Abstract
The prevalence of gastrointestinal helminth infections of dog in Enugu State, South Eastern Nigeria was studied retrospectively and prospectively. In the retrospective study, records of all diagnosed helminth infections of dogs brought to the University of Nigeria Veterinary Teaching Hospital, Nsukka from January, 2006 to September 2013 were collated and analyzed. The prospective study was carried out between October 2013 and July 2014 by examination of 263 faecal samples collected per rectum from dogs presented to a purposively selected Veterinary Clinics in Enugu metropolis and the Veterinary Teaching Hospital of the University of Nigeria, Nsukka. The results of the 8 year retrospective prevalence study gave an overall prevalence of 56.1% and Ancylostoma species as the most prevalent helminth in the study area (33.2%). Mixed infections with more than one helminth parasite species were recorded in 8.6% of the cases. Annual breakdown of the prevalence data showed that the highest prevalence was recorded in 2009. Breed and age of the dogs were found to significantly influence the prevalence. In the prospective study, an overall prevalence of 51.7% was obtained. Ancylostoma spp. was also found most often in the study area, with a prevalence rate of 33.6%. Mixed infections with more than one helminth parasite species were found in 16.3% of the cases. A strong association was obtained between prevalence and breed of the dogs and also between prevalence and season. Due to the zoonotic nature of most of the encountered parasites and the close association between children and dogs, routine deworming, proper management of dogs and adequate personal hygiene is therefore recommended.
Keywords: Gastrointestinal helminth, Prevalence, Dogs, Nigeria
1. Introduction
Parasitic helminths are among the most commonly encountered disease causing agents in dogs all over the world especially regarding pathologies of the intestinal tract (Zelon, 2003, Ramírez-Barrios et al., 2004). The parasites affects dogs of all ages, including both kernel and free-roam dogs. Sometimes, dogs could be infected without apparent evidence of the parasites' presence (Overgaauw and Boersema, 1998, Endrias et al., 2010). Infections by several species of these helminths impede the successful rearing of dogs resulting in losses manifested by lowered resistance to other infectious agents, poor growth, weight loss, reduced work and feed efficiency, general ill health and sometimes death if untreated (Soulsby, 1982).
Some helminth parasites of dogs are zoonotic causing many diseases in man like hydatidosis caused by Echinococcus species, visceral larva migrans caused by Toxocara canis and cutaneous larva migrans caused by Ancylostoma species. Since dogs live in close proximity to humans, contamination of man's food, water and hands with infective stages of these gastrointestinal (GI) helminths can lead to these infections with serious consequences. Some parasites like Echinococcus granulosus also use food animals as intermediate hosts in which they cause great economic loss through organ condemnation during meat inspection (Gracey, 1986, Urquhart et al., 1996).
The prevalence of helminth parasites has been shown to vary considerably from one geographic region to another depending on the genera of helminth involved, the animal species, and local environmental conditions such as humidity, temperature, rainfall, vegetation, and management practices (Robertson et al., 2000, Oliveira-Sequeira et al., 2002). It is therefore, necessary for periodic surveillance of the prevalence of these parasites within a given locality for successful formulation and implementation of an effective worm control strategy. Available literature on the prevalence of GI helminth infection of dogs in Enugu State is limited to a work done by Okoye et al. (2011) on stray dogs and others done several years ago, which include those of Chiejina and Ekwe (1986), Anene et al. (1996) and Onyenwe and Ikpegbu (2004). Thus, this study aims to determine retrospectively and prospectively the prevalence of GI helminth infections of dogs in Enugu State, South Eastern Nigeria.
2. Materials and methods
2.1. Study area and study population
The study area comprised two major urban towns of Enugu State, namely, Nsukka and Enugu metropolis representing two of the 3 senatorial/geographical zones of the state. Household dogs, including those brought to two purposively selected Veterinary clinics in these towns namely, the University of Nigeria Veterinary Teaching Hospital (UNVTH), Nsukka and Eva Veterinary Clinics, Enugu were included in the study. Dogs aged up to 12 months were classified as young while those above 12 months were regarded as adults (Bone, 1988).
2.2. Study design
Both retrospective and prospective prevalence studies were carried out. In the retrospective study, records of all diagnosed helminth infections of dogs brought to the University of Nigeria, Veterinary Teaching Hospital, Nsukka from January 2006 to September 2013 were collated and analyzed. The prospective study was carried out between October 2013 and July 2014 by examination of faecal samples collected per rectum from the dogs. Samples were placed in appropriately labeled, leakproof containers and transported to the laboratory for immediate examination while samples to be preserved were placed in 10% formol saline. Information regarding the sex, breed and age of the dogs was documented. Each faecal sample was examined qualitatively using the formol-ether concentration (Allen and Ridley, 1970) and salt floatation techniques (MAFF, 1977). The results were considered as positive when at least one parasite egg is present (Lorenzini et al., 2007). Parasite stages recovered were identified using standard morphological criteria (Soulsby, 1982). Infections with more than one species of helminth parasite (polyparasitism) were referred to as mixed infection.
