Abstract
As peri-prosthetic infection is one of the most devastating complications associated with implant placement, we have reasoned that such infection can be largely subverted by development of antibacterial implants. Our previous work demonstrated that covalent coupling of vancomycin to titanium alloy prevented colonization by the gram-positive pathogens, Staphylococcus aureus and Staphylococcus epidermidis. Some orthopaedic devices, including permanent prosthesis anchors, and most dental implants are transcutaneous or transmucosal and can be prone to colonization by Gram-negative pathogens. We report here the successful covalent coupling of the broad-spectrum antibiotic, tetracycline (TET), to titanium surfaces (Ti-TET) to retard Gram-negative colonization. Synthetic progress was followed by changes in water contact angle, while the presence of TET was confirmed by immunofluorescence. Ti-TET actively prevented colonization in the presence of bathing Escherichia coli, both by fluorescence microscopy and direct counting. Finally, the Ti-TET surface supported osteoblastic cell adhesion and proliferation over a 72 hour period. Thus, this new surface offers a powerful means to protect transcutaneous implants from adhesion of Gram-negative pathogens, decreasing the need for replacement of this hardware.
Introduction
Implant failure due to microbial infection is a painful, debilitating, and costly complication that affects both orthopaedic and dental implant recipients7, 25. These implant-associated infections are difficult to eradicate and can require additional surgical procedures to treat the infection and replace contaminated hardware. Currently, the most successful means of treating implant infections, which are now attributed to the presence of biofilm 13, 14 involves irrigation and debridement with subsequent loss of bone stock and, often, removal of the implant23. The implant serves as an ideal surface for bacterial colonization, because bacteria adhere to the surface, produce a biofilm matrix11 and organize into a sessile community of bacteria that express a phenotype that is distinct from their non-adherent planktonic counterparts12. Importantly, antibiotic resistance is common in biofilm-encased bacteria as the matrix may form a physical barrier to antibiotic penetrance as well as to immune surveillance. In addition, as antibiotics target cellular functions associated with rapidly growing planktonic bacteria, the reduced metabolism/growth rates of adherent bacteria blunt antibiotic effectiveness13, 26, so that bacteria contained within this biofilm become insensitive to levels of antibiotics that would be lethal in a non-adherent state28. We have been investigating means of rendering implant surfaces resistant to bacterial colonization and biofilm formation and by inference, preventing implant-associated infections. While Staphylococci can account for up to 60–70% of microbes cultured from infected periprosthetic tissue in orthopaedics, up to 10% of infections are associated with Gram-negative species10, 29. Furthermore, the main pathogens in transcutaneous applications, such as fracture fixation rods, permanent prosthesis anchors and dental implant placement, are Gram-negative microbes9. Considering recent data that suggests that even so-called “aseptic” loosening may have a bacterial component16, effective prevention and treatment for biofilms on implant surfaces may be more important for minimizing implant complications than simple infection rates would suggest.
We previously described the in vitro and in vivo efficacy of the surface-tethered glycopeptide antibiotic, vancomycin (VAN). This surface inhibited colonization and biofilm formation by Staphylococcus aureus 1–4, 17, 20 and Staphylococcus epidermidis 2, however, like the antibiotic itself, this new surface was less effective against Gram-negative strains of bacteria. In this manuscript, we asked if the strategy we have used in the past could be used to tether antibiotics with broader coverage. As a first step, we have synthesized a surface displaying tetracycline, a broad-spectrum antibiotic that is active against Gram-negative and some Gram-positive pathogens. As in our previous work with vancomycin1–4, 17, 20, tetracycline is tethered via membrane-soluble linkers to a silylated self-assembled monolayer on titanium alloy (Ti90Al6V4) (Ti) surfaces. We show that this new, tetracycline-bound Ti surface (Ti-TET) has antibacterial properties against a common Gram-negative pathogen, Escherichia coli, and permits osteoblast adhesion and proliferation.
