Abstract
Halicephalobus gingivalis is a saprophytic nematode parasite that causes a rare form of fatal meningoencephalomyelitis in equids, humans, and ruminants. This nematode has neurotropic activity, but has also been found in the kidney, liver, lungs, optic nerves and even heart of its host. Despite the zoonotic potential and severity of the disease, the epidemiology, pathogenesis, life cycle, and risk factors are poorly understood. Cases have been reported from several countries in Europe countries and North America but none is recorded in Africa except Egypt. This review looks at the historical overview, morphology, diagnosis, treatment and summary of reported cases in humans and equids. We recommend the parasitic helminthic infection in the differential list of meningoencephalitis involving humans and animals worldwide despite its rareness.
Keywords: Meningoencephalitis, Equids, Humans, Halicephalobus
1. Introduction
Nematodes (round worms) are among the most diverse taxa in the animal kingdom with over 26,000 species described (Hugot et al., 2001). Nematodes are the most abundant soil metazoans (Bernard, 1992). Parasitic species may infect either animal (vertebrates and invertebrates) or man. Despite their diversity in terms of lifestyles, nematodes share a general common anatomy and physiology.
Halicephalobiasis is a helminthic infection caused by Halicephalobus gingivalis. The latter is a free-living soil saprophytic nematode known to cause opportunistic infections in the form of fatal meningoencephalomyelitis mainly in equines. Halicephalobus gingivalis, also known as Micronema deletrix; Halicephalobus deletrix belongs to the nematode Order Rhabditida, Family Paragrolaimidae. Currently, there are eight described species of Halicephalobus, and only H. gingivalis has been reported to infect humans and equines - predominantly in horses (Anderson et al., 1998) and to a lesser extent zebras (Equus grevyi) (Isaza et al., 2000) and donkeys (Schmitz and Chaffin, 2004). Recently, H. gingivalis has been reported to cause bovine meningoencephalomyelitis in ruminants (calves), where parasites were identified morphologically and diagnosis confirmed by molecular analysis of the large subunit (LSU) and small subunit (SSU) rRNA genes of the parasite (Enemark et al., 2016). Other species within the genus Halicephalobus include H. limuli; H. similigaster; H. minutum; H. parvum; H. palmaris; H. intermedia and H. laticauda (Anderson et al., 1998).
The organism is commonly referred to by its generic name, because the species are difficult to distinguish morphologically (Ondrejka et al., 2010) although, the shape of the tail and descriptions of the reproductive tract seem to distinguish H. gingivalis from other species in the genus. Only parasitic female adults, larvae and eggs have been isolated from parasitized hosts, confirming that H. gingivalis can reproduce parthenogenetically, although how H. gingivalis infects human and equine hosts is still largely speculative (Papadi et al., 2013). In the environment, H. gingivalis has been isolated from horse manure and compost (Steel et al., 2010).
Human infections are very rare, but all cases described to date involved fatal meningoencephalitis (Lim et al., 2015) and of the reported equine cases only four horses have survived (Dunn et al., 1993, Pearce et al., 2001, Schmitz and Chaffin, 2004, Muller et al., 2008). The success of this parasite in causing its pathology may be unconnected to its mode of reproduction. Current reports have demonstrated the presence of this parasite in localities where it was presumed to be absent. This review aims to provide more light on this silent but potentially zoonotic parasite causing fatal meningoencephalitis in humans and equids around the world and the need to develop a diagnostic tool to diagnose the infection antemortem as majority of the diagnosis has been at postmortem.
2. Parasite biology
2.1. Historical perspective
Stefanski (1954) was the first to accurately describe the nematode. He found the worms in a granuloma in the gingivae of a horse in Poland. He first placed the species in the genus Rhabditis. It was later transferred to the genus Tricephalobus by Dougherty (1955). Sudhaus (1976) placed gingivalis in Trilabiatus. Later, Andrassy (1952) placed the species in Halicephalobus where it remains till date. Anderson and Bemrick (1965) described Micronema deletrix from a nasal tumour of a horse in Minnesota, USA. The clinical pathology of this case was later described by Johnson and Johnson (1966) who reported the nematodes mainly in large granulomas in the maxillae. Andrassy (1952) and Geraert et al. (1988) in reviews of the genus Halicephalobus apparently regarded H. deletrix as a possible synonym of H. gingivalis. Although they gave no reasons for this possibility it can probably be assumed that it was because H. gingivalis and H. deletrix were both from lesions in horses.
2.2. Morphology
The typical morphologic features of Halicephalobus deletrix are the distinctive rhabditiform esophagus with corpus, isthmus, and bulb and their reproductive tracts are didelphic with dorsiflexed ovaries at the posterior end. Only female worms are found in tissue. Adult female worms are 250 to 460 μm by 15 to 25 μm. The larvae stages are smaller and have the same features as the fully developed worms but lack a reproductive system. The larvae are 250 to 300 μm long and 15 to 20 μm wide, with a rhabditoid esophagus (70 to 90 μm long). Eggs are oval, elongated, thin-shelled and colorless 40 to 55 μm by 20 to 25 μm in size (Lim et al., 2015). This nematode characteristically lacks lateral or cuticular alae. The cuticle of the worm is thin and has fine striations (Gardiner et al., 2000).
