Abstract
Traumatic brain injury (TBI) is a major cause of death and disability worldwide. Despite intense investigation, no neuroprotective agents for TBI have yet translated to the clinic. Recent efforts have focused on identifying potential therapeutic targets that underlie the secondary TBI pathology that evolves minutes to years following the initial injury. Oxidative stress is a key player in this complex cascade of secondary injury mechanisms and prominently contributes to neurodegeneration and neuroinflammation. NADPH oxidase (NOX) is a family of enzymes whose unique function is to produce reactive oxygen species (ROS). Human post-mortem and animal studies have identified elevated NOX2 and NOX4 levels in the injured brain, suggesting that these two NOXs are involved in the pathogenesis of TBI. In support of this, NOX2 and NOX4 deletion studies have collectively revealed that targeting NOX enzymes can reduce oxidative stress, attenuate neuroinflammation, promote neuronal survival, and improve functional outcomes following TBI. In addition, NOX inhibitor studies have confirmed these findings and demonstrated an extended critical window of efficacious TBI treatment. Finally, the translational potential, caveats, and future directions of the field are highlighted and discussed throughout the review.
Abbreviations: CCI, Controlled cortical impact; NOX, NADPH oxidase; ROS, reactive oxygen species; TBI, traumatic brain injury; WT, wild-type
Keywords: NADPH oxidase, NOX, NOX2, NOX4, NOX1, Traumatic brain injury, TBI, FPI, CCI, Controlled cortical impact, CHI, ROS, Oxidative stress, Microglia, Apocynin, gp91ds-tat
1. Introduction
Traumatic brain injury (TBI) is the leading cause of death and disability in young adults in the United States [1]. It accounts for approximately 2.8 million annual visits to the emergency department, hospitalizations, and deaths [2], [3], [4]. In recent years, TBI has gained recognition as an acute trauma that can progress into a chronic disorder [5], [6]. For instance, TBI in military veterans has been associated with a 60% increase in the risk of dementia [7]. Furthermore, a growing body of evidence suggests that even mild TBI can have detrimental cognitive consequences in the long-term [3]. Unfortunately, the heterogeneity of trauma based on injury location and severity, as well as patient age and associated comorbidities, has posed a significant challenge in the development of effective therapies for TBI [8]. As a consequence, current neurocritical care still focuses primarily on prevention of TBI and stabilizing TBI patients [9].
Further complicating the search for appropriate TBI treatments is the fact that a complex cascade of secondary injury mechanisms develops following the primary mechanical injury [10], [11], [12], [13]. These secondary pathological mechanisms include edema, ischemia, neuroinflammation, and hypoxia. They evolve over minutes to years after the injury, and can ultimately lead to neuronal cell death (even extending into the initially healthy surrounding tissue) [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]. A number of clinical trials have attempted to target some of these pathological mechanisms identified in preclinical animal studies. However, these trials have collectively failed, possibly due to a lack of variety in animal models utilized, rigor and reproducibility across species, or suboptimal drug administration in the trials [26], [27], [28]. Hence, there remains a strong need for rigorous animal studies to understand other injury mechanisms following TBI, which hopefully can lead to effective therapeutic targets for brain injury.
1.1. Oxidative stress in TBI
In recent years, there has been growing evidence that oxidative stress contributes significantly to secondary injury in TBI pathology. For instance, many studies have shown that oxidative stress plays a key role in the development of cerebral edema, inflammation, and the secondary neuronal damage found post-TBI [5], [11], [29], [30], [31], [32], [33]. Although reactive oxygen species (ROS) have vital physiological functions [34], [35], [36], pathological conditions, such as brain injury, can quickly shift the ROS/antioxidant balance in favor of ROS and significantly impact the severity and progression of TBI [5], [8], [37]. Since previous animal studies of promising ROS scavengers and ROS degrading agents in other neurodegenerative disorders have failed to translate to the clinic [38], [39], it has been suggested that targeting the generation of ROS may be a more successful avenue of therapy for brain injury [5]. Of the many enzymes that produce ROS in the cell, nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase; NOX) is the only family of enzymes with the sole purpose of producing ROS, whereas others generate ROS as a byproduct [40], [41]. While NOX enzymes undoubtedly contribute to physiological functions in the brain [42], [43], many laboratories have focused on enhancing our understanding of their pathological role in brain injury [5], [44], [45]. With evidence suggesting that chronic activation of NOX is detrimental and can even exacerbate the primary injury [6], NOX enzymes have emerged as a potential therapeutic target for TBI.
1.2. The NADPH oxidase enzymes
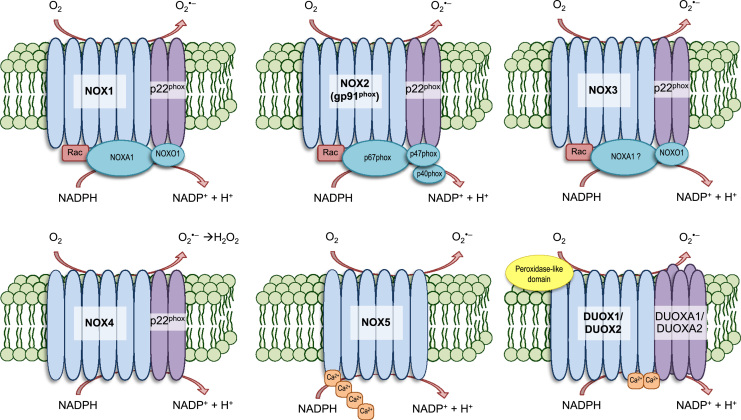
Initially discovered and characterized as the ROS-generating enzyme in phagocytes responsible for the “respiratory burst,” NOX enzymes consume oxygen to generate superoxide and hydrogen peroxide that can go on to produce other forms of ROS, such as hydroxyl and peroxynitrite [43], [44], [46], [47]. To date, seven transmembrane isoforms of the NOX enzyme (Fig. 1) have been identified in non-phagocytic cells, each having binding sites for heme, FAD, and NADPH [48], [49], [50], [51], [52], [53]. NOX1–5 and dual oxidase (DUOX) 1–2 are distributed broadly throughout various tissues and cells, but often times a single isoform is heavily concentrated in specific tissues [44]: NOX1 in the colon [54], [55], NOX2 in phagocytes [56], NOX3 in the inner ear [57], [58], NOX4 in the kidneys [59], [60], NOX5 in the spleen and testis [61], [62], and DUOX1/2 in the thyroid [49], [63]. NOX isoforms are expressed in various brain regions (forebrain, midbrain and hindbrain) and cell types (neurons, astrocytes, and microglia) [44], [64]. Activation of NOX/DUOX enzymes may include several steps involving phosphorylation and translocation of cytosolic subunits, if required, to the membrane where they join transmembrane subunits to form the active complex that transfers an electron from NADPH to O2, producing superoxide [44], [48], [49], [50], [51], [52], [53], [65], [66], [67]. Ma et al. summarizes the expression of NOX isoforms in different brain regions and their involvement in brain injury and neurodegenerative diseases [5]. The most studied and heavily implicated isoform in the context of TBI is NOX2. In addition, recent studies also support an emerging role for NOX4 [5], [31]. Unfortunately, not every isoform has been extensively characterized and studied in the pathogenesis of TBI, but the current existing literature supports the potential translation of NOX targeting therapies for treatment of TBI, as will be discussed in the subsequent sections below.
Fig. 1.
Structure of active NOX and DUOX enzymes. NOX and DUOX enzymes have a primary function to generate ROS. Several components make up the active transmembrane enzymes of each NOX/DUOX isoform. NOX1-5 and DUOX1-2 are shown here. NOX 1-3 are the most structurally similar, each requiring cytosolic subunits for activation. It is believed that the NOX4 isoform is constitutively active, yet inducible, and its generated superoxide is rapidly converted into hydrogen peroxide. NOX5 and the DUOX enzymes are reportedly sensitive to cellular Ca2+ concentrations. Though not pictured, activation of NOX isoforms may require phosphorylation of different sites within each subunit.
