Abstract
Background:
Allium genus with 750 species is the most diverse genus in the Amaryllidaceae family. Historically, Allium species have been used as medicinal plants, especially for prevention and treatment of cardiovascular diseases and considered as valuable sources of phytonutrients. Phytochemical investigation of Allium tripedale, locally called “Anashq,” which is an edible plant of the “Zagros” region (west of Iran) was conducted in the present study.
Materials and Methods:
Air-dried bulbs of the plant were extracted in a four-step extraction method with increasing polarity using hexane, chloroform, chloroform–methanol (9:1), and methanol. Chloroform-methanol (9:1) extract was fractionated by medium-pressure liquid chromatography on a RP-18 column using a linear gradient solvent system of H2O to MeOH. Phenolic-rich fractions were subjected to the final isolation and purification of the constituents by reversed-phase high-performance liquid chromatography method. Structure elucidation of the compounds was performed through comprehensive methods including 1D-and 2D-NMR and mass spectroscopy.
Results:
Two cinnamic acid derivatives were isolated from the bulbs of A. tripedale; using spectroscopic methods, their chemical structures were determined as 6,7-dimethoxy N-trans-caffeoyltyramine (1) and N-trans-feruloyltyramine (2).
Conclusion:
Cinnamic acid derivatives are pharmacologically active phenolic compounds, which have been isolated from different Allium species. Isolation of these compounds from A. tripedale is reported for the first time in this study and could be used as a chemical basis for explanation of the plant biological and pharmacological activities.
Keywords: Allium tripedale, Anashq, cinnamic acid derivatives, phytochemistry, structure elucidation
Introduction
Allium species are very important edible plants, and they have always been considered as edible vegetables and medicinal plants all over the world.[1,2] Historically, these plants have been mainly used for the prevention and treatment of cardiovascular disease, hypertension, and diabetes.[3,4,5]
Allium genus with more than 750 species is the most diverse plant genus in the Amaryllidaceae family and spreads around the globe although the place of origin is supposed to be the central Asia where they were used from about 3000 BC. Iran accounts as one of the main resources of Allium plants with the most distribution of this genus in the west, Zagros Mountains, and also northeast of the country.[3,6,7]
Alliums are also main sources of phytonutrients. These plants are valuable sources of important secondary plant metabolites including steroidal saponins and sapogenins, flavonoids, and organosulfur compounds,[1,2] and numerous studies have been conducted on the isolation and identification of these compounds from different Allium species in recent years. Cinnamic acid derivatives are valuable phenolic compounds isolated from different Alliums and have been demonstrated to bear several biological activities including antifungal,[8] antioxidant,[9] anti-inflammatory,[10] and anticancer[11] effects.
Allium tripedale is an edible plant grows wildly in the mountainous areas of Iran, especially west and northwest regions.[12] This plant is locally called “Anashq” in the west of Iran and grows wildly in Zagros mountainous regions, and its leaves which have a very strong odor and taste are used widely as an edible vegetable and to make dishes. A. tripedale is also considered as a medicinal plant and it has been traditionally used for the treatment of various infectious conditions; some of its pharmacological effects including antifungal, antidiabetic, and hepatoprotective activity have been recently demonstrated.[13,14]
As a part of our research program on phytochemical investigation of different Allium species, identification of main phenolic constituents of the plant was conducted and this paper reports the isolation and identification of two main cinnamic acid derivatives from the bulbs of A. tripedale for the first time.
Materials and Methods
General experimental procedures
Medium-pressure liquid chromatography (MPLC) was performed by a Buchi Gradient System C-605 apparatus using glass columns of LiChroprep® RP-18 (25–40 μm) and C-660 Buchi fraction collector. Thin-layer chromatography (TLC) performed on SiO2 plates with n-BuOH: CH3 COOH: H2O (60:15:25 v/v/v) (BAW) as a mobile phase and cerium sulfate in 2N H2 SO4 and natural product (NP) as reagents for visualizing the spots.
H and C NMR spectra recorded by Bruker 400 MHz (H at 400 MHz and C at 100 MHz) spectrometer, using solvent signal for calibration (CD3 OD: δH = 3.31, δC = 49.0). Distortionless enhancement by polarization transfer (DEPT) experiments was used to determine the multiplicities of C NMR resonances.
