Abstract
Nigeria has the heaviest burden of lymphatic filariasis (LF) in sub-Saharan Africa, which is caused by the parasite Wuchereria bancrofti and transmitted by Anopheles mosquitoes. LF is targeted for elimination and the national programme is scaling up mass drug administration (MDA) across the country to interrupt transmission. However, in some regions the co-endemicity of the filarial parasite Loa loa (loiasis) is an impediment due to the risk of severe adverse events (SAEs) associated with the drug ivermectin. To better understand factors influencing LF elimination in loiasis areas, this study conducted a cross-sectional survey on the prevalence and co-distribution of the two infections, and the potential demographic, landscape, human movement, and intervention-related risk factors at a micro-level in the South West zone of Nigeria. In total, 870 participants from 10 communities on the fringe of a meso-endemic loiasis area of Osun State were selected. LF prevalence was measured by clinical assessment and using the rapid immunochromatographic test (ICT) to detect W. bancrofti antigen. Overall LF prevalence was low with ICT positivity ranging from 0 to 4.7%, with only 1 hydrocoele case identified. Males had significantly higher ICT positivity than females (3.2% vs 0.8%). Participants who did not sleep under a bed net had higher ICT positivity (4.0%) than those who did (1.3%). ICT positivity was also higher in communities with less tree coverage/canopy height (2.5–2.8%) than more forested areas with greater tree coverage/canopy height (0.9–1.0%). In comparison, loiasis was determined using the rapid assessment procedure for loiasis (RAPLOA), and found in all 10 communities with prevalence ranging from 1.4% to 11.2%. No significant difference was found by participants' age or sex. However, communities with predominately shrub land (10.4%) or forested land cover (6.2%) had higher prevalence than those with mosaic vegetation/croplands (2.5%). Satellite imagery showed denser forested areas in higher loiasis prevalence communities, and where low or no ICT positivity was found. Only one individual was found to be co-infected. GPS tracking of loiasis positive cases and controls also highlighted denser forested areas within higher loiasis risk communities and the sparser land cover in lower-risk communities. Mapping LF-loiasis distributions against landscape characteristics helped to highlight the micro-heterogeneity, identify potential SAE hotspots, and determine the safest and most appropriate treatment strategy.
Keywords: Neglected tropical diseases, NTDs, Lymphatic filariasis, Elephantiasis, Lymphoedema, Hydrocoele, Loiasis, Loa loa, Tropical eye worm, RAPLOA, Severe adverse events, SAEs, Data loggers, Landscape, Land cover, Nigeria, Sub-Saharan Africa
1. Introduction
Lymphatic filariasis (LF) is a mosquito-borne parasitic neglected tropical disease (NTD) targeted for global elimination by the year 2020 as part of the Global Programme to Eliminate LF (GPELF) (World Health Organization, 2017). LF is caused by three parasitic worms: Wuchereria bancrofti, Brugia malayi, and B. timori, with W. bancrofti causing over 90% of the infections. The major vectors of W. bancrofti are the Culex, Anopheles, and Aedes mosquitoes. The World Health Organization (WHO) estimates that over 120 million people are infected with LF in tropical and subtropical areas of the world. Approximately 40 million suffer from disabling clinical manifestations, primarily lymphoedema and elephantiasis, and/or hydrocoele (World Health Organization, 2013). One of the GPELF's main strategies is to interrupt transmission through mass drug administration (MDA) using three combinations of anthelminthic medicines: albendazole plus diethylcarbamazine (DEC); albendazole plus ivermectin (onchocerciasis co-endemic areas), or the alternative strategy of albendazole twice yearly plus vector control (Loa loa or loiasis co-endemic areas) (World Health Organization, 2016).
Since the launch of GPELF in 2000, there has been significant scale-up of MDA across all regions of the world (World Health Organization, 2010). However, at the halfway point in 2010 it was highlighted that the WHO's African Region, which accounts for approximately one third of the global burden of LF, had several challenges and was behind global targets. The main barriers to MDA implementation included incomplete mapping due to logistical challenges, instability, conflict and/or loiasis co-endemicity. The latter related to the risk of severe adverse events (SAEs), mostly neurological, associated with the use of ivermectin in individuals with high L. loa microfilariae (mf) loads in the blood (Boussinesq, 2006, Gardon et al., 1997, Kelly-Hope et al., 2014, Twum-Danso, 2003). The African Programme for Onchocerciasis Control (APOC), launched in 1995, relied on the community-directed treatment with ivermectin (CDTI) strategy to control the disease by targeting areas where the prevalence of skin nodules was ≥ 20% determined by Rapid Epidemiological Mapping of Onchocerciasis (REMO) (Noma et al., 2002). APOC experienced many challenges in expanding this strategy safely in loiasis areas due to the risk SAEs, and where encephalopathic cases were reported (Twum-Danso, 2003, Zouré et al., 2014, Zouré et al., 2011). The GPELF drew on the APOC experiences to help develop a provisional strategy and practical approach for LF elimination in the loiasis endemic countries, which take CDTI and vector control coverage into account (Kelly-Hope et al., 2017b, World Health Organization, 2012).
In Africa, Nigeria has the heaviest LF burden with an estimated 120 million people at risk (Okorie et al., 2013). In 2013, the Nigerian National LF Elimination Programme planned to scale-up MDA based on recent national mapping results, and the use of microstratification overlap mapping (MOM) to delineate L. loa co-endemicity, CDTI, and insecticide-treated net/long-lasting insecticidal mosquito net (ITN/LLIN) distributions to protect from the main Anopheles vectors (Okorie et al., 2013, Okorie et al., 2011). Initial programme work demonstrated successful integration of ITNs with MDA in Central Nigeria (Blackburn et al., 2006, Eigege et al., 2013), links with the malaria programme and co-implementation strategies (Federal Ministry of Health, 2013) and that community-wide distribution of LLINs can reduce transmission alone in loiasis co-endemic areas in South East Nigeria (Richards et al., 2013). However, in some LF-loiasis co-endemic areas more refined mapping and definition of risk factors were important where there was uncertainty about the risk of SAEs and if CDTI or alternative intervention strategies should be used. In Nigeria, the CDTI strategy was adopted in 1997, and currently more than 45 million people are being treated in more than 36,000 communities during the annual MDA (Federal Ministry of Health, 2017).
In Nigeria, the rapid assessment procedure for loiasis (RAPLOA) helped to define the distribution throughout the country (Takougang et al., 2002, Wanji et al., 2005, Zouré et al., 2011). Endemicity was found to be generally low, and closely associated with southern tropical rain forests that support the main vector, Chrysops spp. habitats (Kelly-Hope et al., 2017a). Understanding LF-loiasis co-distribution at a micro-level, delineating risk in relation to forested areas and how people move in and around these areas, may provide insights into where SAEs are more likely to occur, and if the new Test-and-not-Treat (TNT) strategy should be used to help minimize risk (D'Ambrosio et al., 2015, Kamgno et al., 2016, Pion et al., 2016a). The TNT strategy involves using the LoaScope (a mobile phone-based video microscope) to rapidly quantify L. loa mf in individuals during MDA, in order to exclude those with high mf levels and therefore at risk of SAEs. Micro-mapping methods have been used to delineate SAE and environmental risk factors in onchocerciasis-loiasis and LF-onchocerciasis-loiasis areas within relatively defined geographical parameters (Brito et al., 2017, Kelly-Hope et al., 2015, Kelly-Hope et al., 2014, Tekle et al., 2011).
Therefore, to build on these new mapping approaches and better understand factors associated with LF elimination in a loiasis endemic area, this study aimed to examine the prevalence and co-distribution of the two diseases and the potential demographic, landscape, and intervention-related risk factors in a co-endemic area in the South West zone of Nigeria. A secondary aim was to examine the geographical movement and estimated exposure times of individuals infected with loiasis.
2. Methods
2.1. Study area and site selection
This study was conducted in Osun State in the South West zone of Nigeria (Fig. 1A) where there have been reports of LF (Cano et al., 2014, Okorie et al., 2013) and loiasis (Adeoye et al., 2008, Ogunba, 1972, Zouré et al., 2011). Osun State is approximately 14,875 km2 in size with a human population of approximately 3.4 million. The state consists of three senatorial districts (i.e. central, east, west) and is divided into 30 local government areas (LGAs). The economy is mainly based on agriculture and cottage industries. Osun State has a tropical moist broadleaf forested area, and the climate is tropical with the rainy season occurring between April and October each year. Photos of a typical forested community with houses made from mud with iron or zinc roofing are shown in Fig. 2.
