Abstract
Background
Although 5-Flourouracil(5-FU) is used as the first-choice treatment for advanced hepatocellular carcinoma (HCC), it is associated with acquired and intrinsic resistance. Hyperactivation of mTOR signaling has been linked to tumorigenesis and chemoresistance in HCC. The aim of this study was to evaluate and compare the antitumor effects of mTORC1 inhibitor everolimus and mTORC1/2 inhibitor AZD8055 and to examine the interaction between 5-FU and mTORC1/2 inhibitor in HCC.
Material/Methods
Using cultured HCC cells and mouse xenograft, the antitumor effects of everolimus and AZD8055 were analyzed as mono- and combination therapy with 5-Flourouracil.
Results
TSC2-deficient HCC cell lines were more sensitive to everolimus and AZD8055. AZD8055, but not everolimus, potently prevented cells from transitioning from G1 phase to S phase in TSC2-high-expressing HCC cells. AZD8055 reduced phosphorylation of both mTORC1 and mTORC2 substrates. In contrast, everolimus reduced the phosphorylation of mTORC1 substrates, but increased the phosphorylation of AKT. Notably, AZD8055, but not everolimus, synergistically enhanced the efficacy of 5-FU via reversing 5-FU-induced upregulation of P-glycoprotein (P-gp). The synergistic antitumor effect of AZD8055 and 5-FU was also observed in a HCC xenograft mouse model.
Conclusions
TSC2 in HCC is a promising efficacy-predicting biomarker for the treatment of mTORC1/2 inhibitor. AZD8055 showed stronger antitumor activity than everolimus in TSC2-high-expressing HCC cells. Moreover, the combination of mTORC1/2 inhibitor with 5-FU appears to be a promising option for HCC patients refractory to chemotherapy.
MeSH Keywords: Carcinoma, Hepatocellular; Fluorouracil; P-Glycoprotein; TOR Serine-Threonine Kinases
Background
Hepatocellular carcinoma (HCC) is a potentially lethal cancer with a mortality rate ranking second among all cancers globally, mainly due to its high rate of metastasis and recurrence [1]. Curative surgical therapy can be applied only to early-stage HCC patients, while the long-term prognosis of patients who have undergone curative surgical therapy remains unsatisfactory owing to its high rate of relapse [2,3]. Although 5-FU is used as the first-choice treatment for advanced HCC, it showed limited efficacy in clinical trials and is associated with acquired and intrinsic resistance [4]. The multikinase inhibitor sorafenib is a novel targeted agent recently introduced for HCC treatment, and it has shown transient and modest efficacy in clinical studies [5]. Thus, there is an urgent need to explore alternative therapeutic approaches to improve outcomes. Combination therapy based on traditional chemotherapeutic agents and small-molecule inhibitors that selectively target cancer cells is a potentially promising approach.
The PI3K/AKT/mTOR pathway plays a pivotal role in cancers, including HCC [6,7]. The mTOR pathway, with aberrant activation occurring in up to 50–60% of HCC cases, causes hepatocarcinogenesis, and is associated with less-differentiated tumors, early recurrence, and poor prognosis [8,9]. mTOR consists of 2 functionally and structurally distinct complexes named mTORC1 and mTORC2 [10]. mTORC1 can directly phosphorylate downstream targets of S6K1 and 4E-BP1, which enhance the translation of a variety of mRNAs. The TSC1/TSC2 complex is a key negative regulator of mTORC1 [11]. Phosphorylation of TSC2 by AKT or other kinases inactivates TSC2, which leads to unrestrained kinase activity of mTORC1. mTORC2, which phosphorylates AKT and related kinases, contributes to action cytoskeleton organization and protein synthesis. Recent data suggest that activation of AKT via mTORC2 in response to mTORC1 inhibition contributes to drug resistance in some cases [12]. Therefore, it is conceivable that targeting both mTORC1 and mTORC2 in cancer would provide a more effective antitumor activity.
