Abstract
Babesiosis is an economically important tick-borne apicomplexan protozoan disease of cattle in tropical and subtropical regions. In the present study, Rhipicephalus microplus engorged female ticks were collected from 135 apparently healthy cattle from different agro-climatic zones of Punjab, India, to investigate the carrier status of Babesia bigemina infection in vector tick by using microscopy and PCR based assays. PCR when applied on DNA extracted from the egg masses harvested from ticks showed 1.48% (2/135) samples as positive, whereas 4.44% (6/135) samples were positive when product of primary PCR was used as template in nPCR. Further, among the DNA samples isolated from the unfed larval stages that emerged from egg masses laid by ticks, only 1.48% (2/135) samples were detected as positive for B. bigemina in PCR, while 7.41% (10/135) samples were detected positive in nPCR assay. Statistically, non-significant (p > 0.05) difference in prevalence rates was observed across different agro-climatic zones and between different age groups of cattle from which engorged ticks were collected. It can, thus, be concluded that prevalence of B. bigemina in the vector tick, R. microplus in Punjab state of India indicates an endemic status of the organism and a further study is needed for the management and control of the bovine babesiosis.
Keywords: Babesia bigemina; Cattle; PCR; nPCR; Prevalence, Rhipicephalus microplus; Tick
1. Introduction
Bovine babesiosis is an important tick-borne intra-erythrocytic apicomplexan protozoan disease worldwide caused by the parasites of genus Babesia, infecting a wide range of domesticated and wild cattle (McCrosker, 1981). The major species of genus Babesia infecting cattle in tropics and subtropics are B. bigemina and B. bovis with B. bigemina having wider distribution (OIE, 2010), and is transmitted by cattle fever tick, Rhipicephalus microplus (Murrell et al., 2001). The transmission of Babesia organism to a susceptible bovine host depends on the successful infection of the tick midgut epithelium through the sexual stages of the parasite (Howell et al., 2007).With the reports of replacement of multi-host ticks by the one host tick, there is an increasing concern over the diseases transmitted by the common one host tick, R. microplus, particularly babesiosis and anaplasmosis in Punjab state, India (Singh and Rath, 2013).
The most commonly encountered clinical signs induced by these parasites include high grade fever, anemia, hemoglobinuria, ataxia, and sometimes death (Bock et al., 2004). A characteristic feature of Babesia infection is that animals which recover from acute infection become carriers, creating a potential source of infection to healthy susceptible population. Babesiosis is traditionally diagnosed by identification of the parasites in Giemsa stained peripheral blood smear, but it is often very difficult to detect these latent infections microscopically due to low levels of parasitaemia. Alternatively, indirect fluorescent antibody test (IFAT) and enzyme-linked immunosorbent assay (ELISA) are widely used for detection of antibodies against Babesia infections in cattle. Further, for detection of Babesia organism in vector ticks, its parasitic forms like ookinetes and sporozoites have been observed under microscope using different staining methods. Giemsa stained hemolymph of adult ticks along with egg and larval squashes have also been used for diagnosis in ticks (Bock et al., 2004, Oliveira-Sequeira et al., 2005). However, the main drawbacks of microscopic detection of Babesia spp. in blood smears, hemolymph of adult ticks, and in tick egg and larval squashes are the low sensitivity and the difficulty of differentiating between the species involved (Bock et al., 2004). Similarly, sero-diagnostics suffers from the problem of cross-reactivity and sometimes low antigen output.
Therefore, application of molecular biology techniques as PCR-based assays to study the epidemiology of babesiosis is very helpful but still incipient. These assays are characterized by high sensitivity and specificity as has been verified by several authors for the detection of infection both in the vertebrate hosts and ticks (Oliveira-Sequeira et al., 2005, Smeenk et al., 2000, Gayo et al., 2003, Oliveira et al., 2008, Tavassoli et al., 2013). However, in India, there are only few published reports regarding the use of molecular methods for the detection of B. bigemina in cattle (Singh et al., 2007, Silva et al., 2009, Chaudhry et al., 2010, Singh et al., 2013) and vector tick (Ravindran et al., 2006). Therefore, the present study was undertaken with the objective of determining the molecular prevalence of B. bigemina infection in vector tick, R. microplus in Punjab, India.
