Abstract
The present study deals with the green synthesis of silver nanoparticle from the aqueous leaf extracts of Leucas aspera and Hyptis suaveolens as reducing agent and to investigate the larvicidal activity of synthesized silver nanoparticles. The synthesized silver nanoparticles were characterized by Ultraviolet and visible absorption spectroscopy (UV), Fourier transform-infrared spectroscopy (FT-IR), X-ray spectroscopy (XRD), Field emission scanning electron microscope (FESEM) and High-resonance transmission electron microscopy (HRTEM) analysis. The nanoparticles are spherical, hexagonal, triangular and polyhedral in shape and the size of the Silver nanoparticles (AgNPs) of L. aspera was found to be in the range of 7–22 nm and AgNPs of H. suaveolens was 5–25 nm. Larvicidal bioassay with synthesized AgNPs synthesized from L. aspera and H. suaveolens extract, showed 100% mortality at 10 mg/L against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus with LC50 of 4.02, 4.69, 5.06 mg/L and LC90 of 11.22, 12.09, 12.74 mg/L and LC50 of 4.63, 4.04, 3.52 mg/L and LC90 of 12.07, 10.99, 09.61 respectively. These results suggest that the synthesized AgNPs of L. aspera and H. suaveolens have the potential to be used as an ideal eco-friendly agent for the control of the mosquito larvae.
Keywords: Leucas aspera, Hyptis suaveolens, Silver nanoparticles, HRTEM analysis, Larvicidal activity
Graphical abstract
1. Introduction
Nanotechnology concerns with the development of experimental processes for synthesis of nanoparticles of different sizes, shapes and controlled disparity. This provides an efficient control over many of the physical and chemical properties with potential applications in pharmaceuticals and medicine (Dubey et al., 2009). To date, metallic nanoparticle are mostly prepared from noble metals viz., platinum (Pt), gold (Au), silver (Ag) and lead (Pb), among these Ag is the metal of choice in the field of biological system (Elumalai et al., 2015).
The nanoparticles, synthesized through green processes, are known as green nanoparticles (GNPs) (Sumit and Nayak, 2012, Abhijith and Thakur, 2012). GNPs are widely applied in the field of medicine such as chemical biosensing, imaging, drug delivery and therapeutic labeling (Kohler et al., 2001) and are applied in the commercially available consumer products such as skin-creams, wound dressing, dental bonding agents, toothpastes and cosmetic products.
The biogenic reduction of metal ion to base metal is quite rapid, readily conducted at room temperature and pressure. Plant extracts may act both as reducing agents and stabilizing agents in the synthesis of nanoparticles and is known to influence the characteristics of nanoparticles (Kumar and Yadav, 2009).
Insecticide applications, although highly effective against the target vector species control, yet are facing a threat due to the development of resistance to chemical insecticides resulting in rebounding vectorial capacity (Liu et al., 2006). Vector control requires new and improved mosquito control methods that are cheap, ecofriendly and non poisonous to non target organisms.
The use of silver nanoparticles as mosquito larvicide is well established (Patil et al., 2012, Haldar et al., 2013, Suman et al., 2013, Velu et al., 2015). In recent studies, potential mosquito larvicidal activity of synthesized AgNPs from plant extracts is well-documented (Suman et al., 2013, Marimuthu and Govindarajan, 2010, Thirunavukkarasu et al., 2011). Silver nanoparticles using leaf extract of E. prostrata was reported to have larvicidal activity against filariasis and malarial vectors (Rajkumar and Abdul Rahuman, 2011). Green synthesized silver nanoparticles using aqueous root extract of Delphinium denudatum showed potent larvicidal activity against second instar larvae of dengue vector Aedes aegypti (Suresh et al., 2014). Silver nanoparticles synthesized by using aqueous extracts from leaves and green berries of Solanum nigrum were reported to show larvicidal activity against the larvae of Culex quinquefasciatus and Anopheles stephensi (Rawani et al., 2013).
Leucas aspera (Willd) belonging to Lamiaceae family is known for its medicinal properties and the leaves are used in traditional medicine for treating dyspepsia, cough, cold, painful swelling, fevers, ulcers and chronic skin eruptions (Chopra et al., 2002). The leaves are used as insecticide and mosquito repellent in rural areas (Sadhu et al., 2003, Maheswaran et al., 2008) and as a natural pesticide against A. stephensi (Karunamoorthi and Bekele, 2009) and also exhibits larvicidal activity against A. aegypti, A. stephensi and C. quinquefasciatus.
Hyptis suaveolens (L.) Poit is commonly known as Wilayati tulsi, a rigid sweetly aromatic herb belonging to the family Lamiaceae. The leaves are used to treat cancer (Mabberley, 1990) and anti-fertility (Oliver-Bever, 1986). Some species of Hyptis have been shown to possess insecticidal properties and larvicidal properties against C. quinquefasciatus (Elumalai et al., 2013).
The AgNPs which are less likely to cause ecological damage have been identified as a potential replacement of synthetic chemical insecticides; hence the need to use green synthesized AgNPs for the control of disease vectors. The present study focuses on the larvicidal activity of synthesized silver nanoparticles (AgNPs) from aqueous extracts of L. aspera and H. suaveolens leaf extract against the larvae of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus.
2. Materials and methods
2.1. Plant collection
Leucas aspera and Hyptis suaveolens weeds are found growing as dense clumps along the roadsides of Chennai, Tamilnadu, India (Fig. 1). L. aspera and H. suaveolens were identified and authenticated by Prof. P. Jayaraman and deposited at the plant Anatomy Research (PARC/2013/2109) West Tambaram, Chennai-600 045, Tamil Nadu, India.
Fig. 1.

Weeds selected for green synthesis of silver nanoparticles.
2.2. Selection of mosquito species
The mosquito species selected for the present study were A. stephensi, A. aegypti, and C. quinquefasciatus. A. stephensi is vector of malaria in India and larvae of these species are generally found in distinctly different habitat. A. aegypti (Linnaeus), the yellow fever mosquito spreads dengue fever, chikungunya, yellow fever and other diseases. A. aegypti is a vector for transmitting several tropical fevers. C. quinquefasciatus is the vector of West Nile which causes encephalitis or meningitis affecting the brain tissue resulting in permanent neurological damage.
2.3. Preparation of plant extract and synthesis of AgNPs
The leaves of L. aspera and H. suaveolens were washed thoroughly in tap water in order to remove dust particles and rinsed briefly in deionized water. The aqueous solution was prepared by taking 10 g of washed and finely cut leaves in a 250 mL Erlenmeyer flask along with 100 mL of deionized water and the mixture was boiled at 60 °C for 15 min. This extract was filtered through nylon mesh (spectrum), followed by millipore hydrophilic filter (0.22 m) and used for further experiments (Parashar et al., 2009).
2.4. Synthesis of silver nanoparticles
Fresh extract was used for the reduction of Ag+ ions to Ag°, where in 2 mL of extract was added to 98 mL of aqueous silver nitrate (1 mM) solution and incubated at 28 °C for 60 min. The bio-reduced AgNPs were analyzed using UV–visible spectroscopy.