2.3. Data analysis
Data obtained from the prevalence studies were analyzed using descriptive statistics and the results summarized as percentages. The Chi-square (x2) test was used to assess difference in the prevalence of the infection between sexes, breeds and age groups. In all the analyses, probabilities ≤ 0.05 were considered significant.
3. Results
3.1. Retrospective prevalence study
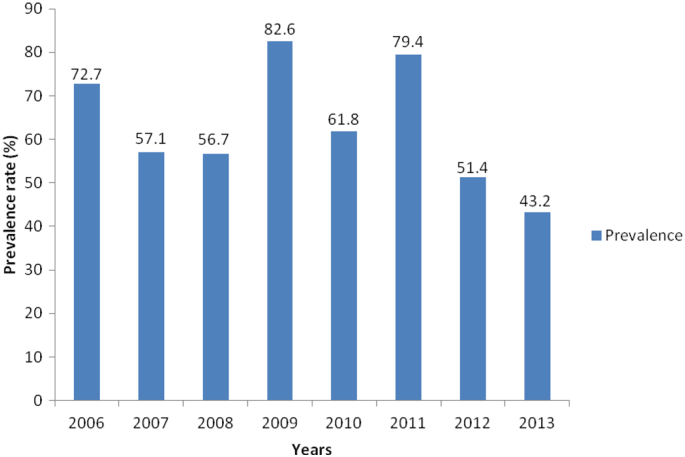
The results of the 8 years retrospective prevalence study are summarized in Table 1. Out of the 376 dogs examined for helminth parasites between 2006 and 2013, 211 representing 56.1% were positive for different species of helminths with lower and upper 95% confidence limits of 51.1 and 56.1% respectively. Four helminth parasites namely, hookworm (Ancylostoma spp.), Toxocara spp., Dipylidium caninum and Trichuris vulpis were identified in the study with the following prevalence rates 33.2%, 5.9%, 4.0% and 0.5% respectively (Table 2). Mixed infections with more than one helminth parasite species were recorded in 8.6% of the cases, of which 7.0% and 1.6% represent dogs infected with two and three different parasite species respectively. Annual breakdown of the prevalence data as presented in Fig. 1 showed that the highest prevalence was recorded in 2009 (82.6%). This was followed closely by 2011 (79.4%), 2006 (72.7%) and 2010 (61.8%) in that order. The lowest recorded prevalence of 43.2% was in 2013. When the prevalence rates were analyzed by sex, it was observed (Table 3) that male dogs had slightly higher prevalence of infection (56.6%) than the female dogs (54.6%) but the difference was not significant (P > 0.05). On breed basis (Table 3), the local breeds of dog had higher prevalence of infection (62.5%) than their exotic (48.0%) counterpart and the difference was significant (P = 0.28). Also, dogs under 12 months old had significantly higher prevalence (62.9%) than dogs over 12 months of age (46.4%) as shown in Table 3 and the difference was significant (P = 0.016). The result in Fig. 2 showed that prevalence rates of 52.9 and 50.4% were recorded for the rainy and dry season months respectively.
Table 1.
Overall prevalence rates of gastrointestinal helminth infections of dogs in Enugu State, Nigeria.
| Study | Number examined | Number positive | Prevalence (%) | Lower 95% CL | Upper 95% CL |
|---|---|---|---|---|---|
| Retrospective | 376 | 211 | 56.1 | 51.1 | 61.0 |
| Prospective | |||||
| Nsukka area | 153 | 84 | 54.9 | 47.0 | 62.6 |
| Enugu area | 110 | 52 | 47.3 | 38.2 | 56.5 |
| Overall prospective | 263 | 136 | 51.7 | 45.7 | 57.7 |
Table 2.
