Abstract
Background and Aims
Previous studies have not addressed a fundamental component of a food addiction disorder: the compulsive relationship between eating and potentially positively reinforcing foods. We aimed to evaluate the association between food consumption and food addiction.
Design, Setting, and Participants
We conducted cross-sectional analyses merging data from the Nurses’ Health Study(n=58,625) and Nurses’ Health Study II (n=65,063), two prospective cohort studies of female nurses in the United States.
Measurements
Diet was assessed in 2006–2007 using a food frequency questionnaire, and food addiction was assessed in 2008–2009 using the Modified Yale Food Addiction Scale.
Findings
The prevalence of food addiction was 5.4%. The odds of food addiction were strongest among nurses consuming 5+ servings/week (compared with <1 serving/month) of hamburgers (MVOR 4.08; 95% CI, 2.66–6.25), French fries (MVOR, 2.37; 95% CI, 1.59–3.51) and pizza(MVOR, 2.49; 95% CI, 1.67–3.69). Consumption of red/processed meat, low/no fat snacks/desserts, and low calorie beverages was positively associated with food addiction, while consumption of refined grains, sugar-sweetened beverages and fruit and vegetables was inversely associated with food addiction.
Conclusions
This epidemiologic study is the largest to examine food consumption and food addiction. Food addiction was positively associated with consumption of many hypothesized positively reinforcing foods that include a combination of carbohydrates and fats such as snacks/desserts, “fast foods” and candy bars. However, it was inversely or not associated with certain sweet foods, refined grains, and sugar-sweetened beverages, which is consistent with literature suggesting that carbohydrates (without other ingredients) are less associated with food addiction. Longitudinal analyses will help untangle the temporal order between food consumption and food addiction, as some relationships in our analyses were difficult to interpret due to the cross-sectional design.
Keywords: Food addiction, feeding behavior, epidemiology, diet, nutrition, eating, fast foods, psychology
Introduction1
In 1956, physician and researcher Theron Randolph introduced the construct of food addiction, theorizing that certain foods had “addictive” potential(1). Since then, the concept of food addiction has existed predominantly in popular culture. Self-help books such as A Substance Called Food (1989)(2) and Food Junkies: The Truth about Food Addiction (2012) (3), and self-help groups such as Food Addicts Anonymous, Overeaters Anonymous, and Food Addicts in Recovery Anonymous, were created for and by individuals who self-identify as food addicts and compulsive eaters. It is hypothesized that highly palatable foods full of fat, salt, and sugar might be linked to addictive-like eating (4, 5), overeating, and obesity.(6) However, food addiction has not been classified by the Diagnostic Statistical Manual of Mental Disorders (DSM) as a mental disorder, or more specifically, as a substance -use disorder, and scientific evidence (7–11)has only recently emerged on the construct of food addiction.
Since 2009, when researchers first validated a food addiction tool to measure food addiction in humans(12), more than fifty peer -reviewed scientific papers have examined the prevalence of food addiction, the reliability and validity of food addiction scales, and potential correlates of the condition in several populations. Recent findings suggest that the prevalence of food addiction in the general population ranges from 5 to 10%(9, 13, 14), that food addiction is distinct from other related disorders such as binge eating disorder(15, 16), and that it is positively associated with body mass index (BMI)(13, 17, 18), binge eating behaviors (16, 19), depression (16, 19), food cravings(20, 21) and impulsivity(20).
This body of research, however, has largely not addressed what is central to a food addiction disorder—the compulsive relationship between eating and specific foods that fail to perform a necessary biological function (22). Currently, it is unclear whether certain types of foods are positively reinforcing, and therefore core to the internal dysfunction of food addiction. Evidence from animal studies support a biological basis for the food addiction construct, demonstrating similarities to models of drug addiction (4, 23–34). In these studies, animals that consume large quantities of sugar and foods high in sugar and/or fat over time exhibit dependency symptoms such as tolerance (a need for markedly increased amounts of palatable food to achieve a desired effect) (35–41)and withdrawal ( chattering teeth, forepaw tremor, and head shakes when deprived of sugar) (35, 42). In addition, one study observed that animals continue to eat palatable foods despite experiencing adverse consequences (e.g., an electric shock) (23). These animal studies suggest that consumption of certain types of foods may be positively reinforcing and associated with addictive eating behaviors.
Recent studies in humans (18, 43–45)examined the relationship between food consumption and food addiction. This research has focused primarily on the relationship between food addiction and nutrients; overall, results have been heterogeneous. Among 652 adults in Canada (18)food addiction was not associated with carbohydrate consumption, yet a positive association was observed with protein and fat intake. When limited to 116 overweight/obese individuals (43), food addicted compared with non-food addicted participants consumed statistically significantly more grams per day of sugar and saturated, trans, and monounsaturated fats than food addiction non-obese subjects. A study (44) in Australia found that among 462 adults, food addiction symptom scores were positively associated with percent of energy from energy-dense, nutrient-poor foods (e.g., candy, baked sweet products); however, sugar and carbohydrate intake were not associated with food addiction diagnosis. In another study among 70 children in the United States, food addiction symptom count was only positively associated with dinner calorie intake.(45)Using the two largest epidemiologic studies to date, we extend this preliminary research by examining the association between consumption of hypothesized potentially reinforcing foods and food addiction in over 120,000 women.
Methods
Study Populations
The Nurses’ Health Study (NHS)and Nurses’ Health Study II ( NHSII) are prospective cohort studies conducted in the United States. Beginning in 1976, 121,700 female registered nurses, married and 30–55 years old were enrolled into NHS(46). NHSII began in 1989, enrolling 116,430 nurses who were 25–42 years old at baseline. Every two years participants receive questionnaires about their demographics, medical history and lifestyle, and response rates have been at least 90%. The Human Research Committees of Brigham and Women’s Hospital and Massachusetts Eye and Ear Infirmary approved the studies(46). The current analyses used diet data collected in 2006 (NHS) and 2007 (NHSII) and food addiction data collected in 2008 (NHS) and 2009 (NHSII). As food addiction was only measured once in each cohort, our analyses were cross-sectional.
Ascertainment of Food and Beverage Items and Food Groups
The NHS first collected dietary information in the 1980, 1984, and 1986 follow-up questionnaires, and every four years since. The NHSII first collected diet information in 1991, and every four years since(47). In our cross-sectional analyses, we examined average consumption of food sand beverages from the 2006 NHS and 2007 NHSII 131-item food frequency questionnaire(FFQ). The FFQ is a primary tool for measuring nutrient and food intake in epidemiologic studies and has been shown to have good reliability and validity(48, 49).
For each food and beverage item, participants selected from nine response options ranging from never or <1/month to 6+ servings/day for frequency of intake. Of the 131 items, we identified 39 potentially positively reinforcing food items from published animal(23, 50) and human research (12, 34, 51, 52)on food addiction and binge eating, discussions with food addiction investigators, and published reports of individuals identifying as food addicts and compulsive overeaters (2, 53). These foods included, but are not limited to, hamburgers (12, 51, 52), French fries(12, 52), milk chocolate(12, 23, 50–52), pasta (12, 51), and sugar-sweetened beverages(12, 51, 52). We also examined consumption of several fruits and vegetables as a contrast to positively reinforcing foods(51, 52). We created nine food groups (red/processed meats, snacks, sweets and desserts, refined grains, fruits and vegetables, no/low fat snacks and sweets, no/low fat dairy, low calorie beverages, and sugar-sweetened beverages) by merging consumption of over 85 individual food items into groups based on previous epidemiologic studies that have used these food groups to study nutrition and disease(54, 55).
Ascertainment of Food Addiction
We assessed food addiction using a modified version of the Yale Food Addiction Scale (mYFAS)(Supplemental Table 1) in the NHS2008 and NHSII2009 questionnaires. The mYFAS has nine of 25 items in the original Yale Food Addiction Scale. There is one item for each of the seven diagnostic symptoms for eating-related substance dependence as defined by the DSM-IV, and two items assess impairment and distress (Supplemental Table 2). A participant meets criteria for food addiction if she has three or more of seven substance-use dependence symptoms and experiences impairment or distress in the past year (13). Previous research has shown that food addiction diagnosis as measured by the mYFAS has marginal to good internal reliability in college students (α=0.75) (13)and in a community-based sample (α=0.63 to 0.84) (56), and substantial test-retest reliability (K=0.73, 95% CI, 0.48–0.88) over a two-week period in a community-based sample (56). Although the long-term stability of the mYFAS has not been examined, a recent study (57)found that food addiction diagnosis measured by the Yale Food Addiction Scale had moderate stability (K=0.50, 95% CI, 0.23–0.77) over an 18-month period. In the current study, 1,461 (2.5%) of NHS and 5,194 (8.0%) of NHSII met the criteria for food addiction.
Exclusions
After excluding women who did not fill out questionnaires on diet or food addiction in 2006 and 2008 or 2007 and 2009, we excluded women who were missing entire sections of the FFQ (n=654), did not provide sufficient food addiction information (n=3,634), were missing calorie intake (n=3,945) or smoking (n=585), or had extreme values for other covariates (n=23). Nurses who responded to the food addiction items on the questionnaire were slightly older (~ 4 years) than those who did not respond. However, we did not observe differences by calorie intake, alcohol and smoking habits or depression scores for this same comparison. After these exclusions, our final sample included 123,688 women, which was over 93% of the available cohort.
Statistical Analysis
We examined frequency distributions for categorical variables and means for continuous variables to compare differences in possible food addiction risk factors (e.g., depression) stratified by food addiction status and cohort. We estimated odds ratios (OR) and 95% confidence intervals (CI)using age -and multivariable -adjusted logistic regression to determine whether food and beverage item intake based on categories of servings (<1/month, 1–3/month, 1/week, 2–4/week and ≥5–6/week) and food groups based on servings/day were associated with food addiction status. Our first multivariable models were adjusted for age, alcohol consumption, current smoking, depression, and total energy intake. Our second multivariable models were additionally adjusted for all food items or food groups in the corresponding table. Because we hypothesized that BMI changes could occur as a consequence of reinforcing food intake and simultaneously a consequence of food addiction, BMI could act as a collider and conditioning on it could introduce collider bias(58). For example, if bacon intake increases BMI and food addiction also increases BMI, then conditioning on BMI could induce a false negative association between bacon and food addiction. Thus our main analyses were not adjusted for BMI.
