Abstract
Background:
Metadoxine (pyridoxine pyrrolidone carboxylate) is considered to be a beneficial agent for the treatment of experimental hepatotoxicity due to alcohol, CCl4, and bile duct ligation. Hence, the therapeutic effect of metadoxine and N-acetylcysteine (NAC) as reference drug was investigated in mice exposed to acute hepatotoxicity induced by a single oral toxic dose of acetaminophen (650 mg/kg).
Materials and Methods:
Metadoxine (200 and 400 mg/kg) and NAC (300 mg/kg) were given orally (p. o.), 2 h after acetaminophen administration. Serum aminotransferases, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), total bilirubin, hepatic glutathione (GSH), and malondialdehyde (MDA) levels were determined for evaluating the extent of hepatotoxicity due to acetaminophen and its protection by metadoxine.
Results:
Findings indicated that metadoxine significantly reduced the level of serum ALT, AST, and ALP but not total bilirubin which increased by acetaminophen intoxication. Administration of metadoxine also attenuated oxidative stress by suppressing lipid peroxidation (MDA) and prevented the depletion of reduced GSH level which caused by acetaminophen toxicity. Besides, metadoxine ameliorated histopathological hepatic tissue injury induced by acetaminophen.
Conclusion:
In most parameters examined, the effect of metadoxine was comparable to NAC. Hence, metadoxine could be considered as a beneficial therapeutic candidate to protect against acute acetaminophen hepatotoxicity.
Keywords: Acetaminophen, hepatoprotection, liver injury, metadoxine, mice
Introduction
The liver is a vital organ that performs a wide variety of functions necessary for survival. It plays a major role in all metabolic processes and is highly specialized for detoxification of various agents or xenobiotics which makes it highly susceptible to injury.[1] Drug-induced liver injury (DILI) is an uncommon adverse drug reaction which leads to liver failure and the most common reason for a liver transplant. One of the most prevalent drugs implicated in DILI is acetaminophen.[2]
Acetaminophen is a widely used nonprescription (over-the-counter); analgesic and antipyretic medication and its effectiveness in relieving pain and fever has been widely known. It is safe and well tolerated at recommended doses, however, in high doses might cause liver damage, ranging from mild to severe liver failure, and even death.[3] Acetaminophen is mainly metabolized through hepatic sulfation and glucuronidation pathways; furthermore, a small proportion of it metabolized by cytochrome P450 to a highly reactive intermediate, N-acetyl-para-benzoquinone imine (NAPQI), that is conjugated with reduced glutathione (GSH) to nontoxic substances.[4] Due to over doses, excessive production of NAPQI caused by saturation of the conjugation pathways leads to rapid depletion of GSH depots. Consequently, NAPQI binds to critical macromolecules within the cells that results in mitochondrial oxidative stress, liver function impairment, massive centrilobular necrosis, and finally, liver failure.[4]
N-acetylcysteine (NAC) is a proven antidote to treat acetaminophen poisoning for several decades. It acts by replenishing GSH supply but its side effects including nausea, vomiting, and anaphylaxis led researchers to find more safe and effective remedies.[5]
Metadoxine (pyridoxine pyrrolidone carboxylate) is an ion pair between pyridoxine (vitamin B6) and pyrrolidone carboxylate. Metadoxine is considered to be a beneficial treatment for alcoholic liver disease.[6] It accelerates the clearance of ethanol and acetaldehyde from the body and diminishes the damaging effect induced by ethanol.[7] Metadoxine is effectively able to preserve GSH reservoir and prevents lipid peroxidation, collagen disposition, and tumor necrosis factor-alpha (TNF-α) secretions induced by ethanol and acetaldehyde in hepatocytes and hepatic stellate cells in culture.[8] Experimental studies in ethanol-administered rats have indicated that metadoxine restores liver GSH content and prevents reduction of hepatic adenosine triphosphate (ATP) concentration caused by ethanol.[7,9,10] Moreover, metadoxine may be useful for treating nonalcoholic steatohepatitis due to inhibition of causative factors that are involved in oxidative stress.[11] In CCl4-chronic administration model of liver fibrosis, animals treated with CCl4 and metadoxine concurrently had less severe fibrosis and inflammation with normal serum levels of immunoreactive prolyl hydroxylase in comparison to rats treated only with CCl4.[12] Therefore, this study for the first time was aimed to examine the probable protective effects of metadoxine in hepatotoxicity induced by acetaminophen in mice, and the mechanisms are involved.
