Abstract
p38α is a redox sensitive MAPK activated by pro-inflammatory cytokines and environmental, genotoxic and endoplasmic reticulum stresses. The aim of this work was to assess whether p38α controls the antioxidant defense in the liver, and if so, to elucidate the mechanism(s) involved and the age-related changes. For this purpose, we used liver-specific p38α-deficient mice at two different ages: young-mice (4 months-old) and old-mice (24 months-old). The liver of young p38α knock-out mice exhibited a decrease in GSH levels and an increase in GSSG/GSH ratio and malondialdehyde levels. However, old mice deficient in p38α had higher hepatic GSH levels and lower GSSG/GSH ratio than young p38α knock-out mice. Liver-specific p38α deficiency triggered a dramatic down-regulation of the mRNAs of the key antioxidant enzymes glutamate cysteine ligase, superoxide dismutase 1, superoxide dismutase 2, and catalase in young mice, which seems mediated by the lack of p65 recruitment to their promoters. Nrf-2 nuclear levels did not change significantly in the liver of young mice upon p38α deficiency, but nuclear levels of phospho-p65 and PGC-1α decreased in these mice. p38α-dependent activation of NF-κB seems to occur through classical IκB Kinase and via ribosomal S6 kinase1 and AKT in young mice. However, unexpectedly the long-term deficiency in p38α triggers a compensatory up-regulation of antioxidant enzymes via NF-κB activation and recruitment of p65 to their promoters. In conclusion, p38α MAPK maintains the expression of antioxidant genes in liver of young animals via NF-κΒ under basal conditions, whereas its long-term deficiency triggers compensatory up-regulation of antioxidant enzymes through NF-κΒ.
Abbreviations: Akt, Protein kinase B; AP-1, Activator protein-1; ASK1, Apoptosis signal-regulating kinase 1; ATF2, activating transcription factor 2;; ChIP, Chromatin immunoprecipitation;; DEN, Diethyl nitrosamine;; EGFR, Epidermal growth factor receptor; G6PDH, Glucose-6-phosphate dehydrogenase; GCLc, Glutamate cysteine ligase catalytic subunit; GSH, Reduced glutathione;; GSSG, Oxidized glutathione;; Gstm1, Glutathione S-transferase mu 1; HPLC, High-performance liquid chromatography; Hsp, Heat shock protein; IKK, IƙB Kinase; IL, Interleukin; JNK, c-Jun N-terminal kinase; LPS, Lipopolysaccharide;; MAPK, mitogen activated protein kinase; MEF, Mouse embryonic fibroblasts; MK2, MAP-activated protein kinase 2;; MKK, MAPK kinase; MKP-1, MAPK phosphatase-1; NEM, N-ethyl maleimide;; NF-ƙB, Nuclear factor kappa B; Nrf2, Nuclear factor erythroid 2-related factor-2; PGC-1α, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; ROS, Reactive oxygen species;; RSK1, Ribosomal S6 kinase1; SOD1, Cu/Zn-superoxide dismutase; SOD2, Mn-superoxide dismutase; TBP, TATA-binding protein; TNF-α, Tumor necrosis factor-alpha; Trx, Thioredoxin
Keywords: Nuclear factor ƙB, Glutathione, Glutamate cysteine ligase, Superoxide dismutase 1, Superoxide dismutase 2, And catalase
Graphical abstract
Highlights
-
•
The p38α/NF-κB axis maintains the physiological antioxidant defense in the liver.
-
•
Long-term deficiency of p38α up-regulates antioxidant enzymes through NF-κΒ.
-
•
p38α/NF-κΒ activation in young animals does not up-regulate pro-inflammatory genes.
1. Introduction
p38α is a redox sensitive mitogen activated protein kinase (MAPK) activated by environmental, genotoxic and endoplasmic reticulum stresses, and by pro-inflammatory cytokines, in addition to oxidative stress [1], [2]. It is one of the major MAPKs critically involved in the regulation of cell proliferation, differentiation, migration, apoptosis, and senescence as well as in inflammation [2], [3]. The family of p38 MAPKs comprises four isoforms (p38α, p38β p38γ, and p38δ), and p38α is ubiquitously expressed in mammalian cells [2], [4], [5], [6]. It is activated by the upstream MAPKKs MKK3 and MKK6, and under certain conditions by MKK4 [7]. It may be also activated by autophosphorylation [8] or by oxidative-induced inactivation of certain protein phosphatases [9]. Substrates of p38α comprise transcription factors, such as p53 and activating transcription factor 2 (ATF2), and protein kinases, such as MAPK-activated protein kinase 2 (MK2) [10], [11]. There is evidence that p38α may activate nuclear factor kappa B (NF-κB), being involved in the up-regulation of pro-inflammatory cytokines, such as TNF-α and IL-1β [12].
Hydrogen peroxide activates p38α in endothelial cells and alveolar epithelial cells [13], [14], [15]. Under pathological conditions, an excess of reactive oxygen species (ROS) may activate p38α leading either to cell dysfunction or cell death by apoptosis [7], [16], [17], [18], [19]. In contrast oxidative stress triggers dephosphorylation of p38α in the metabolic syndrome by JNK-induced upregulation of phosphatase MKP-1, which exhibits a great affinity for p38α as substrate [20].
The major mechanism responsible for the activation of p38α MAPK by oxidative stress is through the MAP3K5 apoptosis signal-regulating kinase 1 (ASK1) [21], which dissociates from its inhibitory binding protein thioredoxin (Trx) upon thiol oxidation [21], [22]. Moreover, mitochondrial ROS may activate p38 MAPK by inducing dissociation of the complex Trx-ASK1 [23]. Aging is associated with chronic oxidative stress and over-activation of p38α [24], [25]. Indeed, mitochondrial chronic oxidative stress is considered a hallmark of cellular aging [24], [26], [27], [28]. The ASK1/Trx-ASK1 ratio increases with age in mouse liver, which would explain the enhanced age-related p38 MAPK activity in the liver [23], [29]. The intracellular redox state is essential for the control of cell fate and seems to be tightly regulated during aging. It has been reported that p38α inhibition down-regulates the expression of two anti-oxidant enzymes, glutathione peroxidase and thioredoxin, in breast and colon cancer cells in vitro [30], but the regulation of the antioxidant defense system by p38α under physiological conditions has been scarcely studied.
p38α mediates the increase in peroxiredoxin I activity induced by lipopolysaccharide (LPS) in microglial cells [31]. On the other hand, liver-specific p38α deficiency leads to decreased phosphorylation of Hsp27 (Hsp25 in the murine model) in old mice triggering severe impairment of the actin cytoskeleton [32], and to decreased Hsp25 expression in diethyl nitrosamine (DEN)-treated mice, which was responsible for glutathione depletion and accumulation of ROS in this experimental model [33]. Although p38α lowers ROS accumulation during liver injury, the role of p38α in the regulation of the antioxidant defense in absence of liver injury has not been investigated yet. Hence, our aim was to assess whether p38α controls the antioxidant defense in the liver; and if so to elucidate the mechanism(s) involved and the age-related changes.
