Abstract
Giardiasis is considered the most common intestinal parasitic disease in humans worldwide. In Cuba, this infection has particularly a strong clinical impact on the child population. Giardia duodenalis is a highly diverse protozoan, which comprises a complex of eight morphologically identical genetic assemblages, further divided into sub-assemblages. The present study used triose phosphate isomerase (tpi) and small-subunit ribosomal RNA (SSU rRNA) genes as genetic markers for the identification of G. duodenalis assemblages and sub-assemblages in correlation with clinical and epidemiological data in children attended at the Paediatric Hospital “William Soler” and at Pedro Kouri Institute, between 2015 and 2016. A prevalence of 8% of G. duodenalis infection was recorded in stool samples after concentration techniques from 68 children out of 847 analysed. A 100% detection of Giardia DNA was achieved by a SSU-rRNA PCR, whereas DNA from 63 of 68 (92.6%) was successfully amplified by tpi-PCR. By this assemblage-specific tpi-PCR 32 (50.8%) assemblage B, 17 (27.0%) assemblage A and 14 (22.2%) mixed infection (A + B) were identified. Assemblage B was significantly (P < 0.02) more frequently found in children with diarrhoea. Sequence analysis of the tpi gene of Giardia isolates from symptomatic children showed that assemblage A belonged to the sub-assemblage AII, and 4 sub assemblages BIV and 1 sub assemblage BIII were also recorded. Only 2 discordant genotyping results were observed by phylogenetic comparison of SSU-rRNA and tpi sequences. Further studies with novel molecular tools for a better discrimination at the sub-assemblage level are needed to identify the dynamics of spread of giardiasis and to verify possible correlations between Giardia genetic diversity and clinical manifestation.
Keywords: Giardia duodenalis, Assemblages, Children, tpi, SSU-rRNA, Sequences, Cuba
1. Introduction
Giardiasis is a worldwide parasitic infection caused by Giardia duodenalis (syn. G. intestinalis, G. lamblia). This flagellate causes one of the most frequently diagnosed intestinal protozoal infections reported, especially in areas of developing countries (Feng and Xiao, 2011). Annually, there are > 200 million cases of notified giardiasis according to the World Health Organization (WHO) and due to the negative impact on growth and cognitive development in childhood, giardiasis was included in the WHO's ‘Neglected Diseases Initiative’ (Savioli et al., 2006).
Giardiasis is a frequently intestinal parasitic infection which contributes to approximately 5% of gastroenteritis incidences in developed countries and as high as 15–55% in developing countries (Alum et al., 2012). In Cuba, infection by Giardia represents one of the most important intestinal parasitic infections in children (Núñez et al., 2003).
Although this parasite was not considered pathogenic until 1978, currently is accepted that the infection by Giardia ranges from asymptomatic cyst passage and acute diarrhoea to a syndrome of chronic diarrhoea, weight loss, and malabsorption. The pathogenic mechanisms of this disease are now considered multifactorial, including apoptosis of enterocytes, loss of epithelial-barrier function, hypersecretion of chloride ions, inhibition of brush-boarder enzymes, and malabsorption of glucose, water, and sodium ions (Ankarklev et al., 2010).
A considerable amount of data has shown that G. duodenalis should be considered as a species complex, whose members show little variation in their morphology, and they can be assigned to at least eight distinct genetic groups or assemblages (A to H) based on protein or DNA polymorphisms (Cacciò and Ryan, 2008, Ryan and Cacciò, 2013). Studies in the molecular characterization of Giardia carried out in humans and animals at several loci, including glutamate dehydrogenase (gdh), β-giardin, small subunit ribosomal RNA (SSU rRNA), and the triose phosphate isomerase (tpi) genes, have revealed that two major genetic groups or assemblages, designed as A and B are responsible for causing the majority of human infections (Feng and Xiao, 2011).
Although several clinical and epidemiological studies of giardiasis have been conducted in Cuba (Núñez et al., 2003, Escobedo et al., 2007, Cañete et al., 2012, Escobedo et al., 2016), there are few works which have been addressed to the molecular analyses of this intestinal parasite associated with the symptomatology manifested in children (Pelayo et al., 2008, Puebla et al., 2014, Jerez-Puebla et al., 2015). The previous studies made by Pelayo et al. (2008) and Puebla et al. (2014) in a group of children from La Habana, have found that children harbouring assemblage B of Giardia were more likely to have symptomatic infections than children with isolates from assemblage A.
In the present study, we performed a molecular characterization of G. duodenalis isolated from children, addressing the SSU-rRNA and the triose phosphate isomerase (tpi) genes, which are among the most commonly genetic markers used for genotyping studies. In particular, we determined the prevalence of different G. duodenalis assemblages among children, and the associations with clinical and epidemiological data collected. Furthermore we assessed the intra-assemblage level of genetic variation at the different loci for assemblages A and B.
2. Materials and methods
2.1. Study design
A descriptive cross-sectional study was conducted in 847 children remitted to the Paediatric Hospital “William Soler” with gastrointestinal disturbances and from kindergartens and primary schools located in the municipalities of Boyeros, Arroyo Naranjo and La Lisa from the province of La Habana. As part of an intestinal parasite surveillance addressed by the Ministry of Public Health, which takes place every year all over the country, the present investigation was performed in the Parasitology Department from the Academic Paediatric Hospital “William Soler” and the Institute of Tropical Medicine “Pedro Kouri” in the period between January 2015 and March 2016.