Materials and Methods
Chemicals
Acetaminophen powder was obtained as a kind gift from Abidi Pharmaceutical Company (Tehran, Iran). Tris-hydroxymethyl aminomethane, 2-thiobarbituric acid (TBA), 5, 5-dithionitrobenzoic acid (DTNB), and trichloroacetic acid (TCA) were purchased from Merck Company (Germany). Tween 80 was obtained from Scharlau (Spain). Pyridoxine hydrochloride, 2-pyrrolidone-5-carboxylic acid, NAC, 1, 1-3, 3-tetramethoxypropane, and GSH powder were purchased from Sigma-Aldrich (USA). The kits for liver biochemistry assay (aminotransferase [ALT], aspartate transaminase [AST], alkaline phosphatase [ALP], and total bilirubin) were obtained from Pars Azmun Co. (Tehran, Iran). All other chemicals used were of analytical grade.
Animals
Male albino mice weighing 20-25 g purchased from animal house of Isfahan University of Medical Sciences, Isfahan, Iran. Mice were housed in stainless steel cages on wood bedding at a temperature of 25°C ± 3°C with 12 h light/12 h dark cycles. Animals fed with standard laboratory diet and water ad libitum. All animal housing, handling and working were executed according to the animal handling protocol approved by local Ethics Committee at Isfahan University of Medical Sciences (IR. MUI. REC.1394.3.787).
Experimental design
Mice were randomly divided into five groups of at least six animals. For all experiments, food was withdrawn 16 h before acetaminophen administration. Drug doses were guided by the literature and adjusted through pilot trials.[4,5]
The treatments were as follow: the first group received oral normal saline (5 ml/kg) through a gastric tube. The second group received a single oral toxic dose of acetaminophen (650 mg/kg prepared in 1% Tween 80 in distilled water), the third group received acetaminophen (650 mg/kg), while 2 h later, a single oral dose of NAC (300 mg/kg) diluted in distilled water was administered. The fourth and fifth groups received acetaminophen (650 mg/kg), while 2 h later, a single oral dose of metadoxine (200 mg/kg) (pyridoxine HCl [114 mg] + pyrrolidone carboxylate [86 mg]) or 400 mg/kg (pyridoxine HCl [228 mg] + pyrrolidone carboxylate [172 mg]) was administered, respectively.
Sample preparation and biochemical studies
Twenty hours later, mice were anesthetized with ether. Blood was drawn by cardiac puncture and added to sterile tubes allowed to clot then centrifuged at 3000 rpm for 10 min to obtain serum.
Serum AST, ALT, ALP levels and total bilirubin were measured by an autoanalyzer (BT 3000, Italy) using Pars Azmun commercial diagnostic kits, and the results were expressed in U/L (total bilirubin was expressed as g/L). After collecting blood samples, the liver of mice carefully separated and washed with ice-cooled saline. A section of each mouse liver was fixed in 10% phosphate-buffered formalin for histopathologic evaluation. Remaining sections of the livers were frozen immediately in liquid nitrogen and stored at −70°C for preparing liver homogenates.[13]
Estimation of glutathione
The liver GSH contents were measured by determining nonprotein sulfhydryl contents with the Ellman's reagent. Two hundred milligram of the liver was weighed and homogenized in 8 mL of cooled ethylenediaminetetraacetic acid solution (0.02 M) in an ice bath. Then, 5 mL of liver homogenate was transferred to new tube and added 4 mL of distilled water and 1 mL of 50% TCA. The mixture was shaken vigorously for 10 min and then centrifuged (15 min at 4°C). Then, 2 mL of supernatant was added to 4 mL of Tris buffer (pH 8.9) and 100 μL of DTNB solution (0.01 M in methanol). The samples were shaken to obtain a homogeneous mixture. The solution absorbance was read within 5 min of the addition of DTNB at 412 nm against a reagent blank with no homogenate.[14]
Lipid peroxidation
The level of lipid peroxidation in mice liver was determined by the TBA reactive substance test.
