Abstract
There is a hypothesis that Mass drug administration (MDA) of ivermectin and albendazole for the treatment of onchocerciasis and lymphatic filariasis could have an impact on the burden of soil-transmitted helminthiasis (STH) in MDA communities. We, therefore, assessed the burden of STH (Ascaris lumbricoides, Trichuris trichiura, and hookworm) infections in nine communities from 3 LGAs (two MDA local government areas (LGAs) and one control LGA) in Kebbi State, Nigeria after 5-years (2010–2015) of MDA for onchocerciasis and/or lymphatic filariasis. We also administered questionnaire to obtain demographic information and history of MDA in the past five years. The three LGAs are Bagudo (Ivermectin MDA); Zuru (Ivermectin/Albendazole MDA) and Dandi (No MDA). The study was a cross sectional survey. The total number of people that complied with provision of stool samples and questionnaire were 1357 persons; stool samples collected were examined for STH infections in the three LGAs. Zuru LGA had the highest prevalence of STH (41.89, 95% CI: 37.08–46.81) followed by Dandi LGA (24.66, 95% CI: 20.69–28.97) and Bagudo LGA (3.36, 95% CI: 1.97–5.32). Prevalence of STH infection was not significantly different among age group and sex. Geometric mean intensity per gram of infection for both A. lumbricoides and Hookworm were highest in Zuru LGA with (1.16 GMI, 95% CI: 0.97–1.36) and (1.49 GMI, 95% CI: 1.29–1.70) respectively. Treatment coverage was less than 65% from 2010 to 2013 in the intervention LGAs. The study shows that STH is still a public health problem in Zuru LGA (IVM + ALB) and requires MDA of albendazole for STH control to continue, while Dandi LGA (No MDA history) requires MDA with albendazole to scale up treatment for STH control.
Keywords: Assessment, Soil-transmitted helminthiasis, Mass drug administration, Onchocerciasis, Lymphatic filariasis, Control
1. Introduction
The soil transmitted helminthiasis (STH), caused by Ascaris lumbricoides, Trichuris trichiura and hookworms (Necator americanus, and Ancyclostoma duodenale), are endemic in Nigeria (Hotez and Kamath, 2009, Hotez et al., 2012, Oluwole et al., 2015). These infections are common where there are poor hygiene practices, including limited environmental sanitation, unsafe water sources, inadequate toilet facilities, and poor faecal disposal methods, coupled with poverty and low household income (Ekpo et al., 2008, Strunz et al., 2014). The recent global commitment to control or eliminate NTDs by 2020 (Bergquist et al., 2015) has necessitated the mobilization of resources and funding for NTDs such as the Preventive Chemotherapy and Transmission control (PCT) NTDs in all endemic countries including Nigeria (Seddoh et al., 2013, Lenk et al., 2016). In Nigeria, these include Onchocerciasis, Lymphatic Filariasis (LF), Schistosomiasis, STH, and Trachoma (FMOH, 2012). Implementation of Mass drug administration for the control of onchocerciasis and lymphatic filariasis (LF) has been on going in the country since 2000. However, nationwide implementation of control activities for STH has been limited. MDA for soil transmitted helminths has not commenced in all endemic communities in Nigeria. In a bit to scale up MDA for soil transmitted helminths in all endemic countries, a programmatic question was asked; “is there a need to establish MDA for STH in communities where MDA for onchocerciasis or Lymphatic filariasis(LF) is currently ongoing?” This question was since ivermectin, a drug given for the control of onchocerciasis has been shown to be effective against STH parasites (Moncayo et al., 2008) and albendazole which is provided in combination with ivermectin for the control of LF is a World Health Organization recommended drug for the control of STH(WHO, 2016). Therefore, expectation is that communities, where MDA for onchocerciasis or LF is ongoing, should enjoy a reduction in morbidity effect resulting from STH burden (Tchuem Tchuenté, 2011). The impact of several years MDA of ivermectin and albendazole for the control of LF on the dynamics and decrease of STH infections has been reported in several countries (De Rochars et al., 2004, Ndyomugyenyi et al., 2008, Montresor et al., 2008, Stothard et al., 2009) and in Eastern Nigeria (Gutman et al., 2010). However, conducting assessments in the likelihood of scaling up STH control is not available in Nigeria. To this end, we have conducted a preliminary assessment of the burden of STH in three purposefully selected LGAs in Kebbi state, Northern Nigeria where MDA for onchocerciasis or LF has been ongoing for at least 5 consecutive years in two of the LGAs and while MDA for STH is about to commence in the third LGA.