Epidemiological and clinical data from each Giardia-positive children were recorded in standard questionnaires that had been completed by the parents, or caregivers, of the patients, following informed consent of their agreement to participate in this investigation. These surveillance data included information about some epidemiological variables (sex, age, residing area, ethnic group) and general health status of the participants (i.e. symptoms related to intestinal parasitic infections such as diarrhoea, nausea, vomiting, flatulence, fatigue, loss of weight, abdominal pain and a history of receiving anthelmintic treatment).
All cases in which co-infection with other intestinal parasites of medical importance was diagnosed, were not included in the investigation correlating infecting assemblages and clinical picture developed in children.
2.2. Stool sample collection and DNA extraction
Fresh faecal samples were collected in 50-mL screw-caps clearly labelled containers. Three stool samples were examined from 847 children for intestinal parasites by a wet smear stained with Lugol's iodine and followed by formalin ethyl acetate concentration techniques (Garcia, 2001). In parallel, these samples were processed by the brine flotation technique for the detection of parasite eggs. The diarrhoeic stool samples were stained by modified acid-fast for the diagnosis of the following intestinal coccidians: Cryptosporidium spp., Cyclospora cayetanensis and Cystoisospora belli (Garcia, 2001).
All Giardia-positive cyst/trophozoites were preserved in 2.5% potassium dichromate and stored at − 20 °C for further molecular analysis at the Institute of Tropical Medicine “Pedro Kourí”.
Giardia cysts were purified and concentrated from faecal samples in a sucrose gradient with a specific gravity of 0.85 M, following the protocol described elsewhere (Babaei et al., 2011). The cyst wall was disrupted by 5–8 freeze–thaw cycles in liquid nitrogen alternated with a 95 °C water bath.
Whole DNA was extracted directly from the specimens following the conventional DNA extraction method of phenol/chloroform/isoamyl alcohol (PCI). Briefly, purified cysts were mixed with 300 μL of buffer lysis (50 mM Tris–HCl, pH 7.5; 25 mM EDTA, 25 mM NaCl), and 1% of sodium dodecyl sulphate (SDS). After adding 100 μg/mL of proteinase K, the suspension was incubated at 56 °C for 2 h. The DNA lysate was then treated with phenol/chloroform/isoamyl alcohol (24:24:1), followed by chloroform/isoamyl alcohol (24:1) according to Sambrook and Russell (2001). The DNA was precipitated by the addition of 1 mL chilled ethanol and stored at − 20 °C until use. The dried DNA was re-suspended in 50 μL distilled water and used as a template for PCR.
2.3. Amplification of tpi and SSU-rRNA genes
Amplification of the tpi gene was performed as a single PCR according to Bertrand et al. (2005). The PCR reaction included 5 μL of DNA template and was done with 1 unit of GoTaq® DNA Polymerase (Applied Biosystems, USA) in a total volume of 25 μL comprising 5.0 μL of 5 × PCR buffer (Applied Biosystems, USA), 0.2 mM of each deoxynucleoside triphosphate (dNTPs) (Applied Biosystems, USA), and 0.4 μM of A-for and B-for forward primers and 0.4 μM of A-rev and B-rev; reverse primers. Cycling parameters were 15 min at 95 °C (initial heat activation step), followed by 50 cycles of 30 s at 94 °C, 30 s at 62 °C, and 30 s at 72 °C, with a final extension of 7 min at 72 °C. A 148-bp fragment of the assemblage A gene and 81-bp fragment of assemblage B gene were obtained with these primers.
DNA from axenic cultures of G. duodenalis strains WB-C6 (assemblage A) and GS (Assemblage B), were used as positive controls, while ultrapure H2O instead of DNA was included in negative control PCRs.
The DNA amplification products from the SSU rRNA and the tpi PCR were visualized on 2% agarose gels stained with 0.5 μg/mL of ethidium bromide (Sambrook and Russell, 2001).
For sequence analysis of the tpi gene, a nested PCR approach was done by amplification of 530-bp segment of the tpi gene according to Sulaiman et al. (2003). For the primary PCR, a PCR product of 605 bp was amplified by using primers AL3543 and AL3546. The PCR reaction included 5 μL of DNA template and was done with 1 unit of GoTaq® DNA Polymerase (Applied Biosystems, USA) in a total volume of 25 μL comprising 5.0 μL of 5 × PCR buffer (Applied Biosystems, USA), 0.2 mM of each deoxynucleoside triphosphate (dNTPs) (Applied Biosystems, USA), and 0.5 μM of primers. The reactions were performed for 35 cycles (94 °C for 45 s, 50 °C for 45 s, and 72 °C for 60 s) in a BIO RAD T100™ thermocycler, with an initial hot start (94 °C for 5 min) and a final extension (72 °C for 10 min).
For the secondary PCR, a fragment of 530 bp was amplified by using primers AL3544 and AL3545 and 2.5 μL of primary PCR reaction. The conditions for the secondary PCR were identical to the primary PCR. The PCR products were analysed by 2.0% agarose gel electrophoresis and visualized after ethidium bromide staining (Sulaiman et al., 2003).
Giardia DNA was detected in a nested SSU-rRNA PCR by using specific primers RH11 and RH4 to amplify a 130 bp product from the SSU-rRNA gene (Hopkins et al., 1997) and the primers GiarF and GiarR for the secondary PCR (Read et al., 2002) as previously described.
PCR reactions were performed in a total volume of 25 μL, with the primary PCR reaction mixture containing 1unit GoTaq® DNA Polymerase (Applied Biosystems, USA) with 5.0 μL of 5 × PCR buffer GeneAmp® PCR buffer kit (Applied Biosystems, USA), 0.5 μM of RH11 and RH4 primers, 0.2 mM of deoxynucleotide triphosphates (dNTPs) and 1.25 μL of dimethyl sulfoxide (DMSO). The thermocycler (BIO RAD T100™, Singapore) conditions consisted of 96 °C for 5 min for 1 cycle, 96 °C for 45 s, a50°C for 30 s and 72 °C for 45 s for 35 cycles followed by 72 °C for 7 min.