2.3. Life cycle
The life cycle of Halicephalobus sp. is poorly understood. But it is believed that the adult female worm reproduce asexually by parthenogenesis. Asexual reproduction is usually considered as an evolutionary dead end, and difficulties for asexual lineages to adapt to a fluctuating environment are anticipated due to the lack of sufficient genetic plasticity (Castagnone-Sereno and Danchin, 2014). This mode of reproduction is common among nematodes of the order Tylenchida and Rhabditida. When a female reproduces using parthenogenesis, she has no need for a male. This means that a parthenogenetic female can spend more time and energy seeking food and shelter while such resources are readily available. Without the need for males, parthenogens can reproduce faster than species that reproduce sexually. In fact, a group of parthenogenetic females can produce a certain number of offspring with only half as many parents as a similarly sized group of sexually reproducing animals. Surprisingly, only the females - usually found in association with eggs and immature larvae - have been identified and characterized from tissue samples till date (Lim et al., 2015).
2.4. Phylogenetics of Halicephalobus gingivalis
A recent study carried out by Pintore et al. (2017) revealed that the H. gingivalis isolates belonged to Lineage 3, which includes other isolates from Japan and USA. Only a few sequences are available on public databases, with little information on date of sample collection and location. However, the sequence similarity data and phylogenetic analysis confirm that there is no correlation between location and genetics in H. gingivalis based on 28S rRNA gene data. Furthermore, there was no apparent correlation between lineages and clinical manifestation of H. gingivalis, although the number of available sequences is limited (Nadler et al., 2003). Notably, the only two H. gingivalis sequences available from human cases are those recently reported in Australia and Germany (Lim et al., 2015, Monoranu et al., 2015). Both were classified by phylogenetic analysis as belonging to Lineage 1. Sequencing of more isolates and the analysis of multiple loci will help in establishing a correlation between phylogenetic clustering, geographic correlation, clinical signs and the zoonotic potential of H. gingivalis. Genetic variation study was used to distinguish the H. gingivalis isolated from Danish calves to published isolates by comparing the large subunit (LSU) rRNA and the small subunit (SSU) rRNA genes of H. gingivalis, which was found to have a genetic variation of 0.5% - 4.4% and 0.7–8.6%, respectively (Enemark et al., 2016).
3. Epidemiology
3.1. Host range
Though, this worm is known to be saprophytic and free-living in humus soil. It has been reported to infect and become parasitic in horses, humans, zebras, donkeys, and recently in ruminants (cattle) where it causes fatal granulomatous and neurological lesions. A schematic presentation of host range is presented in Fig. 1 below.
Fig. 1.
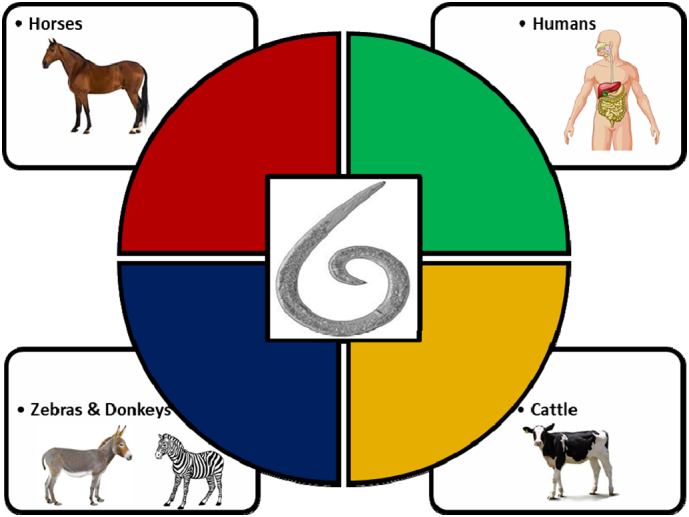
Reported host organism infected by Halicephalobus gingivalis.
3.2. Distribution
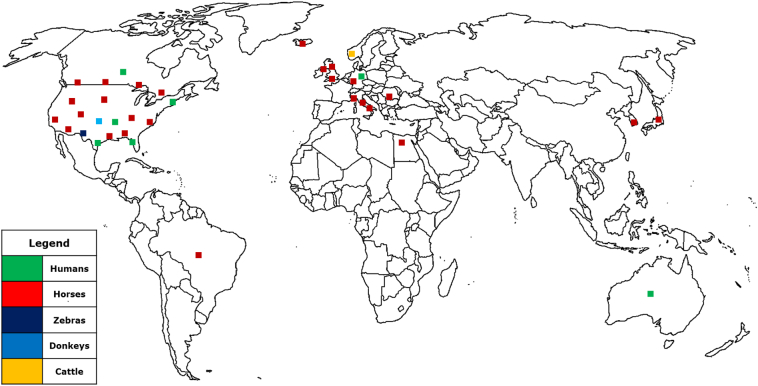
It has a worldwide distribution but majority of the reports in humans and equids so far have been in Europe, North America and North East Asia. Only one documented report was from Africa. Geographical spread is presented in the map below (Fig. 2) and in Table 1, Table 2 below.
Fig. 2.
World map showing the geographical distribution of Halicephalobiasis in animals and humans.
