Abstract
Hundreds of millions of people are infected with helminths and intestinal protozoa, particularly children in low- and middle-income countries. Preventive chemotherapy is the main strategy to control helminthiases. However, rapid re-infection occurs in settings where there is a lack of clean water, sanitation and hygiene. In August and September 2014, we conducted a cross-sectional epidemiological survey in 56 communities of three departments of south-central Côte d’Ivoire. Study participants were invited to provide stool and urine samples. Stool samples were examined for helminth and intestinal protozoa infections using the Kato-Katz technique and a formalin-ether concentration method. Urine samples were subjected to a filtration method for the diagnosis of Schistosoma haematobium. Information on sociodemographic characteristics, knowledge, attitude, practices and beliefs with regard to hygiene, sanitation and intestinal parasitic diseases were collected using a questionnaire administered to household heads. Multivariable logistic regression models were employed to analyse associations between parasite infections and risk factors. Overall, 4,305 participants had complete parasitological and questionnaire data. Hookworm was the predominant helminth species (21.2%), while Ascaris lumbricoides, Trichuris trichiura, Schistosoma mansoni and S. haematobium showed prevalences below 10%. Infections with pathogenic intestinal protozoa (e.g. Entamoeba histolytica/E. dispar and Giardia intestinalis) were similarly prevalent in the three departments. Hookworm infection was associated with open defecation and participants' age and sex. Entamoeba coli infection was negatively associated with the use of tap water at home (odds ratio (OR) = 0.66; p = 0.032). Disposal of garbage in close proximity to people’s home was positively associated with G. intestinalis (OR = 1.30; p = 0.015). Taken together, helminth and intestinal protozoa infections affected a considerable proportion of rural dwellers in south-central Côte d’Ivoire at the onset of a cluster-randomised intervention trial. Our results will serve as baseline to monitor the effect of a package of interventions, including preventive chemotherapy, sanitation and health education on re-infection with helminths and intestinal protozoa.
Trial registration: ISRCTN53102033 (date assigned: 26 March 2014)
Keywords: Côte d’Ivoire, Integrated control, Intestinal protozoa, Sanitation and hygiene, Schistosomiasis, Soil-transmitted helminthiasis
1. Introduction
Intestinal parasitic diseases due to infections with helminths (e.g. soil-transmitted helminthiasis (STH) and schistosomiasis) and intestinal protozoa (e.g. amoebiasis and giardiasis) are widespread in tropical and subtropical regions, where climatic, ecological, socioeconomic and hygienic conditions favour their transmission (Hotez et al., 2014; Utzinger et al., 2012). More than a billion people are affected by STH, schistosomiasis and intestinal protozoa infections, causing an estimated 26.1 million disability-adjusted life years (GBD 2015 DALYs and HALE Collaborators, 2015; Pullan et al., 2014). School-aged children in low- and middle-income countries are at highest risk of infection, and hence, developing morbidity (Nematian et al., 2004; Ostan et al., 2007).
For the control of STH and schistosomiasis, the World Health Organization (WHO) recommends preventive chemotherapy, which is the periodic treatment with albendazole or mebendazole against STH and praziquantel against schistosomiasis, mainly targeting school-aged children (WHO, 2006). Preventive chemotherapy primarily aims at reducing worm loads, and hence, reducing associated morbidity (WHO, 2011). However, preventive chemotherapy does not protect from re-infection (Hotez et al., 2008; Jia et al., 2012). To sustain control and move towards elimination, it is necessary to complement preventive chemotherapy with other measures, such as interventions improving water, sanitation and hygiene (WASH) and information, education and communication (IEC) (Grimes et al., 2014; Jia et al., 2012; McManus et al., 2014; Strunz et al., 2014; Ziegelbauer et al., 2012).
In Côte d’Ivoire, STH, schistosomiasis, giardiasis and amoebiasis are of considerable public health relevance (Ouattara et al., 2010; Yapi et al., 2016). Coverage of improved water and sanitation is low among rural populations. In turn, open defecation is common (Schmidlin et al., 2013). In 2011 and 2012, a pilot study was implemented to evaluate the effect of an intervention package to reduce re-infection with helminths and intestinal protozoa and to initiate changes in hygiene and defecation behaviour (Hürlimann et al., 2018). Results were promising, and hence, a research project was launched in August 2013, designed as a cluster-randomised trial to be conducted in 56 communities of three departments in south-central Côte d’Ivoire. The aim was to document the effect of an integrated control approach, consisting of preventive chemotherapy, community-led total sanitation (CLTS) and health education, on re-infection with helminths and intestinal protozoa and diarrhoeal incidence. Here, we focus on the baseline situation before implementing the aforementioned cluster-randomised trial and describe the epidemiology of helminthiases and intestinal protozoa infections.
2. Material and methods
2.1. Ethics approval and consent to participate
Institutional approval of the study protocol was granted by the research commission of the Centre Suisse de Recherches Scientifiques en Côte d’Ivoire (CSRS). Ethical clearance was granted by the ethics committees of Basel (EKBB, reference no. 300/13) and Côte d’Ivoire (reference no. 76-MSLS-CNER-dkn). Local authorities (village chiefs) and community members were informed on the objectives, procedures, and potential risks and benefits of the study. Written informed consent of each participant was obtained (for children aged below 18 years, consent was given by parents or legal guardians). It was emphasised that participation was voluntary and withdrawal from the study was possible anytime without further obligations.
All members of the 56 communities received a single oral dose of albendazole (400 mg for participants aged >2 years and 200 mg for children aged 1–2 years, respectively) against STH. A single 40 mg/kg oral dose of praziquantel against schistosomiasis was administered to community members aged 5 years and above in localities where the prevalence of schistosomiasis was greater or equal to 5%, while individual case treatment was applied in localities with lower prevalences. Drug administration was implemented by the ‘Programme National de Lutte contre les Maladies Tropicales Négligées à Chimiothérapie Préventive’ (PNLMTN-CP) in collaboration with personnel from local health districts and our research team.
2.2. Study area and population
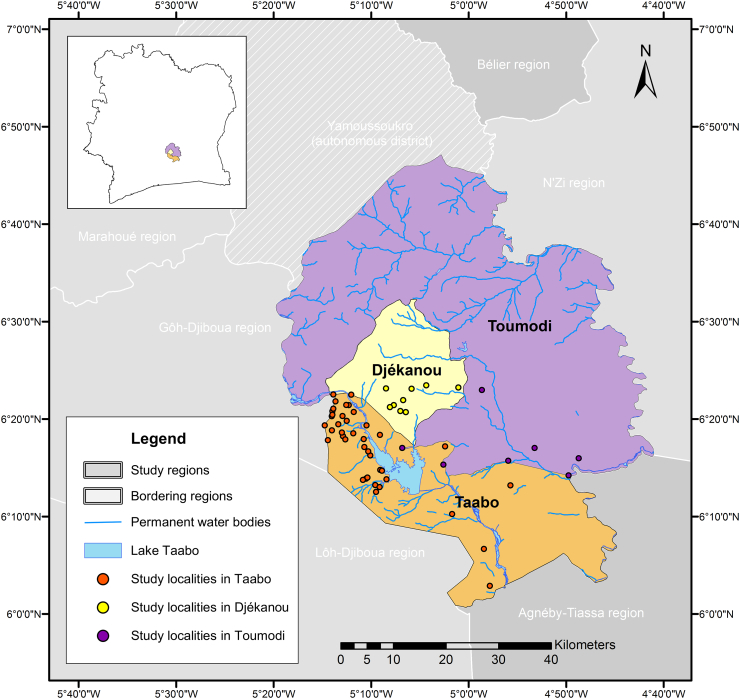
The study was conducted in August and September 2014 in the departments of Taabo, Djékanou and Toumodi, located in the south-central part of Côte d’Ivoire. The department of Taabo belongs to the Agnéby-Tiassa region, while Djékanou and Toumodi are part of the Bélier region (Fig. 1). The area of the three departments is drained by the tributaries of the Bandama and N’Zi rivers. The former crosses the Taabo department and is impounded by a large dam creating Lake Taabo that is used for hydroelectric power production (N'Goran et al., 1997). Additionally, there are seasonal streams that are usually dried out between November and February. The study zone is characterised by a forest savannah ecology (Koffi et al., 2013) and a tropical climate with a recent tendency to a single rainy season (March to July) (Bassa et al., 2016). The mostly rural population is engaged in subsistence farming (e.g. banana, cassava, maize and yams) and cultivation of cacao, coffee and rubber for cash. In communities living in close proximity to Lake Taabo, fishery constitutes an important livelihood activity.
Fig. 1.
Study area. The cross-sectional study was carried in August and September 2014 in 56 communities of three departments in south-central Côte d'Ivoire: seven localities were in the Toumodi department, nine localities in the Djékanou department and the remaining 40 localities in the Taabo department.