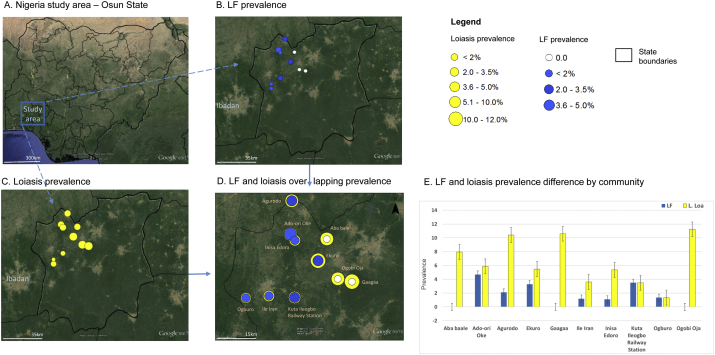
Fig. 1.
Nigeria study area LF and loiasis prevalence.
A. Nigeria study area – Osun State
B. LF prevalence
C. Loiasis prevalence
D. LF and loiasis overlapping prevalence
Fig. 2.
Photo of study area showing typical houses and nearby forested areas.
The northwest region of Osun State was chosen as the study area as it was endemic for both LF and loiasis, and close to the forested areas which are habitats of Chrysops spp. (Kelly-Hope et al., 2017a). To determine the prevalence and co-distribution of the two diseases and the potential demographic, landscape, and intervention-related risk factors, a micro-mapping survey was conducted in 10 communities covering an area approximately 60 km2 over a four-week period in May–June 2014. Communities were selected using a 15 km grid overlay map, and geo-referenced using the geographical information system (GIS) software (ArcGIS 10, ESRI, Redlands, CA). This method helped to demarcate a 5 to 15 km distance between each community, ensuring that prevalence was measured at regular intervals and that a variety of landscape characteristics were incorporated across the study area.
2.2. Sampling and field logistics
The survey sample size was based on the WHO guidelines for LF mapping (Gyapong and Remme, 2001, World Health Organization, 2011a), which includes sampling up to 100 individuals aged > 15 years from each community. Convenience sampling was used to invite individuals to participate in the survey at a highly-frequented location in the community, which was typically around the local health centre or chief's house. Only individuals who had lived in the community for five or more years were included to better determine if the infections were acquired through local transmission.
Local researchers from the Institute for Advanced Medical Research and Training, University of Ibadan, Osun State NTD staff and the South West NTD Zonal Coordinator visited each of the 10 communities the week before the study began to inform community leaders of the survey and sensitize the communities. The team included two research leads, two field assistants to help administer the surveys and two medically trained phlebotomists. When available, the local LGA coordinator assisted with the mobilization of survey participants. The field team was based in the city of Ibadan, and departed early each morning to reach the study communities to conduct the surveys.
2.3. LF prevalence
To determine the LF prevalence, rapid diagnosis was carried out using the BinaxNow Filariasis immunochromatographic test (ICT) cards (Alere Scarborough Inc., ME, USA). From each participant, 100 μl of finger-prick blood was collected and transferred to the ICT card to detect the presence of W. bancrofti circulating filarial antigen (CFA) (World Health Organization, 2011a). The ICT cards were pre-tested for quality using a positive control, and used per the instructions of the manufacturer. The presence of the W. bancrofti CFA was recorded for each participant and the percentage of positive individuals was calculated for each community.
Information on LF morbidity was also obtained during the survey. Participants were shown pictures of LF clinical manifestations, including lymphoedema (limb swelling) and hydroceles (scrotal swelling) and asked if they were suffering from any of the symptoms (World Health Organization, 2013). Participants who indicated they had clinical symptoms were examined and their condition confirmed by a medical officer. The number of clinical cases was recorded for each community.
2.4. Loiasis prevalence
To determine the prevalence of L. loa, the RAPLOA survey method was used (Takougang et al., 2002, Wanji et al., 2005, Zouré et al., 2011) and conducted on the same participants as the LF survey. RAPLOA is a rapid clinical survey method based on the history of eye worm. Information is obtained from a simple survey relating to an individual's experience of eye worm, which is confirmed by a photograph showing the L. loa adult worm and the duration of the most recent episode being between 1 and 7 days.
RAPLOA questions include: Have you ever experienced or noticed worms move along the white of your eye? (yes/no); Have you ever had the condition in this picture? (yes/no); and How long (in days) did the worm stay before disappearing? (between 1 and 7; yes/no). Loiasis was confirmed when an individual answered positively to all three questions. This is known as the restricted definition of loiasis, which has been correlated with high L. loa mf rates and risk of SAEs (Addiss et al., 2003). The percentage of individuals with loiasis was calculated for each community. Those with a prevalence between 20 and 40% were considered at moderate risk, and those with > 40% at high risk of SAEs.
2.5. Demographic and intervention related risk factors
To identify potential risk factors associated with filarial prevalence a short semi-structured questionnaire was administered to the survey participants at the time of the sero-prevalence and clinical assessments. Information on participants' sex (male/female), age (number), occupation (title), and frequency of visits into the surrounding forested areas (never/daily/weekly/other and reasons) was collected to determine demographic characteristics that may increase risk of transmission exposure. In addition, information on participants' usage of interventions was collected and included: the intake of ivermectin for onchocerciasis (yes/no); the number of times ivermectin had been taken (number); as well as bed net ownership (yes/no) and usage (slept under night before - yes/no).
2.6. Landscape characteristics
To examine the relationship between disease prevalence and the local landscape, particularly forested areas associated with the risk of loiasis, the communities were categorized per land cover, tree canopy cover and forest canopy height.
Data were obtained from remote sensing sources including the i) GlobCover Land Cover map with 300 m resolution, which was developed as part of the GlobalCover 2009 Project and provides a detailed portrait of the earth (GlobCover, 2010); ii) Tree cover map for year 2000, defined as percentage of canopy closure for all vegetation taller than 5 m in height at 500 m resolution (Hansen et al., 2013); and iii) Forest canopy height, based on a global map of canopy height at 1 km spatial resolution, using 2005 data from space borne light detection and ranging (lidar) (Simard et al., 2011).These data are among the best high resolution sources available in the public domain; however, it is acknowledged that there are some limitations with comparisons between layers as the resolution and time periods vary.
The GPS coordinates of the main location where the surveys were conducted in each community were recorded, i.e. around health clinic or chief's house. Using the ArcGIS software Geoprocessing (Buffer) and Spatial Analyst Tools (Zonal Statistics) (ESRI, Redlands, CA), a 5 km buffer (radius) around this central location was created to account for vectors flight range (Kelly-Hope et al., 2017a). Related raster data on landscape characteristics were then extracted and summarised to identify the main type of land cover, mean percentage of tree cover and mean forest canopy height within the community area (Fig. 5A–F), and how they were associated with LF and loiasis prevalence.
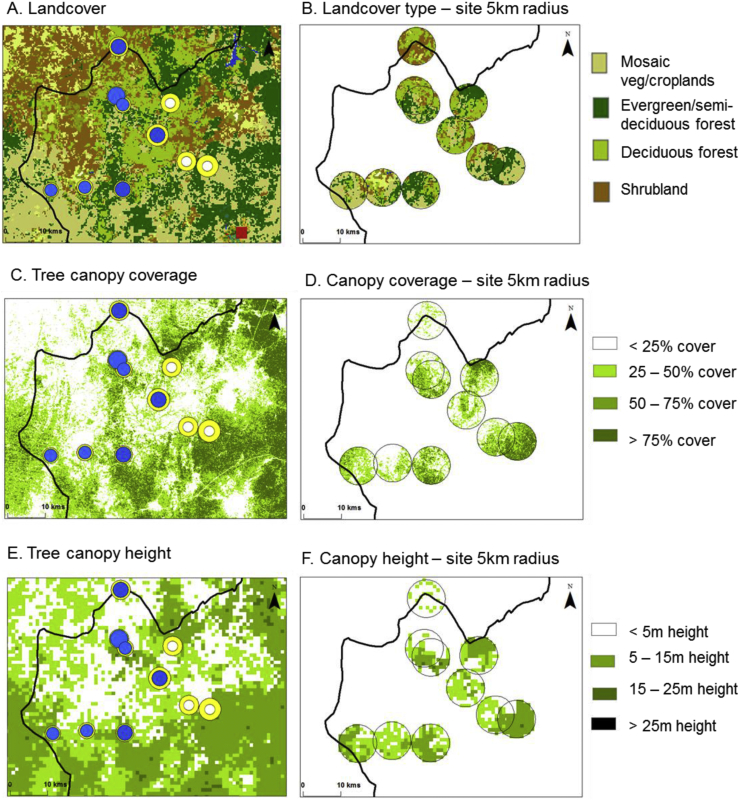
Fig. 5.
Landscape characteristics by study sites (5 km buffer).