3.3. Mode of infection and predilection sites
The method of infection is unclear but it is presumed to be contamination of oral mucosa or skin wounds (Anderson et al., 1998). It is presumed that the nematodes enter the tissues through wound lesions and are carried to other parts of the body through haematogenous route in blood and lymph (Henneke et al., 2014) or through the optic nerve – optic tract and that the contamination of ocular wounds would be the entrance (Rames et al., 1995). Transmammary infection from mare to foal has been reported once (Wilkins et al., 2001). The larvae has been demonstrated in urine samples (Taulescu et al., 2016), and a fly (Musca autumnalis) has been proposed as carrier of larvae (Anderson et al., 1998). Viable Halicephalobus organisms have been detected and isolated from in semen (Kinde et al., 2000), though no transmission via this route has been proven, it is another possible source of infection. H. gingivalis gets access to the central nervous system via blood vessels, causing necrosis and inflammation due to visceral migration (Kreuder et al., 1996). The brain is the most commonly involved site, followed by other organs such as the kidneys, oral and nasal cavities, lymph nodes, spinal cord and adrenal glands. Infections involving the heart, stomach, liver, ganglion and bone have also been reported (Spalding et al., 1990, Kreuder et al., 1996). Massive intracranial invasion in horses is acute and of short duration (Jubb and Huxtable, 1993).
4. Diagnosis
Diagnosis of Halicephalobus sp. infection in humans and animals involves a combination of various methods. So far, no specific antemortem diagnosis technique is available for specific diagnosis of the disease in humans and animals. But a combination of magnetic resonance imaging (MRI), haematology and serum biochemical parameters has been evaluated in previous report. Furthermore, gross and histopathological lesions have been observed from affected tissues/organs in infected animals and humans at postmortem. Molecular technique such as Polymerase chain reaction (PCR) in detecting parasite DNA in brain tissues collected at post mortem have been specific. So far, no specific antemortem diagnostic technique has been developed for diagnosis. Therefore, there is an urgent need to develop a tool which will be rapid and specific for the diagnosis of suspected cases of meningoencephalitis.
5. Treatment
Anthelmintic therapy has been used with limited effect to alleviate signs of infection with H. gingivalis in horses (Rames et al., 1995, Pearce et al., 2001, Ferguson et al., 2008), and in clinical cases involving the CNS treatment was particularly inadequate (Ferguson et al., 2008, Umlauf et al., 2012). Treatment responses in previous cases in animals, shows that most affected animals deteriorated (Ruggles et al., 1993, Trostle et al., 1993, Rames et al., 1995, Kinde et al., 2000, Isaza et al., 2000 and Ferguson et al., 2008) despite treatment and the presence of live worms at autopsy suggests that the anthelmintic treatment was ineffective. None of the reported human cases received anthelmintics (Hoogstraten and Young, 1975, Shadduck et al., 1979, Gardiner et al., 1981, Ondrejka et al., 2010 and Lim et al., 2015). In vitro susceptibility testing using microagar larval developmental tests (MALDTs) has been used to assess the effects of thiabendazole and ivermectin on the hatching rate and larval development of H. gingivalis (Fonderie et al., 2012). Thiabendazole at concentrations of 10 to 100 μg/ml showed a dose-dependent inhibition effect on the hatchability of eggs. However, no inhibition of larval development was observed. Thus, H. gingivalis appears to have some intrinsic tolerance of ivermectin, but larval development can be temporarily suppressed at 2 μg/ml. Pharmacokinetic studies have showed that ivermectin rarely enters the CNS since it is actively removed by the P-glycoprotein, an abundant transporter protein in the brain. This can result in an undetectable CSF level, despite a parenteral dose of 200 μg/kg of body weight, which is often used for disseminated S. stercoralis infection (Nau et al., 2010). Treatment has not been described in human cases, but pharmacokinetic studies suggest that treatment with ivermectin or thiabendazole administered parenterally may not be effective because of poor killing effect per se and inability to achieve therapeutic levels in CNS (Fonderie et al., 2012).
6. Prevention and control
No specific control measures have so far been developed. This is because limited information is available concerning the epidemiology of the infection. Overall, it is recommended that good pasture management and prevention of horses from marsh or swampy environment. Humans working in soils and handling stable waste should wash hands regularly with regular deworming.
7. Pathological findings
The predominant inflammatory cells are plasmocytes and lymphocytes, occasional eosinophils and numerous macrophages, giant cells and epithelioid cells (Rames et al., 1995). Similar inflammation was observed, in systemic infections, in the kidneys (Isaza et al., 2000, Kinde et al., 2000), lymph nodes, uterus and heart (Isaza et al., 2000), testicles (Kinde et al., 2000), stomach, lungs, joints, adrenal gland (Simpson et al., 1988), prepuce (Dunn et al., 1993), bones (Kreuder et al., 1996, Teifke et al., 1998) and the spinal cord, the roots of the peripheral nerves and the cauda equina (Johnson et al., 2001). The predominant localization of the rhabditiform larvae is perivascular. Many authors report the presence of larvae, adult worms and eggs in the granulomatous lesions of H. gingivalis (Lim et al., 2015, Ondrejka et al., 2010). This fact would be a criterion to differentiate it from Strongyloides sp. Besides the different morphology, eggs and adult parasites are not seen in the cutaneous lesions of the horse (Dunn et al., 1993).