Initially, the study was planned to comprise 56 localities in the department of Taabo alone, because of the presence of the Taabo health and demographic surveillance system (HDSS) that monitors a population of about 42,000 people since 2008 (Koné et al., 2015). However, the number of appropriately sized communities in the Taabo department was lower than expected and consequently the study was extended into the adjacent Djékanou and Toumodi departments to obtain the required number of localities. To that end, our study involved 40 localities in the Taabo department, nine in the Djékanou department and seven in the Toumodi department (Fig. 1). In each locality, households were selected as follows: six transects were set from the centre of the community in different directions towards the edge of the village and, subsequently, on each transect five households with at least one child aged 5–15 years were selected. Thus, a total of 30 households were chosen per locality. In small hamlets comprising less than 30 households, all households were invited to participate. In each household, all school-aged children (5–15 years), one adolescent or adult (aged >15 years) and one preschool-aged child (<5 years) were selected and invited to participate.
2.3. Collection of stool and urine samples
In each study community, project-associated community health workers (CHWs) and field enumerators from the Taabo HDSS provided participants with two plastic containers; one for stool and one for urine collection, the day before the field team’s visit. All participating household members were asked to return the filled containers the next morning (between 08:00 and 12:00 h). On the day of collection, the research team labeled each container with a unique identification code and stored them in racks to be transferred to nearby laboratories at the general hospitals of Taabo and Djékanou, the community health centre of Kpouébo in the department of Toumodi and a mobile field laboratory set up at the dispensary of Léléblé in the Taabo department.
2.4. Laboratory procedures
Stool samples were processed by the Kato-Katz technique (Katz et al., 1972). In brief, duplicate thick smears were prepared on microscope slides using standard 41.7 mg templates. The slides were allowed to clear for 30–45 min before examination under a microscope by one of eight experienced laboratory technicians. The number of eggs of each helminth species (e.g. Schistosoma mansoni, Ascaris lumbricoides, Trichuris trichiura and hookworm) was recorded and multiplied by a factor of 24 to obtain the number of eggs per gram of stool (EPG). A portion of stool (1–2 g) was preserved in 10 ml of sodium acetate-acetic acid-formalin (SAF) solution for further diagnostic work-up. Urine samples that were positive for microhaematuria from reagent strip testing (Hemastix, Siemens Healtheare; Zurich, Switzerland) were subjected to a filtration method for quantification of Schistosoma haematobium eggs (Plouvier et al., 1975).
SAF-preserved stool samples were transferred to the Université Félix Houphouët-Boigny (Abidjan, Côte d’Ivoire). An ether concentration method was employed and samples examined for helminths and intestinal protozoa by experienced laboratory technicians (Utzinger et al., 2010). The number of species-specific helminth eggs was recorded, whereas intestinal protozoa cysts and trophozoites were recorded semi-quantitatively based on occurrence per slide or field of views at a magnification of x400 or x500 (Utzinger et al., 2010).
2.5. Questionnaire survey
A questionnaire was administered to all selected households on the same day as stool and urine samples were collected. The questionnaire was readily adapted from an instrument previously used in a pilot study conducted in the same setting (Schmidlin et al., 2013). It aimed at household heads and included questions on demographic factors (e.g. age, sex, ethnicity and education), socioeconomic indicators (e.g. possession of household assets) and reported or observed WASH indicators (e.g. sources of water for household use, open defecation, use of latrines and waste disposal near the house).
2.6. Statistical analysis
Parasitological data were double-entered in Microsoft Excel and cross-checked in EpiInfo version 3.5.4 (Centers for Disease Control and Prevention; Atlanta, USA). Questionnaire data were collected using open data kit (ODK). Note that ODK is an open source software for electronic data collection (http://opendatakit.org/) that we operated on mobile Android devices. Data were uploaded daily on a server. Statistical analyses were performed with STATA version 11.0 (Stata Corporation; College Station, USA).
For each study participant, the arithmetic mean of the number of species-specific helminth egg counts was calculated based on the duplicate Kato-Katz thick smear readings. At the community level, the geometric mean egg counts were calculated. Helminth infection intensities were classified according to WHO guidelines, as follows: for S. mansoni infection three categories were used, light infection (1–99 EPG), moderate infection (100–399 EPG) and heavy infection (≥400 EPG); for STH, light, moderate and heavy infections were 1–1,999 EPG, 2,000–3,999 EPG and ≥4,000 EPG for hookworm; 1–4,999 EPG, 5,000–49,999 EPG and ≥50,000 EPG for A. lumbricoides; and 1–999 EPG, 1,000–9,999 EPG and ≥10,000 EPG for T. trichiura (WHO, 2002). S. haematobium infection was categorised into light (1–49 eggs/10 ml of urine) and heavy (≥50 eggs/10 ml of urine) intensity (WHO, 2002).
Participants were stratified into six age groups (<5, 5–9, 10–14, 15–19, 20–24 and ≥25 years). The socioeconomic index was calculated using principal component analysis (PCA) via an asset-based approach and stratified into wealth quartiles (most poor, very poor, poor and least poor) (Filmer and Pritchett, 2001). Univariate analysis (x2 and Fisher’s exact test, as appropriate) was used for comparison between groups. Significant associations between parasite infections, sociodemographic factors and WASH indicators (e.g. use of latrine, open defecation and water sources) were assessed by multivariable logistic regression. Models were adjusted for parasite species, age, sex, ethnicity and wealth quartiles. For all statistical analyses, a p-value below 0.05 was considered as significant.
3. Results
3.1. Characteristics of the study population
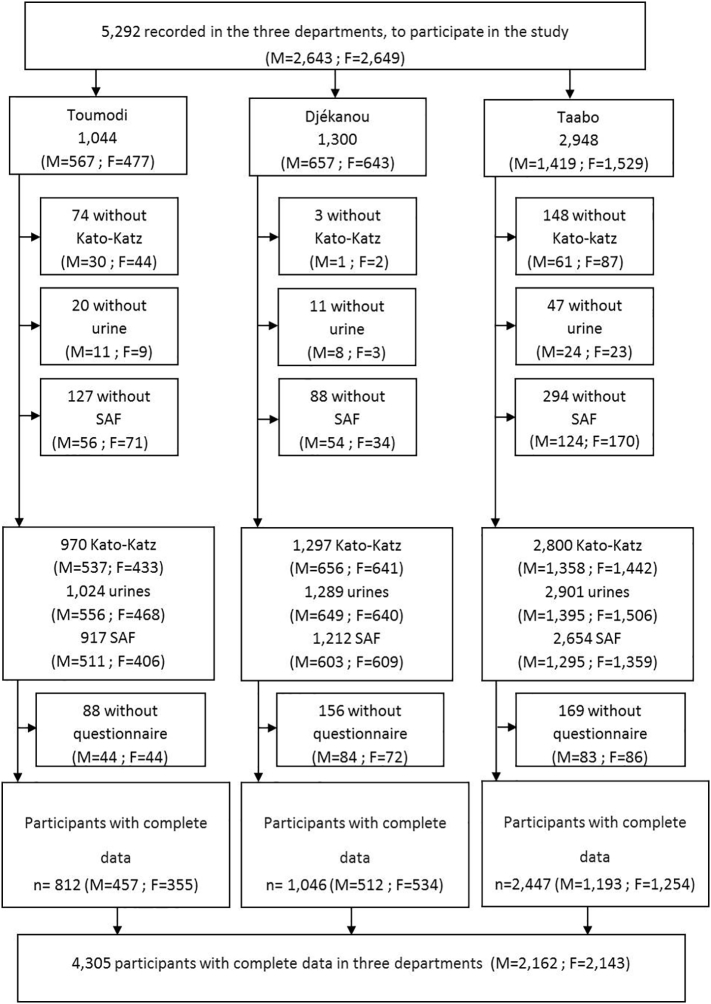
A total of 4,305 participants (2,162 males and 2,143 females) had complete parasitological and questionnaire data, and hence, were included for all subsequent analyses (Fig. 2). There were 339 (7.9%) preschool-aged children (<5 years), 3,183 (73.9%) school-aged children (5–15 years) and 783 (18.2%) adolescents and adults (>15 years). The majority (2,447) of participants lived in the Taabo department, while 1,046 and 812 participants were from the Djékanou and Toumodi departments, respectively.
Fig. 2.
Flow chart detailing the participation of individuals (F = female, M = male) in the parasitological and questionnaire survey and final sample used for analysis. The cross-sectional study was carried in August and September 2014 in 56 communities of three departments in south-central Côte d'Ivoire.
3.2. Parasite infections
The prevalence of species-specific helminth and intestinal protozoa infection is shown in Table 1. Individual helminth infections were generally low (<5%), except for hookworm that showed a prevalence of 35.3% in Djékanou and 34.2% in Toumodi departments, respectively. In Taabo department, the overall hookworm prevalence was 10.9%. We noted a few hot spots of schistosomiasis with prevalences above 20% and up to 43% in some villages of Toumodi and Taabo departments.
Table 1.