Prevalence rate of different GI helminth species of dogs brought to the University of Nigeria Veterinary Teaching Hospital, Nsukka between 2006 and 2013.
| Helminth species | No positive | Prevalence (%) | Lower 95% CL | Upper 95% CL |
|---|---|---|---|---|
| Ancylostoma spp. Only | 125 | 33.2 | 28.7 | 38.2 |
| Toxocara spp. Only | 22 | 5.9 | 3.9 | 8.7 |
| Dipylidium caninum only | 15 | 4.0 | 2.4 | 6.5 |
| Trichuris vulpis only | 2 | 0.5 | 0.1 | 1.9 |
| Ancylostoma spp. + Toxocara spp. | 16 | 4.3 | 2.6 | 6.8 |
| Ancylostoma spp. + Dipylidium caninum | 9 | 2.4 | 1.3 | 4.5 |
| Ancylostoma spp. + Trichuris vulpis | 1 | 0.3 | 0.0 | 1.5 |
| Ancylostoma spp. + Toxocara + Dipylidium | 5 | 1.3 | 0.6 | 3.1 |
| Ancylostoma spp. + Toxocara spp. + Trichuris vulpis | 1 | 0.3 | 0.0 | 1.5 |
Fig. 1.
Annual prevalence rates of gastrointestinal helminth infection of dogs brought to the University of Nigeria Veterinary Teaching Hospital between 2006 and 2013.
Table 3.
The prevalence rates of GI helminth infections of dogs brought to the University of Nigeria Veterinary Teaching Hospital, Nsukka between 2006 and 2013 on basis of breed, sex and age.
| No exam (%) | Positive cases | Negative cases | Prevalence rates (%) | |
|---|---|---|---|---|
| Breed | ||||
| Local | 200 | 125 | 75 | 62.5 |
| Exotic | 179 | 86 | 93 | 48.0 |
| Sex | ||||
| Female | 183 | 100 | 83 | 54.6 |
| Male | 196 | 111 | 85 | 56.6 |
| Age | ||||
| < 1 year | 213 | 134 | 79 | 62.9 |
| > 1 year | 166 | 77 | 89 | 46.4 |
Fig. 2.
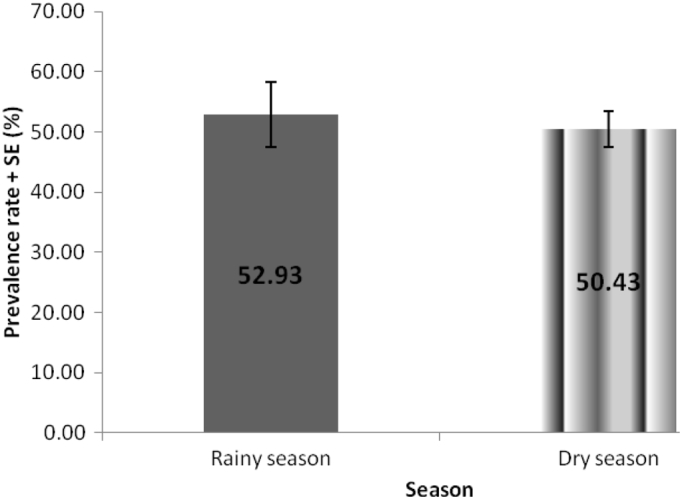
Mean rainy and dry season prevalence of parasitic helminths of dogs brought to the University of Nigeria Veterinary Teaching Hospital, Nsukka between 2006 and 2013.
3.2. Prospective prevalence study
Table 1 also presents the result of the overall prospective prevalence study of gastrointestinal helminth parasites in dogs from Enugu metropolis and Nsukka areas of Enugu State. The study recorded a total of 136 (51.7%) positive cases out of the 263 dogs examined with 95% confidence intervals between 45.7 and 57.7%. Specifically, prevalence rates of 47.2 (95% CL: 38.2 to 56.5%) and 54.9% (95% CL: 47.0 to 62.6%) of the dogs from Enugu metropolis and Nsukka respectively were positive for gastrointestinal helminth parasites. Monthly prevalence of infection as presented in Fig. 3, showed a progressive increase in the overall prevalence of infection from October 2013 through July 2014 whereby the highest prevalence (93.8%) was recorded in July followed closely by 83% in June. A mean prevalence of 68.59 ± 10.48 was recorded for the rainy season months (April to October) while 26.49 ± 6.73 was recorded for the dry season (November to March) months as shown in Fig. 4.
Fig. 3.
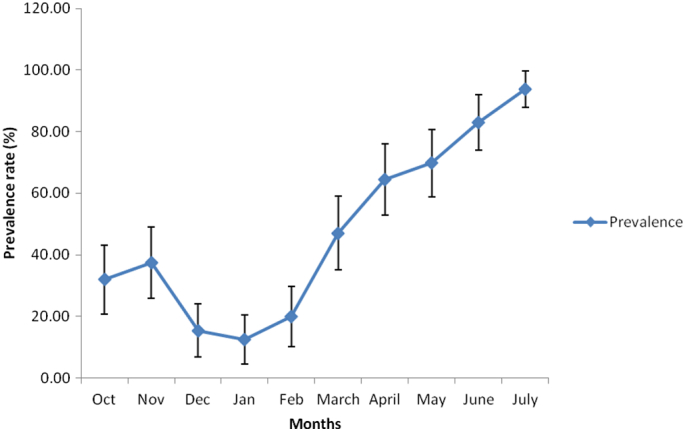
Monthly prevalence of parasitic helminth infection of dogs in Nsukka and Enugu areas of Enugu State and their 95% confidence interval.
