Abstract
Background
Pyronaridine–artesunate is a novel artemisinin-based combination therapy. The efficacy and safety of pyronaridine–artesunate were compared with artemether–lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in children.
Methods
This phase III open-label randomized controlled non-inferiority trial was conducted in Western Kenya. Children aged 6 months to ≤ 12 years with a bodyweight > 5 kg and microscopically confirmed P. falciparum malaria were randomly assigned in a 1:1 ratio to orally receive pyronaridine–artesunate or artemether–lumefantrine, dosed according to bodyweight, for 3 days.
Results
Of 197 participants, 101 received pyronaridine–artesunate and 96 received artemether–lumefantrine. The day-28 adequate clinical and parasitological response in the per-protocol population, PCR-corrected for reinfections, was 98.9% (93/94, 95% CI 94.2–99.8) for pyronaridine–artesunate and 96.4% (81/84, 95% CI 90.0–98.8) for artemether–lumefantrine. Pyronaridine–artesunate was found to be non-inferior to artemether–lumefantrine: the treatment difference was 2.5% (95% CI − 2.8 to 9.0). Adverse events occurred in 41.6% (42/101) and 34.4% (33/96) of patients in the pyronaridine–artesunate group and the artemether–lumefantrine group, respectively. No participants were found to have alanine or aspartate aminotransferase levels > 3 times the upper limit of normal.
Conclusions
Pyronaridine–artesunate was well tolerated, efficacious and non-inferior to artemether–lumefantrine for the treatment of uncomplicated P. falciparum malaria in Kenyan children. Results are in line with previous reports and inclusion of pyronaridine–artesunate in paediatric malaria treatment programmes should be considered.
This study is registered at clinicaltrials.gov under NCT02411994. Registration date: 8 April 2015. https://clinicaltrials.gov/ct2/show/NCT02411994?term=pyronaridine–artesunate&cond=Malaria&cntry=KE&rank=1
Electronic supplementary material
The online version of this article (10.1186/s12936-018-2340-3) contains supplementary material, which is available to authorized users.
Keywords: Plasmodium falciparum, Malaria, Paediatric, Pyronaridine–artesunate, Artemether–lumefantrine, Kenya
Background
Plasmodium falciparum malaria still has high morbidity and mortality rates. The World Health Organization (WHO) estimated that 216 million malaria cases and 445,000 deaths, mainly due to P. falciparum, occurred worldwide in 2016 [1]. Most endemic countries have adopted artemisinin-based combination therapy (ACT) as the first-line treatment for P. falciparum malaria, as recommended by the WHO [2]. However, resistance against commonly used ACT medicines is rising in South-East Asia and the potential spread to African countries is a major concern [3, 4].
New drugs are under development that offer possible alternatives to currently used ACT medicines. One of these alternatives is pyronaridine–artesunate (PA), which is a fixed-dose combination therapy developed by Shin Poong Pharmaceuticals (South Korea), in partnership with Medicines for Malaria Venture (MMV) [5]. PA received a positive scientific opinion from the European Medicines Agency (EMA) [6] and has some advantages over the frequently used artemether–lumefantrine combination (AL): it does not require fatty food for optimal absorption, it needs to be taken only once per day (instead of twice) and the longer half-life (13.2 days for pyronaridine compared to 3.2 days for lumefantrine [7, 8]) may prevent early reinfection.
In phase III studies, PA was found to be well tolerated and efficacious for the treatment of uncomplicated P. falciparum malaria and the blood stage of Plasmodium vivax malaria [9–15]. Polymerase chain reaction (PCR)-corrected cure rates were > 95% on day 28 in the per-protocol populations of these studies and > 93% on day 42 [9, 10, 14, 15]. Safety and efficacy were maintained after retreatment of multiple malaria episodes [15]. However, in a recent study in Western Cambodia, in an area known for high prevalence of artemisinin resistance, cure rates just below the 90% WHO-recommended efficacy threshold were found at day 42 [2, 16]. The reason for this lower efficacy remains to be investigated, as the study area knows very limited use of pyronaridine and cross-resistance between partner drugs seems unlikely according to a WHO meeting report of the Technical Expert Group on Drug Efficacy and Response [17].
One study specifically included children ≤ 12 years of age [14], but most participants in other studies evaluating the efficacy and safety of PA were adults and older children, who are expected to have acquired some antimalarial immunity [10, 15]. This immunity could improve treatment outcomes, especially for partly effective drugs [18, 19]. As young children are most vulnerable to malaria and adverse events may be more serious in this patient group [20], it is important to pay special attention to the efficacy and safety of PA in children [15, 21].
Malaria management in children improves with the use of paediatric formulations [22]. Child-friendly soluble PA granules were developed to facilitate easier swallowing, in addition to the tablet formulation for children ≥ 20 kg and adults. Pharmacokinetics were shown to be similar between the PA tablet and granule formulation [23]. Non-inferiority of PA granules to AL was demonstrated in a phase III study evaluating the treatment efficacy for uncomplicated P. falciparum malaria in children ≤ 12 years of age from various endemic settings [14], and confirmed by (a subgroup of) Sagara et al. [15]. However, to make future implementation decisions, more studies evaluating the efficacy and safety of PA in children are warranted [13].
In this phase III trial, the efficacy and safety of PA was compared with AL for the treatment of uncomplicated P. falciparum malaria in Kenyan children aged ≤ 12 years. The primary objective was to test non-inferiority of PA to AL based on an adequate clinical and parasitological response (ACPR) on day 28, PCR-corrected for reinfections.
