Abstract
Inflammation has been implicated in a variety of retinal diseases. The immunoproteasome plays a critical role in controlling inflammatory responses, but whether activation of immunoproteasome contributes to angiotensin II (Ang II)-induced retinopathy remains unclear. Hypertensive retinopathy (HR) was induced by infusion of Ang II (3000 ng/kg/min) in wild-type (WT) and immunoproteasome subunit LMP10 knockout (KO) mice for 3 weeks. Changes in retinal morphology, vascular permeability, superoxide production and inflammation were examined by pathological staining. Our results showed that immunoproteasome subunit LMP10 expression and its trypsin-like activity were significantly upregulated in the retinas and serum of Ang II-infused mice and in the serum from patients with hypertensive retinopathy. Moreover, Ang II-infused WT mice showed an increase in the central retinal thickness, vascular permeability, reactive oxygen species (ROS) production and inflammation compared with saline controls, and these effects were significantly attenuated in LMP10 KO mice, but were aggravated in mice intravitreally injected with rAAV2-LMP10. Interestingly, administration of IKKβ specific inhibitor IMD-0354 remarkably blocked an Ang II-induced increase in vascular permeability, oxidative stress and inflammation during retinopathy. Mechanistically, Ang II-induced upregulation of LMP10 promoted PTEN degradation and activation of AKT/IKK signaling, which induced IkBα phosphorylation and subsequent degradation ultimately leading to activation of NF-kB target genes in retinopathy. Therefore, this study provided novel evidence demonstrating that LMP10 is a positive regulator of NF-kB signaling, which contributes to Ang II-induced retinopathy. Strategies for inhibiting LMP10 or IKKβ activity in the eye could serve as a novel therapeutic target for treating hypertensive retinopathy.
Keywords: Angiotensin II, Retinopathy, Immunoproteasome LMP10, Vascular permeability, Oxidative stress, Inflammation
Graphical abstract
Highlights
-
•
Immunosubunit LMP10 is increased in the retinas of Ang II-infused mice.
-
•
Ang II-induced retinopathy is attenuated in LMP10 knockout mice.
-
•
LMP10 overexpression in mice aggravates Ang II-induced retinopathy.
-
•
IKKβ inhibitor IMD-0354 blocks Ang II-induced retinopathy.
1. Introduction
Hypertension is one of the most important risk factors that may affect ocular structure and function, and has been linked to a wide range of major eye diseases [1]. More specifically, high blood pressure decreases choroidal circulatory flow and increases intraocular pressure and microvascular abnormalities [1]. Currently, hypertensive retinopathy can be found in 3–14% of adult individuals aged > 40 years [2]. Increasing evidence suggests that angiotensin II (Ang II), the major effector peptide of the rennin-angiotensin system (RAS), has a fundamental role in the pathogenesis of retinal vascular diseases. While blocking of angiotensin type 1 receptor (AT1R) by inhibitors or intraocular administration of AAV-ACE2/Ang-(1–7) has been found to improve pathology in ocular diseases, such as early retinopathy and diabetic retinopathy [3], [4], a critical role for Ang II has been demonstrated in the formation of retinopathy. Therefore, it is important to identify mechanisms that mediate retinopathy in response to Ang II stress.
The 26 S proteasome, the major proteolysis machinery in eukaryotic cells, has a critical role in the regulation of cell survival and the cell cycle, gene expression, signal transduction and protein quality control [5], [6]. The 20 S catalytic core of the proteasome contains three standard catalytic subunits β1 (PMSB6), β2 (PMSB7) and β5 (PMSB5). After stimulation of cytokines, such as FN-γ, the standard subunits can be replaced with the inducible subunits, such as LMP2 (β1i), LMP10 (β2i or MECL), and LMP7 (β5i), which form the core of the immunoproteasome [7]. LMP10 is one of the immunoproteasome catalytic subunits and has trypsin-like activity. Several studies have indicated that hypertensive stimuli, such as Ang II or high-salt, can provoke upregulation of the immunosubunits, including LMP10 and LMP7 in the heart, and knockout of LMP10 significantly attenuated DOCA-salt-induced hypertension and cardiac remodeling in mice [8]. Moreover, the increased expression and activity of immunosubunits are also observed in retinopathy [9], whereas inhibiting immunoproteasaome activity improves this disease [10]. However, the functional role of LMP10 in regulating Ang II-induced retinopathy remains unknown.
In this study, we showed for the first time that the LMP10 level and its trypsin-like activity in the retina or blood were markedly upregulated in Ang II-infused mice and in patients with hypertensive retinopathy. Deficiency of LMP10 or inhibition of IKKβ by specific inhibitors significantly attenuated Ang II-induced retinopathy, while this change was aggravated in rAAV2-LMP10-injected mice. These effects were associated with LMP10-mediated degradation of PTEN and IkBα and activation of AKT-IKKβ-NF-kB signaling target genes. Thus, our results demonstrated that LMP10 contributes to Ang II-induced retinopathy.
2. Methods
An expanded Methods section is available in the online-only Data Supplement.
2.1. Animal experiments
Wild-type (WT) mice and LMP10 knockout (KO) mice (males, 8–10-weeks-old) were infused with saline or angiotensin II (Ang II, Sigma-Aldrich, St. Louis, MO) at a dose of 3000 ng/kg/min using osmotic mini-pumps (Alzet MODEL1004,0.11 μl/hour,28 days; DURECT, Cupertino, CA) for 3 weeks, as previously described [11], [12]. IKKβ inhibitor IMD-0354 (S2864, Selleck) was administered intraperitoneally (30 mg/kg/d) beginning 1 day before Ang II infusion and continued for 3 weeks [13], [14]. All investigations were approved by the Animal Care and Use Committee of Dalian Medical University and conformed to the US National Institutes of Health Guide for the Care and Use of Laboratory and the ARRIVE guidelines [15].