Methods
Ti-TET synthesis
Titanium alloy (Ti90Al6V4 (Ti)) rods (1mm diameter, 1 cm in length; Goodfellow, Cambridge, UK) or polished Ti alloy discs (6 mm thick, 12 mm dia; kindly provided by Stryker Osteonics, Mahwah NJ) were used for antibacterial or cell culture experiments, respectively. Ti was cleaned by immersion in Alconox overnight with shaking, rinsed in H2O and then extensively rinsed with distilled deionized H2O (diH2O). To create an even hydroxyl layer22, the samples were subjected to 3 successive autoclave cycles while submerged in diH2O. Autoclaved samples were rinsed 3X with diH20, dimethylformamide (DMF), and toluene, dried under vacuum, incubated with 5% (v/v) aminopropyltriethoxysilane/toluene (APTS) under anhydrous conditions, RT, 2 h, washed in N, N-dimethylformamide (DMF) and dried overnight 5. The Fmoc-aminoethoxyethoxyacetate (Fmoc-AEEA) linkers were coupled to the silanized samples using activation by O-(7-azabenzo-triazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU), 30 min, RT. The linker was deprotected with 20% piperidine in DMF, RT 30 min, and this linker addition was repeated three additional times to create a surface displaying four sequential linkers. After the final deprotection step, samples were rinsed with tetrahydrofuran (THF) and diH2O and covered with a 55 mM solution of triethylamine in THF (~13 mL). Primary amines were measured with the ninhydrin assay 20, and a ~5-fold excess (i.e., if 1 × 10−5 moles of amines are available, ~5 × 10−5 moles were used) of succinic anhydride and dimethylaminopyridine (DMAP) were added to the triethylamine (TEA)/THF solution, followed by stirring for 24 h. After washing 3X with THF and diH2O, samples were covered with 5 ml of diH2O containing 7 × 10−5 moles of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC) and 3.6 × 10−5 moles of N-hydroxy succinimide (NHS) with stirring for 30 min; ~ 1 × 10−5 moles of tetracycline-HCl was then added in 4 mL diH2O. 4.3 mL of TEA (~30 mmoles) was then added in a drop-wise fashion and the reaction stirred for 24 h. Tetracycline-tethered Ti surfaces (Ti-TET) were rinsed with diH2O and ethanol and then stored in 75% ethanol (EtOH), 4°C protected from light.
Characterization of the Ti-TET surfaces
The surface charge of the Ti discs was determined using a 10μL droplet of diH2O suspended from the tip of a pre-calibrated pipette. The test surface was raised on a platform so that the droplet contacted and then dispersed on the surface. The platform was then retracted and the image of the settled droplet captured immediately with a digital camera (Nikon D70S) positioned perpendicular to the surface. This image was then enlarged, and the angle between the lines drawn from the base and tangent of the droplet was measured (n=3, significance determined by one-way ANOVA with a Tukey Post-Hoc Test).
Detection of Tetracycline on the Ti surface
Ti-TET rods were incubated with a rabbit anti-tetracycline antibody (US Biological, T2965-05) or a sheep anti-tetracycline antibody (Randox, PAS9747). Antibody dilutions and incubations were performed in 10% fetal bovine serum (FBS) for 2 h, RT, followed by six rinses with phosphate buffered saline (PBS). Rods were then incubated with AlexaFluor 488-anti-rabbit (Life Technologies) or anti-sheep (Life Technologies) IgG in 10% FBS for one hour, and washed 6 times with PBS. Fluorescence was visualized by laser scanning confocal microscopy.
Bacterial cultures and incubation of surfaces
E. coli (ATCC® 25922™) was the primary test species; in addition E. coli bearing a Tet binding protein (Tet(M)) linked to green fluorescent protein (E. coli MC4100/pTGM) was used in several experiments 8. Bacteria from a single colony were grown overnight in Luria-Bertani (LB) broth and diluted to 104 CFU/mL by comparison to a 0.5 McFarland standard (a turbidity standard where an absorbance of 0.1 ~108 bacteria/mL). Prior to inoculation, all surfaces were sterilized by multiple washes in 70% ethanol (EtOH) washes followed by 30 min incubation in 70% EtOH. Surfaces were then washed in sterile PBS, pH 7.4, with a final incubation in sterile PBS for a minimum of 30 min. For each experiment, control (n = 3) and experimental pins (n = 3) prepared as above were used and each experiment repeated at least 3 times. Pins were incubated with 1 mL of E. coli for 4 h at 37°C in a 24-well tissue culture plate, rinsed 6 times with LB broth, and adherent bacteria recovered by sonication in 1 ml 1% Tween 80 for 5 min. Recovered bacteria were diluted, plated on Petri Films (3M), and counted after 24 h at 37°C. Results were analyzed using a paired t-test.
Bacterial adhesion/viability assays were performed on Ti-TET pins that had been incubated with E. coli and washed as above. The bacteria that remained adherent were fluorescently stained with the LIVE/DEAD® BacLight™ Viability Kit (green = viable, red=dead; Invitrogen Life Science, Carlsbad, CA), 30 min room temperature (RT) in the dark, washed 6 times with PBS and visualized by laser scanning confocal microscopy (Olympus Fluoview 300, Olympus America, Center Valley, Pennsylvania).
Statistical analysis
All experiments were performed independently at least 3 times. Data are presented as means ± standard errors. All statistical analyses were performed on normal equally variant data, unless indicated otherwise in the legend, using a student’s t-test or one or two way analysis of variance (ANOVA) with a Tukey multiple comparison procedure. An alpha of 0.05 was considered significant.