Methods
Ethics statement
This randomized controlled non-inferiority trial was conducted at St. Jude’s Clinic, Mbita, Western Kenya from October 2015 to June 2016 and from January to August 2017, in accordance with Good Clinical Practice, regulatory requirements and the Declaration of Helsinki (2013). Ethical approval was obtained from the Ethical Review Committee of the Kenya Medical Research Institute (KEMRI) (NON-SSC no. 479) and the Expert Committee on Clinical Trials of the Kenyan Pharmacy and Poisons Board (PPB). The trial protocol was registered at clinicaltrials.gov under NCT02411994. The study had a Data and Safety Monitoring Board (DSMB). Written informed consent from a parent or legal guardian was required for participation; assent was sought from children able to understand the study.
Patients
Children were eligible to participate if they were 6 months to 12 years of age, weighed at least 5 kg, lived within 10 km from the study clinic and had microscopically confirmed P. falciparum mono-infection with 1000–200,000 asexual parasites/µL. Exclusion criteria were complicated or severe malaria, non-P. falciparum or mixed Plasmodium infection, a history of hepatic and/or renal impairment, any clinically significant illness other than malaria, anaemia with a haemoglobin (Hb) concentration < 6 g/dL, severe malnutrition, treatment with anti-malarial therapy in the previous 2 weeks, known hypersensitivity to artemisinins, previous participation in this study, current participation in other anti-malarial drug intervention studies or not being available for follow-up.
Randomization and masking
Participants were randomized 1:1 to PA or AL using a computer-generated randomization schedule, provided by the sponsor. The code linking to the treatment was kept in sequentially numbered sealed opaque envelopes. Participants were allocated in order of enrollment to the treatment in the next available envelope. Drugs were administered by pharmacy personnel aware of group assignments. Clinical and parasitological assessments were performed by study staff members blinded to treatment allocation (until completion of data analysis). The sponsor remained blinded to treatment allocation as well.
Treatment
Study drugs were given orally for 3 days (0, 1 and 2) and were dosed according to body weight (Additional file 1). PA (Shin Poong Pharmaceutical Company, Seoul, South-Korea) was given once daily, directly observed at the study clinic. The tablet form (for children ≥ 20 kg) contained 180 mg pyronaridine–tetraphosphate and 60 mg artesunate per tablet. Granules (for children < 20 kg) were provided in sachets and contained 60 mg pyronaridine–tetraphosphate and 20 mg artesunate per sachet. Oral suspensions were prepared immediately before dosing, whereby granules were stirred into 50 mL lemonade. Residual drug was given by adding 100 mL of water or lemonade to the dosing cup. AL (Novartis, Basel, Switzerland) contained 20 mg artemether and 120 mg lumefantrine per tablet and was taken twice daily. AL was provided in tablet formulation, crushed and prepared like PA granules for children unable to swallow the tablets. The morning dose of AL was administered directly observed at the study clinic. The evening dose was given to the parent/guardian to administer at home. On every day following AL treatment, pharmacy personnel asked the parent or guardian whether study drugs were administered in the evening. All participants received their drugs with food (mandazi—a type of fried bread) or milk, as recommended for AL to optimize absorption. Mandazi was also provided for the AL evening dose. Participants who vomited within 30 min of receiving the first dose were given a repeat dose. Vomiting after repeat dosing or any subsequent dose led to withdrawal from the study and administration of rescue treatment as per local guidelines.
Procedures
Finger-prick blood samples were collected at screening and on day 0, 1, 2, 3, 7, 14, 28 and 42. Giemsa-stained thick and thin blood smears were prepared according to WHO guidelines [24]. Slides were read by local expert microscopists. A slide was considered negative when 100 high-power fields were examined at 1000× magnification and no parasites were observed. Parasitaemia was determined from thick smears by counting the number of parasites against 200 leukocytes, with the assumption of 8000 leukocytes/µL blood. In case the number of parasites after counting 200 leukocytes was < 100, counting continued up to 500 leukocytes.
Hb was determined on day 0, 3, 7 and 28 by HemoCue (Ängelholm, Sweden). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were retrospectively measured on day 3 and 7. If ALT and/or AST levels were > 3× the upper limit of normal (ULN) on day 3 and/or day 7, ALT and AST were measured for day 0 and 28 as well. Bilirubin was also measured in these particular cases. If bilirubin levels were > 2× ULN, alkaline phosphatase (ALP) was measured in order to identify potential Hy’s law cases. Due to logistic constraints, ALT and AST were only measured for the first 150 participants (see "Results" section).
To distinguish between reinfections and recrudescences after parasite reappearance, nested PCR-based genotyping was performed at the Academic Medical Center (Amsterdam, The Netherlands). Using the msp1, msp2 and glurp genes, a recrudescence was defined as at least one matching allelic band in all markers between the baseline sample and the sample at the day of reappearance [25].
Outcomes
Efficacy outcomes were based on WHO definitions [26]. The primary efficacy outcome was adequate clinical and parasitological response (ACPR) on day 28, corrected for reinfection by genotyping. Secondary efficacy outcomes were ACPR on day 28 without correction for reinfection, ACPR on day 42 with and without correction by genotyping, recrudescence and reinfection rates over 42 days, parasite clearance time (defined as time from first dose to a parasitaemia, determined by two consecutive negative readings 7–25 h apart), fever clearance time (defined as time from first dose to apyrexia, determined by two consecutive normal readings 7–25 h apart), and the proportion of patients with parasite or fever clearance on day 1, 2 and 3. Safety outcomes were incidence of (serious) adverse events, severe anaemia (Hb < 6 g/dL) and the occurrence of hepatotoxicity events (defined as ALT and/or AST > 3× the ULN on day 3 and/or 7).