Everolimus, a highly selective mTORC1 inhibitor, can bind with the intracellular receptor FK506-binding protein (FKBP-12) and form the everolimus-FKBP12 complex to block the activation of mTORC1 [13]. However, a recent study demonstrated that selective mTORC1 inhibition results in the feedback activation of AKT at Ser473 via mTORC2, which limits the antitumor efficacy of this strategy. Therefore, mTOR ATP-competitive inhibitors, which are able to suppress both mTORC1 and mTORC2 complexes-mediated signaling, have been developed recently. AZD8055, a novel ATP-competitive inhibitor of mTORC1/2, has been clinically tested for various tumors, including HCC [14,15]. To realize the full clinical potential of mTOR kinase inhibitors, a greater understanding of the molecular and cellular mechanisms of action of these compounds is needed. Furthermore, the ability of cancer cells to become resistant to 5-FU remains a significant hurdle to its clinical efficacy. To date, little is known about whether the combination 5-FU with mTORC1/C2 inhibitor can maximize efficacy and therapeutic index. In this study, we evaluated and compared the antitumor activity of the mTORC1 inhibitor everolimus with the dual mTORC1/2 inhibitor AZD8055, and observed the interaction between the mTORC1/2 inhibitor and 5-FU resistance.
Material and Methods
Reagents and antibodies
Everolimus, AZD8055, and 5-FU were purchased from Selleck Chemicals (Houston, TX, USA). All primary antibodies used for Western blot and immunoprecipitation were obtained from Cell Signaling Technology, with the exception of TSC2 (Abcam, Cambridge, UK) and GAPDH (Proteintech, IL, USA). All secondary antibodies were purchased from Jackson ImmunoResearch (Philadelphia, PA, USA).
Cell lines
The human HCC cell lines SNU398, PLC/PRF5, SMMC7721, and BEL7402 were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). These cell lines were routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL, USA) at 37°C in a humidified incubator containing 5% CO2.
Western blot analysis
Western blot analysis was performed as previously described [16]. Briefly, HCC cells were washed 3 times with PBS and lysed with RIPA Lysis Buffer (Santa Cruz Biotechnology, CA, USA) containing a protease inhibitor mixture (Roche Corp., Basal, Swiss) and phosphatase inhibitors (Roche Corp., Basal, Switzerland). The proteins were separated by SDS-PAGE and we transferred the protein to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked and washed. Primary antibodies were applied and then horseradish-peroxidase–conjugated secondary antibodies were added. The proteins were detected with an enhanced chemiluminescence reagent (Millipore Corp., MA, USA).
Cell viability assay
The cell viability was measured by CCK-8 assay (Dojindo, Kumamoto, Japan) according to the manufacturer’s protocol. Briefly, HCC cells were seed onto a 96-well plate for 24 h. Then, various concentrations of the compounds were added into the wells. After treatment for 48 h, 10 μl of CCK-8 reagent was added and incubated for an additional 2 h. The absorbance was read at 450 nm using a microplate reader (Thermo, MD, USA). Everolimus and AZD8055 dose-response curves and IC50 (half maximal inhibitory concentration) values were calculated using GraphPad Prism software (San Diego, CA, USA).
Cell cycle analysis
Cells, treated with either 0.1% DMSO or various concentrations of everolimus or AZD8055 for 24 h, were harvested and fixed in 70% ethanol overnight at 4°C. Cells were then stained with RNase A and propidium iodide (PI) according to the manufacturer’s protocol (BD Biosciences, MD, USA). Flow cytometry (Beckman Coulter, USA) was used to measure the fluorescence-stained cells. Data acquisition and analysis were conducted using Cell Quest (BD Biosciences, MD, USA).
Drug combination analysis
Multiple drug-effect analysis was preformed to evaluate the effects of combined drug treatment, as previously described [17]. The results of the CCK-8 assay were calculated using the following equation: combination index (CI)=(D)1/(Dx)1+(D)2/(Dx)2, where (Dx)1 and (Dx)2 are the concentrations of drug 1 and drug 2 alone that achieve effect fa; (D)1 and (D)2 are the concentrations of drug 1 and drug 2 in combination that give the same effect fa. A CI <1 indicates synergism; CI=1 and CI >1 indicate an additive effect and antagonism, respectively.