Prevalence of helminth and intestinal protozoa infections in three departments, south-central Côte d’Ivoire in August and September 2014.
| Parasite species |
Department |
|||||
|---|---|---|---|---|---|---|
| Toumodi (n = 812) |
Djékanou (n = 1,046) |
Taabo (n = 2,447) |
||||
| Infected | % (95% CIa) | Infected | % (95% CIa) | Infected | % (95% CIa) | |
| Helminths | ||||||
| Schistosoma haematobium | 57 | 7.0 (5.4–9.0) | 23 | 2.2 (1.4–3.3) | 82 | 3.4 (2.7–4.1) |
| Schistosoma mansoni | 18 | 2.2 (1.3–3.5) | 8 | 0.8 (0.3–1.5) | 94 | 3.8 (3.1–4.7) |
| Hookworm | 278 | 34.2 (31.0–37.6) | 369 | 35.3 (32.4–38.3) | 266 | 10.9 (9.7–12.2) |
| Trichuris trichiura | 4 | 0.5 (0.1–1.3) | 13 | 1.2 (0.7–2.1) | 51 | 2.1 (1.6–2.7) |
| Ascaris lumbricoides | 3 | 0.4 (0.1–1.1) | 1 | 0.1 (0.0–0.5) | 8 | 0.3 (0.1–0.6) |
| Intestinal protozoa | ||||||
| Entamoeba coli | 331 | 40.8 (37.4–44.2) | 443 | 42.4 (39.3–45.4) | 955 | 39.0 (37.1–41.0) |
| Endolimax nana | 179 | 22.0 (19.2–25.1) | 223 | 21.3 (18.9–24.0) | 440 | 18.0 (16.5–19.6) |
| Giardia intestinalis | 105 | 12.9 (10.7–15.4) | 139 | 13.3 (11.3–15.5) | 320 | 13.1 (11.8–14.5) |
| Blastocystis hominis | 74 | 9.1 (7.2–11.3) | 93 | 8.9 (7.2–10.8) | 231 | 9.4 (8.3–10.7) |
| Iodamoeba bütschlii | 68 | 8.4 (6.6–10.5) | 75 | 7.2 (5.7–8.9) | 211 | 8.6 (7.5–9.8) |
| Entamoeba histolytica/E. dispar | 46 | 5.7 (4.2–7.5) | 45 | 4.3 (3.2–5.7) | 149 | 6.1 (5.2–7.1) |
| Chilomastix mesnili | 42 | 5.2 (3.8–6.9) | 52 | 5.0 (3.7–6.5) | 113 | 4.6 (3.8–5.5) |
| Entamoeba hartmanni | 8 | 1.0 (0.4–1.9) | 18 | 1.7 (1.0–2.7) | 46 | 1.9 (1.4–2.5) |
Confidence interval.
The prevalences of intestinal protozoa infections were similar between the three departments, varying from 1.0% to 42.4%, depending on the species. The most common intestinal protozoa were non-pathogenic species, Entamoeba coli and Endolimax nana, with overall prevalences of 40.2% and 19.6%, respectively. Giardia intestinalis had a prevalence of about 13% in all three departments.
3.3. Intensity of infection
Helminth infection intensities were quite similar in the three departments. However, a marked difference was observed for hookworm infection, where a considerably lower infection intensity was observed in the Taabo department (1.6 EPG, 95% confidence interval (CI): 1.5–1.7 EPG), compared to Toumodi (5.1 EPG, 95% CI: 4.3–6.0 EPG) and Djékanou (5.8 EPG, 95% CI: 4.9–6.7 EPG). In terms of intensity categories, helminth infections were primarily of light intensity in all three departments (Table 2). Regarding pathogenic intestinal protozoa, almost all Entamoeba histolytica/E. dispar-infected participants had a light or moderate intensity infection, while one-third of the G. intestinalis-positive participants were heavily infected.
Table 2.
Helminth and intestinal protozoa infection intensities, stratified by study departments, from August to September 2014.
| Parasite species |
Department |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Toumodi (n=812) |
Djékanou (n=1,046) |
Taabo (n=2,447) |
|||||||
| Light (%) | Moderate (%) | Heavy (%) | Light (%) | Moderate (%) | Heavy (%) | Light (%) | Moderate (%) | Heavy (%) | |
| Helminths | |||||||||
| Schistosoma haematobium | 45 (78.9) | N/Aa | 12 (21.1) | 20 (87.0) | N/Aa | 3 (13.0) | 64 (78.0) | N/Aa | 18 (22.0) |
| Schistosoma mansoni | 9 (50.0) | 6 (33.3) | 3 (16.7) | 8 (100) | 0 | 0 | 60 (63.8) | 19 (20.2) | 15 (16.0) |
| Hookworm | 269 (96.8) | 6 (2.2) | 3 (1.1) | 347 (94.0) | 10 (2.7) | 12 (3.3) | 260 (97.7) | 5 (1.9) | 1 (0.4) |
| Trichuris trichiura | 2 (50.0) | 2 (50.0) | 0 | 12 (92.3) | 1 (7.7) | 0 | 38 (74.5) | 9 (17.6) | 4 (7.8) |
| Ascaris lumbricoides | 3 (100) | 0 | 0 | 1 (100) | 0 | 0 | 5 (62.5) | 3 (37.5) | 0 |
| Intestinal protozoa | |||||||||
| Entamoeba coli | 116 (35.0) | 167 (50.5) | 48 (14.5) | 181(40.9) | 173 (39.1) | 89 (20.1) | 320 (33.5) | 458 (48.0) | 177 (18.5) |
| Endolimax nana | 79 (44.1) | 79 (44.1) | 21 (11.7) | 110 (49.3) | 99 (44.4) | 14 (6.3) | 216 (49.1) | 172 (39.1) | 52 (11.8) |
| Giardia intestinalis | 27 (25.7) | 45 (42.9) | 33 (31.4) | 32 (23.0) | 61 (43.9) | 46 (33.1) | 73 (22.8) | 147 (45.9) | 100 (31.3) |
| Blastocystis hominis | 21 (28.4) | 38 (51.4) | 15 (20.3) | 39 (41.9) | 42 (45.2) | 12 (12.9) | 75 (32.5) | 117 (50.6) | 39 (16.0) |
| Iodamoeba bütschlii | 29 (42.6) | 27 (39.7) | 12 (17.6) | 22 (29.3) | 35 (46.7) | 18 (24.0) | 69 (32.7) | 99 (46.9) | 43 (20.4) |
| Entamoeba histolytica/E. dispar | 22 (47.8) | 21 (45.7) | 3 (6.5) | 28 (62.2) | 16 (35.6) | 1 (2.2) | 68 (45.6) | 71 (47.7) | 10 (6.7) |
| Chilomastix mesnili | 16 (38.1) | 19 (45.2) | 7 (16.7) | 18 (34.6) | 28 (53.8) | 6 (11.5) | 49 (43.4) | 46 (40.7) | 18 (15.9) |
| Entamoeba hartmanni | 3 (37.5) | 5 (62.5) | 0 | 7 (38.9) | 8 (44.4) | 3 (16.7) | 21 (45.7) | 20 (43.5) | 5 (10.9) |
Helminth infection intensity categories are based on eggs per gram of stool (EPG) and 10 ml of urine for S. haematobium and defined according to World Health Organization guidelines (WHO, 2002). Intestinal protozoa infection intensities were recorded based on a semi-quantitative method distinguishing between light (1-5 cysts or trophozoites per slide); moderate (1 cyst or trophozoite per observation field at a magnification of ×400 or 500); and heavy (more than 1 cyst or trophozoite per observation field at a magnification of ×400 or 500) (Utzinger et al., 2010).
Not applicable
3.4. Parasite infection status, stratified by age and sex
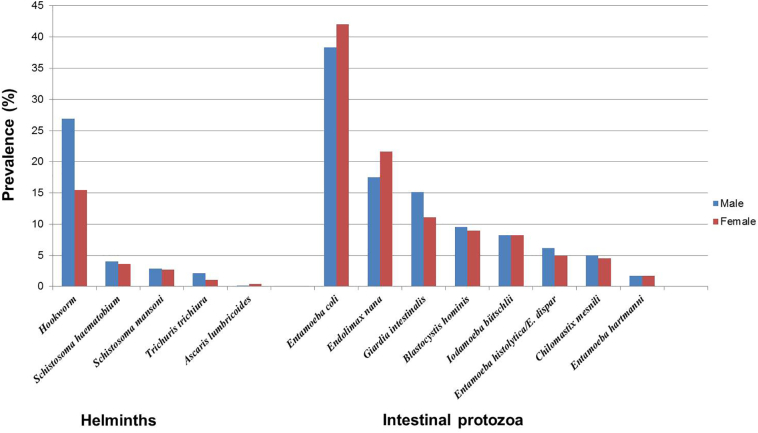
Fig. 3, Fig. 4 show the prevalence of intestinal protozoa and helminth infections, stratified by age groups and sex in the three departments. Males were significantly more often infected with hookworm (x2 = 83.42; p < 0.001) and T. trichiura (x2 = 7.04; p = 0.008) than females. We found the same pattern with G. intestinalis (x2 = 15.63; p < 0.001). In contrast, females were more likely to be infected with E. coli than males (42.0% versus 38.3%; x2 = 6.28; p = 0.012) and E. nana (21.6% versus 17.5%; x2 = 11.36; p = 0.001). In all three departments, males were significantly more infected with hookworm compared to females (p < 0.001 for all three departments). More detailed information pertaining to parasite infection frequencies, stratified by sex, are provided in the Appendix Table A.1.