Fig. 4.
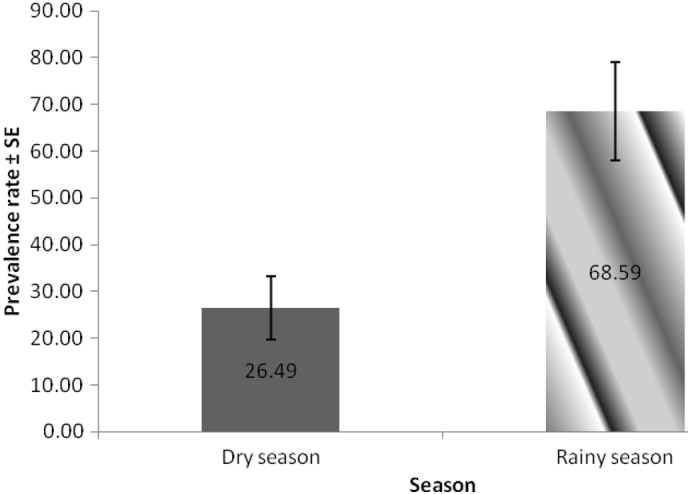
Mean monthly dry and rainy season prevalence of parasitic helminths of dogs in Nsukka and Enugu areas of Enugu state.
The results as presented in Table 4, Table 5 showed that three species of nematode (Ancylostoma, Toxocara and Trichuris) and one species of cestode (D. caninum) were encountered in the prospective study. The result in Table 4 showed that Ancylostoma spp. was found most often in Enugu area, with a prevalence rate of 33.6%, followed by Toxocara spp. and D. caninum which had prevalence rates of 20 and 13.6%, respectively. T. vulpis occurred only in mixed infection and was the least encountered with 3.6% prevalence. Mixed infections with one or more helminth parasite species as presented also in Table 4, showed that 12 dogs (10.9%) were infected with two different helminth parasite species while 6 (5.5%) dogs were infected with three or more different species. Similarly, the result in Table 5 showed also Ancylostoma species as the most prevalent species with a rate of 41.2% followed by Dipylidium, Toxocara and Trichuris which had rates of 17, 9.2, and 3.2% respectively. The prevalence of single species infection had Ancylostoma as most prevalent (24.8%) followed by Toxocara (7.3) and Dipylidium (5.9%) in this order. Trichuris vulpis was not encountered in a single species infection. The prevalence rates of dogs infected with two species of helminthes was 13.1% while 5.9% was the rate for dogs infected with more than two species.
Table 4.
Prevalence rates of GI helminth species of dogs in Enugu area of Enugu state.
| Helminth species | No. positive | Prevalence rate (%) |
|---|---|---|
| Ancylostoma | 19 | 17.3 |
| Toxocara | 10 | 9.1 |
| D. caninum | 5 | 4.5 |
| T. vulpis | 0 | 0.0 |
| Ancylostoma + D. caninum | 4 | 3.6 |
| Ancylostoma + Toxocara | 6 | 5.5 |
| Ancylostoma + T. vulpis | 2 | 1.8 |
| Ancylostoma + Toxocara + D. caninum | 4 | 3.6 |
| Ancylostoma + Toxocara + Dipylidium + T. vulpis | 2 | 1.8 |
| Toxocara + D. caninum only | 0 | 0 |
Table 5.
Prevalence rates of GI helminth species of dogs in Nsukka area of Enugu state.
| Helminth species | No. positive | Prevalence rate (%) |
|---|---|---|
| Ancylostoma | 38 | 24.8 |
| Toxocara | 11 | 7.3 |
| D. caninum | 9 | 5.9 |
| T. vulpis | 0 | 0.0 |
| Ancylostoma + D. caninum | 10 | 6.5 |
| Ancylostoma + Toxocara | 7 | 4.6 |
| Ancylostoma + T. vulpis | 2 | 1.3 |
| Ancylostoma + Toxocara + D. caninum | 3 | 2 |
| Ancylostoma + Toxocara + Dipylidium + T. vulpis | 3 | 2 |
| Toxocara + D. caninum only | 1 | 0.7 |
3.3. Breed, sex and age related prevalence
Table 6 illustrates the prevalence of GI helminth in dogs from both Enugu and Nsukka area with respect to breeds, sex and age. On the basis of breed the result showed that the local breed had higher prevalence rates of helminth infections (30.0%) than the exotic (11.4%) and the cross (10.3%) breeds. Chi-Square (x2) analysis showed a strong association (P = 0.001) between breed and prevalence of helminth infection. The results in Table 6 also showed that the male dogs had higher prevalence of infection (27.8%) than the females (24.0%) but this difference was not statistically significant (P > 0.05). The prevalence recorded on age basis (Table 6) showed that the adult dogs (> 1 year of age) had significantly (P < 0.05) higher prevalence (30.8%) than the young ones (20.9%). A logistic regression was performed to ascertain the effects of breed, sex and age on the likelihood of acquiring GI helminth infection. The logistic regression model was statistically significant (P < 0.0001) for breed. Local breeds were 2.44 times more likely to acquire the infection than the exotic breeds.