2. Methodology
2.1. Study area
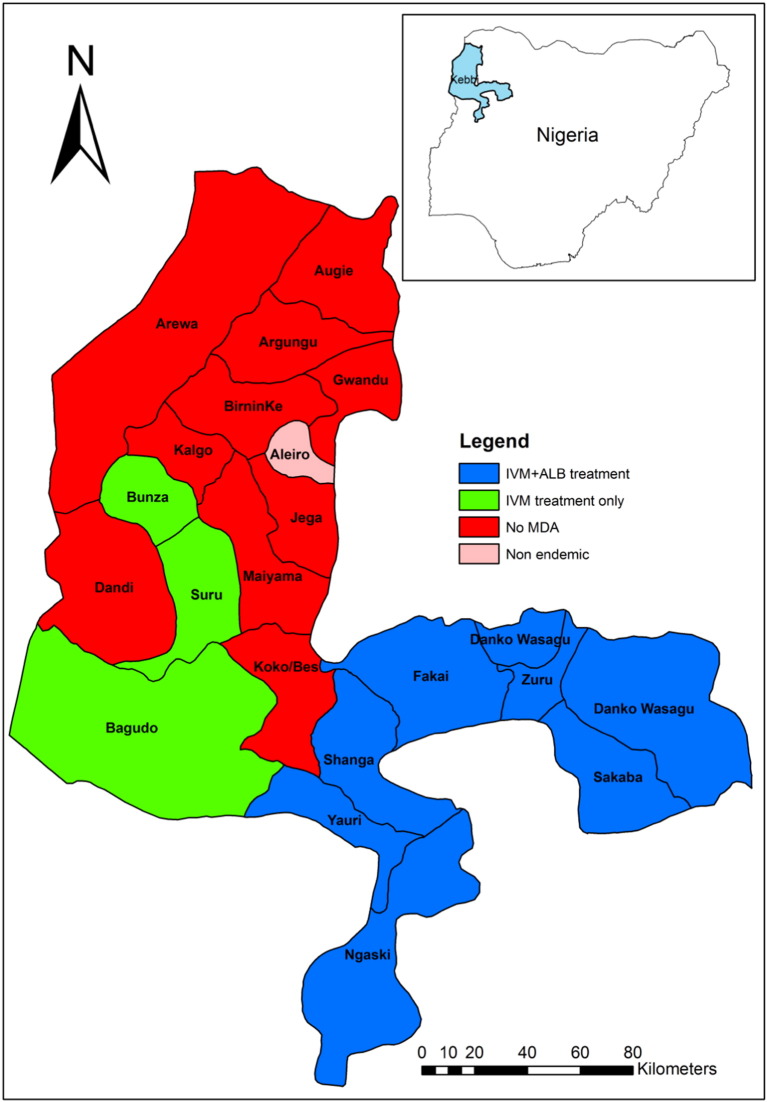
Kebbi State is in North-Western Nigeria (Fig. 1). It is endemic for both onchocerciasis and LF with a prevalence range of 1–30% and 2–58% respectively (FMoH, 2012). There are twenty-two LGAs in the State; nine of them are endemic for both onchocerciasis and LF, while eleven are endemic for LF only. Mass Drug Administration (MDA) of Ivermectin for the control of onchocerciasis in Kebbi State started in 1997. In 2010, Ivermectin and Albendazole were administered for the treatment of LF in 7 LGAs where it co-exists with onchocerciasis. Therefore, MDA for both diseases has been ongoing for 5 years where the two diseases co-exist in Kebbi (Fig. 1). Treatment data shows that many communities had received up to ten rounds of treatment with Ivermectin only and up to five rounds of treatment with Ivermectin + Albendazole. Bagudo, Dandi, and Zuru LGAs where selected for the study.
Fig. 1.
Map of Kebbi state showing implementation of MDA by LGAs with Nigeria (inset).
2.2. Study design and population
The study was a retrospective randomized controlled trial coupled with a cross-sectional survey. The LGAs in Kebbi state were grouped into three based on their history and type of MDA. One LGA was randomly selected from each MDA group. We randomly selected three communities from each LGA as shown below in Table 1.
Table 1.
Selection of LGAs and study communities in Kebbi State.
| Criteria for selection | LGA selected | Communities | Year MDA treatment started in the LGA |
|---|---|---|---|
| Communities treated with Ivermectin only | Bagudo LGA | Giris Hausawa | MDA with Ivermectin started in 1997 for onchocerciasis |
| Kali | |||
| Kabaka | |||
| Communities treated with Ivermectin and Albendazole | Zuru LGA | Isgogo | MDA for onchocerciasis started in 1997 and Albendazole was added in 2010 for LF |
| Selchi | |||
| Tadurga | |||
| Communities without any history of MDA | Dandi LGA | Geza | No history MDA |
| Fingillah | |||
| Kyangakwai |
All community members with residency of 2 years and above who are willing to participate and able to provide a stool sample were included in the study. Children less than two years' old, sick individuals, pregnant, nursing mothers within seven days of delivery and individuals whose residency in the communities were less than two years were excluded from the study.
2.3. Sampling size determination
The sample size was calculated using estimated LGA population of 5000, with an expected prevalence of STH to be 20% (FMOH, 2015), the desired precision of ± 5% and a design effect for 1 for random sampling. Thus, a sample size of 370 persons was computed and adjusted to 400. The sample size was determined using the method of Yamane (1967) as follows
n = N/1 + Ne2
N: total population
n: require a sample size
e: the error at 95% CI
However, to cater for non-compliance, we set our sample size at 500 samples per LGA.
2.4. Community mobilization and ethical issues
Before the activity, we visited the LGAs and communities that were selected for the study to mobilize the community leaders and community members about the activity. Ethical clearance for the study was obtained from the Kebbi State Ministry of Health with an approval reference number KSHREC 101.1/2013. Verbal informed consent was also obtained from participants who volunteered to take part in the study after explaining the aim and objectives of the study to them in Hausa language. Participation was voluntary. After the study, we treated all participants and members of the communities selected for the study with Mectizan® (Merck Sharp & Dohme (MSD)) and Albendazole (GlaxoSmithKline (GSK)). Both drugs were donated to the National Onchocerciasis and Lymphatic filariasis programme of the Federal Ministry of Health (FMOH) Nigeria. The drugs were administered through mass drug administration to communities using the community directed Intervention (CDI) as recommended by the FMOH, Nigeria.
