Abstract
Background
The relatively low incidence of duodenal gastrointestinal stromal tumors (GISTs) and the unique anatomy make the surgical management and outcomes of this kind of tumor still under debate. Thus, this study aimed to explore the optimal surgical strategy and prognosis of duodenal GISTs.
Methods
A total of 300 cases of duodenal GISTs were obtained from our center (37 cases) and from case reports or series (263 cases) extracted from MEDLINE. Clinicopathological features, type of resections and survivals of duodenal GISTs were analyzed.
Results
The most common location of duodenal GISTs was descending portion (137/266, 51.5%). The median tumor size was 4 cm (0.1–28). Most patients (66.3%) received limited resection (LR). Pancreaticoduodenectomy (PD) was mainly performed for GISTs with larger tumor size or arose from descending portion (both P < 0.05). For both the entire cohort and tumors located in the descending portion, PD was not an independent risk factor for disease-free survival (DFS) and disease-specific survival (DSS) (both P > 0.05). Duodenal GISTs were significantly different from gastric GISTs with respect to tumor size, mitotic index and NIH risk category (all P < 0.05). The DFS and DSS of duodenal GISTs was significantly worse than that of gastric GISTs (both P < 0.05).
Conclusions
LR was a more prevalent surgical procedure and PD was mainly performed for tumors with larger diameter or located in descending portion. Type of resection was not an independent risk factor for the prognosis of duodenal GISTs. Prognosis of duodenal GISTs was significantly worse than that of gastric GISTs.
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4485-4) contains supplementary material, which is available to authorized users.
Keywords: Duodenum, Gastrointestinal stromal tumor, Features, Surgery, Prognosis
Background
Gastrointestinal stromal tumor (GIST) is the commonest mesenchymal tumor in alimentary tract representing an annual incidence of 10 cases per million people worldwide [1]. While this kind of tumor could originate from the interstitial Cajal cells (ICC) throughout the entire alimentary tract, GISTs are mostly found in the stomach (60–70%), small intestine (20–30%) and colorectum (10%) [2]. Notably, only 1–5% GISTs occurred in the duodenum [3]. Thus, the research on duodenal GIST was lacking due to its rare incidence.
To date, complete resection without lymph node clearance is the standard curative treatment for primary localized GISTs [4, 5]. However, the optimal surgical procedure for duodenal GISTs is not well defined due to their complex anatomy around the pancreaticoduodenal region [6–8]. The limited resection (LR) is reported to be a technically feasible and oncologically sound procedure for duodenal GISTs, while the pancreaticoduodenectomy (PD) is also warranted in some cases due to the anatomical considerations of the proximity of critical structures, including the papilla, pancreas and biliary and pancreatic ducts [9–12]. However, the survival impact of surgical procedure on duodenal GISTs still remains controversial [6, 13, 14].
Thus, the current study aimed to investigate the optimal surgical strategy and prognosis of duodenal GISTs based on the largest sample size so far.
Methods
Thirty-seven cases of duodenal GISTs which were diagnosed and treated in our center from May 2010 to November 2016, and 263 cases of duodenal GISTs reported in the literature were enrolled into this study. Literature published in English from 1st January 2000 to 1st January 2017 were searched in the database of MEDLINE using the following keywords: (GIST OR gastrointestinal stromal tumor OR gastrointestinal stromal tumour OR GISTs OR gastrointestinal stromal tumors OR gastrointestinal stromal tumours OR extragastrointestinal stromal tumor OR extragastrointestinal stromal tumors OR extragastrointestinal stromal tumour OR extragastrointestinal stromal tumours) AND (duodenum OR duodenal). The research resulted in 101 eligible case reports or series [8, 10, 12, 15–112] including 263 cases of duodenal GISTs. Finally, a total of 300 cases of duodenal GISTs were identified in our study (Additional file 1). In addition, the clinicopathological features and prognosis of duodenal GISTs were compared with 378 gastric GISTs which were diagnosed and treated from May 2010 to November 2016 in our center. This study was approved by the Ethics Committee of Xijing Hospital, and written informed consents were obtained from the patients.
Clinicopathological factors including age, gender, preoperative symptoms, anatomical location, surgical procedure, resection margin, tumor size, mitotic index, morphology, immunohistochemistry, genomic mutation, National Institutes of Health risk category (NIH), adjuvant therapy and survival data were collected. The GISTs were classified as very low, low, intermediate and high risk following the modified protocol of NIH risk classification reported by Joensuu [113].
For survival analysis, the exclusion criteria were listed as follows (Both for duodenal and gastric GISTs): 1) accompanied with other malignant tumors or GISTs in other locations; 2) with distant metastasis or tumor rupture; 3) with neoadjuvant therapy; 4) not received R0 resection; 5) without follow-up records. Because of data acquisition, completeness of data is limited. Finally, a total of 202 patients of duodenal GISTs and 253 patients of gastric GISTs were included for survival analysis.
Data were processed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL). Numerical variables were expressed as the mean ± SD unless otherwise stated. Discrete variables were analyzed using the Chi-square test or Fisher’s exact test. Risk factors for survival were identified by univariate analysis and Cox proportional hazards regression model was used for multivariate analysis. Evaluation for disease-free survival (DFS) and disease-specific survival (DSS) were obtained by the Kaplan-Meier method and differences between curves were compared using log-rank test. The P-values were considered to be statistically significant at the 5% level.
Results
The clinicopathological characteristics of 300 duodenal GISTs were summarized in Table 1. There were 143 male (49.1%) and 148 female (50.8%). The patient age ranged from 7 to 84 years (mean, 56 years; median, 57 years). The most common symptom was bleeding (128/300, 42.7%) followed by abdominal pain (56/300, 18.7%). Descending portion was the most common site (137/266, 51.5%), followed by horizontal portion (65/266, 24.4%), superior portion (42/266, 15.8%) and ascending portion (22/266, 8.3%). R0 resection was performed for the 91.7% of the patients. There were only 2 patients that underwent R1 or R2 resection. One hundred and ninety-nine (66.3%) patients received LR and 78 (26.0%) patients received PD. The tumors ranged from 0.1 cm to 28 cm (mean: 5.6 cm; median: 4 cm) in maximum diameter. The mitotic index of 59 (24.6%) patients exceeded 5/50 high-power field (HPF). One hundred and twenty-seven patients (49.2%) were classified as high risk by the NIH risk category, and 104 patients (40.3%) were at low risk. A total of 13 (4.3%) patients received neoadjuvant therapy and 37 (12.3%) patients received imatinib therapy after surgery.
Table 1.
Clinicopathological characteristics of 300 cases of duodenal GISTs
| Characteristics | Parameters |
|---|---|
| Age (∑ = 267) | |
| ≤ 60 | 161 (60.3%) |
| > 60 | 106 (39.7%) |
| Gender (∑ = 291) | |
| Male | 143 (49.1%) |
| Female | 148 (50.8%) |
| Symptoms (∑ = 300) | |
| Bleeding or anemia | 128 (42.7%) |
| Abdominal pain | 56 (18.7%) |
| Abdominal mass | 11 (3.7%) |
| Abdominal discomfort | 11 (3.7%) |
| Anorexia | 6 (2.0%) |
| Othersaa | 36 (12.0%) |
| Anatomical location (∑ = 266) | |
| Superior portion | 42 (15.8%) |
| Descending portion | 137 (51.5%) |
| Horizontal portion | 65 (24.4%) |
| Ascending portion | 22 (8.3%) |
| Surgical procedure (∑ = 300) | |
| Limited resection* | 199 (66.3%) |
| Pancreaticoduodenectomy | 78 (26.0%) |
| Not available | 13 (4.3%) |
| No surgery | 10 (3.3%) |
| Resection margin (∑ = 300) | |
| R0 | 275 (91.7%) |
| R1/2 | 2 (0.7%) |
| Not available/No surgery | 23 (7.7%) |
| Tumor size (∑ = 277) | |
| ≤ 2 cm | 34 (12.3%) |
| 2–5 cm | 135 (48.7%) |
| 5–10 cm | 73 (26.4%) |
| > 10 cm | 35 (12.6%) |
| Mitotic index (∑ = 240) | |
| ≤ 5 | 181 (75.4%) |
| > 5 | 59 (24.6%) |
| Morphology (∑ = 160) | |
| Spindle | 148 (92.5%) |
| Epithelioid | 1 (0.6%) |
| Mixed | 11 (6.9%) |
| Immunohistochemistry | |
| CD117 (∑ = 288) | 284 (98.6%) |
| DOG-1 (∑ = 41) | 40 (97.6%) |
| CD34 (∑ = 167) | 126 (75.4%) |
| Genomic mutation (∑ = 41) | |
| KIT | 31 (75.6%) |
| PDGFRA | 1 (2.4%) |
| KIT and PDGFRA | 5 (12.2%) |
| Wild type | 4 (9.8%) |
| NIH risk category (∑ = 258) | |
| Very low | 25 (9.7%) |
| Low | 104 (40.3%) |
| Intermediate | 2 (0.8%) |
| High | 127 (49.2%) |
| Neoadjuvant therapy (∑ = 300) | |
| No | 287 (95.7%) |
| Yes | 13 (4.3%) |
| Adjuvant therapy (∑ = 300) | |
| No | 263 (87.7%) |
| Yes | 37 (12.3%) |
| Follow-up (∑ = 202, month) | |
| Mean ± SD | 39.3 ± 39.4 |
| Median (range) | 25.0 (13.0, 58.5) |
| Survival rate (∑ = 202) | |
| 1−/3−/5−/10-year DFS | 94.4%/75.2%/64.4%/46.5% |
| 1−/3−/5−/10-year DSS | 99.5%/93.4%/80.9%/54.5% |
aLimited resection included wedge resection, segmental resection or enucleation
GIST: gastrointestinal stromal tumor; NIH: National Institutes of Health; SD: standard deviation
Survival data of 202 patients with duodenal GISTs were eventually selected for analysis using exclusion criteria described in the methods section (Table 1). The median follow-up time was 25.0 months (mean: 39.3 months). As shown in Fig. 1, the 1−/3−/5−/10-year DFS of duodenal GISTs was 94.4, 75.2, 64.4 and 46.5%, respectively. The 1−/3−/5−/10-year DSS was 99.5, 93.4, 80.9 and 54.5%, respectively.