2. Materials and methods
2.1. Animals
Liver- specific p38α-deficient mice were generated by combining p38α floxed alleles [34] and the Afp-Cre transgene as described [35]. Animals were distributed in wild-type and knock-out mice at two different ages: young (4 months-old) and old (24 months-old). Four to six mice were used for each experimental group.
All mice received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals (NIH publication 86–23 revised 1985). The study was approved by the Ethics Committee of Animal Experimentation and Welfare of the University of Valencia (Valencia, Spain). Mice were euthanized under anesthesia with isoflurane 3–5% and once they were unconscious they were exsanguinated. Death was confirmed by cervical dislocation.
2.2. Determination of GSH and GSSG
Frozen liver samples were homogenized in phosphate saline buffer (PBS) with 10 mM N-ethyl maleimide (NEM). Perchloric acid (PCA) was then added to obtain a concentration of 4% and centrifuged at 15,000 g for 15 min at 4 °C. The concentrations of GSH and GSSG were determined in the supernatants by high-performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS). The chromatographic system consisted of a Micromass QuatroTM triple-quadrupole mass spectrometer (Micromass, Manchester, UK) equipped with a Zspray electrospray ionization source operating in the positive ion mode with a LC-10A Shimadzu (Shimadzu, Kyoto, Japan) coupled to the MassLynx software 4.1 for data acquisition and processing. Samples were analyzed by reverse-phase HPLC with a C18 Mediterranea SEA column (Teknokroma, Barcelona, Spain). The mobile phase consisted of the following gradient system (min/%A/%B) (A, 0.5% formic acid; B, isopropanol/acetonitrile 50/50; 0,5% formic acid): 5/100/0, 10/0/100, 15/0/100, 15.10/100/0, and 60/100/0. The flow rate was set at 0.2 ml/min. Positive ion electrospray tandem mass spectra were recorded using the following conditions: capillary voltage 3.5 kV, source temperature 120 °C, nebulization and cone gases were set at 500 and 30 L/h, respectively. Argon at 1.5610–3 mbar was used as the collision gas for collision-induced dissociation. An assay based on LC-MS/MS with multiple reaction monitoring was developed using the transitions m/z, cone energy (V), collision energy (eV) and retention time (min) for each compound that represents favorable fragmentation pathways for these protonated molecules (Table 1). Calibration curves were obtained using twelve-point (0.01–100 mmol/l) standards (purchased from Sigma-Aldrich, St Louis, USA) for each compound. The concentrations of metabolites were expressed as nmol/mg of protein.
Table 1.
Transitions and retention times for analytes determined by LC–MS/MS.
| Analyte | Cone (V) | Collision (eV) |
Transition (m/Z) |
Retention time (min) |
|---|---|---|---|---|
| GS-NEM | 30 | 15 | 433>304 | 4.32 |
| GSSG | 30 | 25 | 613>355 | 1.46 |
2.3. Determination of malondialdehyde
Lipid peroxidation was assessed by the measurement of malondialdehyde levels in liver tissue according to Wong et al. [36] by HPLC. This method is based in the reaction of malondialdehyde with thiobarbituric acid (TBA) to yield the adduct MDA-TBA, which is determined specifically by HPLC.
Liver tissue was homogenized in phosphate buffer. Derivatization was performed mixing homogenized sample with 2 M sodium acetonitrile buffer pH 3.5 with TBA 0.2%. The mixture was heated in a boiling-water bath for 60 min at 95 ⁰C. Then, 50 mM potassium phosphate pH 6.8 was added. The samples were centrifuged at 13000 rpm for 5 min at 4 ⁰C and the supernatant was mixed 1:1 with 50 mM potassium phosphate pH 3.5.
Kromasil® 100–5C8, 15 × 0,46 cm column (Teknocroma, Barcelona, España) and 50 mM potassium phosphate pH 6.8 with acetonitrile (83/17) were used as stationary and mobile phase, respectively. The flow rate was 1 ml/minute and 532 nm was fixed for the peak detection for 4.5 min.
2.4. Western-blotting
Liver tissues were frozen at − 80 °C until homogenization in Hepes lysis buffer (100 mg/ml) on ice. The Hepes lysis buffer contained 75 mM NaCl, 750 μM magnesium chloride, 25 mM Hepes (pH 7.4), 500 μM EGTA, 5% glycerol, 0,5% Igepal, 1 mM dithiothreitol, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, and 1 mM sodium orthovanadate. A protease inhibitor cocktail (Sigma) containing AEBSF, aprotinin, bestatin, leupeptin, pepstatin A, and E-64 was added just before its use at a concentration of 5 µl/ml. The homogenates were sonicated with a Branson Sonicator SLPe for 30 sec (2 sec each pulse) at 30% of amplitude and centrifuged at 15,000 g for 15 min at 4 °C. In case of nuclei isolation, a slight modification of the nuclei isolation method described by [32] was used. Chemiluminescence was detected with a charge-coupled device camera (Biorad ChemiDoc XRS+ Molecular Imager and LAS-3000, Fujifilm) using the Luminata Classico Western HRP Substrate (Millipore, Billerica, USA).