2.5. Data collection
Due to the Hausa culture popularly known as “bar shiga” (meaning; A man is not permitted to enter the house), a house to house data collection was not feasible because 70% of the research team members were men. Hence, data collection was centralized at either a school or the village head's house. Each participant that consented to participate in the study was administered a questionnaire to collect information on their demography including sex, age and history of individual MDA and deworming drugs treatment in the last five years (2010–2014). We assessed the treatment coverage using the formula as follows
where
A = number of participants that claimed to have received treatment in that years.
B = number of participants that enrolled for the study.
Each participant was given a clean sheet of paper and one universal sample bottle with an identification number and an in-built spoon for collection of fresh, uncontaminated faecal sample. One faecal sample was obtained from each participant and 10 ml of Sodium acetate-Acetic acid-Formalin solution (SAF) was poured into each faecal sample immediately they were submitted by the participants to arrest further biological activity of the parasite. The samples were then taken to a nearby laboratory for processing and microscopic examination. Due to the drawback of Kato Katz for stool examination in areas with low intensity of infection (Glinz et al., 2010). Approximately 1 g each of faecal samples were processed using SAF-Ether concentration methods and examined for detection and estimation of helminth ova. All faecal samples were processed within 48 hours of collection. We used the World Health Organization standard diagnostic bench-aid for STH species identification.
2.6. Data entry and analysis
We used EpiData programme (Christiansen and Lauritsen, 2010) for data entering. Only complete data (questionnaire + faecal samples) were included in the analysis. Analysis done includes descriptive and chi-square statistics. Since egg count data were not normally distributed and skewed towards low egg counts, we use logarithmic transformation and geometric means for faecal egg density per gram. We compared the geometric means of egg count across the three LGAs using non-parametric Kruskal Wallis test and employed, Mann Whitney U test to compare geometric means of egg count between sex and the two age groups. We run all analysis in STATA version 12 (Stata Corp LP, USA).
3. Results
3.1. Study adherence
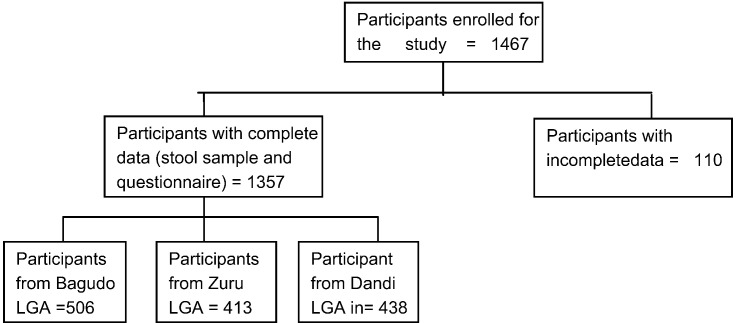
Participants that enrolled and took part in the study were 1467, but only 1357 of them provided stool sample and completed the study questionnaire. A total of 506 (37.29%) were from Bagudo (IVM only), 413 (30.43%) were from Zuru LGA (IVM + ALB) and 438 (32.28) were from Dandi LGA (no history of MDA) (Fig. 2).
Fig. 2.
Flow chart on study adherence.
3.2. Treatment history on mass drug administration programmes
Treatment pattern varied at the three LGA with percentage number of people treated ranging from 13.01% in Dandi LGA to 93.08% in Bagudo LGA (Table 2). Although in Dandi LGA (without a history of MDA), 13.10% of the participants who received treatment got it from another local government in 2015. Treatment history in three LGAs in the last five years (2010–2014) reveal that from year 2010 till 2012 treatment coverage in the two MDA LGAs were below 50% but increases tremendously from 2013 to 2014 (Table 2).
Table 2.
Treatment History in the three LGAs selected in Kebbi State.
| Variables | Bagudo (IVM only) n = 506 | Zuru (IVM + ALB) n = 413 | Dandi (No MDA) n = 438 |
|---|---|---|---|
| Treated with MDA before | |||
| Yes | 471 (93.08%) | 380 (92.01%) | 57 (13.01%) |
| No | 35 (6.92%) | 33(7.99%) | 381 (86.99%) |
| History of MDA in the last five years | |||
| MDA in 2010 | 115 (22.73) | 49 (11.86) | Not applicable |
| MDA in 2011 | 181 (35.77) | 83 (20.09) | Not applicable |
| MDA in 2012 | 244 (48.22) | 153 (37.05) | Not applicable |
| MDA in 2013 | 313 (61.86) | 335 (81.11) | Not applicable |
| MDA in 2014 | 469 (92.69) | 399 (96.61) | Not applicable |
3.3. Prevalence and intensity of STH infections
Prevalence of STH was highest (41.89, 95% CI: 37.08–46.81) in Zuru LGA followed by Dandi LGA which had a prevalence of (24.66, 95% CI: 20.69–28.97) while Bagudo LGA had the least prevalence of 3.36% for STH (Table 3). The most common STH parasite infection in the study population as shown in Table 2 is Hookworm (13.63, 95% CI: 11.85–15.57) while the least common one is T. trichuira (0.29, 95% CI: 0.08–0.75) (Table 3)
Table 3.