Fig. 1.
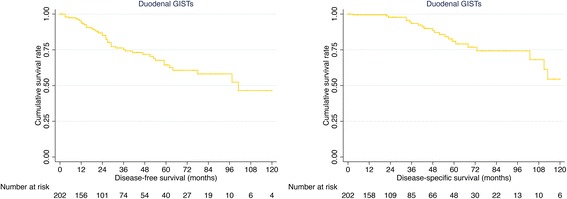
DFS and DSS of duodenal GISTs
The clinicopathological characteristics of duodenal GISTs received different surgical procedures were compared in Table 2, the tumors underwent PD were mainly located in descending portion (52/77, 88.1%), and had larger diameter, higher mitotic index and higher NIH risk category (all P < 0.001). Prognostic factors for duodenal GISTs according to univariate and multivariate analysis were summarized in Table 3. Surgical procedure, tumor size, mitotic index and NIH risk category were risk factors for both DFS and DSS (all P < 0.05). Patients underwent LR had a higher 5-year DFS (78.6% vs 35.1%, P < 0.001) and DSS (83.9% vs 72.9%, P = 0.008) than patients underwent PD according to Kaplan-Meier analysis (Fig. 2). However, multivariate analysis showed that surgical procedure was not an independent prognostic factor (P > 0.05).
Table 2.
Comparison of clinicopathological factors of duodenal GISTs according to surgical procedure
| Characteristics | Limited resection (n = 200) | Pancreaticoduodenectomy (n = 77) | P value |
|---|---|---|---|
| Age | 0.782 | ||
| ≤ 60 | 101 (60.5%) | 48 (62.3%) | |
| > 60 | 66 (39.5%) | 29 (37.7%) | |
| Gender | 0.205 | ||
| Male | 100 (51.3%) | 32 (42.7%) | |
| Female | 95 (48.7%) | 43 (57.3%) | |
| Anatomical location | < 0.001 | ||
| Superior portion | 37 (19.7%) | 3 (5.1%) | |
| Descending portion | 73 (38.8%) | 52 (88.1%) | |
| Horizontal portion | 56 (29.8%) | 4 (6.8%) | |
| Ascending portion | 22 (11.7%) | 0 | |
| Tumor size | < 0.001 | ||
| ≤ 2 cm | 28 (15.1%) | 3 (4.1%) | |
| 2–5 cm | 102 (54.8%) | 25 (33.8%) | |
| 5–10 cm | 37 (19.9%) | 32 (43.2%) | |
| > 10 cm | 19 (10.2%) | 14 (18.9%) | |
| Mitotic index | < 0.001 | ||
| ≤ 5 | 144 (83.2%) | 32 (52.5%) | |
| > 5 | 29 (16.8%) | 29 (47.5%) | |
| Morphology | 0.146 | ||
| Spindle | 100 (94.3%) | 39 (86.7%) | |
| Epithelioid | 0 | 1 (2.2%) | |
| Mixed | 6 (5.7%) | 5 (11.1%) | |
| NIH risk category | < 0.001 | ||
| Very low | 23 (12.8%) | 2 (2.8%) | |
| Low | 87 (48.6%) | 15 (21.1%) | |
| Intermediate | 2 (1.1%) | 0 | |
| High | 67 (37.4%) | 54 (76.1%) | |
| Neoadjuvant therapy | 0.472 | ||
| No | 194 (97.0%) | 73 (94.8%) | |
| Yes | 6 (3.0%) | 4 (5.2%) | |
| Adjuvant therapy | 0.362 | ||
| No | 165 (82.5%) | 67 (87.0%) | |
| Yes | 35 (17.5%) | 10 (13.0%) |
GIST: gastrointestinal stromal tumor; NIH: National Institutes of Health
Table 3.
Univariate and multivariate analysis of prognostic factors for duodenal GISTs
| Prognostic factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| β | Hazard ratio (95% CI) | P value | β | Hazard ratio (95% CI) | P value | |
| DFS | ||||||
| Age | 0.480 | 1.616 (0.874–2.987) | 0.126 | |||
| Gender | −0.148 | 0.862 (0.470–1.582) | 0.632 | |||
| Anatomical location | 0.108 | 1.114 (0.744–1.666) | 0.601 | |||
| Surgical procedure | 1.517 | 4.559 (2.510–8.281) | < 0.001 | |||
| Tumor size | 1.441 | 4.224 (2.769–6.442) | < 0.001 | 1.406 | 4.082 (1.979–8.416) | < 0.001 |
| Mitotic index | 2.049 | 7.759 (3.751–16.048) | < 0.001 | 1.294 | 3.648 (1.375–9.680) | 0.009 |
| NIH risk category | 1.457 | 4.294 (2.394–7.702) | < 0.001 | |||
| Adjuvant therapy | 0.327 | 1.387 (0.514–3.742) | 0.518 | |||
| DSS | ||||||
| Age | 0.338 | 1.403 (0.596–3.300) | 0.438 | |||
| Gender | 0.324 | 1.383 (0.587–3.257) | 0.459 | |||
| Anatomical location | 0.107 | 1.113 (0.649–1.909) | 0.697 | |||
| Surgical procedure | 1.082 | 2.952 (1.274–6.837) | 0.012 | |||
| Tumor size | 1.629 | 5.100 (2.640–9.853) | < 0.001 | 1.339 | 3.816 (1.743–8.354) | 0.001 |
| Mitotic index | 1.719 | 5.580 (2.277–13.674) | < 0.001 | |||
| NIH risk category | 1.035 | 2.815 (1.547–5.123) | 0.001 | |||
| Adjuvant therapy | −1.388 | 0.249 (0.033–1.877) | 0.178 | |||
GIST: gastrointestinal stromal tumor; NIH: National Institutes of Health;
DFS: disease-free survival; DSS: disease-specific survival; CI: confidence interval
Fig. 2.
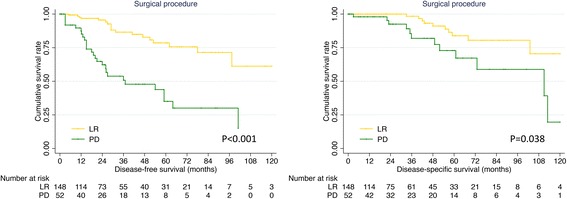
DFS and DSS of duodenal GISTs stratified by surgical procedure
Since more than half of duodenal GIST occur at the descending portion, we specifically studied the clinicopathological features of these GISTs based on the type of resection in Table 4. A higher prevalence of large tumor, high mitotic index and high risk category was observed in the descending tumors received PD (all P < 0.05). Univariate analysis showed that surgical procedure, tumor size, mitotic index and NIH category were risk factors for both DFS and DSS (Table 5, all P < 0.05). As shown in Fig. 3, LR brought a more favorable 5-year DFS (77.8% vs 48.2%, P = 0.002) and DSS (83.9% vs 69.3%, P = 0.011) than PD. However, multivariate analysis showed that surgical procedure was not an independent prognostic factor (Table 5, P > 0.05).
Table 4.
Comparison of clinicopathological factors of descending duodenal GISTs according to surgical procedure
| Characteristics | Limited resection (n = 73) | Pancreaticoduodenectomy (n = 52) | P value |
|---|---|---|---|
| Age | 0.160 | ||
| ≤ 60 | 31 (54.4%) | 30 (68.2%) | |
| > 60 | 26 (45.6%) | 14 (31.8%) | |
| Gender | 0.976 | ||
| Male | 27 (42.9%) | 22 (43.1%) | |
| Female | 36 (57.1%) | 29 (56.9%) | |
| Tumor size | 0.035 | ||
| ≤ 2 cm | 12 (17.4%) | 2 (4.0%) | |
| 2–5 cm | 33 (47.8%) | 19 (38.0%) | |
| 5–10 cm | 17 (24.6%) | 20 (40.0%) | |
| > 10 cm | 7 (10.1%) | 9 (18.0%) | |
| Mitotic index | < 0.001 | ||
| ≤ 5 | 50 (86.2%) | 25 (54.3%) | |
| > 5 | 8 (13.8%) | 21 (45.7%) | |
| Morphology | 0.165 | ||
| Spindle | 35 (97.2%) | 27 (84.4%) | |
| Epithelioid | 0 | 1 (3.1%) | |
| Mixed | 1 (2.8%) | 4 (12.5%) | |
| NIH risk category | 0.001 | ||
| Very low | 10 (15.4%) | 1 (2.1%) | |
| Low | 28 (43.1%) | 11 (22.9%) | |
| Intermediate | 0 | 0 | |
| High | 27 (41.5%) | 36 (75.0%) | |
| Neoadjuvant therapy | 0.401 | ||
| No | 69 (94.5%) | 51 (98.1%) | |
| Yes | 4 (5.5%) | 1 (1.9%) | |
| Adjuvant therapy | 0.777 | ||
| No | 33 (80.5%) | 30 (83.3%) | |
| Yes | 8 (19.5%) | 6 (16.7%) |
GIST: gastrointestinal stromal tumor; NIH: National Institutes of Health
Table 5.