Two microlitres from the first PCR were used as template in the secondary PCR with forward GiarF, and reverse GiarR primers under the same amplification conditions with the exception that 55 °C instead of 50 °C was applied as annealing temperature (Read et al., 2002).
2.4. Giardia phylogenetic analysis
In order to assess the genetic variability of Giardia isolates from symptomatic children, PCR-sequencing-based genotyping using marker genes tpi and SSU-rRNA was performed.
PCR products were purified using the QIAquick® PCR Purification kit (QIAGEN Ltd.), and were sequenced in both directions with the respective forward and reverse primers using the Beckman Coulter Genomics sequencing system (Essex, United Kingdom).
The sequences obtained were aligned using the BioEdit v7.0.1 package, then compared with gene sequences of Giardia available from the NCBI server, using the basic local alignment search tool (BLAST) (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine the Giardia assemblage, sub-assemblage and sub-type.
A phylogenetic tree for tpi and SSU-rRNA was constructed based on the neighbour-joining algorithm. Distance-based analyses were conducted using Kimura 2-parameter distance estimates including alignments obtained from ClustalW using the Molecular Evolutionary Genetics Analysis version 6.06 (MEGA 6) (Tamura et al., 2013). Bootstrap proportions were calculated by the analysis of 1000 replicates of the phylogenetic tree.
SSU-RNA reference sequences used for G. duodenalis were sub assemblage AI: M54878, AII: AF199446, AIII: DQ100287, sub assemblage BIII: AF199447 and BIV: AF113898. Trichomonas tenax U37711 was used as outgroup. Reference sequences for tpi gene from G. duodenalis were sub assemblage AI: L02120, AII: U57897, AIII: DQ650647, sub assemblage BIII: AF069561, BIV: AF069560; assemblage C: AY228641, assemblage D: DQ246216, assemblage E: KF891311 and assemblage G: EU781013. Giardia ardeae AF069564, was used as outgroup.
2.5. Statistical analysis
The Epi-Info 6.04 (Centers for Disease Control and Prevention; Atlanta, GA, USA) and EPIDAT 3.1 statistical programmes were used for the selection of the sampled population and the analyses of data derived from questionnaires and parasitological examinations, respectively. Chi-square test and proportion tests were employed to compare categorical variables. The Fisher's exact test was used when required by data scarcity.
The medians of age in years, was compared using the Kruskal-Wallis non parametric test. The P values < 0.05 were considered as statistically significant for all tests. The odds ratio (OR) with 95% confidence interval (CI) were performed as measures of association.
2.6. Ethics statement
The study was approved by the Ethics Committee of the “Pedro Kourí” Institute, under the following name: Genetic characterization of G. duodenalis isolates from symptomatic and asymptomatic children and its clinical and epidemiological correlation (reference CEI-IPK-15-13).
All parents and/or tutors of participating children were informed about the study objectives and voluntary written consent was sought and obtained before inclusion.
3. Results
3.1. Prevalence and identification of G. duodenalis assemblages and mixed assemblages
From 847 children examined, a total of 68 (8.0%) scored positive for Giardia by microscopy. Co-infection with other parasites was found in 11 children (4 cases with Entamoeba histolytica/E. dispar, 2 Enterobius vermicularis, 2 Trichuris trichiura, 1 Ascaris lumbricoides, 1 Cyclospora cayetanensis, 1 Cryptosporidium spp.).
PCR products of the expected size were successfully amplified by the SSU-rRNA in 100% of microscopically positive cases, and in 63/68 (92.6%) by the assemblage specific tpi PCR.
The PCR-tpi classified 32 (50.8%) isolates as assemblage B and 17 (27.0%) as assemblage A, whereas in 14 (22.2%) cases mixed infections (A + B) were recorded.
3.2. Associated epidemiological and clinical factors for Giardia duodenalis assemblage infections
The association of G. duodenalis assemblages causing infections and sociodemographic characteristics are shown in Table 1. No statistically difference was found between assemblage of Giardia and the variables sex, educational institution, residence (urban or rural) and age (P > 0.05).
Table 1.
Association between Giardia duodenalis assemblages and epidemiological data related to giardiasis in studied children.
| Characteristics | Assemblage B (n = 32) |
Assemblage A (n = 17) |
Mixed assemblages A + B (n = 14) |
P values | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Sex | |||||||
| Male | 18 | 56.3 | 13 | 76.5 | 7 | 50.0 | P = 0,26 |
| Female | 14 | 43.7 | 4 | 23.5 | 7 | 50.0 | P = 0,26 |
| Institution | |||||||
| Kindergarten | 17 | 53.1 | 7 | 41.2 | 5 | 35.7 | P = 0,49 |
| School | 10 | 31.3 | 7 | 41.2 | 5 | 35.7 | P = 0,79 |
| House | 5 | 15.6 | 3 | 17.6 | 4 | 28.6 | P = 0,58 |
| Residing area | |||||||
| Urban | 28 | 87.5 | 16 | 94.1 | 12 | 85.7 | P = 0,71 |
| Rural | 4 | 12.5 | 1 | 5.9 | 2 | 14.3 | P = 0,68 |
| Age | |||||||
| Mean value (± SD) | 4.6 (± 3.7) | 5.4 (± 4.3) | 4.4 (± 2.9) | P = 0,71a | |||
| Median | 3.0 | 4.0 | 4.0 | P = 0,6759 | |||
| Interval | 1–16 | 1–13 | 1–13 | ||||
Kruskal-Wallis test.