2.2. Intravitreous injections of recombinant adenoviral vectors in mice
Recombinant adeno-associated virus serotype 2 (rAAV2) expressing GFP (rAAV2-GFP) and LMP10 (rAAV2-LMP10) were produced by Vigenebio (Shangdong, China). Four microliters of rAAV2-GFP or rAAV2-LMP10 (2.4 × 1012 pfu/ml/per eye) were injected into the intravitreal space of the right eye, just posterior to the limbus, as previously described [16]. Evaluation of infection efficiency and the expression of rAAV2-GFP/LMP10 was performed 2 weeks after injection.
2.3. Fluorescence angiography
Mice were anesthetized with of 2.5% tribromoethanol (0.020 ml/g; Sigma-Aldrich, UK). Eyes were dilated with one drop of Compound Tropicamide Eye Drops (Mydrin-P; Santen Pharmaceutical, Osaka, Japan) and Carbomer Eye Gel (Dr. Gerhard Mann, Germany) were applied. Using retinal imaging system (OPTO-RIS, Optoprobe, Canada), images were collected immediately after fluorescein administration, then every minute thereafter for 5 min. After capture, the images were arranged according to capture time and data were compared for the mice at the same time interval using the Image J software program [17].
2.4. Histopathological examination
All animals were anesthetized by an overdose of pentobarbital (100 mg/kg, ip). The eyes were quickly removed and fixed in 4% paraformaldehyde, embedded in paraffin and sectioned (5 µm). The eye sections were stained with hematoxylin and eosin (H&E) and immunohistochemistry with antibody against ionized calcium binding adapter molecule 1 (Iba1, 1:500, Wako, Osaka, Japan) [18].
2.5. Immunostaining
The freshly frozen eye sections (6 µm thick) were stained with dihydroethidine (DHE, 1 μM in PBS) for 30 min at 37 ℃ as described previously [18]. The eye sections were also permeabilized with 0.3% Triton X-100/PBS and were then incubated at 4 ℃ overnight with Dylight 594 Labeled Griffonia Simplicifolia Lecin I (GSL I) isolectin B4 (DL-1207 1:50, Vector laboratories lnc.), followed by incubation with DAPI (1:10000, Sigma) for 5 min at room temperature [19].
2.6. Sample collection of patients
The study included 31 normal normotensive individuals, 31 patients with hypertension (HP) and 30 with hypertensive retinopathy (HR). Written informed consent was signed by all subjects prior to participation in the study. The study protocol was approved by the Institutional Ethics Committee of First Affiliated Hospital of Dalian Medical University (No. LCKY2016-31) and conformed with the principles outlined in the Declaration of Helsinki.
2.7. Statistical Analyses
All values in the text and figures were tested with SPSS 19.0. Comparisons were made with a one-way ANOVA (S-N-K and Bonferroni method) or independent T-test as appropriate. Values of P < 0.05 were considered statistically significant.
3. Results
3.1. LMP10 expression and its proteasome activity were increased in Ang II-treated mice and patients with hypertensive retinopathy
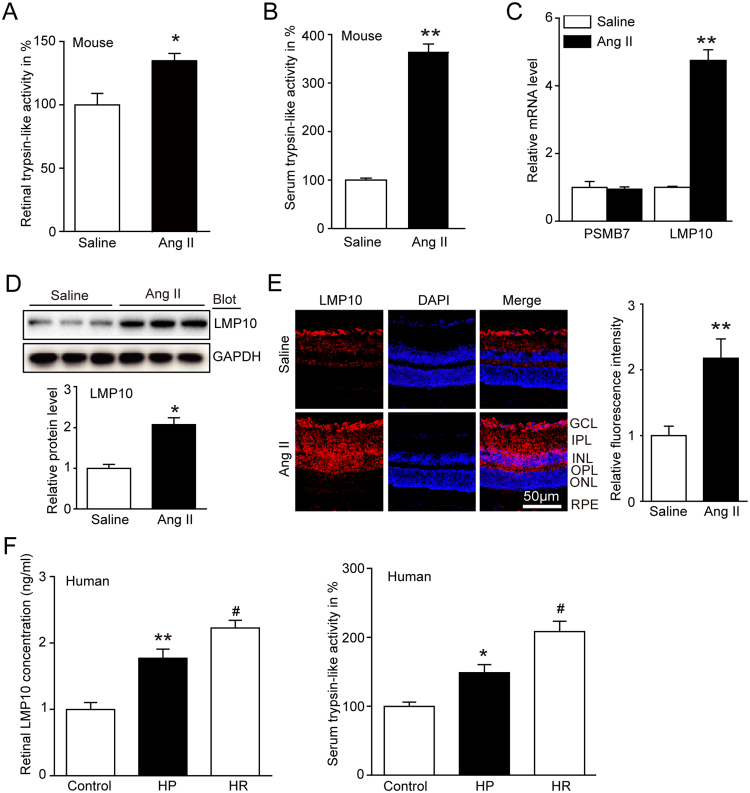
To investigate the functional role of LMP10 in regulating hypertensive retinopathy, we first measured retina and serum proteasome trypsin-like activity, which was displayed by standard subunit PMSB7 and immunosubunit LMP10. We found that the trypsin-like activity in serum and retinal tissues was significantly increased in Ang II-treated mice compared with saline control mice (Fig. 1A and B). To identify which subunit contributes to increased proteasome activity, we performed qPCR analysis. We found that LMP10 expression rather than PMSB7 was markedly upregulated in Ang II-infused retinas compared with saline control mice (Fig. 1C). The LMP10 protein expression was also remarkably upregulated in Ang II-treated retinas (Fig. 1D), indicating that Ang II-induced proteasome activity was due to LMP10. Moreover, immunostaining revealed that LMP10 protein was mainly localized in ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL) and retinal pigment epithelium (RPE) of retinas, and highly induced by Ang II infusion (Fig. 1E). Importantly, we measured serum LMP10 concentrations and trypsin-like activity in human samples, and found that both LMP10 levels and trypsin-like activity (Fig. 1F) were significantly higher in patients with hypertension (HP, n = 31) or hypertensive retinopathy (HR, n = 30) compared to normal controls (n = 31) (Fig. 1F, Appendix A, Appendix A).