2.5. UV- visible spectral analysis
1 mL of sample was withdrawn at time intervals and surface plasmon resonance of silver nanoparticles was characterized using a UV–vis spectrophotometer (Shimadzu 1601 model, Japan) at a resolution of 1 nm between 200 and 800 nm. Furthermore, the final nano-colloidal solution was subjected to repeated centrifugation (twice) to get rid of any un-interacted biological molecules at 10,000 rpm for 20 min in Remi Research Centrifuge. The final pellet was collected, dried in vacuum desiccators and stored for future use. From the synthesized NPs different test concentrations of aqueous solutions were prepared.
2.6. Fourier transform-infrared (FT-IR) spectroscopy
For FT-IR (PERKIN ELMER-SPECTRUM ONE) studies, the powdered sample of AgNPs was prepared by centrifuging the synthesized AgNPs solution at 10,000 rpm for 20 min. The solid residue obtained is then washed with deionized water to remove any unattached biological moieties to the surface of the nano particles, which are not responsible for biofunctionalization and capping. The resultant residue is then dried completely and the powder obtained is used for FT-IR measurements. The scanning range was 4000–450 cm− 1at a resolution of 4 cm− 1.
2.7. X-ray diffraction studies
The formation and quality of compounds were checked using X'Pert Pro Materials Research diffractometer system. The X ray diffraction (XRD) pattern was measured by drop coated films of AgNO3 on glass plate and employed with a characteristic radiation in the range of 20–90° at a scan rate of 0.05°/min with a time constant of 2 s, CuKα radiation and amplitude wave k = 1.5418 Å, with a 40-kV voltage and 30 mA current. The full-width at half-maximum (FWHM) from three different peaks were used in Scherrer's equation to determine the average crystallite size of the nanoparticles.
2.8. Field emission scanning electron microscope (FESEM) analysis
Morphological analysis was done using Hitachi SU6600 FE- SEM machine. Thin films of the sample were prepared on a carbon coated copper grid by just dropping a very small amount on the grid, extra solution was removed using a blotting paper and then the films on the SEM grid were allowed to dry by placing it under the mercury lamp for 5 min.
2.9. High-resonance transmission electron microscopy (TEM) of silver nanoparticles
The transmission electron microscopy (TEM) images were obtained using JEOL model 3010 instrument operated at 200 kV and a beam current of 104.1 μA. Sample for this analysis were prepared by coating the aqueous AgNPs on carbon coated copper grids (300 mesh size) by slow evaporation and then allowed to dry in vacuum at 25 °C overnight.
2.10. Mosquito larvicidal bioassay
The larvae of A. aegypti, A. stephensi and C. quinquefasciatus were collected from rice fields and stagnant water areas of Kancheepuram and identified in the Zonal Entomological Research Centre, Vellore, Tamilnadu to start the colony. The larvae were reared in the laboratory according to the method of Kamaraj et al. (2009). One gram of aqueous leaf extract was first dissolved in 100 mL of distilled water (stock solution). From the stock solution, 100 mg/l was prepared with dechlorinated tap water for bioassay test. The larvicidal activity was assessed according to the procedure of WHO (1996) with slight modifications. For bioassay test, larvae were taken in five batches of 20 in 199 mL of water and to which 1.0 mL of aqueous leaf extract (6.25, 12.5, 25, 50 and 100 mg/L) and were added. The numbers of dead larvae were counted after 24 h of exposure, and the percentage mortality was reported from the average of five replicates.
2.11. Dose–response bioassay
The experimental media in which 100% mortality of larvae occurred were selected for dose–response bioassay. Synthesized AgNPs toxicity test was performed by placing 20 mosquito larvae in 200 mL of sterilized double distilled water with the nanoparticles. The synthesized nanoparticle solutions were diluted using double distilled water as a solvent according to the desired concentrations (10, 8, 6, 4 and 2 mg/L). Each test included a set of control groups (silver nitrate and distilled water) with five replicates for each individual concentration. Mortality was assessed after 24 h to determine the acute toxicities against fourth instar larvae of A. aegypti, A. stephensi, and C. quinquefasciatus. To avoid settling of particles especially at higher doses, all treatments were sonicated for an additional 5 min prior to the addition of mosquito larvae. This additional sonication appeared to significantly decrease the settling of particles.
2.12. Statistical analysis
The average larval mortality data were subjected to probit analysis for calculating LC50, LC90 as described by (Busvin, 1971), and other statistics at 95% fiducial limits of upper confidence limit and lower confidence limit and chi-square values were calculated using the software developed by Reddy et al. (1992). Results with p < 0.05 were considered to be statistically significant (SPSS 11.5).
3. Results and discussion
The development of easy, reliable and eco-friendly method helps to increase the interest in the synthesis and application of nanoparticles that are beneficial for mankind. Biosynthesis of NPs is advantageous over chemical and physical methods as it is cost effective and environmentally friendly method since it is not necessary to use high pressure, energy, temperature and toxic chemicals.
3.1. UV–vis analysis of phyto-synthesized solutions
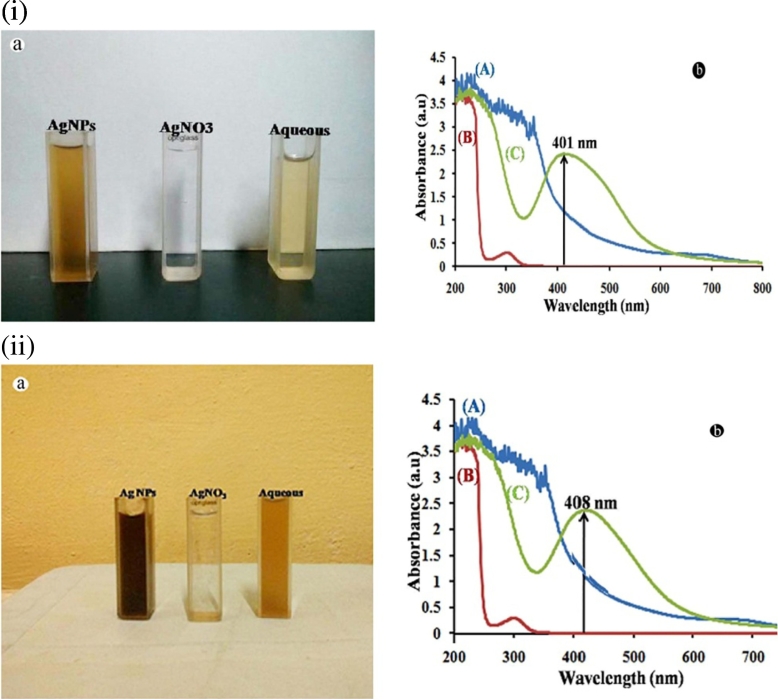
The aqueous extracts of L. aspera and H. suaveolens when mixed in aqueous solution of silver ion complex, pure Ag+ ions was reduced to Ag° and was monitored by measuring UV–vis spectrum of the reaction media. Here, the sharp bands were centered at 401 nm in L. aspera AgNPs extract and at 408 nm in H. suaveolens synthesized AgNPs extracts clearly indicate the presence of silver nanoparticles (Fig. 2 i b and ii b).