Fig. 3.
Prevalence of investigated parasites in 4,305 community members, stratified by sex. The cross-sectional study was carried in August and September 2014 in 56 communities of three departments in south-central Côte d'Ivoire.
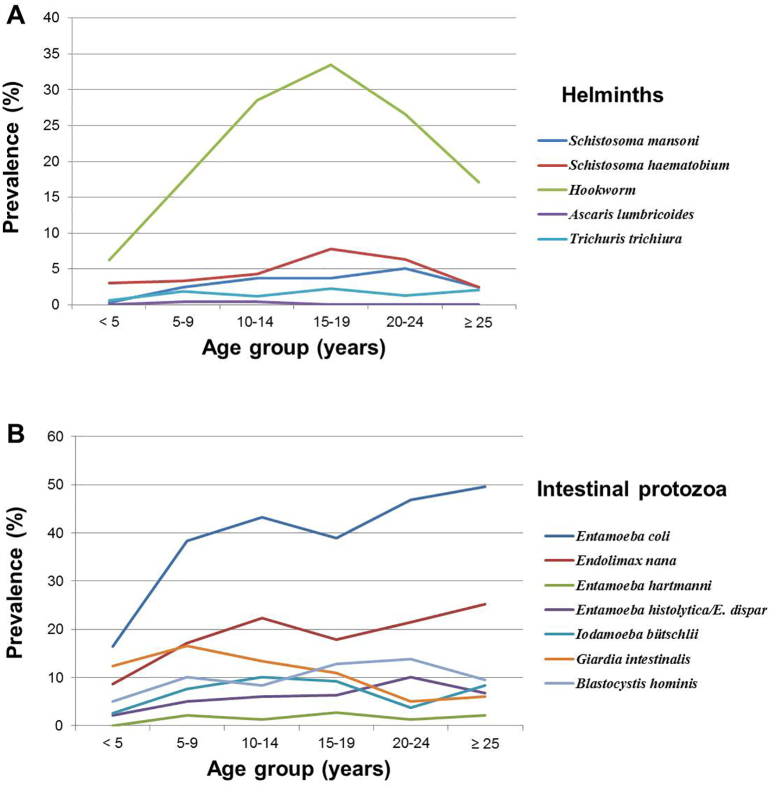
Fig. 4.
Age-prevalence curves of helminths (A) and intestinal protozoa (B) infections of the study population (n=4,305). The cross-sectional study was carried in August and September 2014 in 56 communities of three departments in central Côte d’Ivoire.
Appendix Table A.1.
Prevalence (%) of parasitic infections by sex in the three departments.
| Parasite species | Total | Toumodi |
P | Total | Djékanou |
P | Total | Taabo |
P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | f | m | f | m | f | |||||||
| Helminths | ||||||||||||
| Schistosoma haematobium | 57 (7.0) | 37 (8.1) | 20 (5.6) | 0.173 | 23 (2.2) | 12 (2.3) | 11 (2.1) | 0.754 | 82 (3.4) | 37 (3.1) | 45 (3.6) | 0.503 |
| Schistosoma mansoni | 18 (2.2) | 15 (3.3) | 3 (0.8) | 0.019a | 8 (0.8) | 2 (0.4) | 6 (1.1) | 0.174 | 94 (3.8) | 46 (3.9) | 48 (3.8) | 0.971 |
| Hookworm | 278 (34.2) | 181 (39.6) | 97 (27.3) | <0.001a | 369 (35.2) | 223 (43.6) | 146 (27.3) | <0.001a | 266 (10.9) | 177 (14.8) | 89 (7.1) | <0.001a |
| Trichuris trichiura | 4 (0.5) | 1 (0.2) | 3 (0.8) | 0.206 | 13 (1.2) | 8 (1.6) | 5 (0.9) | 0.361 | 51 (2.1) | 36 (3.0) | 15 (1.2) | 0.002a |
| Ascaris lumbricoides | 3 (0.4) | 1 (0.2) | 2 (0.6) | 0.422 | 1(0.1) | 0 (0.0) | 1 (0.2) | 0.327 | 8 (0.3) | 3 (0.3) | 5 (0.4) | 0.524 |
| Intestinal protozoa | ||||||||||||
| Entamoeba coli | 331 (40.8) | 179 (39.2) | 152 (42.8) | 0.294 | 443 (42.4) | 204 (39.8) | 239 (44.8) | 0.108 | 955 (39.0) | 445 (37.3) | 510 (40.7) | 0.088 |
| Endolimax nana | 179 (22.0) | 87 (19.0) | 92 (25.9) | 0.019a | 223 (21.3) | 94 (18.9) | 126 (23.6) | 0.066 | 440 (18.0) | 195 (16.3) | 245 (19.5) | 0.040a |
| Giardia lamblia | 105 (13.0) | 66 (14.4) | 39 (11.0) | 0.145 | 139 (13.3) | 83 (16.2) | 56 (10.5) | 0.006a | 320 (13.1) | 178 (14.9) | 142 (11.3) | 0.008 |
| Blastocystis hominis | 74 (9.1) | 45 (9.2) | 29 (8.2) | 0.410 | 93 (8.9) | 41 (8.1) | 52 (9.7) | 0.326 | 231 (9.4) | 119 (10.0) | 112 (8.9) | 0.378 |
| Iodamoeba bütschlii | 68 (8.4) | 38 (8.3) | 30 (8.5) | 0.945 | 75 (7.2) | 40 (7.8) | 35 (6.6) | 0.430 | 211 (8.6) | 100 (8.4) | 111 (8.9) | 0.679 |
| Entamoeba histolytica/dispar | 46 (5.7) | 32 (7.0) | 14 (3.9) | 0.061 | 45 (4.3) | 22 (4.3) | 23 (4.3) | 0.993 | 149 (6.1) | 81 (6.8) | 68 (5.4) | 0.158 |
| Chilomastix mesnili | 42 (5.2) | 27 (5.9) | 15 (4.2) | 0.283 | 52 (5.0) | 21 (4.1) | 31 (5.8) | 0.205 | 113 (4.6) | 61 (5.1) | 52 (4.1) | 0.255 |
| Entamoeba hartmanni | 8 (1.0) | 3 (0.7) | 5 (1.4) | 0.282 | 18 (1.7) | 8 (1.6) | 10 (1.9) | 0.700 | 46 (1.9) | 25 (2.1) | 21 (1.7) | 0.443 |
Toumodi (n = 812; m = 457, f = 355), Djékanou (n = 1046; m = 512, f = 534), Taabo (n = 2,447; m = 1,193, f = 1,254) m: male, f: female.
Pearson's X2 test.
Regarding age, significant differences were found for hookworm (x2 = 134.47; p < 0.001), S. mansoni (x2 = 16.17; p = 0.006), S. haematobium (x2 = 16.17; p = 0.006) and seven intestinal protozoa species (G. intestinalis, E. histolytica/E. dispar, E. coli, E. nana, Entamoeba hartmanni, Iodamoeba bütschlii and Blastocystis hominis) diagnosed with an ether concentration method using SAF-fixed stool samples. Participants aged 15–19 years showed the highest infection prevalence for hookworm and S. haematobium. Further details pertaining to parasite infection frequencies, stratified by age group, are presented in the Appendix Table A.2.
Appendix Table A.2.
Prevalence (%) of parasitic infections by age in the three departments.