Immunohistochemical analysis
Harvested tumors were fixed in 4% formaldehyde solution in PBS, and embedded in paraffin and sliced into 5-μm-thick sections. Immunohistochemical staining was carried out using a standard protocol [14,15]. For Ki-67, only nuclear immunoreactivity was considered positive. The proliferation index was the percentage of Ki-67-positive cells among at least 500 cells per field.
Evaluation of in vivo tumor growth in subcutaneous implantation models of HCC
Male BALB/c nu/nu mice (4–6 weeks old) were purchased from the Animal Center of the Chinese Academy of Science and housed in specific pathogen-free conditions. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23 revised 1985).
BALB/c nu/nu mice were injected with SMMC7721 cells (5×107/mL contained in PBS) subcutaneously to establish subcutaneous tumors. The subcutaneous SMM7721 cells were allowed to grow for 1 week to reach a tumor size of approximately 50 to 100 mm3. The mice were then randomized into 4 groups: the control group; the AZD8055 group, orally administered 5 mg/kg in vehicle every other day; the 5-FU group, administered via the intraperitoneal injection with 50 mg/kg 5-FU in saline solution every other day; and the combination group, administered 5 mg/kg AZD8055 and 50 mg/kg 5-FU every other day. The tumor dimensions and body weights of all mice were monitored every 3 days and the mice were killed 2 weeks later. Tumor volume was calculated in mm3 as follows: ab2/2 (with a and b representing the largest and smallest dimensions collected at each measurement). Solid tumors were excised, weighed, and either processed for paraffin embedding or snap frozen and stored at −80°C.
Statistical analysis
The data were calculated as the mean ± standard deviation (SD) from at least 3 independent experiments. Statistical analyses were performed using SPSS 15.0 for Windows (SPSS, Inc., Chicago, IL). A p-value <0.05 was considered statistically significant.
Results
TSC2-deficient HCC cell lines were more sensitive to everolimus and AZD8055
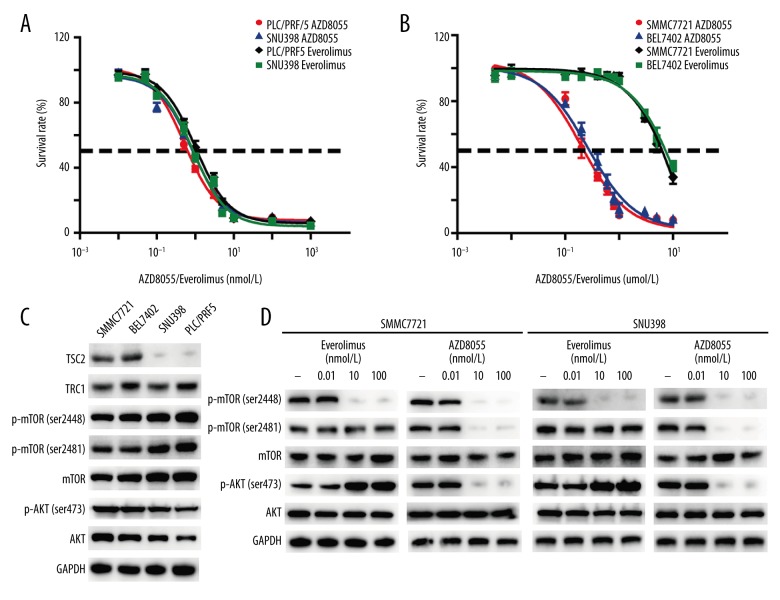
To characterize the activity of mTORC1 inhibitor everolimus and mTORC1/2 inhibitor AZD8055 against HCC, we performed an in vitro antiproliferation screen across a panel of HCC cell lines. The neoplastic activity of cancer cells was assessed after 48 h of treatment with everolimus and AZD8055, and the IC50 values were calculated. As shown in Figure 1A and 1B, both everolimus and AZD8055 effectively inhibited the growth of PLC/PRF5 and SNU398 cells in a concentration-dependent manner, while lower nanomolar concentrations of AZD8055 were required to inhibit SMMC7721 and BEL7402 cell proliferation in comparison with everolimus.
Figure 1.
