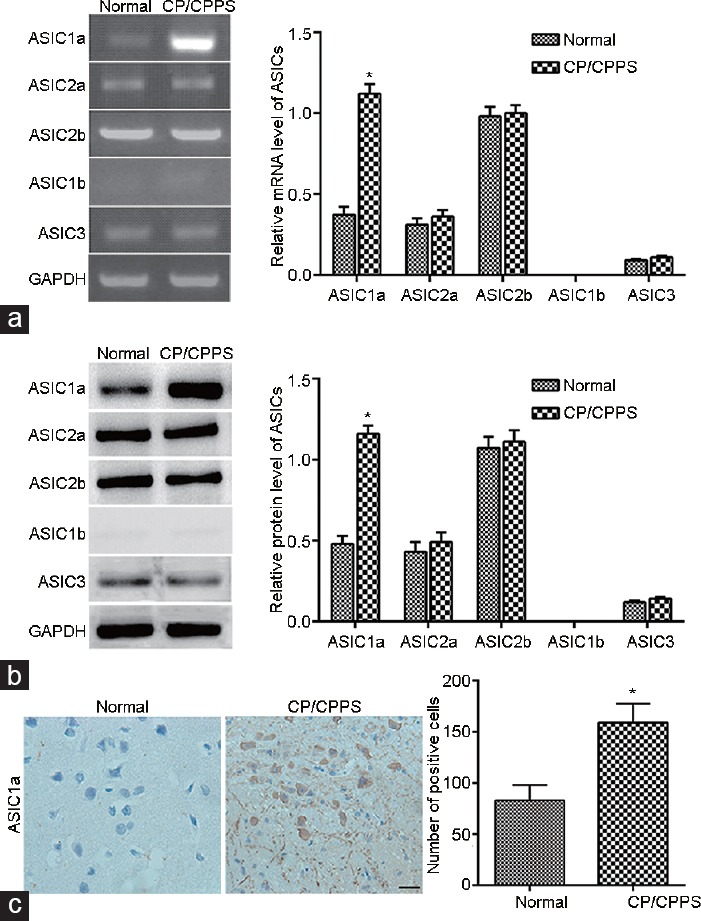
ASIC1a was significantly upregulated in the chronic prostatitis rat model as compared with control rats. (a) The mRNA level of ASIC1a was significantly upregulated in the CP rat model as compared with the normal rats. (b) The protein expression of ASIC1a was significantly upregulated in the CP rat model as compared with the normal rats. (c) Immunohistochemical analysis validated that the protein expression level of ASIC1a was significantly upregulated in the CP rat model. n= 25 in each group. *P< 0.05, CP/CPPS versus control. ASICs: acid-sensing ion channels; CP/CPPS: chronic prostatitis/chronic pelvic pain syndrome.
The effects of ASIC1a on the Ca2+ concentration in the dorsal horn neurons of rat spinal cord
The neurons in the L5-S2 spinal dorsal horn were isolated and were used in the following study, as the L6 and S1 segments received the major nociceptive input from the prostate.10 To explore the effects of ASIC1a on the Ca2+ ion concentration in the dorsal horn neurons of rat spinal cord, the Ca2+ intensity was determined before, during, and after exposure to an acid solution. The results (Figure 2a-2d) showed that the [Ca2+]i intensity in the acid-treated dorsal horn neurons exposed to Ca2+-free extracellular solution had no significant changes. However, the [Ca2+]i intensity in the acid-treated dorsal horn neurons exposed to extracellular Ca2+-containing solution was rapidly increased. Notably, the [Ca2+]i intensity in the acid-treated dorsal horn neurons was decreased in the presence of the ASIC1a antagonist PcTx-1. These results indicated that acid-induced ASIC1a expression increased [Ca2+]i concentration in the dorsal horn neurons of rat spinal cord.
Figure 2.