Univariate and multivariate analysis of prognostic factors for the descending duodenal GISTs
| Prognostic factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| β | Hazard ratio (95% CI) | P value | β | Hazard ratio (95% CI) | P value | |
| DFS | ||||||
| Age | 0.487 | 1.628 (0.632–4.193) | 0.313 | |||
| Gender | −0.375 | 0.687 (0.279–1.694) | 0.415 | |||
| Surgical procedure | 1.473 | 4.361 (1.597–11.909) | 0.004 | |||
| Tumor size | 1.445 | 4.240 (2.073–8.672) | < 0.001 | 1.721 | 5.590 (2.144–14.570) | < 0.001 |
| Mitotic index | 1.696 | 5.453 (2.065–14.400) | 0.001 | |||
| NIH risk category | 1.456 | 4.288 (1.594–11.531) | 0.004 | |||
| Adjuvant therapy | 0.544 | 1.724 (0.367–8.103) | 0.491 | |||
| DSS | ||||||
| Age | 0.276 | 1.318 (0.432–4.019) | 0.627 | |||
| Gender | 0.008 | 1.008 (0.316–3.218) | 0.989 | |||
| Surgical procedure | 1.519 | 4.569 (1.260–16.569) | 0.021 | |||
| Tumor size | 2.142 | 8.515 (2.496–29.053) | 0.001 | 1.976 | 7.213 (2.138–24.338) | 0.001 |
| Mitotic index | 1.567 | 4.792 (1.428–16.087) | 0.011 | |||
| NIH risk category | 1.143 | 3.136 (1.150–8.552) | 0.026 | |||
| Adjuvant therapy | −3.181 | 0.042 (0.000–212.916) | 0.465 | |||
GIST: gastrointestinal stromal tumor; NIH: National Institutes of Health;
DFS: disease-free survival; DSS: disease-specific survival; CI: confidence interval
Fig. 3.
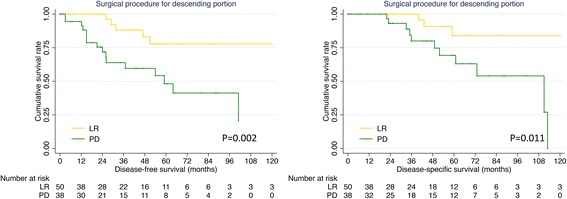
DFS and DSS of descending duodenal GISTs stratified by surgical procedure
The clinicopathological characteristics of 300 duodenal GISTs including age, gender, tumor size, mitotic index, morphology and NIH risk category were compared with 378 gastric GISTs from out center (Table 6). The tumor size, mitotic index and NIH risk category were significantly different between the two groups (all P < 0.001).
Table 6.
Comparison of clinicopathological characteristics between duodenal and gastric GISTs
| Characteristics | Duodenum (n = 300) | Stomach (n = 378) | P value |
|---|---|---|---|
| Age | 0.791 | ||
| ≤ 60 | 161 (60.3%) | 224 (59.3%) | |
| > 60 | 106 (39.7%) | 154 (40.7%) | |
| Gender | 0.826 | ||
| Male | 143 (49.1%) | 189 (50.0%) | |
| Female | 148 (50.9%) | 189 (50.0%) | |
| Tumor size | < 0.001 | ||
| ≤ 2 cm | 34 (12.3%) | 126 (33.5%) | |
| 2–5 cm | 135 (48.7%) | 138 (36.7%) | |
| 5–10 cm | 73 (26.4%) | 86 (22.9%) | |
| > 10 cm | 35 (12.6%) | 26 (6.9%) | |
| Mitotic index | < 0.001 | ||
| ≤ 5 | 181 (75.4%) | 225 (61.1%) | |
| > 5 | 59 (24.6%) | 143 (38.9%) | |
| Morphology | 0.825 | ||
| Spindle | 148 (92.5%) | 341 (93.9%) | |
| Epithelioid | 1 (0.6%) | 2 (0.6%) | |
| Mixed | 11 (6.9%) | 20 (5.5%) | |
| NIH risk category | < 0.001 | ||
| Very low | 25 (9.7%) | 105 (28.5%) | |
| Low | 104 (40.3%) | 97 (26.3%) | |
| Intermediate | 2 (0.8%) | 87 (23.6%) | |
| High | 127 (49.2%) | 80 (21.7%) |
GIST: gastrointestinal stromal tumor; NIH: National Institutes of Health
In order to analyze the prognosis of duodenal and gastric GISTs, survivals of 202 duodenal GISTs were compared to those of 253 gastric GISTs according to the exclusion criteria of survival analysis. The univariate and multivariate analysis showed that location was an independent risk factor for DFS and DSS (P < 0.001, Table 7). As shown in Fig. 4, the 5-year DFS (64.4% vs 94.9%, P < 0.001) and DSS (80.9% vs 92.6%, P = 0.049) of duodenal GISTs were significantly worse than that of gastric GISTs.
Table 7.
Univariate and multivariate analysis of prognostic factors for duodenal and gastric GISTs
| Prognostic factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| β | Hazard ratio (95% CI) | P value | β | Hazard ratio (95% CI) | P value | |
| DFS | ||||||
| Age | −0.084 | 0.920 (0.517–1.638) | 0.776 | |||
| Gender | −0.393 | 0.675 (0.384–1.186) | 0.172 | |||
| Location | −2.105 | 0.122 (0.057–0.259) | 0.000 | −2.122 | 0.120 (0.054–0.266) | < 0.001 |
| Tumor size | 1.451 | 4.268 (2.932–6.213) | 0.000 | 1.417 | 4.124 (2.526–6.733) | < 0.001 |
| Mitotic index | 1.283 | 3.608 (1.868–6.970) | 0.000 | 0.928 | 2.528 (1.225–5.219) | 0.012 |
| NIH risk category | 1.813 | 6.128 (3.254–11.544) | 0.000 | |||
| DSS | ||||||
| Age | 0.387 | 1.473 (0.748–2.898) | 0.263 | 0.759 | 2.136 (1.040–4.384) | 0.039 |
| Gender | 0.279 | 1.322 (0.668–2.614) | 0.423 | |||
| Location | −0.718 | 0.488 (0.236–1.010) | 0.049 | −1.066 | 0.344 (0.164–0.725) | 0.005 |
| Tumor size | 1.297 | 3.658 (2.304–5.807) | 0.000 | 1.386 | 3.999 (2.408–6.641) | < 0.001 |
| Mitotic index | 1.202 | 3.327 (1.574–7.032) | 0.002 | |||
| NIH risk category | 1.094 | 2.985 (1.821–4.895) | 0.000 | |||
GIST: gastrointestinal stromal tumor; NIH: National Institutes of Health;
DFS: disease-free survival; DSS: disease-specific survival; CI: confidence interval
Fig. 4.

Comparison of DFS and DSS between duodenal and gastric GISTs
Discussion
The current study represented the largest number of duodenal GISTs to date. We found that LR was a more prevalent surgical procedure and PD was mainly performed for tumors with larger diameter or located in descending portion. Type of resection was not an independent risk factor for the prognosis of duodenal GISTs. Prognosis of duodenal GISTs was significantly worse than that of gastric GISTs.
GISTs are thought to derive from the interstitial cells of Cajal (ICC) [114], the pacemaker cells of gastrointestinal tract [115, 116]. A recent study found that the type of ICC distributed in proximal duodenum is very similar to that in stomach, and its distal duodenal pattern is more identical to that in jejunoileum [117]. Moreover, they found that ICC of circular muscle are only distributed in the proximal duodenum and are absent in the distal portion. In our study, most tumors located in the proximal portion of duodenum (superior and descending portion), which was consistent with the previous literature [1, 9, 13, 118]. This distribution characteristics may attribute to the distribution of ICC in this region. However, this remains to be further investigated.
Surgical strategy of duodenal GISTs remains challenging, owing to the unique anatomy of duodenum [91]. Complete surgical resection with sufficient margin and without intraoperative tumor rupture remains as the curative treatment for GISTs [2, 119]. Tumor size, location and invasion of adjacent organs are generally considered for the choice of surgery for duodenal GISTs [13, 120]. A few studies proponing PD as a routine procedure argued that an extensive surgery is always required in the pancreaticoduodenal region to obtain a clear margin and achieve a good oncological outcome [7, 13, 121]. On the other hand, LR, a less demanding procedure, could obviously decrease the perioperative morbidity and brings a parallel [121, 122] or better survival compared with PD [14]. A meta-analysis suggested LR as the routine choice for the duodenal GISTs whenever technically feasible, due to the good oncological outcomes and lower morbidity brought by this procedure compared with PD [118]. However, these results were all based on small samples. In our study, PD was mainly performed for GISTs with larger tumor size, higher risk-category or arose from descending portion. Although PD was associated with poorer survival of patients, surgical procedure was not an independent prognostic factor for duodenal GISTs. The survival disadvantage of PD observed in our study may be due to the higher-risk tumors distributed in the PD group.
