Abstract
Background and objectives
Dengue is an emerging and re-emerging infectious disease, transmitted by mosquitoes. It is mostly prevalent in tropical and sub-tropical regions of the world, particularly, in Asia-Pacific region. To understand the epidemiology and spatial distribution of dengue, a retrospective surveillance study was conducted in the state of Andhra Pradesh, India during 2011–2013.
Material and methods
District-wise disease endemicity levels were mapped through geographical information system (GIS) tools. Spatial statistical analysis such as Getis-Ord Gi* was performed to identify hot spots and cold spots of dengue disease. Similarly self organizing maps (SOM), a datamining tool was also applied to understand the endemicity patterns in study areas.
Results
The analysis shows that districts of Warangal, Karimnagar, Khammam and Vizianagaram are reported as hot spot regions whereas Adilabad and Nizamabad reported as cold spots for dengue. The SOM classify 23 districts in 03 major (07 sub) clusters. These SOM clusters were projected in the geographical space and based on the disease/cases intensity the districts were characterized into low, medium and high endemic areas.
Conclusion
This visualization approach, SOM-GIS helps the public health officials to identify the disease endemic zones and take real time decisions for disease management.
Keywords: Dengue, Epidemiology, Geographical information system, Self organizing maps, Andhra Pradesh
1. Introduction
Dengue fever (DF) and its severe form, the dengue hemorrhagic fever (DHF), is a re-emerging arboviral disease of great public health importance (WHO, 2009). Currently, DF is rapidly spreading in the world, particularly in tropical and sub-tropical regions. From the past 50 years dengue case incidence has multiplied 30 fold, with about 30–54.7% of the world's population (2.05–3.47 billion) are at risk from > 100 countries (Brady et al., 2012). An estimated 50–100 million DF infections occur per annum globally, with half a million DHF cases requiring hospitalization with over 20,000 deaths (WHO, 2009). Dengue is caused by dengue virus (DENV) which causes a spectrum of illness ranging from undifferentiated fever, asymptomatic or mild febrile illness, DF, DHF and dengue shock syndrome (DSS) (Chastel, 2012). DENV considered as the most important arbovirus, the most severe cases are caused by four distinct serotypes: DENV-1, DENV-2, DENV-3, and DENV-4 are now circulating in Asia, Africa and the Americas (Weaver and Vasilakis, 2009, Mohammed et al., 2010). (See Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8.)
Fig. 1.
Map showing the study area, Andhra Pradesh state in India.
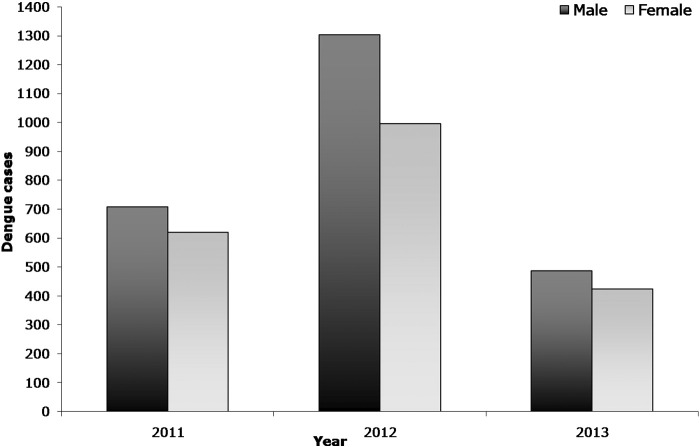
Fig. 2.
Year and gender wise distribution of dengue cases in Andhra Pradesh, India.
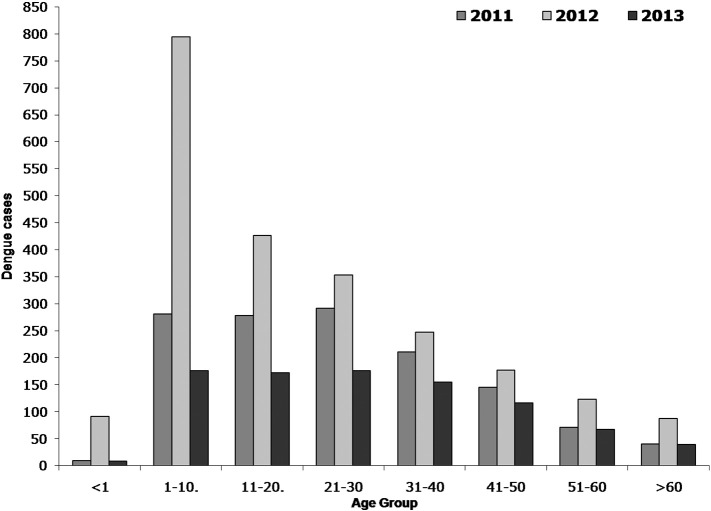
Fig. 3.