| Parasite species |
Total | Toumodi |
Total | Djékanou |
Total | Taabo |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <5 years | 5–9 years | 10–14 years | 15–19 years | 20-24 years | ≥25 years | p | <5 years | 5–9 years | 10–14 years | 15–19 years | 20–24 years | ≥25 years | p | <5 years | 5–9 years | 10–14 years | 15-19 years | 20–24 years | ≥25 years | p | ||||
| Helminths | ||||||||||||||||||||||||
| Schistosoma haematobium | 57 (7.0) | 3 (6.8) | 17 (6.2) | 27 (8.1) | 4 (8.9) | 1 (14.3) | 5 (4.7) | 0.767 | 23 (2.2) | 1 (1.1) | 5 (1.3) | 13 (3.6) | 4 (6.3) | 0 (0.0) | 0 (0.0) | 0.018a | 82 (3.4) | 6 (2.9) | 29 (3.2) | 24 (3.1) | 9 (8.3) | 4 (7.6) | 10 (2.5) | 0.032a |
| Schistosoma mansoni | 18 (2.2) | 0 (0.0) | 2 (0.7) | 9 (2.7) | 3 (6.7) | 0 (0.0) | 4 (3.8) | 0.086 | 8 (0.8) | 1 (1.1) | 2 (0.5) | 3 (0.8) | 1 (1.6) | 1 (5.3) | 0 (0.0) | 0.215 | 94 (3.8) | 0 (0.0) | 33 (3.7) | 43(5.5) | 4 (3.7) | 3(5.7) | 11 (2.8) | 0.008a |
| Hookworm | 278 (34.2) | 4 (9.1) | 77 (27.9) | 137 (41.0) | 23 (51.1) | 3 (42.9) | 34 (32.1) | <0.0013a | 369 (35.3) | 8 (8.9) | 115 (29.6) | 175 (49.0) | 33 (51.6) | 7 (36.8) | 31 (24.4) | <0.001a | 266 (10.9) | 9 (4.4) | 78 (8.7) | 108 (13.8) | 17 (15.6) | 11 (20.8) | 43 (10.8) | <0.001a |
| Trichuris trichiura | 4 (0.5) | 0 (0.0) | 1 (0.4) | 2 (0.6) | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0.594 | 13 (1.2) | 2 (2.2) | 7 (1.8) | 2 (0.6) | 0 (0.0) | 0 (0.0) | 2 (1.6) | 0.521 | 51 (2.1) | 0 (0.0) | 21 (2.3) | 14 (1.8) | 4 (3.7) | 1 (1.9) | 11 (2.8) | 0.202 |
| Ascaris lumbricoides | 3 (0.4) | 0 (0.0) | 2 (0.7) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.884 | 1 (0.1) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.890 | 8 (0.3) | 0 (0.0) | 3 (0.3) | 5 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.435 |
| Intestinal protozoa | ||||||||||||||||||||||||
| Entamoeba coli | 331 (40.7) | 8 (18.2) | 102 (37.0) | 143 (42.8) | 17 (37.8) | 4 (57.1) | 57 (53.8) | 0.001a | 443 (42.4) | 11 (12.2) | 157 (40.4) | 177 (49.6) | 27 (42.2) | 10 (52.6) | 61 (48.0) | <0.001a | 955 (39.0) | 37 (18.1) | 341 (37.9) | 318 (40.6) | 41 (37.6) | 23 (43.4) | 195 (49.0) | <0.001a |
| Endolima x nana | 179 (22.0) | 8 (18.2) | 42 (15.2) | 82 (24.6) | 11 (24.4) | 0 (0.0) | 36 (34.0) | 0.001a | 223 (21.3) | 4 (4.4) | 81 (20.8) | 96 (26.9) | 12 (18.8) | 5 (26.3) | 25 (19.7) | <0.001a | 440 (18.0) | 17 (8.3) | 146 (16.2) | 151 (19.3) | 16 (14.7) | 12 (22.7) | 98 (24.6) | <0.001a |
| Giardia lamblia | 105 (12.9) | 5 (11.4) | 42 (15.2) | 50 (15.0) | 5 (11.1) | 0 (0.0) | 3 (2.8) | 0.020a | 139 (13.3) | 11 (12.2) | 66 (17.0) | 45 (12.6) | 5 (7.8) | 2 (10.5) | 10 (7.9) | 0.080 | 320 (13.1) | 26 (12.7) | 151 (16.8) | 102 (13.0) | 14 (12.8) | 2 (3.8) | 25 (6.3) | <0.001a |
| Blastocystis hominis | 74 (9.1) | 2 (4.6) | 27 (9.8) | 29 (8.7) | 3 (6.7) | 1 (14.3) | 12 (11.3) | 0.775 | 93 (8.9) | 3 (3.3) | 39 (10.0) | 32 (9.0) | 7 (10.9) | 4 (21.1) | 8 (6.3) | 0.113 | 231(9.4) | 12 (5.9) | 92 (10.2) | 63 (8.1) | 18 (16.5) | 6 (11.3) | 40 (10.1) | 0.031a |
| Iodamoeba bütschlii | 68 (8.4) | 3 (6.8) | 22 (8.0) | 33 (9.9) | 4 (8.9) | 0 (0.0) | 6 (5.7) | 0.722 | 75 (7.2) | 1 (1.1) | 25 (6.4) | 35 (9.8) | 6 (9.4) | 0 (0.0) | 8 (6.3) | 0.050 | 211 (8.6) | 5 (2.4) | 73 (8.1) | 81 (10.3) | 10 (9.1) | 3 (5.7) | 39 (9.8) | 0.013a |
| Entamoeba histolytica/dispar | 46 (5.7) | 1 (2.3) | 11 (4.0) | 19 (5.7) | 3 (6.7) | 2 (28.6) | 10 (9.4) | 0.032a | 45 (4.3) | 1 (1.1) | 14 (3.6) | 18 (5.0) | 5 (7.8) | 0 (0.0) | 7 (5.5) | 0.270 | 149 (6.1) | 5 (2.4) | 53 (5.9) | 53 (6.8) | 6 (5.5) | 6 (11.3) | 26 (6.5) | 0.145 |
| Chilomastix mesnili | 42 (5.1) | 0 (0.0) | 11 (4.0) | 22 (6.6) | 3 (6.7) | 0 (0.0) | 6 (5.7) | 0.393 | 52 (5.0) | 2 (2.2) | 16 (4.1) | 17 (4.8) | 4 (6.3) | 2 (10.5) | 11 (8.7) | 0.206 | 113 (4.6) | 8 (3.9) | 45 (5.0) | 33 (4.2) | 3 (2.8) | 2 (3.8) | 22 (5.5) | 0.772 |
| Entamoeba hartmanni | 8 (1.0) | 0 (0.0) | 3 (1.1) | 3 (0.9) | 1 (2.2) | 0 (0.0) | 1 (0.9) | 0.938 | 18 (1.7) | 0 (0.0) | 10 (2.6) | 3 (0.8) | 2 (3.1) | 0 (0.0) | 3 (2.4) | 0.282 | 46 (1.9) | 0 (0.0) | 19 (2.1) | 13 (1.7) | 3 (2.8) | 1 (1.9) | 10 (2.5) | 0.335 |
Toumodi (n = 812; >5 years = 44, 5–9 years = 276, 10–14 years = 334, 15–19 years = 45, 20–24 years = 7, ≥25 years = 106), Djékanou (n = 1,046; <5 years = 90, 5–9 years = 389, 10–14 years = 357, 15–19 years = 64, 20–24 years = 19, ≥25 years = 127), Taabo (n = 2,447; <5 years = 205, 5–9 years = 899, 10–14 years = 783, 15–19 years = 109, 20–24 years = 53, ≥25 years = 398).
Pearson's X2 test.
3.5. Associations between parasite infections, sociodemographic factors and WASH indicators
All significant associations (p < 0.05) between parasite infections, sociodemographic factors and WASH indicators, as determined by logistic regression analyses, are presented in Table 3, Table 4. Our results showed that being infected with S. mansoni and S. haematobium was significantly associated with ethnic groups. In terms of relationships between parasite infections and WASH, the multivariable models revealed a positive association between hookworm and open defecation (OR = 1.28; p = 0.014). Furthermore, E. coli was negatively associated with tap water use (OR = 0.66; p = 0.032). G. intestinalis was statistically positively associated with household's waste disposal occurring near the household (OR = 1.32; p = 0.010).
Table 3.
Significant associations between helminth infections, socio-demographic factors and WASH indicators in study participants (n = 4,305) from 56 communities of three departments in south-central Côte d'Ivoire, from August to September 2014.
| Parasite | Association | ORb (95% CIa) | p-valuec |
|---|---|---|---|
| Schistosomasis | |||
| Schistosoma mansoni | |||
| 10–14 years | 9.59 (1.30–70.93) | 0.027 | |
| 15–19 years | 12.45 (1.50–103.26) | 0.020 | |
| 20–24 years | 21.18 (2.19–205.23) | 0.008 | |
| Ethnics group from Mali (Dioula/Bozoh/Kadô) | 35.11 (7.35–167.81) | <0.001 | |
| Ethnics group from Burkina Faso (Mossi/Groussi/Tronka) | 4.79 (1.81–12.64) | 0.002 | |
| Poor | 2.84 (1.30–6.20) | 0.009 | |
| Least poor | 8.52 (4.11–17.69) | <0.001 | |
| Well | 3.44 (1.85–6.40) | <0.001 | |
| River | 0.27 (0.11–0.66) | 0.004 | |
| Schistosoma haematobium | |||
| 15–19 years | 3.14 (1.32–7.47) | 0.010 | |
| Ethnics group from Mali (Dioula/Bozoh/Kadô) | 4.01 (1.38–11.69) | 0.011 | |
| Pump | 0.39 (0.22–0.70) | 0.001 | |
| Tap water | 0.16 (0.04–0.72) | 0.017 | |
| Soil-transmitted helminthiasis (STH) | |||
| Hookworm | |||
| Schistosoma haematobium | 1.73 (1.18–2.54) | 0.005 | |
| Trichuris trichiura | 2.30 (1.34–3.92) | 0.002 | |
| Ascaris lumbricoides | 4.57 (1.30–16.05) | 0.018 | |
| Entamoeba coli | 1.20 (1.01–1.42) | 0.033 | |
| Female | 0.49 (0.41–0.57) | <0.001 | |
| 5–9 years | 3.15 (1.96–5.04) | <0.001 | |
| 10–14 years | 6.27 (3.93–10.02) | <0.001 | |
| 15–19 years | 7.43 (4.33–12.77) | <0.001 | |
| 20-24 years | 6.33 (3.17–12.65) | <0.001 | |
| ≥25 years | 3.49 (2.11–5.76) | <0.001 | |
| Ethnics group from Mali (Dioula/Bozoh/Kadô) | 0.40 (0.20–0.80) | 0.009 | |
| Ethnics group from Burkina Faso (Mossi/Groussi/Tronka) | 0.34 (0.20–0.58) | <0.001 | |
| Least poor | 0.52 (0.40–0.68) | <0.001 | |
| Tap water | 0.37 (0.20–0.68) | 0.001 | |
| Pump | 1.34 (1.01–1.78) | 0.040 | |
| River | 0.57 (0.42–0.74) | <0.001 | |
| Latrine | 0.77 (0.61–0.97) | 0.028 | |
| Open defecation | 1.28 (1.05–1.55) | 0.014 | |
| Ascaris lumbricoides | |||
| Giardia intestinalis | 4.84 (1.34–17.55) | 0.016 | |
Reference group of explanatories: intestinal parasites = non-infected; sex = male, age group = <5; ethnic group = local ethnic group (Baoulé); wealth quartile = most poor; source of water for household = not use; use of latrine = no; open defecation = no.