Fig. 2.
(і): (a) Shows colour change from light yellow to be brown before and after the process of phytoreduction of Ag + to AgNPs of L.aspera (b) UV- visible spectrum.
(іі): (b) Shows colour change from light yellow to brown before and after the process of phytoreduction of Ag + to AgNPs of H. suaveolens (b) UV- visible spectrum.
Earlier reports stated that maximum absorbance occurs due to the presence of silver particle (Sathishkumar et al., 2009). The peak area increased with the increase in reaction time. The possible reason for this observation could be due to the bioreduction of silver ion by the biomolecules present in the leaf extracts (Huang et al., 2007, Jagtap and Bapat, 2013). L. aspera and H. suaveolens aqueous leaf extracts and 1 mM AgNO3 solution showed no colour change and these results indicate that abiotic reduction of AgNO3 did not occur under the reaction conditions. It is generally recognized that UV–vis spectroscopy could be used to examine the size and shape of controlled nanoparticles in aqueous suspension (Srivastava et al., 2010).
3.2. FT-IR analysis of synthesized silver nanoparticles
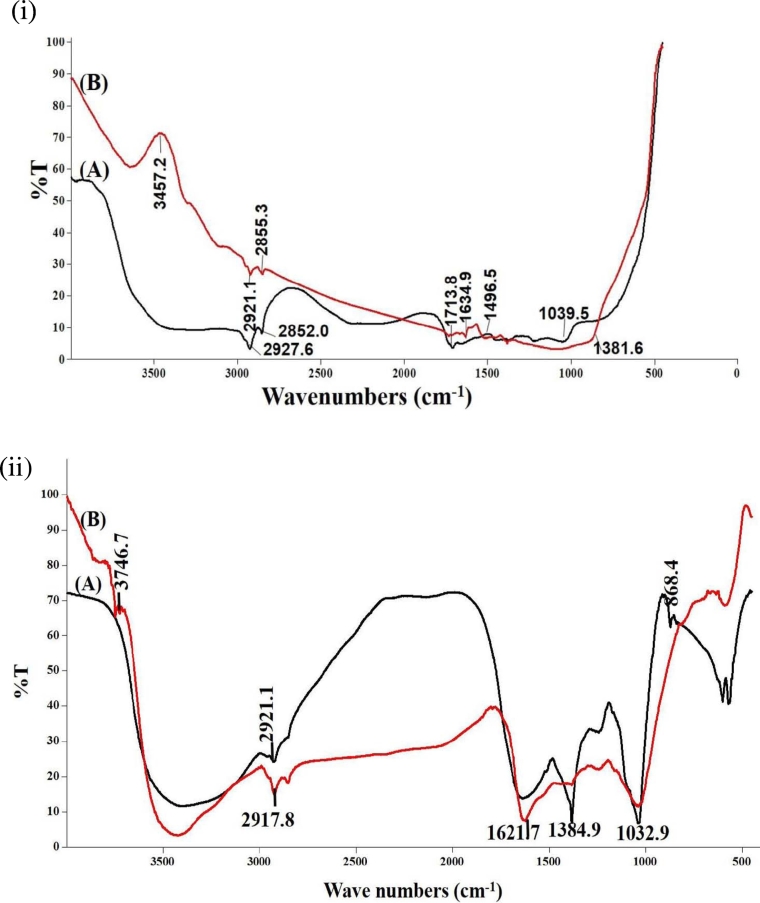
FT-IR spectroscopy is used to probe the chemical composition of the surface of the silver nanoparticles and to identify the possible biomolecules responsible for the reduction of the Ag+ ions and the capping of the bioreduced silver nanoparticle synthesized by the leaf extracts. The FT-IR spectrum of L. aspera and H. suaveolens extracts mediated silver nanoparticles are shown in Fig. 3.
Fig. 3.
(і): FTIR spectrums of L. aspera leaf extract (A) Aqueous (B) Green synthesized AgNPs.
(іі): FTIR spectrums of H. suaveolens leaf extract (A) Aqueous (B) Green synthesized AgNPs.
The FT-IR spectroscopy measurements were carried out to identify the biomolecules in the leaf extracts that were bound specifically on the synthesized silver nanoparticles. In L. aspera the band at 3457.2 cm− 1 corresponds to intermolecular O—H stretching vibrations of (phenolic and alcoholic compound), 2921.1 cm− 1 and 2855.3 cm− 1C-H asymmetric and symmetric stretches (alkenes), 1634.9 cm− 1 is due to N—H bend of amides and 1039.5 cm− 1 is assigned to C—O stretching of alcohols, carboxylic acid, esters and ethers. H. suaveolens AgNPs revealed peaks at 2917.8 cm− 1 due to C—H asymmetric and symmetric stretch of alkenes and the peak, 1621.7 cm− 1 is due to C O stretching of ketones and a peak at 1032.9 cm− 1 due to C—O stretch of ethers. Present data strongly indicate the involvement of polyphenols, carboxyl groups, amino groups and amino acid residues in silver nanoparticle synthesis. As mentioned earlier, L. aspera and H. suaveolens leaves have a rich source of bioactive compounds such as tannins, phenols, flavonoids, saponin, quinone, protein, carbohydrates, cyanin and terpenoids (Elumalai et al., 2013). It is known that proteins can easily bind to silver nanoparticles through either free amine groups or cystein residues in the proteins which ultimately stabilizes the silver nanoparticles (Gole et al., 2001). Based on the report by Wu et al. (2010) the functional groups C-O, C-OH, NH and COO from amino acid and proteins has strong affinity to bind metals to produce highly stable NPs. This indicates that AgNPs synthesized from the extract are surrounded by some protein and metabolites such as terpenoids that have amines, alcohols, carboxylic acid, esters and ether as functional groups. Another report has assigned that the peak at 1634 cm− 1 is due to –C = C– aromatic stretching. This suggests that silver NPs might be capped by water soluble secondary plant metabolites like flavonoids.
Stabilization of AgNPs by biological components is known to interact with metal salts via these functional groups and mediate their reduction to nanoparticles. According to Basavaraja et al. (2008), the carboxyl groups of amino acids and protein have the ability to link metals and thus prevent their agglomeration. A similar observation was noticed in biological synthesis of AgNPs using leaf extract of Mimusops elengi (Prakash et al., 2013), Ocimum sanctum (Subba Raoa et al., 2013), Jatropha curcas seed extract (Bar et al., 2009) and Banana peel extract (Bankar et al., 2010).