Age wise distribution of dengue cases in Andhra Pradesh, India.
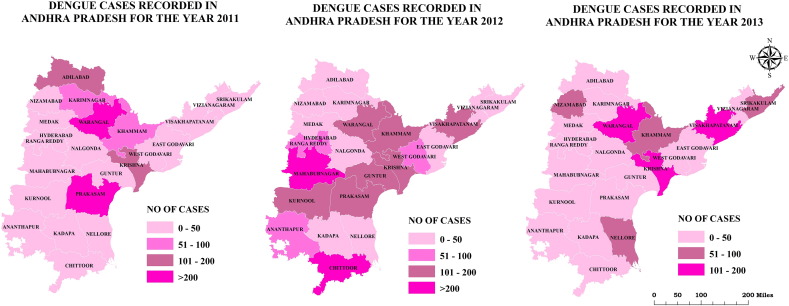
Fig. 4.
Spatial distribution of dengue cases in Andhra Pradesh from 2011 to 2013.
Fig. 5.
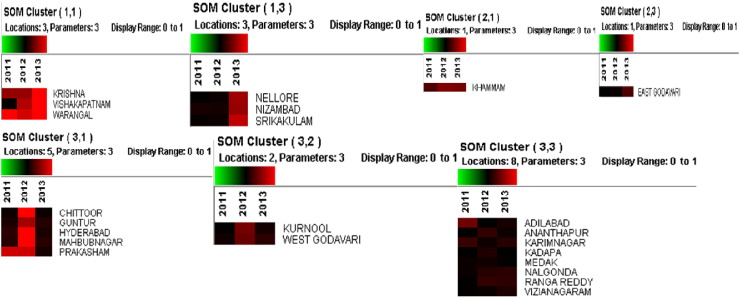
Dengue clusters identified in Andhra Pradesh using Self-Organizing Maps.
Fig. 6.
Geographic distribution of clusters in Andhra Pradesh using Self-Organizing Maps.
Fig. 7.
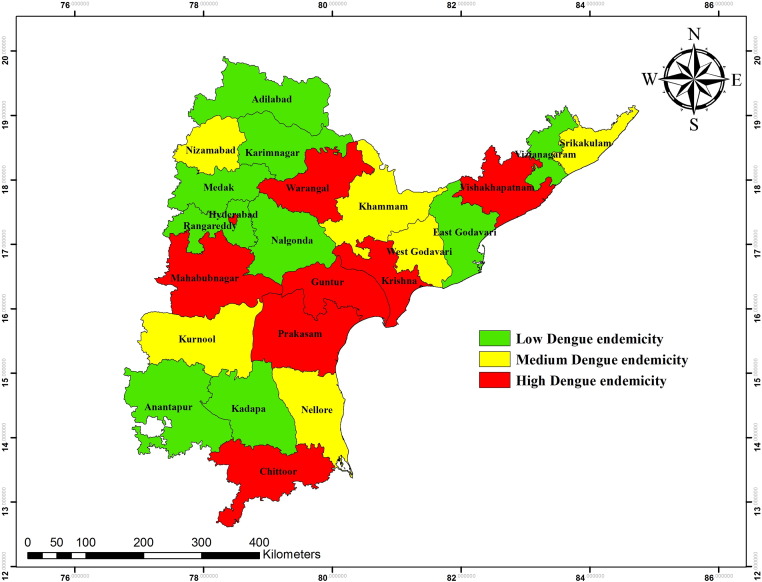
SOM classified dengue endemic areas in Andhra Pradesh, India.
Fig. 8.