Briefly, 500 mg of the liver tissue was homogenized in 5 ml of KCL (1.15%). Then, 0.5 mL of 10% liver homogenate was pipetted into a new tube and added 3 mL of 1% phosphoric acid, and 1 mL of 1% TBA. The mixture was shaken and then heated in boiling bath water for 45 min. After cooling, 4 ml of n-butanol was added to reaction mixture and shaken intermittently for 5 min. Samples were centrifuged (3000 g for 5 min) and the absorbance of butanol phase was read at 532 nm using an Unicom 2100 ultraviolet spectrophotometer.[15,16]
Statistical analysis
Results were expressed as mean ± standard error of mean. All statistical analyses were performed using one-way analysis of variance. For multiple comparisons, Tukey's post hoc test was used. P < 0.05 was considered statistically significant.
Results
Results of the present investigation showed that a single toxic dose of acetaminophen significantly increased serum activities of ALT, AST, and ALP (P < 0.001) when compared with the control acetaminophen group [Table 1]. The acetaminophen group showed slightly (not significant) increase in serum total bilirubin concentration (P > 0.05), as presented in Table 1.
Table 1.
Effect of metadoxine and N-acetylcysteine on serum alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and total bilirubin levels in mice treated with hepatotoxic dose of acetaminophen
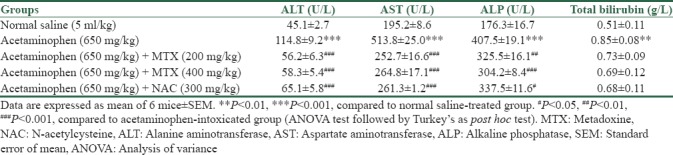
Treatment with NAC and different doses of metadoxine (200 and 400 mg/kg) exhibited a significant reduction in the levels of AST (P < 0.001), ALT (P < 0.001), and ALP (at least P < 0.05) as compared to acetaminophen control group. With NAC and metadoxine, serum ALT and AST levels were restored back to nearly normal values [Table 1]. Total bilirubin concentration, elevated by acetaminophen, exhibited a slight decrease (P > 0.05) in response to the treatment with metadoxine and NAC [Table 1].
Liver damage was associated with oxidative stress evidenced by significant elevation of tissue thiobarbituric acid-reactive substances (TBARS) (malondialdehyde [MDA]) levels with significant reductions in tissue GSH contents [Table 2]. Administration of acetaminophen in toxic dose significantly increased MDA (P < 0.001), the final product of lipid peroxidation, and significantly decreased tissue reduced GSH (P < 0.001). Administration of NAC and metadoxine was accompanied with a significant decrease in the levels of MDA and also effectively prevented GSH reservoir decline in liver tissue, as shown in Table 2.
Table 2.
Effect of metadoxine and N-acetylcysteine on two markers of oxidative stress in livers of acetaminophen-intoxicated mice

Histopathological results
Histopathological examination of liver sections obtained from normal group showed normal hepatic architecture as shown in Figure 1a. The liver of acetaminophen-intoxicated group exhibited severe histopathological changes, such as centrilobular hepatic necrosis, fatty changes, sinusoidal congestion, ballooning degeneration, and inflammatory cell infiltration as shown in Figure 1b and c.
Figure 1.
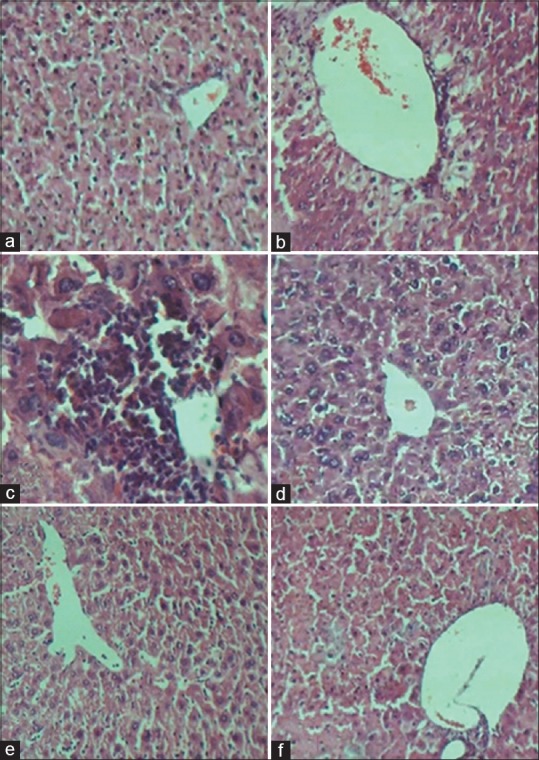
(a) Normal saline-treated group; liver showing normal architecture. (b and c) Acetaminophen-induced liver toxicity group; Liver exhibited severe centrilobular hepatic necrosis, fatty changes, ballooning degeneration, and inflammatory cell infiltration. (d) Acetaminophen + N-acetylcysteine group; showing hepatocytes are normal with slightly congested central vein, mild fatty change, moderate inflammation, and a remarkably reduction in necrosis while other alterations are seen as in acetaminophen group. (e) Acetaminophen + metadoxine 200 mg/kg group, showing the hepatocytes are normal with slightly congested central vein, mild inflammation, mild fatty change, and notable reductions in necrosis while other alterations are similar to acetaminophen group. (f) Acetaminophen and metadoxine 400 mg/kg group, showing the hepatocytes are normal with slightly congested central vein, mild vacuolization around central vein, and a considerable reduction in necrosis and inflammation. H and E, ×20 magnification was used with the exception of picture C for which magnification of ×40 was applied