Confidence interval.
Adjusted odds ratio (all models are adjusted for other parasite species, sex, age, ethnicity and wealth quartiles).
Only significant categories at 0.05 levels are shown.
Table 4.
Significant associations between intestinal protozoa infections, socio-demographic factors and WASH indicators in study participants (n = 4,305) from 56 communities of three departments in south-central Côte d'Ivoire, from August to September 2014.
| Parasite | Association | ORb (95% CIa) | p-valuec |
|---|---|---|---|
| Entamoeba coli | |||
| Endolimax nana | 3.18 (2.69–3.75) | <0.001 | |
| Giardia intestinalis | 0.80 (0.65–0.98) | 0.031 | |
| Entamoeba histolytica/dispar | 2.25 (1.70–3.00) | <0.001 | |
| Blastocystis hominis | 1.32 (1.05–1.64) | 0.016 | |
| Chilomastix mesnili | 5.41 (3.81–7.68) | <0.001 | |
| Iodamoeba bütschlii | 2.60 (2.04–3.31) | <0.001 | |
| Female | 1.16 (1.01–1.32) | 0.034 | |
| 5–9 years | 2.71 (1.96–3.74) | <0.001 | |
| 10–14 years | 3.04 (2.20–4.20) | <0.001 | |
| 15–19 years | 2.56 (1.68–3.90) | <0.001 | |
| 20–24 years | 3.39 (1.93–5.97) | <0.001 | |
| ≥25 years | 3.89 (2.75–5.49) | <0.001 | |
| Ethnics group from North Côte d’Ivoire (Senoufo/Tagbana/Lobi) | 0.44 (0.24–0.81) | 0.009 | |
| Pump | 0.76 (0.60–0.95) | 0.016 | |
| Tap water | 0.66 (0.45–0.97) | 0.032 | |
| Giardia lamblia | |||
| Endolimax nana | 0.62 (0.47–0.82) | 0.001 | |
| Female | 0.74 (0.61–0.89) | 0.001 | |
| 5–9 years | 1.46 (1.02–2.09) | 0.037 | |
| ≥25 years | 0.52 (0.33–0.84) | 0.007 | |
| Disposal of garbage near home | 1.32 (1.07–1.63) | 0.010 | |
| Entamoeba histolytica/E. dispar | |||
| Ascaris lumbricoides | 5.67 (1.14–28.19) | 0.034 | |
| Female | 0.74 (0.56–0.97) | 0.030 | |
| 10–14 years | 2.57 (1.16–5.69) | 0.020 | |
| 15–19 years | 2.90 (1.13–7.43) | 0.026 | |
| 20–24 years | 5.10 (1.75–14.85) | 0.003 | |
| ≥25 years | 2.77 (1.21–6.32) | 0.016 | |
| Ethnics group from North Côte d'Ivoire (Senoufo/Tagbana/Lobi) | 3.00 (1.29–7.00) | 0.011 | |
Reference group of explanatories: intestinal parasites = non-infected; sex = male; age group = <5; ethnic group = local ethnic group (Baoulé); wealth quartile = most poor; source of water for household = non-use; disposition of garbage near the household = no.
Confidence interval.
Adjusted odds ratio (all models are adjusted for other parasite species, sex, age, ethnicity and wealth quartiles).
Only significant categories at 0.05 levels are shown.
4. Discussion
We present the baseline epidemiological situation of helminthiases (STH and schistosomiasis) and intestinal protozoa infection among more than 4,000 individuals before the implementation of a cluster-randomised trial in three departments of south-central Côte d’Ivoire. The most prevalent intestinal helminth infection was hookworm (913 among 4,305 individuals infected; 21.2%). While more than a third of the study population in Toumodi and Djékanou were infected with hookworm, the respective prevalence in Taabo was considerably lower (10.9%). This observation might be explained by prior interventions in Taabo, in the frame of research and control targeting neglected tropical diseases. This includes annual treatment with albendazole plus ivermectin for the control/elimination of lymphatic filariasis, IEC and CLTS conducted by researchers and the Taabo HDSS staff (Fürst et al., 2012; Glinz et al., 2017; Hürlimann et al., 2014; Hürlimann et al., 2018; Lo et al., 2017; Righetti et al., 2013; Schmidlin et al., 2013). Of note, control/elimination activities for lymphatic filariasis, schistosomiasis and STH by the Ministry of Health in Côte d’Ivoire are currently ongoing in all three departments.
Males, particularly those aged 15–19 years, were most commonly infected with hookworm. Life style of adolescents and young adults put them at risk of becoming infected with helminths trough professional activities. Indeed, in the study area, cocoa farming is a main economic activity of the local population (Bassa et al., 2016; Hürlimann et al., 2018). It is important to note that control of STH currently focuses on school-aged children, as they are at highest risk of morbidity. Hence, control is mainly done through the education platform or might be combined with vaccination campaigns for infants (Anon, 2016). It follows that individuals aged 15 years and above are neglected by preventive chemotherapy campaigns. There is a pressing need to address this issue, particularly in view of equity, cost-effectiveness and aspirations to move from morbidity control to elimination (Lo et al., 2016; Lo et al., 2017).
Schistosomiasis was observed in all three departments with a prevalence of up to 40% in some focal areas. Interestingly, we noted an increase in the overall prevalence of S. mansoni from 1.3% in 2013 (Schmidlin et al., 2013) to 3.8% in the current study (2014/2015) in the Taabo department. The occurrence of S. mansoni in the study area is quite recent and appears to expand as we observed new foci (Fürst et al., 2012; N’Goran et al., 1997). For instance, a high prevalence was observed in Ahouaty (43.1%) and a moderate prevalence in N'Denou (20.9%), two neighbouring localities, downstream from Lake Taabo in close proximity to the Bandama River. In contrast to the increase of S. mansoni, it seems that S. haematobium is well under control; while a high prevalence was observed in the early 1990s in villages around Lake Taabo (73%) and in the late 1990s in Taabo village (up to 90%) (N'Goran et al., 2001), we now found an overall prevalence of 3.4%. It is conceivable that preventive chemotherapy with praziquantel, coupled with IEC and social and economic development, explains this decline. Yet, the fact that S. mansoni foci emerged might as well indicate a change in the intermediate host snail population ecology with Biomphalaria pfeifferi becoming the predominant snail species (Southgate, 1997).
The prevalence of intestinal protozoa infection was similar in all three departments. Pathogenic intestinal protozoa were observed at comparable levels as in previous studies (Hürlimann et al., 2014). G. intestinalis remains the predominant pathogenic intestinal protozoon with an overall prevalence above 10% in all three departments. Meanwhile, non-pathogenic intestinal protozoa species, namely E. coli and E. nana, showed the highest prevalence, corroborating previous studies conducted in different parts of Côte d’Ivoire (Coulibaly et al., 2012; Ouattara et al., 2010; Traoré et al., 2011). Although these are non-pathogenic protozoa, it highlights the high faecal contamination of the community surroundings (Savichtcheva and Okabe, 2006).