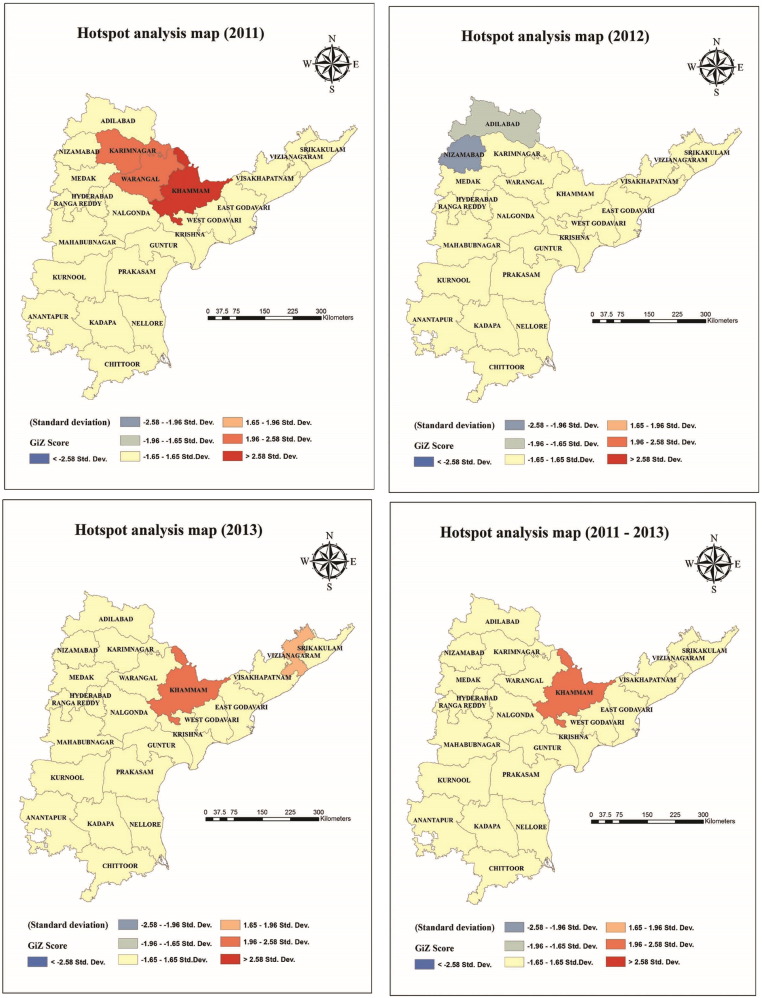
Spatial clustering of dengue cases in 2011, 2012 and 2013. The dark and light red colours indicate hotspots of dengue cases (Z score Getis-Ord > 2.58 statistically significant), blue and light blue color represents cold spot areas (Z score Getis-Ord <− 2.58 statistically significant).
According to Bhatt et al. (2013), there were 96 million with apparent dengue infections globally during the year 2010 and Asian region contributes around 70% (67 million infections) of this burden (Bhatt et al., 2013). Dengue is among the greatest disease burdens in Southeast Asia, and has been hyper endemic for decades. During the last decade, Southeast Asia has the highest reported dengue incidence with an estimated 50–100 million annual cases and number of epidemics occurring every three to five years (WHO-SEARO, 2011, World Health Organization (WHO), 2012). From this region around 1.3 billion people are at risk of dengue and is the leading cause of hospitalization and death among children (Gubler, 2002a). The dengue epidemics can cause significant economic, social and health toll. The WHO regions, Southeast Asia and the Western Pacific represent about 75% of the current global burden of dengue (Shepard et al., 2013). The burden of dengue is approximately 1300 disability-adjusted life years (DALYs) per million populations in the endemic countries in Asia and the Americas (Gubler and Meltzer, 1999). Similarly, the endemicity levels of dengue fever reflects the distribution of its primary urban mosquito vector Aedes aegypti (Gubler, 2002a) to new geographical area, warm and humid climate, increased population density, water storage pattern in houses, storage of junk in open spaces, including tyres, coconut shells etc. that trap rain water and introduction of new serotype of the virus.
Dengue infection has been known to be endemic in India for over two centuries as a benign and self-limited disease. Since the mid 1990s, epidemics of dengue have become more frequent in many parts of India. In recent years, the disease has changed its course manifesting in the severe form as DHF and with increasing frequency of outbreaks (Gupta et al., 2006). India contributes around 34% (33 million infections) of the global dengue cases during the year 2010 (Bhatt et al., 2013). Dengue has been an urban disease but now has spread to rural areas of India as well (Arunachalam et al., 2004). A total of 82,327 dengue cases have been reported during 1998–2009 and 213,607 cases observed from 2010 to 2014. Thus the number of dengue cases has increased tremendously during last five years. Dengue fever is a major problem in Andhra Pradesh, India. In recent years, the dengue outbreaks are gradually increasing year by year in various districts of Andhra Pradesh. The rise of these dengue cases are driven by complex interactions between hosts, vectors and viruses that are influenced by environmental, climatic, demographic and socio-economic factors. Apart from this other determinants in dengue fever emergence are human population growth, accelerated urbanization, increased international transport, lack of proper public health infrastructure as well as a lack of effective vector control and disease surveillance system (Rigau-Pérez et al., 1998, Gubler, 2002b, Hales et al., 2002, Mackenzie et al., 2004, Chaturvedi and Nagar, 2008). The epidemiology of dengue is complex and remains poorly understood. Many field observations have raised questions against widely accepted epidemiological characteristics of dengue (Chastel, 2012, Bhatia et al., 2013). It is thus imperative to properly understand the evolving pattern and trend of dengue, as it is crucial in determining the success of prevention and control programs (Bhatia et al., 2013).