In fact, the argument about LR and PD for duodenal GISTs mainly focused on tumors located in the descending portion. To date, study focused on this issue is lacking. In our study, PD was mainly performed for the descending GISTs. And, due to the particularly anatomic features of the duodenal descending portion, we then investigated the survival impact of surgical procedure for this subgroup of GISTs. The results showed that patients with descending GISTs underwent PD had larger tumor size and poorer DFS and DSS than those of patients underwent LR. However, multivariate analysis revealed that surgical procedure was not an independent prognostic factor.
Although our study indicated that type of resection was not associated with the prognosis of duodenal GISTs, the conclusion should be interpreted cautiously. For example, PD was the only choice to achieve a clearance margin when tumors were too large or close to the anatomically disadvantageous region. Thus, it is meaningless to compare the clinical impact of different types of resection without consideration of size and location of tumor. These two procedures could be compared only when the tumor is not large enough and is distant from the critical structures. However, to date, there is no more detailed study published. It is also a limitation in our study that the information of tumor location and involvement of the pancreaticoduodenal complex could not be extracted from published literatures.
Beside tumor size and mitotic index, tumor location is also reported as a key prognostic factor for GISTs [123, 124]. There are three main risk-stratification methods used to estimate the prognosis of GIST after surgery: NIH consensus criteria [125], Armed Forces Institute of Pathology (AFIP) criteria [126] and modified NIH criteria [113]. The latter two both include tumor site but only the AFIP criteria stratifies site into stomach, duodenum, jejunum and rectum while the modified NIH criteria only encompasses stomach and non-stomach. Even though, the comparison of survival between duodenal GISTs and GISTs from other sites was still rare due to the extremely low incidence [65]. Thus, we compared the prognosis of duodenal GISTs to gastric GISTs from our center. The univariate and multivariate analysis revealed that the DFS and DSS of duodenal were significantly worse than those of gastric GISTs. However, a recently nation-wide study [127] extracting GIST cases from Surveillance, Epidemiology, and End Results (SEER) database showed that gastric and small intestine GISTs had similar outcomes. This contrary result might because duodenal GIST was not analyzed separately from the small intestine GIST in their study which could lead to a bias. Actually, there is also a deficiency in current study, that the number of gastric GISTs in our study was relatively small compared to the large number of duodenal GISTs.
There are some other limitations in current study. Firstly, it is a retrospective single-center study and the completeness of systematic data is limited. Till now, the survival impact of surgical procedure on duodenal GISTs is still controversial, mainly because the lack of more accurate description of location of tumors in previous studies, which could result in a bias. Although the current study contained the largest number of duodenal GISTs, it still failed to make up this deficiency. Thus, a multi-center randomized control trial is needed to clarify this question. Secondly, due to the small size of small intestinal and colorectal GISTs in our center, the prognosis of duodenal GISTs were only compared to that of gastric GISTs.
Conclusions
The most common symptom of duodenal GISTs was bleeding. Descending portion was the most frequent tumor site. LR was a more prevalent surgical procedure and PD was mainly performed for tumors with larger diameter or located in descending portion. But type of resection was not an independent risk factor for the prognosis of duodenal GISTs. Thus, the choice of surgical strategy of duodenal GISTs prevalently depended on tumor size and location. Prognosis of duodenal GISTs was significantly worse than that of gastric GISTs.
Additional file
Table S1. The comparison of clinicopathological features of duodenal GISTs between our center and published data. Table S2. The comparison of clinicopathological features of duodenal GISTs between published data and the entire cohort. Figure S1. The comparison of survival. We analyzed our own data (37 cases) and compared to the published combined data (263 cases). Then compared the 263 cases to the total combined 300 cases. The results showed that there was no significant difference in the results of the two comparisons. (DOCX 7148 kb)
Acknowledgments
We wish to thank Xingbin Hu for his help with the revision of manuscript.
Funding
This study was supported in part by grants from the National Natural Scientific Foundation of China [NO. 31100643, 31570907, 81572306, 81502403, XJZT12Z03]. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing of this manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DFS
Disease-free survival
- DSS
Disease-specific survival
- GISTs
Gastrointestinal stromal tumors
- ICC
Interstitial Cajal cells
- LR
Limited resection
- PD
Pancreaticoduodenectomy
- SEER
Surveillance, Epidemiology, and End Results
Authors’ contributions
ZL, GZZ and JQL conceived the study and drafted the manuscript. SSL, GHX and QW collected the data and participated in drafting the manuscript. MG and XL performed statistical analysis. HWZ designed and supervised the study. All authors read and approved the final manuscript. All authors contributed to the writing of the manuscript and provided final approval of the manuscript. All authors have read and approved the final version of this manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Xijing Hospital, and written informed consent was obtained from the patients in our center.
Competing interests
There are no financial or other relations that could lead to a conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4485-4) contains supplementary material, which is available to authorized users.
Contributor Information
Zhen Liu, Email: doctorliuzhen@msn.com.
Gaozan Zheng, Email: 136697876@qq.com.
Jinqiang Liu, Email: ljq679@163.com.
Shushang Liu, Email: 1437167914@qq.com.
Guanghui Xu, Email: xuguanghui8@126.com.
Qiao Wang, Email: 594100214@qq.com.
Man Guo, Email: 1246504365@qq.com.
Xiao Lian, Email: lxlx119@126.com.
Hongwei Zhang, Phone: +86-029-84771531, Email: zhanghwfmmu@126.com.
Fan Feng, Phone: +86-029-84771531, Email: surgeonfengfan@163.com.
References
- 1.Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27(5):625–641. doi: 10.1097/00000478-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Connolly EM, Gaffney E, Reynolds JV. Gastrointestinal stromal tumours. Br J Surg. 2003;90(10):1178–1186. doi: 10.1002/bjs.4352. [DOI] [PubMed] [Google Scholar]
- 3.Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol. 2009;19(1):58–69. doi: 10.1016/j.annepidem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, Le Cesne A, McClure J, Maurel J, Nupponen N, Ray-Coquard I, Reichardt P, Sciot R, Stroobants S, van Glabbeke M, van Oosterom A, Demetri GD. Panelists Gcm. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST consensus conference of 20-21 march 2004, under the auspices of ESMO. Ann Oncol. 2005;16(4):566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 5.Singer S, Rubin BP, Lux ML, Chen CJ, Demetri GD, Fletcher CD, Fletcher JA. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol. 2002;20(18):3898–3905. doi: 10.1200/JCO.2002.03.095. [DOI] [PubMed] [Google Scholar]
- 6.Tien YW, Lee CY, Huang CC, Hu RH, Lee PH. Surgery for gastrointestinal stromal tumors of the duodenum. Ann Surg Oncol. 2010;17(1):109–114. doi: 10.1245/s10434-009-0761-5. [DOI] [PubMed] [Google Scholar]
- 7.Goh BK, Chow PK, Kesavan S, Yap WM, Wong WK. Outcome after surgical treatment of suspected gastrointestinal stromal tumors involving the duodenum: is limited resection appropriate? J Surg Oncol. 2008;97(5):388–391. doi: 10.1002/jso.20954. [DOI] [PubMed] [Google Scholar]
- 8.Goh BK, Chow PK, Ong HS, Wong WK. Gastrointestinal stromal tumor involving the second and third portion of the duodenum: treatment by partial duodenectomy and roux-en-Y duodenojejunostomy. J Surg Oncol. 2005;91(4):273–275. doi: 10.1002/jso.20311. [DOI] [PubMed] [Google Scholar]