Table 6.
Prevalence rates of GI helminth infections of dogs in relation to breed, sex and age of the dogs in both Enugu and Nsukka areas of Enugu State.
| No. exam (%) | No. infected | Prevalence (%) | |
|---|---|---|---|
| Breed | |||
| Local | 119 | 79 | 30.0 |
| Exotic | 85 | 30 | 11.4 |
| Cross breed | 59 | 27 | 10.3 |
| Sex | |||
| Female | 112 | 63 | 24.0 |
| Male | 151 | 73 | 27.8 |
| Age | |||
| > 1 year | 146 | 81 | 30.8 |
| < 1 year | 117 | 55 | 20.9 |
4. Discussion
The results obtained in this study have demonstrated that dogs in Enugu State are commonly infected with one or more GI helminth species. According to the eight year retrospective study, the overall prevalence of GI helminth parasite infections in the dogs was 56.1%, which is higher but comparable to the prevalence rate of 49.8% reported by Onyenwe and Ikpegbu (2004) in a nine year (1985–1993) retrospective study, in the same study area. The prevalence recorded in the present study is much higher than the overall (16.13%) and dry season (3.14%) prevalence rates recorded by Mbaya et al. (2008) in a ten-year retrospective study of dogs brought to the Veterinary Teaching Hospital of the University of Maiduguri. However, the rainy season prevalence (50.02%) recorded by Mbaya et al. (2008) was comparable to the overall prevalence recorded in the present study. The wide disparity between the prevalence rates recorded in the present study and the overall and dry season prevalence rates recorded by Mbaya et al. (2008) is understandable, given that the present study was carried out in the humid zone of eastern Nigeria with ambient temperatures of 22 to 28 °C and average 7 months of rainfall per year. The study by Mbaya et al. (2008) was done in the arid region of northern Nigeria with very high ambient temperatures (35–45 °C) and an average 3 months of rainfall per year. Climatic conditions in the humid zone are more favourable for the development and survival of the infective stages of some helminths in the environment, than in the arid region (Chiejina, 1986). In this study, there was a disparity in the prevalence rates recorded for the various years which ranged between 43.2% in 2013 and 82.6% in 2009. This disparity was attributed to variability of climatic conditions such as rainfall and temperature which are known to influence the development and survival of pre-parasitic stages of helminths parasites (Andersen et al., 1970, Soulsby, 1982, Hansen and Perry, 1994).
In the prospective prevalence study, an overall prevalence rate of 51.7% was recorded, of which 47.2% and 54.9% were attributed to Enugu and Nsukka areas respectively. These prevalence rates are comparable to each other and to that obtained in the retrospective study and 68.5% reported by Anene et al. (1996) in the same study area in 1994. However, they are lower than the reports of 86.97% by Ugochukwu and Ejimadu (1985) in Calabar and 81.9% by Anosike et al. (2004) in Owerri. Higher prevalence rates have also been reported in other studies outside Nigeria, such as Komatangi (2005) in Cameroon, Minnaar et al. (2002) in South Africa and Dagmawi et al. (2012) in Ethiopia who reported prevalence of 88.5%, 76%, and 97.8% respectively.