7.1. Gross lesions and neurological changes due to Halicephalobiasis in equids
Infections caused by Halicephalobus sp. are seldomly encountered in equine veterinary practices. Infections in horses cause granulomatous lesions and extensive tissue destruction that most commonly affect the central nervous system (CNS), skin, and kidneys (Ondrejka et al., 2010). The clinical manifestations include nasal tumor involvement (Anderson and Bemrick, 1965), osteomyelitis (Kreuder et al., 1996). granulomatous prepuce infection (Muller et al., 2008), granulomatous nephritis (Akagami et al., 2007), meningoencephalitis (Akagami et al., 2007), and occasionally disseminated disease (Ruggles et al., 1993). Several equine cases involving the CNS were presented with ataxia, all of which culminated in euthanasia or death by overwhelming infection (Bryant et al., 2006, Brojer et al., 2000). Furthermore, changes in the brain such as lymphohistiocytic meningoencephalitis were most prominent in the thalamus, internal capsules, and midbrain. Nematodes were occasionally seen in the tunica media, small arteries and meninges. The nematodes from the affected horse were cultured and identified morphologically as Halicephalobus gingivalis (Brojer et al., 2000). A summary of the reported cases so far of the infection in horses from 1987 to 2017 are presented in Table 1 below.
Table 1.
Reported cases of Halicephalobus gingivalis infection in equids between 1972 and 2017.
| Year | Specie | Country | Clinical presentation | Diagnosis | Reference |
|---|---|---|---|---|---|
| 1972 | Horse | Egypt | Encephalitis | Post mortem | Ferris et al. (1972) |
| 1987 | Horse | United Kingdom | Encephalitis | Post mortem, morphological | Blunden et al. (1987) |
| 1990 | Horse | United States | Encephalitis, spinal cord lesions | Post mortem, morphological | Spalding et al. (1990) |
| 1992 | Horse | Scotland | Encephalitis and renal abscess | Post mortem, morphological | Angus et al. (1992) |
| 1993 | Horse | United States | Encephalitis, osteomyelitis | Morphological | Ruggles et al. (1993) |
| 1993 | Horse | United States | Encephalitis | Morphological | Trostle et al. (1993) |
| 1993 | Horse | United States | Prostitis | Morphological | Dunn et al. (1993) |
| 1995 | Horse | United States | Encephalitis | Morphological | Rames et al. (1995) |
| 1998 | Horse | Germany | Osteomyelitis gingivitis | Morphological | Teifke et al. (1998) |
| 2000 | Horses | United States | Encephalitis, nephritis | Morphological | Kinde et al. (2000) |
| 2000 | Grevy's zebra (Equus grevyi) | United States | Ocular infection | Morphological | Isaza et al. (2000) |
| 2000 | Horse | Canada | Encephalitis | Post mortem, morphological | Brojer et al. (2000) |
| 2001 | Horse | United States | Encephalitis | Post mortem, morphological | Wilkins et al. (2001) |
| 2001 | Horse | United States | Encephalitis | Post mortem, morphological | Johnson et al. (2001) |
| 2001 | Horse | Canada | Encephalitis | Morphological | Pearce et al. (2001) |
| 2004 | Donkey | United States | Renal abscess | Morphological | Schmitz and Chaffin (2004) |
| 2006 | Horse | United States | Encephalitis | Post mortem, morphological | Bryant et al. (2006) |
| 2007 | Horse | Japan | Encephalitis, nephritis | PCR | Akagami et al. (2007) |
| 2007 | Horse | Brazil | Neurological sign | Post-mortem, histopathology | Vasconcelos et al. (2007) |
| 2008 | Horse | Switzerland | Prostitis | Morphological | Muller et al. (2008) |
| 2008 | Horse | Canada | Encephalitis, mandibular abscess | Morphological | Ferguson et al. (2008) |
| 2011 | Horse | United Kingdom | Encephalitis | Post mortem, morphological | Hermosilla et al. (2011) |
| 2011 | Horse | Canada | Encephalitis | Post mortem, morphological | Sponseller et al. (2011) |
| 2012 | Horse | Iceland | Encephalitis | Post mortem, morphological | Eydal et al. (2012) |
| 2012 | Horse | Italy | Encephalitis | Post mortem, morphological | Di Francesco et al. (2012) |
| 2014 | Horse | South Korea | Encephalitis | PCR | Jung et al. (2014) |
| 2016 | Horse | Romania | Encephalitis | PCR, morphological | Taulescu et al. (2016) |
| 2016a | Cattle | Denmark | Encephalomyelitis | PCR, morphological | Enemark et al. (2016) |
| 2017 | Horse | Italy | Encephalitis | PCR, morphological | Pintore et al. (2017) |
An exception on the list- this is the first and so far, the only reported case of Halicephalobus gingivalis infection in dairy cattle.