Sample size
Assuming a day-28 ACPR of 95% for both treatment regimens, 201 children per intervention arm (402 in total) would provide 91% power to demonstrate non-inferiority of PA compared to AL, with a non-inferiority margin of 7%. After correcting for 10% drop-out, target recruitment was 447 participants. Unfortunately, target recruitment was not reached (see results section) and 197 participants were included, which resulted in a reduction of power to 62% to demonstrate non-inferiority of PA to AL with a non-inferiority margin of 7%.
Statistical analysis
The intention-to-treat population consisted of all randomized participants who received any amount of study medication, and was the same as the safety population. The per-protocol population included participants who received a full course of study medication, had a known day-28 primary efficacy endpoint and had no major protocol violation. The primary efficacy outcome was evaluated in the per-protocol population. Non-inferiority was demonstrated if the lower limit of the 2-sided 95% confidence interval (CI) for the difference in day-28 cure rate was greater than − 7%. This confidence interval was calculated using the Newcombe-Wilson method without continuity correction. Similar analyses were performed for uncorrected ACPR on day 28, corrected and uncorrected ACPR on day 42 and for the intention to-treat-population. Additionally, Kaplan–Meier analyses were used to compare recrudescence and reinfection rates between intervention groups (log-rank test). Participants without the event (recrudescence or reinfection) or with major protocol deviations were censored at the last available parasite assessment date (before the deviation). Parasite and fever clearance times were also evaluated using Kaplan–Meier estimates and compared between groups (log-rank test). Here, all participants without confirmed clearance on day 3 were censored. Statistical analyses were performed in Stata (version 14.2, Stata Corporation, Texas, USA).
Results
Participants
A total of 197 patients were included and randomized, 101 to PA and 96 to AL (Fig. 1). Of the 101 participants in the PA-group, 60 received tablets and 41 received granules. Less participants were included than initially planned. Most likely explanations were exceptional drought during the study period, many low-density infections and more mixed infections than expected [9.8% (63/644) of all microscopy positive patients at screening had a P. falciparum/Plasmodium malariae mixed infection]. All participants received at least one dose of study medication and were included in the intention-to-treat analysis. Most patients (89.8%) completed day 28. Slightly more patients were excluded from the per-protocol population in the AL group (12.5%) compared to the PA group (6.93%), because more participants in the AL group moved away or withdrew consent (Fig. 1). The most common reason for exclusion from the per-protocol population was missing data for the primary outcome. Baseline characteristics were similar between intervention groups (Table 1).
Fig. 1.
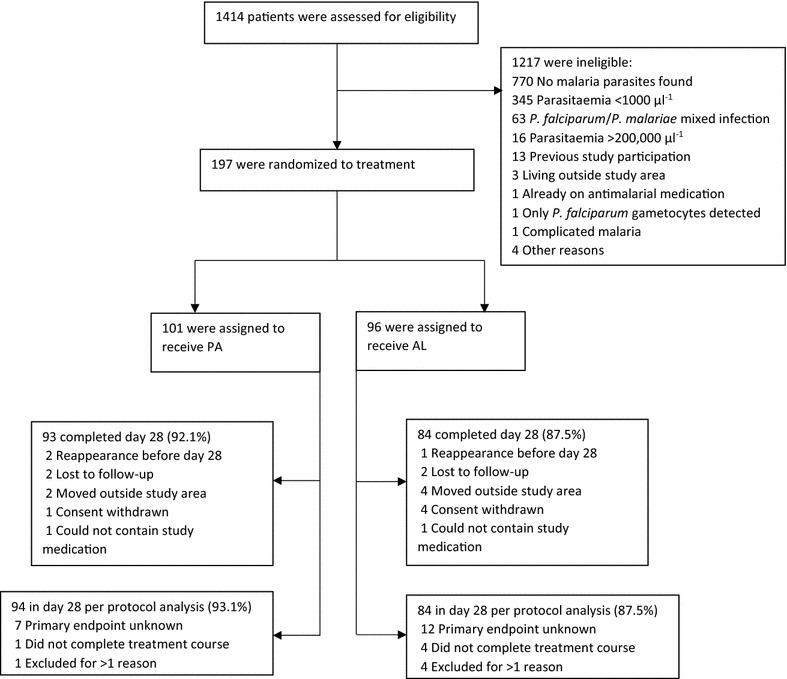
Participant flow. Number of patients screened, randomized and included in the per-protocol population. Some patients had more than one reason for exclusion from the per-protocol population
Table 1.