2. Materials and methods
2.1. Geographical area
The study was conducted from June 2011 to May 2013 in the northwestern Punjab state of India, covering a total area of 50,362 km2 located between 29″30′N to 32″32′N latitude and 73″55′E to 76″50′ E longitudes. The state has been divided into 5 major agro-climatic zones i.e. Central Plain Zone, Undulating Zone, Western Zone, Western plain Zone and Sub-mountain Zone. Punjab has an inland subtropical location and its climate is continental, being semi-arid to sub-humid. Summers are very hot and winters very cold with annual temperatures in range from 1 °C to 46 °C (min to max) with average annual rainfall of 565.9 mm. These environmental conditions provide favorable conditions for the survival and propagation of ticks. The major tick species infesting cross-bred cattle of the region are R. microplus and Hyalomma anatolicum (Singh and Rath, 2013, Haque et al., 2011).
2.2. Sample collection
Engorged adult female R. microplus ticks were collected from 135 cattle of different agro-climatic zones of Punjab reared under intensive production system with periodic use of chemical acaricides (cypermethrin, deltamethrin, amitraz and ivermectin) as the only tick control measure adopted in the region. A total of 10 fully engorged females ticks were collected from each animal in separate vials, closed with muslin cloth to allow air and moisture exchange and brought to the laboratory. Ticks were washed, kept individually in labeled plastic tubes covered with muslin cloth and kept in desiccators placed in BOD incubator maintained at 28 ± 1 °C and 85 ± 5% relative humidity for oviposition.
2.3. Maintenance and processing of tick
Hemolymph was collected from four ticks collected from each individual animal at 8–10 days post incubation by removing both distal legs and blotting the exuding droplet of hemolymph on separate clean microscopic glass slide. Eight hemolymph smears were made from the ticks collected from each animal, fixed in methanol for 2–3 min, stained with Giemsa and observed under oil immersion microscope for presence of any parasitic stage. The remaining engorged female ticks were incubated till completion of oviposition. The eggs laid by all the ticks collected from each animal were pooled. Approximately, 25–30 eggs from the pooled egg mass were utilized for the preparation of egg squash in duplicate, fixed in methanol for 2–3 min, stained with Giemsa and observed under oil immersion microscope for presence of any parasitic stage. A small proportion of pooled egg mass was transferred to a microcentrifuge tube and kept at − 20 °C for DNA isolation. The remaining major portion of egg mass was transferred to labeled plastic tubes covered with muslin cloth and kept in desiccators placed in BOD incubator maintained at 28 ± 1 °C and 85 ± 5% relative humidity for hatching of larvae. Around 300–400, 10–14 day old unfed larvae harvested from the pooled eggs were stored in freezer at − 20 °C and utilized for DNA extraction.
2.4. Genomic DNA isolation from tick larvae and eggs
Whole-genomic DNA was isolated from egg samples and unfed larvae of R. microplus using QIAamp® DNA mini kit (QIAGEN, GmbH, Germany) following the manufacturer's recommendations with minor modifications. In brief, approximately 100 eggs/larvae (kept at − 20 °C) were triturated in 170 μl phosphate buffer saline (pH = 7.2) and homogenate was transferred into the 1.5 ml micro centrifuge tube to which 40 μl of proteinase-K and 150 μl of ATL buffer was added and vortexed thoroughly. The homogenous suspension was incubated overnight at 56 °C, vortexed at intervals and then centrifuged for 30 s at 5000 rpm. Then, 200 μl of AL buffer was added to the lysate, vortexed and incubated at 70 °C for 20 min. Further, 200 μl of ethanol was added to the lysate, vortexed thoroughly and centrifuged at 5000 rpm for 30 s. The mixture was transfered to QIAamp spin column and centrifuged at 8000 rpm for 1.5 min. Thereafter, 2 washings were given with wash buffers (AW1 and AW2) at 8000 rpm for 2 min and 14,000 rpm for 3.5 min, respectively. Finally, genomic DNA was eluted in 150 μl of elution buffer and stored at − 20 °C till further use.
