Abstract
Background
Reducing drug resistance in tumor cells has become an important issue for cancer treatment. The purpose of this study was to investigate whether IL-22 was involved in lung cancer cell resistance to paclitaxel (PTX), and to explore the underlying molecular mechanism.
Material/Methods
Non-small cell lung cancer (NSCLC) cell line A549 and the drug resistant cell line A549/PTX were used in the present study. The inhibitory rate of PTX on A549 and A549/PTX cell proliferation was determined by MTT assay and the half-maximal inhibitory concentration (IC50) value was calculated. The expression level of IL-22 was detected using Western blot and qRT-PCR. To elucidate the mechanism by which IL-22 is involved in PTX resistance, a stable IL-22-silenced A549/PTX cell line was generated by using IL-22-siRNA. Cell apoptosis was analyzed by flow cytometry, and the c-Jun N-terminal kinase (JNK) signal pathway was determined using Western blot analysis.
Results
We found that IL-22 expression level was markedly higher in A549/PTX cells than in A549 cells, and IL-22 gene knockdown significantly enhanced the cell proliferation inhibition rate of PTX to A549/PTX cells and decreased the IC50 value of PTX to A549/PTX cells, indicating IL-22 was involved in cell PTX resistance. Our findings also suggest that IL-22 knockdown notably increased PTX induced apoptosis in A549/PTX cells. Moreover, the results showed that p-JNK and Caspase 3 expression were significantly increased in IL-22 knockdown A549/PTX cells, while Bcl-2 expression was significantly decreased.
Conclusions
IL-22 is involved in A549 cell resistance to PTX through regulating cell apoptosis via the JNK signaling pathway.
MeSH Keywords: Apoptosis; Carcinoma, Non-Small-Cell Lung; Drug Resistance; Paclitaxel
Background
Lung cancer is the most common malignancy in the world, with the highest rate of mortality and a very low survival rate [1]. According to GLOBOCAN data, about 1.8 million patients were diagnosed with lung cancer worldwide in 2012, accounting for about 13% of the total number of new cases of cancer [2]. In China, lung cancer is reported to have the highest morbidity and mortality among all cancers [3]. Non-small cell lung cancer (NSCLC) is the most frequent malignant lung cancer, with a high percentage of distant metastases in initial staging [4]. The rate of brain metastasis has been reported to be up to 43% [5, 6]. Cough, shortness of breath, weight loss, loss of appetite, and chest tightness seriously impair the quality of life [7]. The current treatment method for NSCLC is mainly surgical resection combined with chemotherapy and radiotherapy [8]. However, there are many postoperative complications after surgical resection, such as tumor cells metastases and systemic inflammatory response [9,10]. It is well known that, with the administration of chemotherapeutic agents, cancer cells can undergo adaptive changes and update their drug resistance ability, thus reducing the efficacy of chemotherapy drugs [11]. In recent years, target therapy using RNAi as a novel method has attracted much attention as it reduces the risk of target-related adverse effects, particularly for specific diseases [12–15].
Interleukin-22 (IL-22), a member of the IL-10 cytokine family, only acts on interleukin-22 receptor 1 (IL-22-R1)-positive epithelial cells [16]. As a pro-inflammatory factor, IL-22 is involved in the pathogenesis of rheumatoid arthritis and inflammatory bowel disease [17,18]. In addition, more and more studies have revealed the abnormal expression of IL-22 in various malignant tumor tissues or cell lines, and it can act as both an oncogene and a tumor suppressor gene. Zhang et al. suggested that IL-22 production improved the survival of human lung cancer cells and resistance to chemotherapy via up-regulating the expression of antiapoptotic proteins [19]. Bi et al. found that administration of IL-22 promoted tumor cell proliferation, migration, and invasion. Furthermore, they suggested that knockdown of IL-22R1 by siRNA completely eliminated the changes of NSCLC cell proliferation and migration caused by IL-22 treatment [20]. Moreover, studies have revealed that the expression level of IL-22 in tumor tissue and serum was related to the drug resistance of FOLFOX [21]. These findings indicate that IL-22 plays a critical role in the development and progression of NSCLC, and the level of IL-22 might be related to chemotherapy resistance.