A. Land cover
B. Land cover type – site 5 km radius
C. Tree canopy coverage
D. Canopy coverage – site 5 km radius
E. Tree canopy height
F. Canopy height – site 5 km radius
2.7. Loiasis case tracking – GPS data logger pilot study
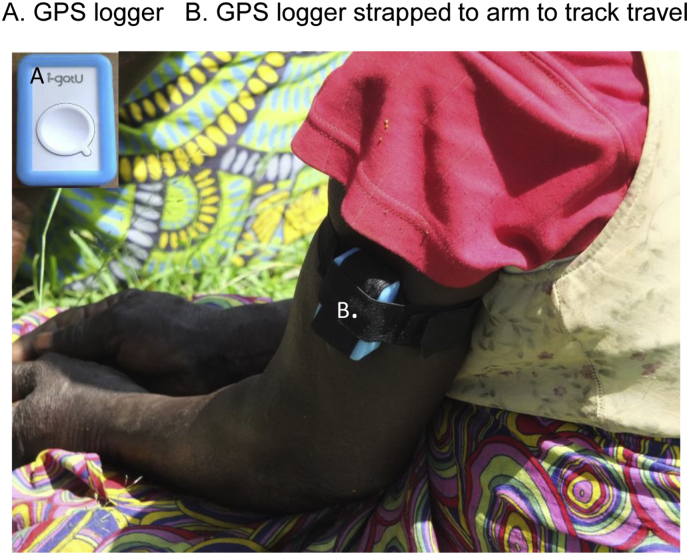
To begin to understand how human movement in and around forested areas may influence the risk of loiasis, the use of GPS data loggers (I-gotU GT-120, Mobile Action, UK) was piloted by recording and mapping the movement of participants with loiasis. In total, 20 participants with loiasis were randomly selected and invited to wear a GPS data logger over a period of 4 days. One participant without loiasis per community was also selected as a control for comparison; however, for this pilot they were not matched by age, sex or occupation. The field methods followed those used in another study (Seto et al., 2012). Briefly, the data loggers were programmed to record the participant's location at five-minute intervals for the four-day study period. The device was attached to a Velcro wristband and participants were instructed to wear it during all times that they were awake (Fig. 4A–B). The data logger information was downloaded to a computer and converted into GPX files. The average speed and distance travelled by each participant was recorded and the geographic-specific data mapped on Google™ earth and using ArcGIS 10 (ESRI, Redlands, CA).
Fig. 4.
GPS logger on participant arm.
A. GPS logger
B. GPS logger strapped to arm to track travel
2.8. LF entomological – Preliminary work
To better understand the potential LF mosquito vectors in the study area, a small entomological follow-up survey was conducted between July and September 2014 in the three ICT positive communities of Ekuro, Ogburo and Ado Ori Oke, which are in different locations with varying prevalences and landscape characteristics (Table 1).
Table 1.
Summary of each study community, location and landscape characteristics.
| LGA | Community | Longitude | Latitude | Land cover class (majority) | Tree coverage (mean - %) | Forest canopy height (mean - m) |
|---|---|---|---|---|---|---|
| Ayedire | Ile Iran | 7.6038 | 4.1879 | Mosaic vegetation/croplands | 22.1% | 9.1 |
| Kuta Ileogbo Railway Station | 7.5984 | 4.2803 | Broadleaved evergreen or semi-deciduous forest | 35.6% | 13.2 | |
| Ede South | Gaagaa | 7.6554 | 4.4855 | Broadleaved evergreen or semi-deciduous forest | 45.7% | 16.7 |
| Ogobi Oja | 7.6659 | 4.4347 | Broadleaved evergreen or semi-deciduous forest | 33.1% | 10.0 | |
| Egbedore | Aba bale | 7.8080 | 4.3952 | Open broadleaved deciduous forest | 35.6% | 13.1 |
| Ekuro | 7.7302 | 4.3640 | Open broadleaved deciduous forest | 27.5% | 9.5 | |
| Ejigbo | Ado-ori Oke | 7.8254 | 4.2652 | Open broadleaved deciduous forest | 29.9% | 6.5 |
| Agurodo | 7.9443 | 4.2700 | Closed to open shrub land | 19.4% | 2.5 | |
| Inisa Edoro | 7.8036 | 4.2806 | Open broadleaved deciduous forest | 34.5% | 9.1 | |
| Iwo | Ogburo | 7.5969 | 4.1063 | Mosaic vegetation/croplands | 32.8% | 13.1 |
Adult mosquitoes were collected using CDC light traps and Pyrethrum Spray Catch (PSC) in 23 to 26 selected houses of each community over a three-night period. The number of houses sampled depended on the number of houses whose occupants consented to the study. CDC light traps were set up indoors between 18.00 and 06.00 h in selected houses and placed close to the feet of a person sleeping under a bed net at a height of 150 cm. The PSCs were conducted between 06.00 and 08.00 h. Prior to the collection, all food, animals and small furniture were removed from the room. All mosquitoes knocked down were collected with forceps, placed in a labelled petri-dish on a layer of damp cotton wool and filter paper, and kept in labelled paper cups for identification and further analysis in the laboratory.
Mosquitoes were first identified to Genus level using morphological keys and placed in labelled eppendorf tubes containing drierite for further processing. Species identification and detection of W. bancrofti DNA in mosquitoes was then conducted. DNA was extracted from the mosquitoes using the Qiagen DNeasy tissue kit (QiagenDNeasy® kit; Mississauga, Canada). Culex mosquitoes were grouped into 5 pools of 20 mosquitoes per pool. Anopheles mosquitoes were analysed individually. Anopheles mosquitoes were identified by PCR (Scott et al., 1993). Identification of W. bancrofti parasite DNA in the mosquitoes was also conducted by PCR (Ramzy et al., 1997, Weil and Ramzy, 2007).
2.9. Data management and statistical analysis
Survey data were recorded on pre-printed forms in the field and entered onto Microsoft Excel™ 2010. Data were analysed using Excel™ and IBM Statistical Package for the Social Sciences (SPSS) (version 24) (SPSS Inc., Chicago, IL, USA). Participant responses were grouped into categorical variables based on frequency of the responses to survey questions. Analysis was conducted using the Fisher's exact test, with P-values generated to determine differences between the variables. A P-value of ≤ 0.05 was considered statistically significant.
2.10. Ethics, consent and patient referrals
Ethical approval was granted by the Research Ethics Committee, Liverpool School of Tropical Medicine, UK, as well as by the University of Ibadan/University College Hospital (UI/UCH) Ethics Review Committee and the Osun State Ministry of Health, Nigeria. Participants were informed about the purpose of the study in Yoruba and English, and requested to give written consent indicating their willingness to participate in the study. Participants found to be positive for LF and loiasis were informed of preventive measures and referred to the local health clinic. The community leaders, local clinics, and state Ministry of Health were informed of the survey results to help facilitate care and treatment in each of the communities, and to raise awareness of the risk associated with both filarial infections.
3. Results
The survey was conducted across the 10 selected communities from 5 LGAs including Ile Iran and Kuta Ileogbo Railway Station (Ayedire LGA); Gaagaa and Ogobi Oja (Ede South LGA); Aba bale and Ekuro (Egbedore LGA); Ado-ori Oke, Agurodo and Inisa Edoro (Ejigbo LGA) and Ogburo (Iwo LGA). The LGAs, communities, geographic coordinates of the central location where the surveys were conducted and the landscape characteristics are summarised in Table 1.
A total of 870 participants were included in the survey, ranging from 74 to 96 participants per community. It was not possible to attain the target 100 participants per community who had lived in the community for 5 years or more during the study period because some communities were small, disbanded and/or the adults were working away from home. In total, there were 349 males (40.1%) and 521 females (59.9%) included in the survey. The mean age was 49.9 years (range 15 to 100 years), which was similar for males (49.0 years) and females (50.5 years).
3.1. LF prevalence, risk factors and entomology
Overall, LF prevalence was low with only 15 participants (1.7%; n = 870) found to be ICT positive. No participant reported they had lymphoedema, but one male reported he had a hydrocele (aged 62 years, Agurodo community). Another reported he had an operation to remove a hydrocele three years prior (aged 50 years, Gaagaa). A summary of LF prevalence by demographic characteristics is presented in Table 2.
Table 2.