The results of the retrospective and prospective studies showed that breed had significant effect on the prevalence of intestinal helminthosis in the dogs. Helminth infections were encountered more in the local breeds of dogs than their exotic and cross breed counterparts. This finding is similar to that of Awoke et al. (2011) who reported prevalence rates of 10.7 and 4.0% in local and cross breeds of dogs respectively in Gondar region, Addis Ababa. Onyenwe and Ikpegbu (2004) reported significantly higher prevalence in cross breeds over the local and exotic breeds. Anosike et al. (2004) did not find any significant breed difference in their study. The higher prevalence rate observed in this study among the local breeds over the exotic and cross breeds is likely to be attributable largely to the difference in their managements rather than a reflection of the breed susceptibility. Although the exotic and cross breeds may be less immune to the parasites than the local breeds, they are usually kept in confinement and well fed thus, less exposed to helminth infection and less vulnerable to the disease. They are also given good and prompt veterinary care whenever the need arises. The local breeds of dogs, which are usually less treasured by their owners, are allowed to roam from place to place in search of food and games (Aiyedun and Olugasa, 2012) thus getting exposed to the infective stages of the different helminths and intermediate hosts as the case may be. Some of them are also used for hunting, which increases their risk of infection. Generally, canine health management is generally very poor among the owners of the local dog breeds with little or no anthelmintic intervention except when the animal is obviously sick.
In this study, both the retrospective and prospective studies showed no significant effect of sex on the prevalence of the intestinal helminths, although the infection was encountered more in the male dogs to which our result agrees with the reports of Sowemimo and Asaolu (2008) and Awoke et al. (2011). It however, disagrees with the study by Anosike et al. (2004) in Owerri, Imo state which reported that the male dogs were significantly more infected than the females. Anosike et al. (2004) attributed their result to the fact that the males are more free-ranging than the females. The higher prevalence rate observed in the present study among the female dogs over their male counterpart was attributed to hormonal influence on immunity such as the peri-parturient relaxation of the immunity in pregnant and lactating females (Soulsby, 1982).
This present study revealed that age had a significant effect on the prevalence of the GI helminth infection in the dogs. In the retrospective study, significantly higher GI prevalence was observed in young dogs than in adult dogs. This finding agrees with the report of Bobade et al. (1984) in Kafanchan (Plateau state, Nigeria) where the highest prevalence was recorded among young dogs. The findings in the retrospective study could be due to the fact that the younger dogs are most susceptible to the infection as well as the mode of the infection whereby dogs are infected right from birth. On the contrary, the prevalence was significantly higher in adult than in young dogs in the prospective study, a finding which agrees with the report of Ezeokoli (1984) in Zaria as well as that of Yacob et al. (2007) in Ethiopia. The significantly higher prevalence observed in adult than in young dogs in the present study may be due to the fact that dog owners tend to give more attention to the younger dogs including deworming and restriction of movement. Furthermore, adult dogs are usually carriers and do not manifest obvious clinical sign of the infection while the young ones easily show clinical disease and thus attract the attention of the owners. In contrast, a study conducted in Addis Ababa by Awoke et al. (2011) observed no statistical difference between the prevalence rate of GI helminth infection in young and adult dogs.
Concurrent infection with more than one species of helminths in the same dog was very common in both the retrospective and prospective studies. The study identified Ancylostoma spp. as the most common helminth parasite of dog in the study area, followed by Toxocara spp. and D. caninum. This is attributable to the high fecundity of the female Ancylostoma species leading to heavy contamination of the environment with hookworm eggs and larvae, as well as their high infectivity to dogs of all age (Soulsby, 1982). Toxocara species on the other hand, though highly fecund is mostly seen in young animals as adults are relatively resistant to the infection (Bowman et al., 2003). This is of great public health importance because of its implication in the etiology of visceral larva migrans (VLM) in man especially among children who are traditional playmates with puppies. It is important to note that the two very important zoonotic helminth parasites namely, Ancylostoma spp. and Toxocara spp. observed in the present study had high prevalence rates. Many other studies (Chiejina and Ekwe, 1986, Thompson et al., 1986, Onwuliri et al., 1993, Anene et al., 1996) have also observed the occurrence and high prevalence rates of such zoonotic parasites. The presence of different helminth parasite species in a single host, as well as high prevalence of these parasites in the study area require serious attention due to the pathogenic impact of the parasites to the dogs and their zoonotic importance to humans. Woodruff (1975) had observed that zoonotic dog helminths possibly have more deleterious effects in humans than is commonly appreciated. This is because, it is often difficult to diagnose zoonotic helminth infections in humans since the worms rarely reach maturity in humans and hence do not produce eggs that can assist in diagnosis. It therefore, becomes very essential to monitor not only the rate of infections in dogs but also the efficacy of their treatments on constant basis.
In conclusion, the present study showed that intestinal helminthosis is very common in Enugu State, some of which are zoonotic and all classes of dogs were commonly affected. The prevalence as reported in the present and other studies in Nigeria shows that Helminthosis is the most commonly encountered infection in dogs in Nigeria when compared to other endemic diseases of dog, such as rabies and trypanosomosis. It is therefore recommended that public education on the proper care of dogs including veterinary care, personal hygiene by dog owners and handlers, and prevention of zoonotic parasitic diseases is of great importance in the study area.