PTX, a chemotherapy drug, can be effective against malignant NSCLC [22], and the most encouraging effects are observed in cancer chemotherapy for refractory ovarian cancer [23]. But there is also growing evidence suggesting that cancer cells are resistant to PTX during treatment [24], thereby reducing the therapeutic effect. Therefore, research on the mechanism of anti-cancer drug resistance is very important.
In the present study, we aimed to determine whether IL-22 plays a role in drug resistance of PTX, and to explore the possible molecular mechanism by which IL-22 functions in PTX resistance, in an effort to provide a theoretical basis for improving the clinical efficacy of PTX in the treatment of NSCLC.
Material and Methods
Cell culture
The human lung adenocarcinoma epithelial cell line A549 was supplied by the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin mix solution (Sigma, Poole, United Kingdom). The PTX-resistant cell line (A549/PTX) was purchased from KeyGEN biotech (Nanjing, China) and grown in complete 1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin mix solution, and 100 ng/mL PTX (Sigma, Poole, United Kingdom). Both cell lines were maintained at 37°C with 5% CO2.
Transfection of IL-22-siRNA
For cell transfection, Lipofectamine2000 transfection reagent (Invitrogen) was used. IL-22-siRNA or the negative control siRNA (Santa Cruz, CA, USA) was transiently transfected into A549/PTX cells using 20 μL transfection reagent (Santa Cruz, CA, USA) following the manufacturer’s instructions. After incubation for 48 h, the cells were used for the following analyses.
MTT assay
The cell proliferation inhibition rate of PTX to A549 and A549/PTX cells was measured by MTT assay. Briefly, after washing with PBS, the A549 and A549/PTX cells were re-suspended in a final concentration of 1×104/mL in RPMI-1640 medium. Cells were then seeded into a 96-well plate and cultured at 37°C for 24 h, then the cells were treated with various concentrations of PTX (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL) for 48 h. Subsequently, MTT (5 mg/mL) was added to each well and incubated at 37°C for 4 h. Finally, DMSO was used to terminate the reaction. A SynergyTM 2 Multi-function Microplate Reader (Bio-Tek Instruments, Winooski, VT, USA) was used to detect the optical absorbance (OD) at 490 nm. Cell proliferation inhibition rate and the IC50 value were calculated. Experiments were done in triplicate.
Western blot analysis
For protein level detection, Western blot analysis was performed. Total cellular proteins were extracted by using RIPA buffer (Sigma). The protein concentration was detected using Bio-Rad protein assay (Bio-Rad Laboratories). Protein extracts were subjected to electrophoresis on a 12% SDS-PAGE and then electrophoretically transferred onto nitrocellulose membranes. Subsequently, the membranes were blocked with 5% skim milk at room temperature for 1 h, and then successively incubated with specific antibodies for 3 h. After washing 3 times with TBS-Tween 20, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. At the end of the experiment, an enhanced chemiluminescence detection system (Super Signal West Dura Extended Duration Substrate; Pierce Chemical) was used out to visualize the protein bands.
qRT-PCR
Total RNA was extracted from A549 and A549/PTX cells using TRIzol regent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer’s protocol. RNA was quantified by measuring 260 nm/280 nm using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Inc.). The PrimeScript reverse transcription reagent kit (Takara Biotechnology Co., Ltd., Dalian, China) was used to synthesize cDNAs according to the manufacturer’s instructions. Subsequently, the TaqMan Universal PCR Master Mix kit (Thermo Fisher Scientific, Inc.) was used to analyze the cDNAs. The amplification conditions were 95°C for 10 min, followed by 37 cycles of 95°C for 10 s and 56°C for 60 s. Primer sequences for qPCR were as follows:
GAPDH-Forward: 5′CTTTGGTATCGTGGAAGGACTC3′;
GAPDH-Reverse: 5′GTAGAGGCAGGGATGATGTTCT3′;
IL-22-Forward: 5′GCTTGACAAGTCCAACTTCCA3′;
IL-22-Reverse: 5′GCTCACTCATACTGACTCCGT3′.
GAPDH was used as an internal control. The 2−ΔΔCq method was used to quantify relative gene expressions [25].
Flow cytometry assay
Annexin V/propidium iodide double-staining method was used to analyze cell apoptosis. At 48 h after cell transfection, cells were treated with 5 μl PTX for 24 h. Then, cells were labeled with annexin V-FITC and propidium iodide (PI) for cell apoptotic rate detection following the manufacturer’s instructions. Flow cytometry (Becton Dickinson, New Jersey, USA) was performed to analyze the cells. Each experiment was independently repeated at least 3 times.