Effect of everolimus and AZD8055 on HCC cell proliferation and mTORC1/2 signaling in vitro. (A, B) SNU398, PLC/PRF5, BEL7402, and SMMC7721 cells were treated with different concentrations of everolimus and AZD8055 for 48 h in DMEM containing 10% FBS. The cell viability was determined by CCK-8 assay. Each point represented the mean ±SD for 3 independent experiments. IC50 was calculated by nonlinear regression analysis using GraphPad Prism software. (C) Western blot analysis was performed to detect the expression profile of main effectors of mTORC1/2 signaling in HCC cells. (D) Western blot analysis was conducted to measure the effect of everolimus and AZD8055 on the main effectors of mTORC1/2 signaling in HCC cells. Cells were starved in medium containing 1% FBS for 12 h before adding 0.1% DMSO or the indicated concentrations of everolimus and AZD8055. After incubation for 5 h, cells were lysed for Western blot analysis.
We next analyzed the effects of everolimus and AZD8055 on the mTOR signaling pathway. Western blot analysis demonstrated that TSC2 protein was essentially nondetectable in PLC/PRF5 and SNU398 cells, but it was highly expressed in SMMC7721 and BEL7402 cells (Figure 1C). No significant differences in expression of TSC1, p-mTOR (Ser2481; Ser2448), and p-AKT(Ser473) were observed in those HCC cell lines. As shown in Figure 1D, the phosphorylation of mTOR on Ser2481, the marker of mTORC2 complex activity, and its substrate AKT(Ser473) were completely inhibited by AZD8055. In contrast, everolimus inhibited mTORC1 activity (phosphorylation of mTOR on Ser2448) without affecting mTORC2 activity (Ser2481), and also markedly increased AKT phosphorylation on Ser473. Therefore, AZD8055 efficiently inhibits both the mTORC1 and mTORC2 signaling cascades in HCC cells, whereas everolimus only inhibits mTORC1 and does not inhibit mTORC2, even at relatively high doses.
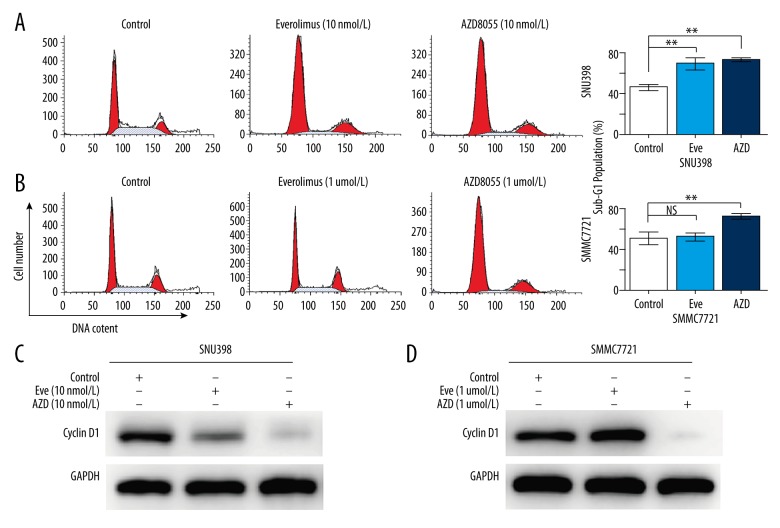
AZD8055, but not everolimus, induced G1-phase arrest in TSC-high-expressing HCC cells
We further investigated the mechanism underlying the increased cytotoxicity of AZD8055 compared with everolimus. After treatment with various doses of everolimus or AZD8055 for 24 h, the cell cycle in different HCC cells was analyzed. In TSC2-deficient SNU398 cells, both everolimus and AZD8055 significantly increased the percentage of cells in the G1-phase, whereas they decreased the percentage of cells in the S phase (Figure 2A). Cyclin D1 is a critical regulator of the G1–S phase transition. Upregulation of cyclin Dl results in rapid growth of a subset of HCC. Western blot analysis showed that cyclin D1 expression in TSC2-deficient SNU398 cells was reduced by everolimus and AZD8055 treatment (Figure 2C). However, in TSC2-high-expressing SMMC7721 cells, AZD8055 clearly increased the number of G0–G1 phase cells and decreased the cyclin D1 expression, whereas everolimus exerted no obvious cell cycle suppressive action, even at micromolar concentrations (Figure 2B, 2D).