Summary of LF and loiasis prevalence and risk factors.
| Variable |
Total |
LF |
Loiasis |
|||||
|---|---|---|---|---|---|---|---|---|
| + ve | (%) | P value | + ve | Loa (%) | P value | |||
| Demographic characteristics | ||||||||
| LGA | Ayedire | 169 | 4 | 2.4% | 6 | 3.6% | ||
| Ede South | 174 | 0 | 0 | 19 | 10.9% | |||
| Egbedore | 179 | 3 | 1.7% | 12 | 6.7% | |||
| Ejigbo | 274 | 7 | 2.6% | 20 | 7.3% | |||
| Iwo | 74 | 1 | 1.4% | 0.321 | 1 | 1.4% | 0.023* | |
| Community | Ile Iran | 83 | 1 | 1.2% | 3 | 3.6% | ||
| Kuta Ileogbo Railway | 86 | 3 | 3.5% | 3 | 3.5% | |||
| Gaagaa | 85 | 0 | 0 | 9 | 10.6% | |||
| Ogobi Oja | 89 | 0 | 0 | 10 | 11.2% | |||
| Aba bale | 88 | 0 | 0 | 7 | 8.0% | |||
| Ekuro | 91 | 3 | 3.3% | 5 | 5.5% | |||
| Ado-ori Oke | 85 | 4 | 4.7% | 5 | 5.9% | |||
| Agurodo | 96 | 2 | 2.1% | 10 | 10.4% | |||
| Inisa Edoro | 93 | 1 | 1.1% | 5 | 5.4% | |||
| Ogburo | 74 | 1 | 1.4% | 0.178 | 1 | 1.4% | 0.121 | |
| Sex | Male | 349 | 11 | 3.2% | 18 | 5.2% | ||
| Female | 521 | 4 | 0.8% | 0.014* | 40 | 7.7% | 0.166 | |
| Age group | 15–50 | 409 | 7 | 1.7% | 21 | 5.1% | ||
| 51–100 | 460 | 8 | 1.7% | 37 | 8.0% | |||
| No answer | 1 | 0 | 0 | 1.0 | 0 | 0 | 0.162 | |
| Occupation | Farmer | 354 | 8 | 2.3% | 19 | 5.4% | ||
| Trader | 256 | 1 | 0.4% | 18 | 7.0% | |||
| Farmer/trader/other | 73 | 1 | 1.4% | 7 | 9.6% | |||
| Student | 80 | 2 | 3.8% | 5 | 6.3% | |||
| Other | 107 | 3 | 1.9% | 0.234 | 9 | 8.4% | 0.638 | |
| Forest visits | Never | 82 | 1 | 1.2% | 5 | 6.1% | ||
| Daily | 496 | 7 | 1.4% | 28 | 5.6% | |||
| Weekly/monthly | 292 | 7 | 2.4% | 0.63 | 25 | 8.6% | 0.301 | |
| Intervention history | ||||||||
| Ivermectin | Never | 253 | 6 | 2.4% | 13 | 5.1% | ||
| 1–4 rounds | 437 | 7 | 1.6% | 32 | 7.3% | |||
| 5 + rounds | 145 | 2 | 1.4% | 13 | 9.0% | |||
| Does not know | 35 | 0 | 0 | 0.776 | 0 | 0 | 0.207 | |
| Bed net own | Yes | 745 | 10 | 1.3% | 53 | 7.1% | ||
| No | 125 | 5 | 4.0% | 0.051* | 5 | 4.0% | 0.246 | |
| Bed net sleep | Yes | 502 | 5 | 1.0% | 34 | 7.8% | ||
| No | 243 | 5 | 2.1% | 19 | 6.8% | |||
| Does not own | 125 | 5 | 4.0% | 0.058 | 5 | 4.0% | 0.382 | |
| Total | 15 | 1.7% | 58 | 6.7% | ||||
Denotes statistically significant difference with P value ≤ 0.05.
3.1.1. Demographic characteristics
The prevalence varied across the LGAs and communities, as shown in Fig. 1B. The LGAs with the highest prevalence were Ayedire and Ejigbo and included the communities of Ado-ori Oke (4.7%; n = 85) and Kuta Ileogbo Railway Station (3.5%; n = 86). The communities of Gaagaa and Ogobi Oja in Ede South LGA and community of Aba bale in Egedbore LGA reported no positive cases (in contrast, these three communities were found to have among the highest loiasis prevalence – see next section).
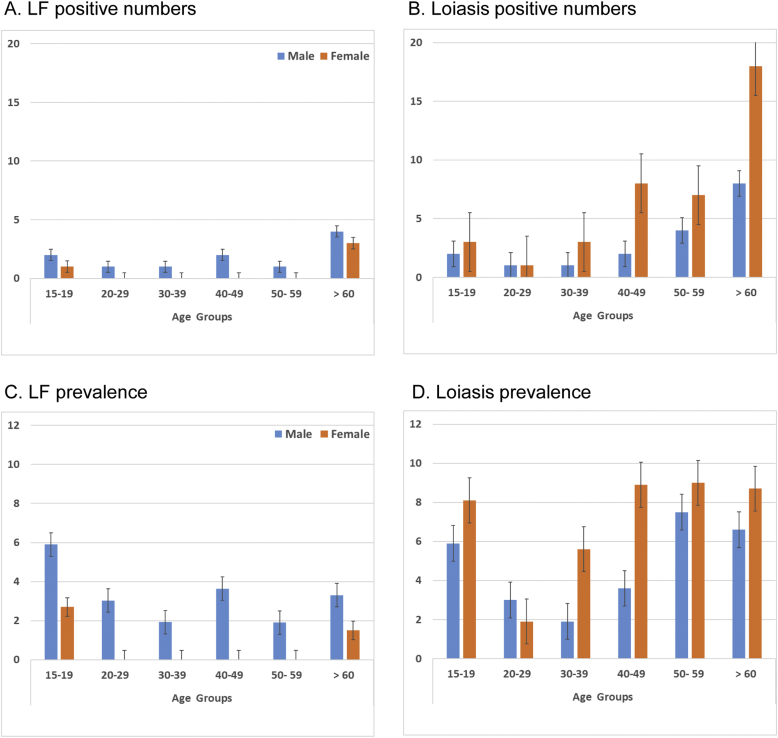
Males were found to have significantly higher LF prevalence than females (3.2% vs 0.8%; P = 0.014) (Table 2). There was no difference found between participants aged below or above the mean age of 49.9 years, and no distinct pattern across the different decadal age groups; however, no female participants aged between 20 and 60 years were found to be ICT positive as shown in Fig. 3A & C.
Fig. 3.
LF and loiasis number of positive cases and prevalence by age group and sex.
A. LF positive numbers
B. Loiasis positive numbers
C. LF prevalence
D. Loiasis prevalence
The most common occupational groups were farmers (40.7%; n = 354), traders (29.4%; n = 256), combination of farmer/trader/other work, as well as students (9.2%; n = 8) and other groups, which included trades such as carpenters, tailors, and professional groups such as teachers, doctors, and pastors. Of the occupational groups, farmers (2.3%; n = 354) and students (3.8%; n = 8) were found to have the highest LF prevalence, while traders had the lowest (0.4%; n = 256).
The frequency in which participants visited forested areas varied with nearly 10% stating that they never did, while more than half visited on a daily basis (57.0%; n = 496). One third visited less frequently on a weekly, monthly, or seasonal basis (33.6%; n = 292) mainly to farm, which included harvesting palm nut and making palm nut oil. There was no significant difference in LF prevalence between participants who stated they visit the forest at least one time per month versus those who stated they never visit the forest.
3.1.2. Intervention history
Overall, approximately one third of participants (29.1%; n = 870) stated they had never taken ivermectin for onchocerciasis, and of these, 6 participants were ICT positive (2.4% prevalence) (Table 2). This compares to the 437 participants (50.2%) who had taken 1–4 rounds with 7 ICT positive participants (1.6% prevalence), and the 145 participants (16.7%) who had ≥ 5 rounds of ivermectin with 2 ICT positive participants (1.4% prevalence). A small proportion (4%) did not know if they had taken the drug, and were all ICT negative. No significant differences were found between the ivermectin groups.
Overall, most participants stated they owned a bed net (85.6%; n = 870) and had a significantly lower prevalence (1.3%) compared to those who did not own a bed net (4.0% prevalence) (P = 0.051) (Table 2). A significantly higher proportion of females (92.3%) stated that they owned a bed net compared with males (75.5%) (P = 0.000).
Of those who owned a bed net, when asked if they slept under it the night before, two thirds of participants stated they had (67.4%; n = 745) and were found to have a lower LF prevalence (1.0%) than those 243 participants who did not (2.1%), as well as to those 125 participants who did not own a net at all (4.0%). The difference in LF prevalence between the bed net groups was approaching statistical significance (P = 0.058).
3.1.3. Landscape characteristics
The most dominant type of land cover for each community (5 km radius) included mosaic vegetation/croplands, closed to open shrub land, closed to open broadleaved evergreen/or semi-deciduous forest and open broadleaved deciduous forest (Table 1). No significant difference in LF prevalence was found between the main classifications as shown in Table 3.
Table 3.