The following antibodies were used: antibody against Nrf2 (1/1000) (Abcam, Cambridge, UK), antibody against PGC-1α (Cell Signaling Technology, Danvers, USA), antibody against AP-1 (1/1000) (Cell Signaling Technology, Danvers, USA), antibody against p-p65 Ser536 (1/1000) (Cell Signaling Technology, Danvers, USA), antibody against p65 (1/1000) (Cell Signaling Technology, Danvers, USA), antibody against p-p38α Thr180/Tyr182 (1/1000) (Cell Signaling Technology, Danvers, USA), antibody against p38α (1/1000) (Santa Cruz Biotechnology, Santa Cruz, CA), antibody against p-IKKα/β Ser173/180 (1/1000) (Cell Signaling Technology, Danvers, USA), antibody against IKKα (1/1000) (Cell Signaling Technology, Danvers, USA), antibody against IKKβ (1/1000) (Cell Signaling Technology, Danvers, USA), antibody against p-RSK1 Ser3880 (1/1000) (Cell Signaling Technology, Danvers, USA), antibody against RSK1 (1/500) (Cell Signaling Technology, Danvers, USA), antibody against p-Akt Ser473(1/1000) (Cell Signaling Technology, Danvers, USA), antibody against Akt (1/1000) (Cell Signaling Technology, Danvers, USA), antibody against Hsp27 (1/1000) (Santa Cruz Biotechnology, Santa Cruz, CA), antibody against phospho-Hsp27 (1/1000) (Cell Signaling Technology, Danvers, USA), antibody against p53 (1/500) (Cell Signaling Technology, Danvers, USA), antibody against TBP (1/1000) (Abcam, Cambridge, UK) and antibody against α-tubulin (1/1000) (Sigma-Aldrich, St. Louis, MO).
2.5. RT-PCR
A small piece (approximately 30 mg) of liver was excised and immediately immersed in RNA-later solution (Ambion, Foster City, CA, USA) to stabilize the RNA. Total RNA was isolated using Trizol (Sigma-Aldrich, St Louis, USA). The cDNA for amplification in the PCR assay was constructed by reversion transcription reaction using Revertaid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, USA). Real-time PCR was performed using an iQTM5 multicolor real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The threshold cycle (CT) was determined and the relative gene expression was expressed as follows: fold change= 2−Δ(ΔCT), where ΔCT=CT target − CT housekeeping, and Δ(ΔCT)= ΔCT treated − ΔCT control. The specific primers used are shown in Table 2 with the exception of TaqMan probes. TaqMan probes (Thermo Fisher Scientific, Massachusetts, USA) of Cxcl1 (Mm04207460_m1), Il-1β (Mm00434228_m1), Tnf-α (Mm00443260_g1) and Rplp0 (Mm00725448_s1) (housekeeping) were employed for mRNA cytokine determination.
Table 2.
Oligonucleotides used for RT-PCR.
| Gene | Direct/Reverse oligonucleotide |
|---|---|
| Catalase | 5´- GGAGCAGGTGCTTTTGGATA − 3´ |
| 5´-GAGGGTCACGAACTGTGTCA − 3´ | |
| Sod1 | 5´- TTTTTGCGCGGTCCTTTC − 3´ |
| 5´-CCATACTGATGGACGTGGAA − 3´ | |
| Sod2 | 5´- GGCCAAGGGAGATGTTACAA −3´ |
| 5´- GAACCTTGGACTCCCACAGA − 3´ | |
| Gclc | 5´- CCATCACTTCATTCCCCAGA − 3´ |
| 5´- GATGCCGGATGTTTCTTGTT − 3´ | |
| Gankyrin | 5´- ACTAGAACTGATCAGGACAGCAGA − 3´ |
| 5´- AGCGGAGGCAGCAATATG − 3´ | |
| Il−1α | 5´- TGACCTGCAACAGGAAGTAAAA − 3´ |
| 5´- TTTCTGGCAACTCCTTCAGC − 3´ | |
| G6pdh | 5´- ATGGGTGCATCGGGTGAC − 3´ |
| 5´- GAAGGGCTCACTCTGCTTTC − 3´ | |
| Tbp | 5´- CAGCCTTCCACCTTATGCTC − 3´ |
| 5´- CCGTAAGGCATCATTGGACT −3´ |
2.6. ChIP
Chromatin immunoprecipitation (ChIP). EZ-Magna ChIP™ HiSens Chromatin Immunoprecipitation Kit (Millipore, California, USA) was used to DNA immunoprecipitation from frozen liver tissue as described by the manufacturer. Briefly, 37% formaldehyde was used to crosslink 100 mg of liver tissue for 10 min. Chromatin was isolated and sonicated for 35 min. Magnetic beads provided by the kit and antibodies against NF-kappa-B p65 subunit and H3K4me3 (Millipore, California, USA) were used for immunoprecipitation. Immunoprecipitated DNA was analyzed using real-time PCR. Primers were designed according to the consensus binding sequence for NF-kappa-B GGGACTTTCC [37] to evaluate the recruitment of p65 to the promoter region of Gclc, Sod1, Sod2, Cxcl1, Tnf-α and catalase genes (Table 3). Immunoprecipitation without antibody was performed as a negative control.
Table 3.
Oligonucleotides used for ChIP.
| Gene | Direct/Reverse oligonucleotide |
|---|---|
| Catalase | 5´- TGCACCGCTGTTTATGTGAT − 3´ |
| 5´- AAAGTGGCCTGGATTGACTG − 3´ | |
| Sod1 | 5´- TAACCCCAGCAACTGAGAGG − 3´ |
| 5´- GGGACTCCCCAGATAGCATT − 3´ | |
| Sod2 | 5´- GAAGGTCACTCCGGGCATAA −3´ |
| 5´- CTCCTGCTTCTGGGAAACCC − 3´ | |
| Gclc | 5´- AACTCCAGCTGTGCCAAGTT − 3´ |
| 5´- GGGTGATGAATGACCCTGTT − 3´ | |
| Cxcl1 | 5´- ACTTTGGGGCAAAAAGCAAA − 3´ |
| 5´- GCCCCTTTTATGCTCGAAAC − 3´ | |
| Tnf-α | 5´- CTCCCAGAGACATGGTGGAT − 3´ |
| 5´- CACCCTCCCACTCCTAAACA − 3´ |
2.7. Statistical analysis
Results are expressed as mean ± standard deviation (S.D.). Statistical analysis was performed in two steps. One-way analysis of variance (ANOVA) was performed first. When the overall comparison of groups was significant, differences between individual groups were investigated by the Scheffé test. Statistical differences are indicated in the figure legends.
3. Results
3.1. p38α-deficiency triggers glutathione depletion and lipid peroxidation in the liver of young mice but not in old mice
The liver of young p38α knockout mice exhibited oxidative stress and lipid peroxidation when compared with young wild-type mice as evidenced by the decrease in GSH levels together with the increase in GSSG/GSH ratio and malondialdehyde levels (Fig. 1). Old wild-type mice exhibited diminished GSH levels and a remarkable increase in malondialdehyde levels in the liver when compared with young wild-type mice. However, unexpectedly old mice deficient in p38α had higher hepatic GSH levels and lower GSSG/GSH ratio than young p38α knock-out mice, and also lower hepatic malondialdehyde levels than old wild-type (Fig. 1).