To test for a linear trend in the odds of food addiction with increasing consumption of each food item, we included a continuous variable with values corresponding to each category of consumption (0–4). The coefficient of the continuous term was evaluated using the Wald test; we considered P values <0.05 as statistically significant. We also examined interaction between food group consumption and depression, alcohol consumption, current smoking status, BMI, and diabetes. To test for multiplicative interaction, we evaluated the coefficient for the cross-product term representing the main effect for food group consumption (servings/day, continuous) and the stratification factor(binary )using the Wald test. We additionally calculated ORs for the association between food group and food addiction within each stratification factor.
As we assumed that the biological effects of food intake on food addiction were similar between cohorts, we merged NHS and NHSII for our main analyses and examined the heterogeneity of associations using the Q statistic( a standard statistical test used for harmonizing data to determine the presence of between-study heterogeneity and whether combining data is justified)(59). Overall we observed little heterogeneity by cohort; we present results for food groups stratified by cohort in Supplemental Table 3.
We performed statistical analyses using SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
The majority of women in both cohorts were married, Caucasian, non-smokers, and non-heavy drinkers (Table 1). Approximately 63% to 66% of obese nurses (BMI kg/m2≥30) met criteria for food addiction. Women with depression or who consumed a higher number of calories were more likely to be classified with food addiction, whereas women who were older, smoked or drank alcohol were less likely.
Table 1.
Characteristics of Nurses in the Nurses’ Health Studies in 2008 and 2009 (n = 123,688)
| NHS (n = 58,625) | NHSII (n = 65,063) | ||||
|---|---|---|---|---|---|
|
| |||||
| Food Addiction, No. (%) | |||||
|
| |||||
| Yes | No | Yes | No | ||
| Food Addiction | No | 57,164 (97.5) | 59,869 (92.0) | ||
| Yes | 1,461 (2.5) | 5,194 (8.0) | |||
|
| |||||
| Age (years) | 45–59 | 4,266 (82.1) | 48,263 (80.6) | ||
| 60–74 | 1,133 (77.5) | 32,307 (56.5) | 928 (17.9) | 11,606 (19.4) | |
| 75–87 | 328 (22.5) | 24,857 (43.5) | |||
|
| |||||
| Martial Statusa | Never married | 397 (7.8) | 2,898 (5.0) | ||
| Separated/divorced | 172 (12.0) | 4,133 (7.3) | 811 (16.0) | 7,270 (12.4) | |
| Widowed | 310 (21.7) | 16,440 (29.0) | 146 (2.9) | 1,725 (3.0) | |
| Married | 950 (66.3) | 36,140 (63.7) | 3,710 (73.3) | 46,678 (79.7) | |
|
| |||||
| Race/Ethnicity | Other/Unknown | 77 (5.3) | 3,397 (5.9) | 158 (3.0) | 1,793 (3.0) |
| African American | 9 (0.6) | 552 (1.0) | 38 (0.7) | 659 (1.10) | |
| Hispanic | 6 (0.4) | 330 (0.6) | 60 (1.2) | 779 (1.3) | |
| Asian | 2 (0.1) | 399 (0.7) | 23 (0.4) | 827 (1.4) | |
| Caucasian | 1,367 (93.6) | 52,486 (91.8) | 4,915 (94.6) | 55,811 (93.2) | |
|
| |||||
| Current Smoking (cig/day) | 0 | 1,415 (96.9) | 54,215 (94.8) | 4,978 (95.8) | 56,264 (94.0) |
| 1–14 | 30 (2.1) | 1,724 (3.0) | 125 (2.4) | 2,022 (3.4) | |
| 15+ | 16 (1.1) | 1,225 ( 2.1) | 91 (1.8) | 1,583 (2.6) | |
|
| |||||
| Alcohol Consumption (g/day) | 0 | 752 (51.5) | 24,975 (43.7) | 2,145 (41.3) | 19,816 (33.1) |
| > 0–15 | 584 (40.0) | 24,041 (42.1) | 2,605 (50.2) | 32,007 (53.5) | |
| > 15 | 125 (8.6) | 8,148 (14.3) | 444 (8.6) | 8,046 (13.4) | |
|
| |||||
| Depression (physician diagnosis) | No | 1,173 (80.3) | 53,388 (93.4) | 3,273 (63.0) | 50,247 (83.9) |
| Yes | 288 (19.7) | 3,776 (6.6) | 1,921 (37.0) | 9,622 (16.1) | |
|
| |||||
| Body Mass Indexa (kg/m2) | <=24.9 | 136 (9.4) | 26,187 (46.4) | 484 (9.4) | 26,228 (44.2) |
| 25–29.9 | 400 (27.7) | 18,859 (33.4) | 1,283 (24.9) | 18,152 (30.6) | |
| 30+ | 906 (62.8) | 11,382 (20.2) | 3,382 (65.7) | 14,917 (25.2) | |
|
| |||||
| Mean Calories (kcal/day)b (SD) | 1,773.1 (579.9) | 1,648.7 (532.3) | 1,882.3 (596.5) | 1,802.8 (554.1) | |
Abbreviations: cig = cigarettes; g = grams; kcal = kilocalories; kg = kilograms; m = meters; NHS = Nurses’ Health Study; SD = standard deviation
Frequencies do not add up to column totals due to missing data; 1,908 missing marital status; 1,372 missing body mass index
This information was collected from 2006 and 2007 food frequency questionnaires
In Table 2, we examined associations (reported as ORs (95% CIs))between consumption of potentially positively reinforcing foods and beverages and food addiction. After controlling for confounders( multivariable model a), we observed the highest odds of food addiction for nurses who reported foods typically consumed as fast foods 5+/week compared with <1/month: hamburgers 4.08 (2.66–6.25), French fries2.37 (1.59–3.51), and pizza2.49 (1.67–3.69); all P’s for trend <0.0001. Bacon, beef as a main dish, popcorn, lean hamburgers, potato/corn chips, popcorn, pretzels, candy bars, candy without chocolate, milk chocolate, white bread, and butter consumption was also positively and significantly associated with food addiction (ORs ranged from 1.13 for pretzels to 1.95 for candy bars). Crackers, cake, store-bought cookies, doughnuts, ice cream, pie, sweet rolls/coffee cakes, pasta, and white potato intake was not associated with food addiction. We observed inverse associations between dark chocolate, homemade cookies, white rice and full fat cheese consumption and food addiction. In addition, when we compared nurses consuming sugar-sweetened beverages 5+/week to <1/month, we observed a strong inverse association with food addiction (0.56 (0.52–0.61)); in contrast, we found a strong positive association between low calorie beverage consumption and food addiction (2.38 (2.24–2.54)). After additionally controlling for all food items in the table, the positive significant associations for hamburgers, candy bars, milk chocolate, butter, pizza, and low calorie beverages remained. Inverse associations were attenuated, but remained significant.
Table 2.
Odds Ratios and 95% Confidence Intervals for Consumption of Potentially Positively Reinforcing Foods and Beverages (2006 and 2007) and Food Addiction (2008 and 2009) in NHS and NHS II (n = 123,688)
| OR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Number of Servings | |||||||
|
| |||||||
| Food Item | <1/month | 1–3/month | 1/week | 2–4/week | ≥5–6/week | Ptrend* | |
| Processed and Red Meats | |||||||
| Bacon | No. of cases | 2938 | 2340 | 993 | 318 | 66 | |
| Age-adjusted | 1.00 | 1.01 (0.96–1.07) | 1.10 (1.02–1.18) | 1.19 (1.06–1.35) | 1.62 (1.25–2.10) | <0.001 | |
| Multivariablea | 1.00 | 1.01 (0.95–1.07) | 1.08 (1.00–1.16) | 1.13 (1.00–1.28) | 1.56 (1.20–2.03) | 0.001 | |
| Multivariableb | 1.00 | 0.95 (0.89–1.01) | 0.96 (0.88–1.04) | 0.93 (0.82–1.06) | 1.12 (0.86–1.47) | 0.16 | |
|
| |||||||
| Hamburgers | No. of cases | 3461 | 1688 | 1164 | 310 | 32 | |