7.2. Gross lesions and neurological changes due to Halicephalobiasis in Humans
Involvement of the brain is one of the most outstanding clinical manifestations of this disease in humans with fatal consequences. The most consistent observation relates to the central nervous system notably meningoencephalomyelitis. Clinical symptoms common to these cases include change in mental status, lethargy, and fever. Cerebrospinal fluid studies were reported in 4 of the 7 previous patients and showed an elevated white blood cell count with a lymphocytic predominance. The distribution of the disease has consistently included the meninges and parenchyma, with a myelitis component recognized in half of the patients (Ondrejka et al., 2010). Juvenile forms of the nematodes have also been found in pulmonary capillaries in sections of the lungs, but it is believed that the worms were carried to the lungs from the CNS not the reverse (Hoogstraten and Young, 1975). The worm has been reported in the liver and lungs with infiltration of inflammatory cells and no cellular response respectively (Gardiner et al., 1981). Other gross pathologic findings include edema, focal hemorrhage, and variable evidence of necrosis. The typical histopathologic pattern is that of predominantly perivascular acute and chronic inflammation, including multinucleated giant cells. Most reports describe regions of gliosis or glial cellular proliferation. In some instances, a more intense inflammatory infiltrate occasionally accompanied by granulomatous inflammation has been observed in association with the organisms themselves. Biopsy performed on the right temporal lobe showed chronic active meningoencephalitis with focal chronic granulomatous vasculitis (Ondrejka et al., 2010). Mild cerebral atrophy and chronic ischemic changes have also been recorded using computed tomography and magnetic resonance imaging respectively of the brain of an affected human. Results of an electroencephalogram of an affected patient showed moderate diffuse slowing of electrocerebral activity, suggestive of encephalopathy of toxic, metabolic or degenerative nature (Papadi et al., 2013). Furthermore, microscopic examination of brain, spinal cord, and meninges also showed extensive inflammation and nematodes morphologically consistent with Halicephalobus sp. (Papadi et al., 2013). Finally, gross lesions that have been observed include restricted lateral ventricles, sub-facial herniation of the right enlarged hemisphere, and symmetrical cerebellar herniation. A summary of reported cases so far of the infection in humans from 1975 to 2017 are presented in Table 2 below.
Table 2.
Reported human cases of Halicephalobus gingivalis infection between 1975 and 2017.
| Year | Demography | City/country | Clinical presentation | Diagnosis | Reference |
|---|---|---|---|---|---|
| 1975 | 5 years old, male | Manitoba, Canada | Meningoencephalitis, mandibular injuries | Post mortem | Hoogstraten and Young (1975) |
| 1979 | 47 years old, male | Texas, United States | Meningoencephalitis, brainstem signs | Post mortem | Shadduck et al. (1979) |
| 1981 | 54 years old, male | Washington, United States | Bilateral internuclear ophthalmoplegia. | Post mortem | Gardiner et al. (1981) |
| 2010 | 39 years old, female | Kentucky, United States | Meningoencephalitis, bilateral ring enhancing lesions | Post mortem | Ondrejka et al. (2010) |
| 2013 | 65 years old, female | Florida, United States | Encephalopathy, blurring of vision | Post mortem | Papadi et al. (2013) |
| 2014 | 74 years old, female | Eyre Peninsula of South Australia | Meningoencephalitis, frontoparietal meningitis | Post mortem | Lim et al. (2015) |
| 2015 | 49 years old, no sex indicated | Würzburg, Germany | Meningoencephalitis | Post mortem | Monoranu et al. (2015) |
8. Current situation in Africa
To the best of our knowledge, only one report so far was made in Egypt (Ferris et al., 1972). So far, no reported case of halicephalobiasis has been made in west and east Africa. That does not mean it may not have happened or occurred in these regions. Poor state of disease surveillance and diagnosis may mask the situation on ground. It is therefore expedient that proper institutional framework be put in place to curtail and prevent diseases generally of equine. Findings from close conversation with horse owners in Nigeria underscore the need for a collaborative and synergistic effort to improve proper disease prevention, diagnosis, and management in equine and by extension to prevent zoonotic outbreaks in humans.
9. Final perspective
Diseases have no boundary and the International movement of humans and horses could facilitate the transmission of diseases. The horse racing and polo sports are becoming popular in Nigeria, and in most African countries, where horses are transported from a part of the country or continent to another. In Nigeria, horse riding and polo sports are mainly undertaken by the wealthy. Huge amount of money are spent annually to import horses from different parts of the world, most especially from Europe and North America. Equine veterinary practitioners are few in Nigeria and most of the horse owners depend on services from local quacks or veterinary assistants for the management of their horses. From the current situation with equine practice, many diseases of horses are either misdiagnosed or not properly managed. There is the need for a more concrete and collaborative efforts by the local authorities to put adequate measures in place to prevent or forestall any undesirable consequences. Halicephalobiasis, although extremely rare, could be a fatal disease that may result to mortality and morbidity in both in equines and humans, respectively. We recommend that Halicephalobiasis should be considered as a potential cause of parasitic meningoencephalitis in cases of infection affecting equines and humans in worldwide and proper diagnosis should be intensified.