Fig. 1.
Levels of GSH (A) and GSSG (B), GSSG/GSH ratio (C) and malondialdehyde levels (D) in the liver of young WT and p38α KO mice and in old WT and p38α KO mice. The number of samples per group was 4–6. Statistical significance is indicated as * p < 0.05 WT vs. KO; #p < 0.05 and ##p < 0.01 old vs. young.
3.2. p38α-deficiency causes a dramatic down-regulation of antioxidant enzymes in the liver of young mice but their up-regulation in old mice
In order to explain the changes in glutathione redox status observed upon p38α deficiency and aging, the expression levels of the mRNAs encoding the catalytic subunit of glutamate cysteine ligase (Gclc) as well as the antioxidant enzymes superoxide dismutase 1 (Sod1), Sod2, and catalase were measured in the liver of young and old wild-type and liver specific p38α knockout mice. A dramatic decrease in the mRNA expression levels of Gclc, Sod1, Sod2, and catalase was found in the liver of young p38α deficient mice when compared with young wild-type animals (Fig. 2). However, in old wild-type mice the expression of the four enzymes markedly diminished when compared with the young wild-type group (Fig. 2). Strikingly, old mice deficient in p38α exhibited up-regulation of Gclc, Sod1, Sod2, and catalase in comparison with old wild-type mice (Fig. 2).
Fig. 2.
mRNA relative expression of Gclc (A), Sod1 (B), Sod2 (C) and catalase (D) vs Tbp in the liver of young WT and KO mice and in the liver of old WT and p38α KO mice. The number of samples per group was 4–6. Statistical significance is indicated as *p < 0.05 and * *p < 0.01 WT vs. KO; #p < 0.05 and ##p < 0.01 old vs. young.
3.3. p38α-deficiency reduces nuclear translocation of phopho-p65 in the liver of young mice but induces its nuclear translocation in old mice
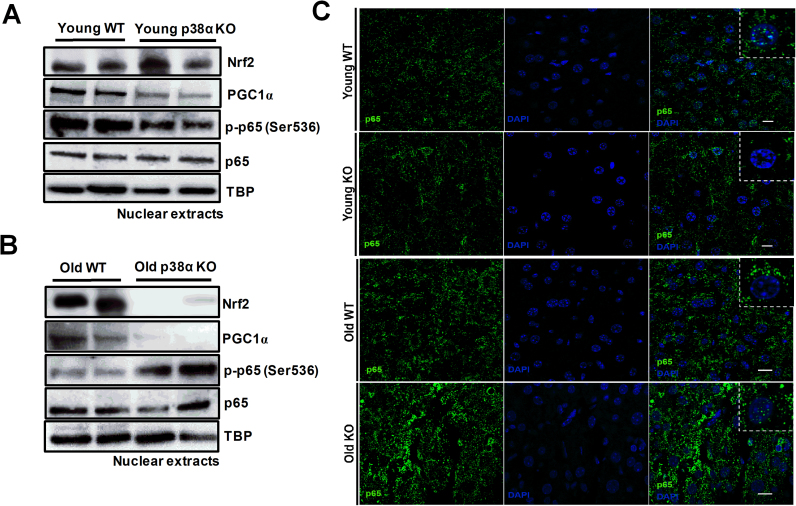
To assess whether Nrf-2, PGC-1α, and NF-κB were involved in the down-regulation of antioxidant enzymes observed upon p38α deficiency in young mice as well as in the age-related up-regulation, nuclear levels of Nrf-2, PGC-1α, and phospho-p65 were measured as an index of their nuclear translocation and activation. Nrf-2 nuclear levels did not change significantly in the liver of young mice upon p38α deficiency, but nuclear levels of PGC-1α and phospho-p65 decreased in the knockout mice (Fig. 3A and S1A). Nrf-2 levels clearly diminished upon p38α deficiency in the liver of old mice (Fig. 3B and S1B). As in young animals, nuclear PGC-1α significantly diminished in the liver of old mice upon p38α deficiency (Fig. 3B and S1B). However, it is noteworthy that nuclear levels of phopho-p65 increased in the liver of old knock-out mice when compared with old wild-type mice (Fig. 3B and S1B).
Fig. 3.
Representative image of western blotting of nuclear levels of Nrf-2, PGC-1α, p-p65 (Ser536), p65, and TBP (TATA-binding protein) as loading control in the liver of young WT and p38α KO mice (A) and in the liver of old WT and p38α KO mice (B). The number of samples per group was 4. Representative images of immunohistochemistry of p65 in liver histological sections (C). Scale bar = 30 µm; selected squares with magnification 200%.
Moreover, immunohistochemistry analysis confirmed that nuclear translocation of p65 was higher in the liver of young wild-type mice as well as in old p38α knockout mice, showing these latter animals remarkably the highest levels of p65 in the nuclei of hepatocytes (Fig. 3C).
As p38α deficiency has been previously related with down-regulation of Hsp25 [33] that could be responsible for glutathione depletion, we also measured the protein levels of HSP25 and the mRNA levels of its target gene glucose 6-phosphate dehydrogenase, but no significant change was found in any of these parameters upon p38α deficiency in the liver of young mice (see Supplementary Fig. S3).
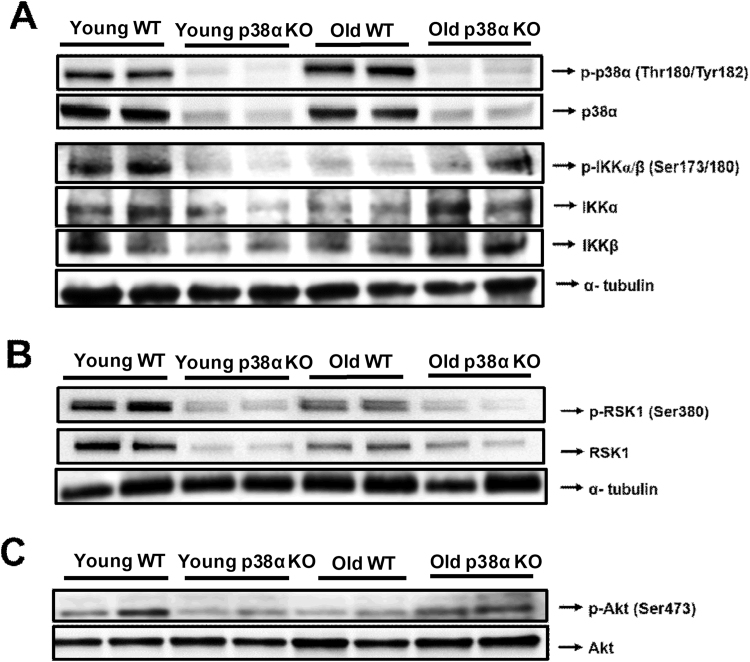
3.4. p38α-deficiency abrogates phosphorylation of IKK, RSK1 and AKT in the liver of young mice but promotes IKK and AKT phosphorylation in old mice
As phospho-p65 levels were higher in the nuclei of livers from young wild-type and old p38α knockout mice, we investigated the status of the canonical pathway of NF-κB activation via phopho-IKK. Indeed, phospho-IKKα/β levels were lower in livers of young p38α knockout mice than in young wild-type mice, whereas in contrast phopho-IKKα/β levels were higher in livers of old p38α knockout mice than in old wild-type mice (Fig. 4A; Supplementary Fig. S2A).