Statistical analysis
All quantitative data are displayed as the mean ± standard deviation (SD). Student’s t-test or ANOVA was carried out to make comparisons between groups. For all statistical analyses, a value of p<0.05 was identified as statistically significant.
Results
A549 and A549/PTX cell line culture results
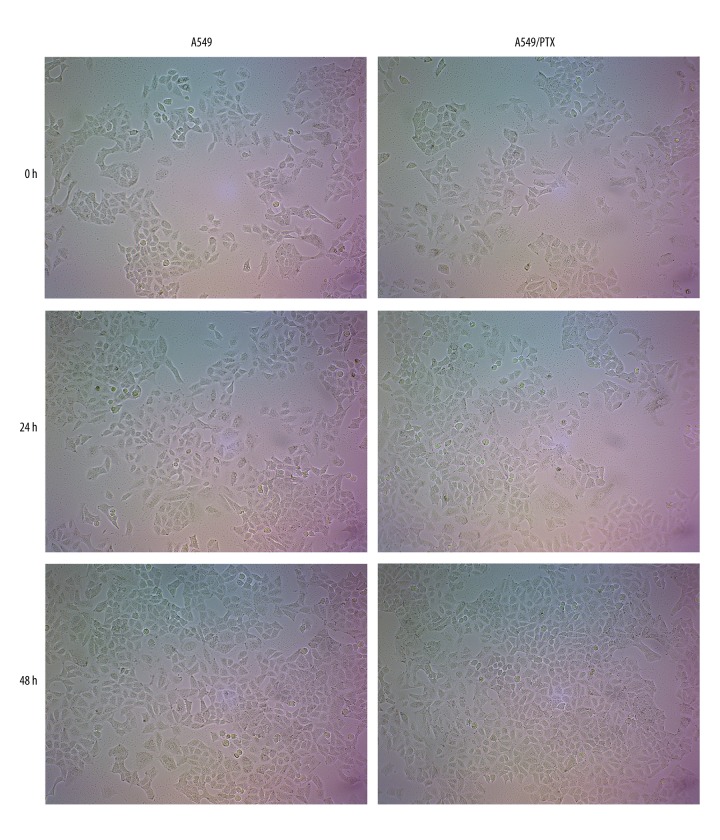
A549 and A549/PTX cells were chosen to perform our present investigation. Cell growth can be judged by cell morphology. According to Figure 1, under the 10× optical microscope, A549 and A549/PTX cell appearance was regular spindle and adherent growth. After 24 h, the number of cells increased significantly and cell morphology was normal. After 48 h, the cells reached almost 95% confluent state. These results suggested that all cells remained healthy under our culture conditions.
Figure 1.
A549 and A549/PTX cell culture results. Cell morphology was observed and photographed under the 10× optical microscope.
A549/PTX was confirmed to be PTX-resistant
A549 and A549/PTX cells were treated with different concentrations of PTX (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL) for 24 h, then cell proliferation inhibition rate was detected and IC50 value was calculated. As shown in Figure 2, PTX inhibited A549 and A549/PTX cell proliferation in a dose-dependent manner. The IC50 value of PTX to A549 cells was 39.97 μM, while it was 60.40 μM in A549/PTX cells. The data indicated that A549/PTX cells were resistant to PTX treatment.
Figure 2.
Cell proliferation inhibition rate and IC50 between A549 and A549/PTX cells. A549 and A549/PTX cells were treated with different concentrations of paclitaxel (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL) for 24 h. Then, cell proliferation inhibition rate was detected by MTT assay, and the IC50 value was calculated.
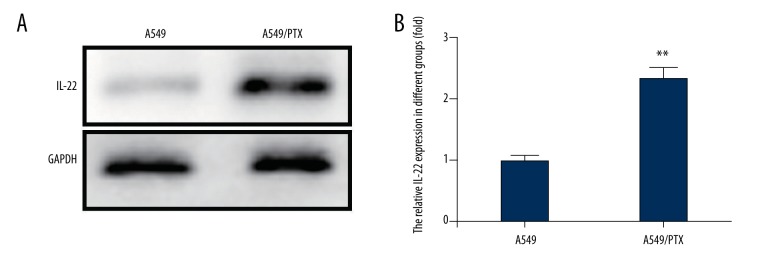
IL-22 was significantly up-regulated in A549/PTX cells
Previous studies have shown that IL-22 plays critical roles in tumor progression. To study whether IL-22 is involved in lung cancer cell resistance to PTX, we detected the IL-22 expression level in A549 and A549/PTX cells. As shown in Figure 3, the protein and mRNA levels of IL-22 in A549/PTX cells were significantly higher than in A549 cells. This result was consistent with the previous reported findings. Thus, IL-22 might be closely associated with PTX resistance in A549/PTX cells.