- 9.Lee SY, Goh BK, Sadot E, Rajeev R, Balachandran VP, Gonen M, Kingham TP, Allen PJ, D'Angelica MI, Jarnagin WR, Coit D, Wong WK, Ong HS, Chung AY, DeMatteo RP. Surgical strategy and outcomes in duodenal gastrointestinal stromal tumor. Ann Surg Oncol. 2017;24(1):202–210. doi: 10.1245/s10434-016-5565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung JC, Chu CW, Cho GS, Shin EJ, Lim CW, Kim HC, Song OP. Management and outcome of gastrointestinal stromal tumors of the duodenum. J Gastrointest Surg. 2010;14(5):880–883. doi: 10.1007/s11605-010-1170-6. [DOI] [PubMed] [Google Scholar]
- 11.Gervaz P, Huber O, Morel P. Surgical management of gastrointestinal stromal tumours. Br J Surg. 2009;96(6):567–578. doi: 10.1002/bjs.6601. [DOI] [PubMed] [Google Scholar]
- 12.Yang WL, Yu JR, Wu YJ, Zhu KK, Ding W, Gao Y, Shen QY, Lv KZ, Zhang Q, Yang XJ. Duodenal gastrointestinal stromal tumor: clinical, pathologic, immunohistochemical characteristics, and surgical prognosis. J Surg Oncol. 2009;100(7):606–610. doi: 10.1002/jso.21378. [DOI] [PubMed] [Google Scholar]
- 13.Johnston FM, Kneuertz PJ, Cameron JL, Sanford D, Fisher S, Turley R, Groeschl R, Hyder O, Kooby DA, Blazer D, 3rd, Choti MA, Wolfgang CL, Gamblin TC, Hawkins WG, Maithel SK, Pawlik TM. Presentation and management of gastrointestinal stromal tumors of the duodenum: a multi-institutional analysis. Ann Surg Oncol. 2012;19(11):3351–3360. doi: 10.1245/s10434-012-2551-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Shou CH, Yu JR, Yang WL, Liu XS, Yu H, Gao Y, Shen QY, Zhao ZC. Prognostic characteristics of duodenal gastrointestinal stromal tumours. Br J Surg. 2015;102(8):959–964. doi: 10.1002/bjs.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto R, Kato S, Maru T, Ninomiya R, Ozawa F, Beck Y, Abe K, Tamaru J, Nagoshi S, Yakabi K. The coexistence of Somatostatinoma and gastrointestinal stromal tumor in the duodenum of a patient with Von Recklinghausen's disease. Internal medicine (Tokyo, Japan) 2016;55(6):617–622. doi: 10.2169/internalmedicine.55.5761. [DOI] [PubMed] [Google Scholar]
- 16.Valli PV, Valli C, Pfammatter T, Bauerfeind P. Life-threatening bleeding of a duodenal gastrointestinal stromal tumor in a teenager: a rare case report. Endoscopy international open. 2016;4(12):E1244–E1246. doi: 10.1055/s-0042-115936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugase T, Takahashi T, Nakajima K, Hirota S, Masuzawa T, Nishida T, Kimura Y, Miyazaki Y, Makino T, Kurokawa Y, Yamasaki M, Takiguchi S, Mori M, Doki Y. Clinicopathological characteristics, surgery and survival outcomes of patients with duodenal gastrointestinal stromal tumors. Digestion. 2016;94(1):30–36. doi: 10.1159/000447665. [DOI] [PubMed] [Google Scholar]
- 18.Palomeque Jimenez A, Rubio Lopez J, Perez Cabrera B, Jimenez Rios JA. Partial duodenectomy as a therapeutic option in multiple duodenal gastrointestinal stromal tumour associated with neurofibromatosis type 1. Gastroenterologia y hepatologia. 2017;40(8):534–536. doi: 10.1016/j.gastrohep.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Kumar T, Gupta B, Das P, Jain D, Jain HA, Madhusudhan KS, Dash NR, Gupta SD. Combined presence of multiple gastrointestinal stromal tumors along with duodenal submucosal somatostatinoma in a patient with neurofibromatosis type 1. Indian j pathology microbiol. 2016;59(3):359–361. doi: 10.4103/0377-4929.188123. [DOI] [PubMed] [Google Scholar]
- 20.Katagiri H, Sakamoto T, Shimaguchi M, Lefor AT, Kubota T, Mizokami K, Kishida A. Giant gastrointestinal stromal tumor arising from the fourth portion of the duodenum. Surgery. 2016;159(2):665–667. doi: 10.1016/j.surg.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Crown A, Biehl TR, Rocha FG. Local resection for duodenal gastrointestinal stromal tumors. Am J Surg. 2016;211(5):867–870. doi: 10.1016/j.amjsurg.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Chung JC, Kim HC, Hur SM. Limited resections for duodenal gastrointestinal stromal tumors and their oncologic outcomes. Surg Today. 2016;46(1):110–116. doi: 10.1007/s00595-015-1163-x. [DOI] [PubMed] [Google Scholar]
- 23.Boselli C, Cirocchi R, Gemini A, Barberini F, Grassi V, Avenia S, Polistena A, Sanguinetti A, Pironi D, Santoro A, Tabola R, Avenia N. Urgency surgical treatment for duodenal GISTs: analysis of aged patients and review of the literature. Aging Clin Exp Res. 2017;29(Suppl 1):1–6. doi: 10.1007/s40520-016-0641-3. [DOI] [PubMed] [Google Scholar]
- 24.Okasha HH, Amin HM, Al-Shazli M, Nabil A, Hussein H, Ezzat R. A duodenal gastrointestinal stromal tumor with a large central area of fluid and gas due to fistulization into the duodenal lumen, mimicking a large duodenal diverticulum. Endoscopic ultrasound. 2015;4(3):253–256. doi: 10.4103/2303-9027.163018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mrak K, Liegl-Atzwanger B, Haybaeck J, Petritsch W, Mischinger HJ, Kornprat P. Surgical Management of Duodenal Gastrointestinal Stromal Tumors: a case report. Anticancer Res. 2015;35(11):6321–6324. [PubMed] [Google Scholar]
- 26.Mikityanskiy Y, Marshak JE, Stavropoulos SN, Friedel DM. Images of the month: excavated duodenal gastrointestinal stromal tumor diagnosed via standard endoscopic biopsy. Am J Gastroenterol. 2015;110(7):964. doi: 10.1038/ajg.2014.402. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A, Jakhmola CK, Chauhan SS, Singh A. Atypical presentation of gastrointestinal stromal tumor masquerading as a large duodenal cyst: a case report. Int J Surg Case Rep. 2015;9:123–126. doi: 10.1016/j.ijscr.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karaahmet F, Hamamci M, Coskun Y, Akinci H, Yuksel I. Hidden duodenal gastrointestinal stromal tumor. Endoscopy. 2015;47(Suppl 1):UCTN:E176. doi: 10.1055/s-0034-1391499. [DOI] [PubMed] [Google Scholar]
- 29.Jarczyk G, Bereziak L, Jackowski M. Massive gastrointestinal bleeding caused by a gastrointestinal stromal tumour of the third part of the duodenum treated by means of emergency partial duodenal resection. Przeglad gastroenterologiczny. 2015;10(3):181–184. doi: 10.5114/pg.2015.49106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginori A, Scaramuzzino F, Marsili S, Tripodi S. Late hepatic metastasis from a duodenal gastrointestinal stromal tumor (29 years after surgery): report of a case and review of the literature. Int J Surg Pathol. 2015;23(4):317–321. doi: 10.1177/1066896915573571. [DOI] [PubMed] [Google Scholar]
- 31.Castro-Pocas FM, Araujo TP, Silva JD, Lopes CA, Miguel MS. Duodenal gastrointestinal stromal tumor and endoscopic ultrasound. Revista espanola de enfermedades digestivas : organo oficial de la Sociedad Espanola de Patologia Digestiva. 2015;107(12):759–760. [PubMed] [Google Scholar]
- 32.Ueda K, Hijioka M, Lee L, Igarashi H, Niina Y, Osoegawa T, Nakamura K, Takahashi S, Aishima S, Ohtsuka T, Takayanagi R, Ito T. A synchronous pancreatic neuroendocrine tumor and duodenal gastrointestinal stromal tumor. Internal medicine (Tokyo, Japan) 2014;53(21):2483–2488. doi: 10.2169/internalmedicine.53.2694. [DOI] [PubMed] [Google Scholar]
- 33.Slavik T, du Plessis J, Sparaco A, van der Merwe SW. Duodenal gastrointestinal stromal tumor with epithelioid and neural features mimicking a primary pancreas head neuroendocrine tumor. Pancreas. 2014;43(3):482–483. doi: 10.1097/MPA.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 34.Patil M, Sheth KA, Adarsh CK, Manjunath S, Devarbhavi H. Duodenal gastrointestinal stromal tumor presenting as massive gastrointestinal bleed. Indian j gastroenterology : offic j Indian Soc Gastroenterol. 2014;33(2):192–194. doi: 10.1007/s12664-013-0384-4. [DOI] [PubMed] [Google Scholar]
- 35.Parisi A, Desiderio J, Trastulli S, Grassi V, Ricci F, Farinacci F, Cacurri A, Castellani E, Corsi A, Renzi C, Barberini F, D’Andrea V, Santoro A, Cirocchi R. Robotic pancreaticoduodenectomy in a case of duodenal gastrointestinal stromal tumor. World j surgical oncol. 2014;12(1):372. doi: 10.1186/1477-7819-12-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuyama K, Fujikawa T, Kuramitsu S, Tanaka A. Successful treatment of bleeding large duodenal gastrointestinal stromal tumour in a patient under dual antiplatelet therapy after recent drug-eluting coronary stent implantation. BMJ case reports. 2014;2014. PMID: 24777088. 10.1136/bcr-2014-204462. [DOI] [PMC free article] [PubMed]