Baseline characteristics of study participants at enrolment
| Pyronaridine–artesunate (n = 101) |
Artemether–lumefantrine (n = 96) |
|
|---|---|---|
| Male sex, n (%) | 53 (52.5) | 48 (50.0) |
| Mean age, years (SD) [range] | 6.9 (2.9) [1.6–12] | 6.4 (3.3) [0.9–12] |
| Age category, years, n (%) | ||
| ≤ 1 | 0 | 2 (2.1) |
| > 1 to < 5 | 31 (30.7) | 31 (32.3) |
| 5–12 | 70 (69.3) | 63 (65.6) |
| Mean weight, kg (SD) [range] | 23.0 (8.4) [10–44] | 22.4 (9.2) [8.5–45] |
| Mean hemoglobin, g/dL (SD) [range] | 11.8 (1.9) [6.2–15.8] | 11.9 (2.1) [6.8–16.4] |
| Mean temperature, °C (SD) [range] | 37.5 (1.2) [35.1–39.8] | 37.3 (1.2) [35.2–39.9] |
| Fever (temperature > 37.5 °C), n (%) | 53 (52.5) | 40 (41.7) |
| Geometric mean asexual parasitaemia, µL−1 (95% CI) | 24,420.6 (18,837.6–31,658.3) | 23,672.5 (18,576.2–30,167.0) |
| Gametocyte prevalence-microscopy, n (%) | 2 (1.98) | 4 (4.17) |
Efficacy
PCR-corrected ACPR on day 28 in the per-protocol population, the primary efficacy outcome, was 98.9% (95% CI 94.2–99.8) in the PA group and 96.4% (95% CI 90.0–98.8) in the AL group. The treatment difference was 2.5 percentage points (95% CI − 2.8 to 9.0) and non-inferiority of PA compared to AL was demonstrated (Table 2). On day 42, the PCR-corrected ACPR in the per-protocol population was 94.8% (95% CI 87.4–98.0) in the PA group and 91.0% (95% CI 81.8–95.8) in the AL group, with a treatment difference of 3.8 (95% CI − 5.1 to 13.5). The uncorrected ACPR on day 28 was 91.6% (95% CI 84.3–95.7) in the PA group and 88.2% (95% CI 79.7–93.5) in the AL group. On day 42, the uncorrected ACPR was 77.9 (95% CI 68.1–85.4) in the PA group and 72.7 (95% CI 61.9–81.4) in the AL group.
Table 2.
Adequate clinical and parasitological response (ACPR) in the per-protocol population
| Pyronaridine–artesunate | Artemether–lumefantrine | Difference (95% CI) | |
|---|---|---|---|
| Day 28 | |||
| PCR-corrected ACPR, n/Nb | 93/94 | 81/84 | |
| % (95% CI) | 98.9 (94.2 to 99.8) | 96.4 (90.0 to 98.8) | 2.5 (− 2.8 to 9.0)a |
| Total no. of failures | 1 (1.1) | 3 (3.6) | |
| No. with early treatment failure | 0 | 0 | |
| No. with late clinical failure | 0 | 0 | |
| No. with late parasitological failure | 1 (1.1) | 3c (3.6) | |
| No. with missing data | 7 (7.4) | 12 (14.3) | |
| Uncorrected ACPR, n/N | 87/95 | 75/85 | |
| % (95% CI) | 91.6 (84.3 to 95.7) | 88.2 (79.7 to 93.5) | 3.3 (− 5.7 to 12.8) |
| Total no. of failures | 8 (8.4) | 10 (11.8) | |
| No. with early treatment failure | 0 | 0 | |
| No. with late clinical failure | 1 (1.1) | 1 (1.2) | |
| No. with late parasitological failure | 7 (7.4) | 9 (10.6) | |
| No. with missing data | 6 (6.4) | 11 (12.9) | |
| Day 42 | |||
| PCR-corrected ACPR, n/Nb | 73/77 | 61/67 | |
| % (95% CI) | 94.8 (87.4 to 98.0) | 91.0 (81.8 to 95.8) | 3.8 (− 5.1 to 13.5) |
| Total no. of failures | 4 (5.2) | 6 (9.0) | |
| No. with early treatment failure | 0 | 0 | |
| No. with late clinical failure | 1 (1.3) | 0 | |
| No. with late parasitological failure | 3c (3.9) | 6d (9.0) | |
| No. with missing data | 24 (31.2) | 29 (43.3) | |
| Uncorrected ACPR, n/N | 67/86 | 56/77 | |
| % (95% CI) | 77.9 (68.1 to 85.4) | 72.7 (61.9 to 81.4) | 5.2 (− 8.0 to 18.4) |
| Total no. of failures | 19 (22.1) | 21 (27.3) | |
| No. with early treatment failure | 0 | 0 | |
| No. with late clinical failure | 5 (5.8) | 2 (2.6) | |
| No. with late parasitological failure | 14 (16.3) | 19 (24.7) | |
| No. with missing data | 15 (17.4) | 19 (24.7) | |
Data are n (%) unless otherwise indicated
Participants with a reinfection before day 28 were included in the day-28 per-protocol population for the uncorrected analysis. In the PCR-corrected analysis, however, patients with a reinfection before day 28 were excluded from the analysis because data were missing on day 28 (and there was no established recrudescence). On day 42, the per-protocol population was defined similarly
aNon-inferiority of pyronaridine–artesunate to artemether–lumefantrine is demonstrated if the lower limit of the 95% confidence interval (CI) of the difference in ACPR is > − 7%
bPCR-corrected for reinfection by msp1, msp2 and glurp genotyping
cOne of the late parasitological failures had indeterminate genotyping and was marked as recrudescence
dTwo of the late parasitological failures had indeterminate genotyping and were marked as recrudescence
In the intention-to-treat population, day-28 PCR-corrected ACPR was 92.1% (95% CI 85.1–95.9) in the PA group and 84.4% (95% CI 75.8–90.3) in the AL group. Day-42 PCR-corrected ACPR was 72.3% (95% CI 62.9–80.1) in the PA group and 63.5% (95% CI 53.6–72.5) in the AL group. All outcomes for the intention-to-treat population are shown in Table 3.
Table 3.