2.5. PCR assays
The primary and nested PCR assays were carried out using the sequences of oligonucleotide primers specific for B. bigemina as described by Figueroa et al. (1992). The sequences of the primers are as follows:
- Primary PCR
- BiIA: 5′ -CAT CTA ATT TCT CTC CAT ACC CCT CC- 3′
- BiIB: 5′ -CCT CGG CTT CAA CTC TGA TGC CAA AG- 3′
- Nested PCR
- BiIAN 5′ -CGC AAG CCC AGC ACG CCC CGG TGC- 3′
- BiIBN 5′ -CCG ACC TGG ATA GGC TGT GTG ATG- 3′
Two rounds of PCR in a final volume of 25 μl were carried out in a PCR thermal cycler (Eppendorf, Germany). In the primary PCR assay, the master solution consisted of 2.5 μl of 10XPCR buffer (MBI Fermentas), 0.5 μl of 10 mM dNTP mix (MBI Fermentas), 2.0 μl of 25 mM MgCl2 (MBI Fermentas), 1.0 U of recombinant Taq DNA polymerase (MBI Fermentas), 1 μl each (15 pmol) of the external forward and reverse primers and 5 μl of template DNA isolated from field samples. The volume was made up to 25 μl with nuclease-free water. The cycling conditions were as: Initial denaturation at 94 °C for 5 min, 37 cycles of denaturation at 94 °C for 1 min, annealing at 59 °C for 1 min, extension at 72 °C for 1 min and the final extension was performed at 72 °C for 10 min. In order to carry out the nested PCR assay, the PCR conditions were the same as described above, except annealing temperature of 70 °C and 1 μl of the primary PCR product was used as template and amplified with 1 μl (15 pmol), each of the internal forward and reverse primers. The PCR products, primary as well as nested, were checked for amplification by electrophoresis on a 1.5% and 1.75% agarose gel, respectively and visualized using gel documentation system (Syngene, UK). In order to check the specificity of the assay, genomic DNA of Theileria annulata, Anaplasma marginale and Trypanosoma evansi isolated from the microscopically positive cases by standard protocols were also employed in the PCR to see the amplification, if any.
2.6. Statistical analysis
Statistical analysis was performed on data by SPSS 13.0 software by applying Chi-Square test and statistical differences (p < 0.01 and p < 0.05) between various groups were calculated.
3. Results
A total of 135 tick samples were collected from cross-bred cattle of different agro-climatic zones of Punjab, India and screened for the presence of B. bigemina organism in adult and subsequent egg and larval stages. On hemolymph examination of engorged female tick (135) in quadruplicate, none of the smears revealed any developmental stage of B. bigemina. Also, none of the Giemsa stained egg squashes prepared from pooled egg masses (135) in duplicate revealed any stages of the protozoa. Concentration of the extracted DNA from egg and larval samples was found to be above 50 ng/μl. Purity of the extracted DNA was estimated by observing the ratio of OD260 and OD280 which was found to be 1.7 to 2.
Primary PCR when applied with DNA extracted from the pooled egg masses as template showed 1.48% (2/135) samples to be positive, whereas 4.44% (6/135) samples were positive when product of PCR was used as template in nested PCR (nPCR) (Fig. 1, Fig. 2). Further, among the DNA isolated from larval samples, only 1.48% (2/135) samples were detected as positive for B. bigemina in PCR, while 7.41% (10/135) samples were detected as positive in nPCR (Fig. 1, Fig. 2).
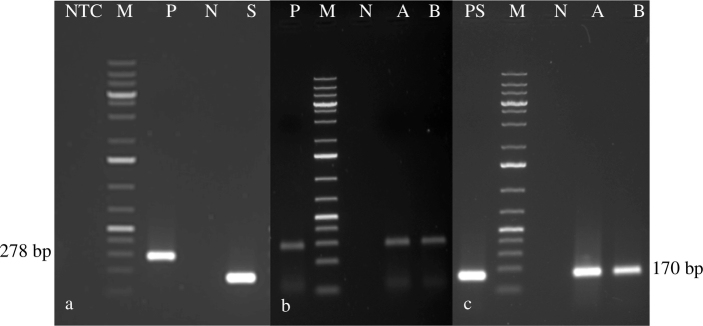
Fig. 1.