Figure 2.
Effects of everolimus and AZD8055 on cell cycle progression in HCC cells. (A, B) Cell cycle was determined by flow cytometry assay after treatment with either 0.1% DMSO or the indicated concentrations of everolimus and AZD8055 for 24 h. (C, D) Western blot analysis was performed for Cyclin D1 (a critical regulator of the G1–S transition) expression in HCC cells treated with 0.1% DMSO or the indicated concentrations of everolimus and AZD8055 for 24 h. Data are reported as the mean ±SD. * P<0.05 and ** P<0.01 versus control.
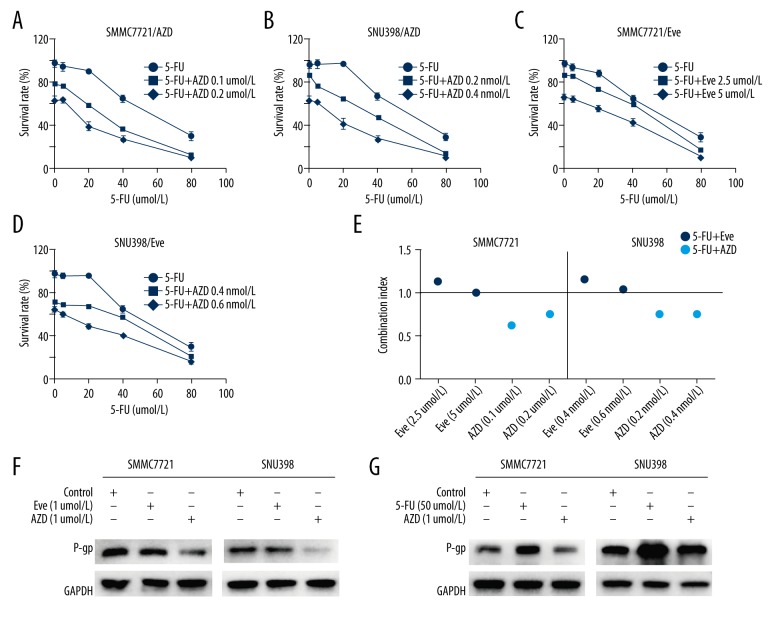
AZD8055 synergistically enhances the efficacy of 5-FU via reversing 5-FU-induced upregulation of P-gp
Because AZD8055 showed significant cell cycle suppressive action, we explored whether the combination of AZD8055 and chemotherapeutic agent 5-FU would lead to an additive or synergistic effect. Drug combination index (CI) analysis is a generalized method for analyzing the effects of multiple drugs and identifying addition, synergism, and antagonism. For this purpose, TSC2-deficient SNU398 cells and TSC2-high-expressing SMMC7721 cells were incubated with increasing concentrations of either 5-FU alone or with 5-FU in combination with sub-toxic concentrations of AZD8055 or everolimus. The dose-response curves clearly demonstrated marked inhibition of cell growth when SNU398 and SMMC7721 cells were treated with the combination of 5-FU and AZD8055 in comparison with single-agent treatment with 5-FU (CI values ranging from 0.62 to 0.76; Figure 3A, 3B, 3E). However, treatment of SNU398 and SMMC7721 cells with 5-FU in combination with everolimus resulted mainly in additive to slightly antagonistic cytotoxicity (CI values ranging from 1.01 to 1.17; Figure 3C–3E).
Figure 3.
AZD8055, but not everolimus, synergizes the efficacy of 5-FU via reversing the upregulation of P-gp induced by 5-FU. (A–D) Cell viability of HCC cells treated with AZD8055, everolimus, 5-FU, the combination of AZD8055 and 5-FU, or the combination of everolimus and 5-FU for 48 h was determined by CCK-8 assay. (E) The combinatorial effects of treatments were determined by combination index (CI) values. The CI values <1, =1 and >1 indicate synergy, additivity, and antagonism, respectively. (F, G) Western blot analysis was performed for P-gp expression in HCC cells treated with 0.1% DMSO or the indicated concentrations of everolimus and AZD8055 for 24 h.