The purpose of the present study is to examine the spatial distribution of dengue cases and identify hot spots of dengue in Andhra Pradesh by applying the spatial cluster analysis techniques such as Getis-Ord Gi* and self organizing maps (SOM) using Geographical Information System (GIS) tools.
2. Methods
2.1. Study area
Andhra Pradesh (Fig. 1), the third largest state in India is located in the South-eastern part, with an area of 275,000 sq·km (12°41′ and 22° longitude and 77° and 84°40′ latitude). It is the biggest and most populous state in the south of India. According to 2011 census, it has a total population of 84,580,777 of which male and female are 42,442,146 and 42,138,631, respectively. Climatically, it has three seasons: summer (March to June), rainy (July to September) and winter (October to February). The state receives maximum rainfall from the Southwest monsoon followed by Northeast monsoon. Its landscape is filled with 24% of unevenly distributed forests.
2.2. Epidemiological data
The epidemiological data on dengue cases was obtained from the Directorate of Health, Government of Andhra Pradesh from 2011 to 2013, which consists of information on dengue patients belonging to 23 districts of Andhra Pradesh.
2.3. Ethics statement
The study received ethical approval from the CSIR-Indian Institute of Chemical Technology ethical committee affiliated with the Ministry of Science and Technology, Government of India. The data was analyzed anonymously; here no particular patient by name was involved.
2.4. Geographical information system (GIS) mapping dengue
To conduct a GIS-based analysis of the spatial distribution of dengue was mapped at 1:25,000 scale by district-wise by using a GIS technique in ArcGIS 10.2 software (ESRI Inc., USA).
2.5. Data analysis
2.5.1. Hotspot detection
Getis-OrdGi * statistics identifies different spatial clustering patterns like hot spots, high risk and cold spots over the entire study area with statistical significance (Osei and Duker, 2008, Getis and Ord, 1992). The result expresses the Z-score and p-value of the calculated Gi *, in comparison with the normal distribution of the statistics calculated by simulation (Feser et al., 2005). These values represent the statistical significance of the spatial clustering of values, given the conceptualization of spatial relationships and the scale of analysis (distance parameter). The output from Gi * statistic identifies spatial clusters of high values (hot spots) and spatial clusters of low values (cold spots). High risk areas are at lower significance level in comparison to hot spots. For hot spot analysis, the spatial relationship is ‘fixed distance’ with a defined threshold limit or distance band. The distance band which exhibits the highest spatial autocorrelation (peak Z value) is taken for analysis. With fixed distance band option, a moving window conceptual model of spatial interactions is imposed onto the data where each feature is analyzed within the context of those neighboring features within the specified distance band (Bhunia et al., 2013, Saxena et al., 2012). This hot spot study provides precise identification and geographical location of hot and cold spots of dengue cases that have any spatial relationship with the different districts in Andhra Pradesh in order to facilitate evidence to enable the policy decisions. The statistical significance was set at p < 0.01.
2.5.2. Cluster analysis through self organizing map (SOM)
SOM algorithm is also known as Kohonen map or Self Organizing feature map, is an unsupervised neural network based on competitive learning (Kohonen, 1988, Kohonen, 2001). SOM is used to map multidimensional data into a space of lower dimensionality (Tom Germano, 1999). In SOM the neurons are organized in lattice, either with one or two dimensional array and are associated with weights of the same dimension of the input data (Murty et al., 2008). The machine learning is accomplished by first choosing an output neuron that most closely matches the presented input pattern, then determining a neighborhood of excited neurons around the winner, and finally updating all of the excited neurons (Vesanto and Alhoniemi, 2000). This process iterates and fine tunes and it is called self organizing. An illustration of the flow chart of the SOM application and detailed SOM algorithm was discussed in earlier papers (Murty et al., 2011, Savarapu et al., 2013).
SOM, a data-mining tool, has been widely applied in various engineering and health applications. The output of SOM gives clustering patterns, which have been applied to a wide range of classification problems in public health and other related fields (Murty et al., 2011). SOM has been used as a tool to recognize patterns with data sets measuring public health outcome, social status and economic variables and the geographical variation (Basara and Yuan, 2008). Recently SOM was applied in geospatial context to study cases of adult asthma and insulin resistance syndrome (Oyana et al., 2008, Valkonen et al., 2002).