2D heteronuclear multiple bond correlation (HMBC), optimized for[2,3] JCH of 8 Hz, was used for determination of two- and three-bond heteronuclear 1H–13 C connectivities while 2D heteronuclear single-quantum coherence (HSQC), interpulse delay set for 1JCH of 130 Hz, and COSY were used for determination of one-bond heteronuclear 1H–13C connectivities and homonuclear 1H-1H connectivities, respectively. ESIMS spectra were prepared by Shimadzu LCMS 2010 EV, using methanol as the solvent.
Plant material
The whole plant of A. tripedale was collected from Khorramabad, Lorestan province, on April 2014. The plant was identified by botanist and a voucher specimen (No. 3579) deposited at the Department of Pharmacognosy, Faculty of Pharmacy, Isfahan University of Medical Sciences, Iran.
Extraction and isolation
Air-dried bulbs of A. tripedale were finely powdered by means of a mill, and the powder (1650 g) was extracted at room temperature in a four-step extraction method with increasing solvent polarity using the solvents; hexane, chloroform, chloroform-methanol (9:1), and methanol. Extraction was done using maceration method, performing each step four times with 5 L of solvent.
The chloroform-methanol (9:1) extract was concentrated by rotary evaporator, yielded a dried extract (12 g) which was then fractionated by MPLC on a RP-18 column (36*460 mm) using a linear gradient solvent system of H2O: MeOH (90:10) to MeOH (100%). Fractions were analyzed by TLC (SiO2, BAW 60:15:25 v/v/v) and visualized by 1% cerium sulfate in 2N H2SO4 or NP reagents, and similar fractions were mixed together.
Based on TLC and preliminary NMR analysis, fraction 8 was rich in phenolic compounds, which subjected again to MPLC with same conditions and resulted in two impure white compounds. Final purification by recrystallization yielded the pure compounds 1 (ATCM-8-4; 7 mg) and 2 (ATCM-8-5; 20 mg), respectively.
Results
Final purification of two main phenolic-rich fractions resulted in the isolation of two pure phenolic compounds, finally identified as cinnamic acid derivatives.
Identification of compounds
Compound (1) showed a pseudo molecular ion peak at m/z 327.15 [M-H] in the negative-ion ESIMS, indicated the presence of a nitrogen atom in the chemical structure.
Nineteen carbon signals were observed in 13CNMR spectrum that using the information from DEPT experiment, were determined as 7 aromatic methines, 2 aliphatic methines, 2 methylenes, 2 methoxys, and 6 quaternary sp2 carbon signals [Table 1], suggested that the molecular formula is C19H21NO4. Four of the quaternary carbons had downfield chemical shifts in 13CNMR spectra (δC 169.22, 149.80, 149.30, 156.90) which were concluded to be oxygenated.
Table 1.
1H and 13CNMR data of compound (1) and (2) (400 MHz, 100 MHz, CD3OD)
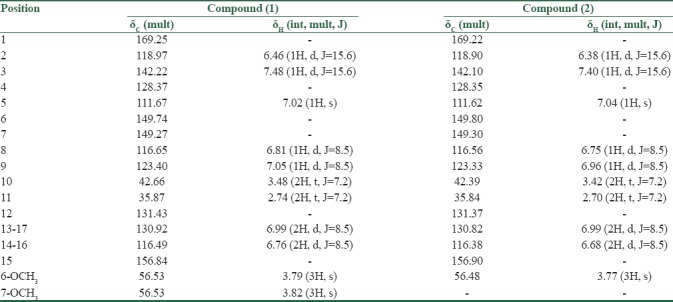
Analysis of 1HNMR spectra and correlating its information with that of 13CNMR spectra resulted to the identification of two aromatic rings, involving one p-substituted (2H doublets; δH6.76 and 6.99, j = 8.5 Hz) and one m-and p-substituted (2H doublets; δH6.82 and 7.05, j = 8.5 and a broad singlet; δH7.03) ring [Table 1]. Two sp2 methines with the downfield chemical shifts of proton and carbon signals (1H doublets; δH6.46 and 7.48, j = 15.7) indicated the existence of a di-substituted double bond conjugated with one of the aromatic rings in the structure. The attachment of two methoxy groups to the structure was concluded from the existence of the methoxy signals at 1H and 13CNMR spectra (3H singlets; δH3.79 and 3.82; δC56.53) [Table 1]. Finally, determining the heteronuclear 1H–13 C connectivities by HSQC and HMBC experiment, the assignments were confirmed and the chemical structure of (1) defined as 6,7-dimethoxy N-trans-caffeoyltyramine [Figure 1].