Fig. 1.
The trypsin-like activity and LMP10 expression were upregulated in Ang II-infused mice and in hypertensive retinopathy patients. A and B, Measurement of trypsin-like activity in the retinas and serum of wild-type (WT) mice 3 weeks after saline or Ang II infusion (n = 8 mice per group). C, qPCR analysis for the mRNA expression of PSMB7 and LMP10 in the retinas (n = 8 mice per group). D, Immunoblotting analysis of LMP10 (upper) and quantification of the protein bands (lower) (n = 3 mice per group). GAPDH as an internal control. E, Immunostaining of the LMP10 (red) in the retinal sections (left) and quantification of fluorescence intensity (right, n = 4 mice per group). Nuclei were counterstained with DAPI (blue). Scale bar, 50 µm. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. F, Measurement of the LMP10 concentration by ELISA assay and the trypsin-like activity in serum of normal controls (n = 31) and hypertension (HP) (n = 31) or hypertensive retinopathy (HR) (n = 30) patients. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01 versus saline or normal controls; #P < 0.05 versus hypertension (HP) group.
3.2. Deficiency of LMP10 improves retinal morphology and vascular permeability induced by angiotensin II
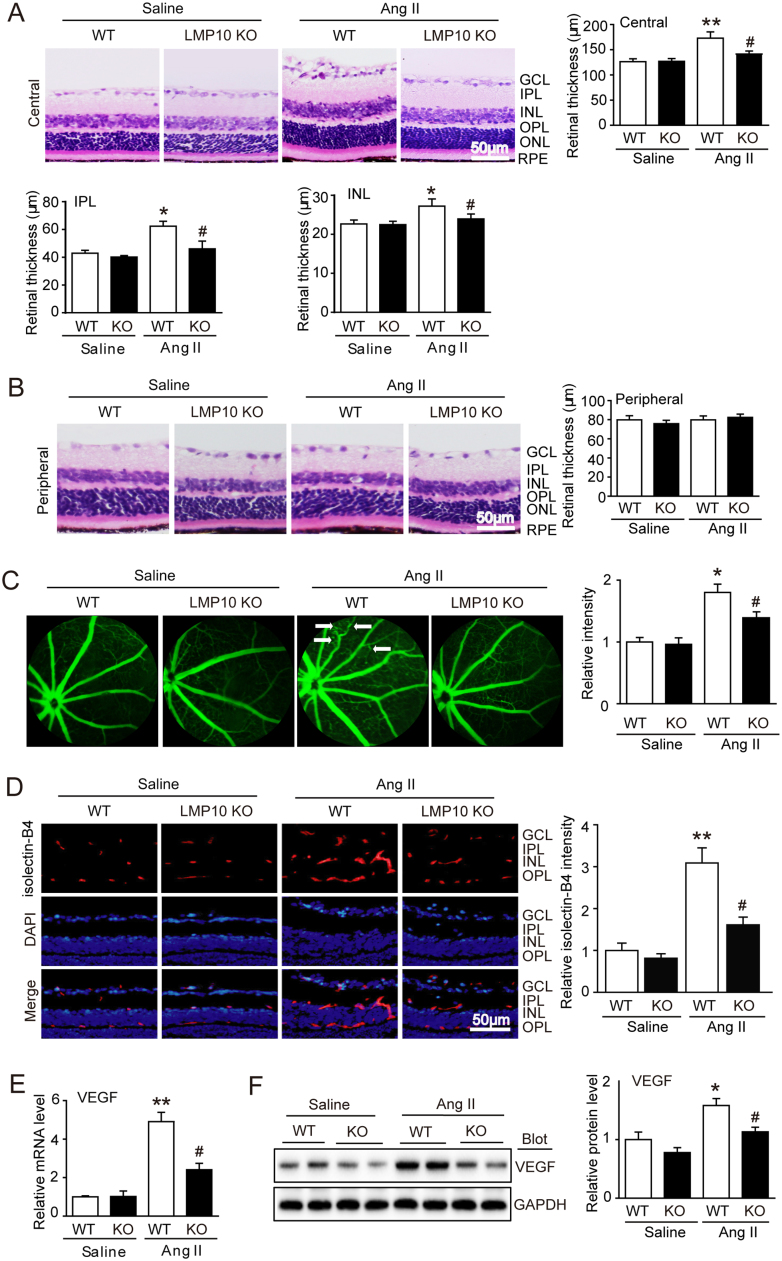
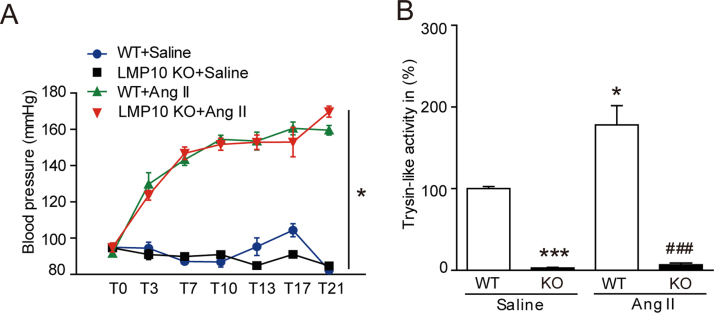
We then determine the effect of LMP10 on retinal morphology induced by Ang II in wild-type (WT) and LMP10 knockout (KO) mice. After 3 weeks of Ang II infusion, the systolic blood pressure (SBP) was remarkably increased in both WT and PSMB10 KO mice, but there was no statistically significant difference between the two groups (Fig. S1A). Knockout of LMP10 significantly reduced trypsin-like activity compared with WT controls after saline or Ang II infusion (Fig. S1B). Moreover, H&E staining showed that Ang II infusion for 3 weeks markedly increased the thickness of central retinas, especially the inner plexiform layer (IPL) and inner nuclear layer (INL) in the WT mice, but this increase was markedly reduced in LMP10 KO mice (Fig. 2A). The peripheral retinal morphology was similar between the two groups after saline or Ang II infusion (Fig. 2B).