Fig. 4.
Representative image of western blotting of p-p38α (Thr180/Tyr182), p38α, p-IKKα/β (Ser173/180), IKKα and IKKβ in the liver of young WT and p38α KO mice and in the liver of old WT and p38α KO mice. α-tubulin was used as a loading control (A). Representative image of western blotting of p-Rsk1 (Ser380) and Rsk-1 in the liver of young WT and p38α KO mice and in the liver of old WT and p38α KO mice. α-tubulin was used as a loading control (B). Representative image of western blotting of p-Akt (Ser473) and Akt in the liver of young WT and p38α KO mice and in the liver of old WT and p38α KO mice (C). The number of samples per group was 4.
The alternative pathway responsible for NF-κB activation via RSK1 and AKT [38], [39], [40], [41] was also investigated. Interestingly, phospho-RSK1 and phospho-AKT levels were higher in the liver of young wild-type mice than in young p38α knockout mice (Fig. 4B and C; see densitometries in Supplementary Figs. S2B and C). However, no increase in phospho-RSK1 levels was found in the liver of old p38α knockout mice (Fig. 4B and S3B), whereas phospho-AKT levels were higher in the liver of old knockout mice compared with old wild-type mice (Fig. 4C; see densitometries in Supplementary Fig. S3C).
3.5. p38α-deficiency abrogates recruitment of p65 and H3K4me3 to promoters of antioxidant genes in the liver of young mice but induces their recruitment in old mice
In order to confirm the implication of NF-κB in the regulation of antioxidant genes upon p38α deficiency and aging, ChIP assays were performed to assess p65 recruitment to the promoters of Gclc, Sod1, Sod2, and catalase. As it has been previously reported the regulation of the expression of antioxidant genes by PGC1-α in mouse liver, the ChIP assay was not performed for PGC1-α [42], [43]. p38α deficiency markedly reduced the recruitment of p65 to the promoters of the four genes in the liver of young mice (Fig. 5A). In contrast, long term p38α deficiency induced p65 recruitment to the promoters of Gclc, Sod1, and catalase when compared with old wild-type mice (Fig. 5B).
Fig. 5.
Histograms showing the recruitment of p65 in the promoter regions of Gclc, Sod1, Sod2 and catalase in the liver of young WT and p38α KO mice (A) and in the liver of old WT and p38α KO mice (B), measured by chromatin immunoprecipitation (ChIP) assay. The number of samples per group was 4. Statistical significance is indicated as * *p < 0.01 WT vs. KO.
The recruitment of p65 to the promoters of antioxidant genes in livers of young wild-type mice and old p38α knockout mice was paralleled by detection of the euchromatin marker H3K4me3. Indeed, Fig. 6A shows that H3K4me3 was found in the promoters of Gclc, Sod1, Sod2, and catalase in the liver of young wild-type mice, but clearly diminished in young p38α knockout mice. However, similarly to p65, long-term p38α deficiency resulted in strong H3K4me3 signal in the promoters of Gclc, Sod1, Sod2, and catalase (Fig. 6B).
Fig. 6.
Histograms showing chromatin immunoprecipitation (ChIP) assay of the histone modification H3K4me3 in the promoter regions of Gclc, Sod1, Sod2 and catalase in the liver of young WT and p38α KO (A) and in the liver of old WT and p38α KO mice (B). The number of mice per group was 4. Statistical significance is indicated as **p < 0.01 WT vs. KO.
3.6. p38α-deficiency did not affect pro-inflammatory gene expression in the liver of young mice
As NF-κB generally controls the expression of pro-inflammatory genes, we assessed whether NF-kB activation associated with p38α deficiency resulted in pro-inflammatory gene up-regulation. Thus, the mRNA expression levels of Tnf-α, Cxcl-1 (mouse analogue of interleukin 8) and interleukin 1β (Il1-β) were measured. Strikingly, the enhanced phospho-p65 levels found in the nuclei of liver cells from young wild-type mice was not associated with up-regulation of Tnf-α, Cxcl-1 or Il1-β mRNAs (Fig. 7A and Supplementary Fig. S3). Old mice exhibited higher expression levels of Tnf-α mRNA than young mice in the liver, and this increase was higher in p38α knockout mice (Fig. 7). No changes with age or with p38α deficiency were found in the recruitment of p65 to the promoter of Cxcl-1, whereas in the case of Tnf-α, p65 recruitment increased with age but without differences between wild-type and p38α knockout livers (Fig. 7B and C).
Fig. 7.
mRNA relative expression of Tnf-α and Cxcl-1 vs Rplp0 in the liver of young WT and p38α KO mice and in the liver of old WT and p38α KO mice (A). Histograms showing the recruitment of p65 in the promoter regions of Tnf-α and Cxcl-1 in the liver of young WT and p38α KO mice (B) and in the liver of old WT and p38α KO mice (C), measured by chromatin immunoprecipitation (ChIP) assay. The number of samples per group was 4–6 for RT-PCR and 4 for ChIP assay. Statistical significance is indicated as * p < 0.05 WT vs. KO; #p < 0.05 old vs. young.
Additionally, protein levels of p53 and mRNA levels of Il1-α and gankyrin were measured as they have been previously related with p38α signaling and cell fate, particularly in oncogenesis. p53 protein levels as well as Il1-α and gankyrin mRNA levels increased with age, but no significant changes were found between old wild-type mice and old p38aα-knock out mice (see Supplementary Fig. S3).