Adequate clinical and Parasitological response (ACPR) in the intention-to-treat population
| Pyronaridine–artesunate (n = 101) |
Artemether–lumefantrine (n = 96) |
Difference (95% CI) | |
|---|---|---|---|
| Day 28 | |||
| PCR-corrected ACPR, n/Na | 93/101 | 81/96 | |
| % (95% CI) | 92.1 (85.1 to 95.9) | 84.4 (75.8 to 90.3) | 7.7 (− 1.4 to 17.1) |
| Total no. of failures | 8 (7.9) | 15 (15.6) | |
| No. with missing data | 6 (5.9) | 11 (11.5) | |
| No. with early treatment failure | 0 | 0 | |
| No. with late clinical failure | 0 | 0 | |
| No. with late parasitological failure | 1 (1.0) | 3b (3.1) | |
| No. with reinfection < day 28 | 1 (1.0) | 1 (1.0) | |
| Uncorrected ACPR, n/N | 87/101 | 75/96 | |
| % (95% CI) | 86.1 (78.1 to 91.6) | 78.1 (68.9 to 85.2) | 8.0 (− 2.7 to 18.8) |
| Total no. of failures | 14 (13.9) | 21 (21.9) | |
| No. with missing data | 6 (5.9) | 11 (11.5) | |
| No. with early treatment failure | 0 | 0 | |
| No. with late clinical failure | 1 (1.0) | 1 (1.0) | |
| No. with late parasitological failure | 7 (6.9) | 9 (9.4) | |
| Day 42 | |||
| PCR-corrected ACPR, n/Na | 73/101 | 61/96 | |
| % (95% CI) | 72.3 (62.9 to 80.1) | 63.5 (53.6 to 72.5) | 8.7 (− 4.3 to 21.4) |
| Total no. of failures | 28 (27.7) | 35 (36.5) | |
| No. with missing data | 15 (14.9) | 19 (19.8) | |
| No. with early treatment failure | 0 | 0 | |
| No. with late clinical failure | 1 (1.0) | 0 | |
| No. with late parasitological failure | 3b (3.0) | 6c (6.3) | |
| No. with reinfection < day 42 | 9 (8.9) | 10 (10.4) | |
| Uncorrected ACPR, n/N | 67/101 | 56/96 | |
| % (95% CI) | 66.3 (56.7 to 74.8) | 58.3 (48.3 to 67.7) | 8.0 (− 5.5 to 21.1) |
| Total no. of failures | 34 (33.7) | 40 (41.7) | |
| No. with missing data | 15 (14.9) | 19 (19.8) | |
| No. with early treatment failure | 0 | 0 | |
| No. with late clinical failure | 5 (5.0) | 2 (2.1) | |
| No. with late parasitological failure | 14 (13.9) | 19 (19.8) | |
Data are n (%) unless otherwise indicated
aPCR-corrected for reinfection by msp1, msp2 and glurp genotyping
bOne of the late parasitological failures had indeterminate genotyping and was marked as recrudescence
cTwo of the late parasitological failures had indeterminate genotyping and were marked as recrudescence
Kaplan–Meier estimates of recrudescence in the intention-to-treat population were 5.31% (95% CI 2.00–13.7) in the PA group and 8.47% (95% CI 3.85–18.1) in the AL group (through day 42). Estimates of reinfection were 18.1% (95% CI 11.3–28.4) and 23.8% (95% CI 13.4–40.1) for PA and AL, respectively. No difference in recrudescence (P = 0.41, log-rank test) or reinfection (P = 0.75, log-rank test) rates was found between the study groups (Fig. 2).
Fig. 2.

Kaplan–Meier estimates for a rate of recrudescence and b rate of reinfection (in the intention-to-treat population). Black markers represent censored cases. Participants without the event (recrudescence or reinfection) were censored at the last available parasite assessment date
Kaplan–Meier estimates of parasite clearance at day 3 were 97.0% (95% CI 92.2–99.2) in the PA group and 97.5% (95% CI 92.3–99.5) in the AL group. There was no difference between PA and AL in the parasite clearance time (P = 0.17, log-rank test) (Fig. 3a). Median time to parasite clearance and the proportion of patients with clearance on day 1, 2 and 3 are presented in Additional file 2.
Fig. 3.
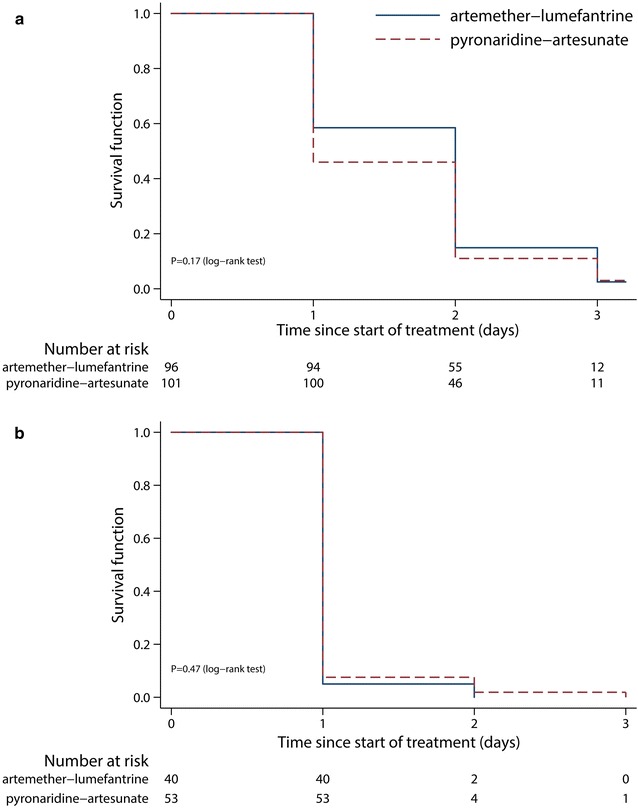
Kaplan–Meier estimates for a time to parasite clearance (intention-to-treat population) and b time to fever clearance. Participants without the event (parasite or fever clearance) were censored at the last available parasite or fever assessment date
All patients were fever-free on day 3. Fever clearance time was similar between intervention groups (P = 0.47, log-rank test) (Fig. 3b). Median time to fever clearance and the proportion of patients with fever clearance on day 1, 2 and 3 are presented in Additional file 2.