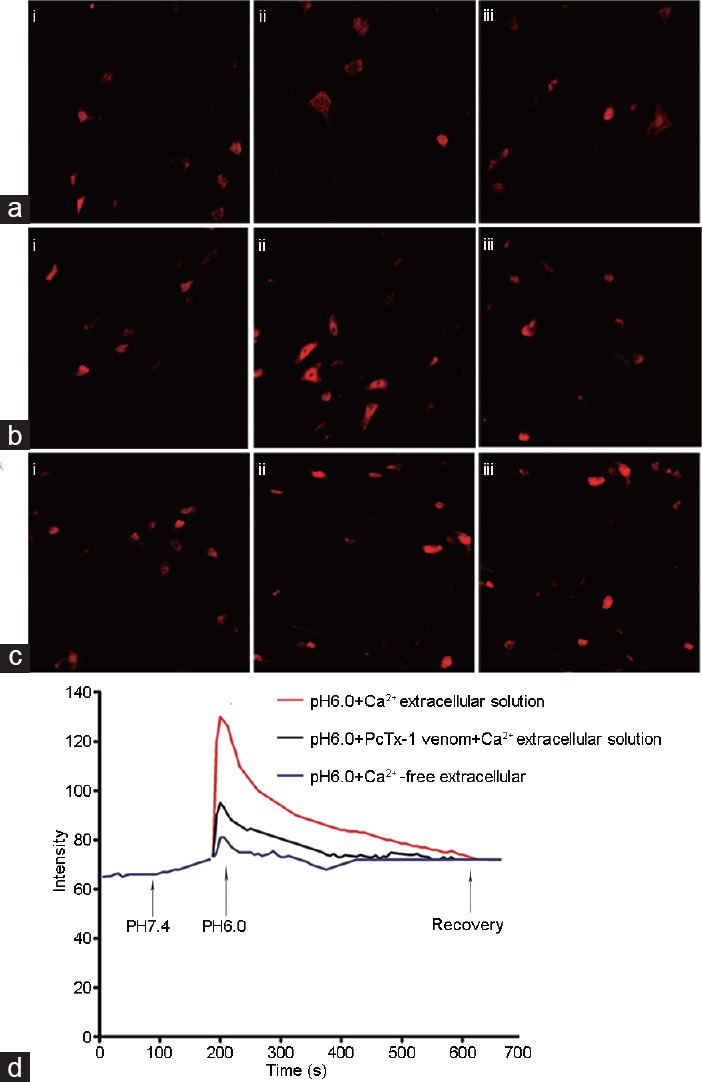
Effect of ASIC1a on [Ca2+]i in acid-treated dorsal horn neurons of rat spinal cord. (a) [Ca2+]i intensity in the acid-treated dorsal horn neurons exposed to Ca2+-free extracellular solution. (b) [Ca2+]i intensity in the acid-treated dorsal horn neurons exposed to extracellular Ca2+-containing solution. (c) [Ca2+]i intensity in the acid-treated dorsal horn neurons in the presence of PcTx-1. (d) Summary of data demonstrating changes in [Ca2+]i intensity in the dorsal horn neurons of rat spinal cord. (i) before exposure to acid solution; (ii) when the pH was decreased to 5.9; (iii) a few minutes after exposure to the acid solution (n = 7 per treatment). ASICs: acid-sensing ion channels.
The effects of ASIC1a on the levels of neurogenic inflammation-related factors in the dorsal horn neurons of rat spinal cord
ASIC1a was knocked down to explore the effects of ASIC1a on neurogenic inflammation-related factors in the dorsal horn neurons of rat spinal cord. The transfection efficiency was first determined by Western blotting analysis (Figure 3a). We also found that the levels of TNF-α, IL-2, IL-6, IL-10, and IFN-γ were significantly increased in dorsal horn neurons exposed to acid (Figure 3b-3f). ASIC1a knockdown significantly decreased the levels of the pro-inflammatory cytokines TNF-α, IL-2, IL-6, and IFN-γ in the acid-treated dorsal horn neurons but had no effect on the level of the anti-inflammatory cytokine IL-10. Notably, these results were comparable to the results from acid-treated dorsal horn neurons treated with PcTx-1.
Figure 3.