Figure 3.
IL-22 expression in A549 and A549/PTX cells. (A) The IL-22 protein expression level in A549 and A549/PTX cells was detected using western blot; (B) Relative mRNA expression level of IL-22 was measured by qRT-PCR. ** p<0.05.
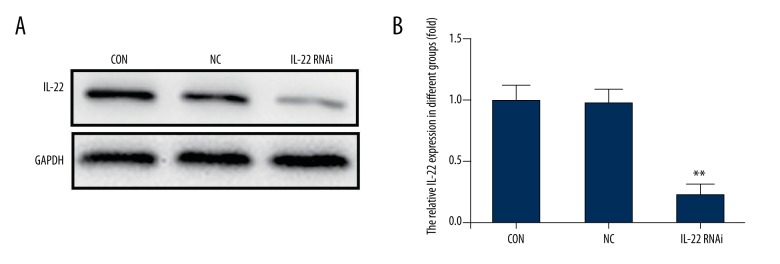
To explore how IL-22 participates in drug resistance, IL-22 was knocked-down in A549/PTX cells by transfection with IL-22-siRNA. The transfection efficiency was determined by Western blot assay and qRT-PCR. The Western blotting results suggested that after transfection with IL-22-siRNA, the protein expression level of IL-22 was significantly down-regulated in A549/PTX cells. No significant difference was found between the control group and the negative control group. The results of qRT-PCR were consistent with the Western blotting results (Figure 4).
Figure 4.
IL-22 was knocked-down in A549/PTX cells. A549/PTX cells were transfected with IL-22-siRNA and the negative control siRNA respectively. Cells without any treatment were used as the control group. At 48 h after cell transfection, the expression level of IL-22 was determined. (A) The IL-22 protein expression level in A549/PTX cells was detected using Western blot; (B) Relative mRNA expression level of IL-22 was measured by qRT-PCR. ** p<0.05. Con – control; NC – negative control.
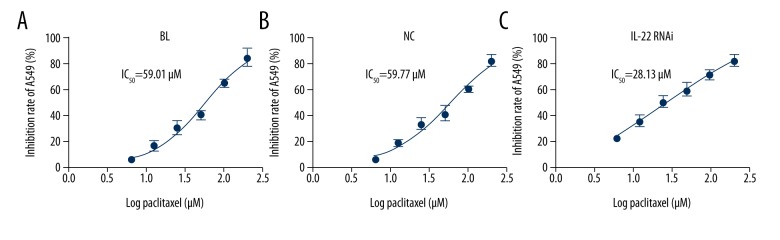
IL-22 knockdown proved the sensitivity of A549/PTX cells to PTX
After specific treatment, cell proliferation inhibition rate and IC50 value of PTX to A549/PTX cells were determined. Compared with the negative control group and control group, the cell proliferation inhibition rate was notably enhanced in the IL-22 knockdown group. The IC50 values of PTX to A549/PTX cells in the 3 groups were 59.01, 59.77, and 28.13 μM, respectively (Figure 5), indicating that the sensitivity of A549/PTX to PTX was significantly improved after transfection of IL-22-siRNA.
Figure 5.
Effect of IL-22 on the cell proliferation inhibition rate and IC50 of PTX to A549/PTX cells. At 48 h after cell transfection, A549/PTX cells were treated with different concentrations of paclitaxel (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL) for 24 h, then cell proliferation inhibition rate was detected by MTT assay, and the IC50 value was calculated. ** p<0.05. Con – control; NC – negative control.