2. Elevated expression of NOX enzymes in TBI
2.1. Clinical correlations
Several groups have examined the role of NOX isoforms in human TBI pathology, and the clinical and post-mortem human data support NOX involvement in TBI. In humans, TBI increases the expression of NOX2 in circulating monocytes 1 day post-injury (dpi), suggesting that TBI can induce systemic inflammatory responses [68]. Sampling the cerebral cortex from post-mortem human brains revealed peak NOX2 expression in neurons and astrocytes between 6 and 24 h post-injury and peak NOX4 expression in neurons between 1 and 2 dpi [69], [70]. This increased level of NOX2 was associated with increased DNA oxidation [70]. Furthermore, higher expression of NOX2 and NOX4 in the brain correlated with increased severity of TBI as measured by the Glasgow coma scale [69]. Another study using TBI post-mortem human brain samples reported that NOX2 expression was also detected in microglia [70]. Finally, cortices from athletes diagnosed with chronic traumatic encephalopathy also showed higher expression of NOX4 and p22phox (a subunit of NOX 1–4 isoforms) [71].
2.2. Animal studies – NOX2
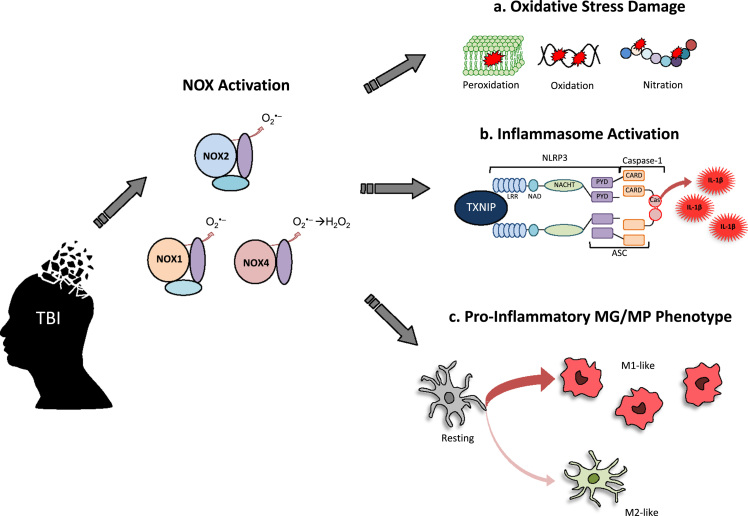
Animal studies have extensively evaluated NOX elevation and associated pathology following TBI across rodent species and various models of focal and diffuse brain injury. Increases in NOX expression post-injury, which is exacerbated in aged mice [72], are frequently accompanied by evidence of increased ROS production, oxidative stress damage, lesion volume, and cell death that persist throughout the first year following TBI [6], [73], [74], [75], [76], [77]. Of all the NOX isoforms, NOX2 is the most studied in TBI and is found mostly in neurons and microglia following injury [78]. Several groups have shown that TBI-induced NOX2 is associated with increased oxidative damage [77], neuroinflammation [79], and microglial activation (Fig. 2) [77], [80], [81], [82].
Fig. 2.
NOX involvement in secondary TBI pathology. TBI induces activation of NOX enzymes (in particular, NOX1, NOX2, and NOX4) to produce ROS. The activated NOX induces secondary TBI pathology that can exacerbate the primary injury. A few prominent examples are depicted here: a. production of oxidative stress damage (peroxidation of lipids, oxidation of DNA, nitration of amino acids), b. generation of pro-inflammatory cytokines (IL-1β) by a TXNIP-mediated activation of NLRP3 inflammasome, and c. regulation of MG/MP phenotype towards the pro-inflammatory M1-like phenotype. Abbreviations: NLRP3 – nucleotide oligomerization domain (NOD)-like receptors containing Pyrin domain-3; TXNIP – thioredoxin interacting protein; LRR – leucine rich repeats; NAD – NACHT-associated domain; NACHT - domain present in NAIP, CIITA, HET-E and telomerase associated protein; PYD – pyrin domain; CARD – caspase recruitment domain; Cas – caspase-1; ASC - apoptosis associated speck-like protein containing a CARD; IL-1β – interleukin-1 beta; MG/MP – microglia/macrophage.
Our laboratory and others demonstrated that NOX activity and NOX2 expression in the cortex and hippocampal CA1 region increases rapidly following controlled cortical impact (CCI) with an early peak at 1 h post-injury and a secondary peak from 1 to 4 dpi [77], [83]. Others have characterized the mRNA expression, protein expression, and activity of NOX2 post-TBI in mice and rats and found NOX2 expression and NOX activity to be increased at 1 dpi [72], [73], [74], [76], [78], [84], [85]. Furthermore, translocation of other NOX isoform subunits to the plasma membrane has also been demonstrated for p47phox, p67phox, and p40phox in the injured brain as early as 6 h post-injury [76], [86], which further supports NOX activation early after TBI. NOX2 activation persists at 7 dpi, when the majority of NOX2 and p22phox expression is found in Iba1+ microglia near the lesion [72], [78], [87]. It has even been suggested that NOX activity increases in the contralateral cortex at 7 dpi [73], indicating that global brain inflammation can occur at this time-point. Microglia that persist at 1 month after TBI in the injured hemisphere highly express NOX2 [78], [81]. Furthermore, an acute injury such as TBI can lead to chronic activation of microglia, and their elevated expression of NOX2 at 3–4 months [72], [88] and even 1 year after the initial injury [6]. This finding suggests that NOX2 may play a role in the chronic inflammation that occurs following TBI.
Interestingly, a series of recent studies demonstrated that microglia shift toward a pro-inflammatory state (M1-like) that express high levels of NOX2 at 7 dpi, which is associated with increased cortical and hippocampal neurodegeneration [82], [89]. It's worthwhile to note that NOX2 is also highly activated in infiltrating macrophages following TBI, suggesting the possibility of peripheral involvement in TBI pathology [90]. Though some anti-inflammatory (M2-like) microglia are present at 7 dpi, they are reduced by 5 weeks and reportedly absent at 12 weeks and 1 year post-TBI [6]. Since the generally M1-like microglia isolated from the injured mouse cortex at 7 dpi are neurotoxic [82], it's strongly possible that these NOX2-expressing microglia contribute to the progressive lesion expansion seen in TBI at chronic time-points. In support of this possibility, microglia isolated from NOX2-/- mice after TBI had a significantly attenuated neurotoxicity on surrounding neurons [82]. This reduced neurotoxicity could be due to reduced generation of damaging cytokines, as NOX2-/- mice had a profoundly attenuated expression of TNF-α and IL-1β [82]. Taken as a whole, these animal studies demonstrate that NOX2 is upregulated in the injured cortex after TBI, and thus could play an important role in TBI pathogenesis and post-injury neuroinflammation.
2.3. Animal studies – other NOX isoforms
Although the majority of TBI studies have focused on NOX2, other isoforms have also been examined, and their expression has been characterized in the post-TBI brain [78]. Of the ones studied, NOX3 appears unresponsive to injury, showing little change in its neuronal expression after TBI [78]. Several studies have investigated involvement of NOX1 in TBI, showing that an increased NOX activity at 1 dpi was accompanied by increased NOX1 expression [75], [91], [92]. At this early time-point, the increased NOX1 expression was accompanied by evidence of elevated oxidative damage, such as lipid peroxidation and protein nitration [75], [91], [92]. NOX1 is induced after both single and repeated blast injuries, with NOX1 mRNA upregulated as early as 6 h post-injury, followed by a peak NOX1 protein expression at 2–8 dpi [91]. TBI-induced NOX1 correlates with instability of the blood brain barrier (BBB) in the cerebral microvessels, suggesting that NOX1 is associated with cerebrovascular injury [91].