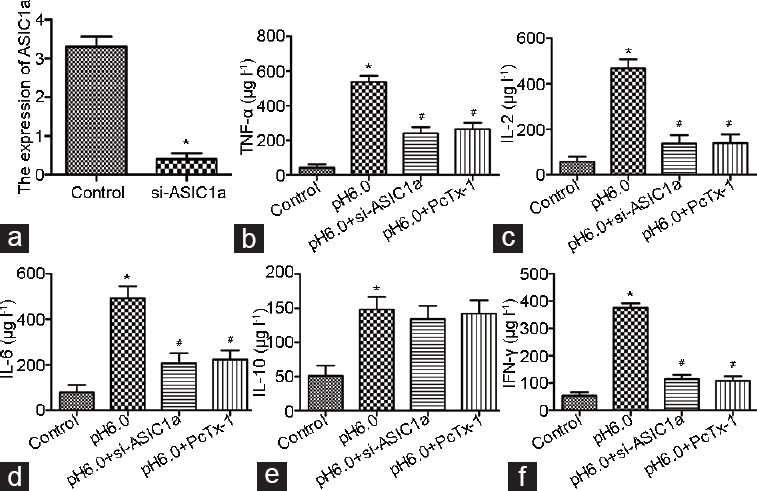
The effects of ASIC1a on the levels of neurogenic inflammation-related factors in the dorsal horn neurons of rat spinal cord. (a) The transfection efficiency of si-ASIC1a was determined by Western blotting analysis. The effects of ASIC1a knockdown on the levels of (b) TNF-α, (c) IL-2, (d) IL-6, (e) IL-10, and (f) IFN-γ in the acid-treated dorsal horn neurons, which were comparable to the results from the acid-treated dorsal horn neurons treated with PcTx-1. *P < 0.05, si-ASIC1a group or the acid-treated group (pH 6.0) versus control; and #P < 0.05, pH 6.0+ si-ASIC1a group or pH 6.0+ PcTx-1 group versus the acid-treated group (pH 6.0). ASICs: acid-sensing ion channels; TNF: tumor necrosis factor; IL: interleukin; IFN: interferon.
The effects of ASIC1a on the expression of p38/MAPK signaling pathway in the dorsal horn neurons of rat spinal cord
As shown in Figure 4, the protein expression of p-p38 in the acid-treated dorsal horn neurons was significantly increased in a time-dependent manner, indicating that acid-induced ASIC1a increases p-p38 expression (Figure 4a). However, the protein expression of p-p38 in the acid-treated dorsal horn neurons was significantly decreased in the presence of the ASIC1a antagonist PcTx-1 (Figure 4b). The protein expression of p-p38 in the acid-treated dorsal horn neurons was also significantly decreased in the presence of the Ca2+ chelator BAPTA-AM, which was comparable to the group that was treated with the p38-MAPK inhibitor (SB203580) (Figure 4c).
Figure 4.
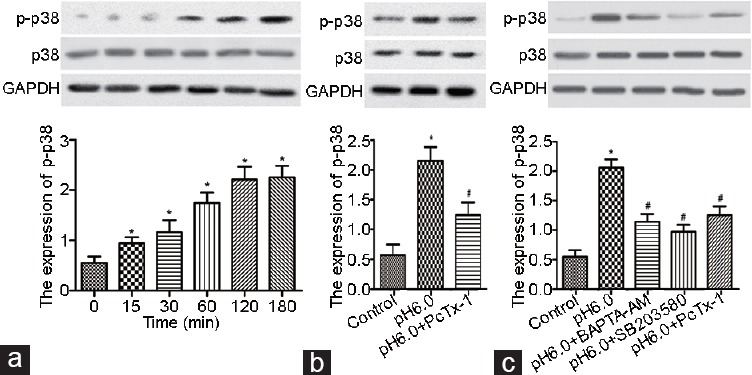
The effects of ASIC1a on the activity of the p38/MAPK signaling pathway in the dorsal horn neurons of rat spinal cord. (a) The protein expression of p-p38 in the acid-treated dorsal horn neurons was significantly increased in a time-dependent manner. (b) The protein expression of p-p38 was significantly decreased in the acid-treated dorsal horn neurons in the presence of the ASIC1a antagonist PcTx-1. (c) The protein expression of p-p38 in the acid-treated dorsal horn neurons was significantly decreased in the presence of the Ca2+ chelator BAPTA-AM or p38-MAPK inhibitor (SB203580). *P < 0.05, acid-treated group (pH 6.0) versus control; and #P < 0.05, pH 6.0+ PcTx-1 group or pH 6.0+ BAPTA-AM group or pH 6.0+ SB203580 versus acid-treated group (pH 6.0).