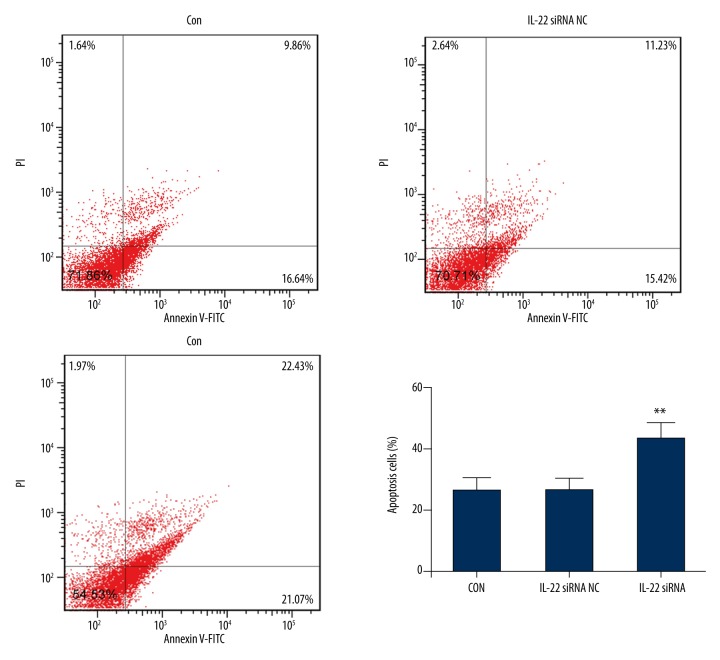
IL-22 knockdown increased apoptosis in A549/PTX cells
To investigate the influences of IL-22 on cell apoptosis of A549/PTX cells, Annexin V-FITC/PI double-staining was performed. The findings suggested that compared with the control group and the negative control group, the apoptosis rate of IL-22-siRNA group was markedly increased (p<0.05). No significant difference was found between the control group and the negative control group (Figure 6).
Figure 6.
IL-22 knockdown increased apoptosis in A549/PTX cells. At 48 h after cell transfection, A549/PTX cells were treated with different concentrations of paclitaxel (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL) for 24 h, then cell apoptosis (early apoptosis + late apoptosis) was analyzed by FCM. ** p<0.05. Con – control; NC – negative control.
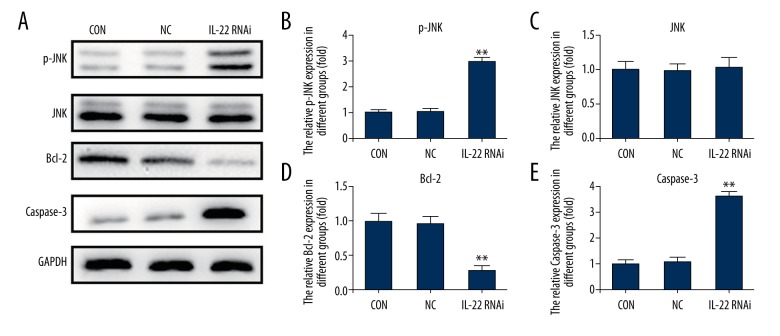
IL-22 modulated JNK signaling pathway
To explore the underlying molecular mechanism of the role of IL-22 cell PTX resistance, we assessed the JNK signaling pathway. The results showed that IL-22-siRNA transfection activated the JNK signaling pathway, as p-JNK expression was notably increased in IL-22 knockdown A549/PTX cells. Moreover, we found that compared with the control group and the negative control group, Caspase 3 expression was significantly increased in IL-22 knockdown A549/PTX cells, while Bcl-2 expression was significantly decreased (Figure 7).
Figure 7.
IL-22 modulated JNK signaling pathway. At 48 h after cell transfection, A549/PTX cells were treated with different concentrations of paclitaxel (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL) for 24 h, then the JNK signaling pathway was analyzed. (A) The protein expression level of p-JNK, Caspase3, and Bcl-2 in A549/PTX cells was detected using Western blot; (B–E) p-JNK, Caspase3, and Bcl-2 protein expression was quantified and expressed as fold of the control; ** p<0.05. Con – control; NC – negative control.
Discussion
Drug resistance is the major factor that affects the efficacy of chemotherapy in patients with lung cancer. There are many factors that affect the resistance of NSCLC drug resistance, including the increased expression of resistant multidrug (MDR) gene and multidrug resistance-associated protein (MRP) genes and their products, as well as the decreased expression of Topoisomerase II (Topo II) and Glutathione (GSH) and elevation of the glutathione S transferase (GST) system. In addition, studies have shown that the abnormal expression of related factors in cell signal transduction, DNA repair abnormality in tumor cells, and the aberrant expression of other related genes are closely related to lung cancer drug resistance [26,27].