Results
Tetracycline can be covalently coupled to Ti surfaces
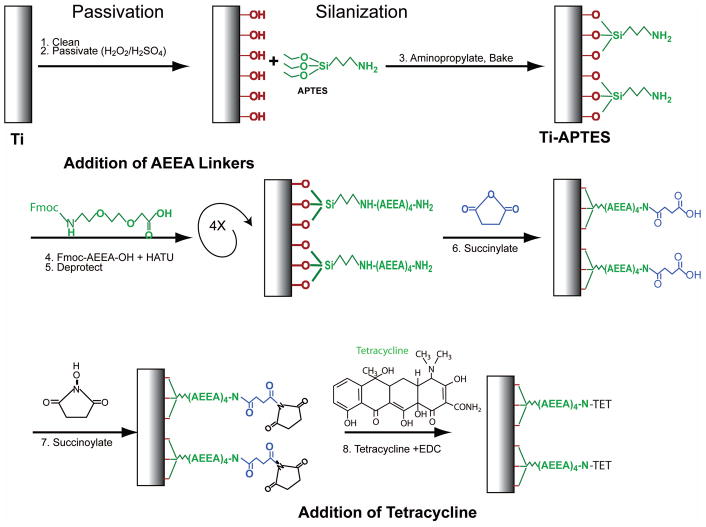
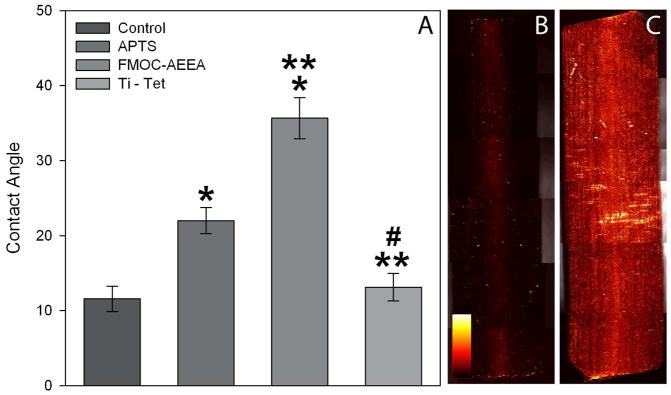
The Ti surface was modified by production of a self-assembled monolayer of aminopropyltriethoxysilane (APTES) on the oxidized metal surface followed by addition of linkers and tetracycline (TET, Fig. 1). We reasoned that each chemical coupling ought to alter the surface tension of the Ti coupon. We therefore recorded surface contact angles for nine samples after aminopropylation, addition of the fourth linker, and coupling of TET (Fig. 2A). Addition of APTES caused a statistically significant increase in contact angle compared to the starting surface (control cleaned Ti). Addition of Fmoc-AEEA linkers caused another statistically significant increase in contact angle. Finally, coupling of TET to the final AEEA linker (Ti-TET) significantly decreased the contact angle to levels similar to the starting Ti surface.
Figure 1.
Attachment scheme of Tetracycline to Titanium Alloy. See materials and methods section for specific explanation of synthesis steps.
Figure 2. Evidence of chemical modification of surfaces.
(A) Contact Angle Analysis: Using the sessile drop technique, surface contact angles of a 10μL droplet of dH2O was measured on control Ti-OH discs (following autoclaving), silanized Ti discs (APTS), silanized Ti discs with attached Fmoc-AEEA linkers (Fmoc-AEEA) and the completed covalently tethered tetracycline (Ti-TET). Data are presented as means and standard error. A one-way analysis of variance test was used and a statistically significant difference (p < 0.05) was found between control and both APTS and Fmoc-AEEA (*), between APTS and both Fmoc-AEEA and Ti-TET (**), as well as between Fmoc-AEEA and Ti-TET (#). Immunohistological Analysis: Ti control rods (B) and those tethered with tetracycline (C) were each examined using an antibody specific against the 4-dimethylamino group of tetracycline. Note the minimal fluorescence on the control rods in comparison to the extensive level of fluorescence on Ti-TET rods. When a similar assay was performed using an antibody specific to the 2-(amino-hydroxy-methylidene) group of tetracycline (the theoretical binding site), minimal fluorescence was observed on both surfaces. Fluorescence is presented as an intensity map (see legend in B).
We next determined if TET could be detected on Ti-TET surfaces. We used two separate antibodies to tetracycline. The first antibody, purchased from US biologicals, exhibited only low levels of fluorescence for both the control and Ti-TET (data not shown), Although the manufacturer could not confirm the epitope, we hypothesized that this antibody might include the portion of the tetracycline molecule targeted for tethering to the surface, the carboxamide group. To test this theory, we identified an antibody produced by Randox that recognizes the 4-dimethylamino site in TET. When stained with this second antibody, the control surface showed little fluorescence (Fig. 2B), while the Ti-TET surfaces showed a bright fluorescence (Fig. 2C). Thus, when exposed to an antibody that recognizes a portion of the tetracycline molecule far from the carboximide group, the presence of tetracycline on the surface is easily demonstrated.