Fig. 2.
Deficiency of LMP10 attenuates Ang II-induced retinal thickness and vascular permeability. A, Representative H&E staining of central retinal sections and quantification of central retinal thickness of wild-type (WT) and LMP10 knockout (KO) mice 3 weeks after saline or Ang II infusion (n = 10 mice per group). B, Representative H&E staining of peripheral retinal sections (left) and quantification of retinal thickness (n = 10 mice per group). C, Representative angiograms for retinal vessels and quantification of fluorescence intensity. White arrows showed perivascular fluorescein leakage (right, n = 6 mice per group). D, Representative isolectin B4 staining of central retinal sections for endothelial cell proliferation (left) and quantification of fluorescence intensity (right, n = 6 mice per group). Nuclei were counterstained with DAPI (blue). Scale bar, 50 µm. E, qPCR analysis of VEGF mRNA expression in the retinas (n = 6 mice per group). F, Immunoblotting analysis of VEGF expression (left) in the retinas and quantification of protein bands (right) (n = 4 mice per group). GAPDH as an internal control. Data are the mean ± SEM. *P < 0.05, **P < 0.01 versus saline-infused WT mice; #P < 0.05 versus Ang II-infused WT mice. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
Since vascular permeability is the hallmark of retinopathy, we next tested whether knockout of LMP10 influences retinal vascular permeability and endothelial cell proliferation. Fluorescence angiography showed retinal vascular permeability reflected by typically diffuse pale green areas (white arrow), arteriolar narrowing and tortuosity in Ang II-infused WT retinas compared with the saline controls, whereas this effect was attenuated in LMP10 KO mice (Fig. 2C). Moreover, isolectin B4 staining revealed that Ang II-induced endothelial cell proliferation in WT inner retinas was significantly reduced in LMP10 KO mice (Fig. 2D). In addition, Ang II-induced upregulation of VEGF (a critical driver for angiogenic and vascular permeability) at both mRNA and protein levels in WT retinas was also reduced in LMP10 KO mice (Fig. 2E and F). These results indicate that LMP10 is involved in Ang II-induced vascular permeability in this model.
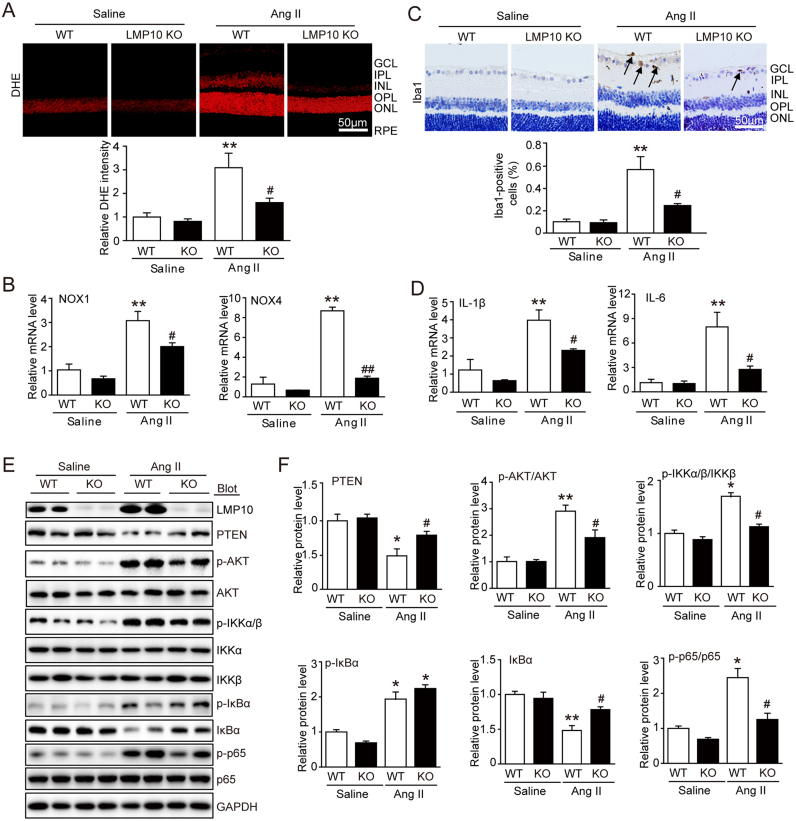
3.3. Knockout of LMP10 suppresses angiotensin II-induced retinal oxidative stress and inflammation
Oxidative stress and inflammation have been recognized as central drivers of vessel growth in retinopathy. We therefore tested the effect of LMP10 on superoxide production and activation of NADPH oxidase in Ang II-infused retinas. DHE staining showed that Ang II infusion strongly increased DHE intensity of ROS levels in WT mice, whereas this increase was remarkably blocked in LMP10 KO mice (Fig. 3A). Since NADPH oxidase isoforms of NOX1 and NOX4 are the major source of ROS generation in retinas, we then analyzed mRNA expression of NOX1 and NOX4 by qPCR analysis. In keeping with the decrease in ROS levels, the expression of retinal NOX1 and NOX4 were also significantly reduced in LMP10 KO mice compared with WT animals after Ang II treatment (Fig. 3B).
Fig. 3.