The clinical data and the association with infections by G. duodenalis assemblages are shown in Table 2. Assemblage B of Giardia was significantly (P < 0.02) more associated with diarrhoea in children. No correlation was found between other symptoms and signs with specific assemblage infections compared with the analysed groups (P > 0.05).
Table 2.
Association between clinical data and infecting G. duodenalis assemblages in children only infected with Giardia (no mixed intestinal infection).
| Clinical data | Assemblage B (n = 28) |
Assemblage A (n = 13) |
Mixed assemblages A + B (n = 11) |
P values | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Symptomatic children | 24 | 85.7 | 10 | 76.9 | 7 | 63.6 | P = 0,31 |
| Diarrhoea | 13 | 46.4 | 2 | 15.4 | 1 | 9.1 | P = 0,02a |
| Abdominal pain | 13 | 46.4 | 6 | 46.1 | 3 | 27.3 | P = 0,52 |
| Flatulence | 8 | 28.6 | 1 | 7.7 | 5 | 45.5 | P = 0,11 |
| Nausea | 4 | 14.3 | 1 | 7.7 | 2 | 18.2 | P = 0,74 |
| Vomiting | 2 | 7.1 | 4 | 30.8 | 3 | 27.3 | P = 0,11 |
| Headache | 1 | 3.6 | 0 | 0 | 0 | 0 | P = 0,65 |
| Anorexia | 12 | 42.9 | 4 | 30.8 | 1 | 9.1 | P = 0,09 |
| Fever | 4 | 14.3 | 4 | 30.8 | 1 | 9.1 | P = 0,33 |
| Loss of weight | 12 | 42.9 | 4 | 30.8 | 3 | 27.3 | P = 0,58 |
| Fatigue | 6 | 21.4 | 2 | 15.4 | 1 | 9.1 | P = 0,64 |
| Duration of diarrhoea (days) | |||||||
| Mean (± SD) | 4.5 (± 3.5) | 3.2 (± 2.0) | 3.3 (± 0.58) | P = 0,76b | |||
| Median | 3.0 | 3.0 | 3.0 | ||||
| Interval | 1–12 | 1–7 | 3–4 | ||||
Significant difference by Chi squared test.
Kruskal-Wallis test.
3.3. DNA sequence analyses of tpi and SSU-rRNA amplicons and identification of Giardia sub-assemblages
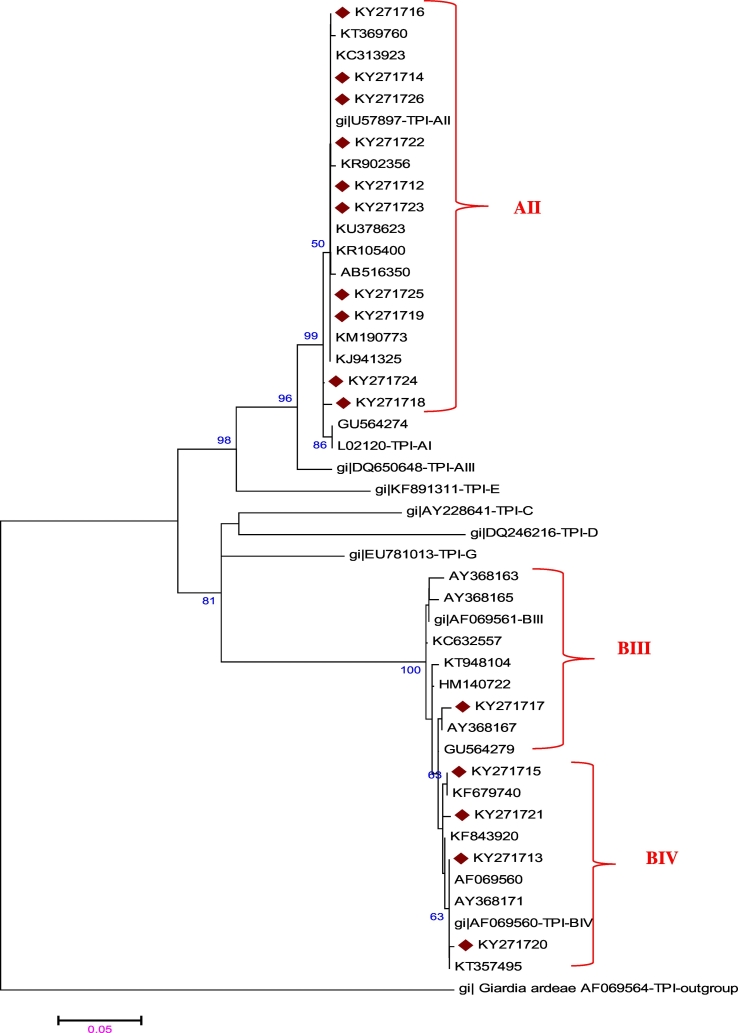
From 22 strong PCR products obtained in the nested PCR-tpi, PCR amplicons from 15 symptomatic children (10 assemblages A and 5 assemblages B) were successfully sequenced. Based on phylogenetic analysis of the tpi gene (Fig. 1), all 10 of the assemblage A isolates were classified as subtype A2, 4 revealed a subtype BIV and 1 BIII (Table 3). A complete coincidence between PCR results and sequence analysis was obtained for the tpi marker gene.
Fig. 1.
Phylogenetic tree constructed from the tpi sequences of Giardia duodenalis isolates. The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. Evolutionary analyses were conducted in MEGA6 and further bootstrap analysis of 1000 replicas.
Table 3.