| Age-adjusted | 1.00 | 1.19 (1.12–1.27) | 1.59 (1.49–1.71) | 2.27 (2.01–2.57) | 5.76 (3.83–8.67) | <0.001 | |
| Multivariablea | 1.00 | 1.15 (1.08–1.23) | 1.46 (1.36–1.57) | 1.83 (1.61–2.08) | 4.08 (2.66–6.25) | <0.001 | |
| Multivariableb | 1.00 | 1.13 (1.06–1.21) | 1.33 (1.23–1.44) | 1.51 (1.32–1.74) | 3.21 (2.02–5.09) | <0.001 | |
|
| |||||||
| Hamburgers (lean) | No. of cases | 1843 | 2140 | 2045 | 599 | 28 | |
| Age-adjusted | 1.00 | 0.94 (0.89–1.01) | 1.18 (1.10–1.25) | 1.58 (1.44–1.74) | 2.13 (1.43–3.19) | <0.001 | |
| Multivariablea | 1.00 | 0.96 (0.90–1.02) | 1.12 (1.04–1.19) | 1.34 (1.21–1.48) | 1.62 (1.07–2.44) | <0.001 | |
| Multivariableb | 1.00 | 1.02 (0.95–1.09) | 1.12 (1.04–1.20) | 1.27 (1.15–1.42) | 1.10 (0.71–1.70) | <0.001 | |
|
| |||||||
| Beef as a Main Dish | No. of cases | 1267 | 2030 | 2299 | 1001 | 58 | |
| Age-adjusted | 1.00 | 0.96 (0.89–1.03) | 1.01 (0.94–1.08) | 1.22 (1.12–1.33) | 1.77 (1.34–2.35) | <0.001 | |
| Multivariablea | 1.00 | 0.97 (0.90–1.04) | 1.01 (0.94–1.09) | 1.15 (1.05–1.26) | 1.54 (1.16–2.06) | 0.001 | |
| Multivariableb | 1.00 | 0.94 (0.87–1.01) | 0.94 (0.87–1.02) | 1.02 (0.92–1.12) | 1.14 (0.84–1.54) | 0.85 | |
|
| |||||||
| Snacks | |||||||
|
| |||||||
| Crackers | No. of cases | 1494 | 1734 | 1450 | 1289 | 688 | |
| Age-adjusted | 1.00 | 0.89 (0.83–0.95) | 0.94 (0.87–1.02) | 0.94 (0.87–1.01) | 1.16 (1.06–1.27) | 0.04 | |
| Multivariablea | 1.00 | 0.89 (0.83–0.96) | 0.94 (0.87–1.01) | 0.90 (0.83–0.98) | 1.06 (0.96–1.17) | 0.98 | |
| Multivariableb | 1.00 | 0.89 (0.82–0.96) | 0.92 (0.85–1.00) | 0.88 (0.81–0.96) | 1.01 (0.91–1.12) | 0.24 | |
|
| |||||||
| French fries | No. of cases | 2882 | 2332 | 1076 | 331 | 34 | |
| Age-adjusted | 1.00 | 1.11 (1.04–1.17) | 1.37 (1.28–1.48) | 1.91 (1.69–2.16) | 3.41 (2.33–4.98) | <0.001 | |
| Multivariablea | 1.00 | 1.07 (1.01–1.13) | 1.25 (1.16–1.35) | 1.53 (1.34–1.74) | 2.37 (1.59–3.51) | <0.001 | |
| Multivariableb | 1.00 | 0.97 (0.91–1.04) | 1.01 (0.92–1.10) | 1.04 (0.90–1.20) | 1.14 (0.74–1.74) | 0.77 | |
|
| |||||||
| Popcorn | No. of cases | 4202 | 1512 | 644 | 233 | 64 | |
| Age-adjusted | 1.00 | 1.05 (0.99–1.11) | 1.28 (1.17–1.40) | 1.39 (1.21–1.59) | 1.78 (1.37–2.31) | <0.001 | |
| Multivariablea | 1.00 | 1.00 (0.94–1.06) | 1.19 (1.09–1.30) | 1.21 (1.05–1.39) | 1.45 (1.11–1.89) | <0.001 | |
| Multivariableb | 1.00 | 0.99 (0.93–1.06) | 1.14 (1.04–1.25) | 1.15 (1.00–1.33) | 1.28 (0.97–1.68) | 0.003 | |
|
| |||||||
| Potato/corn chips | No. of cases | 1876 | 2158 | 1443 | 948 | 230 | |
| Age-adjusted | 1.00 | 1.02 (0.95–1.08) | 1.09 (1.02–1.17) | 1.22 (1.12–1.33) | 1.53 (1.32–1.77) | <0.001 | |
| Multivariablea | 1.00 | 1.02 (0.95–1.09) | 1.08 (1.00–1.16) | 1.14 (1.05–1.25) | 1.38 (1.19–1.61) | <0.001 | |
| Multivariableb | 1.00 | 0.99 (0.93–1.07) | 0.98 (0.90–1.07) | 1.00 (0.91–1.10) | 1.13 (0.97–1.33) | 0.77 | |
|
| |||||||
| Pretzels | 2851 | 1940 | 917 | 639 | 308 | ||
| Age-adjusted | 1.00 | 1.02 (0.96–1.08) | 1.07 (0.99–1.15) | 1.11 (1.02–1.21) | 1.19 (1.06–1.35) | <0.001 | |
| Multivariablea | 1.00 | 1.02 (0.96–1.08) | 1.08 (0.99–1.16) | 1.09 (1.00–1.20) | 1.13 (1.00–1.28) | 0.005 | |
| Multivariableb | 1.00 | 0.99 (0.93–1.05) | 1.00 (0.92–1.09) | 1.03 (0.93–1.13) | 1.02 (0.90–1.17) | 0.85 | |
|
| |||||||
| Sweets and Desserts | |||||||
|
| |||||||
| Cake | No. of cases | 2965 | 2821 | 686 | 165 | 18 | |
| Age-adjusted | 1.00 | 1.12 (1.06–1.18) | 1.37 (1.26–1.49) | 1.57 (1.33–1.85) | 1.37 (0.85–2.22) | <0.001 | |
| Multivariablea | 1.00 | 1.04 (0.98–1.10) | 1.19 (1.09–1.30) | 1.24 (1.05–1.47) | 1.12 (0.69–1.82) | 0.001 | |
| Multivariableb | 1.00 | 1.10 (1.03–1.17) | 1.21 (1.08–1.35) | 1.29 (1.07–1.56) | 0.99 (0.59–1.67) | <0.001 | |
|
| |||||||
| Candy bar | No. of cases | 3647 | 1957 | 725 | 258 | 68 | |
| Age-adjusted | 1.00 | 1.32 (1.25–1.40) | 1.74 (1.60–1.90) | 2.08 (1.82–2.37) | 2.32 (1.80–3.00) | <0.001 | |
| Multivariablea | 1.00 | 1.24 (1.17–1.32) | 1.55 (1.43–1.70) | 1.74 (1.51–2.00) | 1.95 (1.50–2.54) | <0.001 | |
| Multivariableb | 1.00 | 1.16 (1.09–1.24) | 1.33 (1.20–1.46) | 1.41 (1.22–1.64) | 1.61 (1.22–2.14) | <0.001 | |
|
| |||||||
| Candy without chocolate | No. of cases | 3588 | 1819 | 678 | 368 | 202 | |
| Age-adjusted | 1.00 | 1.21 (1.14–1.28) | 1.35 (1.24–1.47) | 1.44 (1.29–1.61) | 1.50 (1.29–1.74) | <0.001 | |
| Multivariablea | 1.00 | 1.15 (1.08–1.22) | 1.24 (1.14–1.35) | 1.27 (1.14–1.43) | 1.28 (1.10–1.49) | <0.001 | |
| Multivariableb | 1.00 | 1.10 (1.03–1.17) | 1.08 (0.98–1.18) | 1.12 (1.00–1.27) | 1.12 (0.96–1.31) | 0.006 | |
|
| |||||||
| Dark chocolate | No. of cases | 3501 | 1692 | 803 | 422 | 237 | |
| Age-adjusted | 1.00 | 1.01 (0.95–1.08) | 1.01 (0.94–1.10) | 0.98 (0.88–1.09) | 0.90 (0.79–1.03) | 0.37 | |
| Multivariablea | 1.00 | 0.98 (0.92–1.04) | 0.95 (0.88–1.03) | 0.88 (0.79–0.98) | 0.77 (0.67–0.89) | <0.001 | |
| Multivariableb | 1.00 | 1.00 (0.94–1.07) | 0.91 (0.83–0.99) | 0.88 (0.79–0.99) | 0.80 (0.69–0.93) | <0.001 | |
|
| |||||||
| Milk chocolate | No. of cases | 2135 | 2164 | 1265 | 736 | 355 | |
| Age-adjusted | 1.00 | 1.21 (1.13–1.28) | 1.49 (1.39–1.60) | 1.75 (1.60–1.91) | 2.05 (1.82–2.31) | <0.001 | |
| Multivariablea | 1.00 | 1.16 (1.09–1.24) | 1.37 (1.27–1.47) | 1.54 (1.40–1.68) | 1.69 (1.49–1.91) | <0.001 | |
| Multivariableb | 1.00 | 1.12 (1.05–1.20) | 1.24 (1.14–1.35) | 1.34 (1.21–1.48) | 1.43 (1.25–1.63) | <0.001 | |
|
| |||||||
| Cookies (homemade) | No. of cases | 3143 | 2192 | 817 | 362 | 141 | |
| Age-adjusted | 1.00 | 0.82 (0.78–0.87) | 0.91 (0.84–0.99) | 0.83 (0.74–0.93) | 0.89 (0.75–1.06) | <0.001 | |
| Multivariablea | 1.00 | 0.79 (0.75–0.84) | 0.83 (0.76–0.90) | 0.74 (0.66–0.83) | 0.75 (0.63–0.90) | <0.001 | |
| Multivariableb | 1.00 | 0.81 (0.76–0.86) | 0.77 (0.71–0.85) | 0.76 (0.67–0.85) | 0.79 (0.66–0.95) | <0.001 | |
|
| |||||||
| Cookies (store) | No. of cases | 2916 | 1819 | 970 | 618 | 332 | |
| Age-adjusted | 1.00 | 1.07 (1.01–1.14) | 1.36 (1.26–1.47) | 1.15 (1.05–1.26) | 1.19 (1.06–1.34) | <0.001 | |
| Multivariablea | 1.00 | 1.02 (0.96–1.09) | 1.23 (1.14–1.33) | 1.02 (0.93–1.11) | 1.00 (0.89–1.13) | 0.06 | |
| Multivariableb | 1.00 | 1.00 (0.93–1.06) | 1.13 (1.03–1.23) | 0.91 (0.82–1.00) | 0.90 (0.79–1.02) | 0.23 | |
|
| |||||||
| Doughnuts | No. of cases | 4335 | 1765 | 425 | 108 | 22 | |
| Age-adjusted | 1.00 | 1.24 (1.17–1.31) | 1.51 (1.36–1.67) | 1.65 (1.35–2.01) | 1.96 (1.26–3.05) | <0.001 | |
| Multivariablea | 1.00 | 1.15 (1.08–1.22) | 1.31 (1.18–1.46) | 1.32 (1.07–1.62) | 1.49 (0.95–2.34) | <0.001 | |
| Multivariableb | 1.00 | 1.06 (1.00–1.14) | 1.09 (0.97–1.23) | 1.01 (0.81–1.26) | 1.01 (0.63–1.62) | 0.08 | |
|
| |||||||
| Ice cream | No. of cases | 2854 | 2280 | 794 | 596 | 131 | |
| Age-adjusted | 1.00 | 1.00 (0.94–1.05) | 1.05 (0.96–1.13) | 1.24 (1.13–1.36) | 1.28 (1.07–1.54) | <0.001 | |
| Multivariablea | 1.00 | 0.95 (0.90–1.01) | 0.92 (0.84–1.00) | 1.01 (0.92–1.11) | 0.94 (0.78–1.13) | 0.26 | |