P-gp has been considered a major hindrance to the successful utilization of cancer chemotherapy [18]. Therefore, we next investigated whether mTORC1/mTORC2 inhibition affects P-gp expression. Western blot analysis demonstrated obvious downregulation of P-gp when the cells were treated with the mTORC1/mTORC2 inhibitor AZD8055, whereas the mTORC1 inhibitor everolimus did not significantly affect the expression of P-gp (Figure 3F). Moreover, the upregulation of P-gp induced by 5-FU was reversed by AZD8055 (Figure 3G).
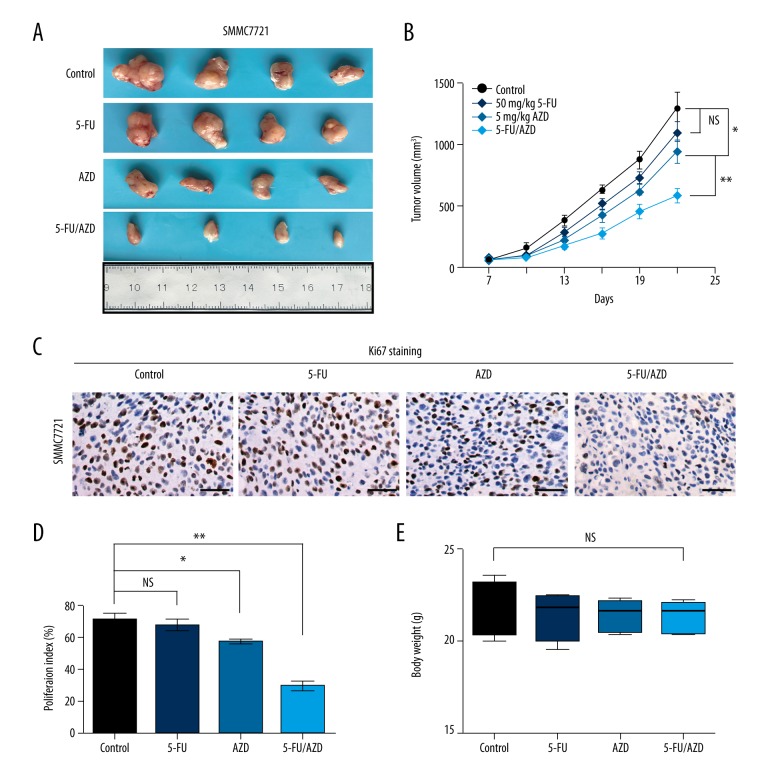
In vivo combination of AZD8055 and 5-FU significantly enhances tumor growth inhibition in HCC
To examine the in vivo efficacy of AZD8055/5-FU, nude mice were inoculated with a representative HCC cell line, SMMC7721, and dosed with AZD8055 and/or 5-FU daily for 14 days as described in Methods. Treatment with 5-FU did not significantly inhibit tumor growth compared with the control group. However, AZD8055 treatment significantly inhibited the tumor growth, with tumor volume reduced by 27% compared with the control group. Importantly, the combined AZD8055 with 5-FU therapy led to maximal tumor suppression with tumor growth reduced more than 55% compared with the control group (Figure 4A, 4B). The level of cell proliferation in different treated tumors was further examined by IHC staining of nucleus Ki67. As shown in Figure 4C and 4D, the combined treatment group also had less nucleus Ki67-positive cells in tumor tissues than either AZD8055 or 5-FU alone. To assess the toxicities mediated by the co-treatment of AZD8055 and 5-FU in vivo, mouse body weight was measured every 3 days and results demonstrated no significant difference among the various groups (Figure 4E).
Figure 4.