Distribution of G. duodenalis assemblages A and B and sub assemblages based on PCR-tpi result and sequences obtained by SSU-rRNA and tpi genes.
| Isolate | Sequence by tpi | GenBank accession no. | Sequence by SSU-rRNA | GenBank accession no. |
|---|---|---|---|---|
| 1 | AII | KY271712 | A | KY271727 |
| 2 | BIV | KY271713 | B | KY271728 |
| 3 | AII | KY271714 | A | KY271729 |
| 4 | BIV | KY271715 | B | KY271730 |
| 5 | AII | KY271716 | B | KY271731 |
| 6 | BIII | KY271717 | A | KY2717132 |
| 7 | AII | KY271718 | A | KY271733 |
| 8 | AII | KY271719 | A | KY271734 |
| 9 | BIV | KY271720 | B | KY271735 |
| 10 | BIV | KY271721 | B | KY271736 |
| 11 | AII | KY271722 | A | KY271737 |
| 12 | AII | KY271723 | A | KY271738 |
| 13 | AII | KY271724 | Not sequenced | |
| 14 | AII | KY271725 | Not sequenced | |
| 15 | AII | KY271726 | Not sequenced |
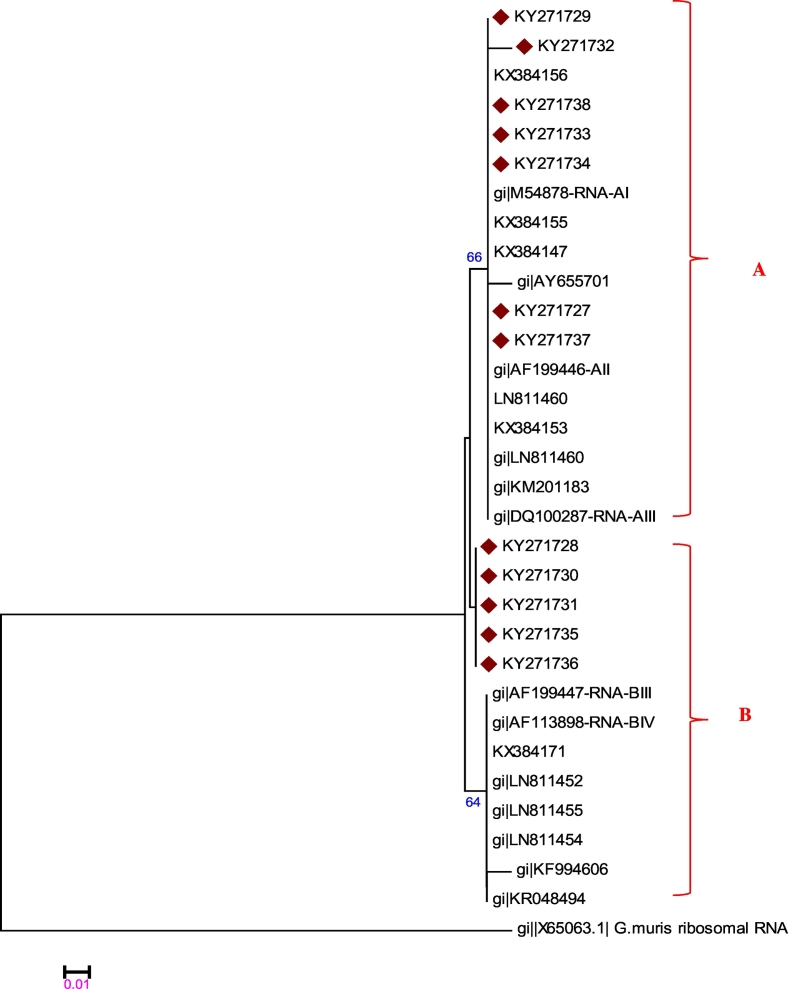
In the case of the SSU-rRNA gene, sequences from 12 out of the same 15 symptomatic children were obtained. The SSU-rRNA-based phylogenetic tree was not able to differentiate between G. duodenalis sub-assemblages (Fig. 2). An agreement of 83.3% (10 out of 12) between sequences results of RNA and tpi genes was recorded (Table 3).
Fig. 2.
Phylogenetic tree constructed from the SSU-rRNA sequences of Giardia duodenalis isolates using Kimura-2 parameter distance estimation method clustered by the NJ method in MEGA 6 and further bootstrap analysis of 1000 replicas.
Sequences obtained at the tpi and SSU rRNA loci were deposited in GenBank under the following accession numbers: KY271712-KYP271726, and KY271727-KYP271738, respectively.
4. Discussion
The present study provides information on the status of Giardia infection among children of Havana, Cuba. The overall prevalence rate of Giardia infection was 8.0%. This is in agreement with previous studies made in the last few years where the prevalence rates among children have been reported from 9 to 22% (Núñez et al., 2003, Escobedo et al., 2008, Cañete et al., 2012, Puebla et al., 2014).
The symptomatology developed in giardiasis is probably determined by several factors, among which the virulence of the parasite, nutritional, and immunological status of the host, the nature of intestinal microbiota, and the presence or absence of other co-pathogens are considered the most important ones (Cotton et al., 2011). In the last years, an approach about how the genetic diversity of the parasite can influence the outcome, as a possible factor involving in differences observed between symptomatic and asymptomatic children, has been ascertained (Kohli et al., 2008).
Molecular characterization of Giardia by several molecular markers is considered a key component in enhancing the understanding on the taxonomy, epidemiology and population genetics of this intestinal parasite (Feng and Xiao, 2011). Actually, several genetic loci are used to characterize Giardia at the genotype and subtype levels, among these, the most commonly used are the SSU rRNA, tpi, gdh and bg genes (Ryan and Cacciò, 2013).