3.3. X- ray diffraction (XRD) of synthesized silver nanoparticles
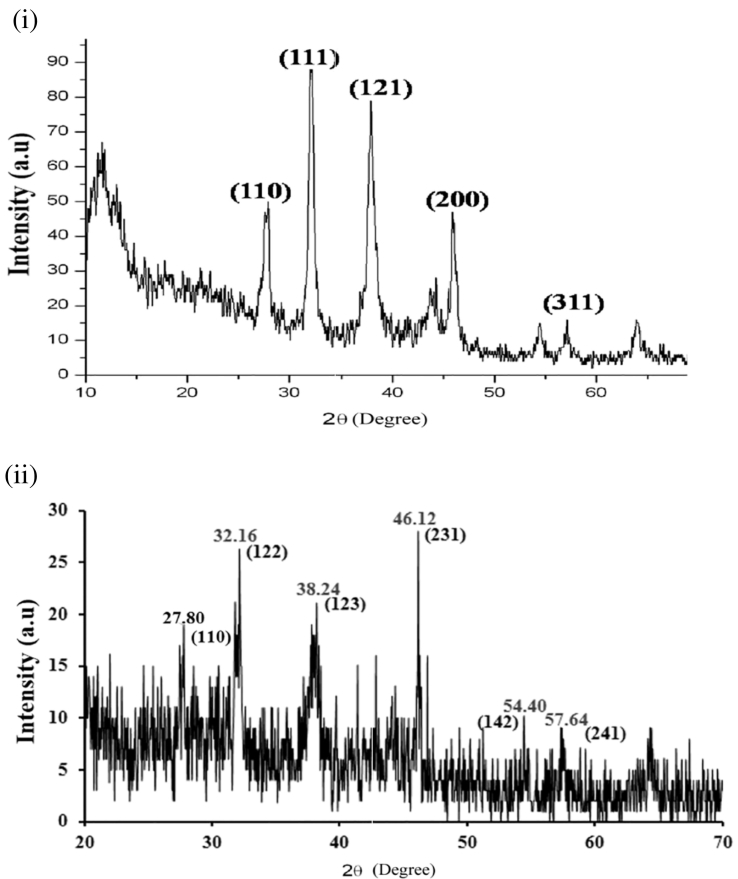
X-ray diffraction was used to confirm the crystalline nature of the particles. Fig. 4(i) shows a representative XRD pattern of the silver nanoparticles synthesized by L. aspera leaf extract after complete reduction of silver. The XRD pattern showed numbers of Bragg reflections that may be indexed on the basis of the face-centered cubic structure of silver. A comparison of the XRD spectrum with the standard confirmed that the silver particles formed were in the form of nanocrystals, as evidenced by the peaks at 2θ values of 27.80°, 32.14°, 38.28°, 46.04° and 57.64° representing the (110), (111), (121), (200) and (311) Bragg's reflections which may be indexed based on the face-centered cubic structure of metallic silver. X-ray diffraction results clearly reveal that the silver nanoparticles formed by the reduction of Ag+ ions by the L. aspera leaf extract are crystalline in nature. Fig. 4(ii) shows XRD pattern of silver nanoparticles obtained with H. suaveolens extract. After reaction, the diffraction peaks at 2θ values of 27.80°, 32.16°, 38.24°, 46.12°, 54.40° and 57.64°, assigned to the (110), (122), (123), (231), (1 4 2) and (241) planes of a faced center cubic (FCC) lattice of silver respectively and suggest that the silver nanoparticles are crystalline in nature (JCPDS File No. 84-0713).
Fig. 4.
(і): XRD pattern of green synthesized AgNPs from aqueous leaf extract of L. aspera.
(іі): XRD pattern of green synthesized AgNPs from aqueous leaf extract of H. suaveolens.
The reduction of the silver ions is moderately rapid at ambient conditions. This is novel and intriguing to material science as the studied leaf biomass has the capability to reduce metal ions at ambient conditions. In order to verify the results of UV–vis analysis, the sample of Ag+ exposed leaf extracts of L. aspera and H. suaveolens were examined by XRD. The XRD pattern showed four intense peaks in whole spectrum of 2θ values ranging from 25 to 60°. XRD pattern clearly illustrates that the silver nanoparticles formed are crystalline in nature. This indicates that the prepared silver nanoparticles may be enriched in (111) facets and thus the (111) plane seems to be preferentially oriented parallel to the surface of the supporting substrate (Germain et al., 2003). The sharpening of the peaks clearly suggests that the particles were in nanoregime. These sharp Bragg peaks might have resulted due to the capping agent stabilizing the nanoparticle. XRD spectrums confirmed the formation of silver nanoparticles (Balaji et al., 2009) and suggest that crystallization of the bioorganic phase occurs on the surface of the silver nanoparticles. The results in the present study are in agreement with the observation of Krishnaraj et al. (2010).
3.4. Morphology of synthesized silver nanoparticles
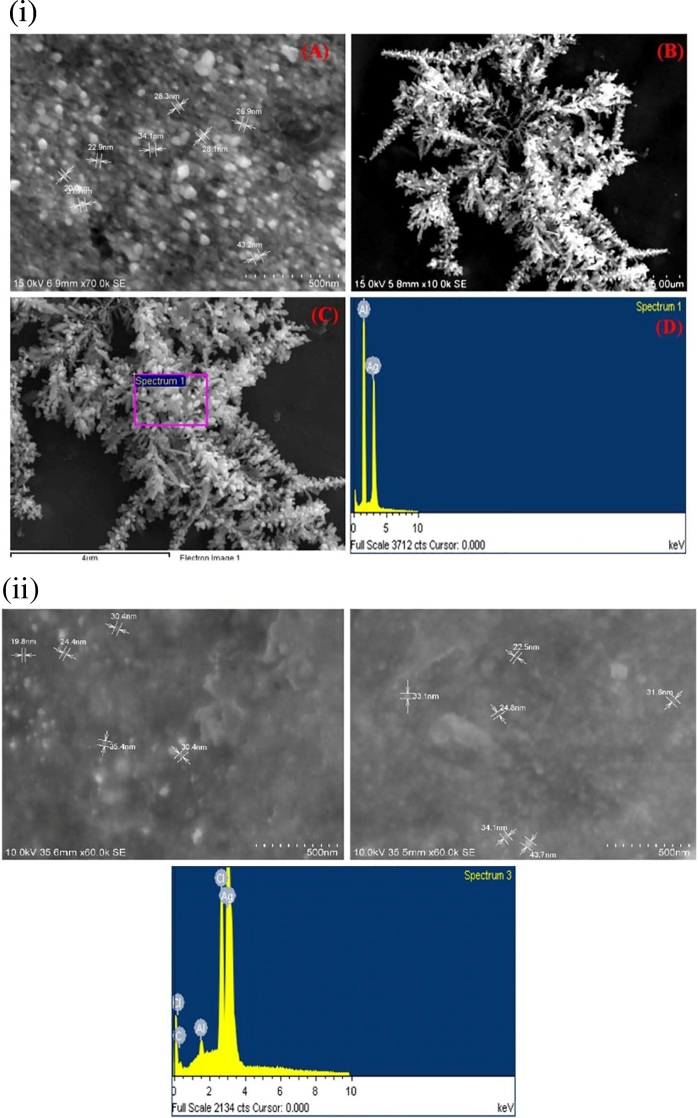
The FESEM technique was applied to determine the nano size and shape of metallic silver particles. The typical FESEM image confirmed the size of the silver nanoparticles and ranged in size of 22–43 nm in the case of L. aspera and 19–35 nm for H. suaveolens extract. FESEM images revealed that the synthesized silver nanoparticles were mostly spherical and quasispherical in H. suaveolens and in L. aspera synthesized AgNPs. The elemental composition of powdered samples was determined using SEM equipped with an EDAX detector. The energy dispersive X-ray analysis (EDAX) shown in Fig. 5, revealed the strong signal in the silver region and confirmed the formation of silver nanoparticles. The EDAX spectrum of synthesized silver nanoparticles clearly exhibited the absence of elemental nitrogen and oxygen peaks and the presence of elemental silver metal (Fig. 5 i, ii). The single sharp peak confirmed the reduction of silver nitrate to silver nanoparticles. These results strongly suggest that L. aspera and H. suaveolens leaf extracts might act as a reducing and stabilizing agent in production of AgNPs. Morones et al. (2005) described that the smaller size makes the nano particles to penetrate the cell membrane easily. Similar studies by Suresh et al. (2014) in Delphinium denudatum AgNPs was reported to be polydispersed, spherical and about 85 nm in size.