SOM and spatial applications have been integrated in GIS to provide a novel approach for the analysis of health reports in different views. In the present study, coupling of the SOM results (i.e. clusters) of disease endemic regions was showed with the GIS, which is first of its kind study on dengue in India and it provides the pattern of disease in different endemic regions. This SOM-GIS methodology helps in disease transmission as well as vector control operations.
3. Results
The epidemiological data shows that 4537 dengue cases were reported in the state of Andhra Pradesh (districts, n = 23) during the period 2011 to 2013. The number of positive dengue cases in 2011 (1328 cases) and 2013 (910 cases) was comparatively lower than that of 2012 (2299 cases). The result shows that majority of the dengue cases were reported in males than in female population (Fig. 2). The data clearly indicates that dengue is quite prevalent in all age groups (Fig. 3) but it was found to be more in the age groups of 1–10, 11–20 and 20–30. Compared to other age groups, these age groups were mostly affected in all three years.
3.1. District level pattern of dengue cases
Based on number of total dengue cases, all districts (n = 23) were grouped into four categories: low endemic areas with dengue cases between 0 and 50; medium endemic areas with cases between 51 and 100; high endemic areas with cases 100 to 200 and very high endemic areas with > 200 cases (Fig. 4). Fig. 4 shows the spatial distribution of dengue cases among districts in Andhra Pradesh from 2011 to 2013. During 2011, the high endemic areas are Krishna and Adilabad and very high endemic areas are Prakasam and Warangal. In the year 2012, Kurnool, Prakasam, Guntur, Krishna, Khammam, Warangal and Vishakhapatnam are high endemic areas whereas Chittoor and Mahabubnagar are very high endemic areas. During 2013, dengue cases are reported in many of the districts, with high endemic areas being Krishna, Warangal and Vishakhapatnam.
3.2. SOM cluster analysis
SOM analyzed dengue data for the years 2011–2013 of all 23 districts of Andhra Pradesh at learning rate from 0.1 to 0.01 for each parameter. The SOM, clusters the data into 03 major clusters (07 sub clusters) which were categorized according to number of cases reported in each district (Fig. 5). The Fig. 6 shows the geographic distribution of the each cluster by district wise. Cluster-1 comprised of two sub clusters (1,1& 1,3) of which the sub cluster 1,1 is characterized as highly endemic cluster (red in color) and cluster 1,3 is medium endemic (maroon in color). Cluster-2 has been divided in two sub clusters (2,1& 2,3) and categorized as medium and low endemic regions (black in color). Similarly, cluster-3 is differentiated in to three sub clusters which are 3,1; 3,2 and 3,3. The cluster 3,1 is highly endemic region followed by clusters 3,2 and 3,3 which are medium and low endemic regions. Based on SOM cluster analysis, the dengue endemic area was classified into low, medium and high and these are geographically mapped using GIS (Fig. 7).
3.3. Hot spot analysis
The localities with high and low clusters of dengue case abundance were identified with the Z-score computed by Getis-Ord Gi* (Fig. 8). In Andhra Pradesh, three hot spot districts in 2011 [Khammam (99% confidence; P < 0.01), Karimnagar and Warangal (95% confidence)], two hot spots areas in 2013 [Khammam (95% confidence) and Vizianagaram (90% confidence)] were identified. Two cold spot districts [Adilabad (90% confidence) and Nizamabad (95% confidence)] were observed in 2012. It is important to note that the standard deviations are very high in hot spot districts as they have different rates of dengue cases.
4. Discussion
In India, the dengue cases are increasing gradually year by year. To understand dengue epidemiology, proper surveillance studies, analysis of epidemiological data, prediction of outbreaks and hot spot analysis are of critical importance. In this study, we examined epidemiological scenario and spatial distribution of dengue through cluster analysis in the state Andhra Pradesh, India during 2011–2013. Our study results revealed that the number of cases infected with dengue was higher among males than in females and these results were similar to other studies (Anker and Arima, 2011, World Health Organization (WHO), 2009). This could be due to the following reasons: males may be more frequently exposed to mosquito bites than females; males may not be wearing protective clothing at the time of mosquito bite and males may be not taken care of precautionary measures such as applying repellents. Additionally, there could be differences in behavioural and lifestyle patterns. Kumar et al., (2001), Dung and Cam (2005) reported that 90% of dengue morbidity was reported in individuals aged between 15 and 25 years. By agreeing the above statement, in our study the higher number of cases was found in the age groups of 1–10 years followed by 11–20 and 21–30 years. This could be due to number of cases reported in these age groups is more in number in this population. In the year 2012, the largest proportion of cases reported in Andhra Pradesh was in individuals aged 1–10 years (around 794 cases of 2299). The highest number of cases in this age group may be due to circulating dengue virus serotype and strain or changes in susceptibility to infection or secondary infection or enhanced disease due to immune status (Halstead, 1997). Dengue can also create lifelong immunity for the individual, so that older persons who have been exposed more often may have more resistance, decreasing the morbidity rate (Toan et al., 2015).