It is known that tpi gene is a polymorphic gene, hence it is a profitable marker for genotyping and subtyping studies in G. duodenalis (Sprong et al., 2009); whereas the SSU rRNA, due to its multi-copy nature, results in greater sensitivity of PCR; however, the potential for discrimination is lower, and differentiation is possible only between assemblages, not between sub-assemblages or subtypes (Ryan and Cacciò, 2013). Because Giardia spp. have a clonal population structure, the use of a typing system based on sequence analysis of a single genetic locus with high sequence heterogeneity, such as tpi, can provide a resolution as high as multilocus sequence typing (Sulaiman et al., 2003). Similar reports were also reported in previous studies (Lalle et al., 2009, David et al., 2011, Mbae et al., 2016).
PCR for the SSU-rRNA gene proved to be the most sensitive method to detect Giardia DNA in all microscopically-Giardia positive stool samples. It has been reported that the Giardia-specific SSU rRNA PCR has a detection limit of 10 pg DNA/μL (Jaros et al., 2011).
Assemblage B of Giardia accounted 50% of infected children using assemblage-specific PCR-tpi, whereas assemblage A and mixed infection A + B had a similar distribution. Similar results in our country reveal a predominance of assemblage B (Puebla et al., 2014, Jerez-Puebla et al., 2015), which agrees with previous reports from other countries around the world (Gelanew et al., 2007, Lebbad et al., 2008, Breathnach et al., 2010). Some reports have suggested that the higher Giardia excretion in assemblage B-infections as detected by microscopy or real-time PCR could explain the predominance of this assemblage in certain areas (Kohli et al., 2008).
Mixed infections were identified in 22.2% of Giardia-infected children. Data collected in our country have shown that mixed infections of Giardia ranges from 17 to 25% (Puebla et al., 2014, Jerez-Puebla et al., 2015). Interestingly, in both studies, the tpi gene was used as one of the molecular markers. It is indeed known that the use of assemblage-specific tpi primers allows the detection of a much higher number of mixed assemblages A and B infections than approaches based on the use of more general primers for PCR (Sulaiman et al., 2003). Besides, the occurrence of mixed infections with several assemblages/subtypes of G. duodenalis reflects the complex circulation of the parasite in the environment and the exposure of humans to multiple sources (Gelanew et al., 2007).
Several studies have described correlations between assemblages and symptoms, but there has been a lack of concordance among the data worldwide. G. duodenalis infection is known to vary widely in clinical manifestation including acute, chronic, and asymptomatic courses (Feng and Xiao, 2011). Among symptomatic children investigated in the present study, a significant association between diarrhoea and infection with assemblage B of Giardia was found. This is in agreement with previous studies made in Havana, Cuba, where assemblage B has been associated with diarrhoea, flatulence and abdominal pain in Giardia-infected children (Puebla et al., 2014, Jerez-Puebla et al., 2015, Jerez-Puebla et al., 2016).
To date, there is still a lack of clear association between the assemblage of Giardia and the clinical outcome, with contradictory results worldwide. Some studies have found an association of diarrhoea with assemblage A infection (Haque et al., 2005, Ajjampur et al., 2009); other studies from various regions also suggest a correlation between the presence of symptoms and infection with assemblage B (Gelanew et al., 2007, Lebbad et al., 2008, Al-Mohammed, 2011). On the contrary, some reports have however found no relation between symptomatology and the infecting assemblage (Kohli et al., 2008).
Considering the level of genetic diversity within G. duodenalis, some authors point out that it will be important to analyse if intra-specific variation of this parasite would underlie differences in host infectivity/disease outcome among different isolates (Ryan and Cacciò, 2013). For that reason, a sequences analysis of tpi and SSU-rRNA genes was performed in some Giardia isolates from symptomatic children.
In the current study, sequences of tpi gene showed that all children infected with assemblage A had probably acquired their parasites through the anthroponotic route, since they harboured sub-assemblage AII, which is rarely found in animals (Sprong et al., 2009). This is similar with other studies where AII was the predominant sub-assemblage in human (Bonhomme et al., 2011, Lebbad et al., 2011). Distribution of subassemblage B showed that BIV accounted 4 out of 5 infected children. Although designation of isolates to subassemblages BIII or BIV is problematic, an accurate system to classify assemblage B isolates that enables comparison between studies is currently not available (Asher et al., 2014). The isolation of anthroponotic assemblages and sub-assemblages B, as well, implicates human as a potential source of the infection. Similar results of predominance of subassemblage AII have been found in our country in previous works (Puebla et al., 2014, Jerez-Puebla et al., 2015). In this study only one sub assemblage BIII was identified in the few samples that were sequenced and 4 sub assemblage BIV were classified according to the percentage of homology with references strains. In a previous research made in Sancti Spiritus, a high prevalence of subassemblage BIII was recorded by PCR-RFLP analysis (Jerez-Puebla et al., 2015).
Even though PCR for the SSU-rRNA gene proved to be the most sensitive method in identifying Giardia in faecal samples, phylogenetic analysis of this gene, as a subtyping tool proved to have a low discriminatory potential, and differentiation is possible only between assemblages, not between sub-assemblages or subtypes (Ryan and Cacciò, 2013).
In the present study, discordant genotyping results between tpi and SSU-rRNA genes were obtained in 2 cases out of 12 by sequences analysis. Although the underlying mechanisms remain uncertain, some factors that could contribute to the incongruent classification of assemblages by different makers include (i) the presence of a mixture of genetically different cysts in a faecal sample in combination with preferential amplification of a particular assemblage-specific marker gene compared to another marker gene indicative for a second assemblage (i.e., a true mixed infection followed by biased PCR amplification) (Wielinga and Thompson, 2007); (ii) meiotic recombination, which suggests the potential of sexual reproduction in Giardia, and (iii) introgression that involves back crossing of hybrids with parental species and retention of ancestral polymorphism that could lead to presence of identical alleles in genetically distinct groups (Ryan and Cacciò, 2013).