Tethered tetracycline inhibits bacterial colonization
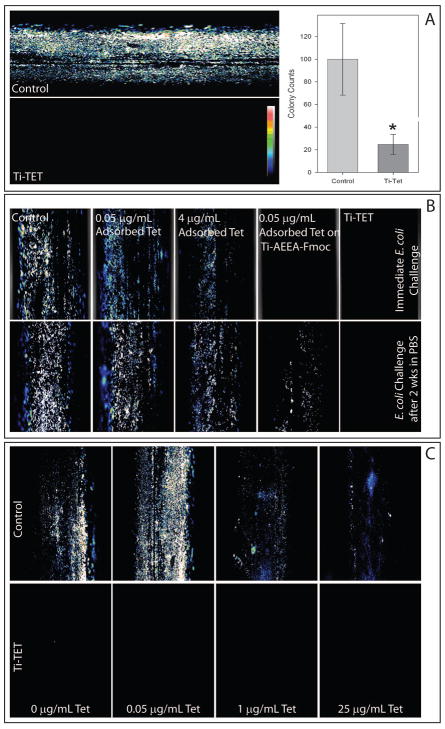
We then asked if the Ti-TET surface could resist bacterial colonization (Fig. 3A). After 24 h incubation with E. coli, control Ti rods were abundantly colonized as evidenced by the high fluorescence. In contrast, little fluorescence was apparent on the Ti-TET rods, indicating minimal levels of colonization. In parallel experiments, adherent E. coli were recovered from Ti-TET surfaces exposed to the bacteria for 24 h And Ti-TET surfaces showed a 4-fold decrease in colony forming units (CFU) in comparison to control surfaces.
Figure 3. Anti-bacterial properties of the Ti-TET surface.
A: Control Ti and Ti-TET rods were incubated with E. coli for 24 h which were then stained using the Live/Dead® BacLight™ Viability Kit and imaged using confocal microscopy. Abundant fluorescence on the control rods indicates the presence of numerous live bacterial colonies. In contrast, the Ti-TET rods exhibit no fluorescence. Fluorescence is presented as an intensity map (see legend on Ti-TET image). Graph demonstrates bacterial colony counts. Control and Ti-TET rods were sonicated in LB broth and that broth serially diluted and plated. E. coli colonies were counted after 16 hours. A paired t-test showed a statistically significant difference between control and Ti-TET (p < 0.05). Data are presented as percentage of control values. Bars are means and standard error. B: TET was adsorbed to control Ti surfaces at concentrations 10 X the theoretical concentration covalently tethered (5 ×10−2 μg/ml) or 4X MIC of TET for E coli (4 μg/ml). In addition, 5 ×10−2 μg/ml TET was also adsorbed to the surface of Ti rods that had undergone synthesis through the addition of Fmoc-AEEA linkers (Ti-AEEA-Fmoc). In the top row, rods were immediately incubated with E coli for 24h following synthesis and the bacteria imaged as described in A. Note that adsorbed tetracycline partially inhibits bacterial colonization, and that 5 ×10−2 μg/ml tetracycline adsorbed to Ti-AEEA-Fmoc completely inhibited bacterial colonization in a similar fashion to Ti-TET. In the bottom panel, rods prepared in parallel with those in the top panel were tested after a two-week incubation in PBS. Adsorbed tetracycline both showed a similar level of colonization as control Ti rods. Rods with 5 ×10−2 μg/ml tetracycline adsorbed to Ti-AEEA-Fmoc showed a reduced level of bacterial colonization. The Ti-TET rods continued to resist bacterial colonization. C: Control Ti rods and Ti-TET rods were exposed to E. coli in combinations with solutions containing 0 μg/mL TET, 0.05 μg/mL TET (10 times the hyopthesized Ti-TET rod concentration), 1μg/mL (the MIC of TET for E. coli), and 25μg/mL (25 times the MIC of TET for E. coli) for 24h following synthesis and the bacteria imaged as described in A. Note that while tremendous increases in TET in the bathing solution does reduce the bacteria colonizing control Ti, at no point does that bathing antibiotic eliminate colonization as the Ti-TET surfaces do at all concentrations.
Tetracycline is covalently tethered, not adsorbed, to titanium
The antibacterial action of the Ti-TET could be due to TET physisorbed onto Ti or covalently bound TET. We compared bacterial colonization of (1) control Ti rods that had been incubated with 0.05 μg/mL TET (based on data from a ninhydrin assay, this amount is ~10 X the amount of TET that could be tethered to a saturated surface), (2) control Ti rods incubated with 4μg/mL TET (4 times the MIC of TET for E. coli) or (3) 0.05 μg/mL TET adsorbed to Ti rods that had been taken through the linker addition steps (Ti-AEEA-Fmoc; the terminal Fmoc was still present) to both control and Ti-TET rods (Fig 3B, top row). After staining with the LIVE/DEAD® BacLight™ kit, confocal microscopy revealed abundant colonization of control rods that had been incubated with E. coli for 24 h, as were rods that had been physisorbed with 0.05 μg/mL TET and with 4 μg/mL TET. Interestingly, no apparent colonization was seen on 0.05 μg/mL TET physisorbed to Ti-AEEA-Fmoc rods. Importantly, Ti-TET rods showed no apparent colonization.