Fig. 5.
(і): FE-SEM and EDAX showing synthesized silver nanoparticles formed from L. aspera.
(іі): FE-SEM and EDAX showing synthesized silver nanoparticles formed from H. suaveolens.
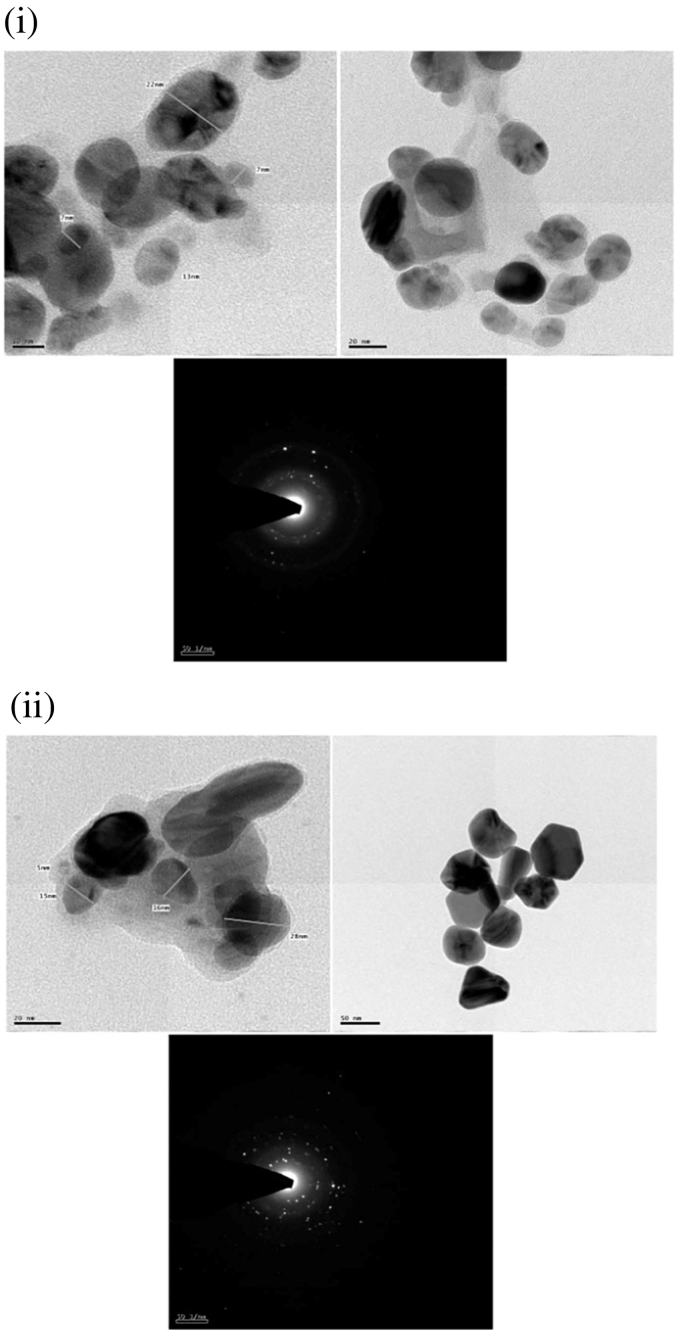
3.5. TEM analysis of synthesized silver nanoparticles
The size and morphology of AgNPs were determined by transmission electron microscopy (TEM) images. TEM analysis was done to analyse the size and the shape of the biogenically stabilized Ag nanoclusters. Fig. 6 i, shows that most of the nano-crystals formed from L. aspera extracts were spherical in shape, largely uniform with a moderate variation in particle size. According to size distribution, most of nanoparticles ranged from 7 to 22 nm. Synthesized AgNPs from fresh leaves of H. suaveolens were spherical, hexagonal, triangular and polyhedral in shape (Fig. 6 ii). The crystal lattice planes with ordered orientation of the lattice fringes are literally visible in one of the particle image and the TEM image of H. suaveolens shows that the particles size ranges from 5 to 25 nm. Furthermore, the SAED pattern proved that the green silver NPs are single crystalline in nature. Selected area electron diffraction (SAED) pattern of one of the spherical particles indicates the face-centered (fcc) crystalline nature of the nanoscale particles. The study reveals that most of the nanocrystals formed were spherical and polyhedral in shape. These results are similar to the observations of Rawani et al. (2013) who reported the spherical, polyhedral shape of AgNPs synthesized from the leaves and berries of Solanum nigrum.
Fig. 6.
(і): Transmission electron microscopy and SAED images of AgNPs derived from L. aspera leaves.
(іі): Transmission electron microscopy and SAED images of AgNPs derived from H. suaveolens leaves.
3.6. Mosquito larvicidal activity of aqueous leaf extracts of L. aspera and H. suaveolens and their synthesized silver nanoparticles
Varying concentrations of L. aspera and H. suaveolens aqueous extract (100, 50, 25, 12.5 and 6.5 mg/L) and synthesized AgNPs (10, 8, 6, 4 and 2 mg/L) were tested against 4th instar of A. aegypti, A. stephensi and C. quinquefasciatus. The LC50 and LC90 along with upper and lower confidence limit values and regression equations are presented in Table 1, Table 2.
Table 1.