| Multivariableb | 1.00 | 0.94 (0.88–1.00) | 0.88 (0.80–0.96) | 0.95 (0.86–1.05) | 0.86 (0.71–1.05) | 0.004 | |
|
| |||||||
| Pie | No. of cases | 3893 | 2289 | 403 | 61 | 9 | |
| Age-adjusted | 1.00 | 0.99 (0.94–1.04) | 1.24 (1.12–1.38) | 1.38 (1.06–1.79) | 2.73 (1.35–5.51) | 0.002 | |
| Multivariablea | 1.00 | 0.91 (0.86–0.96) | 1.04 (0.93–1.16) | 1.02 (0.78–1.33) | 1.94 (0.94–4.01) | 0.20 | |
| Multivariableb | 1.00 | 0.90 (0.84–0.96) | 0.94 (0.82–1.07) | 0.94 (0.71–1.26) | 1.91 (0.86–4.23) | 0.006 | |
|
| |||||||
| Sweet roll/coffee cake (homemade) | No. of cases | 5263 | 1124 | 224 | 37 | 7 | |
| Age-adjusted | 1.00 | 0.92 (0.87–0.99) | 0.97 (0.84–1.11) | 0.94 (0.67–1.31) | 1.16 (0.54–2.51) | 0.08 | |
| Multivariablea | 1.00 | 0.87 (0.81–0.93) | 0.85 (0.74–0.98) | 0.79 (0.57–1.11) | 0.94 (0.43–2.05) | <0.001 | |
| Multivariableb | 1.00 | 0.92 (0.85–0.99) | 0.78 (0.67–0.92) | 0.74 (0.52–1.04) | 0.96 (0.44–2.12) | <0.001 | |
|
| |||||||
| Sweet roll/coffee cake (store) | No. of cases | 4158 | 1784 | 534 | 156 | 23 | |
| Age-adjusted | 1.00 | 1.15 (1.09–1.22) | 1.48 (1.34–1.62) | 1.82 (1.54–2.15) | 1.43 (0.93–2.19) | <0.001 | |
| Multivariablea | 1.00 | 1.08 (1.02–1.14) | 1.28 (1.16–1.41) | 1.45 (1.22–1.72) | 1.11 (0.72–1.71) | <0.001 | |
| Multivariableb | 1.00 | 1.08 (1.01–1.15) | 1.24 (1.11–1.39) | 1.37 (1.14–1.65) | 0.97 (0.62–1.53) | <0.001 | |
|
| |||||||
| Refined Grains | |||||||
|
| |||||||
| Pasta | No. of cases | 547 | 1857 | 2665 | 1441 | 145 | |
| Age-adjusted | 1.00 | 0.82 (0.74–0.90) | 0.77 (0.70–0.85) | 0.79 (0.71–0.87) | 1.10 (0.90–1.33) | 0.01 | |
| Multivariablea | 1.00 | 0.82 (0.74–0.91) | 0.74 (0.67–0.82) | 0.69 (0.62–0.77) | 0.88 (0.72–1.08) | <0.001 | |
| Multivariableb | 1.00 | 0.86 (0.77–0.95) | 0.80 (0.71–0.89) | 0.75 (0.67–0.85) | 0.92 (0.74–1.13) | <0.001 | |
|
| |||||||
| White bread | No. of cases | 2885 | 1320 | 754 | 971 | 725 | |
| Age-adjusted | 1.00 | 1.11 (1.04–1.19) | 1.05 (0.96–1.14) | 1.10 (1.02–1.18) | 1.28 (1.18–1.39) | <0.001 | |
| Multivariablea | 1.00 | 1.11 (1.03–1.18) | 1.03 (0.94–1.12) | 1.05 (0.97–1.13) | 1.13 (1.03–1.23) | 0.02 | |
| Multivariableb | 1.00 | 1.12 (1.04–1.20) | 1.02 (0.93–1.11) | 1.04 (0.96–1.13) | 1.06 (0.96–1.16) | 0.37 | |
|
| |||||||
| White Potato | No. of cases | 730 | 1965 | 2177 | 1557 | 226 | |
| Age-adjusted | 1.00 | 0.96 (0.87–1.04) | 0.95 (0.87–1.03) | 0.93 (0.85–1.01) | 1.16 (1.00–1.36) | 0.71 | |
| Multivariablea | 1.00 | 0.93 (0.85–1.01) | 0.88 (0.80–0.96) | 0.79 (0.72–0.87) | 0.91 (0.77–1.06) | <0.001 | |
| Multivariableb | 1.00 | 0.99 (0.90–1.09) | 0.92 (0.84–1.02) | 0.82 (0.74–0.91) | 0.89 (0.75–1.05) | <0.001 | |
|
| |||||||
| White rice | No. of cases | 2376 | 2721 | 1206 | 327 | 25 | |
| Age-adjusted | 1.00 | 0.91 (0.86–0.96) | 0.90 (0.84–0.97) | 0.85 (0.75–0.95) | 0.54 (0.36–0.81) | <0.001 | |
| Multivariablea | 1.00 | 0.88 (0.83–0.93) | 0.84 (0.78–0.90) | 0.72 (0.64–0.82) | 0.46 (0.31–0.69) | <0.001 | |
| Multivariableb | 1.00 | 0.90 (0.84–0.96) | 0.89 (0.82–0.97) | 0.77 (0.68–0.88) | 0.51 (0.34–0.78) | <0.001 | |
|
| |||||||
| Other | |||||||
|
| |||||||
| Butter | No. of cases | 2807 | 1110 | 639 | 1062 | 1037 | |
| Age-adjusted | 1.00 | 1.04 (0.96–1.11) | 0.98 (0.90–1.08) | 1.02 (0.95–1.10) | 1.16 (1.08–1.25) | 0.002 | |
| Multivariablea | 1.00 | 1.07 (0.99–1.15) | 1.02 (0.94–1.12) | 1.06 (0.98–1.14) | 1.13 (1.05–1.22) | 0.004 | |
| Multivariableb | 1.00 | 1.12 (1.04–1.20) | 1.10 (1.00–1.20) | 1.17 (1.08–1.26) | 1.26 (1.16–1.37) | <0.001 | |
|
| |||||||
| Cheese (full fat) | No. of cases | 2512 | 282 | 523 | 988 | 2350 | |
| Age-adjusted | 1.00 | 0.80 (0.71–0.91) | 0.86 (0.78–0.95) | 0.86 (0.80–0.93) | 0.93 (0.87–0.98) | 0.006 | |
| Multivariablea | 1.00 | 0.85 (0.75–0.97) | 0.90 (0.82–0.99) | 0.90 (0.83–0.97) | 0.90 (0.85–0.96) | 0.001 | |
| Multivariableb | 1.00 | 0.88 (0.77–1.01) | 0.92 (0.83–1.02) | 0.91 (0.84–0.99) | 0.90 (0.84–0.96) | <0.001 | |
|
| |||||||
| Pizza | No. of cases | 1293 | 2843 | 2104 | 380 | 35 | |
| Age-adjusted | 1.00 | 1.09 (1.02–1.17) | 1.28 (1.19–1.38) | 1.87 (1.65–2.11) | 3.59 (2.46–5.25) | <0.001 | |
| Multivariablea | 1.00 | 1.07 (1.00–1.15) | 1.19 (1.10–1.29) | 1.51 (1.33–1.72) | 2.49 (1.67–3.69) | <0.001 | |
| Multivariableb | 1.00 | 1.07 (1.00–1.16) | 1.11 (1.02–1.21) | 1.27 (1.11–1.46) | 1.90 (1.26–2.88) | 0.001 | |
|
| |||||||
| Beverages | |||||||
|
| |||||||
| Sugar-Sweetened Beverages | No. of cases | 4281 | 774 | 446 | 364 | 790 | |
| Age-adjusted | 1.00 | 0.73 (0.68–0.79) | 0.68 (0.62–0.75) | 0.72 (0.64–0.80) | 0.69 (0.64–0.74) | <0.001 | |
| Multivariablea | 1.00 | 0.70 (0.64–0.76) | 0.63 (0.57–0.70) | 0.65 (0.58–0.73) | 0.56 (0.52–0.61) | <0.001 | |
| Multivariableb | 1.00 | 0.75 (0.69–0.82) | 0.69 (0.62–0.76) | 0.71 (0.63–0.80) | 0.63 (0.57–0.69) | <0.001 | |
|
| |||||||
| Low Calorie Beverages | No. of cases | 1540 | 463 | 500 | 544 | 3608 | |
| Age-adjusted | 1.00 | 1.34 (1.20–1.49) | 1.58 (1.42–1.75) | 1.60 (1.44–1.76) | 2.47 (2.32–2.63) | <0.001 | |
| Multivariablea | 1.00 | 1.37 (1.23–1.53) | 1.59 (1.43–1.77) | 1.63 (1.47–1.81) | 2.38 (2.24–2.54) | <0.001 | |
| Multivariableb | 1.00 | 1.31 (1.17–1.46) | 1.48 (1.33–1.65) | 1.46 (1.32–1.62) | 1.93 (1.80–2.07) | <0.001 | |
Abbreviations: CI = Confidence Interval; NHS = Nurses’ Health Study; OR = Odds Ratio
Adjusted for age (continuous), total energy intake (continuous calories in kcal), alcohol (non-drinkers (reference), >0–15 and >15 grams/day), current smoking (no current smoking (reference), 1–14 and ≥15 cigarettes/day), and physician diagnosis of depression (no (reference), yes)
Additionally adjusted for food items in table
P for trend was calculated by including a continuous variable with values corresponding to each category of consumption (i.e., 0–4); multivariable model b included continuous variables for all food items simultaneously
Low Calorie Beverages: Low-calorie beverage with caffeine and Other low-calorie beverage without caffeine
Sugar-Sweetened Beverages: Carbonated beverage with caffeine and sugar, Carbonated beverage with sugar, Other sugared beverages
Analyses of two additional FFQ items were consistent with the “fast food” findings: compared with nurses who ate fried food <1/week the odds of food addiction were almost 3-fold for nurses who ate fried food outside the home 4+/week and for nurses consuming fried food at home daily.
With the exception of no/low fat cookies and water, consumption of all no/low fat snacks and sweets, artificial sugar, and low calorie beverages had significant, positive associations with food addiction (Table 3, multivariable model a). For example, compared with nurses consuming <1/month, we observed the strongest odds of food addiction for those consuming 5+/week of caffeinated low calorie beverages( 2.21 (2.07–2.35)), decaffeinated low calorie beverages (2.00 (1.86–2.14)), artificial sweetener(excluding Splenda) ( 1.71 (1.60–1.83)), and Splenda (1.76 (1.65–1.87)).
Table 3.