We then tested the antibacterial activity of these constructs after incubation in PBS for 2 weeks (Fig. 3B, bottom row). After a 24 h challenge with E. coli, control Ti rods, control rods with 0.05 μg/mL adsorbed TET and those with 4 μg/mL adsorbed TET were colonized to a similar extent, as evidenced by fluorescent staining. Ti-AEEA-Fmoc samples with adsorbed TET were colonized by E. coli, albeit much less abundantly than the colonization apparent in the control rods or the other adsorbed TET. Importantly, no apparent E. coli colonization was observed on the Ti-TET surface. Even after 8 months in PBS, Ti-TET surfaces continue to resist bacterial colonization by E coli (data not shown).
Ti-TET prevents bacterial colonization more effectively than tetracycline in solution
We next asked how the ability of the Ti-TET surface to prevent surface colonization compared with that of TET in solution (Fig. 3C). Without added TET, control surfaces were abundantly colonized with the Gram negative pathogen E. coli, while Ti-TET surfaces showed little, if any, colonization. When control rods were incubated with E. coli in the presence of 0.05 μg/mL TET in solution, abundant colonization was apparent, although the overall bacterial coverage appeared slightly diminished. At both the MIC (1 μg/mL) and 25 times the MIC (25 μg/mL), E. coli colonization of control surfaces was present, but visibly diminished. At no concentration of TET did the Ti-TET surfaces show any apparent colonization. Thus, solution antibiotics displayed a dose-dependent ability to diminish, but not eliminate bacterial colonization of the control surfaces; in contrast, the Ti-TET surfaces showed no apparent bacterial colonization independent of the bathing concentration of TET.
Ti-TET inhibits colonization by TET-resistant E. coli and partially inhibits colonization by S. aureus
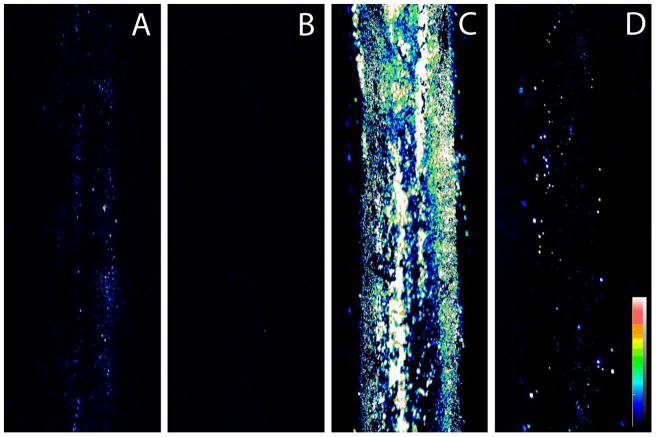
We next determined if tethered TET maintained its antimicrobial specificity. Ti-TET was challenged with the Gram-positive pathogen S. aureus and a strain of the Gram negative E. coli that was resistant to TET. This resistance to TET is dependent on dissociation of TET from the ribosome by the tet(M) protein 15 (E. coli MC4100/pTGM 8). If the TET bound to the Ti was acting via the same mechanisms as solution TET, it would be expected that the TET-resistant E. coli MC4100/pTGM would readily colonize the surface. Also, as TET is a broad spectrum antibiotic, Ti-TET would be expected to retard S. aureus colonization. After a 24 h incubation with E. coli MC4100/pTGM, control rods showed colonies fairly uniformly distributed over the surface of the control rods (Fig. 4A), although the colonization appeared less florid than that observed with E. coli ATCC®25922™. No colonization by E. coli MC4100/pTGM was apparent on the Ti-TET rod (Fig. 4B). When control Ti rods were incubated with S. aureus ATCC®25923™ for 24 h, a large number of colonies were apparent on control Ti rods (Fig. 4C). The Ti-TET surface diminished, but did not totally inhibit S. aureus colonization (Fig. 4D).
Figure 4. Tetracycline Resistant E. coli and S. aureus are prevented from colonizing Ti-TET rods.
Control rods (A) and Ti-TET rods (B) were incubated with TETR E. coli (8 a generous gift of Dr. Lars Hansen) for 4 hrs, the bacteria were then stained using the LIVE/DEAD® BacLight™ Viability Kit and imaged using confocal microscopy. Note that while colony formation of these bacteria is fairly low on control surfaces, it is completely inhibited on the Ti-TET surface. In parallel, S. aureus was incubated with Control (C) and Ti-TET rods for 4 h. The bacteria were then stained using the Live/Dead® BacLight™ Viability Kit and imaged using confocal microscopy. Control rods were abundantly colonized while the Ti-TET rods showed diminished, albeit not absent, S. aureus colonization. Fluorescence is presented as an intensity map, with signal intensity legend shown in D.