Ca2+ chelator BAPTA-AM, p38-MAPK inhibitor SB203580, or ASIC1a antagonist PcTx-1 significantly decrease the levels of neurogenic inflammation-related factors in acid-treated dorsal horn neurons
Although the levels of TNF-α, IL-6, IL-10, and IFN-γ were significantly increased in the dorsal horn neurons exposed to acid, the Ca2+ chelator BAPTA-AM, p38-MAPK inhibitor SB203580, or ASIC1a antagonist PcTx-1 significantly decreased the levels of these neurogenic inflammation-related factors in acid-treated dorsal horn neurons (Figure 5a-d). Notably, these reagents had the same degree of effect on the levels of neurogenic inflammation-related factors in the acid-treated dorsal horn neurons.
Figure 5.
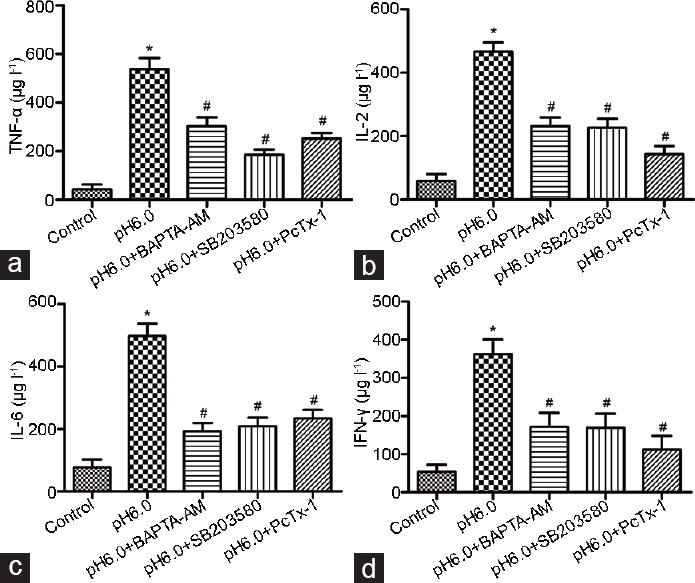
The Ca2+ chelator BAPTA-AM, p38-MAPK inhibitor SB203580, or ASIC1a antagonist PcTx-1 significantly decrease the levels of neurogenic inflammation-related factors in the acid-treated dorsal horn neurons. Ca2+ chelator BAPTA-AM, p38-MAPK inhibitor SB203580, or ASIC1a antagonist PcTx-1 significantly decreased the levels of neurogenic inflammation-related factors (a) TNF-α, (b) IL-6, (c) IL-10, and (d) IFN-γ in the acid-treated dorsal horn neurons. *P < 0.05, acid-treated group (pH 6.0) versus control; and #P < 0.05, pH 6.0+ BAPTA-AM group or pH 6.0+ SB203580 or pH 6.0+ PcTx-1 group versus the acid-treated group (pH 6.0). ASICs: acid-sensing ion channels; TNF: tumor necrosis factor; IL: interleukin; IFN: interferon.