Standardization of primary PCR and nested PCR: NTC is no template control, L is 100 bp plus ladder, P is primary PCR product (278 bp), N is negative control and S is nested PCR product (170 bp), lanes A and B tick egg samples; Amplification of 170-bp fragment of Babesia bigemina by nested PCR: Lane L 100 bp plus DNA ladder, lane P positive control, lane N negative control, lanes A and B tick egg samples.
Fig. 2.
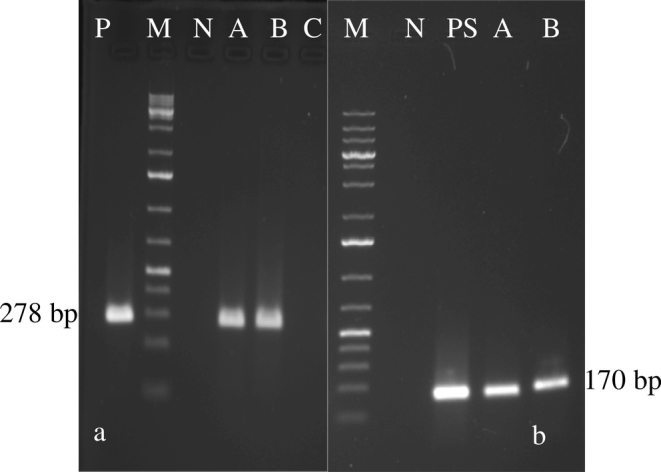
Amplification of 278-bp fragment of Babesia bigemina by primary PCR: Lane L 100 bp plus DNA ladder, lane P positive control, lane N negative control, lanes A–C larval samples; Amplification of 170-bp fragment of Babesia bigemina by nested PCR: Lane L 100 bp plus DNA ladder, lane P positive control, lane N negative control, lanes A and B larval samples.
Of engorged ticks collected from Central Plain Zone of Punjab, only 2.13% (1/47) and 4.25% (2/47) egg masses were seen positive in PCR and nPCR, respectively. On the other hand, 2.13% (1/47) larval samples in PCR and 6.38% (3/47) in nPCR were found to be positive. From Undulating Plain Zone, none of the egg masses was detected positive by PCR, while 4.55% (1/22) were found positive by nPCR. PCR detected all 22 larval samples as negative, while nPCR revealed 9.09% (2/22) as positive. Among tick samples collected from Western Zone, 3.03% (1/33) egg masses harvested were found positive in nPCR and thereafter, 6.06% (2/33) of unfed larval samples harvested were found positive in nPCR. Whereas none of egg masses were found positive in PCR or nPCR from Western Plain Zone, 5.88% (1/17) larval samples were revealed positive in nPCR. Among egg masses and larvae harvested from ticks of Sub-mountain Undulating Zone, 6.25% (1/16) and 12.5% (2/16) in both cases were observed as positive in PCR and nPCR, respectively (Table 1).
Table 1.
Agro-climatic zone wise prevalence of B. bigemina in tick progeny.
| Agro-climatic zone | No. of samples collected | Percentage positivity (%) |
|||
|---|---|---|---|---|---|
| Egg mass |
Unfed larvae |
||||
| PCR | nPCR | PCR | nPCR | ||
| Central Plain | 47 | 1 (2.13) |
2 (4.25) |
1 (2.13) |
3 (6.38) |
| Undulating Plain | 22 | 0 (0.0) |
1 (4.55) |
0 (0.0) |
2 (9.09) |
| Western | 33 | 0 (0.0) |
1 (3.03) |
0 (0.0) |
2 (6.06) |
| Western plain | 17 | 0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (5.88) |
| Sub-mountain undulating | 16 | 1 (6.25) |
2 (12.5) |
1 (6.25) |
2 (12.5) |
| Total | 135 | 2 (1.48) |
6 (4.44) |
2 (1.48) |
10 (7.41) |
| χ2 value (p > 0.05) (b/w zones) | 1.140 | 2.058 | |||
| χ2 value (p < 0.05) (b/w tests) | 15.947* | 5.745* | |||
Among 43 egg masses harvested from engorged female ticks collected from the cattle of 0–6 month age, 2.32% (1/43) were found positive in nPCR, whereas all of these samples were found negative in PCR. After harvesting larvae, 6.98% (3/43) batches were seen positive in nPCR. Among 92 egg masses harvested from ticks collected from the cattle of above 6 months age, 2.17% (2/92) and 5.43% (5/92) were found positive in PCR and nPCR, respectively, and among 92 larval batches, 2.17% (2/92) and 7.61% (7/92) samples were detected as positive in PCR and nPCR, respectively (Table 2).