4. Discussion
p38α MAPK is essential for development in mammals. In fact, p38α deficiency results in mouse embryo lethality due to a placental defect [44], but when p38α is deleted only in the embryos, these complete development and die soon after birth likely due to pulmonary insufficiency caused by insufficient vascularization [6]. Nevertheless, mice with tissue-specific deficiency of p38α are viable and, liver-specific p38α knockout mice in particular exhibit neither liver dysfunction nor damage under basal conditions [33]. However, we previously showed that p38α is required for cytokinesis completion in hepatocytes and accordingly liver-specific p38α deficiency enhances hepatocyte binucleation [35]. Furthermore, long-term p38α deficiency leads to severe impairment of the actin cytoskeleton in hepatocytes of old mice, which seems to be mediated by lack of Hsp25 phosphorylation [32].
Here we show that liver-specific p38α deficiency triggers a dramatic down-regulation of the mRNAs encoding key antioxidant enzymes Gclc, Sod1, Sod2, and catalase in young mice, which is probably mediated by the lack of p65 recruitment to their promoters and by down-regulation of PGC1α, with no change in the nuclear translocation of Nrf-2. The marked decrease in the expression of these antioxidant enzymes leads to oxidative stress evidenced by GSH depletion together with increases in the GSSG/GSH ratio and malondialdehyde levels. The deficiency in key antioxidant enzymes and the associated oxidative stress might contribute to the diminished lifespan that we found in mice with biliary cirrhosis and liver-specific p38α deficiency [35]. As a hypothesis for further studies, we propose that the p38α/NF-κB and p38α/PGC-1α pathways maintain the normal antioxidant defense in the liver under basal physiological conditions, whereas the adaptive up-regulation of the hepatic antioxidant response upon exposure to electrophilic stimuli or oxidative stress would rely mainly on the Nrf-2 pathway [45], [46].
AKT is known to activate NF-κB via IKK [38], and our data is consistent with the p38α-dependent activation of NF-κB occurring in the liver of young mice through both the classical IKK pathway and also via RSK1 and AKT. As total IKKα/β protein levels were also increased in the liver of young mice in comparison with p38α knock out mice, those changes in the phosphorylation might be due to changes in the expression of IKKα/β subunits. We previously reported that p38α is required for the development of hepatomegaly upon chronic cholestasis and its deficiency leads to shorter lifespan likely due to down-regulation of the AKT/mTor pathway [35]. The canonical pathway of RSK1 activation is through ERK1/2 [39], and it has been reported that RSK1-dependent NF-κB activation may be induced by p53 or IL-1β [40], [41]. However, neither p53 protein levels nor IL-1β mRNA were affected by p38α deficiency in the liver of young animals (see Supplementary Fig. S3). Hence, RSK1 phosphorylation may be triggered by p38α in the liver, as previously reported in dendritic cells [39], [47], which seems to be a pathway independent of p53 and IL-1β. Nevertheless, as RSK1 protein levels also increased in the liver of young mice in comparison with p38α knock out mice, those changes in RSK1 phosphorylation might be due to changes in RSK1 expression. Unexpectedly, the activation of NF-κB regulated by p38α leads to up-regulation of antioxidant genes, but not of pro-inflammatory cytokines. This differential up-regulation of NF-κB target genes is intriguing and deserves further investigation.
Unexpectedly, the long-term deficiency of p38α in liver cells triggers a compensatory up-regulation of antioxidant enzymes, which correlates with recruitment of p65 to their promoters, and results in increased GSH levels and lower GSSG/GSH ratio and malondialdehyde levels. The compensatory NF-κB activation observed upon long-term p38α deficiency probably involves both classical IKK and also AKT. Accordingly, phospho-IKK and phospho-AKT levels were higher in the liver of old p38α knockout mice than in old wild-type mice. Strikingly, Nrf-2 nuclear levels were much higher in the liver of wild-type mice than in old p38α knockout mice, but antioxidant gene levels were lower in wild-type mice. The explanation for this age-related loss of efficient transcription driven by Nrf-2 in the liver remains to be elucidated.
Interestingly, the combined deficiency in p38α and IKK-β sensitized hepatocytes to cytokine-induced apoptosis, and accordingly LPS triggered liver failure in mice deficient in both p38α and IKK-β, but not in mice lacking only p38α [25]. Hence, NF-κB compensates p38α deficiency to protect hepatocytes against cytokine-induced cell death [25]. Our results suggest that NF-κB-mediated up-regulation of antioxidant genes might contribute to the protection exerted by NF-κB.
Liver-specific p38α deficiency led to ROS accumulation, which is likely to be due to decreased expression of Hsp25 in DEN-treated mice [33]. Hsp25 is known to increase GSH levels and glutathione peroxidase activity in skeletal myoblasts, leading to lower accumulation of hydrogen peroxide [48]. Hsp25 may also up-regulate glutathione reductase and glucose 6-phosphate dehydrogenase favoring the increase in GSH levels [49]. However, we did not find any significant change upon p38α deficiency in the protein levels of Hsp25, nor in the mRNA expression of its target gene glucose 6-phosphate dehydrogenase (see Supplementary Fig. S3). Consequently, Hsp25 does not seem to be involved in the down-regulation of antioxidant genes triggered by p38α deficiency in the liver of young animals.
p38α inhibits the G1/S and the G2/M checkpoints of the cell cycle, and down-regulates the JNK and EGFR pathways; hence it has been generally considered as a tumor suppressor in normal epithelial cells [2], [41], [50]. The role of p38α as tumor suppressor has been also supported by its ability to mediate oncogene-induced apoptosis or senescence (OIS) in vitro and by the enhanced tumorigenesis induced in mice by p38α deficiency [51], [52], [53], [54], [55], [56], [57], [58]. Accordingly, liver-specific p38α deficiency consistently leads to enhanced hepatocyte proliferation and tumor development in DEN-induced hepatocarcinogenesis in mice [6], [33]. The pro-tumorigenic effect of p38α deficiency in the liver is mainly ascribed to the up-regulation of the JNK pathway [6], accumulation of superoxide and hydrogen peroxide, and release of IL-1α [33]. We found neither changes in the expression of Il-1α or Gankyrin mRNAs nor tumor development in the mice with p38α deficient livers (see Supplementary Fig. S3), suggesting that p38α deficiency alone is not sufficient to induce tumorigenesis.
p38α is activated by oncogene-induced ROS and inhibits tumor initiation by triggering apoptosis [52]. Strikingly, highly tumorigenic cancer cell lines tend to up-regulate Gstm1 and/or 2, which in turn bypass p38α activation and allow the accumulation of high ROS levels [52]. Interestingly, H-RasV12 transformed MEFs deficient in p38α exhibit in the long-term a dramatic increase in ROS levels [52]. Although H-Ras-induced p38α activation seemed to be downstream of ROS. Our findings point to a key role of p38 in the regulation of the expression of genes encoding antioxidant enzymes, which would contribute to modulate ROS levels during tumorigenesis.