Effect of AZD8055 in combination with 5-FU on the growth of HCC xenografts in vivo. (A) The image showed the tumor size of SMMC7721 xenografts after 2-week treatment with 5-FU in the presence or absence of AZD8055. (B) Tumor volumes were measured and recorded every 3 days from initial treatment to tumor harvest. (C) Representative pictures of proliferative cells stained with Ki-67 in tumor tissues. (D) The bar graph shows the mean ±SD of quantification of Ki-67-positive cells from immunohistochemical analysis of tumors. (E) The image showed the body weight of SMMC7721 xenografts treatment with 5-FU in the presence or absence of AZD8055 at the end of the experiment. * P<0.05 and ** P<0.01 versus control. Scale bars: 50 um.
Discussion
Approval of Sorafenib for use in advanced HCC triggered the search for additional molecular agents to further improve patient survival. Unfortunately, none of them (sunitinib, brivanib, and linifanib) have resulted in even modest survival benefits [19]. Therefore, there is an unmet need to develop additional effective agents, especially those with novel mechanisms of action that target hepatocarcinogenesis. mTOR, which is located downstream of the PI3K-AKT pathway, is a key regulator of growth and proliferation of tumor cells [9]. mTOR-activated HCC is associated with a higher incidence of recurrence [8]. Therefore, blockade of the mTOR pathway may be an effective therapeutic strategy in the treatment of HCC. In this study, we found that both the mTORC1 inhibitor everolimus and the mTORC1/2 inhibitor AZD8055 inhibited the growth of SNU398 and PLC/PRF5 cells at very low concentrations. In BEL7402 and SMMC7721 cells, everolimus hardly exerted any anticancer effect. In contrast, AZD8055 was also active in the everolimus-resistant cell lines. TSC2 loss-of-function mutations have been identified in 1 thyroid cancer patient who achieved a complete response that lasted for 18 months when treated with everolimus [20]. In agreement with earlier studies, we found that TSC2 protein were essentially nondetectable in PLC/PRF5 and SNU398 cells, but was highly expressed in SMMC7721 and BEL7402 cells. Our data showing that TSC2-high-expressing HCC cells do not respond to mTORC1 inhibition suggest that mTORC1 inhibition may show a blunted survival benefit within all HCC patients, and suggests the use of a personalized approach that requires patients to be stratified based on TSC2 expression prior to mTORC1 inhibitor treatment.
We sought to further identify whether mTORC1 or mTORC2 participates in the induction of cell cycle arrest by use of AZD8055 and everolimus. Flow cytometric analysis showed that TSC2-deficient HCC cells treated with everolimus and AZD8055 were arrested in the G1 phase of the cell cycle. However, TSC2-high-expressing HCC cells targeted inhibition of mTORC1/2, but not mTORC1, and potently prevented cells from transitioning from the G1 phase to S phase. Cyclin D1 is a critical regulator of the G1–S transition. Upregulation of cyclin D1 results in rapid growth of a subset of HCC. Western blot analysis showed that both everolimus and AZD8055 significantly reduced the cyclin D1 expression in TSC2-deficient PLC/PRF5 and SNU398 cells. However, in TSC2-high-expressing SMMC7721 and BEL7402 cells, AZD8055 also clearly decreased the cyclin D1 expression, whereas everolimus exerted no obvious cell cycle suppressive action. These data suggest that targeting of mTORC1 could only promote G1-phase arrest in TSC2-deficient HCC cells, while G1-phase arrest could be induced by targeting of mTORC1/2 in both TSC2-deficient and high-expressing HCC cells.
To date, 5-FU is still being widely used for the treatment of advanced HCC [4]. Unfortunately, success of a single or combination chemotherapy regimen is mostly transient and modest [21,22]. The present study showed that the combination of AZD8055, but not of everolimus, with 5-FU significantly decreased tumor cell viability compared with single-drug treatment. Multidrug resistance (MDR) is mediated by high expression of ATP-binding cassette (ABC) transporter family members that increase the efflux of chemotherapeutic agents out of cancer cells [18]. P-glycoprotein (P-gp), encoded by the MDR1 gene, is one of the best-studied ABC transporters in drug resistance [23]. In addition, P-gp is thought to render tumors resistant to chemotherapy, and changes in its expression or function can contribute to MDR [24]. Our study shows that inhibition of mTORC1/2 by AZD8055 reversed the overexpression of P-gp induced by 5-FU in HCC cells. However, inhibition of mTORC1 alone by everolimus had limited effect on P-gp expression. These results indicate that the chemosensitization effects on 5-FU induced by mTORC1/2 inhibition can be ascribed to reduction of the P-gp expression.