DISCUSSION
In the present study, we first demonstrated that ASIC1a was significantly upregulated in the CP rat model as compared with the control rats. It is known that ASICs are widely expressed in the central nervous system and associated with pain sensing and mechanoperception in sensory neurons.13 Previous data confirmed that ASIC1a is broadly expressed in all the laminas of the adult spinal cord and contributes to every ASIC current recorded in cultured spinal cord neurons, indicating the importance of the ASIC1a subunit in spinal cord neurons.13 Another previous study demonstrated that homomeric ASIC1a and/or heteromeric ASIC1a + 2b channels are responsible for proton-induced currents in the majority of dorsal horn neurons. More importantly, they found that ASIC1a and ASIC2a expressions were upregulated by peripheral inflammation, suggesting the physiological involvement of ASICs in central pain sensing under physiological and/or pathological conditions.6 Furthermore, another study demonstrated that ASIC1a channels in spinal dorsal horn neurons are essential for inflammation-induced pain hypersensitivity and spinal neuron sensitization.
It is well accepted that neurogenic inflammation plays a role in the pathophysiological mechanism of chronic pain syndromes.8 It can be concluded that the upregulation of ASIC1a in spinal dorsal horn neurons of the CP rat model may result from neurogenic inflammation. Consistently, we found that the levels of TNF-α, IL-2, IL-6, and IFN-γ were significantly increased in dorsal horn neurons exposed to acid, and ASIC1a knockdown or PcTx-1 treatment ameliorated this increase. Our results also demonstrated that the [Ca2+]i in the acid-treated dorsal horn neurons exposed to a Ca2+-containing solution was quickly increased. However, the [Ca2+]i in the acid-treated dorsal horn neurons in the presence of the ASIC1a antagonist PcTx-1 was decreased. Vukicevicand Kellenberger14 suggested that ASIC1a channels in spinal dorsal horn neurons of mice are positioned to respond to acidification associated with persistent synaptic activity after peripheral inflammation, resulting in enhanced Ca2+ signaling. This enhanced firing may in turn facilitate the activation of N-methyl-D-aspartate (NMDA) receptors, leading to the activation of mitogen-activated protein kinases and Ca2+/calmodulin-stimulated adenylyl cyclases, and finally causing activity-dependent central sensitization.15,16 However, Gao et al.17 indicated thatNMDA receptor activation can further sensitize the cells to acidic conditions by inducing ASIC1a phosphorylation. Thus, it is likely that ASIC1a channels may function together with NMDA receptors in activity-dependent central sensitization underlying pain hypersensitivity. We also found that acid-induced ASIC1a increases p-p38 expression. However, the protein expression of p-p38 in the acid-treated dorsal horn neurons was significantly decreased in the presence of the ASIC1a antagonist PcTx-1. To date, six distinct groups of MAPKs have been characterized in mammals: p38, extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), ERK3/4, ERK5, and ERK7/8.18 p-p38 MAPK is the active form of p38 MAPK, which was reported to be increased in the spinal cord after peripheral inflammation.19 Moreover, intrathecal administration of a p38 MAPK inhibitor into the spinal cord has been shown to effectively reduce pain behavior associated with the peripheral inflammation.20,21,22 Further investigation is required to determine the exact effect of the MAPK signaling pathway on the spinal cord of rats suffering from the peripheral inflammatory pain.
Notably, the levels of p-p38 and neurogenic inflammation-related factors in the acid-treated dorsal horn neurons were significantly decreased in the presence of PcTx-1, BAPTA-AM, or SB203580. To some extent, this suggests that the p38 MAPK signaling pathway may be involved in the role of ASIC1a in neurogenic inflammation-induced pain in patients with CP. ASIC1a antagonists may therefore be promising therapeutic agents with anti-inflammatory and analgesic effects in patients with symptoms of pain from CP.
AUTHOR CONTRIBUTIONS
SF designed the project; ZYH, LZ, and JZ performed the experiments; YFZ, ST, and XSZ analyzed the data; CZL drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This research was supported in part by grants from the National Natural Science Foundation of China (No. 81400757); National College Students Innovation and Entrepreneurship Training Program (No. 201510366009); and Anhui Provincial Natural Science Foundation (1508085QH171).