Summary of LF and loiasis prevalence and intervention and landscape characteristics.
| Variable | Total | LF | Loiasis | |||||
|---|---|---|---|---|---|---|---|---|
| Landscape characteristics | ||||||||
| Land cover | Mosaic vegetation - cropland | 157 | 2 | 1.3% | 4 | 2.5% | ||
| Shrub land | 96 | 2 | 2.1% | 10 | 10.4% | |||
| Deciduous forest | 357 | 8 | 2.2% | 22 | 6.2% | |||
| Evergreen/semi-deciduous forest | 260 | 3 | 1.2% | 0.756 | 22 | 8.5% | 0.046* | |
| Tree coverage (%) | < 33% of total area | 355 | 10 | 2.8% | 23 | 6.5% | ||
| > 33% of total area | 515 | 5 | 1.0% | 0.060 | 35 | 6.8% | 0.891 | |
| Forest canopy height (mean) | < 10 m of total area | 448 | 11 | 2.5% | 28 | 6.3% | ||
| > 10 m of total area | 422 | 4 | 0.9% | 0.118 | 30 | 7.1% | 0.684 | |
| Total | 15 | 1.7% | – | 58 | 6.7% | |||
The mean percentage of tree coverage ranged from 19.4% to 45.7% (overall mean 32.5%; Table 1), and when comparing the LF prevalence above/below the mean, communities with less than average tree coverage were found to have a higher LF prevalence (2.8%) than those with above average tree coverage (1.0%) with differences approaching statistical significance (P = 0.060) (Table 3).
The mean forest canopy height ranged from 2.5 m to 16.7 m (overall mean 10.3 m; Table 1), and when comparing the LF prevalence above/below the mean, communities with less than average forest canopy height were found to have a higher LF prevalence (2.5%) than those with above average canopy height (0.9%), which were not found to be significant different (Table 3).
3.1.4. Entomological study
A total of 157 mosquitoes comprising of Anopheles (64.3%), Culex (35.0%) and Aedes (0.6%) were collected using both PSC and CDC light traps (Table 4). Out of the 101 Anopheles mosquitoes collected, 98 (97.0%) were identified as Anopheles gambiae while 3 (2.9%) were identified as Anopheles arabiensis. Only 1 An. gambiae mosquito was positive for W. bancrofti DNA (Table 5).
Table 4.
Number and mosquito species compositions collected by different collection methods.
| Community |
Pyrethroid spray catch (PSC) |
CDC light trap |
||||||
|---|---|---|---|---|---|---|---|---|
| Anopheles | Culex | Aedes | Total | Anopheles | Culex | Aedes | Total | |
| Ekuro | 37 | 1 | 0 | 38 | 7 | 0 | 0 | 7 |
| Ogburo | 26 | 0 | 1 | 27 | 30 | 0 | 0 | 30 |
| Ado-ori Oke | 1 | 34 | 0 | 35 | 0 | 20 | 0 | 20 |
| Total | 64 | 35 | 1 | 100 | 37 | 20 | 0 | 57 |
Table 5.
Anopheles mosquito identification and W. bancrofti detection.
|
An. gambiae |
An. arabiensis |
Culex sp |
||||
|---|---|---|---|---|---|---|
| Community | No. analysed | No. positive (%) | No. analysed | No. positive (%) | No. analysed | No. positive (%) |
| Ekuro | 44 | 1 (2.3) | 0 | 0 | 37 | 0 |
| Ogburo | 54 | 0 | 2 | 0 | 26 | 0 |
| Ado-ori Oke | 0 | 0 | 1 | 0 | 37 | 0 |
| Total | 98 | 1 (1.0) | 3 | 0 | 100 | 0 |
3.2. Loiasis prevalence, risk factors and data logger maps
Overall, loiasis prevalence determined by RAPLOA was 6.7% with 58 participants answering yes to all three RAPLOA questions. In total, 149 participants (17.1%) stated they had experienced a worm moving in their eye (question 1). Of those participants, 79 confirmed the worm with the picture provided (question 2) and of those, 58 stated that the worm was in their eye for 1 to 7 days (question 3). A summary of prevalence by demographic characteristics, intervention history and landscape characteristics is shown in Table 2.
3.2.1. Demographic characteristics
The loiasis prevalence varied across the LGAs and communities as shown in Fig. 1C. Overall, significant differences were found between the different LGAs (P = 0.023) (Table 2). The LGAs with the highest loiasis prevalence were Ede South (10.9%) and Ejigbo (7.3%) and included the high prevalence communities of Ogobi Oja (11.2%), Gaagaa (10.6%), and Agurodo (10.4%). The lowest was in Iwo LGA, Ogburo community (1.4%) and in Ayedire LGA, in Ile Iran (3.6%), and Kuta Ileogbo Railway Station (3.5%) communities. Overall, there appeared to be small geographical differences between loiasis and LF prevalence patterns, with higher loiasis communities in the eastern part of the study area and higher LF communities in the western region of the study areas as highlighted in Fig. 1D–E. Further, only one participant was found to be both loiasis and ICT positive. The participant was a 64 year old female from Ado-ori Oke community, who had lived in the community for 37 years, visited the forest weekly to farm, reported to have only taken ivermectin twice and did not own a bet net.
Females (40/521; 7.7%) were found to have higher numbers of positive loiasis cases and prevalence than males (18/349; 5.2%); however, the differences were not significant (Table 2). There was also no difference found between participants aged below or above the mean age of 49.9 years, however there was a small increasing trend with age for both females and males from the 30–39 year age group onwards as shown in Fig. 3B and D.
Loiasis prevalence was found not to vary significantly between the different occupational groups, with the farmers/trader/other group (9.6%) reporting the highest, and farmers the lowest (5.3%). Similarly, there was no distinct pattern in prevalence between the forest visit groups (Table 2).
3.2.2. Intervention history
Of those who had never taken ivermectin for onchocerciasis (n = 253), 13 were loiasis positive (5.1% prevalence), which was not significantly different to those who had taken 1–4 rounds (7.3% prevalence), and those who had ≥ 5 rounds of ivermectin (9.0% prevalence).
Similarly, no significant differences were found between the prevalence of those who owned a bed net (7.1% n = 745) and those who did not (4.0%; n = 125), or those participants who slept under the bed net the night before (7.8%; n = 502) and those who did not (6.8%; n = 243) (Table 2).
3.2.3. Landscape characteristics
There was a significant difference in loiasis prevalence between the four main classification groups of mosaic vegetation/croplands (2.5%), shrub land (10.4%), deciduous forest (6.3%) and evergreen/semi-deciduous forest (8.5%), but not when comparing the loiasis prevalence above/below the mean tree coverage or forest canopy height as shown in Table 3.
3.2.4. Loiasis GPS data logger pilot study
In total, eight loiasis positive participants and eight control participants were included in the GPS data logger analysis, as data from Ado-ori Oke and Inisa Edoro were not used due to devices not recording correctly. For loiasis positive participants, there were four males and four females, with an average age of 55 years and main occupations reported as farmer, trader and pastor. For the control participants, there were six males and two females, with an average age of 52 years and main occupations reported as farmer, trader, pastor and nurse. The participants travelled by foot as well as local transport including cars and motorcycles. All movement was included in the analysis.
Fig. 6A shows the locations where the 16 participants worked or travelled over the 4 days, highlighting the wide area covered by both groups. Overall, the average speed (km/h), and distance (km) were similar for the loiasis positive (9.1 km/h and 17.4 km), and control (10.2 km/h and 15.2 km) participants. There were similar trends in the speed and distance among the males (loiasis 7.9 km/h; 15.4 km and control 10.8 km/h; 14.9 km) and females (loiasis 10.3 km/h; 19.5 km and control 7.7 km/h; 16.7 km).
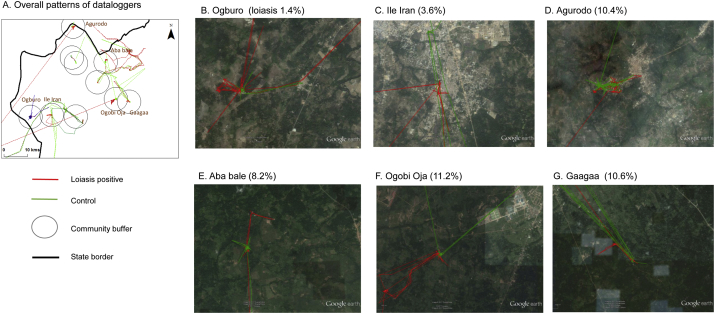
Fig. 6.
GPS data logger loiasis case and control patterns for the study area and six communities.