At baseline, gametocyte prevalence by microscopy was 1.98% (95% CI 0.54–6.93) for PA and 4.17% (95% CI 1.63–10.2) for AL (Table 1). On day 7, 3.00% of participants in the PA group (95% CI 1.03–8.45) and 1.09% (95% CI 0.19–5.91) of participants in the AL group had microscopically detectable gametocytes.
Safety
Adverse events occurred in 41.6% of patients in the PA group and 34.4% of patients in the AL group (Table 4). One patient in the PA group was excluded on day 0 and one patient in the AL group was excluded on day 1, due to repeated vomiting. There were no serious adverse events observed during the study.
Table 4.
Summary of adverse events in the intention-to-treat population
| Pyronaridine–artesunate (n = 101) | Artemether–lumefantrine (n = 96) | |
|---|---|---|
| Adverse event leading to discontinuation of study drug | ||
| Vomiting | 1 (0.99) | 1 (1.04) |
| Adverse event of any cause | ||
| Patients with at least 1 event | 42 (41.6) | 33 (34.4) |
| Headache | 10 (9.90) | 10 (10.4) |
| Vomiting | 10 (9.90) | 5 (5.21) |
| Cough | 18 (17.8) | 15 (15.6) |
| Abdominal pain | 3 (2.97) | 6 (6.25) |
| Anorexia | 4 (3.96) | 6 (6.25) |
| Diarrhoea | 2 (1.98) | 4 (4.17) |
| Chills | 2 (1.98) | 0 |
| Fatigue | 4 (3.96) | 1 (1.04) |
| Myalgia | 1 (0.99) | 1 (1.04) |
| Nasopharyngitis | 3 (2.97) | 4 (4.17) |
| Dizziness | 1 (0.99) | 0 |
| Skin rash | 2 (1.98) | 1 (1.04) |
| Dark urine | 1 (0.99) | 1 (1.04) |
| Ear pain | 0 | 1 (1.04) |
| Chest pain | 0 | 2 (2.08) |
| Throat pain | 1 (0.99) | 0 |
| Neck pain | 0 | 1 (1.04) |
Data are n (%)
No patients had instances of post-baseline ALT or AST levels > 3 times the ULN. No potential Hy’s law cases were identified. Mean ALT levels on day 3 were 11.0 U/L (n = 66, SD: 6.3) in the PA group and 11.8 U/L (n = 61, SD: 8.6) in the AL group. On day 7, mean ALT levels were 11.7 U/L (n = 65, SD: 7.5) in the PA group and 11.2 U/L (n = 60, SD: 6.0) in the AL group. Mean AST levels on day 3 were 19.6 U/L (n = 62, SD: 7.6) in the PA group and 21.2 U/L (n = 52, SD: 8.9) in the AL group. On day 7, mean AST levels were 21.0 U/L (n = 62, SD: 8.0) in the PA group and 21.5 U/L (n = 63, SD: 8.2) in the AL group (Additional file 3).
The lowest Hb value measured was 6.2 g/dL. Similar Hb changes from baseline were found in the two intervention groups. Mean Hb concentrations on day 3 compared to baseline decreased 0.8 and 0.6 g/dL for the PA and the AL group, respectively, and recovered by day 28 (Additional file 3).
Discussion
Treatment of uncomplicated P. falciparum malaria in Kenyan children aged ≤ 12 years with PA tablets or granules resulted in a PCR-corrected ACPR of 98.9% on day 28 in the per-protocol population, which is above the 95% standard set by the WHO in 2010 for the selection of a new or alternative anti-malarial [27]. However, the newest 2015 WHO guidelines for the treatment of malaria do not specifically state this 95% threshold [2]. PCR-corrected ACPR in the AL-group was 96.4% and non-inferiority of PA to AL was demonstrated. These and day 42 PCR-corrected efficacy estimates were consistent with previously reported ACPR rates in adults and children with P. falciparum malaria living in areas without artemisinin resistance [9, 10, 14].
The ACPR estimates in the uncorrected analysis, however, were lower compared to reports from Tshefu et al. [10] and Rueangweerayut et al. [9], but very similar to those presented reported by Kayentao et al. [14], due to a higher number of reinfections. This may be explained by differences in transmission rates between the studies or by age differences (mean age 6.9 and 4.9 years in the current study and Kayentao et al. [14], respectively, versus 17.2 and 25 years in the other studies [9, 10]). Better immunity to P. falciparum in the older population might have contributed to a longer prophylactic effect [14].
No difference in the rate of recrudescence was found between study groups. Reinfections were anticipated to occur later in the PA group compared to the AL group, as observed in a previous trial, due to the longer half-life of pyronaridine [10]. However, no differences in reinfection rates were found between treatment groups, similar to the findings of Kayentao et al. [14]. This disagreement may be related to differences in the patient population or, in the present study, to the sample size.
Both treatments rapidly cleared P. falciparum and no difference in parasite clearance time was observed between the two study groups, in contrast to previous trials where parasite clearance time was faster with PA [9, 10, 14]. All patients were fever free on day 3 and no difference in fever clearance time was observed, in line with a previous study [10], while another trial showed faster fever clearance with PA [14].