The lack of clean water and improved sanitation, is an impediment for transmission interruption of intestinal parasite infections in the surveyed communities (Schmidlin et al., 2013). Multivariable logistic regression analyses revealed significant associations between parasite infections and hygiene indicators, after adjusting for sex, age, ethnicity and wealth quartiles. For example, we found a positive association between hookworm infection and open defecation. Open defecation is a common practice in the surveyed communities (Schmidlin et al., 2013) and a major risk factor in the spread of intestinal parasites, particularly hookworm (Esrey et al., 1991; Fewtrell et al., 2005; Vercruysse et al., 2001). Furthermore, we found that the use of tap water at home is associated with lower odds of S. haematobium, hookworm and E. coli. These findings are consistent with a previous study carried out in southern Côte d’Ivoire that revealed that regular consumption of tap water is associated with a lower odds of E. histolytica/E. dispar, E. coli and E. nana (Ouattara et al., 2010). Finally, the disposal of waste near the household was associated with a higher odds of G. intestinalis. Lack of appropriate hygiene conditions leads to a contaminated environment, which contributes to the spread of parasites via the faecal-oral route. In particular, it has been suggested that flies and cockroaches can carry intestinal protozoa cysts, bacteria and viruses from faeces in the environment to food (Pai et al., 2003). Furthermore, some authors have shown that G. intestinalis is a parasite of rats (Reedyk and Scott, 2001) and garbage dumps near and around the villages are places where rats proliferate. Rats could thus contaminate these places with their faeces containing G. intestinalis cysts, whereas flies and cockroaches would, in turn, contaminate the food.
In the current epidemiological baseline survey, we used a comprehensive approach to assess risk factors of parasite infections through parasitological examinations and a household questionnaire. We employed four different diagnostic techniques to identify and quantify helminths and intestinal protozoa among people of all age groups. This is of relevance, since co-infections are frequent in rural settings, but studies mostly focus on single or restricted groups of parasites (e.g. STH or schistosomiasis) in specific age group (e.g. school-aged children).
There are, however, some limitations of the study that are offered for discussion. Although we used duplicate Kato-Katz thick smears, we only collected one stool sample from each participant, thus, the true parasite prevalence is higher due to day-to-day variation in helminth egg output and intestinal protozoa cyst and trophozoite output (Booth et al., 2003; Knopp et al., 2008). In future studies, more sensitive diagnostic tools such as the FLOTAC technique (Coulibaly et al., 2016; Cringoli et al., 2010), molecular diagnosis (e.g. polymerase chain reaction (PCR) (Verweij et al., 2007)) should be envisaged, particularly in view of future shifts from morbidity control to transmission interruption of schistosomiasis and STH. Furthermore, our questionnaire was administered at the household level. Some questions pertaining to hygiene practices might have yielded more specific information if asked at the individual level.
Our results confirm that lack of appropriate sanitation facilities is associated with helminth and intestinal protozoa infections. Integrated control approaches that couple ongoing control and elimination efforts through preventive chemotherapy with improved sanitation and health education have the potential to sustainably control and eliminate these diseases. Of note, in the past, ordnidazole, secnidazole and tinidazole have been identified as drug candidates allowing treatment of amoebiasis (amoebic dysentery) and/or giardiasis with a single oral application. Such a treatment scheme holds promise for cost-effective control, particularly if integrated with ongoing STH and schistosomiasis control and elimination strategies. However, the evidence-base, particularly the safety and efficacy of these is insufficient (Escobedo and Cimerman, 2007). Other drugs, such as nitaxozanide, have been tested in the field but results were disappointing against major pathogenic intestinal protozoa (Speich et al., 2013). Furthermore, potential drug interactions with currently used drugs against schistosomiasis and STH would need to be evaluated in detail.
Data from this study will serve as a baseline reference to assess the effect of a package of interventions, including preventive chemotherapy, CLTS and health education on helminth and intestinal protozoa infections and diarrhoea incidence. Results and experiences from our subsequent cluster-randomised trial will be important for the national control programmes targeting neglected tropical diseases and might influence public health actions elsewhere in sub-Saharan Africa.
Conflict of interest
There is no conflict of interest.
References
- Anon Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2015. Releve Epidemiol. Hebd. 2016;91:585–595. [PubMed] [Google Scholar]
- Bassa F.K., Ouattara M., Silué K.D., Adiossan L.G., Baikoro N., Koné S., N’Cho M., Traoré M., Bonfoh B., Utzinger J., N'Goran E.K. Epidemiology of malaria in the Taabo health and demographic surveillance system, south-central Côte d'Ivoire. Malar. J. 2016;15:9. doi: 10.1186/s12936-015-1076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth M., Vounatsou P., N'Goran E.K., Tanner M., Utzinger J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Côte d'Ivoire. Parasitology. 2003;127:525–531. doi: 10.1017/s0031182003004128. [DOI] [PubMed] [Google Scholar]
- Coulibaly J.T., Fürst T., Silué K.D., Knopp S., Hauri D., Ouattara M., Utzinger J., N’Goran E.K. Intestinal parasitic infections in schoolchildren in different settings of Côte d'Ivoire: effect of diagnostic approach and implications for control. Parasit. Vectors. 2012;5:135. doi: 10.1186/1756-3305-5-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly J.T., Ouattara M., Becker S.L., Lo N.C., Keiser J., N'Goran E.K., Ianniello D., Rinaldi L., Cringoli G., Utzinger J. Comparison of sensitivity and faecal egg counts of Mini-FLOTAC using fixed stool samples and Kato-Katz technique for the diagnosis of Schistosoma mansoni and soil-transmitted helminths. Acta Trop. 2016;164:107–116. doi: 10.1016/j.actatropica.2016.08.024. [DOI] [PubMed] [Google Scholar]
- Cringoli G., Rinaldi L., Maurelli M.P., Utzinger J. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010;5:503–515. doi: 10.1038/nprot.2009.235. [DOI] [PubMed] [Google Scholar]
- Escobedo A.A., Cimerman S. Giardiasis: a pharmacotherapy review. Expert. Opin. Pharmacother. 2007;8:1885–1902. doi: 10.1517/14656566.8.12.1885. [DOI] [PubMed] [Google Scholar]
- Esrey S.A., Potash J.B., Roberts L., Shiff C. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull. World Health Organ. 1991;69:609–621. [PMC free article] [PubMed] [Google Scholar]
- Fewtrell L., Kaufmann R.B., Kay D., Enanoria W., Haller L., Colford J.M. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect. Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- Filmer D., Pritchett L.H. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- Fürst T., Silué K.D., Ouattara M., N’Goran D.N., Adiossan L.G., N'Guessan Y., Zouzou F., Koné S., N’Goran E.K., Utzinger J. Schistosomiasis, soil-transmitted helminthiasis, and sociodemographic factors influence quality of life of adults in Côte d'Ivoire. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2015;388(2016):1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinz D., Wegmüller R., Ouattara M., Diakité V.G., Aaron G.J., Hofer L., Zimmermann M.B., Adiossan L.G., Utzinger J., N'Goran E.K., Hurrell R.F. Iron fortified complementary foods containing a mixture of sodium iron EDTA with either ferrous fumarate or ferric pyrophosphate reduce iron deficiency anemia in 12- to 36-month-old children in a malaria endemic setting: a secondary analysis of a cluster-randomized controlled trial. Nutrients. 2017;9 doi: 10.3390/nu9070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes J.E.T., Croll D., Harrison W.E., Utzinger J., Freeman M.C., Templeton M.R. The relationship between water, sanitation and schistosomiasis: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobson J. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Alvarado M., Basáñez M.-G., Bolliger I., Bourne R., Boussinesq M., Brooker S.J., Brown A.S., Buckle G., Budke C.M., Carabin H., Coffeng L.E., Fèvre E.M., Fürst T., Halasa Y.A., Jasrasaria R., Johns N.E., Keiser J., King C.H., Lozano R., Murdoch M.E., O’Hanlon S., Pion S.D.S., Pullan R.L., Ramaiah K.D., Roberts T., Shepard D.S., Smith J.L., Stolk W.A., Undurraga E.A., Utzinger J., Wang M., Murray C.J.L., Naghavi M. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002865. (e2865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hürlimann E., Yapi R.B., Houngbedji C.A., Schmidlin T., Kouadio B.A., Silué K.D., Ouattara M., N'Goran E.K., Utzinger J., Raso G. The epidemiology of polyparasitism and implications for morbidity in two rural communities of Côte d'Ivoire. Parasit. Vectors. 