Competing interests
The authors declare they have no competing interests.
Acknowledgement
The authors will like to thank the staff of the following establishments for their co-operation: Veterinary Teaching Hospital, University of Nigeria, Nsukka; Eva Veterinary Clinic, Emene Enugu and Veterinary Parasitology and Entomology Laboratory of the University of Nigeria, Nsukka.
References
- Aiyedun J.O., Olugasa B.O. Identification and analysis of dog use, management practices and implications for rabies control in Ilorin, Nigeria. Sok. J. Vet. Sci. 2012;10(2):1–6. [Google Scholar]
- Allen A.V., Ridley D.S. Further observations on the formol-ether concentration technique for faecal parasites. J. Clin. Pathol. 1970;23:545–546. doi: 10.1136/jcp.23.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen F.L., Levine N.D., Boatman P.A. Survival of third stage Trichostrongylus colubriformis larvae on pasture. J. Parasitol. 1970;56:209–232. [Google Scholar]
- Anene B.M., Nnaji T.O., Chime A.B. Internal parasitic infections of dogs in the Nsukka area of Enugu State, Nigeria. Prev. Vet. Med. 1996;27:89–94. [Google Scholar]
- Anosike J.C., Nwoke B.E., Ukaga C.N., Madu N.G., Dozie I.N. Aspects of intestinal helminth parasites of dogs in World bank-assisted Housing Estate, New Owerri, Nigeria. Afr. J. Appl. Zoo. Environ. Biol. 2004;6:25–29. [Google Scholar]
- Awoke E., Bogale B., Chanie M. Intestinal nematode parasites of dogs: prevalence and associated risk factors. Int. J. Anim. Vet. Adv. 2011;3(5):374–378. [Google Scholar]
- Bobade P.A., Oduye O.O., Aghomo H.O. Studies on canine hookworms in Ibadan, Nigeria. Niger. Vet. J. 1984;13:96–101. [Google Scholar]
- Bone J.F. third ed. Prentice-Hall Inc.; New Jersey, USA: 1988. Animal Anatomy and Physiology. [Google Scholar]
- Bowman D.D., Lynn R.C., Eberhard M.L., Alcarez A.A. eighth ed. Elseveir Health Sciences; St. Louis, USA: 2003. Georgis' Parasitology for Veterinarians. [Google Scholar]
- Chiejina S.N. The epizootiology and control of parasitic gastroenteritis of domesticated ruminants in Nigeria. Helminthol. Abst. 1986;55:413–429. [Google Scholar]
- Chiejina S.N., Ekwe T.O. Canine Toxocariasis and the associated environmental contamination of urban areas in Eastern Nigeria. Vet. Parasitol. 1986;22(1–2):157–161. doi: 10.1016/0304-4017(86)90019-1. [DOI] [PubMed] [Google Scholar]
- Dagmawi P., Mekonnen A., Abebe F., Berhanu M. Prevalence of gastrointestinal helminthes among dogs and owners perception about zoonotic dog parasites in Hawassa Town, Ethiopia. J. Pub. Health Epidemiol. 2012;4(8):205–209. [Google Scholar]
- Endrias Z., Yohannes S., Berhanu M. Prevalence of helminth parasites of dogs and owners awareness about zoonotic parasites in Ambo town, central Ethiopia. Eth. Vet. J. 2010;14(2):17–30. [Google Scholar]
- Ezeokoli C.D. Studies on prevalence of gastrointestinal parasites in pet dogs in Zaria, Nigeria. Niger. Vet. J. 1984;13:55–57. [Google Scholar]
- Gracey J.F. eighth ed. Baillere Tindall; London: 1986. Meat Hygiene. [Google Scholar]
- Hansen J., Perry B. International Livestock center for Africa; Addis Ababa Ethiopia: 1994. The Epidemiology, Diagnosis and Control of Helminth Parasites of Ruminants; pp. 20–23. [Google Scholar]
- Komatangi M.C. Prevalence of gastrointestinal helminths of dogs in Dschang, Cameroon. J. Cam. Acad. Sc. 2005;5:11–14. [Google Scholar]
- Lorenzini G., Tasca T., De Carli G.A. Prevalence of intestinal parasites in dogs and cats under veterinary care in Porto Alegre, Rio Grande do Sul, Brazil. Braz. J. Vet. Res. Anim. Sci. 2007;44:137–145. [Google Scholar]
- MAFF . Ministry of Agriculture Fisheries and Food. HMSO; London: 1977. Manual of Veterinary Laboratory Diagnostic Techniques. Bulletin Number 18; pp. 5–50. [Google Scholar]