Conclusions
TSC2 in HCC is a promising efficacy-predicting biomarker for the treatment of mTORC1/2 inhibitor. AZD8055 showed stronger antitumor activity compared to everolimus in TSC2-high-expressing HCC cells. Moreover, the combination of mTORC1/2 inhibitor with 5-FU could be a promising treatment strategy for HCC patients who can tolerate chemotherapy.
Acknowledgements
We are grateful to Dr. Liang Fu (Fudan University) for his technical assistance, and we gratefully acknowledge the support we received from the Natural Science Foundation of China for this research.
Footnotes
Conflict of interest
None.
Source of support: This work was funded by the Natural Science Foundation of China (NSFC81773089)
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut. 2014;63(5):844–55. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan X, Li X, Cui L, Wang Q. Preoperative phenacetin metabolism test in the prediction of postoperative liver dysfunction of patients with hepatocellular carcinoma. Med Sci Monit. 2018;24:2607–11. doi: 10.12659/MSM.905228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeda M, Mitsunaga S, Ohno I, et al. Systemic chemotherapy for advanced hepatocellular carcinoma: Past, present, and future. Diseases. 2015;3(4):360–81. doi: 10.3390/diseases3040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–53. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 6.Sahin F, Kannangai R, Adegbola O, et al. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10(24):8421–25. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- 7.Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135(6):1972–83. 1983.e1–11. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L, Huang Y, Li J, Wang Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2010;27(2):255–61. doi: 10.1007/s12032-009-9201-4. [DOI] [PubMed] [Google Scholar]
- 9.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27(13):2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Manning BD. The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem J. 2008;412(2):179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan X, Harkavy B, Shen N, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 13.Houghton PJ. Everolimus. Clin Cancer Res. 2010;16(5):1368–72. doi: 10.1158/1078-0432.CCR-09-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petigny-Lechartier C, Duboc C, Jebahi A, et al. The to reduce the Mcl-1/[Bim and Puma] ratio and to sensitize ovarian carcinoma cells to ABT-737. Mol Cancer Ther. 2017;16(1):102–15. doi: 10.1158/1535-7163.MCT-16-0342. [DOI] [PubMed] [Google Scholar]
- 15.Shao H, Gao C, Tang H, et al. Dual targeting of mTORC1/C2 complexes enhances histone deacetylase inhibitor-mediated anti-tumor efficacy in primary HCC cancer in vitro and in vivo. J Hepatol. 2012;56(1):176–83. doi: 10.1016/j.jhep.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Jia QA, Ren ZG, Bu Y, et al. Herbal compound “Songyou Yin” renders hepatocellular carcinoma sensitive to oxaliplatin through inhibition of stemness. Evid Based Complement Alternat Med. 2012;2012:908601. doi: 10.1155/2012/908601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 18.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett. 2006;580(4):998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: Reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20(8):2072–79. doi: 10.1158/1078-0432.CCR-13-0547. [DOI] [PubMed] [Google Scholar]
- 20.Wagle N, Grabiner BC, Van Allen EM, et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med. 2014;371(15):1426–33. doi: 10.1056/NEJMoa1403352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501–8. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein R, Yu D, Gillmore R, et al. Oxaliplatin/5-fluorouracil in advanced hepatocellular carcinoma: Case report and single-center retrospective review. Future Oncol. 2014;10(13):2007–14. doi: 10.2217/fon.14.108. [DOI] [PubMed] [Google Scholar]
- 23.Takara K, Sakaeda T, Okumura K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr Pharm Des. 2006;12(3):273–86. doi: 10.2174/138161206775201965. [DOI] [PubMed] [Google Scholar]
- 24.Nobili S, Landini I, Mazzei T, Mini E. Overcoming tumor multidrug resistance using drugs able to evade P-glycoprotein or to exploit its expression. Med Res Rev. 2012;32(6):1220–62. doi: 10.1002/med.20239. [DOI] [PubMed] [Google Scholar]