REFERENCES
- 1.Litwin MS, McNaughton-Collins M, Fowler FJ, Jr, Nickel JC, Calhoun EA, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J Urol. 1999;162:369–75. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 2.Bartoletti R, Mondaini N, Pavone C, Dinelli N, Prezioso D. Introduction to chronic prostatitis and chronic pelvic pain syndrome (CP/CPPS) Arch Ital Urol Androl. 2007;79:55–7. [PubMed] [Google Scholar]
- 3.Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–7. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 4.Wenninger K, Heiman JR, Rothman I, Berghuis JP, Berger RE. Sickness impact of chronic nonbacterial prostatitis and its correlates. J Urol. 1996;155:965–8. [PubMed] [Google Scholar]
- 5.Chen Y, Song BO, Jin XY, Xiong EQ, Zhang JH. Possible mechanism of referred pain in the perineum and pelvis associated with the prostate in rats. J Urol. 2005;174:2405–8. doi: 10.1097/01.ju.0000180421.90260.65. [DOI] [PubMed] [Google Scholar]
- 6.Wu LJ, Duan B, Mei YD, Gao J, Chen JG, et al. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279:43716–24. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- 7.Krishtal O. The ASICs: signaling molecules. Modulators? Trends Neurosci. 2003;26:477–83. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 8.Wesselmann U. Neurogenic inflammation and chronic pelvic pain. World J Urol. 2001;19:180–5. doi: 10.1007/s003450100201. [DOI] [PubMed] [Google Scholar]
- 9.Hornick L, Slocumb JC. Treating chronic pelvic pain-focus on pain triggers and neurogenic inflammation. Adv Nurse Pract. 2008;16:44–53. [PubMed] [Google Scholar]
- 10.Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol. 1993;265:326–33. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- 11.Toshiyuki K, Shigeru S, Tadaichi K. Effect of cernitin pollen-extract on experimental nonbacterial prostatitis in rats. Prostate. 2001;49:122–31. doi: 10.1002/pros.1126. [DOI] [PubMed] [Google Scholar]
- 12.Duan B, Wu LJ, Yu YQ, Ding Y, Jing L, et al. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci. 2007;27:11139–48. doi: 10.1523/JNEUROSCI.3364-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron A, Voilley N, Lazdunski M, Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci. 2008;28:1498–508. doi: 10.1523/JNEUROSCI.4975-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vukicevic M, Kellenberger S. Modulatory effects of acid-sensing ion channels on action potential generation in hippocampal neurons. Am J Physiol Cell Physiol. 2004;287:C682–90. doi: 10.1152/ajpcell.00127.2004. [DOI] [PubMed] [Google Scholar]
- 15.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–9. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 16.Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW, et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron. 2002;36:713–26. doi: 10.1016/s0896-6273(02)01019-x. [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, et al. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635–46. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci. 2008;65:3525–44. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y, Fang JQ, Du JY, Fang JF. Effect of electroacupuncture on activation of p38MAPK in spinal dorsal horn in rats with complete Freund's adjuvant-induced inflammatory pain. Evid Based Complement Altern Med. 2012;2012:568273. doi: 10.1155/2012/568273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Huang Y, Yu X, Yue J, Yang N, et al. The influence of p38 mitogen-activated protein kinase inhibitor on synthesis of inflammatory cytokine tumor necrosis factor alpha in spinal cord of rats with chronic constriction injury. Anesth Analg. 2007;105:1838–44. doi: 10.1213/01.ane.0000287660.29297.7b. [DOI] [PubMed] [Google Scholar]
- 21.Sung CS, Wen ZH, Chang WK, Chan KH, Ho ST, et al. Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1β-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. J Neurochem. 2005;94:742–52. doi: 10.1111/j.1471-4159.2005.03226.x. [DOI] [PubMed] [Google Scholar]
- 22.Cao FL, Liu MG, Hao J, Li Z, Lu ZM, et al. Different roles of spinal p38 and c-Jun N-terminal kinase pathways in bee venom-induced multiple pain-related behaviors. Neurosci Lett. 2007;427:50–4. doi: 10.1016/j.neulet.2007.09.005. [DOI] [PubMed] [Google Scholar]