The results of this study indicated that there was a significant variation in the spatial distribution of dengue fever in districts of Andhra Pradesh and that the geographic range of the cases has expanded during the study period. Many of the dengue endemic areas of Andhra Pradesh share interstate borders with Tamilnadu, Karnataka, Orissa and Maharashtra which are also reported as dengue endemic areas (Cecilia, 2014). It has been reported that neighboring villages/districts share similar ecology, human behavior, social patterns and lifestyle, such characters may give rise to a positive or negative association with the occurrence of dengue in neighboring areas (Dietz, 2002, Reiter et al., 2003, Tipayamongkholgul and Lisakulruk, 2011). Apart from mapping in this study, SOM was performed to classify the disease endemicity in to seven clusters at the districts level and these endemic clusters were mapped using spatial tools.
SOM have been proven to be a promising tool for identifying disease clustering in endemic zones (Murty et al., 2008, Vesanto and Alhoniemi, 2000, Murty et al., 2011). The SOM projections shifts the complicated structure from high dimensional arrays into the lower dimensional clusters based on the neighborhood relations, which is important for clustering of disease endemic areas. A total of nine districts out of 23 were reported as low endemic, 06 are medium endemic and 07 are high endemic zones for dengue disease. The three endemic zones proposed in the study were clustered based on SOM analysis (Fig. 7). The integration of SOM and GIS has been successfully designed to produce dynamic visualization which in turn helps public health authorities to make timely decisions for disease management.
Spatial distribution pattern of dengue cases were significantly clustered and identified the dengue hot spots in Andhra Pradesh. Only one consistent dengue hot spot was identified during the study period, i.e., Khammam (95% confidence) (Fig. 8). Hot spots (Karimnagar, Warangal and Khammam) and cold spots (Nizamabad and Adilabad) are mainly located in the Northern regions of the state only. The size of the whole region have relationship with the geographical characteristics of the territory because the hot spot areas are the forest regions. We observed that the population of these regions keep close connections, frequently migrate and rapidly urbanize. Therefore, these regions have emerged as hot spots. Gubler predicted that if global trends of unprecedented population growth, continued globalization and unprecedented urbanization continue as projected there will be continued increase in the severity, frequency, geographical distribution and magnitude of dengue epidemics in the future (Gubler, 2011). All these above factors may contribute to the increased dengue risk in districts of Andhra Pradesh as the state is the tenth largest state by population in India according to census 2011. Preventing or reducing dengue virus transmission depends on the control of the mosquito vectors and interruption of human-vector contacts (Fan et al., 2014).
The study has three major strengths. Firstly, in this study, we have included dengue notification data for the recent years. This kind of recent information will be useful in designing future prevention and control measures. Secondly, we have included all the districts in Andhra Pradesh state in this study for data analysis. Finally, we have conducted hot spot analysis using novel spatial methods such as Getis-Ord Gi* and SOM which are both user-friendly and highly useful in identifying hot spots/clusters of disease. The spatial and temporal maps produced in this study will be useful for local public health officials in making decisions towards dengue management.
There are certain limitations in this study. We do not have the local mosquito density data and therefore unable to analyze the effect of vector control interventions for disease management. We did not include the major factors including climate change, population growth and socioeconomic conditions for the incidence of dengue in this study. This study has only included three years of data as data is unavailable for the previous years. Future attempts need to consider the above points for integrated control of dengue.
In our study, we found spatial and temporal clusters of dengue and hot spot and cold spot regions in the state of Andhra Pradesh, using GIS techniques. Identifying spatial clusters of dengue disease is an essential tool in planning policy and estimating the local resources required and the timescales over which targets can be achieved. These spatial and temporal clusters will also be helpful in informing and supporting highly effective, locally tailored interventions for dengue disease which is highly spatially heterogeneous infection. Similarly, our study also suggests that spatial and temporal analyses of population-based disease surveillance data would be helpful in managing the vector-borne diseases such as dengue, by highlighting where and when limited public health resources should be concentrated.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
We thank Director, Council of Scientific & Industrial Research - Indian Institute of Chemical Technology for his encouragement and support. Thanks to Council of Scientific & Industrial Research (BSC-0121), Govt. of India for funding the project. Dr. Naish acknowledges Governments of Australia and India for the fellowship.