Figure 1.
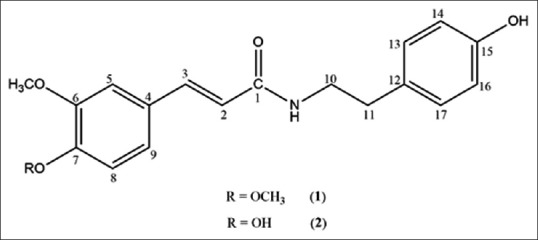
Chemical structure of cinnamic acid derivatives isolated from the bulbs of Allium tripedale
Besides to the great similarity of spectra to that of compound (1), compound (2) had a pseudo molecular ion peak of m/z 3.13.13 [M-H] in the ESIMS spectrum, which together with the 13CNMR data [Table 1] suggested the molecular formula as C18H19NO4. 1H, and 13CNMR spectra were almost completely coincident to those of (1), except for the absence of a singlet (δH3.82) at 1HNMR spectrum, indicated the chemical structure of (2) to possess a methoxy group less than that of (1). Complementary 1D and 2D NMR analysis showed that C7 in (2) was substituted by a hydroxyl group instead of methoxy compared to that of (1) [Table 1]. Regarding these data, the chemical structure of compound (2) was defined as N-feruloyltyramine [Figure 1].
Discussion
Many natural compounds with well-defined biological and pharmacological activities have been isolated and structurally characterized from different medicinal plants. Phenolic compounds are a very interesting group of these naturally occurring compounds with a broad spectrum of biomedical, food, and industrial applications. Allium species have recently attracted great attention due to high content and diversity of their phenolic compounds which due to their abundant use as edible vegetables and medicinal plants could be of great importance for explanation of their protective and medicinal effects on different diseases.
Allium tripedale, locally called “Anashq”, is an important edible plant of “Zagros” region in the west of Iran. The leaves of the plant are used widely as a vegetable, to make dishes and also as a medicinal plant, especially for the treatment of infectious diseases. Pharmacological activities of A. tripedale have been evaluated through different studies and the antifungal effects of the aqueous extract, hepatoprotective effects of hydroalcoholic extract accompanied by reducing aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and blood urea nitrogen, antidiabetic and cholesterol- and triglyceride-lowering effects of hydroalcoholic extract of the plant have been recently demonstrated.[13,14]
A. tripedale has been phytochemically investigated for its volatile organosulfur constituents that resulted to the isolation and identification of three new cysteine-based 1-butenyl derivatives including homoisoalliin and desoxyhomoisoalliin (12). However, nonvolatile secondary metabolites of the plant including phenolic compounds and steroidal saponins have not been studied yet. Hence, as one of the important edible Alliums of Iran, A. tripedale was subjected to phytochemical investigation in this study, especially for its phenolic constituents, resulted to the isolation and identification of two cinnamic acid derivatives from bulbs of the plant.
Cinnamic acid derivatives are natural substances mainly found in fruits, vegetables, and flowers and are a part of our daily dietary intake of phenolic compounds.[15,16] Besides, these compounds are important intermediate molecules in the production of different pharmaceutical ingredients. Cinnamic acid derivatives have been isolated from different plant species; among them, Alliums have been recently shown to be a source of these compounds,[1,2] for example, Allium sativum (N-feruloyltyramine, N-caffeoyltyramine), A. ampeloprasum Subsp. Persicum, Persian leek (Persicoimidate, N-feruloyltyramine, 6,7-dimethoxy N-caffeoyltyramine), and A. fistulosum (N-trans-feruloyl-3′-methoxytyramine, N- cis-feruloyl-3′-methoxytyramine, N-trans-p-coumaroyltyramine).[8,17,18] Biological activities of cinnamic acid and its derivatives have been also the subject of many studies and shown to possess antioxidant, hepatoprotective, anxiolytic, insect repellent, antiviral, antibacterial, antidiabetic, anticholesterolemic, anti-inflammatory, and anticancer effects.[9,10,11]