References
- Akagami M., Shibahara T., Yoshiga T., Tanaka N., Yaguchi Y., Onuki T., Kubo M. Granulomatous nephritis and meningoencephalomyelitis caused by Halicephalobus gingivalis in a pony gelding. J. Vet. Med. Sci. 2007;69(11):1187–1190. doi: 10.1292/jvms.69.1187. [DOI] [PubMed] [Google Scholar]
- Anderson R.V., Bemrick W.J. Micronema deletrix, a saprophagous nematode inhabiting a nasal tumor of a horse. Proc. Helminthol. Soc. Wash. 1965;32:74–75. [Google Scholar]
- Anderson R.C., Linder K.E., Peregrine A.S. Halicephalobus gingivalis (Stefanski, 1954) from a fatal infection in a horse in Ontario, Canada with comments on the validity of H. deletrix and a review of the genus. Parasite. 1998;5:255–261. doi: 10.1051/parasite/1998053255. [DOI] [PubMed] [Google Scholar]
- Andrassy I. Freilebende Nematoden aus dem Bükk-Gebirge. Annales Historico-Naturales Musei Nationalis Hungarica (Series Nova) 1952;2:13–65. [Google Scholar]
- Angus K.W., Roberts L., Archibald D.R., Fraser D.G., Jackson F., Gibbons L.M. Halicephalobus deletrix infection in a horse in Scotland. Vet. Rec. 1992;131:495. doi: 10.1136/vr.131.21.495-a. [DOI] [PubMed] [Google Scholar]
- Bernard E.C. Soil nematode biodiversity. Biol. Fertil. Soils. 1992;14:99–103. [Google Scholar]
- Blunden A.S., Khalil L.F., Webbon P.M. Halicephalobus deletrix infection in a horse. Equine Vet. J. 1987;19(3):255–260. doi: 10.1111/j.2042-3306.1987.tb01399.x. [DOI] [PubMed] [Google Scholar]
- Brojer J.T., Parsons D.A., Linder K.E., Peregrine A.S., Dobson H. Halicephalobus gingivalis encephalomyelitis in a horse. Can. Vet. J. 2000;41(7):559–561. [PMC free article] [PubMed] [Google Scholar]
- Bryant U.K., Lyons E.T., Bain F.T., Hong C.B. Halicephalobus gingivalis-associated meningoencephalitis in a thoroughbred foal. J. Vet. Diagn. Investig. 2006;18:612–615. doi: 10.1177/104063870601800618. [DOI] [PubMed] [Google Scholar]
- Castagnone-Sereno P., Danchin E.G.J. Parasitic success without sex–the nematode experience. J. Evol. Biol. 2014;27(7):1323–1333. doi: 10.1111/jeb.12337. [DOI] [PubMed] [Google Scholar]
- Di Francesco G., Savini G., Maggi A., Cavaliere N., D'Angelo A.R., Marruchella G. Equine meningo-encephalitis caused by Halicephalobus gingivalis: a case report observed during West Nile disease surveillance activities. Vet. Ital. 2012;48:437–442. [PubMed] [Google Scholar]
- Dougherty E.C. The genera and species of the subfamily Rhabditinae Micoletzky, 1922 (Nematoda): a nomenclatorial analysis - including an addendum on the composition of the family Rhabditidae Örley, 1880. J. Helminthol. 1955;29:105–152. [PubMed] [Google Scholar]
- Dunn D.G., Gardiner C.H., Dralle F.R., Thilsted J.P. Modular granulomatous posthitis caused by Halicephalobus (syn. Micronema) sp. in a horse. Vet. Pathol. 1993;30:207–208. doi: 10.1177/030098589303000215. [DOI] [PubMed] [Google Scholar]
- Enemark H.L., Hansen M.S., Jensen T.K., Larsen G., Al-Sabi M.N.S. An outbreak of bovine meningoencephalomyelitis with identification of Halicephalobus gingivalis. Vet. Parasitol. 2016;218:82–86. doi: 10.1016/j.vetpar.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Eydal M., Bambir S.H., Sigurdarson S., Gunnarsson E., Svansson V., Fridriksson S., Sigurdardóttir Ó.G. Fatal infection in two Icelandic stallions caused by Halicephalobus gingivalis (Nematoda: Rhabditida) Vet. Parasitol. 2012;186(3):523–527. doi: 10.1016/j.vetpar.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Ferguson R., van Dreumel T., Keystone J.S., Manning A., Malatestinic A., Caswell J.L., Peregrine A.S. Unsuccessful treatment of a horse with mandibular granulomatous osteomyelitis due to Halicephalobus gingivalis. Can. Vet. J. 2008;49:1099–1103. [PMC free article] [PubMed] [Google Scholar]
- Ferris D.H. Micronema deletrix in equine brain. Am. J. Vet. Res. 1972;33(1):33–38. [PubMed] [Google Scholar]
- Fonderie P., Bert W., Hendrickx F., Houthoofd W., Moens T. Anthelmintic tolerance in free-living and facultative parasitic isolates of Halicephalobus (Panagrolaimidae) Parasitology. 2012;139:1301–1308. doi: 10.1017/S0031182012000558. [DOI] [PubMed] [Google Scholar]
- Gardiner C.H., Koh D.S., Cardella T.A. Micronema in man: third fatal infection. Am. J. Trop. Med. Hyg. 1981;30:586–589. doi: 10.4269/ajtmh.1981.30.586. [DOI] [PubMed] [Google Scholar]