Prevalence of STH by parasite species, sex and age group in the three LGAs selected in Kebbi State, Nigeria.
| Variable | Bagudo (IVM only) Prevalence (95%CI)n |
Dandi (No MDA) Prevalence (95%CI)n |
Zuru (IVM + ALB) Prevalence (95%CI)n |
Total Prevalence (95%CI)n |
|---|---|---|---|---|
| STH | 3.36 (1.97–5.32)506 | 24.66 (20.69–28.97)438 | 41.89 (37.08–46.81)413 | 21.96 (19.78–24.26)1357 |
| Ascaris lumbricoides | 1.19 (0.44–2.56)506 | 15.07 (11.85–18.77)438 | 15.25 (11.92–19.09)413 | 9.95 (8.41–11.67)1357 |
| Hookworm | 2.17 (1.09–3.86)506 | 12.33 (9.40–15.78)438 | 29.06 (24.72–33.69)413 | 13.63 (11.85–15.57)1357 |
| Trichuris trichiura | 0.20 (0.01–1.10)506 | 0.46 (0.06–1.64)438 | 0.24 (0.01–1.34)413 | 0.29 (0.08–0.75)1357 |
| Prevalence by age group | ||||
| STH | ||||
| School age (5–15 years) | 3.72 (1.71–6.94)242 | 24.29 (18.65–30.66)210 | 41.60 (35.42–47.98)250 | 23.36 (20.28–26.67) 702 |
| Adult (> 15 years) | 3.03 (1.32–5.88)264 | 25.00 (19.52–31.14)228 | 42.33 (34.64–50.30)163 | 20.45 (17.43–23.75) 655 |
| p-Value | 0.806 | 0.912 | 0.883 | 0.213 |
| Ascaris lumbricoides | ||||
| School age (5–15 years) | 0.83 (0.10–2.95)242 | 17.14 (12.30–22.93)210 | 12.00 (8.24–16.69)250 | 9.69 (7.60–12.12)702 |
| Adult (> 15 years) | 1.52 (0.41–3.83)264 | 13.16(9.06–18.25)228 | 20.25 (14.36–27.24)163 | 10.23 (8.032–12.81)655 |
| p-Value | 0.687 | 0.285 | 0.023 | 0.786 |
| Hookworm | ||||
| School age (5–15 years) | 2.48 (0.92–5.32)242 | 10.95 (7.07–15.98)210 | 32.0 (26.26–38.17)250 | 15.53 (12.93–18.42)702 |
| Adult (> 15 years) | 1.89 (0.62–4.36)264 | 13.60 (9.43–18.74) 228 | 24.54 (18.15–31.88)163 | 11.60 (9.25–14.31)655 |
| p-Value | 0.764 | 0.468 | 0.103 | 0.039 |
| Prevalence by sex | ||||
| STH | ||||
| Male | 3.32 (1.53–6.21)271 | 26.32 (20.72–32.54)228 | 45.03 (37.84–52.37)191 | 22.46 (19.40–25.76)690 |
| Female | 3.40 (1.48–6.60)235 | 22.86 (17.36–29.14)210 | 39.19 (32.73–45.95)222 | 21.44 (18.38–24.75)667 |
| p-Value | 1.00 | 0.438 | 0.231 | 0.694 |
| Ascaris lumbricoides | ||||
| Male | 1.11 (0.23–3.20)271 | 15.79 (11.31–21.18)228 | 14.66 (9.97–20.49)191 | 9.71 (7.61–12.17)690 |
| Female | 1.28 (0.26–3.69)235 | 14.29(9.85–19.76)210 | 15.77(11.23–21.24)222 | 10.19 (8.00–12.75)667 |
| p-Value | 1.00 | 0.690 | 0.755 | 0.786 |
| Hookworm | ||||
| Male | 2.21 (0.82–4.77)271 | 12.28 (8.32–17.26)228 | 33.51 (26.86–40.68)191 | 14.20 (11.68–17.03)690 |
| Female | 2.13 (0.69–4.90)235 | 12.38 8.25–17.61)210 | 25.23 (19.65–31.47)222 | 13.04 (10.58–15.84)667 |
| p-Value | 1.00 | 1.00 | 0.065 | 0.580 |
n = number examined.
In relation to specific STH parasite, we observed that Zuru had the highest prevalence of A. lumbricoides (15.25, 95% CI: 11.92–19.09) and Hookworm (29.06, 95% CI: 24.1–33.69) while Bagudo LGA (IVM only) had the least prevalence of A. lumbricoides (1.19, 95% CI 0.4–2.56) and hookworm (2.17, 95% CI: 1.09–3.86) (Table 3). Dandi LGA had Ascaris infection prevalence of (15.07, 95% CI: 11.85–18.77) and hookworm prevalence of (12.33, 95% CI: 9.39–15.78).
Geometric mean intensity (GMI) of infection per gram was highest for both A. lumbricoides infection (1.16 GMI, 95% CI: 0.97–1.36) and hookworm infection (1.49 GMI, 95% CI: 1.29–1.70) in Zuru LGA followed by Dandi LGA (i.e. (1.06 GMI 95% CI: 0.90–1.22) and (0.68 GMI, 95% CI: 0.50–0.85) for A. lumbricoides and hookworm infection respectively (Table 4). Bagudo had the least geometric mean intensity of infection for both A. lumbricoides (0.18 GMI, 95%: CI − 0.18–0.55) and hookworm (0.13 GMI, 95% CI: 0.04–0.29)
Table 4.