Reports of NOX4's involvement in TBI have also emerged recently. NOX4 has been characterized in neurons, astrocytes, and microglia following CCI [78]. A recent study from our group found that NOX4 mRNA was significantly upregulated between 1 and 7 dpi, returning to baseline by 14 dpi [93]. Furthermore, Western blot analysis showed elevated NOX4 protein expression at 1, 4, and 7 dpi, and double immunohistochemistry revealed that the elevated NOX4 expression was predominantly located in neurons in the injured cerebral cortex [93]. The elevated NOX4 expression in the cerebral cortex after TBI was correlated with increased oxidative stress damage to DNA, protein and lipids [93]. Likewise, in blast-injured rats, elevated NOX4 and p22phox expression correlated with increased superoxide production [71]. Finally, NOX5 and the DUOXs have not been characterized in TBI, and thus studies to explore their potential role in TBI pathogenesis are needed.
3. Evidence from NOX knockout studies
Perhaps the most definitive evidence we have that implicates a causative role of NOX isoforms in TBI pathology comes from studies utilizing NOX knockout mice. The first report utilizing NOX2-/- mice (gp91-/- mice) in brain injury employed a surgically induced brain injury (SBI) model [94]. Although edema was unaltered by the deletion of NOX2, knockout mice had improved neurological scores following injury as compared with wild-type mice in SBI [94]. In classic models of TBI, such as CCI, the first report of NOX2-/- mice showed reduced contusion area and apoptosis following TBI that was coupled with a reduction in superoxide and peroxynitrite metabolites in the injured cortex [80]. In addition to decreasing lesion volume, deletion of NOX2 improved motor coordination at 7 [95], 14, and 21 dpi [90], indicating that targeting NOX2 may be functionally beneficial. Knockout studies have also confirmed an extensive role of NOX2 in TBI-induced neuroinflammation. For instance, NOX2-/- mice have significantly reduced NLRP3 inflammasome expression and activation in the injured cortex following TBI [79]. This effect in NOX2-/- mice may be explained by a correlated decrease of TBI-induced thioredoxin-interacting protein (TXNIP), which has been implicated to directly link oxidative stress to NLRP3 inflammasome activation [79].
Interestingly, deletion of NOX2 also reduced expression of M1-like markers in microglia/macrophages (MG/MP) in the injured cerebral cortex without altering total number of infiltrating macrophages (CD45hi cells) to the injury site [90]. The shift in MG/MP polarization dynamics towards the M2-like phenotype in injured NOX2-/- mice, which may be a result of enhanced IL-10 and STAT3 signaling [96], was also associated with decreased production of pro-inflammatory cytokines via a down-regulation of the classical NF-κB pathway [82]. As mentioned previously, microglia isolated from NOX2-/- injured mice had attenuated neurotoxicity in cultured neurons [82], reinforcing the notion that NOX2 inhibition is neuroprotective following TBI.
In addition, deletion of NOX4 was also found by our group to attenuate the severity of TBI via reduction of lesion size, oxidative damage, neurodegeneration, and apoptosis [93]. This finding indicates that in addition to NOX2, NOX4 also plays a significant role in the pathology and neurodegeneration that occurs in the injured cortex after TBI. These animal studies utilizing NOX knockouts have been summarized in Table 1. To date, no studies utilizing NOX1-/- or NOX3-/- mice have been conducted in TBI. In addition, development of NOX2/4 double knockout models may be useful for evaluating potentially additive effects of these isoforms in TBI. Finally, cautious interpretation of the knockout animal studies discussed above is necessary due to the global knockout approach, which may have life-long developmental and compensatory confounds in addition to a secondary effect.
Table 1.
Effect of NOX deletion in models of TBI. Overview of published studies utilizing genetic NOX knockout mice in models of TBI. The animal TBI model used, the specific region of interest (ROI) evaluated, and the major findings of these studies have been summarized. To date, only NOX2 and NOX4 knockout mice have been studied. Abbreviations: SBI – surgical brain injury; CCI – controlled cortical impact.
| NOX modulation | Animal model | ROI | Results | Ref. |
|---|---|---|---|---|
| NOX2-/- | SBI | Frontal lobe |
|
[94] |
| NOX2-/- | CCI | Cortex |
|
[80] |
| NOX2-/- | CCI | Cortex |
|
[90] |
| NOX2-/- | CCI | Cortex |
|
[79] |
| NOX2-/- | CCI | Cortex, CA1 hippocampus |
|
[82] |
| NOX2-/- | CCI | Cortex |
|
[95] |
| NOX4-/- | CCI | Cortex |
|
[93] |
4. Evidence from NOX inhibition studies
To circumvent the limitations of global knockout approaches, the role of NOX enzymes have been further studied through utilizing NOX inhibitors to investigate whether acute inhibition of NOX can offer similar benefits following TBI. One of the most studied NOX inhibitors in TBI research is apocynin, a compound isolated from the medicinal plant Picrorhiza kurroa that inhibits NOX2 via preventing the membrane translocation of p47phox and p67phox [97], [98], [99], [100]. Apocynin has also been reported to reduce oxidative damage by scavenging hydrogen peroxide and hypochlorous acid in phagocytic cells [101], [102].
Administration of apocynin, in doses varying from 5 mg/kg to 100 mg/kg, has been documented to decrease NOX2 expression, ROS production, and oxidative damage in animal models of TBI [74], [83], [103], [104]. In animal models of diffuse brain injury via weight drop, pre-treatment with apocynin resulted in neuroprotection of the cortex [77], [83] and hippocampus [77], [103], reduced edema [83], [103], [104], and attenuated neuroinflammation [77], [103] while improving TBI-induced neurological deficits [83], [104]. Further supporting NOX2's prominent role in TBI pathology, gp91ds-tat, a competitive inhibitor specific to NOX2, was also shown to be neuroprotective following TBI [77]. These pre-injury inhibition studies demonstrate that acute targeting of NOX can be beneficial in similar ways to long-term, global knockdown of NOX2. However, the post-injury administration of any potentially therapeutic agent is critical for future clinical application. While investigating a therapeutic role of NOX inhibition in TBI, several groups discovered that the post-TBI administration of apocynin can also decrease NOX2 expression and accompanied oxidative stress in rodents [77], [82], [105]. Administration of apocynin, at a dose as low as 5 mg/kg, within two hours of injury is neuroprotective [77], [106], as shown by attenuated lesion volume [105], reduced neuroinflammation within the first week of injury [77], [106], and decreased expression of Alzheimer's disease (AD)-related proteins [77]. While initial assessment of edema and neuromotor deficits within the first 24 h is conflicted [105], extended assessment found reduced edema within 7 dpi [106], attenuated memory impairment at 7 dpi [105], and improved sensorimotor performance at 7, 14, and 21 dpi [95], [106], [107]. Intriguingly, delayed administration of apocynin at 24 h post-injury also attenuated oxidative damage, reduced NLRP3 inflammasome activation, and promoted microglial polarization towards the M2-like phenotype following TBI in the mouse [79], [82]. A delayed administration of gp91ds-tat at 24 h post-injury was also able to attenuate cognitive deficits [90], further suggesting that the critical window of TBI treatment can be extended to 24 h post-injury. These animal studies utilizing NOX inhibitors and their dosing regimens are summarized in Table 2.
Table 2.