Larvicidal activity of Aqueous, 1 mM silver nitrate solution and synthesized AgNPs of L. aspera leaf extract against fourth instar larvae of A. stephensi, A. aegypti and C. quinquefasciatus.
| Extract | Species | Con (mg/mL) |
Mortality% ± SD | LC50 ± SE (UCL–LCL) (mg/mL) |
LC90 ± SE (UCL–LCL) (mg/mL) |
χ2 (df = 4) |
|---|---|---|---|---|---|---|
| Aqueous | A. stephensi | 100 | 88 ± 1.82 | 26.08 ± 2.15 (30.31–21.85) |
147.61 ± 26.61 (134.89–67.95) |
4.96 |
| 50 | 68 ± 1.67 | |||||
| 25 | 41 ± 1.22 | |||||
| 12.5 | 28 ± 0.84 | |||||
| 6.25 | 19 ± 0.71 | |||||
| A. aegypti | 100 | 90 ± 2.17 | 17.16 ± 1.52 (20.15–14.18) |
101.42 ± 17.07 (134.89–67.95) |
4.16 | |
| 50 | 73 ± 2.51 | |||||
| 25 | 59 ± 3.03 | |||||
| 12.5 | 40 ± 1.48 | |||||
| 6.25 | 26 ± 1.34 | |||||
| C. quinquefasciatus | 100 | 85 ± 2.05 | 33.68 ± 3.01 (39.59–27.78) |
207 ± 44.08 (294.08–121.28) |
9.7 | |
| 50 | 57 ± 1.79 | |||||
| 25 | 39 ± 0.89 | |||||
| 12.5 | 23 ± 1.48 | |||||
| 6.25 | 17 ± 2.05 | |||||
| Silver nitrate | (1 mM solution) | 10 | 65 ± 1.67 | 21.25 ± 5.09 (32.19–12.38) |
73.49 ± 35.72 (142.57–43.42) |
4.99 |
| 8 | 51 ± 2.51 | |||||
| 6 | 32 ± 4.47 | |||||
| 4 | 18 ± 1.30 | |||||
| 2 | 08 ± 0.87 | |||||
| AgNPs | A. aegypti | 10 | 100 ± 0.00 | 4.02 ± 0.21 (4.44–3.59) |
11.22 ± 9.91 (13.16–9.28) |
32.5 |
| 8 | 80 ± 1.55 | |||||
| 6 | 54 ± 1.30 | |||||
| 4 | 41 ± 0.84 | |||||
| 2 | 28 ± 1.00 | |||||
| A. stephensi | 10 | 100 ± 0.00 | 4.69 ± 0.22 (5.13–4.57) |
12.09 ± 1.04 (14.13–10.05) |
40.7 | |
| 8 | 75 ± 1.10 | |||||
| 6 | 45 ± 1.67 | |||||
| 4 | 34 ± 1.66 | |||||
| 2 | 21 ± 1.00 | |||||
| C. quinquefasciatus | 10 | 100 ± 0.00 | 5.06 ± 0.22 (5.51–4.61) |
12.74 ± 1.10 (14.90–10.58) |
49.1 | |
| 8 | 72 ± 1.87 | |||||
| 6 | 39 ± 0.89 | |||||
| 4 | 29 ± 1.14 | |||||
| 2 | 19 ± 0.84 |
Table 2.
Larvicidal activity of Aqueous, 1 mM silver nitrate solution and synthesized AgNPs of H. suaveolens leaf extract against fourth instar larvae of A. stephensi, A. aegypti and C. quinquefasciatus.
| Extract | Species | Con (mg/mL) |
Mortality % ± SD | LC50 ± SE (UCL–LCL) (mg/mL) |
LC90 ± SE (UCL–LCL) (mg/mL) |
χ2 (df = 4) |
|---|---|---|---|---|---|---|
| Aqueous | A. stephensi | 100 | 79 ± 1.22 | 34.33 ± 3.01 (40.24–28.42) |
200.47 ± 40.65 (280.15–120.79) |
0.87 |
| 50 | 62 ± 1.34 | |||||
| 25 | 37 ± 1.67 | |||||
| 12.5 | 23 ± 0.14 | |||||
| 6.25 | 11 ± 1.00 | |||||
| A. aegypti | 100 | 86 ± 2.28 | 21.30 ± 1.86 (24.97–17.64) |
131.88 ± 24.31 (179.54–84.21) |
0.8 | |
| 50 | 75 ± 1.48 | |||||
| 25 | 51 ± 3.36 | |||||
| 12.5 | 36 ± 1.79 | |||||
| 6.25 | 20 ± 1.10 | |||||
| C. quinquefasciatus | 100 | 90 ± 2.12 | 19.25 ± 1.87 (22.91–15.58) |
146.31 ± 30.55 (206.21–86.42) |
5.47 | |
| 50 | 65 ± 2.74 | |||||
| 25 | 59 ± 1.22 | |||||
| 12.5 | 37 ± 2.28 | |||||
| 6.25 | 26 ± 2.21 | |||||
| Silver nitrate | (1 mM solution) | 10 | 65 ± 1.67 | 21.25 ± 5.09 (32.19–12.38) |
73.49 ± 35.72 (142.57–43.42) |
4.99 |
| 8 | 51 ± 2.51 | |||||
| 6 | 32 ± 4.47 | |||||
| 4 | 18 ± 1.30 | |||||
| 2 | 08 ± 0.87 | |||||
| AgNPs | A. aegypti | 10 | 100 ± 0.00 | 4.63 ± 0.22 (5.06–4.19) |
12.07 ± 1.043 (14.12–10.03) |
33.7 |
| 8 | 72 ± 1.30 | |||||
| 6 | 50 ± 0.71 | |||||
| 4 | 36 ± 1.52 | |||||
| 2 | 21 ± 1.51 | |||||
| A. stephensi | 10 | 100 ± 0.00 | 4.04 ± 0.21 (4.46–3.63) |
10.99 ± 0.92 (12.80–9.17) |
29.4 | |
| 8 | 80 ± 0.84 | |||||
| 6 | 56 ± 2.97 | |||||
| 4 | 40 ± 1.56 | |||||
| 2 | 28 ± 1.58 | |||||
| C. quinquefasciatus | 10 | 100 ± 0.00 | 3.52 ± 0.20 (3.92–3.13) |
9.61 ± 0.76 (11.12–8.11) |
24 | |
| 8 | 86 ± 1.10 | |||||
| 6 | 65 ± 1.67 | |||||
| 4 | 46 ± 1.00 | |||||
| 2 | 32 ± 1.48 |
The aqueous extracts of L. aspera were less toxic than the green synthesized silver nanoparticles against three tested mosquito species. The fourth instar larvae of the three mosquito species tested were highly susceptible to L. aspera AgNPs. At 10 mg/L concentration, 100% mortality was recorded after 24 h post treatments. The LC50 and LC90 values of aqueous leaf extract treated against fourth instar larvae of A. stephensi after 24 h post treatment was 26.08; 147.6 mg/L and 17.16; 101.42 mg/L against A. aegypti and 33.68; 207 mg/L against C. quinquefasciatus was observed, while LC50 and LC90 values of synthesized AgNPs treated against fourth instar larvae of A. aegypti was 4.02; 11.22 mg/L against A. stephensi was 4.69; 12.09 mg/L and C. quinquefasciatus was 5.06; 12.74 mg/L. The green synthesized AgNPs of L. aspera showed high toxicity against the treated larvae at very low concentrations.
The aqueous extract of H. suaveolens was less toxic than the green synthesized silver nanoparticles against the three tested mosquito species. The LC50 and LC90 values of aqueous leaf extract treated against fourth instar larvae of A. stephensi after 24 h post treatment was 34.33; 200.47 mg/L and 21.30; 131.88 mg/L against A. aegypti and was 19.25; 146.31 mg/L against C. quinquefasciatus. The LC50 and LC90 values of synthesized AgNPs treated against fourth instars larvae of C. quinquefasciatus was 3.52; 9.61 mg/L against A. stephensi was 4.04; 10.99 mg/L and A. aegypti was 4.63; 12.07 mg/L respectively. The green synthesized AgNPs of H. suaveolens showed high toxicity against the treated larvae at very low concentrations and showed 100% mortality against all the tested species at 10 mg/L concentration.