In recent years, inflammatory factors in the tumor micro-environment have been confirmed to play important roles in the development and progression of tumors [28,29], and this topic has attracted much attention. IL-22, a pro-inflammatory factor, is abnormally expressed in a variety of malignancies, and it has been suggested to be associated with chemotherapy drug resistance [21]. PTX-based chemotherapy is a standard treatment for NSCLC, but treatment often fails due to drug resistance. Therefore, in this study, we assessed whether IL-22 was involved in NSCLC cell resistance to PTX, and explored the underlying molecular mechanism. We first determined that the cell lines were able to grow normally under our culture conditions, and A549/PTX cells were resistant to PTX treatment. Further analysis indicated that IL-22 expression level was significantly higher in A549/PTX cells than in A549 cells. IL-22 gene knockdown significantly promoted the cell proliferation inhibition rate of PTX to A549/PTX cells, decreased the IC50 value of PTX to A549/PTX cells, and increased PTX induced apoptosis in the A549/PTX cells. These findings indicate that IL-22 is involved in cell PTX resistance.
Evidence has revealed that the c-Jun N-terminal kinases (JNK) signaling pathway is activated in tumor cells after PTX treatment, and JNK signaling activation is associated with PTX sensitivity [30,31]. In addition, the binding of IL-22 to its specific receptor IL-22R can induce JNK pathway activation in tumor cells, thus playing a role in regulating the biological function of tumor cells [32,33]. To explore the underlying molecular mechanism of the role IL-22 plays in cell PTX resistance, the JNK signaling pathway was analyzed in the present study. The results suggest that IL-22 knockdown activates the JNK signaling pathway.
We reported the effect of IL-22 on NSCLC paclitaxel resistance for the first time, and the data indicated that IL-22 was involved in A549 cell resistance to PTX through regulating cell apoptosis via the JNK signaling pathway.
Conclusions
IL-22 may serve as a novel therapeutic target in NSCLC treatment in the future, as well as an effective molecular marker to predict the sensitivity of patients to chemotherapy drugs, which can better guide the individual treatment and improve the efficacy of chemotherapy.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Chen WQ, Zhang SW, Zou XN, Zhao P. Cancer incidence and mortality in China, 2006. Chin J Cancer Res. 2011;23:3–9. doi: 10.1007/s11670-011-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Parkin DM, Li LD, et al. Estimation and projection of the national profile of cancer mortality in China: 1991–2010. Br J Cancer. 2011;90:2157–66. doi: 10.1038/sj.bjc.6601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mujoomdar A, Austin JH, Malhotra R, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: Primary tumor size, cell type, and lymph node metastases. Radiology. 2007;242:882–88. doi: 10.1148/radiol.2423051707. [DOI] [PubMed] [Google Scholar]
- 6.Torre LA, Bray F, Siegel RL, et al. Cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 7.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA. 2003;290:2149–58. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 8.Donington JS, Koo CW, Ballas MS. Novel therapies for non-small cell lung cancer. J Thorac Imaging. 2011;26:175–85. doi: 10.1097/RTI.0b013e3182161709. [DOI] [PubMed] [Google Scholar]
- 9.Hattori T, Hamai Y, Takiyama W, et al. Enhancing effect of thoracotomy on tumor growth in rats with special reference to the duration and timing of the operation. Gan. 1980;71:280–84. [PubMed] [Google Scholar]
- 10.Radu DM, Jauréguy F, Seguin A, et al. Postoperative pneumonia after major pulmonary resections: An unsolved problem in thoracic surgery. Ann Thorac Surg. 2007;84:1669–73. doi: 10.1016/j.athoracsur.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 11.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13:714–26. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 12.Fu RG, Sun Y, Sheng WB, Liao DF. Designing multi-targeted agents: An emerging anticancer drug discovery paradigm. Eur J Med Chem. 2017;136:195–211. doi: 10.1016/j.ejmech.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Ulbrich K, Holá K, Šubr V, et al. Covalent and noncovalent approaches, release control, and clinical studies. Chem Rev. 2016;116:5338–31. doi: 10.1021/acs.chemrev.5b00589. [DOI] [PubMed] [Google Scholar]