References
- Anker M., Arima Y. Male–female differences in the number of reported incident dengue fever cases in six Asian countries. Western Pac. Surveill. Response J. 2011;2:17–23. doi: 10.5365/WPSAR.2011.2.1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam N., Murty U.S., Kabilan L., Balasubramanian A., Thenmozhi V., Narahari D., Ravi A., Satyanarayana K. Studies on dengue in rural areas of Kurnool District, Andhra Pradesh. India. J. Am. Mosq. Control Assoc. 2004;20:87–90. [PubMed] [Google Scholar]
- Basara H.G., Yuan M. Community health assessment using self organizing maps and geographic information systems. Int. J. Health Geogr. 2008;7:1. doi: 10.1186/1476-072X-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia R., Dash A.P., Sunyoto T. Changing epidemiology of dengue in South-East Asia. WHO South-East Asia J. Public Health. 2013;2:23–27. doi: 10.4103/2224-3151.115830. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., Myers M.F., George D.B., Jaenisch T., Wint G.R., Simmons C.P., Scott T.W., Farrar J.J., Hay S.I. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunia G.S., Kesari S., Chatterjee N., Kumar V., Das P. Spatial and temporal variation and hotspot detection of kala-azar disease in Vaishali district (Bihar) India. BMC Infectious Diseases. 2013;13:64. doi: 10.1186/1471-2334-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady O.J., Gething P.W., Bhatt S., Messina J.P., Brownstein J.S., Hoen A.G., Moyes C.L., Farlow A.W., Scott T.W., Hay S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecilia D. Current status of dengue and Chikungunya in India. WHO South-East Asia Journal of Public Health. 2014;3:1–6. doi: 10.4103/2224-3151.206879. [DOI] [PubMed] [Google Scholar]
- Chastel C. Eventual role of asymptomatic cases of dengue for the introduction and spread of dengue viruses in non-endemic regions. Front. Physiol. 2012;3:70. doi: 10.3389/fphys.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U.C., Nagar R. Dengue and dengue haemorrhagic fever: Indian perspective. J. Biosci. 2008;33:429–441. doi: 10.1007/s12038-008-0062-3. [DOI] [PubMed] [Google Scholar]
- Dietz R. The estimation of neighborhood effects in the social sciences: An interdisciplinary approach. Soc. Sci. Res. 2002;31:539–575. [Google Scholar]
- Dung N.T., Cam N.N. Dengue fever/dengue hemorrhagic fever in Hanoi–2003. Journal of Vietnam Preventive Medicine. 2005;1:73–77. [Google Scholar]
- Fan J., Lin H., Wang C., Bai L., Yang S., Chu C., Yang W., Liu Q. Identifying the high-risk areas and associated meteorological factors of dengue transmission in Guangdong Province, China from 2005 to 2011. Epidemiol. Infect. 2014;142:634–643. doi: 10.1017/S0950268813001519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feser E., Sweeney S., Renski H. A descriptive analysis of discrete U.S. industrial complexes. J. Reg. Sci. 2005;45:395–419. [Google Scholar]
- Germano T. 1999. Self Organizing Maps; p. 1. March 23. [Google Scholar]
- Getis A., Ord J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992;24:189–207. [Google Scholar]
- Gubler D.J. Epidemic dengue/dengue haemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- Gubler D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 2002;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J. Dengue, urbanization and globalization: The unholy trinty of the 21st century. Tropical Med. Int. Health. 2011;39:3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J., Meltzer M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv. Virus Res. 1999;53:35–70. doi: 10.1016/s0065-3527(08)60342-5. [DOI] [PubMed] [Google Scholar]
- Gupta E., Dar L., Kapoor G., Broor S. The changing epidemiology of dengue in Delhi. India. Virology Journal. 2006;3:92. doi: 10.1186/1743-422X-3-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales S., de Wet N., Maindonald J., Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- Halstead S.B. Epidemiology of dengue and dengue hemorrhagic fever. In: Gubler D., Kuno G., editors. Dengue and dengue hemorrhagic fever. CAB International; Wallingford (UK): 1997. pp. 23–44. [Google Scholar]
- Kohonen T. Springer-Verlag: New York, USA; Berlin, Heidelberg, Germany: 1988. Self-organization and Associative Memory. [Google Scholar]
- Kohonen T. Springer-Verlag; New York, Ny, USA; Berlin, Heidelberg, Germany: 2001. Self-Organizing Maps. [Google Scholar]