Table 2.
Age-wise prevalence of B. bigemina in tick progeny.
| Age group | Percentage positivity (%) |
|||
|---|---|---|---|---|
| Egg masses |
Unfed larvae |
|||
| PCR | nPCR | PCR | nPCR | |
| Calves 0–6 months (n = 43) |
0 (0) |
1 (2.32) |
0 (0) |
3 (6.98) |
| Adult cattle > 6 months (n = 92) |
2 (2.17) |
5 (5.43) |
2 (2.17) |
7 (7.61) |
| Total | 2 (1.48) |
6 (4.44) |
2 (1.48) |
10 (7.41) |
| χ2 (p > 0.05) (b/w age groups) | 0.017 | 0.013 | ||
4. Discussion
As per 18th livestock census 2007, India has a population of about 200 million cattle and Punjab state possesses approximately 17,77,000 cattle, out of which 12,78,000 are cross-bred. Punjab has bagged top position in dairy farming during the recent years, with milk production of about 95,51,000 ton/year which contributes about 7.5% to the overall milk production from the country (Department of Animal Husbandry, Dairying and Fisheries, Ministry of Agriculture, Government of India). Ticks and tick borne diseases pose major health problem of crossbred cattle population of this region with an overall prevalence of ixodid ticks as 58.0% (Singh and Rath, 2013). The major tick species infesting crossbred cattle of Punjab state are R. microplus and Hyalomma anatolicum (Singh and Rath, 2013, Haque et al., 2011). They were ranked high in terms of their impact on the livelihood resource of poor farming communities in developing countries (Perry et al., 2002, Minjauw and Mcleod, 2003). In India, the losses due to tick and tick borne diseases in animals have been estimated to the tune of US$ 498.7 million per annum (Minjauw and Mcleod, 2003).
In the Indian subcontinent, it is assumed that about 80% of the herd resides in the areas endemic for Babesia and Anaplasma infections, hence, need arises to conduct epidemiological surveys in order to identify these areas (Ravindran et al., 2002). In this regard, identification of healthy carrier animals exhibiting low parasitaemia and detection of disease causing agent in vector tick with more sensitive tests becomes the need of the hour. Many PCR based assays have been optimized for the detection of B. bigemina in R. microplus tick using tick hemolymph, eggs and larvae as specimen by various workers worldwide (Oliveira-Sequeira et al., 2005, Smeenk et al., 2000, Gayo et al., 2003, Oliveira et al., 2008, Tavassoli et al., 2013, Mahoney and Mirre, 1971) including India (Ravindran et al., 2006). The present study was undertaken to determine the occurrence B. bigemina organism in vector tick, R. microplus. Although the tick sampling was not much comprehensive in the current study, a comparatively high percentage of the R. microplus progeny was found positive for B. bigemina DNA by nested PCR assay. Statistically, significant (p < 0.05) difference was seen between overall prevalence values recorded by PCR and nPCR. Smeenk et al. (Smeenk et al., 2000), in Zimbabwe, reported relatively high prevalence of B. bigemina DNA in B. microplus as compared to the reports of Mahoney and Mirre (1971). Further, as all Giemsa stained hemolymph smears and egg squashes were found negative for parasitic stages, it indicates that although hemolymph infection may be undetectable, transmission to larval progeny occurs at a level that assures transmission to the bovine host, as has been reported earlier by Howell et al. (2007).