Safety findings were similar to previous reports on PA and pyronaridine and artesunate monotherapy [9–11, 14, 15, 23, 28–30]. Importantly and in contrast to former studies, none of the ALT and/or AST concentrations measured was > 3 times the ULN on day 3 and/or 7. No indication of hepatotoxicity was found.
A limitation of this study is that the initially planned sample size was not reached, which led to a reduction of power. However, PA efficacy estimates were never lower than AL estimates, and for the primary efficacy endpoint the lower limit of the 95% CI was − 2.8, whereas − 7 was the limit for demonstrating non-inferiority (Table 2). This strengthens this studies’ conclusion that PA was non-inferior to AL. The present study included only 2 children ≤ 1 year in the AL group (both had an ACPR at day 28), and more efficacy and safety data on this patient group is still needed for PA [15]. Furthermore, the study was conducted from an outpatient clinic and participants were not hospitalized. It was, therefore, logistically not feasible to collect more than one sample per day, while other studies collected a sample every 8 h for the first 3 days. The latter provides a more detailed insight into parasite and fever clearance dynamics. Concerning hepatotoxicity, ALT and AST were not measured for all participants, due to logistic constraints and because not enough serum was available for all participants. The hepatic enzyme profile is, therefore, not complete.
Conclusions
This study adds much needed data to the efficacy and safety profile of PA in children. PA granules and tablets were shown to be effective and well tolerated for the treatment of uncomplicated P. falciparum malaria in Kenyan children aged ≤ 12 years. These findings are in line with previous reports and inclusion of pyronaridine–artesunate in paediatric malaria treatment programs should be considered.
Additional files
Additional file 1. Weight-based dosing of pyronaridine–artesunate and artemether–lumefantrine.
Additional file 2. Parasite and fever clearance at day 1, 2 and 3.
Additional file 3. Haemoglobin (Hb), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values.
Authors’ contributions
JR, PS, HS and PM were responsible for study concept and design. JR, PS, NM, GO, VO, SO and FC were involved in data collection. JR performed the data analysis and coordinated writing of the manuscript. All authors critically reviewed the paper. All authors read and approved the final manuscript.
Acknowledgements
We thank the team of St. Jude’s Clinic, study participants and their parents/guardians. We thank Dr. Michèle van Vugt, Dr. Tjalling Leenstra and Dr. Mark Ogundo for their work in the data safety and monitoring board (DSMB). We also thank Dr. Sarah Arbe-Barnes (Artemida Pharma) for her organizational role. Finally, we thank Shin-Poong and MMV for their partnership and for providing pyronaridine–artesunate.
Competing interests
The authors declare that they have no competing interests. Shin Poong Pharmaceutical Company (Seoul, South-Korea) provided pyronaridine–artesunate tablets and granules, but had no further role in study design, data collection, data analysis and writing of the report.
Availability of data and materials
The datasets used and/or analyzed during the current study are available on request from the corresponding author.
Consent for publication
No details relating to individual participants are presented in this manuscript.
Ethics approval and consent to participate
Ethical approval was obtained from the Ethical Review Committee of the Kenya Medical Research Institute (KEMRI) (NON-SSC No. 479, registered at clinicaltrials.gov under NCT02411994). Written informed consent from a parent or guardian was required for study participation, assent was sought from children able to understand the study.
Funding
This work was supported by the EU FP7-Health-2013. 0-1 Project “Translation of the direct-on-blood PCR-NALFIA system into an innovative near point-of-care diagnostic for malaria” (DIAGMAL) [Grant Number 601714].
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12936-018-2340-3) contains supplementary material, which is available to authorized users.
References
- 1.WHO. World Malaria Report 2017. Geneva: World Health Organization; 2017. http://www.who.int/malaria/publications/world-malaria-report-2017/report/en/. Accessed 15 Jan 2018.
- 2.WHO. Guidelines for the treatment of malaria. 3rd ed. Geneva: World Health Organization; 2015. http://www.who.int/malaria/publications/atoz/9789241549127/en/. Accessed 15 Jan 2018.
- 3.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. NEJM. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fairhurst RM, Dondorp AM. Artemisinin-resistant Plasmodium falciparum malaria. Microbiol Spectr. 2016;4:El10-0013-2016. [DOI] [PMC free article] [PubMed]
- 5.Medicines for Malaria Venture (MMV). Pyramax (pyronaridine–artesunate). 2017. https://www.mmv.org/access/products-projects/pyramax-pyronaridine–artesunate. Accessed 23 Nov 2017.
- 6.European Medicines Agency. Summary of opinion: Pyramax (pyronaridine–artesunate). http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_for_use_outside_EU/2015/11/WC500196738.pdf. Accessed 10 Dec 2017.