- 37.Donatelli G, Vergeau BM, Roseau G, Meduri B. Unusual presentation of a gastrointestinal stromal tumor of the duodenum mimicking an inflammatory enlargement of a peripancreatic lymph node. Annals gastroenterol quarterly pub Hellenic Soc Gastroenterol. 2014;27(4):410. [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng JM, Tirumani SH, Shinagare AB, Jagannathan JP, Hornick JL, Raut CP, Ramaiya NH. MDCT of primary, locally recurrent, and metastatic duodenal gastrointestinal stromal tumours (GISTs): a single institution study of 25 patients with review of literature. Clin Radiol. 2014;69(2):137–144. doi: 10.1016/j.crad.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Bormann F, Wild W, Aksoy H, Dorr P, Schmeck S, Schwarzbach M. A pancreatic head tumor arising as a duodenal GIST: a case report and review of the literature. Case Rep Med. 2014;2014:420295. doi: 10.1155/2014/420295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw A, Jeffery J, Dias L, Nazir S. Duodenal wedge resection for large gastrointestinal stromal tumour presenting with life-threatening haemorrhage. Case reports in gastrointestinal medicine. 2013;2013:562642. doi: 10.1155/2013/562642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serio G, Zampatti C, Ballabio A, Ricci R, Martini M, Zurleni F. Neurofibromatosis 1 presenting with multiple duodenal GISTS associated with a somatostatin-producing D cell neoplasm. Endocr Pathol. 2013;24(2):100–105. doi: 10.1007/s12022-013-9239-x. [DOI] [PubMed] [Google Scholar]
- 42.Ohi M, Yasuda H, Ishino Y, Katsurahara M, Saigusa S, Tanaka K, Tanaka K, Mohri Y, Inoue Y, Uchida K, Kusunoki M. Single-incision laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor arising from the duodenum. Asian j endoscopic surgery. 2013;6(4):307–310. doi: 10.1111/ases.12059. [DOI] [PubMed] [Google Scholar]
- 43.Mouaqit O, Chbani L, Maazaz K, Amarti A, Ait Taleb K, Oussaden A. A large gastrointestinal stromal tumor of the duodenum treated by partial duodenectomy with roux-en-Y duodenojejunostomy: a case report. J Med Case Rep. 2013;7:184. doi: 10.1186/1752-1947-7-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mokhtare M, Taghvaei T, Tirgar FH. Acute bleeding in duodenal gastrointestinal stromal tumor. Middle East j digestive dis. 2013;5(1):47–51. [PMC free article] [PubMed] [Google Scholar]
- 45.Liang X, Yu H, Zhu LH, Wang XF, Cai XJ. Gastrointestinal stromal tumors of the duodenum: surgical management and survival results. World J Gastroenterol. 2013;19(36):6000–6010. doi: 10.3748/wjg.v19.i36.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoeppner J, Kulemann B, Marjanovic G, Bronsert P, Hopt UT. Limited resection for duodenal gastrointestinal stromal tumors: surgical management and clinical outcome. World j gastrointestinal surgery. 2013;5(2):16–21. doi: 10.4240/wjgs.v5.i2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beham A, Schaefer IM, Cameron S, von Hammerstein K, Fuzesi L, Ramadori G, Ghadimi MB. Duodenal GIST: a single center experience. Int J Color Dis. 2013;28(4):581–590. doi: 10.1007/s00384-012-1432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acar F, Sahin M, Ugras S, Calisir A. A gastrointestinal stromal tumor of the third portion of the duodenum treated by wedge resection: a case report. World j gastrointestinal surgery. 2013;5(12):332–336. doi: 10.4240/wjgs.v5.i12.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh S, Paul S, Khandelwal P, Khichy S. Duodenal GIST presenting as large pancreatic head mass: an uncommon presentation. JOP : J pancreas. 2012;13(6):696–699. doi: 10.6092/1590-8577/1213. [DOI] [PubMed] [Google Scholar]
- 50.Salas NR, Cubillo A. Resected duodenal gastrointestinal stromal tumour with an affected margin and exon 9 mutation: adjuvant therapy. Anti-Cancer Drugs. 2012;23(Suppl):S18–S21. doi: 10.1097/CAD.0b013e3283559fcd. [DOI] [PubMed] [Google Scholar]
- 51.Muroni M, Ravaioli M, Del Gaudio M, Nigri G, D'Angelo F, Uccini S, Ramacciato G. Pancreas-preserving segmental duodenectomy for gastrointestinal stromal tumor of the duodenum and splenectomy for splenic angiosarcoma. Hepatobiliary & pancreatic dis int HBPD INT. 2012;11(3):325–329. doi: 10.1016/s1499-3872(12)60169-6. [DOI] [PubMed] [Google Scholar]
- 52.Lin C, Chang Y, Zhang Y, Zuo Y, Ren S. Small duodenal gastrointestinal stromal tumor presenting with acute bleeding misdiagnosed as hemobilia: two case reports. Oncol Lett. 2012;4(5):1069–1071. doi: 10.3892/ol.2012.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Gendi A, El-Gendi S, El-Gendi M. Feasibility and oncological outcomes of limited duodenal resection in patients with primary nonmetastatic duodenal GIST. J Gastrointest Surg. 2012;16(12):2197–2202. doi: 10.1007/s11605-012-2034-z. [DOI] [PubMed] [Google Scholar]
- 54.Cubas RF, Ballarino EA, Nieto FA, Diaz MD. Local resection of a gastrointestinal stromal tumor of the third portion of the duodenum. Am Surg. 2012;78(1):E22–E23. [PubMed] [Google Scholar]
- 55.Cavaniglia D, Petrucciani N, Lorenzon L, Caterino S, Cavallini M. Partial duodenectomy with end-to-end anastomosis for duodenal gastrointestinal stromal tumor. Am Surg. 2012;78(5):E273–E275. [PubMed] [Google Scholar]
- 56.Yagishita A, Matsubayashi H, Kakushima N, Tanaka M, Takizawa K, Yamaguchi Y, Ono H. Gastrointestinal stromal tumors of the duodenum: a report of four cases. Clin J Gastroenterol. 2011;4(3):162–166. doi: 10.1007/s12328-011-0218-9. [DOI] [PubMed] [Google Scholar]
- 57.Morcos B, Al-Ahmad F. A large gastrointestinal stromal tumor of the duodenum: a case report. J Med Case Rep. 2011;5:457. doi: 10.1186/1752-1947-5-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Machado NO, Chopra PJ, Al-Haddabi IH, Al-Qadhi H. Large duodenal gastrointestinal stromal tumor presenting with acute bleeding managed by a whipple resection. A review of surgical options and the prognostic indicators of outcome. JOP : J pancreas. 2011;12(2):194–199. [PubMed] [Google Scholar]
- 59.Li LF, Tse YH, Ho SL, Yan KW, Lui WM. Duodenal GIST metastasized to skull and orbit managed by surgery: a case report. Asian j surg. 2011;34(4):181–184. doi: 10.1016/j.asjsur.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Kato M, Nakajima K, Nishida T, Yamasaki M, Nishida T, Tsutsui S, Ogiyama H, Yamamoto S, Yamada T, Mori M, Doki Y, Hayashi N. Local resection by combined laparoendoscopic surgery for duodenal gastrointestinal stromal tumor. Diagnostic therapeutic endoscopy. 2011;2011:645609. doi: 10.1155/2011/645609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung JC, Kim HC, Chu CW. Segmental duodenectomy with duodenojejunostomy of gastrointestinal stromal tumor involving the duodenum. J Korean Surg Soc. 2011;80(Suppl 1):S12–S16. doi: 10.4174/jkss.2011.80.Suppl1.S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamaguchi T, Nishizaki D, Kagawa R, Takeda R, Onoyama H, Yosida K. Duodenum-preserving local excision of a gastrointestinal stromal tumor presenting with duodenal bleeding. Am Surg. 2010;76(4):444–446. [PubMed] [Google Scholar]
- 63.Wall ML, Ghallab MA, Farmer M, Durkin DJ. Gastrointestinal stromal tumour presenting with duodenal-jejunal intussusception: a case report. Ann R Coll Surg Engl. 2010;92(7):W32–W34. doi: 10.1308/147870810X12822015504527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohtake S, Kobayashi N, Kato S, Kubota K, Endo I, Inayama Y, Nakajima A. Duodenal gastrointestinal stromal tumor resembling a pancreatic neuroendocrine tumor in a patient with neurofibromatosis type I (von Recklinghausen's disease): a case report. J Med Case Rep. 2010;4:302. doi: 10.1186/1752-1947-4-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miki Y, Kurokawa Y, Hirao M, Fujitani K, Iwasa Y, Mano M, Nakamori S, Tsujinaka T. Survival analysis of patients with duodenal gastrointestinal stromal tumors. J Clin Gastroenterol. 2010;44(2):97–101. doi: 10.1097/MCG.0b013e3181b8e754. [DOI] [PubMed] [Google Scholar]
- 66.Kansakar R, Adhikari S. Local resection of gastrointestinal stromal tumor of the second part of duodenum. JNMA; j Nepal Med Assoc. 2010;50(180):306–308. [PubMed] [Google Scholar]
- 67.Hecker A, Hecker B, Bassaly B, Hirschburger M, Schwandner T, Janssen H, Padberg W. Dramatic regression and bleeding of a duodenal GIST during preoperative imatinib therapy: case report and review. World j surgical oncology. 2010;8:47. doi: 10.1186/1477-7819-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frampton AE, Bong JJ, Kyriakides C, Cohen P, Jiao LR. En bloc resection of the pancreatic head and second part of duodenum for a duodenal gastrointestinal stromal tumor: a multi-media report. JOP : J pancreas. 2010;11(4):396–400. [PubMed] [Google Scholar]