Synthesized AgNPs and aqueous extracts of L. aspera and H. suaveolens showed larvicidal activity against the fourth instar larvae of A. aegypti, A. stephenesi and C. quinquefasciatus. However, highest mortality was found in synthesized AgNPs when compared to aqueous extract against all the three mosquito species tested. These results are comparable to earlier reports of Priyadarshini et al. (2012) who reported the toxicity of AgNPs synthesized using Euphorbia hirta leaves against the first to fourth instar larvae and pupae of the malarial vector, A. stephensi. Similar results were observed by Patil et al. (2012) who reported the bioactivity of AgNPs synthesized from the latex producing plant Pergularia daemia against the larval instars of A. aegypti and A. stephenesi. Gnanadesigan et al. (2011) reported the larvicidal potential of AgNPs synthesized from Rhizophora mucronata leaf extract against the larvae of A. aegypti and C. quinquefasciatus. GNPs synthesized from the aqueous leaf extract of Hibiscus rosasinensis showed potent activity against the larvae of Aedes albopictus (Sareen et al., 2011) and nanoparticles from the fungus have been reported against A. stephensi (Salunkhe et al., 2011).
4. Conclusions
The nano-sized silver particles synthesized using leaves extracts of L. aspera and H. suaveolens were spherical, hexagonal, triangular and polyhedral. The formed silver nanoparticles are highly stable and exhibited significant mosquito larvicidal activity against the larvae of A. aegypti, A. stephensi and C. quinquefasciatus. This reveals the efficacy of AgNPs as a potent larvicidal agent. The surface reactivity facilitated by capping enables these functionalized NPs as promising tool for vector control. Therefore, in combination with mosquito repellents or other vector control measures, such plants synthesized AgNPs may have significant impact on the incidence of malaria and filariasis and can be considered as an important tool in integrated vector control programs. The application of plants synthesized AgNPs being simple and affordable may be useful in preventing dengue, malaria and filariasis.
Acknowledgments
Acknowledgements
The authors are grateful to University Grants Commission (F. No. 35-69/2008 (SR)) dated 20.03.2009 for providing financial assistance to carry out the present investigations. Our thanks are also extended to The Principal, Presidency College, Chennai, for providing infrastructure and research facilities. We acknowledge the support extended by SAIF, IIT Madras for FTIR studies and University of Madras for analyzing the samples by XRD, FE-SEM and TEM.
References
- Abhijith K.S., Thakur M.S. Application of green synthesis of gold nanoparticles for sensitive detection of aflatoxin B1 based on metal enhanced fluorescence. Anal. Methods. 2012;4(12):4250–4256. [Google Scholar]
- Balaji D.S., Basavaraja S., Deshpande R., Mahesh D.B., Prabhakar B.K., Venkataraman A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf. B: Biointerfaces. 2009;68:88–92. doi: 10.1016/j.colsurfb.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Bankar A., Joshi B., Kumar A.R., Zinjarde S. Banana peel extract mediated novel route for synthesis of silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010;368:58–63. [Google Scholar]
- Bar H., Bhui D.K., Gobinda S.P., Sarkar P.M., Pyne S., Misra A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009;348:212–216. [Google Scholar]
- Basavaraja S., Balaji D.F., Lagashetty A., Rajasab A.H., Venkataraman A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. 2008;43:1164–1170. [Google Scholar]
- Busvin R.J. Commonwealth Agric. Bureau, Lond. 1971. A critical review of the techniques for testing insecticides; pp. 263–288. [Google Scholar]
- Chopra R.N., Nair S.L., Chopra I.C. Vol. 23. CSIR; New Delhi: 2002. Glossary of Indian Medicinal Plants. [Google Scholar]
- Dubey M., Bhadauria S., Kushwah B.S. Green synthesis of nanosilver particles from extract ofEucalyptus hybrida (Safeda) leaf. Dig. J. Nanomater. Biostruct. 2009;4:537–543. [Google Scholar]
- Elumalai D., Kaleena P.K., Fathima M., Muttapan M. Evaluation of biological activity of Hyptis suaveolens (L) Poit and Leucas aspera (wild) aganist Culex quinquefasciatus. Int. J. Biomed. Res. 2013;1:7. [Google Scholar]
- Elumalai D., Kaleena P.K., Ashok K., Suresh A., Hemavathi M. Green synthesis of silver nanoparticle using Achyranthes aspera and its larvicidal activity against three major mosquito vectors. Eng. Agric. Environ. Food. 2015;1(8) doi: 10.1016/j.eaef.2015.08.002. [DOI] [Google Scholar]
- Germain M.A., Hatton A., Williams S., Matthews J.B., Stone M.H., Fisher J., Ingham E. Comparison of the cytotoxicity of clinically relevant cobalt-chromium and alumina ceramic wear particles in vitro. Biomaterials. 2003;24(3):469–479. doi: 10.1016/s0142-9612(02)00360-5. [DOI] [PubMed] [Google Scholar]
- Gnanadesigan M., Anand M., Ravikumar S., Maruthupandy M., Vijayakumar V., Selvam S., Dhineshkumar M., Kumaraguru A.K. Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pac J Trop Med. 2011;4:799–803. doi: 10.1016/S1995-7645(11)60197-1. [DOI] [PubMed] [Google Scholar]
- Gole A., Dash C., Ramakrishnaan V., Sainkar S.R., Mandal A.B., Rao M., Sastry M. Pepsin-gold colloid conjugates: Preparation, characterization and enzymatic activity. Langmuir. 2001;17:1674–1679. [Google Scholar]
- Haldar K.M., Haldar B., Chandra G. Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2013;447(7146):859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- Huang J., Li Q., Sun D., Lu Y., Su Y., Yang X., Wanh H., Wang Y., Shao W., He N., Hong J., Chen C. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum canphora leaf. Nanotechnology. 2007;18:1–11. [Google Scholar]
- Jagtap U.B., Bapat V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus lam seed extract and its antibacterial activity. Ind. Crop. Prod. 2013;46:32–137. [Google Scholar]
- Kamaraj C., Bagavan A., Rahuman A.A., Zahir A.A., Elango G., Pandiyan G. Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae) Parasitol. Res. 2009;104:1163–1171. doi: 10.1007/s00436-008-1306-8. [DOI] [PubMed] [Google Scholar]
- Karunamoorthi K., Bekele M. Prevalence of malaria from peripheral blood smears examination: A 1-year retrospective study from the Serbo Health Center, Kersa Woreda, Ethiopia. J. Infect. Public Health. 2009;2(4):171–176. doi: 10.1016/j.jiph.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Kohler J.M., Csaki A., Reichert J. Selective labeling of oligonucleotide monolayers by metallic nanobeads for fast optical readout of DNA chips. Sensors Actuators B Chem. 2001;76(1–3):166–172. [Google Scholar]