Knockout of LMP10 reduces Ang II-induced retinal oxidative stress and inflammation. A, Representative dihydroethydium (DHE) staining of central retinal sections (upper) and quantification of DHE intensity (lower) of wild-type (WT) and LMP10 knockout (KO) mice 3 weeks after saline or Ang II infusion (n = 6 mice per group). B, qPCR analysis of the mRNA levels of NOX1 and NOX4 in the retinas (n = 6 mice per group). C, Representative immunohistochemical staining of calcium-binding adapter molecule 1 (Iba1, arrow) in the retinas (upper) and quantification of Iba-positive cells (lower, n = 6 mice per group). Scale bar, 50 µm. D, qPCR analysis of the mRNA levels of IL-1β and IL-6 in the retinas (n = 3 mice per group). E, Immunoblotting analysis of the protein levels of LMP10, PTEN, p-AKT, AKT, p-IKKα/β, IKKα, IKKβ, p-IkBα, IkBα, p-p65 and p65 in the retinas. F, Quantification of the protein bands (n = 4 mice per group). GAPDH as an internal control. Data are the mean ± SEM. *P < 0.05, **P < 0.01 versus saline-infused WT mice; #P < 0.05, ##P < 0.01 versus Ang II-infused WT mice. IL-1β, interleukin-1β; IL-6, interleukin-6; NOX, NADPH oxidase. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
We next evaluated the contribution of LMP10 to retinal inflammatory responses. Immunohistochemical staining revealed that Ang II infusion caused marked accumulation of Iba1-positive microgalia/macrophages in the inner retinal layer, which was significantly attenuated in LMP10 KO mice (Fig. 3C). Accordingly, qPCR analysis indicated that the levels of IL-1β and IL-6 were markedly lower in LMP10 KO retinas than in WT controls after Ang II infusion (Fig. 3D).
NF-kB has been reported to be a key regulator of proinflammatory cytokines, NADPH oxidase isoforms and VEGF expression at transcriptional levels. We then examined whether LMP10 influences NF-kB activity and its upstream regulators IKK and IkBa [20]. Ang II infusion resulted in a significant increase of p-IKKα/β and p-p65 in WT retinas, which was markedly reduced in LMP10 KO mice. Moreover, Ang II-induced reduction of total IkBa was markedly reversed in LMP10 KO mice (Fig. 3E and F). However, upregulation of p-IkBa induced by Ang II was similar between WT and LMP10 KO mice (Fig. 3E and F), indicating that LMP10 knockout attenuates IKKα/β activation and IkBa degradation resulting in inactivation of NF-kB.