- Mbaya A.W., Aliyu M., Nwosu C.O., Ibrahim U.I., Shallanguwa J.M. A ten-year retrospective study of the prevalence of parasite infections of dogs at the University of Maiduguri Veterinary Teaching Hospital, Nigeria. Nig. Vet. J. 2008;29(2):31–36. [Google Scholar]
- Minnaar W.N., Krecek R.C., Fourie L.J. Helminths from a peri-urban resource limited community in free State Province, South Africa. Vet. Parasitol. 2002;107:343–349. doi: 10.1016/s0304-4017(02)00155-3. [DOI] [PubMed] [Google Scholar]
- Okoye I.C., Obiezue N.R., Okorie C.E., Ofoezie I.E. Epidemiology of intestinal helminth parasites in stray dogs from markets in south-eastern Nigeria. J. Helminthol. 2011;85(4):415–420. doi: 10.1017/S0022149X10000738. [DOI] [PubMed] [Google Scholar]
- Oliveira-Sequeira T.C., Amarante A.F., Ferrari T.B., Nunes L.C. Prevalence of intestinal parasites in dogs from Sao Paulo State, Brazil. Vet. Parasitol. 2002;103:19–27. doi: 10.1016/s0304-4017(01)00575-1. [DOI] [PubMed] [Google Scholar]
- Onwuliri C.O., Anosike J.C., Nkem C.N., Payne V.K. The ecology of animal parasitic nematodes in endemic areas of Jos, Nigerian. J. Appl. Parasitol. 1993;34:131–137. [PubMed] [Google Scholar]
- Onyenwe I.W., Ikpegbu E.O. Prevalence of gastrointestinal helminth parasites (GIHP) of dogs presented at the University of Nigeria Veterinary Teaching Hospital (UNVTH) between 1994 and 2002. Niger. Vet. J. 2004;25(1):21–25. [Google Scholar]
- Overgaauw P.A., Boersema J.H. A survey of Toxocara infections in cat breeding colonies in the Netherlands. Vet. Q. 1998;20:9–11. doi: 10.1080/01652176.1998.9694826. [DOI] [PubMed] [Google Scholar]
- Ramírez-Barrios R.A., Barboza-Mena G., Munoz J., Angulo-Cubillan F., Hernandez E., Gonzalez F., Escalona F. Prevalence of intestinal parasites in dogs under veterinary care in Maracaibo, Venezuela. Vet. Parasitol. 2004;121:11–20. doi: 10.1016/j.vetpar.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Robertson I.D., Irwin P.J., Lymbry A.J., Thompson R.C. The role of companion animals in the emergence of parasitic zoonosis. Int. J. Parasitol. 2000;30:1369–1377. doi: 10.1016/s0020-7519(00)00134-x. [DOI] [PubMed] [Google Scholar]
- Soulsby E.J. seventh ed. Bailliere Tindall; London: 1982. Helminth, Arthropods and Protozoa of Domesticated Animals; pp. 212–252. [Google Scholar]
- Sowemimo O.A., Asaolu S.O. Epidemiology of intestinal helminths parasites of dogs in Ibadan, Nigeria. J. Helminthol. 2008;82:89–93. doi: 10.1017/S0022149X07875924. [DOI] [PubMed] [Google Scholar]
- Thompson D.E., Bundy D.A., Cooper E.S., Schantz P.M. Epidemiological characteristics of Toxocara canis zoonotic infection of children in a Caribbean community. Bull. World Health Organ. 1986;64:283–290. [PMC free article] [PubMed] [Google Scholar]
- Ugochukwu E.I., Ejimadu K.N. Studies on the prevalence of gastro-intestinal helminthes of dogs in Calabar, Nigeria. Int. J. Zoon. 1985;12:214–218. [PubMed] [Google Scholar]
- Urquhart G.M., Armour J., Duncan J.L., Dunn A.M., Jennings F.W. In: Veterinary Parasitology. 2nd ed, editor. Blackwell Science Ltd; Oxford, London: 1996. pp. 19–25. [Google Scholar]
- Woodruff A.W. Toxocara canis and other nematodes transmitted from dogs to men. Br. Vet. J. 1975;131(6):627–632. doi: 10.1016/s0007-1935(17)35131-x. [DOI] [PubMed] [Google Scholar]
- Yacob H.T., Ayele T., Fikru R., Basu A.K. Gastrointestinal nematodes in dogs from Debre Zeit, Ethiopia. Vet. Parasitol. 2007;148:144–148. doi: 10.1016/j.vetpar.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Zelon D.B. Dogs, Humans and Gastrointestinal Parasites: Unraveling Epidemiological and zoonotic Relationships in an endemic Tea-Growing Community in Northeast India. 2003. http://www.lookd/dogs Retrieved from.