Effect of NOX inhibition in models of TBI. Overview of published studies utilizing pharmacological inhibition of NOX enzymes in models of TBI. The dosing regimen of well-cited NOX inhibitors, the model of TBI used, the specific region of interest (ROI) evaluated, and the major findings of each study have been summarized. Abbreviations: SBI – surgical brain injury; CHI – closed-head injury; CCI – controlled cortical impact; mLFPI – moderate lateral fluid percussion injury; MG/MP – microglia/macrophage.
| NOX modulation | Animal model | ROI | Results | Ref. |
|---|---|---|---|---|
| Apocynin 5 mg/kg ip; 30 min pre-SBI | SBI | Frontal lobe |
|
[94] |
| Apocynin 50 mg/kg ip; 30 min pre-TBI | Weight drop (CHI) | Injured hemisphere; cortex |
|
[83] |
| Apocynin 4 mg/kg ip; 20 min pre-TBI | CCI | Cortex; CA1 /CA3 hippocampus |
|
[77] |
| Apocynin 100 mg/kg ip; 15 min pre-TBI | Weight drop (open skull) | CA3 hippocampus |
|
[103] |
| Apocynin 10 mg/kg ip; 5 min or 2 h + 2nd dose at 24 h post-TBI | CCI | Cortex |
|
[95] |
| Apocynin 5 mg/kg sc; 30 min and 24 h post-TBI | mLFPI | Cortex |
|
[105] |
| Apocynin 4 mg/kg ip; 2 h post-TBI | CCI | Cortex; CA1 /CA3 hippocampus |
|
[77] |
| Apocynin 5 mg/kg ip; 23 h post-TBI, qd | CCI | Cortex, CA1 hippocampus |
|
[82] |
| Apocynin 5 mg/kg ip; 23 h post-TBI, qd | CCI | Cortex |
|
[79] |
| Gp91ds-tat; 250 μg/mouse ip; 20 min pre-TBI | CCI | Cortex |
|
[77] |
| Gp91ds-tat; 5 mg/kg ip; 24 h, 48 h, and 72 h post-TBI | CCI | Cortex |
|
[89] |
| Gp91ds-tat; 5 mg/kg ip; 1d, 2d, and 3d post-TBI | CCI | Cortex |
|
[90] |
Mechanistically, current evidence strongly suggests that the beneficial effects of targeting NOX may be mediated in the injured brain through attenuated neuroinflammation and a shift in cellular dynamics towards an anti-inflammatory state [79], [82], [89], [90], [96], [106]. Inhibition of NOX decreased TLR4 and NF-κB signaling and reduced expression of pro-inflammatory cytokines [82], [106]. Similar to NOX2-/- studies [82], [90], [96], NOX inhibition also enhances the M2-like phenotype of MG/MP in the injured cortex after TBI [82], [89], [90]. The benefit of NOX targeting appears to reach beyond reduction of oxidative damage and may be key in limiting chronic neuroinflammation following TBI by reducing microglia activation in the injury site and altering MG/MP inflammatory phenotypes.
5. Therapeutic considerations
5.1. Targeting NOX2
As described above, NOX2 targeting via selective inhibitors or via apocynin has been reported to improve cognitive outcomes following TBI in animal models [83], [90], [95], [105]. In particular, apocynin is attractive for use in TBI treatment due to its ability to reach the brain parenchyma with oral administration, high stability, and low general toxicity even at much higher doses than that used in TBI studies [108]. Apocynin has also been used in clinical trials of inflammatory respiratory conditions and showed efficacy with no adverse effects [109], [110], [111]. Apocynin's broad range of potential therapeutic application across different tissues, such as arthritis [112], renal ischemia [113], vascular disease [114], and chronic obstructive pulmonary disease [115], may cause concern for potential off-target effects of systemically administering apocynin. Although systemic administration of apocynin can limit the extent of TBI, a targeted approach to deliver apocynin to a limited tissue area or organ may be optimal to limit suppression of physiological NOX signaling. Cell-specific targeting of NOX inhibitors, towards activated pro-inflammatory microglia for example, may also provide yet another approach that would limit off-target effects.
Along these lines, the most isoform-selective NOX inhibitor to date, gp91ds-tat (also known as NOX2ds-tat) is a synthetic, small peptide inhibitor that was designed to penetrate cells and prevent the assembly of the active NOX2 complex by inhibiting the association of p47phox with gp91phox [116]. As previously mentioned, several groups have treated injured animals with gp91ds-tat and reported beneficial effects. However, as a peptide, gp91ds-tat has low oral bioavailability and low stability [117], which may hinder its translation to clinical applications. It is also uncertain whether this small peptide can pass through the BBB in mild injuries that may leave the BBB intact. Nonetheless, its high selectivity for NOX2 [118] is attractive for studying NOX2-specific mechanisms underlying TBI pathology.
5.2. Targeting NOX1 and NOX4
The majority of studies presented in this review focus on the role of NOX2 in mediating neuroinflammation and oxidative stress after TBI, with few recent studies implicating a role for NOX1 and NOX4. Although NOX2 seems the obvious choice for translational pursuit, targeting NOX4 may be advantageous if specific inhibitors are developed. Hospitalized TBI patients have increased susceptibility to potentially fatal nosocomial infections [119], [120], [121], [122]. NOX2-/- mice are frequently used to model chronic granulomatous disease, an inherited disorder characterized by recurrent infections due to defects in innate immunity [123], [124], [125]. Considering the elevated risk of infection in TBI patients, NOX2 targeting could potentially exacerbate susceptibility to infections post-hospitalization, though further studies are necessary to evaluate this potential disadvantage. In contrast, NOX4-/- mice are generally healthy and lack gross phenotypes [126], [127], and NOX4 is primarily upregulated in neurons following TBI [93]. These characteristics position NOX4 to be a promising novel target in the treatment of TBI that may avoid potential immune suppression of NOX2-expressing microglia. Furthermore, whether apocynin exerts some effect via inhibition of NOX4 remains unclear. Although apocynin-mediated neuroprotection was lost in NOX2-/- mice [128], similar studies have not been conducted with NOX4-/- mice.
While there is no NOX4-specific inhibitor, a new NOX1/NOX4 dual inhibitor GKT137831 has been developed, which may be worth investigating in TBI. GKT137831 has already been shown to reduce neuronal death and degeneration in a rat model of subarachnoid hemorrhage [129]. Furthermore, orally bioavailable GKT137831 has already been employed in a phase II clinical trial for diabetic nephropathy and primary biliary cholangitis, and appears to be well tolerated with no adverse events (Clinical Trial ID: NCT02010242, NCT03226067). Based on these observations, preclinical studies to evaluate the efficacy of GKT137831 in treatment of TBI appear to be needed, as they would help determine the therapeutic potential of targeting the NOX1 and NOX4 isoforms in TBI pathology.
5.3. Additional considerations and future directions
An important caveat in experimental animal studies on TBI is that the vast majority of animal TBI studies have been conducted in adult male mice of approximately 3 months of age. The lack of age range in the studies is a concern and potentially limiting in translating these basic findings therapeutically to humans. For instance, the highest rates of TBIs are amongst the oldest or youngest age groups who are particularly prone to falling [4]. Thus, NOX involvement should continue to be evaluated to include juvenile and aged mice, especially since one report suggests that aged mice appear to have prolonged NOX expression and worse outcomes after TBI [72]. In addition, as NOX activity and function may vary with gender [130], [131], [132], [133], further pre-clinical studies should evaluate whether NOX inhibition is similarly efficacious in female mice. As TBI is an epidemic that impacts males and females throughout all life stages, perhaps further studies of NOX involvement, and its potential therapeutic targeting should be explored across all ages and genders.