To further learn how LMP10 deficiency inhibits IKKα/β activation, we examined the tumor suppressor PTEN and its downstream target AKT, a critical regulator of IKKα/β activation [21], [22]. Western blot analysis revealed that Ang II infusion markedly decreased PTEN levels but increased AKT activation in the WT retina, and this effect was reversed in LMP10 KO mice (Fig. 3E and F), suggesting that LMP10 promotes PTEN degradation, leading to activation of AKT-IKKα/β signaling. Together, these results suggested that LMP10 deficiency inhibits activation of AKT/IKK/NF-kB signaling by preventing degradation of both PTEN and IkBa in Ang II-induced retinopathy.
3.4. Overexpression of LMP10 aggravates Ang II-Induced vascular permeability, inflammation, and oxidative stress in the retinas
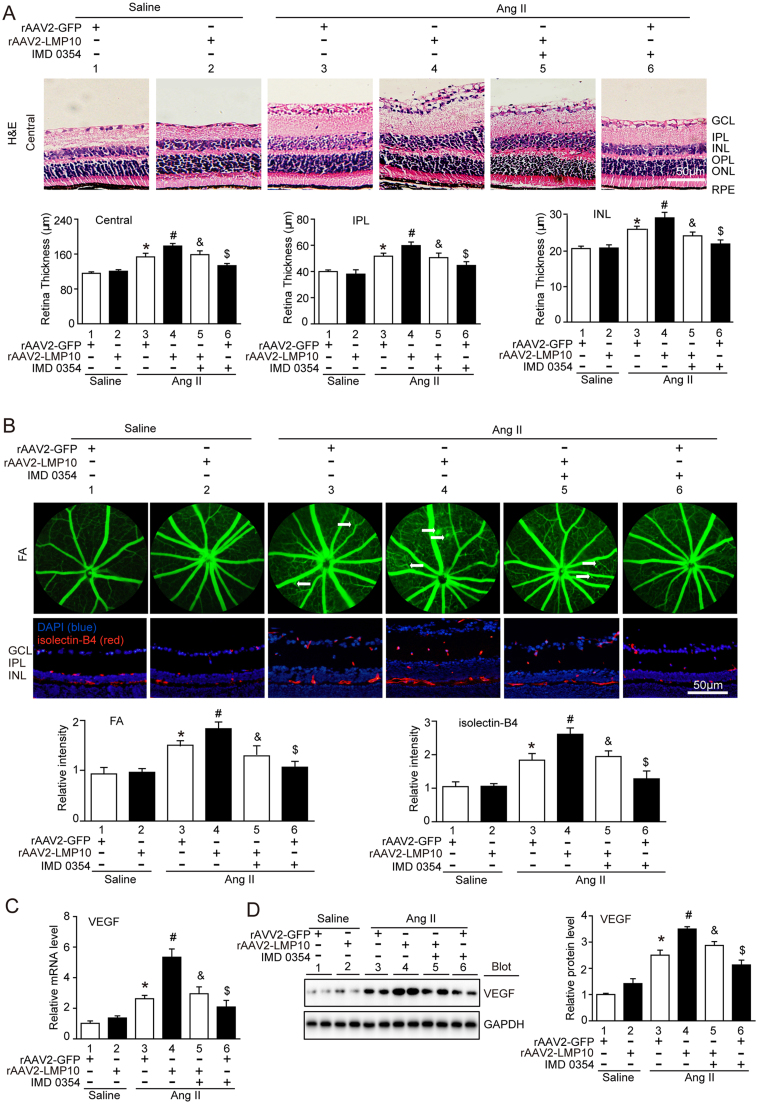
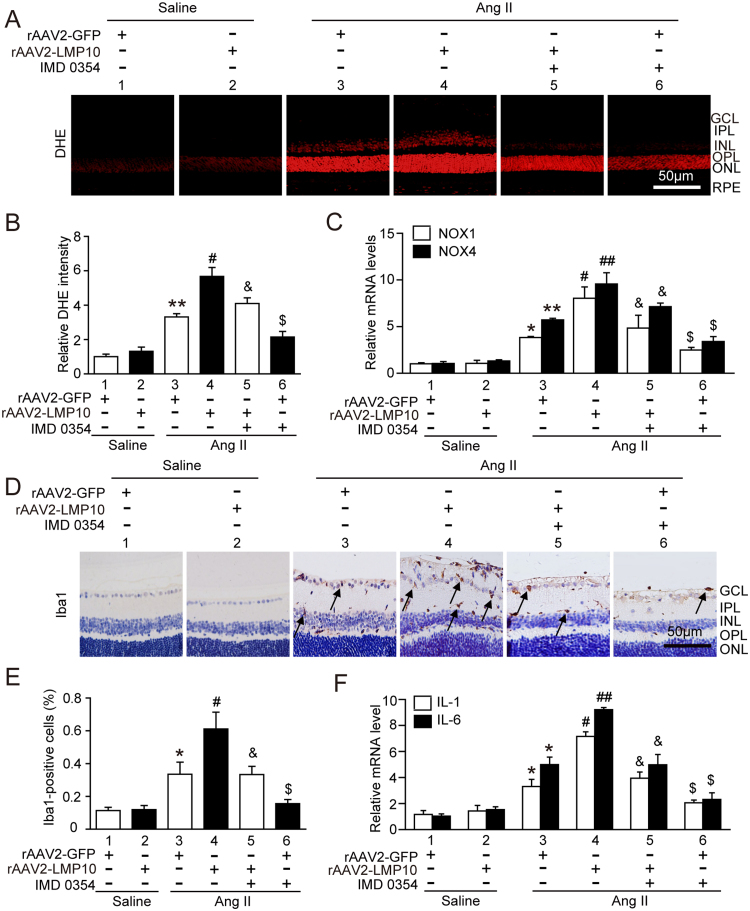
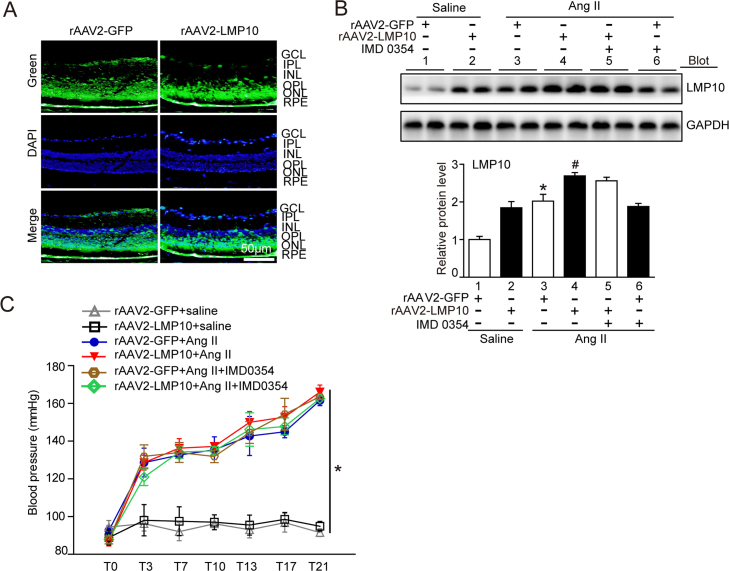
To further assess whether overexpression of LMP10 is sufficient to promote Ang II-induced retinopathy in vivo, we generated rAAV2 overexpressing LMP10 (rAAV2-LMP10) or GFP alone (rAAV2-GFP). Intravitreal injection of WT mice with rAAV2-LMP10 (2.4 × 1012 pfu/ml) resulted in high infection efficiency as indicated by GFP fluorescence intensity (Fig. S2A) and LMP10 expression (about 1.8-fold) in the retinas (Fig. S2B). Mice injected with rAAV2 were then exposed to Ang II or saline infusion for an additional 3 weeks. There was no significant difference in SBP elevation between rAAV2-LMP10- and rAAV2-GFP-injected mice after saline or Ang II infusion (Fig. S2C). After 3 weeks of Ang II infusion, rAAV2-GFP-injected mice showed a significant increase in the central retinal thickness, including the inner plexiform layer (IPL) and inner nuclear layer (INL) (Fig. 4A), vascular permeability, arteriolar narrowing, endothelial cell proliferation (Fig. 4B) and VEGF expression at mRNA and protein levels (Fig. 4C and D), which were further aggravated in rAAV2-LMP10-injected mice (Fig. 4A-D, lanes 4 vs 3). Moreover, Ang II-induced production of ROS (Fig. 5A-B), the infiltration of Iba1-positive macroglia/macrophages and mRNA expression of NOX1, NOX4, IL-1β and IL-6 (Fig. 5C, F) were markedly enhanced in rAAV2-LMP10-injected mice (Fig. 5A-F, lanes 4 vs 3).
Fig. 4.