Odds Ratios and 95% Confidence Intervals for Consumption of Low or No Fat and/or No Sugar Foods and Beverages (2006 and 2007) and Food Addiction (2008 and 2009) in NHS and NHS II (n = 123,688)
| OR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Number of Servings | |||||||
|
| |||||||
| Food Item | <1/month | 1–3/month | 1/week | 2–4/week | ≥5–6/week | Ptrend* | |
| Low and No Fat Snacks | |||||||
|
| |||||||
| Popcorn (fat free) | No. of cases | 3403 | 1694 | 881 | 492 | 185 | |
| Age-adjusted | 1.00 | 1.21 (1.14–1.29) | 1.45 (1.34–1.56) | 1.76 (1.59–1.94) | 2.23 (1.91–2.62) | <0.001 | |
| Multivariablea | 1.00 | 1.19 (1.12–1.26) | 1.38 (1.28–1.49) | 1.65 (1.49–1.83) | 2.04 (1.73–2.40) | <0.001 | |
| Multivariableb | 1.00 | 1.06 (0.99–1.13) | 1.17 (1.08–1.27) | 1.36 (1.23–1.51) | 1.58 (1.34–1.87) | <0.001 | |
|
| |||||||
| Low and No Fat Sweets and Desserts | |||||||
|
| |||||||
| Cookies (no/low fat) | No. of cases | 4978 | 946 | 377 | 244 | 110 | |
| Age-adjusted | 1.00 | 1.15 (1.07–1.24) | 1.39 (1.24–1.55) | 1.27 (1.11–1.45) | 1.21 (0.99–1.47) | <0.001 | |
| Multivariablea | 1.00 | 1.11 (1.03–1.19) | 1.31 (1.17–1.46) | 1.19 (1.04–1.36) | 1.08 (0.89–1.32) | <0.001 | |
| Multivariableb | 1.00 | 0.94 (0.87–1.02) | 1.08 (0.96–1.21) | 1.00 (0.87–1.15) | 0.94 (0.76–1.15) | 0.78 | |
|
| |||||||
| Sherbet/frozen yogurt | No. of cases | 3341 | 1835 | 643 | 624 | 212 | |
| Age-adjusted | 1.00 | 1.11 (1.05–1.18) | 1.13 (1.04–1.24) | 1.31 (1.20–1.43) | 1.43 (1.24–1.65) | <0.001 | |
| Multivariablea | 1.00 | 1.07 (1.01–1.14) | 1.06 (0.97–1.15) | 1.17 (1.07–1.28) | 1.16 (1.00–1.35) | <0.001 | |
| Multivariableb | 1.00 | 0.96 (0.90–1.02) | 0.88 (0.81–0.97) | 0.97 (0.89–1.07) | 0.96 (0.83–1.12) | 0.10 | |
|
| |||||||
| Sweet roll/coffee cake (no/low fat) | No. of cases | 5681 | 744 | 168 | 48 | 14 | |
| Age-adjusted | 1.00 | 1.29 (1.20–1.40) | 1.46 (1.24–1.71) | 1.66 (1.23–2.24) | 2.57 (1.46–4.50) | <0.001 | |
| Multivariablea | 1.00 | 1.21 (1.11–1.31) | 1.28 (1.09–1.51) | 1.35 (0.99–1.83) | 2.16 (1.22–3.85) | <0.001 | |
| Multivariableb | 1.00 | 1.06 (0.98–1.16) | 1.08 (0.91–1.27) | 1.12 (0.82–1.53) | 1.73 (0.95–3.13) | 0.06 | |
|
| |||||||
| Artificial Sweeteners | |||||||
|
| |||||||
| Artificial Sweetener* | No. of cases | 4024 | 545 | 365 | 413 | 1308 | |
| Age-adjusted | 1.00 | 1.51 (1.38–1.66) | 1.67 (1.49–1.87) | 1.59 (1.43–1.77) | 1.81 (1.70–1.94) | <0.001 | |
| Multivariablea | 1.00 | 1.46 (1.33–1.61) | 1.56 (1.40–1.75) | 1.51 (1.35–1.68) | 1.71 (1.60–1.83) | <0.001 | |
| Multivariableb | 1.00 | 1.15 (1.04–1.27) | 1.17 (1.04–1.32) | 1.15 (1.03–1.29) | 1.38 (1.29–1.48) | <0.001 | |
|
| |||||||
| Splenda | No. of cases | 3301 | 625 | 418 | 601 | 1710 | |
| Age-adjusted | 1.00 | 1.52 (1.39–1.66) | 1.72 (1.55–1.91) | 1.85 (1.69–2.03) | 1.91 (1.80–2.03) | <0.001 | |
| Multivariablea | 1.00 | 1.46 (1.33–1.60) | 1.65 (1.48–1.84) | 1.76 (1.60–1.93) | 1.76 (1.65–1.87) | <0.001 | |
| Multivariableb | 1.00 | 1.25 (1.14–1.37) | 1.37 (1.23–1.53) | 1.47 (1.33–1.62) | 1.45 (1.36–1.54) | <0.001 | |
|
| |||||||
| Low Calorie Beverages | |||||||
|
| |||||||
| Low-calorie beverage with caffeine | No. of cases | 2261 | 801 | 533 | 827 | 2233 | |
| Age-adjusted | 1.00 | 1.47 (1.36–1.60) | 1.58 (1.43–1.74) | 1.71 (1.57–1.86) | 2.30 (2.16–2.45) | <0.001 | |
| Multivariablea | 1.00 | 1.49 (1.37–1.62) | 1.58 (1.43–1.75) | 1.71 (1.57–1.86) | 2.21 (2.07–2.35) | <0.001 | |
| Multivariableb | 1.00 | 1.28 (1.17–1.40) | 1.30 (1.17–1.44) | 1.38 (1.27–1.51) | 1.76 (1.65–1.89) | <0.001 | |
|
| |||||||
| Other low-calorie beverage without caffeine | No. of cases | 3287 | 872 | 546 | 705 | 1245 | |
| Age-adjusted | 1.00 | 1.31 (1.21–1.41) | 1.46 (1.33–1.61) | 1.63 (1.50–1.78) | 2.14 (2.00–2.30) | <0.001 | |
| Multivariablea | 1.00 | 1.27 (1.17–1.37) | 1.44 (1.31–1.59) | 1.60 (1.47–1.74) | 2.00 (1.86–2.14) | <0.001 | |
| Multivariableb | 1.00 | 1.03 (0.95–1.12) | 1.12 (1.01–1.24) | 1.25 (1.15–1.38) | 1.48 (1.38–1.60) | <0.001 | |
|
| |||||||
| Water | No. of cases | 432 | 246 | 245 | 431 | 5301 | |
| Age-adjusted | 1.00 | 1.02 (0.87–1.20) | 1.03 (0.87–1.21) | 0.85 (0.74–0.97) | 0.88 (0.80–0.98) | 0.001 | |
| Multivariablea | 1.00 | 0.98 (0.83–1.16) | 0.99 (0.84–1.17) | 0.84 (0.73–0.97) | 0.87 (0.79–0.97) | 0.002 | |
| Multivariableb | 1.00 | 0.95 (0.80–1.12) | 0.94 (0.80–1.12) | 0.81 (0.70–0.93) | 0.90 (0.81–1.00) | 0.04 | |
Abbreviations: CI = Confidence Interval; NHS = Nurses’ Health Study; OR = Odds Ratio
All other artificial sugar except Splenda
Adjusted for age (continuous), total energy intake (continuous calories in kcal), alcohol (non-drinkers (reference), >0–15 and >15 grams/day), current smoking (no current smoking (reference), 1–14 and ≥15 cigarettes/day), and physician diagnosis of depression (no (reference), yes)
Additionally adjusted for food items in table
P for trend was calculated by including a continuous variable with values corresponding to each category of consumption (i.e., 0–4); multivariable model b included continuous variables for all food items simultaneously
Consumption of all fruits, vegetables, and legumes had no or inverse associations with food addiction with the exception of string beans (Table 4, multivariable model a). Compared with nurses consuming <1/month, we observed the lowest odds of food addiction for those consuming corn( 0.57 (0.43–0.76)) and grapes (0.70 (0.61–0.80)) 5+/week.
Table 4.