N-trans-feruloyltyramine and 6,7-dimethoxy N-trans-caffeoyltyramine are known cinnamic acid derivatives, have been previously isolated from an important edible Allium species of Iran, Persian Leek (Allium ampeloprasum Subsp. Persicum), and shown to bear significant antifungal activity, especially against the antagonistic fungi, Trichoderma harzianum.[8] Although the mechanism of action is unclear, the synthesis of these compounds by Alliums may be the reason of their resistance against the plant fungal diseases and could candidate the cinnamic acid derivatives as potential antifungal compounds.[19,20] Recently, antifungal effects of the aqueous extract of A. tripedale against candida albicans were demonstrated which these compounds could at least be responsible for some part of this activity.[14]
N-feruloyltyramine has also been isolated from garlic (Allium sativum) and exhibited to be capable of inhibiting COX enzyme and suppressing P-selectin expression on platelets.[21,22] Similarly, N-caffeoyltyramine was exhibited to inhibit COX enzyme and bear anti-inflammatory effects. It has been isolated from Tribulus terrestris and demonstrated to prevent inflammation process by inhibiting COX-2 enzyme, suppressing COX-2 expression, and inhibiting the synthesis of PGE-2. Since these compounds are potentially safe natural anti-inflammatory compounds which could be candidate for the treatment of inflammatory diseases.[23]
Cinnamic acid derivatives are also potentially potent natural cytotoxic compounds, and their cytotoxic effects have been investigated in numerous studies. N-caffeoyltyramine has been shown to bear potent antiproliferative effects through the induction of cell death, activating caspase-3 and inhibiting protein tyrosine kinases. Recent studies have indicated that the hydroxyl groups of the phenylpropenyl group of the structure are responsible for the potent antiproliferative effects of N-caffeoyltyramine.[24] Substitution of the hydroxyls with methoxyl in 6,7-dimethoxy N-trans-caffeoyltyramine can significantly change the antiproliferative activity which needs more studies for the evaluation of cytotoxic activity.
Conclusion
Two cinnamic acid derivatives were isolated from the bulbs of A. tripedale for the first time in this study. Identification of these compounds is in agreement with previous studies have been done on other Allium species and could be used as a chemical basis for explanation of some of the biological activities observed from this plant, especially its antifungal effects, and to candidate it for investigation of cytotoxic activity.
Financial support and sponsorship
This study was supported by Isfahan University of Medical Sciences for financial and technical supporting of the project.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are grateful to Isfahan University of Medical Sciences for financial supporting of the project.
References
- 1.Fattorusso E, Iorizzi M, Lanzotti V, Taglialatela-Scafati O. Chemical composition of shallot (Allium ascalonicum hort.) J Agric Food Chem. 2002;50:5686–90. doi: 10.1021/jf020396t. [DOI] [PubMed] [Google Scholar]
- 2.Zolfaghari B, Barile E, Capasso R, Izzo AA, Sajjadi SE, Lanzotti V, et al. The sapogenin atroviolacegenin and its diglycoside atroviolaceoside from Allium atroviolaceum. J Nat Prod. 2006;69:191–5. doi: 10.1021/np0503350. [DOI] [PubMed] [Google Scholar]
- 3.Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Kelkar SM, Kaklij GS, Bapat VA. Determination of antidiabetic activity in Allium cepa (onion) tissue cultures. Indian J Biochem Biophys. 2001;38:277–9. [PubMed] [Google Scholar]
- 5.Thomson M, Al-Amin Z, Al-Qattan K, Shaban L, Ali M. Antidiabetic and hypo-lipidemic properties of garlic (Allium sativum) in steretozotocin induced diabetic rats. Int J Diabetes Metab. 2007;15:108–15. [Google Scholar]
- 6.Memariani F, Joharchi MR, Khassanov FO. Allium L. Subgen rhizirideumsensulato in Iran, two new records and a synopsis of taxonomy and phytogeography. J Bot. 2007;13:12–20. [Google Scholar]