- Gardiner C.H., Meyers W.M., Neafie R.C., Marty A.M. Halicephalobiasis. Strongyloidiasis. Angiostrongyliasis cantonensis. Toxocariasis. In: Meyers W.M., Neafie R.C., Marty A.M., Wear D.J., editors. Helminthiases. Vol 1. Armed Forces Institute of Pathology; Washington, DC: 2000. (493–497, 341–347, 375–376, 412–414) [Google Scholar]
- Geraert E., Sudhaus W., Lenaerts L., Bosmans E. Halicephalobus laticauda sp. A nematode found in a Belgian coal mine (Nematoda, Rhabditida) Annales de la Société Royale Zoologique de Belgique. 1988;118:5–12. [Google Scholar]
- Henneke C., Jespersen A., Jacobsen S., Nielsen M.K., McEvoy F., Jensen H.E. The distribution pattern of Halicephalobus gingivalis in a horse is suggestive of a haematogenous spread of the nematode. Acta Vet. Scand. 2014;56(1):56. doi: 10.1186/s13028-014-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosilla C., Coumbe K.M., Habershon-Butcher J., Schöniger S. Fatal equine meningoencephalitis in the United Kingdom caused by the panagrolaimid nematode Halicephalobus gingivalis: case report and review of the literature. Equine Vet. J. 2011;43(6):759–763. doi: 10.1111/j.2042-3306.2010.00332.x. [DOI] [PubMed] [Google Scholar]
- Hoogstraten J., Young W.G. Meningo-encephalomyelitis due to the saprophagous nematode, Micronema deletrix. Can. J. Neurol. Sci. 1975;2:121–126. doi: 10.1017/s0317167100020102. [DOI] [PubMed] [Google Scholar]
- Hugot J.P., Baujard P., Morand S. Biodiversity in helminths and nematodes as a field of study: an overview. Nematology. 2001;3:199–208. [Google Scholar]
- Isaza R., Schiller C.A., Stover J., Smith P.J., Greiner E.C. Halicephalobus gingivalis (Nematoda) infection in a Grevy's zebra (Equus grevyi) J. Zoo Wildl. Med. 2000;31(1):77–81. doi: 10.1638/1042-7260(2000)031[0077:HGNIIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Johnson K.H., Johnson D.W. Granulomas associated with Micronema deletrix in the maxillae of a horse. J. Am. Vet. Med. Assoc. 1966;149:155–159. [PubMed] [Google Scholar]
- Johnson J.S., Hibler C.P., Tillotson K.M., Mason G.L. Radiculomeningomyelitis due to Halicephalobus gingivalis in a horse. Vet. Pathol. 2001;38(5):559–561. doi: 10.1354/vp.38-5-559. [DOI] [PubMed] [Google Scholar]
- Jubb K.V.F., Huxtable C.R. The nervous system. In: Jubb K.V.F., editor. Pathology of domestic animals. 4.ed. V.1. Academic; San Diego: 1993. pp. 267–439. [Google Scholar]
- Jung J.Y., Lee K.H., Rhyoo M.Y., Byun J.W., Bae Y.C., Choi E., Yoon S.S. Meningoencephalitis caused by Halicephalobus gingivalis in a thoroughbred gelding. J. Vet. Med. Sci. 2014;76(2):281–284. doi: 10.1292/jvms.13-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinde H., Mathews M., Ash L., St. Leger J. Halicephalobus gingivalis (H. deletrix) infection in two horses in southern California. J. Vet. Diagn. Investig. 2000;12(2):162–165. doi: 10.1177/104063870001200213. [DOI] [PubMed] [Google Scholar]
- Kreuder C., Kirker-Head C.A., Rose P., Gliatto J. What is your diagnosis? (lameness caused by Micronema deletrix) J. Am. Vet. Med. Assoc. 1996;209(6):1070–1071. [PubMed] [Google Scholar]
- Lim C.K., Crawford A., Moore C.V., Gasser R.B., Nelson R., Koehler A.V., Weldhagen G.F. First human case of fatal Halicephalobus gingivalis meningoencephalitis in Australia. J. Clin. Microbiol. 2015;53:1768–1774. doi: 10.1128/JCM.00032-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monoranu C.M., Müllges W., Keppler M., Brehm K., Ondrejka S.L.…Tannich E., Müller-Hermelink H.K., Tappe D. Open Forum Infectious Diseases. 2 (2) Oxford University Press; 2015. Fatal human meningoencephalitis due to Halicephalobus nematodes, Germany. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S., Grzybowski M., Sager H., Bornand V., Brehm W.A. Nodular granulomatous posthitis caused by Halicephalobus sp in a horse. Vet. Dermatol. 2008;19(1):44–48. doi: 10.1111/j.1365-3164.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- Nadler S.A., Carreno R.A., Adams B.J., Kinde H., Baldwin J.G., Mundo-Ocampo M. Molecular phylogenetics and diagnosis of soil and clinical isolates of Halicephalobus gingivalis (Nematoda: Cephalobina: Panagrolaimoidea), an opportunistic pathogen of horses. Int. J. Parasitol. 2003;33(10):1115–1125. doi: 10.1016/s0020-7519(03)00134-6. [DOI] [PubMed] [Google Scholar]
- Nau R., Sorgel F., Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010;23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrejka S.L., Procop G.W., Lai K.K., Prayson R.A. Fatal parasitic meningoencephalomyelitis caused by Halicephalobus deletrix: a case report and review of the literature. Arch. Pathol. Lab. Med. 2010;134(4):625–629. doi: 10.5858/134.4.625. [DOI] [PubMed] [Google Scholar]