A. Overall patterns of data loggers
B. Ogburo (loiasis 1.4%)
C. Ile Iran (3.6%)
D. Agurodo (10.4%)
E. Aba bale (8.2%)
F. Ogobi Oja (11.2%)
G. Gaagaa (10.6%)
To better understand the environment in which people move around within the study area, a close-up examination of the loiasis positive cases and controls from six communities across different levels of loiasis prevalence and landscapes were visualised using Google Earth (Fig. 6B-G). These images highlight the denser forested areas within the higher loiasis risk communities of Aba bale, Ogobi Oja and Gaagaa. It also highlights the sparser land cover in the lower loiasis risk communities of Ogburo and Ile Iran, where the land cover was predominately mosaic vegetation/croplands with less tree canopy coverage and forest height than the other communities. The GPS data show that loiasis positive participants in Ogburo, Aba bale, Ogobi Oja and Gaagaa were in the forested areas more frequently than the controls; however, the sample size in this pilot study is too small to make any inference.
4. Discussion
This is the first survey in Osun State, Nigeria to determine the presence of two filarial infections based on a combination of WHO LF mapping and RAPLOA survey methods (World Health Organization, 2011a, Zouré et al., 2011). The micro-mapping approach used helped to delineate risk a fine scale and could help to direct targeted standard or alternative intervention strategies. The findings highlight the variation in LF and loiasis prevalence and how their distributions differ to each other within a relatively defined geographical area. Higher LF prevalences were predominately found in communities with low loiasis prevalence, and vice versa, and only one participant was found to have evidence of both infections. This supports findings from other studies highlighting the general low prevalence of W. bancrofti in L. loa areas, and that there is a general disparate or competitive exclusive relationship between different filarial parasitic infections, with little evidence of co-morbidity (Hawking, 1974, Molyneux et al., 2014). The landscape characteristics also appear to differ for each infection.
Overall, LF prevalence was low, with few participants found to be antigen positive and presumably infected or having evidence of clinical conditions. This is key information for the LF programme as the low endemicity suggests that transmission may be readily interrupted. Further, the environmental associations may help to identify risk areas, understand vector ecological habitats and target the most appropriate intervention strategy. An absence of W. bancrofti transmission was found in the more densely forested communities where loiasis was more prevalent, which may be due to some form of parasite competition as noted above, or alternatively, related to the local Anopheles vectors that may not thrive in such shaded canopied areas. Higher W. bancrofti transmission was associated with open and less vegetative landscapes, which may better suit the breeding habitats of the local vectors, including An. gambiae (Wiebe et al., 2017). Follow-up testing for nocturnal W. bancrofti mf in night blood samples was not conducted as it was out of the scope of the study, and because travelling late at night was considered to be difficult and dangerous. This is acknowledged as a limitation of the study, especially given the potential problem of ICT cross-reactivity leading to false positive results in very high-risk L. loa co-endemic areas, which was not published at the time of the study (Bakajika et al., 2014). However, given that the study area was situated on the fringe of a low loiasis endemic area, the ICT was considered still to be a valid tool (Pion et al., 2016b).
Further entomological work in this area will help to determine the extent of LF transmission in local Anopheles species (Okorie et al., 2011), and the impact of bed nets as an additional intervention strategy as recommended by the WHO in LF-loiasis co-endemic areas (Kelly-Hope et al., 2017b, World Health Organization, 2012). It may also help to determine if xenomonitoring has a role in delineating risk and endgame surveillance in GPELF (Pedersen et al., 2009). Bed net ownership and usage were associated with lower LF prevalence, suggesting they are an effective vector control tool, as shown in other studies (Nsakashalo-Senkwe et al., 2017, Rebollo et al., 2015, van den Berg et al., 2013, Richards et al., 2013). However, the LF programme could optimise this impact further by increasing coverage among the male population, as they had a higher prevalence and were less likely than their female counterparts to own a bed net. Understanding the reasons why males have a high risk of LF in this particular area of Nigeria is important. The reason may be related to work or leisure activities, or time spent sleeping outside in bush/forested areas as shown elsewhere, or related to influence of social support (Chesnais et al., 2014, Russell et al., 2015). It may also be related to the use of interventions because males are not targeted by malaria bed net campaigns, which primarily focus on pregnant women and children (although house to house distribution is carried out where possible) (Federal Ministry of Health, 2013). It is foreseeable that the scale-up of universal bed net coverage for malaria may lead to a wider reduction in LF transmission in the future (Kimura, 2011). To help monitor this, it will be essential to continue with strong collaborative links between the malaria and LF programmes (Blackburn et al., 2006, Federal Ministry of Health, 2013, Kelly-Hope et al., 2013, Molyneux and Nantulya, 2004, World Health Organization, 2011b). Key collaborative links between the onchocerciasis and LF programmes will also be important, as well as coordination with the new Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN) (Hopkins, 2016, World Health Organization, 2015).
The additional information collected on ivermectin MDA for onchocerciasis through the CDTI platform provided some insights into drug coverage and potential areas to improve. This is important for the onchocerciasis elimination plan for Osun State, which overall is making good progress (Federal Ministry of Health, 2017). Interestingly, there was lower LF prevalence among those who had participated in more MDA rounds, suggesting that CDTI may have some impact (Kyelem et al., 2003). However, of particular note, was that approximately one third of participants reported that they had never or could not recall taking ivermectin, and around half reported they had taken ivermectin in less than five MDA rounds. Notwithstanding the limitation of recall bias, the seemingly low MDA coverage highlights the need for the LF programme to implement high levels of advocacy and social mobilization to help ensure adequate coverage and impact. This is particularly important in LF-loiasis co-endemic areas where ivermectin MDA has already been implemented, and could help the onchocerciasis elimination programme. The LF programme is recommended to build on this community distribution platform; however, understanding the CDTI duration and coverage, both therapeutic and geographical, is essential to ensure safety as there could still be the risk of SAEs in untreated areas (Kelly-Hope et al., 2017b).
Overall, the loiasis prevalence was low, and within the expected range on the fringe of the meso-endemic area (Zouré et al., 2011). This suggests a limited risk of SAEs. However, identifying any potential hotspots and sub-groups at risk will be essential before the expansion of ivermectin MDA for LF elimination. The highest loiasis prevalence was found in Ede South LGA and communities herein may require more SAE vigilance than other LGAs. Higher transmission was also associated with forested landscapes with greater tree coverage and higher canopies, which better suit the breeding habitats of the main L. loa vector, Chrysops spp. (Kelly-Hope et al., 2017a). Population growth and land-use change has been associated with a decline in L. loa transmission. This was also supported by the land cover maps and satellite images, which highlight the landscape differences between high and low loiasis risk areas. It is important to note that there were some limitations with the environmental data used, as they varied in resolution and the time period covered. This was most notable with the 2000 tree cover map used for the study period; however, modelled high resolution maps indicated minimal forest cover change in the area between 2000 and 2016 (Hansen et al., 2013).
The use of modelled environmental data and satellite imagery provides new ways to understand ecological drivers of transmission, and if conducted on a larger scale, may help to further delineate risk, and implement the safest LF treatment strategy in loiasis co-endemic areas (Cano et al., 2014). Similarly, the Loa Antibody Rapid Test, a new rapid diagnostic test for detecting exposure to L. loa, may be used in the future to refine prevalence maps so that elimination programs can target intervention strategies and reduce the risk of SAEs (Pedram et al., 2017). The additional use of GPS data loggers as a novel approach to mapping geographical patterns may also help to highlight risk areas on a micro-level. Participants were willing to wear tracking devices and these could be further used to identify and map Chrysops spp. breeding sites for targeted vector control and thus transmission suppression (Kelly-Hope et al., 2017a, Seto et al., 2012). The pilot study found that loiasis positive cases and controls moved at similar speeds and distances overall; however, extending this study over a longer period, and including non-forested communities may lead to a better understanding of exposure times and locations associated with high loiasis prevalence.
This study provides key information to the Osun State LF programme on the risk and strategies for LF elimination in loiasis co-endemic areas. It highlights that the overall low transmission may be readily impacted if the CDTI platform is expanded to include albendazole, and efforts to expand bed net coverage and use are continued. The new integrated survey approach was useful to determine the extent of co-endemicity and high risk areas, and will help initiate measures to limit the occurrence or impact of SAEs. It may be that the Test-and-not-Treat strategy can be used in some high risk loiasis communities (D'Ambrosio et al., 2015). These areas can be delineated by forested landscape characteristics and mapping methods and extrapolated across the state to ensure safety and impact.
Acknowledgments
Acknowledgements
We are grateful to the community members who were willing to participate in this study, and the hard work of the supporting field team in Nigeria.
Funding
This work was supported by the grants from the Department for International Development, UK (DFID) to the Centre for Neglected Tropical Diseases (CNTD), Department of Parasitology, Liverpool School of Tropical Medicine, UK for the elimination of lymphatic filariasis as a public health problem. DHM and JRS receive support from the UK DFID as part of the COUNTDOWN NTD to the Liverpool School of Tropical Medicine (grant ID PO 6407) and DHM from GlaxoSmithKline.
Competing interests
None declared.