- 69.Buchs NC, Bucher P, Gervaz P, Ostermann S, Pugin F, Morel P. Segmental duodenectomy for gastrointestinal stromal tumor of the duodenum. World J Gastroenterol. 2010;16(22):2788–2792. doi: 10.3748/wjg.v16.i22.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takeuchi H, Matsumoto T, Kusumoto T, Yoshikawa Y, Muto Y. Duodenal gastrointestinal stromal tumor treated by wedge resection in a patient with Neurofibromatosis type 1: report of a case and review of the Japanese literature. Case reports in gastroenterology. 2009;3(3):343–349. doi: 10.1159/000255019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi Y, Shimizu S, Sakurai S, Kumagai A, Mori S, Fukusato T. Gastrointestinal stromal tumor in the duodenum exhibiting hemangiopericytoma-like histological pattern. Pathol Int. 2009;59(2):98–101. doi: 10.1111/j.1440-1827.2008.02335.x. [DOI] [PubMed] [Google Scholar]
- 72.Matsui T, Mitsui H, Sekigawa K, Kobayashi K, Okubo M, So E, Nakachi K, Hashimoto N, Yamaguchi H, Ishikawa M, Okuda J, Sato K, Terashima H, Kishida Y, Tamura K, Suzuki T. A case of a duodenal gastrointestinal stromal tumor diagnosed with the aid of diffusion-weighted magnetic resonance imaging. Clin J Gastroenterol. 2009;2(6):384–387. doi: 10.1007/s12328-009-0110-z. [DOI] [PubMed] [Google Scholar]
- 73.Adamiak A, Lee CH, Nielsen TO, Webber D, O'Connell JX. Duodenal epithelioid gastrointestinal stromal tumor with prominent granular cell features. Hum Pathol. 2009;40(4):599–602. doi: 10.1016/j.humpath.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 74.Mennigen R, Wolters HH, Schulte B, Pelster FW. Segmental resection of the duodenum for gastrointestinal stromal tumor (GIST) World j surgical oncology. 2008;6:105. doi: 10.1186/1477-7819-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liyanage CA, Abeygunawardhana S, Kumarage S, Deen KI. Duodenum-preserving local excision of a gastrointestinal stromal tumor. Hepatobiliary pancreatic dis int : HBPD INT. 2008;7(2):214–216. [PubMed] [Google Scholar]
- 76.Bhattacharya S, Choudhury AK, Ravi S, Morrissey J, Mathew G. Six years survival on imatinib with no disease progression after diagnosis of metastatic duodenal gastrointestinal stromal tumour: a case report. J Med Case Rep. 2008;2:110. doi: 10.1186/1752-1947-2-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asakawa M, Sakamoto Y, Kajiwara T, Nara S, Esaki M, Shimada K, Hamaguchi T, Kosuge T. Simple segmental resection of the second portion of the duodenum for the treatment of gastrointestinal stromal tumors. Langenbeck's Arch Surg. 2008;393(4):605–609. doi: 10.1007/s00423-007-0243-9. [DOI] [PubMed] [Google Scholar]
- 78.Takeda A, Watanabe Y, Uehara T, Maruyama T, Tanaka H, Matsuzaki H, Arima H, Natsune T, Kudo H, Sakama A, Tohnosu N, Shimada H, Sato H. Successful surgical resection of a huge gastrointestinal stromal tumor of the third portion of the duodenum. J Gastroenterol Hepatol. 2007;22(2):283–284. doi: 10.1111/j.1440-1746.2006.04484.x. [DOI] [PubMed] [Google Scholar]
- 79.Mohiuddin K, Nizami S, Munir A, Memon B, Memon MA. Metastatic duodenal GIST: role of surgery combined with imatinib mesylate. Int seminars surgical oncology : ISSO. 2007;4:9. doi: 10.1186/1477-7800-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ludvigsen L, Toxvaerd A, Mahdi B, Krarup-Hansen A, Bergenfeldt M. Successful resection of an advanced duodenal gastrointestinal stromal tumor after down-staging with imatinib: report of a case. Surg Today. 2007;37(12):1105–1109. doi: 10.1007/s00595-007-3544-2. [DOI] [PubMed] [Google Scholar]
- 81.Lanuke K, Bathe OF. Mack LA. Local excision of duodenal gastrointestinal stromal tumor. J Surg Oncol. 2007;95(3):267–269. doi: 10.1002/jso.20618. [DOI] [PubMed] [Google Scholar]
- 82.Kwon SH, Cha HJ, Jung SW, Kim BC, Park JS, Jeong ID, Lee JH, Nah YW, Bang SJ, Shin JW, Park NH, Kim DH. A gastrointestinal stromal tumor of the duodenum masquerading as a pancreatic head tumor. World J Gastroenterol. 2007;13(24):3396–3399. doi: 10.3748/wjg.v13.i24.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kan EY, Wong LM. Massive occult haemoperitoneum following spontaneous rupture of duodenal gastrointestinal stromal tumour. Australas Radiol. 2007;51:B130–B132. doi: 10.1111/j.1440-1673.2007.01699.x. [DOI] [PubMed] [Google Scholar]
- 84.Gupta N, Schirmer BD, Mishra R, Shami VM. Malignant GIST masquerading as a bleeding duodenal diverticulum. Endoscopy. 2007;39(Suppl 1):E142–E143. doi: 10.1055/s-2007-966245. [DOI] [PubMed] [Google Scholar]
- 85.Chiarugi M, Galatioto C, Lippolis P, Zocco G, Seccia M. Gastrointestinal stromal tumour of the duodenum in childhood: a rare case report. BMC Cancer. 2007;7:79. doi: 10.1186/1471-2407-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akatsu T, Aiura K, Kawachi S, Tanabe M, Shimazu M, Ueda M, Kameyama K, Kitajima M. Duodenal gastrointestinal stromal tumor adjacent to the minor papilla with concomitant pancreatic divisum. Dig Dis Sci. 2007;52(11):3191–3198. doi: 10.1007/s10620-006-9254-6. [DOI] [PubMed] [Google Scholar]
- 87.Stratopoulos C, Soonawalla Z, Piris J, Friend PJ. Hepatopancreatoduodenectomy for metastatic duodenal gastrointestinal stromal tumor. Hepatobiliary pancreatic dis int : HBPD INT. 2006;5(1):147–150. [PubMed] [Google Scholar]
- 88.Sakakura C, Hagiwara A, Soga K, Miyagawa K, Nakashima S, Yoshikawa T, Kin S, Nakase Y, Yamaoka N, Sagara Y, Yamagishi H. Long-term survival of a case with multiple liver metastases from duodenal gastrointestinal stromal tumor drastically reduced by the treatment with imatinib and hepatectomy. World J Gastroenterol. 2006;12(17):2793–2797. doi: 10.3748/wjg.v12.i17.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Juergens KU, Weckesser M, Bettendorf O, Wormanns D. Duodenal somatostatinoma and gastrointestinal stromal tumor associated with neurofibromatosis type 1: diagnosis with PET/CT. AJR Am J Roentgenol. 2006;187(2):W233–W234. doi: 10.2214/AJR.05.1328. [DOI] [PubMed] [Google Scholar]
- 90.Vu HA, Xinh PT, Kikushima M, Zhu Y, Tokuhara M, Tani M, Shimizu T, Saito K, Tokunaga K, Sato Y. A recurrent duodenal gastrointestinal stromal tumor with a frameshift mutation resulting in a stop codon in KIT exon 13. Genes, chromosomes cancer. 2005;42(2):179–183. doi: 10.1002/gcc.20110. [DOI] [PubMed] [Google Scholar]
- 91.Uchida H, Sasaki A, Iwaki K, Tominaga M, Yada K, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. An extramural gastrointestinal stromal tumor of the duodenum mimicking a pancreatic head tumor. J Hepato-Biliary-Pancreat Surg. 2005;12(4):324–327. doi: 10.1007/s00534-005-0985-0. [DOI] [PubMed] [Google Scholar]
- 92.Kurihara N, Kikuchi K, Tanabe M, Kumamoto Y, Tsuyuki A, Fujishiro Y, Otani Y, Kubota T, Kumai K, Kitajima M. Partial resection of the second portion of the duodenum for gastrointestinal stromal tumor after effective transarterial embolization. Int J Clin Oncol. 2005;10(6):433–437. doi: 10.1007/s10147-005-0503-z. [DOI] [PubMed] [Google Scholar]
- 93.Sakakima Y, Inoue S, Fujii T, Hatsuno T, Takeda S, Kaneko T, Nagasaka T, Nakao A. Emergency pylorus-preserving pancreatoduodenectomy followed by second-stage pancreatojejunostomy for a gastrointestinal stromal tumor of the duodenum with an intratumoral gas figure: report of a case. Surg Today. 2004;34(8):701–705. doi: 10.1007/s00595-004-2771-z. [DOI] [PubMed] [Google Scholar]
- 94.Hughes JA, Cook JV, Said A, Chong SK, Towu E, Reidy J. Gastrointestinal stromal tumour of the duodenum in a 7-year-old boy. Pediatr Radiol. 2004;34(12):1024–1027. doi: 10.1007/s00247-004-1298-1. [DOI] [PubMed] [Google Scholar]
- 95.Aksoy NH, Cevikol C, Ogus M, Elpek GO, Gelen T. Adenocarcinoma arising in villous adenoma of the ampulla of Vater with synchronous malignant gastrointestinal stromal tumour of the duodenum: a case report. J Clin Pathol. 2004;57(10):1118–1119. doi: 10.1136/jcp.2004.018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sawaki A, Ohashi K, Yamao K, Inada K, Shimizu Y, Matsuura A, Nakamura T, Suzuki T, Hara K, Okubo K, Ohno R. Effect of a tyrosine kinase inhibitor STI571 in a patient with hepatic metastases from a duodenal gastrointestinal stromal tumor. J Gastroenterol. 2003;38(7):690–694. doi: 10.1007/s00535-002-1124-1. [DOI] [PubMed] [Google Scholar]
- 97.Mussack T, Szeimies U, Arbogast S, Schiemann U, Siebeck M, Hallfeldt K. Extraluminal gastrointestinal stromal tumour in the second portion of the duodenum. Eur J Gastroenterol Hepatol. 2003;15(9):1043–1046. doi: 10.1097/00042737-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi Y, Noguchi T, Takeno S, Uchida Y, Shimoda H, Yokoyama S. Gastrointestinal stromal tumor of the duodenal ampulla: report of a case. Surg Today. 2001;31(8):722–726. doi: 10.1007/s005950170079. [DOI] [PubMed] [Google Scholar]