In addition, most animal studies have focused on acute time frames after TBI (hours to days), and there is a paucity of chronic long-term studies after TBI (e.g. months to years after TBI). This is important, because activated MG/MP are present up to 18 years following a single TBI in the human brain [134], and sustained inflammation over the initial year post-TBI is predictive of global outcome at 6 and 12 months post-TBI [135]. Animal studies have suggested that chronically upregulated neuroinflammation may contribute to the progressive nature of severe TBI [6]. In the long-term, acute inflammatory processes can become self-perpetuating, leading to sustained neuroinflammation that is detrimental [136], [137]. Both NOX2 knockout and inhibition studies described in the above sections have demonstrated strong anti-inflammatory results, presumably via regulation of MG/MP phenotypes. Whether the suppressed neuroinflammation obtained from acutely targeting NOX in animal models can be sustained past the initial weeks following TBI remains unknown, as studies with knockout mice or inhibitors have not extended to these far time-points. Including long-term chronic endpoints in future inhibitor and NOX deletion studies will be critical to resolving this question.
6. Concluding remarks
Despite an abundance of successful pre-clinical studies, the search for an acute neuroprotective drug has yet to deliver promising treatments for TBI. These failures stress the need for innovative therapeutic targets. Though there is significant evidence that NOX enzymes are involved in TBI pathology, many challenges still exist in this pre-clinical phase. Growing the supporting evidence for NOX inhibition across different species, genders, ages, assessment time-points, drug doses, injury sites, and injury models while remaining cognizant of negative data will aid the evaluation of NOX as a target for TBI.
Acknowledgements
The research of the authors discussed in this review was supported by a VA Merit Review Award (5l101BX001117) from the United States Department of Veteran's Affairs, Biomedical Laboratory Research and Development Service.
References
- 1.Rosenfeld J.V., Maas A.I., Bragge P., Morganti-Kossmann M.C., Manley G.T., Gruen R.L. Early management of severe traumatic brain injury. Lancet. 2012;380:1088–1098. doi: 10.1016/S0140-6736(12)60864-2. [DOI] [PubMed] [Google Scholar]
- 2.Finfer S.R., Cohen J. Severe traumatic brain injury. Resuscitation. 2001;48:77–90. doi: 10.1016/s0300-9572(00)00321-x. [DOI] [PubMed] [Google Scholar]
- 3.Nizamutdinov D., Shapiro L.A. Overview of traumatic brain injury: an immunological context. Brain Sci. 2017;7 doi: 10.3390/brainsci7010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor C.A., Bell J.M., Breiding M.J., Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2017;66:1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma M.W., Wang J., Zhang Q., Wang R., Dhandapani K.M., Vadlamudi R.K., Brann D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017;12:7. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loane D.J., Kumar A., Stoica B.A., Cabatbat R., Faden A.I. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes D.E., Kaup A., Kirby K.A., Byers A.L., Diaz-Arrastia R., Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83:312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Leden R.E., Yauger Y.J., Khayrullina G., Byrnes K.R. Central nervous system injury and nicotinamide adenine dinucleotide phosphate oxidase: oxidative stress and therapeutic targets. J. Neurotrauma. 2017;34:755–764. doi: 10.1089/neu.2016.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitaker-Lea W.A., Valadka A.B. Acute management of moderate-severe traumatic brain injury. Phys. Med. Rehabil. Clin. N. Am. 2017;28:227–243. doi: 10.1016/j.pmr.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Prins M., Greco T., Alexander D., Giza C.C. The pathophysiology of traumatic brain injury at a glance. Dis. Models Mech. 2013;6:1307–1315. doi: 10.1242/dmm.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterfield D.A., Reed T.T. Lipid peroxidation and tyrosine nitration in traumatic brain injury: insights into secondary injury from redox proteomics. Proteom. Clin. Appl. 2016;10:1191–1204. doi: 10.1002/prca.201600003. [DOI] [PubMed] [Google Scholar]
- 12.Werner C., Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 13.McGinn M.J., Povlishock J.T. Pathophysiology of traumatic brain injury. Neurosurg. Clin. N. Am. 2016;27:397–407. doi: 10.1016/j.nec.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Maas A.I.R., Stocchetti N., Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 15.Niesman I.R., Schilling J.M., Shapiro L.A., Kellerhals S.E., Bonds J.A., Kleschevnikov A.M., Cui W., Voong A., Krajewski S., Ali S.S. Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice. J. Neuroinflamm. 2014;11:39. doi: 10.1186/1742-2094-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tajiri N., Kellogg S.L., Shimizu T., Arendash G.W., Borlongan C.V. Traumatic brain injury precipitates cognitive impairment and extracellular Abeta aggregation in Alzheimer's disease transgenic mice. PLoS One. 2013;8:e78851. doi: 10.1371/journal.pone.0078851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giunta B., Obregon D., Velisetty R., Sanberg P.R., Borlongan C.V., Tan J. The immunology of traumatic brain injury: a prime target for Alzheimer's disease prevention. J. Neuroinflamm. 2012;9:185. doi: 10.1186/1742-2094-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbonell W.S., Grady M.S. Regional and temporal characterization of neuronal, glial, and axonal response after traumatic brain injury in the mouse. Acta Neuropathol. 1999;98:396–406. doi: 10.1007/s004010051100. [DOI] [PubMed] [Google Scholar]
- 19.Simon D.W., McGeachy M.J., Bayir H., Clark R.S., Loane D.J., Kochanek P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beauchamp K., Mutlak H., Smith W.R., Shohami E., Stahel P.F. Pharmacology of traumatic brain injury: where is the "golden bullet"? Mol. Med. 2008;14:731–740. doi: 10.2119/2008-00050.Beauchamp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaetz M. The neurophysiology of brain injury. Clin. Neurophysiol.: Off. J. Int. Fed. Clin. Neurophysiol. 2004;115:4–18. doi: 10.1016/s1388-2457(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 22.Lotocki G., de Rivero Vaccari J.P., Perez E.R., Sanchez-Molano J., Furones-Alonso O., Bramlett H.M., Dietrich W.D. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. J. Neurotrauma. 2009;26:1123–1134. doi: 10.1089/neu.2008.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harting M.T., Jimenez F., Adams S.D., Mercer D.W., Cox C.S., Jr Acute, regional inflammatory response after traumatic brain injury: implications for cellular therapy. Surgery. 2008;144:803–813. doi: 10.1016/j.surg.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Povlishock J.T., Christman C.W. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J. Neurotrauma. 1995;12:555–564. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- 25.Unterberg A.W., Stroop R., Thomale U.W., Kiening K.L., Pauser S., Vollmann W. Characterisation of brain edema following "controlled cortical impact injury" in rats. Acta Neurochir. Suppl. 1997;70:106–108. doi: 10.1007/978-3-7091-6837-0_33. [DOI] [PubMed] [Google Scholar]