The group received acetaminophen + NAC showed that the hepatocytes were nearly normal with slightly congested central vein. The results showed mild fatty change and activated some inflammatory cells and a remarkably reduction in necrosis and other alterations seen in the acetaminophen group [Figure 1d]. The group administered acetaminophen + metadoxine 200 mg/kg showed normal hepatocytes with slightly congested central vein. The results showed mild inflammation, mild fatty change, and a considerable reduction in necrosis and other alterations seen in the acetaminophen group [Figure 1e]. The group administered acetaminophen + metadoxine 400 mg/kg showed that the hepatocytes were normal with slightly congested central vein, and mild vacuolization around central vein. The results showed a considerable reduction in necrosis, inflammation, and other alterations seen in the acetaminophen group [Figure 1f].
Discussion
Acetaminophen is a commonly used pain reliever and fever reducer which has a safety profile at therapeutic doses; however, hepatotoxicity can occurs following an intentionally or accidentally overdose. Liver injury by acetaminophen is believed to be caused by the biotransformation of acetaminophen to a highly reactive metabolite NAPQI by several isozymes of the cytochrome P450 system.[4]
The elevation in serum levels of hepatic transaminases have been attributed to the damaged structural integrity of cellular membrane which resulted in liver enzyme leakage into the bloodstream. Both ALT and AST enzymes are found in high concentrations in the liver; hence, the presence of these enzymes in serum reflects the severity of the amount of damage that the liver has sustained.[17]
ALP is a nonspecific enzyme widely spread in different tissues. The elevation in the levels of the serum ALP indicates the disturbed excretory function which could be related to some destruction of hepatic cell membrane and impairment in the hepatobiliary duct apparatus.[18,19] Determination of serum total bilirubin is an indicator for the assessment of hepatic function, and elevated levels of bilirubin in the serum indicate hepatobiliary disease and severe disturbance of hepatocellular function.[19]
Results of the present investigation showed that a single oral dose of acetaminophen caused acute liver damage to mice as evidenced by significant increases in serum activities of ALT, AST, ALP and a slight increase but not significantly in the levels of serum bilirubin as compared to normal group. Our results provided a new strong evidence that metadoxine significantly inhibited the acute liver toxicity induced by toxic dose of acetaminophen in mice, as shown by a decrease in serum liver enzyme activities (AST, ALT, and ALP).
In the present study, acute administration of acetaminophen significantly induced the depletion of GSH content compared to the control group while administration of metadoxine prevented depletion of GSH in acetaminophen-intoxicated mice.
The antioxidant activity of metadoxine could be related to its structural characteristics, consisting of two components, pyridoxine (Vitamin B6) and pyrrolidone carboxylate, which participate in the synthesis of GSH. Pyrrolidone carboxylic acid is a cyclic lactam of glutamic acid easily being converted to glutamate following hydrolysis. It is an intermediate in the gamma-glutamyl cycle; a pathway is essential for the synthesis and degradation of GSH.[20,21,22]
Although the exact antioxidant mechanism of pyridoxine (Vitamin B6) is still controversial, it may scavenge nucleophiles and oxygen-derived free radicals and prevents oxidative stress. On the other hand, Vitamin B6 may indirectly play an antioxidant role, acting as a coenzyme in two separate enzymatic reactions in methylation cycle to convert homocysteine first into cystathionine and then converted into cysteine. Cysteine synthesized by this pathway participates in synthesis of GSH.[23]
Results of the present study demonstrated that acute administration of acetaminophen caused oxidative stress in mice liver as demonstrated by significant elevation in hepatic MDA (TBARS) level. The increase in TBARS level is due to overwhelmed antioxidant defense system by excessive production of reactive oxygen and nitrogen species.[24] Treatment with metadoxine could decrease TBARS level and restore GSH content, suggesting that metadoxine had a protection on acetaminophen-induced oxidative damage by its antioxidant activity.