- 99.Zioni T, Dizengof V, Kirshtein B. Laparoscopic resection of duodenal gastrointestinal stromal tumour. J minimal access surgery. 2017;13(2):157–160. doi: 10.4103/0972-9941.195576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mitsui Y, Kagemoto K, Itagaki T, Inoue S, Yamasaki S, Naruse K, Tamura S, Muguruma N, Takayama T. Duodenal gastrointestinal stromal tumor (GIST) with deletion in exon 11 of c-kit treated with emergency pancreaticoduodenectomy due to massive bleeding: a case report. Nihon Shokakibyo Gakkai zasshi Japanese j gastro-enterology. 2015;112(4):690–698. doi: 10.11405/nisshoshi.112.690. [DOI] [PubMed] [Google Scholar]
- 101.Takeda Y, Nakahira S, Katsura Y, Ohmura Y, Kusama H, Kuroda Y, Goto T, Hashimoto T, Kimura K, Matsushita K, Sato Y, Morimoto Y, Ishida T, Nitta K, Kagawa Y, Okishiro M, Takeno A, Sakisaka H, Taniguchi H, Egawa C, Ohzono K, Nakatsuka S, Kato T, Tamura S. A case of recurrent duodenal gastrointestinal stromal tumor resistant to imatinib and sunitinib, successfully treated with regorafenib. Gan to kagaku ryoho Cancer & chemotherapy. 2014;41(12):1545–1547. [PubMed] [Google Scholar]
- 102.Inoue T, Okumura F, Fukusada S, Kachi K, Anbe K, Nishie H, Nishi Y, Mizushima T, Sano H. Two cases of distal duodenal gastrointestinal stromal tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy. Nihon Shokakibyo Gakkai zasshi = The Japanese journal of gastro-enterology. 2013;110(12):2112–2118. [PubMed] [Google Scholar]
- 103.Nishitai R, Manaka D, Uehara M, Hamasu S, Konishi S, Sakamoto K, Yoshino K, Kanto S, Yokoyama D, Kobayashi A, Jinzai Y, Yasuhara Y. Long-term progression-free survival after reduction surgery and postoperative low-dose imatinib administration for multiple liver metastases of duodenal gastrointestinal stromal tumor. Gan to kagaku ryoho Cancer & chemotherapy. 2012;39(6):979–982. [PubMed] [Google Scholar]
- 104.Yamashita K, Hosaka S, Kawamoto S, Yoshida T. A case of ruptured gastrointestinal stromal tumor of the duodenum with intraabdominal bleeding. Gan to kagaku ryoho Cancer & chemotherapy. 2011;38(12):2039–2041. [PubMed] [Google Scholar]
- 105.Okamoto N, Yamafuji K, Takeshima K, Hayashi N, Baba H. A case of gastrointestinal stromal tumor of the duodenum with a huge abscess. Nihon Shokakibyo Gakkai zasshi = The Japanese journal of gastro-enterology. 2011;108(11):1886–1891. [PubMed] [Google Scholar]
- 106.Asami S, Idani H, Kubota S, Kubo S, Kumano K, Kurose Y, Nojima H, Nakano KY, Yoshioka T, Sasaki H, Muro M, Kin H, Takakura N. A case of duodenal gastrointestinal stromal tumor treated by pancreas-sparing partial duodenectomy. Gan to kagaku ryoho Cancer & chemotherapy. 2010;37(12):2786–2788. [PubMed] [Google Scholar]
- 107.Oguri H. A case of multiple gastrointestinal stromal tumors of the duodenum and jejunum with von Recklinghausen's disease in which CT during angiography was useful for localization and diagnosis. Nihon Shokakibyo Gakkai zasshi = The Japanese journal of gastro-enterology. 2009;106(9):1351–1358. [PubMed] [Google Scholar]
- 108.Sakakura C, Kumano T, Mizuta Y, Yamaoka N, Sagara Y, Hagiwara A, Otsuji E. Successful treatment of huge peritoneal metastasis from duodenal gastrointestinal stromal tumor resistant for imatinib mesylate. Gan to kagaku ryoho Cancer & chemotherapy. 2007;34(12):2144–2146. [PubMed] [Google Scholar]
- 109.Cavallini M, Cecera A, Ciardi A, Caterino S, Ziparo V. Small periampullary duodenal gastrointestinal stromal tumor treated by local excision: report of a case. Tumori. 2005;91(3):264–266. doi: 10.1177/030089160509100311. [DOI] [PubMed] [Google Scholar]
- 110.Sakamoto Y, Yamamoto J, Takahashi H, Kokudo N, Yamaguchi T, Muto T, Makuuchi M. Segmental resection of the third portion of the duodenum for a gastrointestinal stromal tumor: a case report. Jpn J Clin Oncol. 2003;33(7):364–366. doi: 10.1093/jjco/hyg063. [DOI] [PubMed] [Google Scholar]
- 111.Otsu H, Oki E, Kawano H, Ando K, Ito S, Sugimachi K, Saeki H, Uchiyama H, Soejima Y, Kawanaka H, Morita M, Sakaguchi Y, Kusumoto T, Ikeda T, Maehara Y. [patient with bulky duodenum GIST became complete resection possible after primary systemic therapy: a case report]. Fukuoka igaku zasshi = Hukuoka acta medica. 2013;104(12):585–588. [PubMed] [Google Scholar]
- 112.Winfield RD, Hochwald SN, Vogel SB, Hemming AW, Liu C, Cance WG, Grobmyer SR. Presentation and management of gastrointestinal stromal tumors of the duodenum. Am Surg. 2006;72(8):719–722. [PubMed] [Google Scholar]
- 113.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39(10):1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 114.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382(9896):973–983. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 115.Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G602–G611. doi: 10.1152/ajpgi.2001.281.3.G602. [DOI] [PubMed] [Google Scholar]
- 116.Ward SM, Sanders KM, Hirst GD. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil. 2004;16(Suppl 1):112–117. doi: 10.1111/j.1743-3150.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 117.Radenkovic G. Two patterns of development of interstitial cells of Cajal in the human duodenum. J Cell Mol Med. 2012;16(1):185–192. doi: 10.1111/j.1582-4934.2011.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chok AY, Koh YX, Ow MY, Allen JC, Jr, Goh BK. A systematic review and meta-analysis comparing pancreaticoduodenectomy versus limited resection for duodenal gastrointestinal stromal tumors. Ann Surg Oncol. 2014;21(11):3429–3438. doi: 10.1245/s10434-014-3788-1. [DOI] [PubMed] [Google Scholar]
- 119.Hohenberger P, Ronellenfitsch U, Oladeji O, Pink D, Strobel P, Wardelmann E, Reichardt P. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010;97(12):1854–1859. doi: 10.1002/bjs.7222. [DOI] [PubMed] [Google Scholar]
- 120.Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7(9):705–712. doi: 10.1007/s10434-000-0705-6. [DOI] [PubMed] [Google Scholar]
- 121.Colombo C, Ronellenfitsch U, Yuxin Z, Rutkowski P, Miceli R, Bylina E, Hohenberger P, Raut CP, Gronchi A. Clinical, pathological and surgical characteristics of duodenal gastrointestinal stromal tumor and their influence on survival: a multi-center study. Ann Surg Oncol. 2012;19(11):3361–3367. doi: 10.1245/s10434-012-2559-0. [DOI] [PubMed] [Google Scholar]
- 122.Duffaud F, Meeus P, Bachet JB, Cassier P, Huynh TK, Boucher E, Bouche O, Moutardier V, le Cesne A, Landi B, Marchal F, Bay JO, Bertucci F, Spano JP, Stoeckle E, Collard O, Chaigneau L, Isambert N, Lebrun-Ly V, Mancini J, Blay JY, Bonvalot S. Conservative surgery vs. duodeneopancreatectomy in primary duodenal gastrointestinal stromal tumors (GIST): a retrospective review of 114 patients from the French sarcoma group (FSG) Euro j surg oncol j Euro Soc Surg Oncol British Assoc Surg Oncol. 2014;40(10):1369–1375. doi: 10.1016/j.ejso.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 123.Rutkowski P, Nowecki ZI, Michej W, Debiec-Rychter M, Wozniak A, Limon J, Siedlecki J, Grzesiakowska U, Kakol M, Osuch C, Polkowski M, Gluszek S, Zurawski Z, Ruka W. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007;14(7):2018–2027. doi: 10.1245/s10434-007-9377-9. [DOI] [PubMed] [Google Scholar]
- 124.Wozniak A, Rutkowski P, Piskorz A, Ciwoniuk M, Osuch C, Bylina E, Sygut J, Chosia M, Rys J, Urbanczyk K, Kruszewski W, Sowa P, Siedlecki J, Debiec-Rychter M, Limon J, Polish Clinical GR. Prognostic value of KIT/PDGFRA mutations in gastrointestinal stromal tumours (GIST): polish clinical GIST registry experience. Ann Oncol. 2012;23(2):353–360. doi: 10.1093/annonc/mdr127. [DOI] [PubMed] [Google Scholar]
- 125.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33(5):459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 126.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 127.Giuliano K, Nagarajan N, Canner J, Najafian A, Wolfgang C, Schneider E, Meyer C, Lennon AM, Johnston FM, Ahuja N. Gastric and small intestine gastrointestinal stromal tumors: do outcomes differ? J Surg Oncol. 2017;115(3):351–357. doi: 10.1002/jso.24514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The comparison of clinicopathological features of duodenal GISTs between our center and published data. Table S2. The comparison of clinicopathological features of duodenal GISTs between published data and the entire cohort. Figure S1. The comparison of survival. We analyzed our own data (37 cases) and compared to the published combined data (263 cases). Then compared the 263 cases to the total combined 300 cases. The results showed that there was no significant difference in the results of the two comparisons. (DOCX 7148 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