References
- Addiss D.G., Rheingans R., Twum-Danso N.A., Richards F.O. A framework for decision-making for mass distribution of Mectizan(R) in areas endemic for Loa loa. Filaria J. 2003;2(Suppl. 1) doi: 10.1186/1475-2883-2-S1-S9. https://doi.org/10.1186/1475-2883-2-S1-S9 S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeoye G.O., Akinsanya B., Otubanjo A.O., Ibidapo C.A., Atalabi T., Okwuzu J., Adejai E.O., Braide E.I. Prevalences of loiasis in Ondo state, Nigeria, as evaluated by the rapid assessment procedure for loiasis (RAPLOA) Ann. Trop. Med. Parasitol. 2008;102:215–227. doi: 10.1179/136485908X267867. [DOI] [PubMed] [Google Scholar]
- Bakajika D.K., Nigo M.M., Lotsima J.P., Masikini G.A., Fischer K., Lloyd M.M., Weil G.J., Fischer P.U. Filarial antigenemia and Loa loa night blood microfilaremia in an area without bancroftian filariasis in the Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 2014;91:1142–1148. doi: 10.4269/ajtmh.14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg H., Kelly-Hope L.A., Lindsay S.W. Malaria and lymphatic filariasis: the case for integrated vector management. Lancet Infect. Dis. 2013;13 doi: 10.1016/S1473-3099(12)70148-2. [DOI] [PubMed] [Google Scholar]
- Blackburn B.G., Eigege A., Gotau H., Gerlong G., Miri E., Hawley W.A., Mathieu E., Richards F. Successful integration of insecticide-treated bed net distribution with mass drug administration in Central Nigeria. Am. J. Trop. Med. Hyg. 2006;75:650–655. [PubMed] [Google Scholar]
- Boussinesq M. Loiasis. Ann. Trop. Med. Parasitol. 2006;100:715–731. doi: 10.1179/136485906X112194. [DOI] [PubMed] [Google Scholar]
- Brito M., Paulo R., Van-Dunem P., Martins A., Unnasch T.R., Novak R.J., Jacob B., Stanton M.C., Molyneux D.H., Kelly-Hope L.A. Rapid integrated clinical survey to determine prevalence and co-distribution patterns of lymphatic filariasis and onchocerciasis in a Loa loa co-endemic area: the Angolan experience. Parasite Epidemiol. Control. 2017;2:71–84. doi: 10.1016/j.parepi.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano J., Rebollo M.P., Golding N., Pullan R.L., Crellen T., Soler A., Kelly-Hope L.A., Lindsay S.W., Hay S.I., Bockarie M.J., Brooker S.J. The global distribution and transmission limits of lymphatic filariasis: past and present. Parasit. Vectors. 2014;7 doi: 10.1186/s13071-014-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnais C.B., Missamou F., Pion S.D., Bopda J., Louya F., Majewski A.C., Fischer P.U., Weil G.J., Boussinesq M. A case study of risk factors for lymphatic filariasis in the Republic of Congo. Parasites & Vectors Vectors. 2014;7:1–12. doi: 10.1186/1756-3305-7-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio M.V., Bakalar M., Bennuru S., Reber C., Skandarajah A., Nilsson L., Switz N., Kamgno J., Pion S., Boussinesq M., Nutman T.B., Fletcher D.A. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigege A., Kal A., Miri E., Sallau A., Umaru J., Mafuyai H., Chuwang Y.S., Danjuma G., Danboyi J., Adelamo S.E., Mancha B.S., Okoeguale B., Patterson A.E., Rakers L., Richards F.O. Long-lasting insecticidal nets are synergistic with mass drug administration for interruption of lymphatic filariasis transmission in Nigeria. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Ministry of Health . 2013. Guidelines for Malaria-Lymphatic Filariasis Co-implementation in Nigeria. [Google Scholar]
- Federal Ministry of Health . 2017. Nigeria Onchocerciasis Elimination Plan. [Google Scholar]
- Gardon J., Gardon-Wendel N., Demanga-Ngangue Kamgno, J., Chippaux J.P., Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- GlobCover . Eur. Sp. Agency., Univ. Cathol. Louvain. 2010. GlobCover land cover maps [WWW Document]http://due.esrin.esa.int/page_globcover.php [Google Scholar]
- Gyapong J.O., Remme J.H.F. The use of grid sampling methodology for rapid assessment of the distribution of bancroftian filariasis. Trans. R. Soc. Trop. Med. Hyg. 2001;95:681–686. doi: 10.1016/s0035-9203(01)90115-4. [DOI] [PubMed] [Google Scholar]
- Hansen M.C., Potapov P.V., Moore R., Hancher M., Turubanova S.A., Tyukavina A., Thau D., Stehman S.V., Goetz S.J., Loveland T.R., Kommareddy A., Egorov A., Chini L., Justice C.O., Townshend J.R.G. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850–853. doi: 10.1126/science.1244693. [DOI] [PubMed] [Google Scholar]
- Hawking F. Africa. World Health Organization. 1–38 WHO/FIL/74.124. 1974. The distribution of human filariasis throughout the world. Part III. [Google Scholar]
- Hopkins A.D. Neglected tropical diseases in Africa: a new paradigm. Int. Health. 2016;8(Suppl. 1):i28–i33. doi: 10.1093/inthealth/ihv077. [DOI] [PubMed] [Google Scholar]
- Kamgno J., Pion S., Bakalar M., Chesnais C., D'Ambrosio M., Kamkumo R.G., Mackenzie C.D., Mehly Ngninzeko M.S., Ngandjui N., Njitchouang G.R., Nwane P., Tchatchueng Mbouga J., Tchinde Toussi A.F., Wanji S., Fletcher D., Nutman T., Klion A., Boussinesq M. American Society of Tropical Medicine 65th Annual Meeting. Atlanta, November 13–17. 2016. Test and not treat (TNT): a safe strategy to provide community-based treatment with ivermectin in Loa loa endemic areas. [Google Scholar]
- Kelly-Hope L.A., Molyneux D.H., Bockarie M.J. Can malaria vector control accelerate the interruption of lymphatic filariasis transmission in Africa; capturing a window of opportunity? Parasit. Vectors. 2013;6 doi: 10.1186/1756-3305-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hope L.A., Cano J., Stanton M.C., Bockarie M.J., Molyneux D.H. Innovative tools for assessing risks for severe adverse events in areas of overlapping Loa loa and other filarial distributions: the application of micro-stratification mapping. Parasit. Vectors. 2014;7 doi: 10.1186/1756-3305-7-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hope L.A., Unnasch T.R., Stanton M.C., Molyneux D.H. Hypo-endemic onchocerciasis hotspots: defining areas of high risk through micro-mapping and environmental delineation. Infect. Dis. poverty. 2015;4 doi: 10.1186/s40249-015-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hope L.A., Paulo R., Thomas B., Brito M., Unnasch T.R., Molyneux D. Loa loa vectors Chrysops spp.: perspectives on research, distribution, bionomics, and implications for elimination of lymphatic filariasis and onchocerciasis. Parasit. Vectors. 2017;10 doi: 10.1186/s13071-017-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hope L.A., Stanton M.C., Zouré H.G.M., Kinvi B.E., Mikhailov A., Tekle A., King J.D. A practical approach for scaling up the alternative strategy for the elimination of lymphatic filariasis in Loa loa endemic countries - developing an action plan. Glob. Heal. Res. Policy. 2017;2 doi: 10.1186/s41256-017-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura E. The Global Programme to Eliminate Lymphatic Filariasis: history and achievements with special reference to annual single-dose treatment with diethylcarbamazine in Samoa and Fiji. Trop. Med. Health. 2011 doi: 10.2149/tmh.2010-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyelem D., Sanou S., Boatin B., Medlock J., Coulibaly S., Molyneux D.H. Impact of long-term ivermectin (Mectizan) on Wuchereria bancrofti and Mansonella perstans infections in Burkina Faso: strategic and policy implications. Ann. Trop. Med. Parasitol. 2003;97:827–838. doi: 10.1179/000349803225002462. [DOI] [PubMed] [Google Scholar]
- Molyneux D.H., Nantulya V.M. Linking disease control programmes in rural Africa: a pro-poor strategy to reach Abuja targets and millennium development goals. BMJ. 2004;328:1129–1132. doi: 10.1136/bmj.328.7448.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux D.H., Mitre E., Bockarie M.J., Kelly-Hope L.A. Filaria zoogeography in Africa: ecology, competitive exclusion, and public health relevance. Trends Parasitol. 2014;30:163–169. doi: 10.1016/j.pt.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Noma M., Nwoke B.E.B., Nutall I., Tambala P.A., Enyong P., Namsenmo A., Remme J., Amazigo U.V., Kale O.O., Sékétéli A. Rapid epidemiological mapping of onchocerciasis (REMO): its application by the African Programme for Onchocerciasis Control (APOC) Ann. Trop. Med. Parasitol. 2002;96(Suppl. 1):S29–S39. doi: 10.1179/000349802125000637. [DOI] [PubMed] [Google Scholar]