- Krishnaraj C., Jagan E.G., Rajasekar S., Selvakumar P., Kalaichelvan P.T., Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B: Biointerfaces. 2010;6:50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Kumar V., Yadav S.K. Plant mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009;84:151–157. [Google Scholar]
- Liu N., Xu Q., Zhu F., Zhang L. Pyrethrid resistance in mosquitoes. Insect Sci. 2006;13:159–166. [Google Scholar]
- Mabberley D.J. Cambridge University Press; London: 1990. The Plant Book; pp. 209–289. [Google Scholar]
- Maheswaran R., Kingsley S., Ignacimuthu S. Proceed Recent Trends Insect Pest Management. Vol. 240. 2008. Larvicidal and repellent activity of Clerodendron phlomides against Culex quinquefasciatus say (Diptera: Culicidae) p. 243. [Google Scholar]
- Marimuthu S., Govindarajan R. Larvicidal and repellent activities of Sida acuta Burm. F. (family: Malvaceae) against three important vector mosquitoes. Asian Pac J Trop Med. 2010:691–695. [Google Scholar]
- Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramfrez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- Oliver-Bever B. Vol. 225. Cambridge University Press; London: 1986. Medicinal Plants in Tropical West Africa. [Google Scholar]
- Parashar U.K., Saxenaa P.S., Srivastava A. Bioinspired synthesis of silver nanoparticles. Dig. J. Nanomater. Biostruct. 2009;4:159–166. [Google Scholar]
- Patil C.D., Borase H.P., Patil S.V., Salunkhe R.B., Salunke B.K. Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex against Aedes aegypti and Anopheles stephensi and nontarget fish Poecillia reticulata. Parasitol. Res. 2012;111(2):555–562. doi: 10.1007/s00436-012-2867-0. [DOI] [PubMed] [Google Scholar]
- Prakash P., Gnanaprakasam P., Emmanuel R., Arokiyaraj S., Saravanan M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi. Linn. For enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B: Biointerfaces. 2013;108:255–259. doi: 10.1016/j.colsurfb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Priyadarshini K.A., Murugan K., Panneerselvam C., Ponarulselvam S., Hwang J.S., Nicoletti M. Biolarvicidal and pupicidal potential of silver nanoparticles synthesized using Euphorbia hirta against Anopheles stephensi Liston (Diptera:Culicidae) Parasitol. Res. 2012;111(3):997–1006. doi: 10.1007/s00436-012-2924-8. [DOI] [PubMed] [Google Scholar]
- Rajkumar G., Abdul Rahuman A. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop. 2011;118:196–203. doi: 10.1016/j.actatropica.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Rawani A., Ghosh A., Chandra G. Mosquito larvicidal and antimicrobial activity of synthesized nano- crystalline silver particles using leaves and green berry extract of Solanum nigrum L (Solanaceae: solanales) Acta Trop. 2013;128:613–622. doi: 10.1016/j.actatropica.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Reddy P.J., Krishna D., Murthy U.S., Jamil K. A microcomputer FORTRAN program for rapid determination of lethal concentration of biocidesin mosquito control. Comput. Appl. Biosci. 1992;8:209–213. doi: 10.1093/bioinformatics/8.3.209. [DOI] [PubMed] [Google Scholar]
- Sadhu S.K., Okuyama E., Fujimoto H., Ishibashi M. Separation of Leucas aspera, a medicinal plant of Bangladesh, guided by prostaglandin inhibitory and antioxidant activities. Chem. Pharm. Bull. 2003;51:595–598. doi: 10.1248/cpb.51.595. [DOI] [PubMed] [Google Scholar]
- Salunkhe R.B., Patil S.V., Patil C.D., Salunke B.K. Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae) Parasitol. Res. 2011;109(3):823–831. doi: 10.1007/s00436-011-2328-1. [DOI] [PubMed] [Google Scholar]
- Sareen S.J., Pillai R.K., Chandramohanakumar N., Balagopalan M. Larvicidal potential of biologically synthesised silver nanoparticles against Aedes Albopictus. Res J Recent Sci. 2011;1:52–56. [Google Scholar]
- Sathishkumar M., Sneha K., Won S.W., Cho C.W., Kim S., Yun Y.S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B: Biointerfaces. 2009;73(2):332–338. doi: 10.1016/j.colsurfb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Santos A., Critchley K., Kim K.S., Podsiadlo P., Sun K., Lee J., Xu C., Lilly G.D., Glotzer S.C., Kotov N.A. Light-controlled self-assembly of semiconductor nanoparticles into twisted ribbons. Science. 2010;12(327):1355–1359. doi: 10.1126/science.1177218. [DOI] [PubMed] [Google Scholar]
- Subba Raoa Y., Venkata S., Kotakadi Prasad T.N.V.K.V., Reddyc A.V., Sai Gopal D.V.R. Green synthesis and spectral characterization of silver nanoparticles from Lakshmi tulasi (Ocimum sanctum) leaf extracts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013;103:156–159. doi: 10.1016/j.saa.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Suman T.Y., Elumalai D., Kaleena P.K., Radhika Rajasree S.R. GC–MS analysis of bioactive components and synthesis of silver nanoparticle using Ammannia baccifera aerial extract and its larvicidal activity against malaria and filariasis vectors. Ind. Crop. Prod. 2013;47:239–245. [Google Scholar]
- Sumit S.L., Nayak P.L. Green synthesis of gold nanoparticles using various extract of a plants and species. Int. J. Sci. Innov. Discov. 2012;3:325–350. [Google Scholar]
- Suresh G., Gunasekar P.H., Kokila D., Dinesh D., Ravichandran N., Ramesh B., Prabhu D., Koodalingam A., Siva G.V. Green synthesis of silver nanoparticles using Delphinium denudatum root extract exhibits antibacterial and mosquito larvicidal activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;127:61–66. doi: 10.1016/j.saa.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu S., Rahuman A.A., Govindasamy R., Marimuthu S., Asokan B., Chidambaram J., Zahir A.A., Elango G., Chinnaperumal K. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol. Res. 2011;108(3):693–702. doi: 10.1007/s00436-010-2115-4. [DOI] [PubMed] [Google Scholar]
- Velu K., Elumalai D., Hemalatha P., Janaki A., Babu M., Hemavathi M., Kaleena P.K. Evaluation of silver nanoparticles toxicity of Arachis hypogaea peel extracts and its larvicidal activity against malaria and dengue vectors. Environ. Sci. Pollut. Res. 2015 doi: 10.1007/s11356-015-4919-3. [DOI] [PubMed] [Google Scholar]
- WHO . 1996. Report of WHO Informal Consultation on the Evaluation and Testing Insecticides. CTD/WHO PES/IC/96.1,69. [Google Scholar]
- Wu C.J., Gaharwar A.K., Schexnailder P.J., Schmidt G. Development of biomedical polymer-silicate nanocomposites; a materials science perspective. Materials. 2010;3(5):2986–3005. [Google Scholar]