- 7.Croft SL, Duparc S, Arbe-Barnes SJ, Craft JC, Shin CS, Fleckenstein L, et al. Review of pyronaridine anti-malarial properties and product characteristics. Malar J. 2012;11:270. doi: 10.1186/1475-2875-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44:697–704. doi: 10.1128/AAC.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Ph D, et al. Pyronaridine–artesunate versus mefloquine plus artesunate for malaria. NEJM. 2012;366:1298–1309. doi: 10.1056/NEJMoa1007125. [DOI] [PubMed] [Google Scholar]
- 10.Tshefu AK, Gaye O, Kayentao K, Thompson R, Bhatt KM, Sesay SSS, et al. Efficacy and safety of a fixed-dose oral combination of pyronaridine–artesunate compared with artemether–lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised non-inferiority trial. Lancet. 2010;375:1457–1467. doi: 10.1016/S0140-6736(10)60322-4. [DOI] [PubMed] [Google Scholar]
- 11.Poravuth Y, Socheat D, Rueangweerayut R, Uthaisin C, Pyae Phyo A, Valecha N, et al. Pyronaridine–artesunate versus chloroquine in patients with acute Plasmodium vivax malaria: a randomized, double-blind, non-inferiority trial. PLoS ONE. 2011;6:e14501. doi: 10.1371/journal.pone.0014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duparc S, Borghini-fuhrer I, Craft JC, Arbe-barnes S, Miller RM, Shin C, et al. Safety and efficacy of pyronaridine–artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials. Malar J. 2013;12:70. doi: 10.1186/1475-2875-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukirwa H, Unnikrishnan B, Kramer C, Sinclair D, Nair S, Tharyan P. Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev. 2014;3:CD006404. doi: 10.1002/14651858.CD006404.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayentao K, Doumbo OK, Pénali LK, Offianan AT, Bhatt KM, Kimani J, et al. Pyronaridine–artesunate granules versus artemether–lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial. Malar J. 2012;11:364. doi: 10.1186/1475-2875-11-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sagara I, Beavogui AH, Zongo I, Soulama I, Borghini-fuhrer I, Fofana B, et al. Safety and efficacy of re-treatments with pyronaridine–artesunate in African patients with malaria: a sub-study of the WANECAM randomised trial. Lancet Infect Dis. 2016;16:189–198. doi: 10.1016/S1473-3099(15)00318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leang R, Canavati SE, Khim N, Vestergaard LS, Borghini-Fuhrer I, Kim S, et al. Efficacy and safety of pyronaridine–artesunate for treatment of uncomplicated Plasmodium falciparum malaria in Western Cambodia. Antimicrob Agents Chemother. 2016;60:3884–3890. doi: 10.1128/AAC.00039-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Minutes of the Technical Expert Group on Drug Efficacy and Response. Geneva: World Health Organization; 2017. http://www.who.int/malaria/mpac/mpac-oct2017-teg-drug-efficacy-response-session3.pdf. Accessed 9 Jan 2018.
- 18.Nosten FH. Pyronaridine–artesunate for uncomplicated falciparum malaria. Lancet. 2010;375:1413–1414. doi: 10.1016/S0140-6736(10)60582-X. [DOI] [PubMed] [Google Scholar]
- 19.Rogerson SJ, Wijesinghe RS, Meshnick SR. Host immunity as a determinant of treatment outcome in Plasmodium falciparum malaria. Lancet Infect Dis. 2010;10:51–59. doi: 10.1016/S1473-3099(09)70322-6. [DOI] [PubMed] [Google Scholar]
- 20.WHO. World malaria report 2016. Geneva: World Health Organization; 2016. http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/. Accessed 20 Dec 2017.
- 21.Kurth F, Bélard S, Basra A, Ramharter M. Pyronaridine–artesunate combination therapy for the treatment of malaria. Curr Opin Infect Dis. 2011;24:564–569. doi: 10.1097/QCO.0b013e32834cabdb. [DOI] [PubMed] [Google Scholar]
- 22.Kurth F, Bélard S, Adegnika AA, Gaye O, Kremsner PG, Ramharter M. Do paediatric drug formulations of artemisinin combination therapies improve the treatment of children with malaria? A systematic review and meta-analysis. Lancet Infect Dis. 2010;10:125–132. doi: 10.1016/S1473-3099(09)70327-5. [DOI] [PubMed] [Google Scholar]
- 23.Ramharter M, Kurth F, Schreier AC, Nemeth J, Von Glasenapp I, Schlie M, et al. Fixed-dose pyronaridine–artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. J Infect Dis. 2008;198:911–919. doi: 10.1086/591096. [DOI] [PubMed] [Google Scholar]
- 24.WHO. Basic malaria microscopy–part I: learner’s guide. 2nd ed. Geneva: World Health Organization; 2010. http://www.who.int/malaria/publications/atoz/9241547820/en/. Accessed 20 Dec 2017.
- 25.WHO. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. Geneva: World Health Organization; 2008. http://www.who.int/malaria/publications/atoz/9789241596305/en/. Accessed 20 Dec 2017.
- 26.WHO. Methods for surveillance of antimalarial drug efficacy. Geneva: World Health Organization; 2009. http://www.who.int/malaria/publications/atoz/9789241597531/en/. Accessed 15 Jan 2018.
- 27.WHO. Guidelines for the treatment of malaria. 2nd ed. Geneva: World Health Organization; 2010. http://apps.who.int/medicinedocs/documents/s19105en/s19105en.pdf. Accessed 16 Jan 2018.
- 28.Ringwald P, Bickii J, Basco L. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet. 1996;347:24–28. doi: 10.1016/S0140-6736(96)91558-5. [DOI] [PubMed] [Google Scholar]
- 29.Ringwald P, Bickii J, Basco LK. Efficacy of oral pyronaridine for the treatment of acute uncomplicated falciparum malaria in African children. Clin Infect Dis. 1998;26:946–953. doi: 10.1086/513942. [DOI] [PubMed] [Google Scholar]
- 30.Price R, van Vugt M, Phaipun L, Luxemburger C, Simpson J, McGready R, et al. Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am J Trop Med Hyg. 1999;60:547–555. doi: 10.4269/ajtmh.1999.60.547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Weight-based dosing of pyronaridine–artesunate and artemether–lumefantrine.
Additional file 2. Parasite and fever clearance at day 1, 2 and 3.
Additional file 3. Haemoglobin (Hb), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values.
Data Availability Statement
The datasets used and/or analyzed during the current study are available on request from the corresponding author.