Odds Ratios and 95% Confidence Intervals for Consumption of Fruits, Vegetables, and Legumes (2006 and 2007) and Food Addiction (2008 and 2009) in NHS and NHS II (n = 123,688)
| OR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Number of Servings | |||||||
|
| |||||||
| Food Item | <1/month | 1–3/month | 1/week | 2–4/week | ≥5–6/week | Ptrend* | |
| Apples | No. of cases | 933 | 1819 | 1377 | 1707 | 819 | |
| Age-adjusted | 1.00 | 1.01 (0.93–1.09) | 0.94 (0.86–1.02) | 0.93 (0.86–1.01) | 0.99 (0.89–1.09) | 0.13 | |
| Multivariablea | 1.00 | 0.98 (0.90–1.06) | 0.88 (0.81–0.96) | 0.85 (0.78–0.93) | 0.85 (0.77–0.94) | <0.001 | |
| Multivariableb | 1.00 | 1.06 (0.97–1.15) | 1.00 (0.92–1.10) | 0.98 (0.90–1.07) | 0.94 (0.85–1.05) | 0.03 | |
|
| |||||||
| Avocado | No. of cases | 4516 | 1418 | 494 | 195 | 32 | |
| Age-adjusted | 1.00 | 0.96 (0.90–1.02) | 0.85 (0.77–0.93) | 0.77 (0.67–0.90) | 0.74 (0.52–1.06) | <0.001 | |
| Multivariablea | 1.00 | 0.97 (0.91–1.03) | 0.85 (0.77–0.94) | 0.77 (0.66–0.89) | 0.70 (0.49–1.01) | <0.001 | |
| Multivariableb | 1.00 | 0.98 (0.92–1.05) | 0.88 (0.80–0.97) | 0.78 (0.67–0.90) | 0.69 (0.48–0.99) | <0.001 | |
|
| |||||||
| Broccoli | No. of cases | 692 | 1698 | 2329 | 1625 | 311 | |
| Age-adjusted | 1.00 | 0.84 (0.76–0.92) | 0.85 (0.78–0.93) | 0.84 (0.77–0.92) | 0.97 (0.85–1.12) | 0.22 | |
| Multivariablea | 1.00 | 0.87 (0.79–0.95) | 0.88 (0.80–0.96) | 0.83 (0.76–0.92) | 0.94 (0.82–1.09) | 0.04 | |
| Multivariableb | 1.00 | 0.94 (0.85–1.03) | 1.01 (0.91–1.11) | 0.96 (0.87–1.06) | 1.00 (0.86–1.15) | 0.68 | |
|
| |||||||
| Corn | No. of cases | 1406 | 2579 | 1944 | 673 | 53 | |
| Age-adjusted | 1.00 | 0.83 (0.77–0.89) | 0.75 (0.70–0.81) | 0.77 (0.70–0.84) | 0.75 (0.57–1.00) | <0.001 | |
| Multivariablea | 1.00 | 0.80 (0.75–0.86) | 0.69 (0.64–0.74) | 0.64 (0.58–0.71) | 0.57 (0.43–0.76) | <0.001 | |
| Multivariableb | 1.00 | 0.85 (0.79–0.91) | 0.75 (0.69–0.81) | 0.70 (0.63–0.77) | 0.58 (0.44–0.78) | <0.001 | |
|
| |||||||
| Grapes | No. of cases | 2993 | 2026 | 800 | 588 | 248 | |
| Age-adjusted | 1.00 | 0.83 (0.78–0.88) | 0.75 (0.70–0.82) | 0.73 (0.67–0.80) | 0.83 (0.73–0.95) | <0.001 | |
| Multivariablea | 1.00 | 0.79 (0.74–0.84) | 0.69 (0.64–0.75) | 0.65 (0.59–0.71) | 0.70 (0.61–0.80) | <0.001 | |
| Multivariableb | 1.00 | 0.82 (0.77–0.87) | 0.73 (0.67–0.80) | 0.69 (0.62–0.75) | 0.72 (0.63–0.83) | <0.001 | |
|
| |||||||
| Peas | No. of cases | 2561 | 2140 | 1480 | 427 | 47 | |
| Age-adjusted | 1.00 | 0.85 (0.80–0.90) | 0.84 (0.79–0.90) | 0.75 (0.67–0.83) | 0.97 (0.72–1.30) | <0.001 | |
| Multivariablea | 1.00 | 0.82 (0.77–0.87) | 0.77 (0.72–0.83) | 0.64 (0.57–0.71) | 0.77 (0.57–1.05) | <0.001 | |
| Multivariableb | 1.00 | 0.88 (0.82–0.93) | 0.85 (0.79–0.91) | 0.69 (0.62–0.78) | 0.78 (0.57–1.07) | <0.001 | |
|
| |||||||
| String beans | No. of cases | 1128 | 1885 | 2188 | 1270 | 184 | |
| Age-adjusted | 1.00 | 0.82 (0.76–0.89) | 0.85 (0.79–0.91) | 0.94 (0.87–1.02) | 1.40 (1.19–1.65) | 0.20 | |
| Multivariablea | 1.00 | 0.83 (0.77–0.90) | 0.83 (0.77–0.90) | 0.87 (0.80–0.95) | 1.22 (1.03–1.44) | 0.23 | |
| Multivariableb | 1.00 | 0.90 (0.83–0.97) | 0.95 (0.88–1.03) | 1.03 (0.94–1.13) | 1.38 (1.16–1.65) | 0.008 | |
Abbreviations: CI = Confidence Interval; NHS = Nurses’ Health Study; OR = Odds Ratio
Adjusted for age (continuous), total energy intake (continuous calories in kcal), alcohol (non-drinkers (reference), >0–15 and >15 grams/day), current smoking (no current smoking (reference), 1–14 and ≥15 cigarettes/day), and physician diagnosis of depression (no (reference), yes)
Additionally adjusted for food items in table
P for trend was calculated by including a continuous variable with values corresponding to each category of consumption (i.e., 0–4); multivariable model b included continuous variables for all food items simultaneously
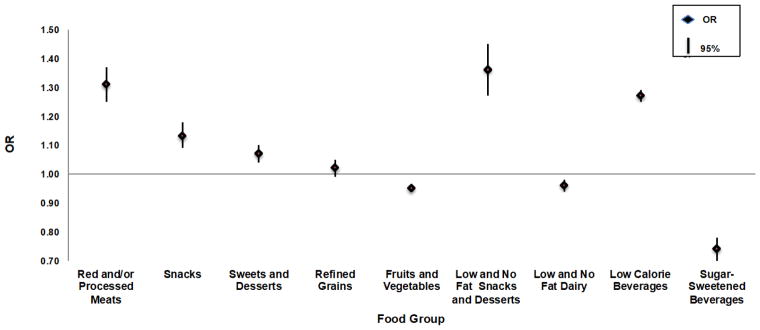
Our food group analyses corroborated our food item analyses (Figure 1). For every serving/day increase of red/processed meats, women had a 31% increased odds of food addiction (95% CI, 1.25–1.37). Similarly, for every serving/day increase of low/no fat snacks and desserts, low calorie beverages, and (regular) snacks, women’s odds of food addiction increased by 36% (95% CI, 1.27–1.45), 27% (95% CI, 1.25–1.29), and 13% (95% CI, 1.09–1.18), respectively. There were no strong associations between sweets and desserts, refined grains, fruits and vegetables, and low/no fat dairy intake and food addiction. However, for every serving/day increase of sugar-sweetened beverage consumption, women had a 26% decreased odds of food addiction (95% CI, 0.70, 0.78). Results were similar when we additionally controlled for all food groups and examined intake by food group quartiles and the 90th versus 10th percentiles of consumption.
Figure 1.
ORs and 95% CIs For Food Group a Consumption (Servings per Day, 2006–2007) and Food Addiction (2008–2009) Among Nurses in the NHS Cohorts (n = 123,688)
Abbreviations: CI = Confidence Intervals; OR = Odds Ratio; NHS = Nurses’ Health Study
Analyses were adjusted for age (continuous), total energy intake (continuous calories in kcal), alcohol (non-drinkers (reference), >0–15 and >15 grams/day), current smoking (no current smoking (reference), 1–14 and ≥15 cigarettes/day), and physician diagnosis of depression (no (reference), yes)
aThe following foods were included in each food group: Red/Processed Meats: Beef or pork hot dogs. Chicken or turkey hot dogs, Bacon (2 slices), Salami, bologna, or other processed meat sandwiches. Other processed meats, e.g., sausage, kielbasa, etc. (2 oz. or 2 small links), Hamburger Lean or extra lean. Hamburger regular, Beef, pork, or lamb as a sandwich or mixed dish, e.g., stew, casserole, lasagna, etc., Pork as a main dish, e.g., ham or chops (4–6 oz.), Beef or lamb as a main dish, e.g., steak, roast (4–6 oz.); Snacks; Crackers, Popcorn, Potato chips or com/tortilla chips. Pretzels, and French fries; Sweets and Desserts: Cake, Candy bars. Candy without chocolate. Homemade Cookies or brownies, Store-bought Cookies or brownies, Dark chocolate. Doughnuts, Milk chocolate, Pie, Ice cream. Homemade sweet roll, coffee cake, or other pastry, and Store-bought sweet roll, coffee cake, or other pastry; Refined Grains: White bread, English muffins, bagels or rolls, Muffins or biscuits. White rice. Pasta, Pancakes or waflles. Crackers, Pretzels, Tortillas; Fruits and Vegetables Apples, apple juice, bananas, grapes, avocados, apricots, blueberries, cantaloupe, grapefruit, other juice, oranges, orange juice, orange juice with calcium, peaches, prunes, prune juice, strawberries, tomatos, tomato juice, tomato sauce, string beans, broccoli, cabbage/coleslaw, cauliflower, brussel sprouts, raw carrots, cooked carrots, corn, mixed vegetables, peas, eggplant, squash, yams, cooked spinach, raw spinach, kale, iceburg lettuce, red leaf lettuce, celery, onions; Low and No Fat Sweets and Snacks. Frozen yogurt, sherbet or low-fat ice cream. Cookies fat free or reduced fat Popcorn fat free or light. Sweet roll, coffee cake or other pastry fat free or reduced fat; Low and No Fat Dairy. Skim milk, 1 or 2% milk, Low-carb, artificially sweetened or plain flavored yogurt. Low or no fat cottage cheese. Low or no fat other cheese; Low Calories Beverages: Low-calorie beverage with caffeine and Other low-calorie beverage without caffeine; Sugar-Sweetened Beverages: Carbonated beverage with caffeine and sugar, Carbonated beverage with sugar, Other sugared beverages
To address the ambiguity of the temporal relationship between food group intake, BMI, and food addiction, we conducted sensitivity analyses in which we additionally controlled for BMI (Supplemental Table 3). Overall, the direction of the associations remained the same. However, the associations between red/processed meat and refined grains consumption and food addiction became inverse. The consequences of controlling for BMI may have introduced bias.
As seen in Supplemental Table 3, a few of the p-values for the test for between studies heterogeneity were statistically significant. In the NHSII cohort, with every increase in servings/day of dessert, women were 12% more likely to have food addiction. We did not see this effect in the NHS cohort, the older cohort. Although statistically significant between studies heterogeneity was observed for the food groups of low/no fat snacks and desserts, low calorie-beverages and sugar-sweetened beverages, risk estimates were in the same direction, although the magnitude of the risk was slightly stronger in one cohort. For example, the effect on food addiction for drinking low calorie beverages among women in NHS was slightly stronger (OR 1.33 (1.28–1.39)) than among women in NHSII (OR 1.25 (1.23–1.27)). In addition, food item consumption-heterogeneity analyses (data not shown) were similar to the food group analyses in that there were no qualitatively meaningful differences between cohorts.
Overall, there were few statistically significant interactions between food addiction and food group consumption by depression, smoking, alcohol, BMI or diabetes (Supplemental Table 3). We found qualitatively and statistically significant interactions between red/processed meat consumption and smoking (p=0.04) and BMI ( p=0.05), fruit and vegetable intake and BMI (p<0.001), low/no fat snack and dessert consumption and diabetes (p=0.02), and low/no fat dairy intake and smoking (p=0.001). In subgroup analyses, non-smokers and overweight nurses had increased odds of food addiction with increased red/processed meat intake, while smokers and non-overweight nurses did not, and smokers had increased odds of food addiction with increased low/no fat dairy intake while non-smokers had decreased odds. Overweight nurses had lower odds of food addiction with increased fruit and vegetable intake while non-overweight nurses did not, and non-diabetic nurses had increased odds of food addiction with increased low/no fat snack and dessert consumption while diabetics did not. There were other statistically significant interactions between food group consumption and certain stratification factors; however, further subgroup analyses revealed only marginal differences in the magnitude, but not the direction of the ORs.
Discussion
Our study was the first to examine the relationship between food and beverage consumption and food addiction. Intake of many positively reinforcing foods (e.g., pizza) was positively associated with food addiction. We also found strong positive associations between no/low fat and artificially sweetened food and low calorie beverage intake and food addiction. Yet, we found no association between food addiction and consumption of certain sweet food items (e.g., cake and ice cream) and refined grains (e.g., pasta and white potato), foods that are similarly metabolized into sugar. We also found inverse associations with homemade cookies, dark chocolate, white rice, full fat cheese, and sugar-sweetened beverage intake, all of which contradict the basic science model of drugs of abuse(60, 61).