- Kumar A., Sharma S.K., Padbidri V.S., Thakare J.P., Jain D.C., Datta K.K. An outbreak of dengue fever in rural areas of northern India. J. Commun. Disord. 2001;33:274–281. [PubMed] [Google Scholar]
- Mackenzie J.S., Gubler D.J., Petersen L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- Mohammed H.P., Ramos M.M., Rivera A., Johansson M., Muñoz-Jordan J.L., Sun W., Tomashek K.M. Travel-associated dengue infections in the United States, 1996 to 2005. J. Transl. Med. 2010;17:8–14. doi: 10.1111/j.1708-8305.2009.00374.x. [DOI] [PubMed] [Google Scholar]
- Murty U.S., Rao M.S., Sriram K., Rao K.M. Applications of self organizing maps (SOM) for prioritization of endemic zones of filariasis in Andhra Pradesh. India. International Journal of Datamining and Bioinformatics. 2011;5:417–427. doi: 10.1504/ijdmb.2011.041557. [DOI] [PubMed] [Google Scholar]
- Murty U.S., Srinivasa Rao M., Misra S. Prioritization of malaria endemic zones using self organizing maps in the Manipur state of India. Informatics for Health and Social Care. 2008;33:170–178. doi: 10.1080/17538150802457687. [DOI] [PubMed] [Google Scholar]
- Osei F.B., Duker A.A. Spatial and demographic patterns of cholera in Ashanti region-Ghana. Int. J. Health Geogr. 2008;7:44. doi: 10.1186/1476-072X-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyana T.J., Boppidi D., Yan J., Lwebuga-Mukasa . Exploration of geographic information systems (GIS)-based medical databases with self-organizing maps (SOM): a case study of adult asthma. In: Bernard L., Friis-Christensen A., H. P., editors. The European Information Society. Springer Berlin Heidelberg; 2008. [Google Scholar]
- Reiter P. Texas lifestyle limits transmission of dengue virus. Emerg. Infect. Dis. 2003;9:86–89. doi: 10.3201/eid0901.020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigau-Pérez J.G., Clark G.G., Gubler D.J., Reiter P., Sanders E.J., Vorndam A.V. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- Savarapu S.K., Misra S., Rao M.S., Murty U.S. Genetic variation and divergence analysis using self-organizing maps (SOM) in silkworm, Bombyx mori L. (Lepidoptera: Bombycidae) genotypes. Biotechnology. 2013;12:189–201. [Google Scholar]
- Saxena R., Nagpal B.N., Das M.K., Srivastava A., Gupta S.K., Kumar A., Jeyaseelan A.T., Baraik V.K. Spatial statistical approach to analyze malaria situation at micro level for priority control in Ranchi district. Jharkhand. Indian J. Med. Res. 2012;136:776–782. [PMC free article] [PubMed] [Google Scholar]
- Shepard D.S., Undurraga E.A., Halasa Y.A. Economic and disease burden of dengue in Southeast Asia. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipayamongkholgul M., Lisakulruk S. Socio-geographical factors in vulnerability to dengue in Thai villages: a spatial regression analysis. Geospat. Health. 2011;5:191–198. doi: 10.4081/gh.2011.171. [DOI] [PubMed] [Google Scholar]
- Toan D.T., Hoat L.N., Hu W., Wright P., Martens P. Risk factors associated with an outbreak of dengue fever/dengue haemorrhagic fever in Hanoi. Vietnam. Epidemiol. Infect. 2015;143:1594–1598. doi: 10.1017/S0950268814002647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkonen V.P., Kolehmainen M., Lakka H.M., Salonen J.T. Insulin resistance syndrome revisited: application of self-organizing maps. Int. J. Epidemiol. 2002;31:864–871. doi: 10.1093/ije/31.4.864. [DOI] [PubMed] [Google Scholar]
- Vesanto J., Alhoniemi E. Clustering of the self organizing map. IEEE Trans. Neural. Netw. 2000;11:586–600. doi: 10.1109/72.846731. [DOI] [PubMed] [Google Scholar]
- Weaver S.C., Vasilakis N. Molecular evolution of dengue viruses: Contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect. Genet. Evol. 2009;9:523–540. doi: 10.1016/j.meegid.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO-SEARO . WHO Regional office for South East Asia (WHO-SEARO); New Delhi: 2011. Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever – revised and expanded edition. xiv-196. [Google Scholar]
- World Health Organization (WHO) Dengue guidelines for diagnosis, treatment, prevention and control. World Health Organization. 2009:1–147. [PubMed] [Google Scholar]
- World Health Organization (WHO) Global strategy for dengue prevention and control 2012–20. World Health Organization. 2012:1–5. [Google Scholar]