Geometric mean intensity (GMI) of STH by parasite species, sex and age group in the three LGAs selected in Kebbi State, Nigeria.
| Variable | Bagudo (IVM only) GMI (g) (95% CI) |
Dandi (No MDA) GMI (g) (95% CI) |
Zuru (IVM + ALB) GMI (g) (95% CI) |
Total GMI (95% CI) |
|---|---|---|---|---|
| Ascaris lumbricoides | 0.18 (− 0.18–0.55) | 1.06 (0.90–1.22) | 1.16 (0.97–1.36) | 1.07 (0.95–1.20) |
| Hookworm | 0.13 (− 0.04–0.29) | 0.68 (0.50–0.85) | 1.49 (1.29–1.70) | 1.17 (1.02–1.33) |
| Trichuris trichiura | – | – | – | – |
| Intensity by age group | ||||
| Ascaris lumbricoides | ||||
| School age (5–15 years) | 0.55 (− 0.86 1.96) | 0.97 (0.77–1.16) | 1.25 (0.94–1.56) | 1.08 (0.91–1.25) |
| Adult (> 15 years) | 0 | 1.17 (0.90–1.44) | 1.09 (0.83–1.35) | 1.06 (0.88–1.25) |
| p-Value | 0.1573 | 0.3949 | 0.5843 | 0.7893 |
| Hookworm | ||||
| School age (5–15 years) | 0.12 (− 0.14–0.37) | 0.59 (0.28–0.90) | 1.62 (1.38–1.87) | 1.32 (1.11–1.53) |
| Adult (> 15 years) | 0.14 (− 0.17–0.45) | 0.74 (0.52–0.96) | 1.23 (0.88–1.59) | 0.96 (0.74–1.18) |
| p-Value | 0.8918 | 0.1126 | 0.0165 | 0.0194 |
| Intensity by sex | ||||
| Ascaris lumbricoides | ||||
| Male | 0.37 (− 0.58–1.31) | 1.10 (0.88–1.33) | 1.34 (1.01–1.68) | 1.17 (0.98–1.36) |
| Female | 0 | 1.01 (0.79–1.24) | 1.03 (0.79–1.26) | 0.98 (0.82–1.14) |
| p-Value | 0.3173 | 0.8407 | 0.1147 | 0.1860 |
| Hookworm | ||||
| Male | 0.23 (0.09–0.56) | 0.75 (0.48–1.03) | 1.30 (1.07–1.53) | 1.08 (0.90–1.26) |
| Female | 0 | 0.59 (0.36–0.82) | 1.72 (1.37–2.06) | 1.28 (1.02–1.54) |
| p-Value | 0.1736 | 0.4760 | 0.1370 | 0.5672 |
GMI (g) = geometrical mean intensity per gram.
Infection with T. trichuira was extremely low with only 4 persons having infection. Low prevalence were observed in all the LGAs with a prevalence of (0.20, 95% CI: 0.05–1.09), (0.24, 95% CI: 0.01–1.34) and (0.46, 95% CI: 0.06–1.64) from Bagudo, Zuru and Dandi LGAs respectively. The intensity of infection per gram was highest in Zuru where the only infected person had ten ova in the faecal sample as compared to one ovum found in those infected in Dandi and Bagudo LGA (Table 4).
Overall prevalence of STH in the study population was higher among school age children (23.36, 95% Cl: 20.28–26.67) with no statistical difference from adult (20.45, 95% Cl: 17.43–23.75). However, within the LGAs, prevalence of STH was higher among adult with no statistical difference from that of the school age children in Dandi and Zuru LGAs (Table 3)
Prevalence of A. lumbricoides was generally higher among adult (10.23, 95% Cl: 8.032–12.81) with no statistical different from the school age (9.69, 95% Cl: 7.60–12.12). This same pattern was observed in Bagudo and Zuru LGA. On the contrary prevalence of A. lumbricoides was higher among the school age children (17.14, 95:Cl 12.30–22.93) but is not statistically different from of adult (13.16, 95% Cl: 9.06–18.25) in Dandi LGA (Table 3).
Overall prevalence of hookworm infection in the study population was higher among school age children (15.53, 95% Cl: 12.93–18.42) than in adult (11.60, 95% Cl: 9.25–14.31) but was not statistically significant. This pattern of prevalence exists within age group in each LGAs (Table 3).
Similarly, there was no statistical difference in the geometric mean intensity of infection with A. lumbricoides within the age group. This was so for the three parasites in the three LGAs (Table 4).
In relation to sex, we observed no statistical difference in the overall prevalence of STH and specific STH parasites (A. lumbricoides and Hookworm infections) within sex in the three LGAs, although it was observed that prevalence was more in males than in females for each of the STH parasites and in the three LGAs (Table 3). This same profile was observed in infection intensity for the three parasites in all the three LGAs (Table 4).