Previous human studies have examined food addiction and food cravings, liking, snacking, and consumption (18, 20, 21, 34, 44, 62–68). Our findings support prior studies observing positive associations between food addiction and fatty food liking(62), processed food liking (62), percent of diet from fat(18, 67), consumption of fat (67), and sweet snacking (20, 21). Our results were also consistent with studies finding no association between food addiction and sugar and carbohydrate consumption,(44)percent of diet from carbohydrates (18, 67), sugar cravings (62, 66), pleasantness ratings of a milkshake (34, 63), reported problems with foods containing mostly sugar without fats or protein (68) and sugar liking(62 ). However, our results were not consistent with prior work suggesting a positive association between food addiction and starchy food cravings(66). Some of these findings contradict previous animal research, which has supported a model of “sugar addiction”(42, 60).
There are a few possible explanations for our findings. First, people meeting criteria for food addiction may not be addicted to sugar in isolation as has been observed in rat studies; rather, the combination of sugar, fat and salt and/or processing level may create the positively reinforcing quality of foods that leads to the most addictive eating(52, 68). Some of our findings support this ‘combination’ theory: certain fatty sweet foods (e.g., candy bars) had strong associations with food addiction, and in our study, consumption of “fast foods”—hamburgers, French fries, and pizza—had the highest odds of food addiction.
Our findings suggest that women with food addiction drink fewer sugar-sweetened beverages and more low calorie beverages than those without food addiction. One possible explanation is reverse causation—people with food addiction may replace their sugar-sweetened beverage and sweet food consumption with “diet” beverages, artificially sweetened foods, and no/low fat foods (69). Recent animal studies have supported the addictive nature of artificially sweetened foods; these studies show that the intense sweetness of artificial sweeteners may surpass the reward of cocaine (70) and may produce sucrose-like rewarding effects and withdrawal (71). As prospective analyses have shown positive associations between sugar-sweetened beverage consumption and long-term weight gain(72) and between BMI and food addiction (13), our inverse association between sugar-sweetened beverage consumption and food addiction is likely non-causal. Due to the cross-sectional nature of our analyses, we could not test whether positively reinforcing food intake(collected in 2006 and 2007) causes food addiction (collected in 2008 and 2009)or conclude that drinking these beverages influences one’s risk of food addiction. However, we used diet data collected before food addiction, so the direction of potential causality would be correct.
The assessment of diet using the FFQ could have led to exposure misclassification (73). Nurses with unusual diets or who eat foods not assessed by the FFQ may appear to eat fewer positively reinforcing foods. It is also possible that women with food addiction may underreport consumption of certain types of positively reinforcing foods, as research suggests that BMI and underreporting are positively associated(74). The FFQ may not adequately capture binge eating behavior patterns (eating large amounts of food within a short period of time). People with food addiction may generally avoid eating the foods for which we found no or inverse associations (e.g., cake, ice cream); however, these foods may be consumed predominantly during less frequent binge episodes(51). In addition, eating patterns for certain foods(e.g., cake, ice cream) may be affected by seasonality and cultural norms, which may make it more difficult to estimate one’s average consumption over the past year; or these foods may appear in a lower intake category due to episodes of binge eating occurring only a few times per year. While these potential sources of error could have biased our effect estimates towards the null, the FFQ is a valid method for assessing long term dietary intake (49).
Our analyses revealed unexpected findings regarding the lack of comorbidity of addictions—i.e., nurses who drank alcohol and smoked cigarettes were less likely to have a food addiction diagnosis. Substance-related disorders involving alcohol, illicit drugs, and nicotine are often comorbid. For example, one study(75) found that the odds of lifetime drug dependence were 15.75 times higher (95% CI 9.59–25.86) among women with lifetime alcohol dependence compared with women without. However, researchers have hypothesized an inverse relationship between current food addiction and current substance-related disorders(76–78). As food and alcohol or food and cigarettes may compete for the same neurotransmitters (e.g., dopamine) in the brain, people with a susceptibility to addictive behavior may not abuse more than one of these substances concurrently. Thus, while lifetime comorbidity may be expected, we expected inverse relationships between current food addiction and current other substance-related disorders. Our data supported this hypothesis: compared with women without food addiction, we found a lower prevalence of substance use (i.e., cigarette smoking and alcohol consumption) among women with food addiction.
Unknown or unmeasured factors may have confounded the relationship between diet and food addiction. For example, we did not account for diet intake at younger ages, which may influence both current food intake and food addiction. In addition, the inverse associations between food addiction and consumption of certain sweet foods, refined grains, and sugar-sweetened beverages could partly be due to confounding by dieting. Women with food addiction may be more likely to diet (79)(as are women with weight concerns and those who engage in binge eating behaviors (80)). Women who engage in dieting behaviors often avoid sugar-sweetened beverages and many sugary sweets, and instead eat no/low fat and artificially sweetened foods(81, 82). Although people typically consume no/low fat and artificially sweetened foods to lose weight, research suggests that overweight individuals who drink non-caloric beverages compensate by consuming additional calories from solid food (83). As we only had dieting behavior information in NHSII, we conducted a sensitivity analysis controlling for dieting in our models; results from this sensitivity analysis were similar to our main models. Overall, we accounted for a wide variety of potential confounders in our analyses, and our adjustment for variables was more comprehensive than most previous food addiction studies.
In addition, we examined a large number of associations, and some of the statistically significant associations may be due to chance. For example, we found some statistically significant p-values for the test for between studies heterogeneity when we examined the association between food group consumption and food addiction by cohort. While certain ORs for food group consumption and food addiction were quantitatively different between cohorts, effect estimates were similar and almost exclusively in the same direction, thus not qualitatively different. Most importantly, there was not a consistent pattern suggesting that food consumption and food addiction would be biologically different between the two cohorts. However, due to the cross-sectional nature of our study and concern about multiple comparisons, these results require replication, especially within different age groups.
Although the NHS cohorts provide an extremely rich source of data, the generalizability of our findings may be limited, as the cohorts are comprised of middle to older-aged female nurses, most of whom are Caucasian. Thus, our findings may not be generalizable to younger individuals, people with a different socioeconomic status, men or non-white populations. However, this study was conducted using the largest cohort to date in over 120,000 women; as most previous studies on food addiction have been conducted in small samples of overweight individuals, our study should be more generalizable to the general public.
Despite these potential limitations, our study has a number of strengths. The NHS cohorts have biennial response rates of 90%, which limits potential selection bias. The large sample size provides ample power to detect main effects and control for many confounders simultaneously. The prospective design also allows for continuous updating of exposures and outcomes, which limits potential misclassification and increases the validity of measures.
This paper was the first to examine the relationship between food consumption and food addiction in a large epidemiologic study. Our analyses make fundamental contributions to assessing the relationship between a new, potentially important addiction and the positively reinforcing substances at play. While our research supported many previously suspected foods as being positively associated with food addiction, some findings did not corroborate a model of sugar addiction. Clinical implications of our results will rely on replication of these findings in prospective studies and whether mental health professionals determine that food addiction is a valid psychiatric diagnosis(84). Longitudinal analyses should further investigate the temporal order between food consumption and food addiction, as some of the relationships examined in the current study were difficult to interpret due to the cross-sectional design.
Supplementary Material
Acknowledgments
The NHS is supported by UM1 CA186107 and DK58845, and NHSII is supported by UM1CA176726 from National Institutes of Health. This work was also supported by grants 5-T32 MH 13043-43 (National Institutes of Mental Health) and T32 DK 91227-5 (National Institutes of Health). We would like to acknowledge the participants and staff of the NHS and NHSII for their valuable contributions and the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School. We would also like to thank Dr. Sharon Schwartz for sharing her wisdom and insights throughout the course of this research.
Footnotes
Abbreviations: BMI, body mass index; CI, Confidence Interval; DSM, Diagnostic Statistical Manual of Mental Disorders; FFQ, Food Frequency Questionnaire; mYFAS, modified Yale Food Addiction Scale; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; OR, Odds Ratio.