- Nsakashalo-Senkwe M., Mwase E., Chizema-Kawesha E., Mukonka V., Songolo P., Masaninga F., Rebollo M., Thomas B., Bockarie M., Betts H., Stothard J.R., Kelly-Hope L. Parasite Epidemiol. Control In Press. 2017. Significant decline in lymphatic filariasis associated with nationwide scale-up of insecticide treated nets in Zambia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunba E.O. Ecology of human loiasis in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1972;66:743–748. doi: 10.1016/0035-9203(72)90088-0. [DOI] [PubMed] [Google Scholar]
- Okorie P.N., McKenzie F.E., Ademowo O.G., Bockarie M., Kelly-Hope L. Nigeria Anopheles vector database: an overview of 100 years' research. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okorie P.N., Ademowo G.O., Saka Y., Davies E., Okoronkwo C., Bockarie M.J., Molyneux D.H., Kelly-Hope L.A. Lymphatic filariasis in Nigeria; Micro-stratification Overlap Mapping (MOM) as a prerequisite for cost-effective resource utilization in control and surveillance. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen E.M., Stolk W.A., Laney S.J., Michael E. The role of monitoring mosquito infection in the Global Programme to Eliminate Lymphatic Filariasis. Trends Parasitol. 2009;25:319–327. doi: 10.1016/j.pt.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Pedram B., Pasquetto V., Drame P.M., Ji Y., Gonzalez-Moa M.J., Baldwin R.K., Nutman T.B., Biamonte M.A. A novel rapid test for detecting antibody responses to Loa loa infections. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pion S.D., Kamgno J., Bakalar M., Bopda J., Chesnais C., D'Ambrosio M., Gonoue Kamkumo R., Mackenzie C., Mbickmen Tchana S., Clotaire Nana Djeunga H., Ngandjui N., Njitchouang G.R., Nwane P., Nutman T.B. American Society of Tropical Medicine 65th Annual Meeting. Atlanta, November 13–17th. 2016. CellScope-Loa: district-wide deployment of a point of care tool for the prevention of post ivermectin serious adverse events in Loa loa endemic areas. [Google Scholar]
- Pion S.D., Montavon C., Chesnais C.B., Kamgno J., Wanji S., Klion A.D., Nutman T.B., Boussinesq M. Positivity of antigen tests used for diagnosis of lymphatic filariasis in individuals without Wuchereria bancrofti infection but with high Loa loa microfilaremia. Am. J. Trop. Med. Hyg. 2016 doi: 10.4269/ajtmh.16-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramzy R.M., Farid H.A., Kamal I.H., Ibrahim G.H., Morsy Z.S., Faris R., Weil G.J., Williams S.A., Gad A.M. A polymerase chain reaction-based assay for detection of Wuchereria bancrofti in human blood and Culex pipiens. Trans. R. Soc. Trop. Med. Hyg. 1997;91:156–160. doi: 10.1016/s0035-9203(97)90205-4. [DOI] [PubMed] [Google Scholar]
- Rebollo M.P., Sambou S.M., Thomas B., Biritwum N.K., Jaye M.C., Kelly-Hope L., Escalada A.G., Molyneux D.H., Bockarie M.J. Elimination of lymphatic filariasis in the Gambia. PLoS Negl. Trop. Dis. 2015;9:1–16. doi: 10.1371/journal.pntd.0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F.O., Emukah E., Graves P.M., Nkwocha O., Nwankwo L., Rakers L., Mosher A., Patterson A., Ozaki M., Nwoke B.E.B., Ukaga C.N., Njoku C., Nwodu K., Obasi A., Miri E.S. Community-wide distribution of long-lasting insecticidal nets can halt transmission of lymphatic filariasis in southeastern Nigeria. Am. J. Trop. Med. Hyg. 2013;89:578–587. doi: 10.4269/ajtmh.12-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C.L., Sallau A., Emukah E., Graves P.M., Noland G.S., Ngondi J.M., Ozaki M., Nwankwo L., Miri E., McFarland D.A., Richards F.O., Patterson A.E. Determinants of bed net use in Southeast Nigeria following mass distribution of LLINs: implications for social behavior change interventions. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.A., Brogdon W.G., Collins F.H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Seto E.Y.W., Sousa-Figueiredo J.C., Betson M., Byalero C., Kabatereine N.B., Stothard J.R. Patterns of intestinal schistosomiasis among mothers and young children from Lake Albert, Uganda: water contact and social networks inferred from wearable global positioning system dataloggers. Geospat. Health. 2012;7:1–13. doi: 10.4081/gh.2012.99. [DOI] [PubMed] [Google Scholar]
- Simard M., Pinto N., Fisher J.B., Baccini A. Mapping forest canopy height globally with spaceborne lidar. J. Geophys. Res. Biogeosci. 2011;116:1–12. [Google Scholar]
- Takougang I., Meremikwu M., Wandji S., Yenshu E.V., Aripko B., Lamlenn S.B., Eka B.L., Enyong P., Meli J., Kale O., Remme J.H. Rapid assessment method for prevalence and intensity of Loa loa infection. Bull. World Health Organ. 2002;80:852–858. [PMC free article] [PubMed] [Google Scholar]
- Tekle A.H., Zoure H., Wanji S., Leak S., Noma M., Remme J.H.F., Amazigo U. Integrated rapid mapping of onchocerciasis and loiasis in the Democratic Republic of Congo: impact on control strategies. Acta Trop. 2011;120(Suppl):S81–90. doi: 10.1016/j.actatropica.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Twum-Danso N.A. Serious adverse events following treatment with ivermectin for onchocerciasis control: a review of reported cases. Filaria J. 2003;2(S3) doi: 10.1186/1475-2883-2-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanji S., Tendongfor N., Esum M., Yundze S.S., Taylor M.J., Enyong P. Combined utilisation of rapid assessment procedures for loiasis (RAPLOA) and onchocerciasis (REA) in rain forest villages of Cameroon. Filaria J. 2005;4:2. doi: 10.1186/1475-2883-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil G.J., Ramzy R.M.R. Diagnostic tools for filariasis elimination programs. Trends Parasitol. 2007 doi: 10.1016/j.pt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Wiebe A., Longbottom J., Gleave K., Shearer F.M., Sinka M.E., Massey N.C., Cameron E., Bhatt S., Gething P.W., Hemingway J., Smith D.L., Coleman M., Moyes C.L. Geographical distributions of African malaria vector sibling species and evidence for insecticide resistance. Malar. J. 2017;16 doi: 10.1186/s12936-017-1734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2010. Global Programme to Eliminate Lymphatic Filariasis: Progress Report 2000–2009 and Strategic Plan 2010–2020. [Google Scholar]
- World Health Organization . Monitoring and Epidemiological Assessment of Mass Drug Administration; Geneva: 2011. Global Programme to Eliminate Lymphatic Filariasis: A Manual for National Elimination Programmes. [Google Scholar]
- World Health Organization Integrated vector management to control malaria and lymphatic filariasis. Wkly Epidemiol. Rec. 2011;86:121–127. [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2012. Provisional Strategy for Interrupting Lymphatic Filariasis Transmission in Loiasis Endemic Countries. [Google Scholar]
- World Health Organization . 2013. Morbidity Management and Disability Prevention in Lymphatic Filariasis: an aide-mémoire for national programme managers. [Google Scholar]
- World Health Organization . 2015. Framework for the Establishment of the Expanded Special Project for Elimination of Neglected Tropical Diseases 29. [Google Scholar]
- World Health Organization Global programme to eliminate lymphatic filariasis: progress report, 2015. Wkly Epidemiol. Rec. 2016;91:73–88. [Google Scholar]
- World Health Organization . World Health Organization; 2017. Programmes and Projects: Lymphatic Filariasis [WWW Document]http://www.who.int/lymphatic_filariasis/en/ URL. [Google Scholar]
- Zouré H.G.M., Wanji S., Noma M., Amazigo U.V., Diggle P.J., Tekle A.H., Remme J.H.F. The geographic distribution of Loa loa in Africa: results of large-scale implementation of the Rapid Assessment Procedure for Loiasis (RAPLOA) PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0001210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouré H.G.M., Noma M., Tekle A.H., Amazigo U.V., Diggle P.J., Giorgi E., Remme J.H.F. The geographic distribution of onchocerciasis in the 20 participating countries of the African Programme for Onchocerciasis Control: (2) pre-control endemicity levels and estimated number infected. Parasit. Vectors. 2014;7:326. doi: 10.1186/1756-3305-7-326. [DOI] [PMC free article] [PubMed] [Google Scholar]