4. Discussion
Operational research to evaluate impact of MDA on NTDs morbidity and prevalence is necessary to monitor progress of control programme and identify challenges to the control of the targeted diseases. This is particularly important with the global focus geared towards elimination or eradication of NTDs in endemic communities (Bergquist et al., 2015). The data obtained on the participants' treatment history shows a continuous increase in the number of persons treated yearly in the MDA LGAs. This is a good result as it shows progress in the control/elimination against the NTDs. A high therapeutic coverage in endemic communities is needed if control and reduction in transmission is to be achieved (Winnen et al., 2002, Bockarie et al., 2013). A commendable increase was observed in the number of persons that received treatment in the two MDA LGAs from year 2012 to 2014. We attributed increase in the number of persons receiving treatment in Zuru LGA to the scale up in the treatment for LF. Before 2012, only selected communities in the LGA were receiving ivermectin treatment for Onchocerciasis but with the introduction of treatment for LF, all communities in the LGA started receiving treatment. This must have led to the high number of persons that received treatment in 2013 and 2014 in Zuru LGA.
Our study has provided information on the status of STH infection in three LGAs studied. Our finding shows that STH is still a major public health problem in Zuru LGA despite ongoing MDA (IVM + MDA) in the area while prevalence of STH in Bagudo LGA is lower than WHO threshold requiring MDA for control. Information on the prevalence of STH in Dandi will serve as a baseline data for future reference since MDA is yet to commence in this LGA before now.
The high prevalence of STH in Zuru LGA despite five years of administration of combine ivermectin and albendazole may be because of continuous re-infection after each MDA treatment rounds. As at the time of the study, the last treatment occurred about 10 months earlier. This time is long enough to allow for re-infection in the communities were exposure to the risk factors such as lack of access to portable water, sanitation facilities and poor hygiene practices are on daily bases. Studies has shown that re-infection with STH could occur within six month with prevalence returning to above 50% its initial level (Jia et al., 2012), therefore, these findings, therefore, support the claim that MDA alone is not sufficient for STH control if the focus is elimination/eradication of the disease in the endemic communities (Tchuem Tchuenté, 2011, Jia et al., 2012) most especially when the exposing risk factors are available. According to Tchuem Tchuenté (2011), three key interventions are essential for a long-term control and elimination of STHs in any endemic settings. These include anthelminthic drug treatment, sanitation, and health education. Tchuem Tchuenté (2011) stated that “Indeed, without an improvement in sanitation and a dramatic change in defecation habits, periodic de-worming cannot attain a stable reduction in transmission.” Hence, the high prevalence of STH in Zuru LGA may be due to lack or poor implementation of the other two-intervention strategy, i.e. sanitation and health education. These two strategies are expected to compliment anthelminthic drug treatment by reducing/preventing re-infection with the STH parasites (Ziegelbauer et al., 2012). However, a low prevalence of STH in Bagudo LGA were ivermectin only is given is expected. Studies have shown the potential of MDA with ivermectin to reduce STH burden in communities where it is being given for onchocerciasis control. (Maegga et al., 2006, Moncayo et al., 2008, Gutman et al., 2010). Among the three common STH parasites, we observed a lower prevalence of T. trichiura in all the three LGAs. This observation is in line with the prediction of Oluwole et al. (2015) on prevalence of T. trichiura in Kebbi state. The very low prevalence of T. trichiura may be due to extreme high temperatures (> 40 °C) usually experienced in the northern part of Nigeria. Ova of T. trichiura cannot withstand extreme temperatures (> 40 °C) in the soil unlike the ova of A. lumbricoides (Brooker et al., 2006). We do not observe a significant different in infection among sex. This is because both sexes have equal exposure to STH infection. We observed a higher prevalence and intensity of STH infection among school age children when compared to infection among adult. This is not abnormal as school aged children are known to be the most at risk of STH infections (WHO, 2002, Oluwole et al., 2015). However, our result shows a significantly higher intensity of hookworm infection among school aged children than adult. This observation is not in line with knowledge from literatures, as intensity of infection with hookworm is expected to increase with age peaking at adulthood (Bethony et al., 2002, Tchuem Tchuenté, 2011). This observation may be due to low socioeconomic status of their parents, who could only afford to buy footwear for themselves but not for their children. Hence, most children in the rural communities particularly those in the northern part of Nigeria walk barefooted, thereby increasing their risk of infection with hookworm whose transmission is majorly through skin penetration. To the best of our knowledge, this study is the first to report on the burden of STH in an area where MDA has been going for at least five years in the Northern part of Nigeria. Studies to assess the burden of the PCT NTD disease in areas where MDA is ongoing in Nigeria will be of interest to global stakeholders in NTDs. This is because WHO has stated that Nigeria accounts for 25% of the NTDs burden in Africa. Information on the progress made towards elimination of the PCT NTDs is useful to ascertain the impact of the commitment made during the London 2012 declaration on NTDs. This study is also the first to provide information on assessment of the burden of STH among school age children and adult in places where MDA is ongoing. Previous similar studies have limited the study to children only (Maegga et al., 2006, Moncayo et al., 2008, Gutman et al., 2010). Although school aged children are most at risk of STH infection, the burden of STH in the adult should be a concern if elimination is the goal.
4.1. Limitation of the study
We could not measure real impact of MDA in the two MDA LGAs since there was no previous baseline data on STH. This is because MDAs was based on the burden of onchocerciasis and lymphatic filariasis.