These results are in agreement with the study carried out by Gutiérrez-Ruiz et al.[8] who reported that metadoxine preserves GSH reservoir and prevents lipid peroxidation in hepatocytes and hepatic stellate cells exposed to ethanol in culture. In addition, Calabrese et al.[7,9] demonstrated that metadoxine conserves hepatic GSH content in ethanol-treated rats.
Metadoxine, in general, possessed hepatoprotective activities in several in vivo and in vitro studies. Annoni et al.[12] revealed that in the chronic bile duct ligation model for inducing liver damage in rat, metadoxine had demonstrated antifibrotic and even antinecrotic properties. It significantly maintained liver glycogen reservoir and preserved enzyme activities raised several folds in the bile duct-ligated group. Fehér et al.[25] studied hepatoprotective potential of metadoxine on ischemia-reperfusion injury in rats. Coadministration of metadoxine and garlic oil eliminated alcoholic steatosis and inhibited both fat accumulation and CYP2E1 induction in rat liver, with restoration of AMP-activated protein kinase activity.[26]
TNF-α and interleukin-1 are pro-inflammatory cytokines, produced in the liver in response to acetaminophen toxicity.[27] TNF-α induction is considered to be one of the earliest events in hepatic injury, triggering a cascade of other cytokines and activate inflammatory cells which leads to various pathological conditions in the liver include apoptosis, necrosis, and tissue remodeling. Neutralization or suppression of TNF-α could be effective in reducing the extent of hepatic injury induced by acetaminophen which needs further studies to be ascertained.[28]
Conclusion
Taken together, it is concluded that metadoxine as an antioxidant agent could be effective in protecting the liver against acetaminophen-induced toxicity. This effect could be reflected in several markers related to liver function and in many aspects; it is similar to NAC, as a standard antidote.
Financial support and sponsorship
This work was financially supported by Vice-chancellor for Research and Technology, Isfahan University of Medical Sciences with project number 394787.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gu X, Manautou JE. Molecular mechanisms underlying chemical liver injury. Expert Rev Mol Med. 2012;14:e4. doi: 10.1017/S1462399411002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17:575–86. doi: 10.1016/j.cld.2013.07.001. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natanzi E, Reza A, Mahmoudian S, Minaeie B, Sabzevari O. Hepatoprotective activity of phloretin and hydroxychalcones against acetaminophen induced hepatotoxicity in mice. Iran J Pharm Res. 2011;7:89–97. [Google Scholar]
- 4.Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28:499–516. doi: 10.1016/j.ccc.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest. 1983;71:980–91. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonghia L, Leggio L, Ferrulli A, Bertini M, Gasbarrini G, Addolorato G, et al. Acute alcohol intoxication. Eur J Intern Med. 2008;19:561–7. doi: 10.1016/j.ejim.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese V, Ragusa N, Rizza V. Effect of pyrrolidone carboxylate (PCA) and pyridoxine on liver metabolism during chronic ethanol intake in rats. Int J Tissue React. 1995;17:15–20. [PubMed] [Google Scholar]
- 8.Gutiérrez-Ruiz MC, Bucio L, Correa A, Souza V, Hernández E, Gómez-Quiroz LE, et al. Metadoxine prevents damage produced by ethanol and acetaldehyde in hepatocyte and hepatic stellate cells in culture. Pharmacol Res. 2001;44:431–6. doi: 10.1006/phrs.2001.0883. [DOI] [PubMed] [Google Scholar]
- 9.Calabrese V, Calderone A, Ragusa N, Rizza V. Effects of metadoxine on cellular status of glutathione and of enzymatic defence system following acute ethanol intoxication in rats. Drugs Exp Clin Res. 1996;22:17–24. [PubMed] [Google Scholar]