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
References
- 1.Randolph TG. The descriptive features of food addiction; addictive eating and drinking. Q J Stud Alcohol. 1956;17(2):198–224. [PubMed] [Google Scholar]
- 2.Arenson G. A Substance Called Food. 2. New York: McGraw-Hill Companies; 1989. [Google Scholar]
- 3.Tarman V, Werdell P. Food Junkies: The Truth about Food Addiction. Toronto, Ontario: Dundurn Press; 2014. [Google Scholar]
- 4.Frascella J, Potenza MN, Brown LL, Childress AR. Shared brain vulnerabilities open the way for nonsubstance addictions: carving addiction at a new joint? Ann N Y Acad Sci. 1187:294–315. doi: 10.1111/j.1749-6632.2009.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss M. Salt Sugar Fat: How the Food Giants Hooked Us. New York: Random House; 2013. [Google Scholar]
- 6.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meule A, Gearhardt AN. Food Addiction in the Light of DSM-5. Nutrients. 2014;6(9):3653–71. doi: 10.3390/nu6093653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelchat ML. Of human bondage: food craving, obsession, compulsion, and addiction. Physiol Behav. 2002;76(3):347–52. doi: 10.1016/s0031-9384(02)00757-6. [DOI] [PubMed] [Google Scholar]
- 9.Pursey KM, Stanwell P, Gearhardt AN, Collins CE, Burrows TL. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients. 2014;6(10):4552–90. doi: 10.3390/nu6104552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownell KD, Gold MS, editors. Food and Addiction: A Comprehensive Handbook. 1. Oxford: Oxford University Press; 2012. [Google Scholar]
- 11.Conference on Eating and Dependence; July; New Haven, Connecticut: Rudd Center for Food Policy and Obesity Yale University; 2007. [Google Scholar]
- 12.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52(2):430–6. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Flint AJ, Gearhardt AN, Corbin WR, Brownell KD, Field AE, Rimm EB. Food-addiction scale measurement in 2 cohorts of middle-aged and older women. Am J Clin Nutr. 2014;99(3):578–86. doi: 10.3945/ajcn.113.068965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gearhardt AN, Roberto CA, Seamans MJ, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale for children. Eat Behav. 2013;14(4):508–12. doi: 10.1016/j.eatbeh.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gearhardt AN, White MA, Masheb RM, Morgan PT, Crosby RD, Grilo CM. An examination of the food addiction construct in obese patients with binge eating disorder. Int J Eat Disord. 2012;45(5):657–63. doi: 10.1002/eat.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gearhardt AN, White MA, Masheb RM, Grilo CM. An examination of food addiction in a racially diverse sample of obese patients with binge eating disorder in primary care settings. Compr Psychiatry. 2013;54(5):500–5. doi: 10.1016/j.comppsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meule A. Food addiction and body-mass-index: a non-linear relationship. Med Hypotheses. 2012;79(4):508–11. doi: 10.1016/j.mehy.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Pedram P, Wadden D, Amini P, Gulliver W, Randell E, Cahill F, et al. Food addiction: its prevalence and significant association with obesity in the general population. PloS one. 2013;8(9):e74832. doi: 10.1371/journal.pone.0074832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burmeister JM, Hinman N, Koball A, Hoffmann DA, Carels RA. Food addiction in adults seeking weight loss treatment. Implications for psychosocial health and weight loss. Appetite. 2013;60(1):103–10. doi: 10.1016/j.appet.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite. 2011;57(3):711–7. doi: 10.1016/j.appet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Davis C, Loxton NJ, Levitan RD, Kaplan AS, Carter JC, Kennedy JL. ‘Food addiction’ and its association with a dopaminergic multilocus genetic profile. Physiol Behav. 2013;118C:63–9. doi: 10.1016/j.physbeh.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Wakefield JC. The concept of mental disorder: diagnostic implications of the harmful dysfunction analysis. World Psychiatry. 2007;6(3):149–56. [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–41. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159(4):1193–9. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44(3):175–80. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 26.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19(4):1709–15. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 27.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 28.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–35. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–21. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Dipatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matheny M, Shapiro A, Tumer N, Scarpace PJ. Region-specific diet-induced and leptin-induced cellular leptin resistance includes the ventral tegmental area in rats. Neuropharmacology. 2011;60(2–3):480–7. doi: 10.1016/j.neuropharm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145(11):4880–9. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 33.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural Correlates of Food Addiction. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139(3):623–8. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bello NT, Guarda AS, Terrillion CE, Redgrave GW, Coughlin JW, Moran TH. Repeated binge access to a palatable food alters feeding behavior, hormone profile, and hindbrain c-Fos responses to a test meal in adult male rats. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R622–31. doi: 10.1152/ajpregu.00087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12(16):3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 38.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–44. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 39.Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry. 1970;126(7):983–7. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- 40.Wojnicki FH, Charny G, Corwin RL. Binge-type behavior in rats consuming trans-fat-free shortening. Physiol Behav. 2008;94(4):627–9. doi: 10.1016/j.physbeh.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin X. The relationship between food addiction and obesity [Health & Mental Health Treatment & Prevention] Ann Arbor: Walden University; 2012. [Google Scholar]
- 42.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10(6):478–88. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 43.Pedram P, Sun G. Hormonal and dietary characteristics in obese human subjects with and without food addiction. Nutrients. 2014;7(1):223–38. doi: 10.3390/nu7010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pursey KM, Collins CE, Stanwell P, Burrows TL. Foods and dietary profiles associated with ‘food addiction’ in young adults. Addictive Behaviors Reports. 2015;2(Supplement C):41–8. doi: 10.1016/j.abrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richmond RL, Roberto CA, Gearhardt AN. The association of addictive-like eating with food intake in children. Appetite. 2017;117:82–90. doi: 10.1016/j.appet.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barton J, Bain C, Hennekens CH, Rosner B, Belanger C, Roth A, et al. Characteristics of respondents and non-respondents to a mailed questionnaire. Am J Public Health. 1980;70(8):823–5. doi: 10.2105/ajph.70.8.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The Channing Laboratory. History: The Nurses’ Health Study. 2012 Available from: http://www.channing.harvard.edu/nhs/?page_id=70.
- 48.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 49.Willett WC. Harvard School of Public Health. Oxford University Press; 1998. Nutritional Epidemiology. [Google Scholar]
- 50.Oswald KD, Murdaugh DL, King VL, Boggiano MM. Motivation for palatable food despite consequences in an animal model of binge eating. Int J Eat Disord. 2011;44(3):203–11. doi: 10.1002/eat.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allison S, Timmerman GM. Anatomy of a binge: food environment and characteristics of nonpurge binge episodes. Eat Behav. 2007;8(1):31–8. doi: 10.1016/j.eatbeh.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PloS one. 2015;10(2):e0117959. doi: 10.1371/journal.pone.0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Overeaters Anonymous. Overeaters Anonymous. 2. Overeaters Anonymous; 2011. [Google Scholar]
- 54.Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS. Prospective study of intake of fruit, vegetables, and carotenoids and the risk of adult glioma. Am J Clin Nutr. 2007;85(3):877–86. doi: 10.1093/ajcn/85.3.877. [DOI] [PubMed] [Google Scholar]
- 55.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72(4):912–21. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 56.Lemeshow AR, Gearhardt AN, Genkinger JM, Corbin WR. Assessing the psychometric properties of two food addiction scales. Eat Behav. 2016;23:110–4. doi: 10.1016/j.eatbeh.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pursey KM, Collins CE, Stanwell P, Burrows TL. The stability of ‘food addiction’ as assessed by the Yale Food Addiction Scale in a non-clinical population over 18-months. Appetite. 2016;96:533–8. doi: 10.1016/j.appet.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 59.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 60.Avena NM. The study of food addiction using animal models of binge eating. Appetite. 2010;55(3):734–7. doi: 10.1016/j.appet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gearhardt AN, Rizk MT, Treat TA. The association of food characteristics and individual differences with ratings of craving and liking. Appetite. 2014;79:166–73. doi: 10.1016/j.appet.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 63.Imperatori C, Fabbricatore M, Innamorati M, Farina B, Quintiliani MI, Lamis DA, et al. Modification of EEG functional connectivity and EEG power spectra in overweight and obese patients with food addiction: An eLORETA study. Brain Imaging Behav. 2014 doi: 10.1007/s11682-014-9324-x. [DOI] [PubMed] [Google Scholar]
- 64.Meule A, Kubler A. Food cravings in food addiction: the distinct role of positive reinforcement. Eat Behav. 2012;13(3):252–5. doi: 10.1016/j.eatbeh.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Meule A, Lutz A, Vogele C, Kubler A. Women with elevated food addiction symptoms show accelerated reactions, but no impaired inhibitory control, in response to pictures of high-calorie food-cues. Eat Behav. 2012;13(4):423–8. doi: 10.1016/j.eatbeh.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Pepino MY, Stein RI, Eagon JC, Klein S. Bariatric surgery-induced weight loss causes remission of food addiction in extreme obesity. Obesity (Silver Spring, Md) 2014;22(8):1792–8. doi: 10.1002/oby.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pedram P, Sun G. Hormonal and Dietary Characteristics in Obese Human Subjects with and without Food Addiction. Nutrients. 2015;7(1) doi: 10.3390/nu7010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Markus CR, Rogers PJ, Brouns F, Schepers R. Eating dependence and weight gain; no human evidence for a ‘sugar-addiction’ model of overweight. Appetite. 2017;114:64–72. doi: 10.1016/j.appet.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 69.Drewnowski A, Rehm CD. The use of low-calorie sweeteners is associated with self-reported prior intent to lose weight in a representative sample of US adults. Nutrition & diabetes. 2016;6:e202. doi: 10.1038/nutd.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PloS one. 2007;2(8):e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ouyang K, Nayak S, Lee Y, Kim E, Wu M, Tallarida CS, et al. Behavioral effects of Splenda, Equal and sucrose: Clues from planarians on sweeteners. Neuroscience letters. 2017;636:213–7. doi: 10.1016/j.neulet.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tylavsky FA, Sharp GB. Misclassification of nutrient and energy intake from use of closed-ended questions in epidemiologic research. Am J Epidemiol. 1995;142(3):342–52. doi: 10.1093/oxfordjournals.aje.a117640. [DOI] [PubMed] [Google Scholar]
- 74.Scagliusi FB, Ferriolli E, Pfrimer K, Laureano C, Cunha CS, Gualano B, et al. Characteristics of women who frequently under report their energy intake: a doubly labelled water study. Eur J Clin Nutr. 2009;63(10):1192–9. doi: 10.1038/ejcn.2009.54. [DOI] [PubMed] [Google Scholar]
- 75.Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54(4):313–21. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- 76.Gearhardt AN, Corbin WR. Body mass index and alcohol consumption: family history of alcoholism as a moderator. Psychol Addict Behav. 2009;23(2):216–25. doi: 10.1037/a0015011. [DOI] [PubMed] [Google Scholar]
- 77.Kleiner KD, Gold MS, Frost-Pineda K, Lenz-Brunsman B, Perri MG, Jacobs WS. Body mass index and alcohol use. J Addict Dis. 2004;23(3):105–18. doi: 10.1300/J069v23n03_08. [DOI] [PubMed] [Google Scholar]
- 78.McIntyre RS, McElroy SL, Konarski JZ, Soczynska JK, Bottas A, Castel S, et al. Substance use disorders and overweight/obesity in bipolar I disorder: preliminary evidence for competing addictions. The Journal of clinical psychiatry. 2007;68(9):1352–7. doi: 10.4088/jcp.v68n0905. [DOI] [PubMed] [Google Scholar]
- 79.Gearhardt AN, Boswell RG, White MA. The association of “food addiction” with disordered eating and body mass index. Eat Behav. 2014;15(3):427–33. doi: 10.1016/j.eatbeh.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schelling S, Munsch S, Meyer AH, Margraf J. Relationship between motivation for weight loss and dieting and binge eating in a representative population survey. Int J Eat Disord. 2011;44(1):39–43. doi: 10.1002/eat.20748. [DOI] [PubMed] [Google Scholar]
- 81.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 Suppl):222s–5s. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 82.Schulze MB, Fung TT, Manson JE, Willett WC, Hu FB. Dietary patterns and changes in body weight in women. Obesity (Silver Spring, Md) 2006;14(8):1444–53. doi: 10.1038/oby.2006.164. [DOI] [PubMed] [Google Scholar]
- 83.Bleich SN, Wolfson JA, Vine S, Wang YC. Diet-beverage consumption and caloric intake among US adults, overall and by body weight. Am J Public Health. 2014;104(3):e72–8. doi: 10.2105/AJPH.2013.301556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mason SM, Flint AJ, Roberts AL, Agnew-Blais J, Koenen KC, Rich-Edwards JW. Posttraumatic Stress Disorder Symptoms and Food Addiction in Women by Timing and Type of Trauma Exposure. JAMA psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.