Overexpression of LMP10 accelerates, whereas IKKβ inhibitor blunts, Ang II-induced retinal thickness and vascular permeability. A, Representative H&E staining of central retinal sections and quantification of central retinal thickness in WT mice injected with rAAV2-GFP (2.4 × 1012 pfu/ml) or rAAV2-LMP10 (2.4 × 1012 pfu/ml) in the presence or absence of IKKβ inhibitor IMD-0354 (30 mg/kg/d) 3 weeks after saline or Ang II infusion (n = 10 mice per group). B, Representative angiograms for retinal vasculature, isolectin B4 (red) staining of central retinal sections and quantification of fluorescence intensity (right, n = 6 mice per group). White arrows showed perivascular fluorescein leakage. Nuclei were counterstained with DAPI (blue). Scale bar, 50 µm. FA, Fluorescence angiography. C, qPCR analysis of VEGF mRNA levels in the retinas (n = 6 mice per group). D, Immunoblotting analysis of VEGF protein levels in the retinas (left). Quantification of the protein bands (right, n = 4 mice per group). GAPDH as an internal control. Data are the mean ± SEM. *P < 0.05 versus rAAV2-GFP-injected mice infused with saline; #P < 0.05 versus rAAV2-GFP-injected mice infused with Ang II; &P < 0.05 versus rAAV2-LMP10-injected mice infused with Ang II; $P < 0.05 versus rAAV2-GFP-injected mice infused with Ang II.
Fig. 5.
Overexpression of LMP10 aggravates, but IKKβ inhibitor suppresses, Ang II-induced retinal oxidative stress and inflammation. A, Representative dihydroethydium (DHE) staining of central retinal sections in WT mice injected with rAAV2-GFP (2.4 × 1012 pfu/ml) or rAAV2-LMP10 (2.4 × 1012 pfu/ml) in the presence or absence of IKKβ inhibitor IMD-0354 (30 mg/kg/d) 3 weeks after saline or Ang II infusion. Scale bar, 50 µm. B, Quantification of DHE intensity in the retinas (n = 6 mice per group). C, qPCR analysis of the mRNA levels of NOX1 and NOX4 in the retinas (n = 6 mice per group). D, Representative immunohistochemical staining of Iba1 (arrow) in the retinas. Scale bar, 50 µm. E, Quantification of Iba1-positive cells (n = 6 mice per group). F, qPCR analysis of the mRNA levels of IL-1β and IL-6 in the retinas (n = 6 mice per group). Data are the mean ± SEM. *P < 0.05, **P < 0.01 versus rAAV2-GFP-injected mice infused with saline; #P < 0.05, ##P < 0.01 versus rAAV2-GFP-injected mice infused with Ang II; &P < 0.05 versus rAAV2-LMP10-injected mice infused with Ang II; $P < 0.05 versus rAAV2-GFP-injected mice infused with Ang II.
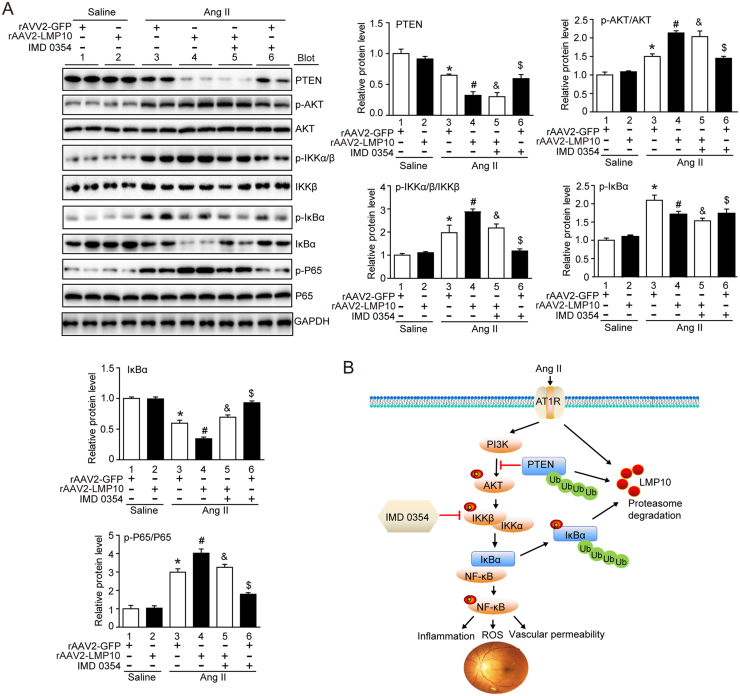
To verify the importance of LMP10 in regulating PTEN-dependent signaling, we evaluated activation of PTEN/AKT and IKK/IkBa/NF-kB in Ang II-treated retinas. Ang II-induced downregulation of PTEN and IkBa in rAAV2-GFP-injected retinas was further reduced in rAAV2-LMP10-injected mice (Fig. 6A, lanes 4 vs 3). In contrast, Ang II-induced increases of p-AKT, p-IKKα/β and p-p65 levels were largely enhanced in rAAV2-LMP10-injected mice compared with rAAV2-GFP controls (Fig. 6A, lanes 4 vs 3). There was no difference in these alterations between the two groups after saline infusion (Fig. 6A, lanes 4 vs 3).
Fig. 6.