2014;7:81. doi: 10.1186/1756-3305-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hürlimann E., Silué K.D., Zouzou F., Ouattara M., Schmidlin T., Yapi R.B., Houngbedji C.A., Dongo K., Kouadio B.A., Koné S., Bonfoh B., N'Goran E.K., Utzinger J., Acka-Douabélé C.A., Raso G. Effect of an integrated intervention package of preventive chemotherapy, community-led total sanitation and health education on the incidence of helminth and intestinal protozoa infections in Côte d'Ivoire. Parasit. Vectors. 2018 doi: 10.1186/s13071-018-2642-x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T.-W., Melville S., Utzinger J., King C.H., Zhou X.-N. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz N., Chaves A., Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. São Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- Knopp S., Mgeni A.F., Khamis I.S., Steinmann P., Stothard J.R., Rollinson D., Marti H., Utzinger J. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl. Trop. Dis. 2008;2 doi: 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffi A.A., Ahoua Alou L.P., Kabran J.-P.K., N’Guessan R., Pennetier C. Re-visiting insecticide resistance status in Anopheles gambiae from Côte d'Ivoire: a nation-wide informative survey. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koné S., Baikoro N., N’Guessan Y., Jaeger F.N., Silué K.D., Fürst T., Hürlimann E., Ouattara M., Séka M.-C.Y., N’Guessan N.A., Esso E.L., Zouzou F., Boti L.I., Gonety P.T., Adiossan L.G., Dao D., Tschannen A.B., von Stamm T., Bonfoh B., Tanner M., Utzinger J., N’Goran E.K. Health & demographic surveillance system profile: the taabo health and demographic surveillance system, Côte d'Ivoire. Int. J. Epidemiol. 2015;44:87–97. doi: 10.1093/ije/dyu221. [DOI] [PubMed] [Google Scholar]
- Lo N.C., Lai Y.-S., Karagiannis-Voules D.-A., Bogoch I.I., Coulibaly J.T., Bendavid E., Utzinger J., Vounatsou P., Andrews J.R. Assessment of global guidelines for preventive chemotherapy against schistosomiasis and soil-transmitted helminthiasis: a cost-effectiveness modelling study. Lancet Infect. Dis. 2016;16:1065–1075. doi: 10.1016/S1473-3099(16)30073-1. [DOI] [PubMed] [Google Scholar]
- Lo N.C., Addiss D.G., Hotez P.J., King C.H., Stothard J.R., Evans D.S., Colley D.G., Lin W., Coulibaly J.T., Bustinduy A.L., Raso G., Bendavid E., Bogoch I.I., Fenwick A., Savioli L., Molyneux D., Utzinger J., Andrews J.R. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: the time is now. Lancet Infect. Dis. 2017;17 doi: 10.1016/S1473-3099(16)30535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus D.P., Bieri F.A., Li Y.-S., Williams G.M., Yuan L.-P., Henglin Y., Du Z.-W., Clements A.C., Steinmann P., Raso G., Yap P., Magalhães R.J.S., Stewart D., Ross A.G., Halton K., Zhou X.-N., Olveda R.M., Tallo V., Gray D.J. Health education and the control of intestinal worm infections in China: a new vision. Parasit. Vectors. 2014;7:344. doi: 10.1186/1756-3305-7-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Goran E.K., Diabate S., Utzinger J., Sellin B. Changes in human schistosomiasis levels after the construction of two large hydroelectric dams in central Côte d'Ivoire. Bull. World Health Organ. 1997;75:541–545. [PMC free article] [PubMed] [Google Scholar]
- Nematian J., Nematian E., Gholamrezanezhad A., Asgari A.A. Prevalence of intestinal parasitic infections and their relation with socio-economic factors and hygienic habits in Tehran primary school students. Acta Trop. 2004;92:179–186. doi: 10.1016/j.actatropica.2004.06.010. [DOI] [PubMed] [Google Scholar]
- N'Goran E.K., Utzinger J., N’Guessan A.N., Müller I., Zamblé K., Lohourignon K.L., Traoré M., Sosthène B.A., Lengeler C., Tanner M. Reinfection with Schistosoma haematobium following school-based chemotherapy with praziquantel in four highly endemic villages in Côte d'Ivoire. Trop. Med. Int. Health TM IH. 2001;6:817–825. doi: 10.1046/j.1365-3156.2001.00785.x. [DOI] [PubMed] [Google Scholar]
- Ostan I., Kilimcioğlu A.A., Girginkardeşler N., Ozyurt B.C., Limoncu M.E., Ok U.Z. Health inequities: lower socio-economic conditions and higher incidences of intestinal parasites. BMC Public Health. 2007;7:342. doi: 10.1186/1471-2458-7-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouattara M., N’Guéssan N.A., Yapi A., N’Goran E.K. Prevalence and spatial distribution of Entamoeba histolytica/dispar and Giardia lamblia among schoolchildren in Agboville area (Côte d'Ivoire) PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai H.-H., Ko Y.C., Chen E.R. Cockroaches (Periplaneta americana and Blattella germanica) as potential mechanical disseminators of Entamoeba histolytica. Acta Trop. 2003;87:355–359. doi: 10.1016/s0001-706x(03)00140-2. [DOI] [PubMed] [Google Scholar]
- Plouvier S., Leroy J.-C., Colette J. A propos d'une technique simple de filtration des urines dans le diagnostic de la bilharziose urinaire en enquête de masse. Med. Trop. 1975:229–230. [Google Scholar]
- Pullan R.L., Smith J.L., Jasrasaria R., Brooker S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit. Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedyk S., Scott N. Bureau Régional de Peace River, ARAP. Fight Against Musk Rats in Ponds-tanks. The Water Quality That Counts. 2001. http://www4.agr.gc.ca/resources/prod/doc/pfra/pdf/muskrt_fra.pdf (4P. Available:)
- Righetti A.A., Adiossan L.G., Ouattara M., Glinz D., Hurrell R.F., N'Goran E.K., Wegmüller R., Utzinger J. Dynamics of anemia in relation to parasitic infections, micronutrient status, and increasing age in south-central Côte d'Ivoire. J. Infect. Dis. 2013;207:1604–1615. doi: 10.1093/infdis/jit066. [DOI] [PubMed] [Google Scholar]
- Savichtcheva O., Okabe S. Alternative indicators of fecal pollution: relations with pathogens and conventional indicators, current methodologies for direct pathogen monitoring and future application perspectives. Water Res. 2006;40:2463–2476. doi: 10.1016/j.watres.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Schmidlin T., Hürlimann E., Silué K.D., Yapi R.B., Houngbedji C., Kouadio B.A., Acka-Douabélé C.A., Kouassi D., Ouattara M., Zouzou F., Bonfoh B., N’Goran E.K., Utzinger J., Raso G. Effects of hygiene and defecation behavior on helminths and intestinal protozoa infections in Taabo, Côte d'Ivoire. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V.R. Schistosomiasis in the Senegal River Basin: before and after the construction of the dams at Diama, Senegal and Manantali, Mali and future prospects. J. Helminthol. 1997;71:125–132. doi: 10.1017/s0022149x00015790. [DOI] [PubMed] [Google Scholar]
- Speich B., Marti H., Ame S.M., Ali S.M., Bogoch I.I., Utzinger J., Albonico M., Keiser J. Prevalence of intestinal protozoa infection among school-aged children on Pemba Island, Tanzania, and effect of single-dose albendazole, nitazoxanide and albendazole-nitazoxanide. Parasit. Vectors. 2013;6:3. doi: 10.1186/1756-3305-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunz E.C., Addiss D.G., Stocks M.E., Ogden S., Utzinger J., Freeman M.C. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traoré S.G., Odermatt P., Bonfoh B., Utzinger J., Aka N.D., Adoubryn K.D., Assoumou A., Dreyfuss G., Koussémon M. No Paragonimus in high-risk groups in Côte d'Ivoire, but considerable prevalence of helminths and intestinal protozoon infections. Parasit. Vectors. 2011;4:96. doi: 10.1186/1756-3305-4-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzinger J., Botero-Kleiven S., Castelli F., Chiodini P.L., Edwards H., Köhler N., Gulletta M., Lebbad M., Manser M., Matthys B., N'Goran E.K., Tannich E., Vounatsou P., Marti H. Microscopic diagnosis of sodium acetate-acetic acid-formalin-fixed stool samples for helminths and intestinal protozoa: a comparison among European reference laboratories. Clin. Microbiol. Infect. 2010;16:267–273. doi: 10.1111/j.1469-0691.2009.02782.x. [DOI] [PubMed] [Google Scholar]
- Utzinger J., Becker S.L., Knopp S., Blum J., Neumayr A.L., Keiser J., Hatz C.F. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med. Wkly. 2012;142:w13727. doi: 10.4414/smw.2012.13727. [DOI] [PubMed] [Google Scholar]
- Vercruysse J., Shaw D.J., De Bont J. Index of potential contamination for schistosomiasis. Trends Parasitol. 2001;17:256–261. doi: 10.1016/s1471-4922(01)01937-7. [DOI] [PubMed] [Google Scholar]
- Verweij J.J., Brienen E.A.T., Ziem J., Yelifari L., Polderman A.M., Lieshout L.V. Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in fecal samples using multiplex real-time PCR. Am. J. Trop. Med. Hyg. 2007;77:685–690. [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2011. Helminth control in school-age children: a guide for managers of control programmes. [Google Scholar]
- WHO . World Health Organization; Geneva: 2006. Preventive chemotherapy in human helminthiasis. [Google Scholar]
- WHO Prevention and control of schistosomiasis and soil-transmitted helminthiasis, World Health Organ. Tech. Rep. Ser. 2002;912(i–vi):1–57. [PubMed] [Google Scholar]
- Yapi R.B., Chammartin F., Hürlimann E., Houngbedji C.A., N'Dri P.B., Silué K.D., Utzinger J., N'Goran E.K., Vounatsou P., Raso G. Bayesian risk profiling of soil-transmitted helminth infections and estimates of preventive chemotherapy for school-aged children in Côte d'Ivoire. Parasit. Vectors. 2016;9:162. doi: 10.1186/s13071-016-1446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelbauer K., Speich B., Mäusezahl D., Bos R., Keiser J., Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]