- 26.Ikonomidou C., Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 27.Maas A.I., Roozenbeek B., Manley G.T. Clinical trials in traumatic brain injury: past experience and current developments. Neurother.: J. Am. Soc. Exp. Neurother. 2010;7:115–126. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein D.G. Embracing failure: what the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015;29:1259–1272. doi: 10.3109/02699052.2015.1065344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdul-Muneer P.M., Schuetz H., Wang F., Skotak M., Jones J., Gorantla S., Zimmerman M.C., Chandra N., Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic. Biol. Med. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdul-Muneer P.M., Chandra N., Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2014;51:966–979. doi: 10.1007/s12035-014-8752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angeloni C., Prata C., Dalla Sega F.V., Piperno R., Hrelia S. Traumatic brain injury and NADPH oxidase: a deep relationship. Oxid. Med. Cell. Longev. 2015;2015:370312. doi: 10.1155/2015/370312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bains M., Hall E.D. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta. 2012;1822:675–684. doi: 10.1016/j.bbadis.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toklu H.Z., Tumer N. Oxidative stress, brain edema, blood-brain barrier permeability, and autonomic dysfunction from traumatic brain injury. In: Kobeissy F.H., editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Frontiers in Neuroengineering; Boca Raton (FL): 2015. [Google Scholar]
- 34.Buetler T.M., Krauskopf A., Ruegg U.T. Role of superoxide as a signaling molecule. News Physiol. Sci.: Int. J. Physiol. Prod. Jt. Int. Union Physiol. Sci. Am. Physiol. Soc. 2004;19:120–123. doi: 10.1152/nips.01514.2003. [DOI] [PubMed] [Google Scholar]
- 35.Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kienhofer D., Boeltz S., Hoffmann M.H. Reactive oxygen homeostasis - the balance for preventing autoimmunity. Lupus. 2016;25:943–954. doi: 10.1177/0961203316640919. [DOI] [PubMed] [Google Scholar]
- 37.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shirley R., Ord E.N., Work L.M. Oxidative Stress and the Use of Antioxidants in Stroke. Antioxidants. 2014;3:472–501. doi: 10.3390/antiox3030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuaib A., Lees K.R., Lyden P., Grotta J., Davalos A., Davis S.M., Diener H.C., Ashwood T., Wasiewski W.W., Emeribe U., Investigators S.I.T. NXY-059 for the treatment of acute ischemic stroke. New Engl. J. Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 40.Altenhofer S., Kleikers P.W., Radermacher K.A., Scheurer P., Rob Hermans J.J., Schiffers P., Ho H., Wingler K., Schmidt H.H. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell. Mol. Life Sci.: CMLS. 2012;69:2327–2343. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armitage M.E., Wingler K., Schmidt H.H., La M. Translating the oxidative stress hypothesis into the clinic: NOX versus NOS. J. Mol. Med. 2009;87:1071–1076. doi: 10.1007/s00109-009-0544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nayernia Z., Jaquet V., Krause K.H. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal. 2014;20:2815–2837. doi: 10.1089/ars.2013.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambeth J.D., Neish A.S. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu. Rev. Pathol. 2014;9:119–145. doi: 10.1146/annurev-pathol-012513-104651. [DOI] [PubMed] [Google Scholar]
- 44.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 45.Gao H.M., Zhou H., Hong J.S. NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol. Sci. 2012;33:295–303. doi: 10.1016/j.tips.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall E.D., Vaishnav R.A., Mustafa A.G. Antioxidant therapies for traumatic brain injury. Neurother.: J. Am. Soc. Exp. Neurother. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wientjes F.B., Segal A.W. NADPH oxidase and the respiratory burst. Semin. Cell Biol. 1995;6:357–365. doi: 10.1016/s1043-4682(05)80006-6. [DOI] [PubMed] [Google Scholar]
- 48.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 49.Dupuy C., Ohayon R., Valent A., Noel-Hudson M.S., Deme D., Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J. Biol. Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 50.Cheng G., Cao Z., Xu X., van Meir E.G., Lambeth J.D. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 51.Geiszt M., Leto T.L. The Nox family of NAD(P)H oxidases: host defense and beyond. J. Biol. Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- 52.Pendyala S., Natarajan V. Redox regulation of Nox proteins. Respir. Physiol. Neurobiol. 2010;174:265–271. doi: 10.1016/j.resp.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 54.Banfi B., Clark R.A., Steger K., Krause K.H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 55.Szanto I., Rubbia-Brandt L., Kiss P., Steger K., Banfi B., Kovari E., Herrmann F., Hadengue A., Krause K.H. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J. Pathol. 2005;207:164–176. doi: 10.1002/path.1824. [DOI] [PubMed] [Google Scholar]
- 56.Sumimoto H., Miyano K., Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- 57.Banfi B., Malgrange B., Knisz J., Steger K., Dubois-Dauphin M., Krause K.H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 58.Paffenholz R., Bergstrom R.A., Pasutto F., Wabnitz P., Munroe R.J., Jagla W., Heinzmann U., Marquardt A., Bareiss A., Laufs J. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geiszt M., Kopp J.B., Varnai P., Leto T.L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. USA. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shiose A., Kuroda J., Tsuruya K., Hirai M., Hirakata H., Naito S., Hattori M., Sakaki Y., Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 61.Banfi B., Molnar G., Maturana A., Steger K., Hegedus B., Demaurex N., Krause K.H. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 62.Fulton D.J. Nox5 and the regulation of cellular function. Antioxid. Redox Signal. 2009;11:2443–2452. doi: 10.1089/ars.2009.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Deken X., Wang D., Many M.C., Costagliola S., Libert F., Vassart G., Dumont J.E., Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 64.Sorce S., Krause K.H. NOX enzymes in the central nervous system: from signaling to disease. Antioxid. Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 65.Accetta R., Damiano S., Morano A., Mondola P., Paterno R., Avvedimento E.V., Santillo M. Reactive oxygen species derived from NOX3 and NOX5 drive differentiation of human Oligodendrocytes. Front. Cell. Neurosci. 2016;10:146. doi: 10.3389/fncel.2016.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bokoch G.M., Diebold B., Kim J.S., Gianni D. Emerging evidence for the importance of phosphorylation in the regulation of NADPH oxidases. Antioxid. Redox Signal. 2009;11:2429–2441. doi: 10.1089/ars.2009.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brandes R.P., Weissmann N., Schroder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic. Biol. Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 68.Liao Y., Liu P., Guo F., Zhang Z.Y., Zhang Z. Oxidative burst of circulating neutrophils following traumatic brain injury in human. PLoS One. 2013;8:e68963. doi: 10.1371/journal.pone.0068963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z., Tian F., Shao Z., Shen X., Qi X., Li H., Wang Z., Chen G. Expression and clinical significance of non-phagocytic cell oxidase 2 and 4 after human traumatic brain injury. Neurol. Sci.: Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2015;36:61–71. doi: 10.1007/s10072-014-1909-z. [DOI] [PubMed] [Google Scholar]