5. Conclusions
In conclusion, p38α MAPK maintains the expression of antioxidant genes in the liver of young animals via NF-κΒ and PGC-1α under basal physiological conditions. However, its long-term deficiency triggers compensatory up-regulation of antioxidant enzymes through NF-κΒ. It is also noteworthy that the p38α-dependent NF-κΒ activation observed in young animals does not result in the up-regulation of pro-inflammatory genes, which deserves further investigation.
Acknowledgments
This work was supported by Grant SAF2012–39694 and SAF2015–71208-R with FEDER funds from the Spanish Ministry of Economy and Competitiveness to J.S. ARN was funded by a grant from the European Commission (ERC 294665). I.F. was recipient of a fellowship from "Programa de Doutorado Sanduíche do Exterior (PDSE)” that belongs to the "Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.02.017.
Appendix A. Supplementary material
Supplementary material
References
- 1.Soga M., Matsuzawa A., Ichijo H. Oxidative stress-induced diseases via the ASK1 signaling pathway. Int. J. Cell Biol. 2012;2012:439587. doi: 10.1155/2012/439587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner E.F., Nebreda A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 3.Shao L., Luo Y., Zhou D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 2014;20:1447–1462. doi: 10.1089/ars.2013.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuadrado A., Nebreda A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 5.Cuenda A., Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Hui L., Bakiri L., Mairhorfer A., Schweifer N., Haslinger C., Kenner L., Komnenovic V., Scheuch H., Beug H., Wagner E.F. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat. Genet. 2007;39:741–749. doi: 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- 7.Wang S., Ding L., Ji H., Xu Z., Liu Q., Zheng Y. The Role of p38 MAPK in the Development of Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17071037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvador J.M., Mittelstadt P.R., Guszczynski T., Copeland T.D., Yamaguchi H., Appella E., Fornace A.J., Ashwell J.D. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat. Immunol. 2005;6:390–395. doi: 10.1038/ni1177. [DOI] [PubMed] [Google Scholar]
- 9.Son Y., Kim S., Chung H.-T., Pae H.-O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 10.Trempolec N., Dave-Coll N., Nebreda A.R. SnapShot: p38 MAPK signaling. Cell. 2013;152 doi: 10.1016/j.cell.2013.01.029. (656–656.e1) [DOI] [PubMed] [Google Scholar]
- 11.Trempolec N., Dave-Coll N., Nebreda A.R. SnapShot: p38 MAPK substrates. Cell. 2013;152 doi: 10.1016/j.cell.2013.01.047. (924–924.e1) [DOI] [PubMed] [Google Scholar]
- 12.Viatour P., Merville M.-P., Bours V., Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem. Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Bundy R.E., Hoare G.S., Kite A., Beach J., Yacoub M., Marczin N. Redox regulation of p38 MAPK activation and expression of ICAM-1 and heme oxygenase-1 in human alveolar epithelial (A549) cells. Antioxid. Redox Signal. 2005;7:14–24. doi: 10.1089/ars.2005.7.14. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen A., Chen P., Cai H. Role of CaMKII in hydrogen peroxide activation of ERK1/2, p38 MAPK, HSP27 and actin reorganization in endothelial cells. FEBS Lett. 2004;572:307–313. doi: 10.1016/j.febslet.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 15.Usatyuk P.V., Vepa S., Watkins T., He D., Parinandi N.L., Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid. Redox Signal. 2003;5:723–730. doi: 10.1089/152308603770380025. [DOI] [PubMed] [Google Scholar]
- 16.Andrusiak M.G., Jin Y. Context specificity of stress-activated mitogen-activated protein (MAP) Kinase Signaling: the story as told by Caenorhabditis elegans. J. Biol. Chem. 2016;291:7796–7804. doi: 10.1074/jbc.R115.711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cederbaum A.I., Lu Y., Wang X., Wu D. Synergistic toxic interactions between CYP2E1, LPS/TNFα, and JNK/p38 MAP kinase and their implications in alcohol-induced liver injury. Adv. Exp. Med. Biol. 2015;815:145–172. doi: 10.1007/978-3-319-09614-8_9. [DOI] [PubMed] [Google Scholar]
- 18.Fiers W., Beyaert R., Declercq W., Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 19.Jha S.K., Jha N.K., Kar R., Ambasta R.K., Kumar P. p38 MAPK and PI3K/AKT signalling cascades inParkinson's disease. Int. J. Mol. Cell. Med. 2015;4:67–86. [PMC free article] [PubMed] [Google Scholar]
- 20.Mendelson K.G., Contois L.R., Tevosian S.G., Davis R.J., Paulson K.E. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc. Natl. Acad. Sci. Usa. 1996;93:12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsukawa J., Matsuzawa A., Takeda K., Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J. Biochem. (Tokyo). 2004;136:261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh C.-C., Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. Fasebj. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006;20:259–268. doi: 10.1096/fj.05-4376com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 25.Heinrichsdorff J., Luedde T., Perdiguero E., Nebreda A.R., Pasparakis M. p38 alpha MAPK inhibits JNK activation and collaborates with IkappaB kinase 2 to prevent endotoxin-induced liver failure. EMBO Rep. 2008;9:1048–1054. doi: 10.1038/embor.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernet J.D., Doles J.D., Hall J.K., Kelly Tanaka K., Carter T.A., Olwin B.B. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 2014;20:265–271. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blau H.M., Cosgrove B.D., Ho A.T.V. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 2015;21:854–862. doi: 10.1038/nm.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sastre J., Pallardó F.V., Viña J. The role of mitochondrial oxidative stress in aging. Free Radic. Biol. Med. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh C.-C., Papaconstantinou J. The effect of aging on p38 signaling pathway activity in the mouse liver and in response to ROS generated by 3-nitropropionic acid. Mech. Ageing Dev. 2002;123:1423–1435. doi: 10.1016/s0047-6374(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 30.Pereira L., Igea A., Canovas B., Dolado I., Nebreda A.R. Inhibition of p38 MAPK sensitizes tumour cells to cisplatin-induced apoptosis mediated by reactive oxygen species and JNK. EMBO Mol. Med. 2013;5:1759–1774. doi: 10.1002/emmm.201302732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S.