- Papadi B., Boudreaux C., Tucker J.A., Mathison B., Bishop H., Eberhard M.E. Halicephalobus gingivalis: a rare cause of fatal meningoencephalomyelitis in humans. Am. J. Trop. Med. Hyg. 2013;88(6):1062–1064. doi: 10.4269/ajtmh.2013.12-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S.G., Bouré L.P., Taylor J.A., Peregrine A.S. Treatment of a granuloma caused by Halicephalobus gingivalis in a horse. J. Am. Vet. Med. Assoc. 2001;219:12–15. doi: 10.2460/javma.2001.219.1735. [DOI] [PubMed] [Google Scholar]
- Pintore M.D., Cerutti F., D'Angelo A., Corona C., Gazzuola P., Masoero L., Casalone C. Isolation and molecular characterisation of Halicephalobus gingivalis in the brain of a horse in Piedmont, Italy. Parasit. Vectors. 2017;10(1):135–139. doi: 10.1186/s13071-017-2070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rames D.S., Miller D.K., Barthel R., Craig T.M., Dziezyc J., Helman R.G., Mealey R. Ocular Halicephalobus (syn. Micronema) deletrix in a horse. Vet. Pathol. 1995;32:540–542. doi: 10.1177/030098589503200514. [DOI] [PubMed] [Google Scholar]
- Ruggles A.J., Beech J., Gillette D.M., Midla L.T., Reef V.B., Freeman D.E. Disseminated Halicephalobus deletrix infection in a horse. J. Am. Vet. Med. Assoc. 1993;203:550–552. [PubMed] [Google Scholar]
- Schmitz D.G., Chaffin M.K. What is your diagnosis? Halicephalobus gingivalis. J. Am. Vet. Med. Assoc. 2004;225:1667–1668. doi: 10.2460/javma.2004.225.1667. [DOI] [PubMed] [Google Scholar]
- Shadduck J.A., Ubelaker J., Telford V.Q. Micronema deletrix in an adult man. Am. J. Clin. Pathol. 1979;72:640–643. doi: 10.1093/ajcp/72.4.640. [DOI] [PubMed] [Google Scholar]
- Simpson R.M., Hoogin E.C., Cho D.Y. Micronema deletrix-induced granulomatous osteoarthritis in a lame horse. J. Comp. Pathol. 1988;99:347–351. doi: 10.1016/0021-9975(88)90056-4. [DOI] [PubMed] [Google Scholar]
- Spalding M.G., Greiner E.C., Green S.L. Halicephalobus (Micronema) deletrix infections in two half-sibling foals. J. Am. Vet. Med. Assoc. 1990;196:1127–1129. [PubMed] [Google Scholar]
- Sponseller B.T., Plattner B.L., Hostetter J.M. Pathology in practice. J. Am. Vet. Med. Assoc. 2011;238(10):1265–1267. doi: 10.2460/javma.238.10.1265. [DOI] [PubMed] [Google Scholar]
- Steel H., de la Peña E., Fonderie P., Willekens K., Borgonie G., Bert W. Nematode succession during composting and the potential of the nematode community as an indicator of compost maturity. Pedobiologia. 2010;53:181–190. [Google Scholar]
- Stefanski W. Rhabditis gingivalis sp. n. parasite trouvé dans un granulome de la gencive chez un cheval. Acta Parasitologica Polonica. 1954;1:329–334. [Google Scholar]
- Sudhaus W. Vergkichende Untersuchungen zur Phylogenie, Systematik, Ökologie, Biologie und Ethologie der Rhab-ditinae (Nematoda) Zoologica. 1976;115:1–229. [Google Scholar]
- Taulescu M.A., Ionicã A.M., Diugan E., Pavaloiu A., Cora R., Amorim I., Roccabianca P. First report of fatal systemic Halicephalobus gingivalis infection in two Lipizzaner horses from Romania: clinical, pathological, and molecular characterization. Parasitol. Res. 2016;115(3):1097–1103. doi: 10.1007/s00436-015-4839-7. [DOI] [PubMed] [Google Scholar]
- Teifke J.P., Schmidt E., Traenckner C.M., Bauer C. Halicephalobus (syn. Micronema) deletrix as a cause of granulomatous gingivitis and osteomyelitis in a horse. Tierarztliche Praxis. Ausgabe G, Grosstiere/Nutztiere. 1998;26(3):157–161. [PubMed] [Google Scholar]
- Trostle S.S., Wilson D.G., Steinberg H., Dzata G., Richard R. Antemortem diagnosis and attempted treatment of (Halicephalobus) Micronema deletrix infection in a horse. Can. Vet. J. 1993;34:117–118. [PMC free article] [PubMed] [Google Scholar]
- Umlauf T.N., Rech R.R., Pellegrini-Masini A.M., Howerth E.W. Pathology in practice. J. Am. Vet. Med. Assoc. 2012;241:703–705. doi: 10.2460/javma.241.6.703. [DOI] [PubMed] [Google Scholar]
- Vasconcelos R.O., Lemos K.R., Moraes J.R.E., Borges V.P. Halicephalobus gingivalis (H. deletrix) in the brain of a horse. Ciência Rural, Santa Maria. 2007;37(4):1185–1187. [Google Scholar]
- Wilkins P.A., Wacholder S., Nolan T.J., Bolin D.C., Hunt P., Bernard W., Piero F. Evidence for transmission of Halicephalobus deletrix (H gingivalis) from dam to foal. J. Vet. Intern. Med. 2001;15(4):412–417. [PubMed] [Google Scholar]