- 70.Schiavone S., Neri M., Trabace L., Turillazzi E. The NADPH oxidase NOX2 mediates loss of parvalbumin interneurons in traumatic brain injury: human autoptic immunohistochemical evidence. Sci. Rep. 2017;7:8752. doi: 10.1038/s41598-017-09202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lucke-Wold B.P., Naser Z.J., Logsdon A.F., Turner R.C., Smith K.E., Robson M.J., Bailes J.E., Lee J.M., Rosen C.L., Huber J.D. Amelioration of nicotinamide adenine dinucleotide phosphate-oxidase mediated stress reduces cell death after blast-induced traumatic brain injury. Transl. Res.: J. Lab. Clin. Med. 2015;166:509–528. doi: 10.1016/j.trsl.2015.08.005. (e501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar A., Stoica B.A., Sabirzhanov B., Burns M.P., Faden A.I., Loane D.J. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niesman I.R., Schilling J.M., Shapiro L.A., Kellerhals S.E., Bonds J.A., Kleschevnikov A.M., Cui W., Voong A., Krajewski S., Ali S.S. Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice. J. Neuroinflamm. 2014;11:39. doi: 10.1186/1742-2094-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Fan X., Tang T., Fan R., Zhang C., Huang Z., Peng W., Gan P., Xiong X., Huang W., Huang X. Rhein and rhubarb similarly protect the blood-brain barrier after experimental traumatic brain injury via gp91(phox) subunit of NADPH oxidase/ROS/ERK/MMP-9 signaling pathway. Sci. Rep. 2016;6:37098. doi: 10.1038/srep37098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdul-Muneer P.M., Conte A.A., Haldar D., Long M., Patel R.K., Santhakumar V., Overall C.M., Pfister B.J. Traumatic brain injury induced matrix metalloproteinase2 cleaves CXCL12alpha (stromal cell derived factor 1alpha) and causes neurodegeneration. Brain Behav. Immun. 2017;59:190–199. doi: 10.1016/j.bbi.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Ansari M.A., Roberts K.N., Scheff S.W. A time course of NADPH-oxidase up-regulation and endothelial nitric oxide synthase activation in the hippocampus following neurotrauma. Free Radic. Biol. Med. 2014;77:21–29. doi: 10.1016/j.freeradbiomed.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Q.G., Laird M.D., Han D., Nguyen K., Scott E., Dong Y., Dhandapani K.M., Brann D.W. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One. 2012;7:e34504. doi: 10.1371/journal.pone.0034504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cooney S.J., Bermudez-Sabogal S.L., Byrnes K.R. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J. Neuroinflamm. 2013;10:155. doi: 10.1186/1742-2094-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma M.W., Wang J., Dhandapani K.M., Brann D.W. NADPH oxidase 2 regulates NLRP3 inflammasome activation in the brain after traumatic brain injury. Oxid. Med. Cell. Longev. 2017;2017:6057609. doi: 10.1155/2017/6057609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dohi K., Ohtaki H., Nakamachi T., Yofu S., Satoh K., Miyamoto K., Song D., Tsunawaki S., Shioda S., Aruga T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J. Neuroinflamm. 2010;7:41. doi: 10.1186/1742-2094-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skovira J.W., Wu J., Matyas J.J., Kumar A., Hanscom M., Kabadi S.V., Fang R., Faden A.I. Cell cycle inhibition reduces inflammatory responses, neuronal loss, and cognitive deficits induced by hypobaria exposure following traumatic brain injury. J. Neuroinflamm. 2016;13:299. doi: 10.1186/s12974-016-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J., Ma M.W., Dhandapani K.M., Brann D.W. Regulatory role of NADPH oxidase 2 in the polarization dynamics and neurotoxicity of microglia/macrophages after traumatic brain injury. Free Radic. Biol. Med. 2017;113:119–131. doi: 10.1016/j.freeradbiomed.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 83.Lu X.Y., Wang H.D., Xu J.G., Ding K., Li T. NADPH oxidase inhibition improves neurological outcome in experimental traumatic brain injury. Neurochem. Int. 2014;69:14–19. doi: 10.1016/j.neuint.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 84.Lu X.Y., Wang H.D., Xu J.G., Ding K., Li T. Pretreatment with tert-butylhydroquinone attenuates cerebral oxidative stress in mice after traumatic brain injury. J. Surg. Res. 2014;188:206–212. doi: 10.1016/j.jss.2013.11.1106. [DOI] [PubMed] [Google Scholar]
- 85.Lu X.Y., Wang H.D., Xu J.G., Ding K., Li T. Deletion of Nrf2 exacerbates oxidative stress after traumatic brain injury in mice. Cell. Mol. Neurobiol. 2015;35:713–721. doi: 10.1007/s10571-015-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang B., Wang B., Cao S., Wang Y. Epigallocatechin-3-gallate (EGCG) attenuates traumatic brain injury by inhibition of edema formation and oxidative stress. Korean J. Physiol. Pharmacol.: Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2015;19:491–497. doi: 10.4196/kjpp.2015.19.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luo T., Wu J., Kabadi S.V., Sabirzhanov B., Guanciale K., Hanscom M., Faden J., Cardiff K., Bengson C.J., Faden A.I. Propofol limits microglial activation after experimental brain trauma through inhibition of nicotinamide adenine dinucleotide phosphate oxidase. Anesthesiology. 2013;119:1370–1388. doi: 10.1097/ALN.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 88.Byrnes K.R., Loane D.J., Stoica B.A., Zhang J., Faden A.I. Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J. Neuroinflamm. 2012;9:43. doi: 10.1186/1742-2094-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar A., Alvarez-Croda D.M., Stoica B.A., Faden A.I., Loane D.J. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J. Neurotrauma. 2016;33:1732–1750. doi: 10.1089/neu.2015.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar A., Barrett J.P., Alvarez-Croda D.M., Stoica B.A., Faden A.I., Loane D.J. NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain Behav. Immun. 2016;58:291–309. doi: 10.1016/j.bbi.2016.07.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abdul-Muneer P.M., Schuetz H., Wang F., Skotak M., Jones J., Gorantla S., Zimmerman M.C., Chandra N., Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic. Biol. Med. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuan J., Wang A., He Y., Si Z., Xu S., Zhang S., Wang K., Wang D., Liu Y. Cordycepin attenuates traumatic brain injury-induced impairments of blood-brain barrier integrity in rats. Brain Res. Bull. 2016;127:171–176. doi: 10.1016/j.brainresbull.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 93.Ma M.W., Wang J., Dhandapani K.M., Brann D.W. Deletion of NADPH oxidase 4 reduces severity of traumatic brain injury. Free Radic. Biol. Med. 2018;117:66–75. doi: 10.1016/j.freeradbiomed.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 94.Lo W., Bravo T., Jadhav V., Titova E., Zhang J.H., Tang J. NADPH oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neurosci. Lett. 2007;414:228–232. doi: 10.1016/j.neulet.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chandran R., Kim T., Mehta S.L., Udho E., Chanana V., Cengiz P., Kim H., Kim C., Vemuganti R. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J. Cereb. Blood Flow. Metab.: Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2017 doi: 10.1177/0271678X17738701. (271678X17738701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barrett J.P., Henry R.J., Villapol S., Stoica B.A., Kumar A., Burns M.P., Faden A.I., Loane D.J. NOX2 deficiency alters macrophage phenotype through an IL-10/STAT3 dependent mechanism: implications for traumatic brain injury. J. Neuroinflamm. 2017;14:65. doi: 10.1186/s12974-017-0843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barbieri S.S., Cavalca V., Eligini S., Brambilla M., Caiani A., Tremoli E., Colli S. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic. Biol. Med. 2004;37:156–165. doi: 10.1016/j.freeradbiomed.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 98.Stolk J., Hiltermann T.J., Dijkman J.H., Verhoeven A.J. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 99.Ximenes V.F., Kanegae M.P., Rissato S.R., Galhiane M.S. The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. Arch. Biochem. Biophys. 2007;457:134–141. doi: 10.1016/j.abb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 100.Gatto G.J., Jr., Ao Z., Kearse M.G., Zhou M., Morales C.R., Daniels E., Bradley B.T., Goserud M.T., Goodman K.B., Douglas S.A. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J. Enzym. Inhib. Med. Chem. 2013;28:95–104. doi: 10.3109/14756366.2011.636360. [DOI] [PubMed] [Google Scholar]
- 101.Heumüller S., Wind S., Barbosa-Sicard E., Schmidt H.H.H.W., Busse R., Schröder K., Brandes R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 102.Petrônio M.S., Zeraik M.L., Fonseca L.Md, Ximenes V.F. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules. 2013;18:2821–2839. doi: 10.3390/molecules18032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi B.Y., Jang B.G., Kim J.H., Lee B.E., Sohn M., Song H.K., Suh S.W. Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res. 2012;1481:49–58. doi: 10.1016/j.brainres.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 104.Song S.X., Gao J.L., Wang K.J., Li R., Tian Y.X., Wei J.Q., Cui J.Z.: Attenuation of brain edema and spatial learning deficits by the inhibition of NADPH oxidase activity using apocynin following diffuse traumatic brain injury in rats. Mol. Med. Rep. 2013;7:327–331. doi: 10.3892/mmr.2012.1147. [DOI] [PubMed] [Google Scholar]