Although relatively higher prevalence was seen in tick progeny harvested from female ticks that fed on adult cattle than those that fed on young cattle, the difference was statistically non-significant. As regards age, higher rate of infection in the egg masses and larvae collected from adult cattle could be related to subclinical infection or the lack of active immunity. The presence of foetal haemoglobin (HbF) in the calves has been cited as possible reason for increased resistance since HbF is considered one of the factors contributing to the high resistance of young cattle against B. bovis infection (Ristic and Levi, 1981). But higher rate of B. bigemina infection in engorged female ticks collected from young calves has also been reported (Oliveira-Sequeira et al., 2005). With respect to agro-climatic zones, comparatively higher prevalence rate, but statistically non-significant, was recorded from tick progeny belonging to Sub-mountain undulating zone and Undulating zone both in PCR and nPCR. A trend of gradual decrease in prevalence values was seen while moving spatially from northeast towards southwest parts of the state. Apart from climatic variation, it could be attributed to sampling fluctuations, seasonal variations and differential distribution of ticks and B. bigemina organism across different zones.
In the present study, the nPCR revealed significantly higher number of samples to be positive in tick progeny than microscopic examination and PCR. Regardless of the high sensitivity and specificity of PCR and nPCR, the lower prevalence in tick stages may be attributed to presence of hard vector tissues which may interfere with the extraction of whole genome DNA from tick eggs and larvae as compared to blood or any other soft tissue. In addition, the variation in the parasitemia levels during the infection course of B. bigemina in vertebrate host and vector tick may affect the detection of parasite DNA (Bock et al., 2004, Bose et al., 1990). Supplemented with the high prevalence in cattle reports from the region, the presence of B. bigemina infection in vector tick detected in primary and nested PCR in present study indicate a situation of forthcoming endemic stability.
5. Conclusion
The use of PCR and nested PCR assays for the parasite infection in vector ticks in the present study appears to be first of its kind in India in general and Punjab state in particular. The presence of B. bigemina in vector tick, R. microplus in Punjab state of India accentuates the importance of a further comprehensive that will likely be very beneficial for management and control programs of the disease.
Acknowledgments
Authors are thankful to The Dean, Postgraduate Studies, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana for providing facilities to carry out the research work.
References
- Bock R., Jackson L., de Vos A., Jorgensen W. Babesiosis of cattle. Parasitology. 2004;129:247–269. doi: 10.1017/s0031182004005190. [DOI] [PubMed] [Google Scholar]
- Bose R., Jacobson R.H., Gale K.R., Waltisbuhl D.J., Wright I.G. An improved ELISA for the detection of antibodies against Babesia bovis using either a native or a recombinant B. bovis antigen. Parasitol. Res. 1990;76:648–652. doi: 10.1007/BF00931081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry Z.I., Suleman M., Younus M., Aslim A. Molecular detection of Babesia bigemina and Babesia bovis in crossbred carrier cattle through PCR. Pak. J. Zool. 2010;42:201–204. [Google Scholar]
- Figueroa J.V., Chieves L.P., Johnson G.S., Buening G.M. Detection of Babesia bigemina infected carriers by polymerase chain reaction amplification. J. Clin. Microbiol. 1992;30:2578–2582. doi: 10.1128/jcm.30.10.2576-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayo V., Romito M., Nel L.H., Solari M.A., Viljoen G.J. PCR based detection of the transovarial transmission of Umguayam Babesia bovis and Babesia bigemina vaccine strains. Onderstepoort J. Vet. Res. 2003;70:197–204. [PubMed] [Google Scholar]
- Haque M., Jyoti J., Singh N.K., Rath S.S., Ghosh S. Epidemiology and seasonal dynamics of Ixodid ticks of dairy animals of Punjab state, India. Indian J. Anim. Sci. 2011;81:661–664. [Google Scholar]