Ti-TET does not inhibit osteoblast-like cell adherence
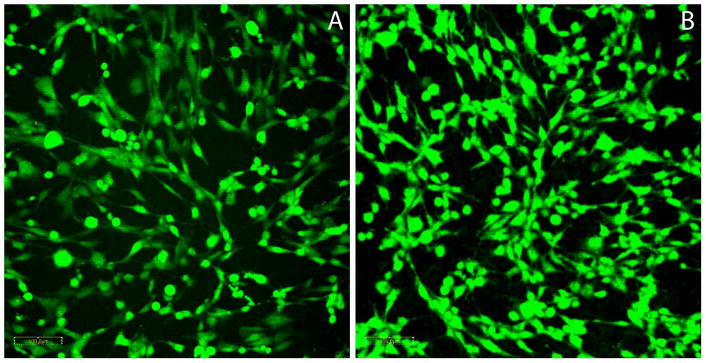
Finally, we asked if the Ti-TET surfaces allowed mammalian cell adhesion and proliferation. Control and Ti-TET discs were seeded with immortalized human fetal osteoblasts (hFOBs), and at 72 h, the cells were stained with Cell Tracker Green, a thiol reactive dye. hFOB cells were observed on both control (Fig. 5A) and Ti-TET (Fig. 5B) discs in similar distribution patterns. No differences in the distribution or proliferation of osteoblasts were observed on the two surfaces.
Figure 5. Viable Cell Growth on the Tethered Antibiotic Surface.
Immortalized human fetal osteoblasts (hFOBs) were growth on control Ti discs (A) and Ti-TET (B) discs. Cells were maintained at 33°C for 3 days, stained with Cell Tracker Green and viewed using laser scanning confocal microscopy. Note the minimal changes in density and morphology in the cells grown on the Ti-TET discs. Magnification is 200X.
Discussion
Bacterial colonization of implanted devices continues to be a cause of implant failure and removal. Whereas Gram positive pathogens such as S. aureus, S. epidermidis and E. faecalis continue to play major roles in deep infection, the Gram negative pathogens become more important for implant failures associated with transcutaneous hardware, such as permanent prosthesis anchors, fracture fixation pins, and dental implants. Our previous work 1–4, 6, 17, 24 has focused on creating antibacterial surfaces for prevention of Gram-positive colonization through tethering of an antibiotic, vancomycin, to the surface of implant materials. The purpose of the current study was to develop a metal surface with a broad-spectrum antibiotic. After forming a SAM with APTES, coupling four linkers that enhance the availability of the TET for insertion into bacterial cells, and finally adding TET, we rendered a titanium surface antimicrobial against Gram-negative bacteria. This type of surface could be useful in preventing bacterial biofilm formation on implants exposed to a broad spectrum of pathogens.
The methodology employed in the current study to tether TET to the Ti surface was based on our previous published syntheses. Thus, a hydroxyl rich layer was produced by hydrothermal aging 22. The advantage of this approach over more conventional acidic techniques is that there is minimal etching of the surface layer With respect to linker length, we reasoned that the 4 rather than 2 coupled AEEA molecules would provide a longer and more flexible arm for insertion of the TET into (and through) the bacterial cell membranes. We hypothesized that TET would be tethered via the carboxamide group following succinoylation of the terminal linker amine. Based on availability of the 4-dimethylamine TET epitope to immunohistochemical detection, it was probable that TET had indeed been coupled to the Ti surface through the carboxamide.
While direct measurement of covalent bond energy between the Ti surface and the APTS was not performed, there were good reasons for considering that strong and stable bonds were formed. First, the Ti-AEEA-Fmoc surface readily supported antibiotic adsorption and release was slow. Moreover, the TET-adsorbed Ti-AEEA-Fmoc surface exhibited considerable biocidal activity when compared to TET adsorbed to a Ti surface. Second, the activity of the Ti-TET remained constant following prolonged exposure to an aqueous environment. Finally, Ti-TET continues to resist bacterial colonization even following eight months of incubation in PBS, suggesting a permanently coupled surface. Thus from a synthesis viewpoint, we can stably attach a bioactive molecule to a Ti surface using reagents and techniques that minimally attack the structure of the metal surface.
Since the use of systemic antibiotics represents the standard of care in response to implant infection, we compared E. coli colonization of Ti-TET rods with colonization of Ti rods, both in the presence and absence of bathing TET. Using two different assays, it was evident that the Ti-TET rod showed little colonization; not unexpectedly, there was robust colonization of the Ti rod. Solution TET concentrations 10- and 25-fold higher than the normal MIC for E. coli reduced the level of bacterial colonization on control rods, but never achieved those low levels observed on the Ti-TET surface. These results emphasize the utility of the Ti-TET surface in preventing bacterial colonization, and by implication, mitigating the first step in establishment of infection.
Ti-TET effectively prevents Gram-negative bacterial colonization. Both by fluorescent staining of bacteria or by colony counting, E. coli colonization is dramatically reduced on the Ti-TET rods. The magnitude of the decrease in colonization on the Ti-TET rod appears much greater by fluorescent detection than by direct counting of recovered adherent E. coli. There are two reasons for this difference: (1) the bacteria adhere less strongly to the modified rods, thus influencing their recovery- inspection of images of the rods plus bacteria support this notion; (2) our previous work showed that tethered antibiotics are, in fact, not highly effective at reducing a population of bacteria in solution 21. Therefore, removal of the bacterium from the TET surface removes the effect of the antibiotic, allowing the bacteria to recover. As the rods exist in an environment rich in bacteria (the surfaces will only affect those bacteria in direct contact with it), they will continue to be populated throughout the duration of the incubation, making the 80% inhibition in adherent bacteria fairly dramatic. That this 80% is sufficient to cause a clinical reduction in infection is suggested by our in vivo experiments with rat 1 and sheep 27.