- 105.Ferreira A.P., Rodrigues F.S., Della-Pace I.D., Mota B.C., Oliveira S.M., Velho Gewehr Cde C., Bobinski F., de Oliveira C.V., Brum J.S., Oliveira M.S. The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: role of inflammatory and oxidative brain damage. Neurochem. Int. 2013;63:583–593. doi: 10.1016/j.neuint.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 106.Feng Y., Cui C., Liu X., Wu Q., Hu F., Zhang H., Ma Z., Wang L. Protective role of Apocynin via suppression of neuronal autophagy and TLR4/NF-kappab signaling pathway in a rat model of traumatic brain injury. Neurochem. Res. 2017;42:3296–3309. doi: 10.1007/s11064-017-2372-z. [DOI] [PubMed] [Google Scholar]
- 107.Loane D.J., Stoica B.A., Byrnes K.R., Jeong W., Faden A.I. Activation of mGluR5 and inhibition of NADPH oxidase improves functional recovery after traumatic brain injury. J. Neurotrauma. 2013;30:403–412. doi: 10.1089/neu.2012.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.t Hart B.A., Copray S., Philippens I. Apocynin, a low molecular oral treatment for neurodegenerative disease. BioMed. Res. Int. 2014;2014:298020. doi: 10.1155/2014/298020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peters E.A., Hiltermann J.T., Stolk J. Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic. Biol. Med. 2001;31:1442–1447. doi: 10.1016/s0891-5849(01)00725-0. [DOI] [PubMed] [Google Scholar]
- 110.Stefanska J., Sokolowska M., Sarniak A., Wlodarczyk A., Doniec Z., Nowak D., Pawliczak R. Apocynin decreases hydrogen peroxide and nitrate concentrations in exhaled breath in healthy subjects. Pulm. Pharmacol. Ther. 2010;23:48–54. doi: 10.1016/j.pupt.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 111.Stefanska J., Sarniak A., Wlodarczyk A., Sokolowska M., Pniewska E., Doniec Z., Nowak D., Pawliczak R. Apocynin reduces reactive oxygen species concentrations in exhaled breath condensate in asthmatics. Exp. Lung Res. 2012;38:90–99. doi: 10.3109/01902148.2011.649823. [DOI] [PubMed] [Google Scholar]
- 112.Pandey A., Kour K., Bani S., Suri K.A., Satti N.K., Sharma P., Qazi G.N. Amelioration of adjuvant induced arthritis by apocynin. Phytother. Res.: PTR. 2009;23:1462–1468. doi: 10.1002/ptr.2803. [DOI] [PubMed] [Google Scholar]
- 113.Altintas R., Polat A., Vardi N., Oguz F., Beytur A., Sagir M., Yildiz A., Parlakpinar H. The protective effects of apocynin on kidney damage caused by renal ischemia/reperfusion. J. Endourol. 2013;27:617–624. doi: 10.1089/end.2012.0556. [DOI] [PubMed] [Google Scholar]
- 114.Virdis A., Gesi M., Taddei S. Impact of apocynin on vascular disease in hypertension. Vasc. Pharmacol. 2016;87:1–5. doi: 10.1016/j.vph.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 115.Stefanska J., Sarniak A., Wlodarczyk A., Sokolowska M., Doniec Z., Bialasiewicz P., Nowak D., Pawliczak R. Hydrogen peroxide and nitrite reduction in exhaled breath condensate of COPD patients. Pulm. Pharmacol. Ther. 2012;25:343–348. doi: 10.1016/j.pupt.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 116.Rey F.E., Cifuentes M.E., Kiarash A., Quinn M.T., Pagano P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ. Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 117.Kim J.Y., Park J., Lee J.E., Yenari M.A. NOX inhibitors - a promising avenue for ischemic stroke. Exp. Neurobiol. 2017;26:195–205. doi: 10.5607/en.2017.26.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Csanyi G., Cifuentes-Pagano E., Al Ghouleh I., Ranayhossaini D.J., Egana L., Lopes L.R., Jackson H.M., Kelley E.E., Pagano P.J. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic. Biol. Med. 2011;51:1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boque M.C., Bodi M., Rello J. Trauma, head injury, and neurosurgery infections. Semin. Respir. Infect. 2000;15:280–286. doi: 10.1053/srin.2000.20935. [DOI] [PubMed] [Google Scholar]
- 120.Dziedzic T., Slowik A., Szczudlik A. Nosocomial infections and immunity: lesson from brain-injured patients. Crit. Care. 2004;8:266–270. doi: 10.1186/cc2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kourbeti I.S., Vakis A.F., Papadakis J.A., Karabetsos D.A., Bertsias G., Filippou M., Ioannou A., Neophytou C., Anastasaki M., Samonis G. Infections in traumatic brain injury patients. Clin. Microbiol. Infect.: Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012;18:359–364. doi: 10.1111/j.1469-0691.2011.03625.x. [DOI] [PubMed] [Google Scholar]
- 122.Harrison-Felix C., Whiteneck G., Devivo M.J., Hammond F.M., Jha A. Causes of death following 1 year postinjury among individuals with traumatic brain injury. J. Head. Trauma Rehabil. 2006;21:22–33. doi: 10.1097/00001199-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 123.Bridges R.A., Berendes H., Good R.A. A fatal granulomatous disease of childhood; the clinical, pathological, and laboratory features of a new syndrome. AMA J. Dis. Child. 1959;97:387–408. [PubMed] [Google Scholar]
- 124.Cachat J., Deffert C., Hugues S., Krause K.H. Phagocyte NADPH oxidase and specific immunity. Clin. Sci. 2015;128:635–648. doi: 10.1042/CS20140635. [DOI] [PubMed] [Google Scholar]
- 125.Segal B.H., Leto T.L., Gallin J.I., Malech H.L., Holland S.M. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine. 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 126.Sirokmany G., Donko A., Geiszt M. Nox/Duox family of NADPH oxidases: lessons from knockout mouse models. Trends Pharmacol. Sci. 2016;37:318–327. doi: 10.1016/j.tips.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 127.Kleinschnitz C., Grund H., Wingler K., Armitage M.E., Jones E., Mittal M., Barit D., Schwarz T., Geis C., Kraft P. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen H., Kim G.S., Okami N., Narasimhan P., Chan P.H. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol. Dis. 2011;42:341–348. doi: 10.1016/j.nbd.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang L., Li Z., Feng D., Shen H., Tian X., Li H., Wang Z., Chen G. Involvement of Nox2 and Nox4 NADPH oxidases in early brain injury after subarachnoid hemorrhage. Free Radic. Res. 2017;51:316–328. doi: 10.1080/10715762.2017.1311015. [DOI] [PubMed] [Google Scholar]
- 130.Miller A.A., Drummond G.R., Mast A.E., Schmidt H.H., Sobey C.G. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke. 2007;38:2142–2149. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- 131.Zhang R., Thor D., Han X., Anderson L., Rahimian R. Sex differences in mesenteric endothelial function of streptozotocin-induced diabetic rats: a shift in the relative importance of EDRFs. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H1183–H1198. doi: 10.1152/ajpheart.00327.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wong P.S., Randall M.D., Roberts R.E. Sex differences in the role of NADPH oxidases in endothelium-dependent vasorelaxation in porcine isolated coronary arteries. Vasc. Pharmacol. 2015;72:83–92. doi: 10.1016/j.vph.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 133.Kander M.C., Cui Y., Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017;21:1024–1032. doi: 10.1111/jcmm.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain: a J. Neurol. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kumar R.G., Boles J.A., Wagner A.K. Chronic inflammation after severe traumatic brain injury: characterization and associations with outcome at 6 and 12 months postinjury. J. Head. Trauma Rehabil. 2015;30:369–381. doi: 10.1097/HTR.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 136.Kumar A., Loane D.J. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 2012;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 137.Lucas S.M., Rothwell N.J., Gibson R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006;147(Suppl 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]