5. Conclusions
The study shows that STH is still a public health problem in Zuru LGA (IVM + ALB) and Dandi LGA (No MDA history) is qualified for MDA for STH control. Therefore there is need for scale up of treatment with albendazole for STH control in Dandi LGA. Provision of complimentary intervention such as safe water, sanitation, and hygiene education (WASH) will reduce transmission of STH in the studied areas. This study has provided information on the burden of STH after five years of mass drug administration for Onchocerciasis and Lymphatic filariasis in two LGAs and one LGA with no history of MDA in Kebbi State, Nigeria.
Support
Sightsavers Nigeria Country Office supported field data collection. However, Sightsavers had no role or influence in study design, data collection, analysis, and interpretation of data, manuscript writing and publication.
Conflict of interest
None.
Acknowledgement
We appreciate the cooperation and support of Kebbi State Ministry of Health to undertake this study. We are grateful to the village heads and their community member for their willingness to provide stool sample for analysis. Our appreciation also goes to the Head of the laboratories of hospitals and Director of primary health care (General hospitals in Dandi, Zuru and Bagudo LGAs) for granting us permission to use their equipment and space in the laboratories for stool processing and examination.
Contributor Information
Akinola Stephen Oluwole, Email: akinolaoluwole@gmail.com.
Sunday Isiyaku, Email: sisiyaku@sightsavers.org.
Christian Nwosu, Email: cnwosu@sightsavers.org.
Adamani William, Email: awilliam@sightsavers.org.
Elizabeth Elhassan, Email: eelhassan@sightsavers.org.
Uwem Friday Ekpo, Email: ufekpo@hotmail.com.
References
- Bergquist R., Yang G., Knopp S., Utzinger J., Marcel Tanner M. Surveillance and response: tools and approaches for the elimination stage of neglected tropical diseases. Acta Trop. 2015;141:229–234. doi: 10.1016/j.actatropica.2014.09.017. (Epub 2014 (accessed date: September 2015)) [DOI] [PubMed] [Google Scholar]
- Bethony J., Chen J., Lin S., Xiao S., Zhan B., Li S., Xue H., Xing F., Humphries D., Yan W., Chen G., Foster V., Hawdon J.M., Hotez P.J. Emerging patterns of hookworm infection: influence of aging on the intensity of Necator infection in Hainan Province, People's Republic of China. Clin. Infect. Dis. 2002;35:1336–1344. doi: 10.1086/344268. http://www.ncbi.nlm.nih.gov/pubmed/12439796. [DOI] [PubMed] [Google Scholar]
- Bockarie M.J., Kelly-Hope L.A., Rebollo M., Molyneux D.H. Preventive chemotherapy as a strategy for elimination of neglected tropical parasitic diseases: endgame challenges. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013;368 doi: 10.1098/rstb.2012.0144. doi.org/10.1098/rstb.2012.0144 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3720042/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S., Clements A.C.A., Bundy D.A.P. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv. Parasitol. 2006;62:221–261. doi: 10.1016/S0065-308X(05)62007-6. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1976253/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen T.B., Lauritsen J.M., editors. EpiData - Comprehensive Data Management and Basic Statistical Analysis System. EpiData Association; Odense Denmark: 2010. http://www.epidata.dk [Google Scholar]
- De Rochars M.B., Direny A.N., Roberts J.M., Addiss D.G., Radday J., Beach M.J., Streit T.G., Dardith D., Lafontant J.G., Lammie P.J. Community-wide reduction in prevalence and intensity of intestinal helminths as a collateral benefit of lymphatic filariasis elimination programs. Am.J.Trop. Med. Hyg. 2004;71:466–470. http://www.ajtmh.org/content/71/4/466.long [PubMed] [Google Scholar]
- Ekpo U.F., Odoemene S.N., Mafiana C.F., Sam-Wobo S.O. Helminthiasis and hygiene conditions of schools in Ikenne, Ogun State, Nigeria. PLoS Negl. Trop. Dis. 2008;2(1) doi: 10.1371/journal.pntd.0000146. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2270794/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- FMoH Nigeria Master Plan for Neglected Tropical Diseases (NTDs) 2013–2017. 2012. http://www.schoolsandhealth.org/Shared%20Documents/Downloads/Nigeria%20Neglected%20Tropical%20Disease%20Control%20Master%20Plan%202013.pdf
- FMOH Report on Epidemiological Mapping of Schistosomiasis and Soil Transmitted Helminthiasis in 19 States and the FCT, Nigeria. 2015. http://www.health.gov.ng/doc/SchistoSTH.pdf
- Glinz D., Silue´ K.D., Knopp S., Lohourignon L.K., Yao K.P., Steinmann P., Rinaldi L., Cringoli G., N'Goran E.K., Utzinger J. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl. Trop. Dis. 2010;4(7) doi: 10.1371/journal.pntd.0000754. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2907416/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman J., Emukah E., Okpala N., Okoro C., Obasi A., Miri E.S., Richards F.O. Effects of annual mass treatment with ivermectin for onchocerciasis on the prevalence of intestinal Helminths. Am.J.Trop. Med. Hyg. 2010;83(3):534–541. doi: 10.4269/ajtmh.2010.10-0033. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2929048/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Kamath A. Neglected tropical diseases in Sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009;3(8) doi: 10.1371/journal.pntd.0000412. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2727001/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Savioli L., Fenwick A. Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control. PLoS Negl. Trop. Dis. 2012;6(2) doi: 10.1371/journal.pntd.0001475. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3289601/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T.-W., Melville S., Utzinger J., King C.H., Zhou X.-N. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta analysis. PLoS Negl. Trop. Dis. 2012;6(5) doi: 10.1371/journal.pntd.0001621. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3348161/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk E.J., Redekop W.K., Luyendijk M., Rijnsburger A.J., Severens J.L. Productivity loss related to neglected tropical diseases eligible for preventive chemotherapy: a systematic literature review. PLoS Negl. Trop. Dis. 2016;10(2) doi: 10.1371/journal.pntd.0004397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegga B.T.A., Malley K.D., Mwiwula V. Impact of ivermectin mass distribution for onchocerciasis control on Ascarislumbricoides among schoolchildren in Rungwe and Kyela Districts, southwest Tanzania. Tanzania Health Research Bulletin. 2006;8(2):70–74. http://www.ajol.info/index.php/thrb/article/view/14275/15935 [Google Scholar]
- Moncayo A.L., Vaca M., Amorim L., Rodriguez A., Erazo S., Oviedo G., Quinzo I., Padilla M., Chico M., Lovato R., Gomez E., Barreto M.L., Cooper P.J. Impact of long-term treatment with ivermectin on the prevalence and intensity of soil-transmitted helminth infections. PLoS Negl. Trop. Dis. 2008;2(9) doi: 10.1371/journal.pntd.0000293. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2553482/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montresor A., Cong D.T., Sinuon M., Tsuyuoka R., Chanthavisouk C., Strandgaard H., Velayudhan R., Capuano C.M., Le Anh T., Tee Dató A.S. Large-scale preventive chemotherapy for the control of helminth infection in Western pacific countries: six years later. PLoS Negl. Trop. Dis. 2008;2(8) doi: 10.1371/journal.pntd.0000278. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2565698/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndyomugyenyi R., Kabatereine N., Olsen A., Magnussen P. Efficacy of ivermectin and albendazole alone and in combination for treatment of soiltransmitted helminths in pregnancy and adverse events: a randomized open label controlled intervention trial in Masindi district, western Uganda. Am.J.Trop. Med. Hyg. 2008;79:856–863. http://www.ajtmh.org/content/79/6/856.long [PubMed] [Google Scholar]
- Oluwole A.S., Ekpo U.F., Karagiannis-Voules D.A., Abe E.M., Olamiju F.O., Isiyaku S., Okoronkwo C. Bayesian geostatistical model-based estimates of soil-transmitted helminth infection in Nigeria, including annual deworming requirements. PLoS Negl. Trop. Dis. 2015;9(4) doi: 10.1371/journal.pntd.0003740. http://journals.plos.org/plosntds/article?id=10.1371%2Fjournal.pntd.0003740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddoh A., Onyeze A., Gyapong J.O., Holt J., Bundy D. Towards an investment case for neglected tropical diseases. Lancet. 2013;9908:1898–1955. [Google Scholar]
- Stothard J.R., French M.D., Khamis I.S., Basanez M.G., Rollinson D. The epidemiology and control of urinary schistosomiasis and soil-transmitted helminthiasis in school children on Unguja Island, Zanzibar. Trans. R. Soc. Trop. Med. Hyg. 2009;103:1031–1044. doi: 10.1016/j.trstmh.2009.03.024. doi.org/10.1016/j.trstmh.2009.03.024 http://www.sciencedirect.com/science/article/pii/S0035920309001229 [DOI] [PubMed] [Google Scholar]
- Strunz E.C., Addiss D.G., Stocks M.E., Ogden S., Utzinger J., Freeman M.C. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11(3) doi: 10.1371/journal.pmed.1001620. http://journals.plos.org/plosmedicine/article?id=10.1371%2Fjournal.pmed.1001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchuem Tchuenté L.A. Control of soil-transmitted helminths in sub-Saharan Africa: diagnosis, drug efficacy concerns and challenges. Acta Trop. 2011;120S:S4–S11. doi: 10.1016/j.actatropica.2010.07.001. doi.org/10.1016/j.actatropica.2010.07.001 http://www.sciencedirect.com/science/article/pii/S0001706X10001828 [DOI] [PubMed] [Google Scholar]
- WHO Prevention and control of schistosomiasis and soil-transmitted helminthiasis: a report of WHO expert committee. WHO Tech Rep Ser. 2002;912:1–57. http://apps.who.int/iris/bitstream/10665/42588/1/WHO_TRS_912.pdf [PubMed] [Google Scholar]
- WHO Soil-transmitted Helminth Infections. 2016. http://www.who.int/mediacentre/factsheets/fs366/en/ Factsheet updated February 2016.
- Winnen M., Plaisier A.P., Alley E.S., Nagelkerke N.J.D., Oortmarssen G.V., Boatin B.A., Habbema J.D.F. Can ivermectin mass treatments eliminate onchocerciasis in Africa? Bull. World Health Organ. 2002;80(5):384–390. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2567795/ [PMC free article] [PubMed] [Google Scholar]
- Yamane Taro. second ed. Harper and Row; New York: 1967. Statistics, an Introductory Analysis. [Google Scholar]
- Ziegelbauer K., Speich B., Mausezahl D., Bos R., Keiser J., Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and metaanalysis. PLoS Negl. Trop. Dis. 2012;9 doi: 10.1371/journal.pmed.1001162. http://journals.plos.org/plosmedicine/article?id=10.1371%2Fjournal.pmed.1001162 [DOI] [PMC free article] [PubMed] [Google Scholar]