- Howell J.M., Ueti M.W., Palmer G.H., Scoles G.A., Knowles D.P. Transovarial transmission efficiency of Babesia bovis tick stages acquired by Rhipicephalus (Boophilus) microplus during acute infection. J. Clin. Microbiol. 2007;45:426–431. doi: 10.1128/JCM.01757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney D.F., Mirre G.B. Bovine babesiosis: estimation of infection rates in the tick vector Boophilus microplus (Canestrini) Ann. Trop. Med. Parasitol. 1971;65:309–317. doi: 10.1080/00034983.1971.11686759. [DOI] [PubMed] [Google Scholar]
- McCrosker . Academic Press, Inc.; New York: 1981. The Global Importance of Babesiosis; pp. 1–24. [Google Scholar]
- Minjauw B., Mcleod A. Centre for Tropical Veterinary Medicine, University of Edinburgh; UK: 2003. Tick-borne Diseases and Poverty, the Impact of Ticks and Tick-borne Diseases on the Livelihoods of Small-scale and Marginal Livestock Owners in India and Eastern and Southern Africa, Research Report, DFID Animal Health Programme. [Google Scholar]
- Murrell A., Campbell N.J.H., Barker S.C. A total-evidence phylogeny of ticks provides insights into the evolution of life cycles and biogeography. Mol. Phylogenet. Evol. 2001;21:244–258. doi: 10.1006/mpev.2001.1018. [DOI] [PubMed] [Google Scholar]
- OIE . 2010. Terrestrial Manual, Bovine Babesiosis. (Chapter 2.4.2.) [Google Scholar]
- Oliveira M.C.S., Oliveira-Sequeira T.C.G., Regitano L.C.A., Alencar M.M., Ne'o T.A., Silva A.M. Detection of Babesia bigemina in cattle of different genetic groups and in Rhipicephalus (Boophilus) microplus tick. Vet. Parasitol. 2008;155:281–286. doi: 10.1016/j.vetpar.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Oliveira-Sequeira T.C.G., Oliveira M.C.S., Araujo J.P., Jr., Amarante A.F.T. PCR-based detection of Babesia bovis and Babesia bigemina in their natural host Boophilus microplus and cattle. Int. J. Parasitol. 2005;35:105–111. doi: 10.1016/j.ijpara.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Perry B.D., Randolph T.F., Mcdermott J.J., Sones K.R., Thornton P.K. International Livestock Research Institute; Nairobi, Kenya: 2002. Investing in Animal Health Research to Alleviate Poverty. [Google Scholar]
- Ravindran R., Mishra A.K., Rao J.R. On the high seroprevalence of bovine babesiosis in Wynad district of Kerala. J. Appl. Anim. Res. 2002;22:43–48. [Google Scholar]
- Ravindran R., Rao J.R., Mishra A.K. Detection of Babesia bigemina DNA in ticks by DNA hybridization using a nonradioactive probe generated by arbitrary PCR. Vet. Parasitol. 2006;141:181–185. doi: 10.1016/j.vetpar.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Ristic M., Levi M.G. A new era of research towards solution of bovine babesiosis. In: Ristic M., Kreier J.P., editors. Babesiosis. Academic Press; San Diego: 1981. pp. 509–562. [Google Scholar]
- Silva M.G., Henriques G., Sanchez C., Marques P.X., Suarez C.E., Oliva A. First survey for Babesia bovis and Babesia bigemina infection in cattle from central and southern regions of Portugal using serological and DNA detection methods. Vet. Parasitol. 2009;166:66–72. doi: 10.1016/j.vetpar.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Singh N.K., Rath S.S. Epidemiology of ixodid ticks in cattle population of various agro-climatic zones of Punjab. Asian Pac J Trop Med. 2013;6:947–951. doi: 10.1016/S1995-7645(13)60169-8. [DOI] [PubMed] [Google Scholar]
- Singh H., Mishra A.K., Rao J.R., Cheema P.S., Sankar M. Polymerase chain reaction for diagnosis of Babesia bigemina. Indian Vet. J. 2007;84:346–348. [Google Scholar]
- Singh H., Jyoti J., Haque M., Singh N.K., Rath S.S. Polymerase Chain Reaction based detection of subclinical bovine babesiosis in Punjab, Indian. J. Anim. Res. 2013;47:543–546. [Google Scholar]
- Smeenk J., Kelly P.J., Wray K., Musuka G., Tress A.J., Jongejan F. Babesia bovis and B. bigemina DNA detected in cattle and ticks from Zimbabwe by polymerase chain reaction. J. S. Afr. Vet. Assoc. 2000;71:21–24. doi: 10.4102/jsava.v71i1.671. [DOI] [PubMed] [Google Scholar]
- Tavassoli M., Tabatabaei M., Mohammadi M., Esmaeilnejad B., Mohamadpour H. PCR-based detection of Babesia spp. infection in collected ticks from cattle in West and North-West of Iran. J. Arthropod. Borne Dis. 2013;7:132–138. [PMC free article] [PubMed] [Google Scholar]