In summary, the present study provides information on the distribution of Giardia assemblage's infection in paediatric population associated with some epidemiological and clinical parameters in Cuban children. The use of molecular epidemiological tools and particularly subtyping tools is important in understanding the epidemiology and dynamic transmission of Giardia infection thus contributing to an improved knowledge about the characteristics of this parasitic disease.
5. Conclusions
The high proportion of assemblage B of Giardia detected in this study among children confirms previous reports in our country. Mixed-assemblage infections had a high frequency. The only correlation observed between symptoms and assemblages was that diarrhoea was more commonly found in children infected with assemblage B. Subassemblage AII and BIV were most frequently identified in the population of children studied. Studies to be carried out with a higher number of samples from humans and animals and based on different sub-typing tools including high throughput next-generation sequencing will increase our understanding in the epidemiology and dynamic transmission of Giardia.
Acknowledgments
This study was in part supported by a grant from the Swiss National Science Foundation (Grant Nos. 31003A_163230 and 31003A_138353). We would like to express our special thanks to children's families for participation in this study, and to the staff at William Soler Paediatric Hospital for assistance and cooperation.
References
- Ajjampur S.S., Sankaran P., Kannan A. Giardia duodenalis assemblages associated with diarrhea in children in South India identified by PCR-RFLP. Am.J.Trop. Med. Hyg. 2009;80:16–19. [PMC free article] [PubMed] [Google Scholar]
- Ankarklev J., Jerlström-Hultqvist J., Ringqvist E., Troell K., Svärd S.G. Behind the smile: cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 2010;8(6):413–422. doi: 10.1038/nrmicro2317. [DOI] [PubMed] [Google Scholar]
- Al-Mohammed H.I. Genotypes of Giardia intestinalis clinical isolates of gastrointestinal symptomatic and asymptomatic Saudi children. Parasitol. Res. 2011;108:1375–1381. doi: 10.1007/s00436-010-2033-5. [DOI] [PubMed] [Google Scholar]
- Alum A., Sbai B., Asaad H., Rubino J.R., Khalid Ijaz M. ECC-RT-PCR: a new method to determine the viability and infectivity of Giardia cysts. Int. J. Infect. Dis. 2012;16(5):e350–e353. doi: 10.1016/j.ijid.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Asher A.J., Holt D.C., Andrews R.M., Power M.L. Distribution of Giardia duodenalis assemblages A and B among children living in a remote indigenous community of the northern territory, Australia. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei Z., Oormazdi H., Rezaie S., Rezaeian M., Razmjou E. Giardia intestinalis: DNA extraction approaches to improve PCR results. Exp. Parasitol. 2011;128:159–162. doi: 10.1016/j.exppara.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Bertrand I., Albertini L., Schwartzbrod J. Comparison of two target genes for detection and genotyping of Giardia lamblia in human feces by PCR and PCR-restriction fragment length polymorphism. J. Clin. Microbiol. 2005;43:5940–5944. doi: 10.1128/JCM.43.12.5940-5944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach A.S., McHugh T.D., Butcher P.D. Prevalence and clinical correlations of genetic subtypes of Giardia lamblia in an urban setting. Epidemiol. Infect. 2010;10:1–9. doi: 10.1017/S0950268810000208. [DOI] [PubMed] [Google Scholar]
- Bonhomme J., Le Goff L., Lemée V., Gargala G., Ballet J.J., Favennec L. Limitations of tpi and bg genes sub-genotyping for characterization of human Giardia duodenalis isolates. Parasitol. Int. 2011;60(3):327–330. doi: 10.1016/j.parint.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Cacciò S.M., Ryan U. Molecular epidemiology of giardiasis. Mol. Biochem. Parasitol. 2008;160:75–80. doi: 10.1016/j.molbiopara.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Cañete R., Díaz M.M., Avalos García R., Martinez M., Ponce F. Intestinal parasites in children from a day care centre in Matanzas City, Cuba. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton J.A., Beatty J.K., Buret A.G. Host parasite interactions and pathophysiology in Giardia infections. Int. J. Parasitol. 2011;41:925–933. doi: 10.1016/j.ijpara.2011.05.002. [DOI] [PubMed] [Google Scholar]
- David E.B., Coradi S.T., Oliveira-Sequeira T.C.G., Ribolla P.E.M., Katagiri S., Guimaraes S. Diagnosis of Giardia infections by PCR-based methods in children of an endemic area. J. Venomous Anim. Toxins. 2011;17:209–215. [Google Scholar]
- Escobedo A.A., Cañete R., Núñez F.A. Intestinal protozoan and helminth infections in the Municipality San Juan y Martínez, Pinar del Río, Cuba. Trop. Dr. 2007;37:236–238. doi: 10.1258/004947507782332991. [DOI] [PubMed] [Google Scholar]
- Escobedo A.A., Alvarez G., González M.E., Almirall P., Cañete R., Cimerman S., Ruiz A., Pérez R. The treatment of giardiasis in children: single-dose tinidazole compared with 3 days of nitazoxanide. Ann. Trop. Med. Parasitol. 2008;102(3):199–207. doi: 10.1179/136485908X267894. [DOI] [PubMed] [Google Scholar]