As TET resistance is not uncommon, we evaluated the efficacy of Ti-TET challenged with resistant organisms. Despite the inherent resistance in these bacteria, we still observed that the Ti-TET surface prevented colonization. We suggest that this could be due to the very high surface concentrations of TET which would overwhelm the resistance mechanisms. Similarly, the colonization of Ti-TET by S. aureus was also inhibited by the Ti-TET surface. These results might suggest that the efficacy of antibiotics is significantly different as a surface layer to that seen in solution.
While the biocompatibility of any modified surface proposed for an implant must necessarily be proven in a multitude of arenas, the first and most important issue relates to the surface interactions. Due to the two-dimensional nature of the implant, and of our experimental model, the ability of our tethered surface to influence events at a distance from the surface is minimal. Even if there were catastrophic failure of the tethered layer on an implant, the effect of that delaminated surface would be minimal as its contents would be rapidly diluted in the bathing fluid surrounding that implant. We have shown this effect on bacteria in this study for TET and in others for vancomycin4. It is for this reason that we have primarily focused on the interaction of these tethered surfaces with adherent eukaryotic cells. The preliminary biocompatibility showed in this study indicates that hFOB cells adherent to the Ti-TET surface exhibited similar shape, similar concentration and a roughly similar metabolic activity. While the Ti-TET surface did not alter eukaryotic cell morphology, clearly clinical use of such surfaces will require far more extensive biocompatibility and toxicological studies.
Taken together, this work demonstrates the potential for covalently tethering a broad-spectrum antibiotic to a material surface which should have activity against important dental pathogens such as Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans 18, 19, as well as environmental pathogens found on the skin. With the ability to tether antibiotics effective against both Gram-positive and Gram-negative organisms, the potential exists to both lower the failure rates associated with many current implant applications, as well as opening the possibility of many other applications.
Acknowledgments
The authors would like to thank Dr. Lars Hansen for the generous gift of the tetracycline-detection strain of E. coli, and Dr. Herbert Jennisen for their contributions and to thank the NIH (grants DE-13319, DE-10875, AR-051303, DE019901, and HD061053) and the Department of Defense (grant DAMD17-03-1-0713) for funding for this study. Results presented are not the statement or policy of the funding agencies.
Reference List
- 1.Antoci V, Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Vancomycin Bound to Ti Rods Reduces Periprosthetic Infection: Preliminary Study. Clinical Orthopaedics & Related Research. 2007;461:88–95. doi: 10.1097/BLO.0b013e318073c2b2. [DOI] [PubMed] [Google Scholar]
- 2.Antoci V, Jr, Adams CS, Parvizi J, Davidson HM, Composto RJ, Freeman TA, Wickstrom E, Zeiger AR, Ducheyne P, Jungkind D, et al. Vancomycin-modified Ti alloy inhibits S. epidermidis biofilm formation: Implications for treatment of periprosthetic infection. Biomaterials. 2008;29:4684–4690. doi: 10.1016/j.biomaterials.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoci V, Jr, Adams CS, Parvizi J, Ducheyne P, Shapiro IM, Hickok NJ. Covalently Attached Vancomycin Provides a Nanoscale Antibacterial Surface. Clinical Orthopaedics & Related Research. 2007;461:81–87. doi: 10.1097/BLO.0b013e3181123a50. [DOI] [PubMed] [Google Scholar]
- 4.Antoci V, Jr, King SB, Jose B, Parvizi J, Zeiger AR, Wickstrom E, Freeman TA, Composto RJ, Ducheyne P, Shapiro IM, et al. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 2007;25:858–866. doi: 10.1002/jor.20348. [DOI] [PubMed] [Google Scholar]
- 5.Antoci V, Jr, King SB, Jose B, Parvizi J, Zeiger AR, Wickstrom E, Freeman TA, Composto RJ, Ducheyne P, Shapiro IM, et al. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 2007;25:858–866. doi: 10.1002/jor.20348. [DOI] [PubMed] [Google Scholar]
- 6.Antoci VJ, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Antibiotics for Local Delivery Systems Cause Skeletal Cell Toxicity In Vitro. Clinical Orthopaedics & Related Research. 2007:462. doi: 10.1097/BLO.0b013e31811ff866. [DOI] [PubMed] [Google Scholar]
- 7.Aslam S, Darouiche R. Antimicrobial therapy for bone and joint infections. Curr Infect Dis Rep. 2009;11:7–13. doi: 10.1007/s11908-009-0002-x. [DOI] [PubMed] [Google Scholar]
- 8.Bahl MI, Hansen LH, Sorensen SJ. Construction of an extended range whole-cell tetracycline biosensor by use of the tet(M) resistance gene. Fems Microbiology Letters. 2005;253:201–205. doi: 10.1016/j.femsle.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 9.Bowen AA, Pascua Garcia MT, Nasimi A. Infections in implantology: from prophylaxis to treatment. Medicina Oral, Patologia Oral y Cirugia Bucal. 2007;12:E323–E330. [PubMed] [Google Scholar]