- 10.Felicioli R, Saracchi I, Flagiello AM, Bartoli C. Effects of pyridoxine-pyrrolidon-carboxylate on hepatic and cerebral ATP levels in ethanol treated rats. Int J Clin Pharmacol Ther Toxicol. 1980;18:277–80. [PubMed] [Google Scholar]
- 11.Fehér J, Lengyel G. A new approach to drug therapy in non-alcoholic steatohepatitis (NASH) J Int Med Res. 2003;31:537–51. doi: 10.1177/147323000303100610. [DOI] [PubMed] [Google Scholar]
- 12.Annoni G, Contu L, Tronci MA, Caputo A, Arosio B. Pyridoxol L,2-pyrrolidon-5 carboxylate prevents active fibroplasia in CCl4-treated rats. Pharmacol Res. 1992;25:87–93. doi: 10.1016/s1043-6618(05)80067-2. [DOI] [PubMed] [Google Scholar]
- 13.Laouar A, Klibet F, Bourogaa E, Benamara A, Boumendjel A, Chefrour A, et al. Potential antioxidant properties and hepatoprotective effects of juniperus phoenicea berries against CCl4 induced hepatic damage in rats. Asian Pac J Trop Med. 2017;10:263–9. doi: 10.1016/j.apjtm.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 15.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 16.Heidari R, Niknahad H, Jamshidzadeh A, Azarpira N, Bazyari M, Najibi A. Carbonyl traps as potential protective agents against methimazole-induced liver injury. J Biochem Mol Toxicol. 2015;29:173–81. doi: 10.1002/jbt.21682. [DOI] [PubMed] [Google Scholar]
- 17.Plaa GL, Charbonneau M. Detection and evaluation of chemically induced liver injury. In: Hayess AW, editor. Principles and Methods of Toxicolog. 5th ed. Massachussets: CRC Press; 2008. pp. 1465–507. [Google Scholar]
- 18.Muthulingam M. Antihepatotoxic effects of boerhaavia diffusa L. on antituberculosis drug, rifampicin induced liver injury in rats. J Pharmacol Toxicol. 2008;3:75–83. [Google Scholar]
- 19.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: A guide for clinicians. CMAJ. 2005;172:367–79. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woolbright BL, Jaeschke H. Mechanisms of acetaminophen-induced liver necrosis. In: Hinson JA, Roberts DW, James LP, editors. Adverse Drug Reactions. Kanzas: Springer; 2010. pp. 369–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Addolorato G, Ancona C, Capristo E, Gasbarrini G. Metadoxine in the treatment of acute and chronic alcoholism: A review. Int J Immunopathol Pharmacol. 2003;16:207–14. doi: 10.1177/039463200301600304. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Bachhawat AK. Pyroglutamic acid: Throwing light on a lightly studied metabolite. Curr Sci. 2012;102:288–97. [Google Scholar]
- 23.Muriel P, Deheza R. Fibrosis and glycogen stores depletion induced by prolonged biliary obstruction in the rat are ameliorated by metadoxine. Liver Int. 2003;23:262–8. doi: 10.1034/j.1600-0676.2003.00837.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsu CC, Cheng CH, Hsu CL, Lee WJ, Huang SC, Huang YC, et al. Role of vitamin B6 status on antioxidant defenses, glutathione, and related enzyme activities in mice with homocysteine-induced oxidative stress. Food Nutr Res. 2015;59:25702. doi: 10.3402/fnr.v59.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehér J, Váli L, Blázovics A, Lengyel G. The beneficial effect of metadoxine (pyridoxine-pyrrolidone-carboxylate) in the treatment of fatty liver diseases. Clin Exp Med. 2009;3:65–79. [Google Scholar]
- 26.Ki SH, Choi JH, Kim CW, Kim SG. Combined metadoxine and garlic oil treatment efficaciously abrogates alcoholic steatosis and CYP2E1 induction in rat liver with restoration of AMPK activity. Chem Biol Interact. 2007;169:80–90. doi: 10.1016/j.cbi.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blazka ME, Elwell MR, Holladay SD, Wilson RE, Luster MI. Histopathology of acetaminophen-induced liver changes: Role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol Pathol. 1996;24:181–9. doi: 10.1177/019262339602400206. [DOI] [PubMed] [Google Scholar]