- Escobedo A.A., Lalle M., Hrastnik N.I., Rodríguez-Morales A.J., Castro-Sánchez E., Cimerman S., Almirall P., Jones J. Combination therapy in the management of giardiasis: What laboratory and clinical studies tell us, so far. Acta Trop. 2016;162:196–205. doi: 10.1016/j.actatropica.2016.06.026. [DOI] [PubMed] [Google Scholar]
- Feng Y., Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011;24(1):110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L.S. Intestinal protoazoa coccidia and microspordia. In: Garcia L., editor. Diagnostic Medical Parasitology. fourth ed. American Society of Microbiology; Washington: 2001. [Google Scholar]
- Gelanew T., Lalle M., Hailu A., Pozio E., Cacciò S.M. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. 2007;102:92–99. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Haque R., Roy S., Kabir M., Stroup S.E., Mondal D., Houpt E.R. Giardia assemblage A infection and diarrhea in Bangladesh. J. Infect. Dis. 2005;192:2171–2173. doi: 10.1086/498169. [DOI] [PubMed] [Google Scholar]
- Hopkins R.M., Meloni B.P., Groth D.M., Wetherall J.D., Reynoldson J.A., Thompson R.C.A. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. J. Parasitol. 1997;83:44–51. [PubMed] [Google Scholar]
- Jaros D., Zygner W., Jaros S., Wedrychowicz H. Detection of Giardia intestinalis assemblages A, B and D in domestic cats from Warsaw, Poland. Pol. J. Microbiol. 2011;60:259–263. [PubMed] [Google Scholar]
- Jerez-Puebla L.E., Núñez F.A., Brito A., Rojas L., Atencio I., Cordoví R. Sub-assemblages distribution of Giardia duodenalis and its association with clinical symptoms in children from Sancti Spíritus, Cuba. J. Coast. Life Med. 2015;3(11):864–868. [Google Scholar]
- Jerez-Puebla L.E., Núñez F.A., Rojas L., Martínez I., Ayllón L., Atencio I., Müller N. Distribution of Giardia duodenalis assemblages by PCR-RFLP of β-Giardin gene in Cuban children. J. Pediatr. Infect. Dis. 2016;1:21–26. [Google Scholar]
- Kohli A., Bushen O.Y., Pinkerton R.C., Houpt E., Newman R.D., Sears C.L. Giardia duodenalis assemblage, clinical presentation and markers of intestinal inflammation in Brazilian children. Trans. R. Soc. Trop. Med. Hyg. 2008;102:718–725. doi: 10.1016/j.trstmh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalle M., Bruschi F., Castagna B., Campa M., Pozio E., Caccio S.M. High genetic polymorphism among Giardia duodenalis isolates from Sahrawi children. Trans. R. Soc. Trop. Med. Hyg. 2009;103:834–838. doi: 10.1016/j.trstmh.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Lebbad M., Ankarklev J., Tellez A., Leiva B., Andersson J.O. Dominance of Giardia assemblage B in Leon, Nicaragua. Acta Trop. 2008;106:44–53. doi: 10.1016/j.actatropica.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Lebbad M., Petersson I., Karlsson L., Botero-Kleiven S., Andersson J.O., Svenungsson B. Multilocus genotyping of human Giardia isolates suggests limited zoonotic transmission and association between assemblage B and flatulence in children. PLoS Negl. Trop. Dis. 2011;5(8) doi: 10.1371/journal.pntd.0001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbae C., Mulinge E., Guleid F., Wainaina J., Waruru A., Njiru Z.K., Kariuki S. Molecular characterization of Giardia duodenalis in children in Kenya. BMC Infect. Dis. 2016;16:135. doi: 10.1186/s12879-016-1436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez F.A., López J.L., De la Cruz A.M., Finlay C.M. Factores de riesgo de la infección por Giardia lamblia en niños en guarderías infantiles de Ciudad de La Habana, Cuba. Cad. Saude Publica. 2003;19:677–682. doi: 10.1590/s0102-311x2003000200036. [DOI] [PubMed] [Google Scholar]
- Pelayo L., Núñez F.A., Rojas L., Furuseth H., Gjerde B., Wilke H. Giardia infections in Cuban children: the genotypes circulating in a rural population. Ann. Trop. Med. Parasitol. 2008;102(7):585–595. doi: 10.1179/136485908X355247. [DOI] [PubMed] [Google Scholar]
- Puebla L.J., Núñez F.A., Fernández Y.A., Fraga J., Rivero L.R., Millán I.A., Valdés L.A. Correlation of Giardia duodenalis assemblages with clinical and epidemiological data in Cuban children. Infect. Genet. Evol. 2014;23:7–12. doi: 10.1016/j.meegid.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Read C., Walters J., Robertson I.D., Thompson R.C. Correlation between genotype of Giardia duodenalis and diarrhoea. Int. J. Parasitol. 2002;32:229–231. doi: 10.1016/s0020-7519(01)00340-x. [DOI] [PubMed] [Google Scholar]
- Ryan U., Cacciò S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013;43:943–956. doi: 10.1016/j.ijpara.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Russell D.W. Cold Spring Harbor Laboratory; New York: 2001. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- Savioli L., Smith H., Thompson R.C.A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 2006;22:203–208. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Sprong H., Cacciò S., van der Giessen J. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl. Trop. Dis. 2009;3(12) doi: 10.1371/journal.pntd.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman I.M., Fayer R., Bern C., Gilman R.H., Trout J.M. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 2003;9:1444–1452. doi: 10.3201/eid0911.030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielinga C.M., Thompson R.C. Comparative evaluation of Giardia duodenalis sequence data. Parasitology. 2007;134:1795–1821. doi: 10.1017/S0031182007003071. [DOI] [PubMed] [Google Scholar]