- 10.Choi JY, Soo Park Y, Cho CH, Seon Park Y, Shin SY, Song YG, Yong D, Lee K, Kim JM. Synergic in-vitro activity of imipenem and sulbactam against Acinetobacter baumannii. Clinical Microbiology and Infection. 2004;10:1098–1101. doi: 10.1111/j.1469-0691.2004.00987.x. [DOI] [PubMed] [Google Scholar]
- 11.Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clinical Orthopaedics & Related Research. 2005:7–11. doi: 10.1097/00003086-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annual Review of Microbiology. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 13.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 14.Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. The Journal Of Clinical Investigation. 2003;112:1466–1477. doi: 10.1172/JCI20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dantley KA, Dannelly HK, Burdett V. Binding Interaction between Tet(M) and the Ribosome: Requirements for Binding. Journal of Bacteriology. 1998;180:4089–4092. doi: 10.1128/jb.180.16.4089-4092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey KE, Riggio MP, Lennon A, Hannah VE, Ramage G, Allan D, Bagg J. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Research and Therapy. 2007;9:1–11. doi: 10.1186/ar2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edupuganti OP, Antoci V, Jr, King SB, Jose B, Adams CS, Parvizi J, Shapiro IM, Zeiger AR, Hickok NJ, Wickstrom E. Covalent bonding of vancomycin to Ti6Al4V alloy pins provides long-term inhibition of Staphylococcus aureus colonization. Bioorganic & Medicinal Chemistry Letters. 2007;17:2692–2696. doi: 10.1016/j.bmcl.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 18.George K, Zafiropoulos GG, Murat Y, Hubertus S, Nisengard RJ. Clinical and microbiological status of osseointegrated implants. Journal of Periodontology. 1994;65:766–770. doi: 10.1902/jop.1994.65.8.766. [DOI] [PubMed] [Google Scholar]
- 19.Hultin M, Gustafsson A, Hallstrom H, Johansson LA, Ekfeldt A, Klinge B. Microbiological findings and host response in patients with peri-implantitis. Clinical Oral Implants Research. 2002;13:349–358. doi: 10.1034/j.1600-0501.2002.130402.x. [DOI] [PubMed] [Google Scholar]
- 20.Jose B, Antoci V, Jr, Zeiger AR, Wickstrom E, Hickok NJ. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chemistry & Biology. 2005;12:1041–1048. doi: 10.1016/j.chembiol.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Ketonis C, Barr S, Shapiro IM, Parvizi J, Adams CS, Hickok NJ. Antibacterial activity of bone allografts: Comparison of a new vancomycin-tethered allograft with allograft loaded with adsorbed vancomycin. Bone. 2011;48:631–638. doi: 10.1016/j.bone.2010.10.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ketonis C, Parvizi J, Adams CS, Shapiro IM, Hickok NJ. Topographic Features Retained after Antibiotic Modification of Ti Alloy Surfaces: Retention of Topography with Attachment of Antibiotics. Clinical Orthopaedics and Related Research«. 2009;467:1678–1687. doi: 10.1007/s11999-009-0828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyad TF, Thornhill T, Estok D. Evaluation and Management of the infected total hip and knee. Orthopaedics. 2008;31:581–588. doi: 10.3928/01477447-20080601-22. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J, Wickstrom E, Zeiger AR, Adams CS, Shapiro IM, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH, Hickok NJ Frank Stinchfield Award. Titanium surface with biologic activity against infection. Clinical Orthopaedics & Related Research. 2004;429:33–38. doi: 10.1097/01.blo.0000150116.65231.45. [DOI] [PubMed] [Google Scholar]
- 25.Shi ZL, Chua PH, Neoh KG, Kang ET, Wang W. Bioactive titanium implant surfaces with bacterial inhibition and osteoblast function enhancement properties. Int J Artif Organs. 2008;31:777–785. doi: 10.1177/039139880803100905. [DOI] [PubMed] [Google Scholar]
- 26.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 27.Stewart S, Barr S, Engiles J, Hickok NJ, Shapiro IM, Richardson DW, Parvizi J, Schaer TP. Vancomycin-Modified Implant Surface Inhibits Biofilm Formation and Supports Bone-Healing in an Infected Osteotomy Model in Sheep: A Proof-of-Concept Study. The Journal of Bone & Joint Surgery. 2012;94:1406–1415. doi: 10.2106/JBJS.K.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subbiahdoss G, Kuijer R, Grijpma DW, van der Mei HC, Busscher HJ. Microbial biofilm growth vs. tissue integration: “The race for the surface” experimentally studied. Acta Biomaterialia. 2009;5:1399–1404. doi: 10.1016/j.actbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerly W, Trampuz A, Ochsner PE. Prosthetic-Joint Infections. New England Journal of Medicine. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]