- 7.Friesen N, Fritsch RM, Blattner FR. Phylogeny and new intrageneric classification of Allium (Alliaceae) based on nuclear ribosomal DNA ITS Sequences. Aliso. 2006;22:372–95. [Google Scholar]
- 8.Sadeghi M, Zolfaghari B, Senatore M, Lanzotti V. Antifungal cinnamic acid derivatives from Persian leek (Allium ampeloprasum subsp. persicum) Phytochem Lett. 2013;6:360–3. [Google Scholar]
- 9.Foti MC, Daquino C, Geraci C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions. J Org Chem. 2004;69:2309–14. doi: 10.1021/jo035758q. [DOI] [PubMed] [Google Scholar]
- 10.Zhang LP, Ji ZZ. Synthesis, antiinflammatory and anticancer activity of cinnamic acids, their derivatives and analogues. Yao Xue Xue Bao. 1992;27:817–23. [PubMed] [Google Scholar]
- 11.Akao Y, Maruyama H, Matsumoto K, Ohguchi K, Nishizawa K, Sakamoto T, et al. Cell growth inhibitory effect of cinnamic acid derivatives from propolis on human tumor cell lines. Biol Pharm Bull. 2003;26:1057–9. doi: 10.1248/bpb.26.1057. [DOI] [PubMed] [Google Scholar]
- 12.Kusterer J, Keusgen M. Cysteine sulfoxides and volatile sulfur compounds from allium tripedale. J Agric Food Chem. 2010;58:1129–37. doi: 10.1021/jf903581f. [DOI] [PubMed] [Google Scholar]
- 13.Paydar S, Jelodar GH, Mohammadi J, Mohammadi N. The effect of hydroalcoholic extract of Nectaroscordum tripedaleon liver and kidney functional parameters in streptozocin-induced diabetic male rats. IJEM. 2016;18(2):112–9. [Google Scholar]
- 14.Panahi J, Havasian MR, Gheitasi S, Pakzad I, Jaliliyan A, Hoshmandfar R, et al. The in vitro inhibitory effects of the aqueous extracts of summer onion on Candida albicans. SJIMU. 2013;21(1):54–9. [Google Scholar]
- 15.He ZD, Qiao CF, Han QB, Cheng CL, Xu HX, Jiang RW, et al. Authentication and quantitative analysis on the chemical profile of Cassia bark (Cortex cinnamomi) by high-pressure liquid chromatography. J Agric Food Chem. 2005;53:2424–8. doi: 10.1021/jf048116s. [DOI] [PubMed] [Google Scholar]
- 16.Wen D, Li C, Di H, Liao Y, Liu H. A universal HPLC method for the determination of phenolic acids in compound herbal medicines. J Agric Food Chem. 2005;53:6624–9. doi: 10.1021/jf0511291. [DOI] [PubMed] [Google Scholar]
- 17.Nishioka T, Watanabe J, Kawabata J, Niki R. Isolation and activity of N-p-coumaroyltyramine, an α-glucosidase inhibitor in welsh onion (Allium fistulosum) Biosci Biotechnol Biochem. 1997;61:1138–41. [Google Scholar]
- 18.Park JB. Isolation and characterization of N-feruloyltyramine as the P-selectin expression suppressor from garlic (Allium sativum) J Agric Food Chem. 2009;57:8868–72. doi: 10.1021/jf9018382. [DOI] [PubMed] [Google Scholar]
- 19.Barile E, Bonanomi G, Antignani V, Zolfaghari B, Sajjadi SE, Scala F, et al. Saponins from Allium minutiflorum with antifungal activity. Phytochemistry. 2007;68:596–603. doi: 10.1016/j.phytochem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Fattorusso E, Lanzotti V, Taglialatela-Scafati O. Antifungal N-feruloyl amides from roots of Allium species. Plant Biosyst. 1999;133:199–203. [Google Scholar]
- 21.Park JB, Schoene N. Clovamide-type phenylpropenoic acid amides, N-coumaroyldopamine and N-caffeoyldopamine, inhibit platelet-leukocyte interactions via suppressing P-selectin expression. J Pharmacol Exp Ther. 2006;317:813–9. doi: 10.1124/jpet.105.097337. [DOI] [PubMed] [Google Scholar]
- 22.Park JB. Quantitation of clovamide-type phenylpropenoic acid amides in cells and plasma using high-performance liquid chromatography with a colorimetric electrochemical detector. J Agric Food Chem. 2005;19:8135–40. doi: 10.1021/jf0516078. [DOI] [PubMed] [Google Scholar]
- 23.Ko HJ, Ahn EK, Oh JS. N-trans-ρ-caffeoyl tyramine isolated from Tribulus terrestris exerts anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 cells. Int J Mol Med. 2015;36:1042–8. doi: 10.3892/ijmm.2015.2301. [DOI] [PubMed] [Google Scholar]
- 24.Park JB, Schoene N. N-caffeoyltyramine arrests growth of U937 and Jurkat cells by inhibiting protein tyrosine phosphorylation and inducing caspase-3. Cancer Lett. 2003;202:161–71. doi: 10.1016/j.canlet.2003.08.010. [DOI] [PubMed] [Google Scholar]