-U., Park Y.-H., Min J.-S., Sun H.-N., Han Y.-H., Hua J.-M., Lee T.-H., Lee S.-R., Chang K.-T., Kang S.W., Kim J.-M., Yu D.-Y., Lee S.-H., Lee D.-S. Peroxiredoxin I is a ROS/p38 MAPK-dependent inducible antioxidant that regulates NF-κB-mediated iNOS induction and microglial activation. J. Neuroimmunol. 2013;259:26–36. doi: 10.1016/j.jneuroim.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Tormos A.M., Rius-Pérez S., Jorques M., Rada P., Ramirez L., Valverde Á.M., Nebreda Á.R., Sastre J., Taléns-Visconti R. p38α regulates actin cytoskeleton and cytokinesis in hepatocytes during development and aging. PloS One. 2017;12:e0171738. doi: 10.1371/journal.pone.0171738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakurai T., He G., Matsuzawa A., Yu G.-Y., Maeda S., Hardiman G., Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ventura J.J., Tenbaum S., Perdiguero E., Huth M., Guerra C., Barbacid M., Pasparakis M., Nebreda A.R. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat. Genet. 2007;39:750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 35.Tormos A.M., Arduini A., Talens-Visconti R., del Barco Barrantes I., Nebreda A.R., Sastre J. Liver-specificp38α deficiency causes reduced cell growth and cytokinesis failure during chronic biliary cirrhosis in mice. Hepatol. Baltim. Md. 2013;57:1950–1961. doi: 10.1002/hep.26174. [DOI] [PubMed] [Google Scholar]
- 36.Wong S.H., Knight J.A., Hopfer S.M., Zaharia O., Leach C.N., Sunderman F.W. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin. Chem. 1987;33:214–220. [PubMed] [Google Scholar]
- 37.Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 38.Madrid L.V., Mayo M.W., Reuther J.Y., Baldwin A.S. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinasep38. J. Biol. Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 39.Anjum R., Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat. Rev. Mol. Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 40.Bohuslav J., Chen L.-F., Kwon H., Mu Y., Greene W.C. p53 induces NF-kappaB activation by an IkappaB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J. Biol. Chem. 2004;279:26115–26125. doi: 10.1074/jbc.M313509200. [DOI] [PubMed] [Google Scholar]
- 41.Xu S., Bayat H., Hou X., Jiang B. Ribosomal S6 kinase-1 modulates interleukin-1beta-induced persistent activation of NF-kappaB through phosphorylation of IkappaBbeta. Am. J. Physiol. Cell Physiol. 2006;291:C1336–C1345. doi: 10.1152/ajpcell.00552.2005. [DOI] [PubMed] [Google Scholar]
- 42.Olmos Y., Sánchez-Gómez F.J., Wild B., García-Quintans N., Cabezudo S., Lamas S., Monsalve M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid. Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherry A.D., Suliman H.B., Bartz R.R., Piantadosi C.A. Peroxisome proliferator-activated receptor γ co-activator 1-α as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. J. Biol. Chem. 2014;289:41–52. doi: 10.1074/jbc.M113.512483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams R.H., Porras A., Alonso G., Jones M., Vintersten K., Panelli S., Valladares A., Perez L., Klein R., Nebreda A.R. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell. 2000;6:109–116. [PubMed] [Google Scholar]
- 45.Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 2015;88:147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 46.Wakabayashi N., Chartoumpekis D.V., Kensler T.W. Crosstalk between Nrf2 and Notch signaling. Free Radic. Biol. Med. 2015;88:158–167. doi: 10.1016/j.freeradbiomed.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaru R., Matthews S.P., Edgar A.J., Prescott A.R., Gomez-Nicola D., Hanauer A., Watts C. The PDK1-Rsk signaling pathway controls langerhans cell proliferation and patterning. J. Immunol. Baltim. Md 1950. 2015;195:4264–4272. doi: 10.4049/jimmunol.1501520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escobedo J., Pucci A.M., Koh T.J. HSP25 protects skeletal muscle cells against oxidative stress. Free Radic. Biol. Med. 2004;37:1455–1462. doi: 10.1016/j.freeradbiomed.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Garrido C., Brunet M., Didelot C., Zermati Y., Schmitt E., Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle Georget. Tex. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 50.Han J., Sun P. The pathways to tumor suppression via routep38. Trends Biochem. Sci. 2007;32:364–371. doi: 10.1016/j.tibs.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Bulavin D.V., Kovalsky O., Hollander M.C., Fornace A.J. Loss of oncogenic H-ras-induced cell cycle arrest and p38 mitogen-activated protein kinase activation by disruption of Gadd45a. Mol. Cell. Biol. 2003;23:3859–3871. doi: 10.1128/MCB.23.11.3859-3871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolado I., Swat A., Ajenjo N., De Vita G., Cuadrado A., Nebreda A.R. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11:191–205. doi: 10.1016/j.ccr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Iwasa H., Han J., Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells Devoted Mol. Cell. Mech. 2003;8:131–144. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- 54.Kwong J., Hong L., Liao R., Deng Q., Han J., Sun P. p38alpha and p38gamma mediate oncogenic ras-induced senescence through differential mechanisms. J. Biol. Chem. 2009;284:11237–11246. doi: 10.1074/jbc.M808327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicke B., Bastien J., Khanna S.J., Warne P.H., Cowling V., Cook S.J., Peters G., Delpuech O., Schulze A., Berns K., Mullenders J., Beijersbergen R.L., Bernards R., Ganesan T.S., Downward J., Hancock D.C. Involvement of MINK, a Ste20 family kinase, in Ras oncogene-induced growth arrest in human ovarian surface epithelial cells. Mol. Cell. 2005;20:673–685. doi: 10.1016/j.molcel.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 56.Wang W., Chen J.X., Liao R., Deng Q., Zhou J.J., Huang S., Sun P. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol. Cell. Biol. 2002;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu Y., Li N., Xiang R., Sun P. Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem. Sci. 2014;39:268–276. doi: 10.1016/j.tibs.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaswen P., Campisi J. Oncogene-induced senescence pathways weave an intricate tapestry. Cell. 2007;128:233–234. doi: 10.1016/j.cell.2007.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material