- 14.Fan JX, Zheng DW, Rong L, et al. Targeting epithelial-mesenchymal transition: Metal organic network nano-complexes for preventing tumor metastasis. Biomaterials. 2017;139:116–26. doi: 10.1016/j.biomaterials.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Li Y, Ahmad A, et al. Targeting miRNAs involved in cancer stem cell and EMT regulation: An emerging concept in overcoming drug resistance. Drug Resist Updat. 2010;13:109–18. doi: 10.1016/j.drup.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: New players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–34. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 17.Zhong W, Zhao L, Liu T, Jiang Z. IL-22-producing CD4+T cells in the treatment response of rheumatoid arthritis to combination therapy with methotrexate and leflunomide. Sci Rep. 2017;7:41143. doi: 10.1038/srep41143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andoh A, Zhang Z, Inatomi O, et al. Interleukin-22, a member of the IL-I0 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–84. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Chen Y, Wei H, et al. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14:6432–39. doi: 10.1158/1078-0432.CCR-07-4401. [DOI] [PubMed] [Google Scholar]
- 20.Bi Y, Cao J, Jin S, et al. Interleukin-22 promotes lung cancer cell proliferation and migration via the IL-22R1/STAT3 and IL-22R1/AKT signaling pathways. Mol Cell Biochem. 2016;415:1–11. doi: 10.1007/s11010-016-2663-8. [DOI] [PubMed] [Google Scholar]
- 21.Wu T, Cui L, Liang Z, et al. Elevated serum IL-22 levels correlate with chemoresistant condition of colorectal cancer. Clin Immunol. 2013;147:38–39. doi: 10.1016/j.clim.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Meng S, Su B, Li W, et al. Integrin-targeted paclitaxel nanoliposomes for tumor therapy. Med Oncol. 2011;28:1180–87. doi: 10.1007/s12032-010-9621-1. [DOI] [PubMed] [Google Scholar]
- 23.von Gruenigen VE, Karlen JR, Waggoner SE. A case of chronic paclitaxel administration in ovarian cancer. Gynecol Oncol. 2003;89:532–35. doi: 10.1016/s0090-8258(03)00145-8. [DOI] [PubMed] [Google Scholar]
- 24.Dia VP, Pangloli P. Epithelial-to-mesenchymal transition in paclitaxel-resistant ovarian cancer cells is downregulated by luteolin. J Cell Physiol. 2017;232:391–401. doi: 10.1002/jcp.25436. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Yano S. Molecular mechanisms of targeted drug resistance in lung cancer and its therapeutic strategy. Nihon Rinsho. 2015;73:1378–83. [PubMed] [Google Scholar]
- 27.He F, Du T, Jiang Q, Zhang Y. Synergistic effect of Notch-3-specific inhibition and paclitaxel in non-small cell lung cancer (NSCLC) cells via activation of the intrinsic apoptosis pathway. Med Sci Monit. 2018;24:3760–69. doi: 10.12659/MSM.902641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blake SJ, Teng MWL. Role of IL-17 and IL-22 in autoimmunity and cancer. Actas Dermosifiliogr. 2014;105:41–50. doi: 10.1016/S0001-7310(14)70017-1. [DOI] [PubMed] [Google Scholar]
- 29.Chew V, Toh HC, Abastado JP. Immune microenvironment in tumor progression: Characteristics and challenges for therapy. J Oncol. 2012;2012:608406. doi: 10.1155/2012/608406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee LF, Li G, Templeton DJ, Ting JP. Paclitaxel (Taxol)-induced gene expression and cell death are both mediated by the activation of c-Jun NH2-terminal kinase (JNK/SAPK) J Biol Chem. 1998;273:28253–60. doi: 10.1074/jbc.273.43.28253. [DOI] [PubMed] [Google Scholar]
- 31.Sunters A, Madureira PA, Pomeranz KM, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–20. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 32.Kim K, Kim G, Kim JY, et al. Interleukin-22 promotes epithelial cell transformation and breast tumorigenesis via MAP3K8 activation. Carcinogenesis. 2014;35:1352–61. doi: 10.1093/carcin/bgu044. [DOI] [PubMed] [Google Scholar]
- 33.Lejeune D, Dumoutier L, Constantinescu S, et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–82. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]