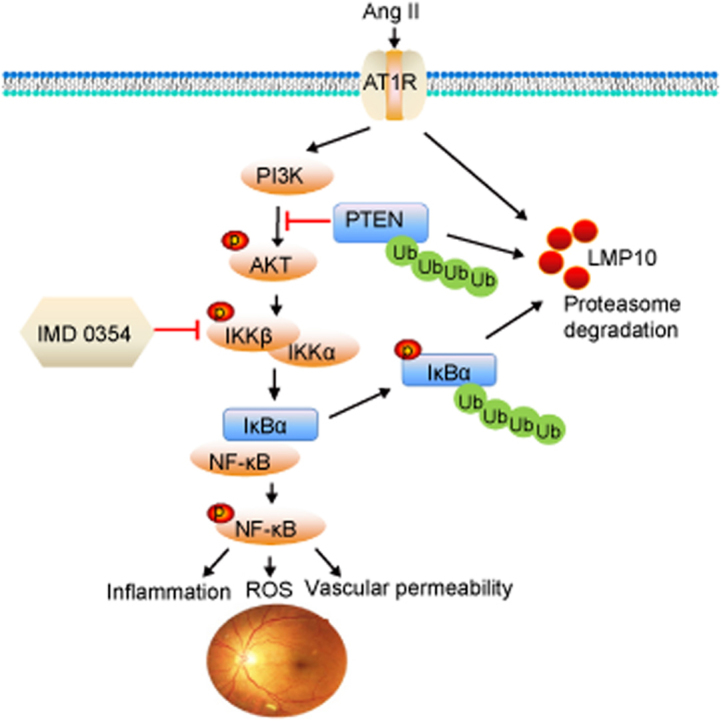
Effect of Overexpression of LMP10 or IKK inhibitor on PTEN-AKT and IKK/IkBα/NF-kB signals in the retinas after Ang II infusion. A, Immunoblotting analysis of the protein levels of PTEN, p-AKT, AKT, p-IKKα/β, IKKβ, p-IkBα, IkBα, p-p65 and p65 in the retinas and quantification of the protein bands (n = 4 mice per group). GAPDH as an internal control. Data are the mean ± SEM. *P < 0.05 versus rAAV2-GFP-injected mice infused with saline; #P < 0.05 versus rAAV2-GFP-injected mice infused with Ang II; &P < 0.05 versus rAAV2-LMP10-injected mice infused with Ang II; $P < 0.05 versus rAAV2-GFP-injected mice infused with Ang II. B, A working model for LMP10 to regulate Ang II-induced signaling pathways and retinopathy. In response to Ang II, increased LMP10 promotes PTEN degradation and activation of AKT/IKK signaling, which induces phosphorylation and subsequent degradation of IkBα, ultimately resulting in activation of NF-kB targets and retinopathy.
3.5. Inhibition of IKK activation blunts retinal vascular permeability, oxidative stress, and inflammation induced by angiotensin II
To test whether IKK/IkBa/NF-kB signaling plays a critical role in LMP10-mediated retinopathy, we applied IKKβ specific inhibitor IMD-0354 to rAAV2-injected WT mice. Systemic administration of IMD-0354 did not cause any noticeable cellular toxicity to the treated eyes as evaluated by histology, and no systemic toxicity was observed. Notably, administration of IMD-0354 to rAAV2-LMP10-injected mice significantly blocked Ang II-induced increases in the central retinal thickness, vascular permeability and VEGF expression at both mRNA and protein levels compared with vehicle control (Fig. 4, lanes 5 vs 4). Ang II-induced upregulation of ROS production and the infiltration of Iba1-positive microglia/macrophages, as well as the mRNA levels of NOX1, NOX4, IL-1β and IL-6, in rAAV2-LMP10-injected retinas were also remarkably lower in IMD-0354-treated mice than in vehicle controls (Fig. 5, lanes 5 vs 4). IMD-0354 had a similar effect in rAAV2-GFP-injected retinas (Fig. 4, Fig. 5, lanes 6 vs 3). Interestingly, IMD-0354 treatment did not significantly affect PTEN and p-AKT levels, but significantly inhibited Ang II-induced enhancement of p-IKKα/β, p-IkBa and p-p65 levels in rAAV2-LMP10- or rAAV2-GFP-injected retinas. The reduction of total IkBa induced by Ang II in rAAV2-injected groups was also markedly reversed by IMD-0354 (Fig. 6, lanes 5 vs 4, lanes 6 vs 3, respectively). There was no difference in these alterations between the two groups after saline infusion (Fig. 4, Fig. 5, Fig. 6).
4. Discussion
In this study, we demonstrated for the first time that LMP10 expression and its trypsin-like activity were significantly increased in Ang II-treated retinas or patients with hypertensive retinopathy. Knockout of LMP10 significantly attenuated Ang II-induced increases in retinal structural remodeling, vascular permeability, oxidative stress and inflammation, but these abnormalities were aggravated by overexpression of rAAV2-LMP10. Interestingly, administration of IKKβ specific inhibitor IMD-0354 markedly blocked Ang II-induced pathological changes. Mechanistically, LMP10 promoted PTEN degradation and caused activation of AKT/IKKβ signaling, which induced IkBa ubiquitination and subsequent degradation, ultimately resulting in activation of NF-kB target genes during retinopathy (Fig. 6B). Thus, this study highlighted the critical role for LMP10 in regulating Ang II-induced retinopathy in mice. Inhibition of LMP10 or IKKβ could serve as a novel therapeutic target for hypertensive retinopathy.
The primary function of the immunoproteasome was originally reported to improve the MHC-I antigen presentation and regulate immune responses [7]. Previous studies showed that the immunosubunits LMP2 (β1i) and LMP7 (β5i) existed in both rat and human retinas [9], and the expression of these immunosubunits was approximately 2-fold higher in the normal retina than in the brain [23], suggesting a critical role in maintaining retinal homeostasis. Moreover, aging and chronic oxidative stress upregulate the expression and proteasome activities of LMP2 (β1i) and LMP7 (β5i) in the retinas and cultured retinal pigment epithelial (RPE) cells [9], [24]. A study we carried out recently indicated that hypertensive stimuli, such as Ang II and high-salt, upregulated the expression of multiple immunosubunits in the heart, and knockout of LMP10 significantly attenuated DOCA-salt-induced hypertension and cardiac remodeling in mice [8]. Here we extended previous findings, and provided new evidence supporting LMP10 playing a critical role in Ang II-induced retinal abnormalities, including vascular permeability, oxidative stress and inflammation (Fig. 2, Fig. 3, Fig. 4, Fig. 5).
Inflammation is a common feature of retinopathy and plays an important role in the initiation and development of retinopathy [1]. Studies have demonstrated that inflammation and ROS can promote endothelial cell (EC) proliferation, migration, and tube formation [25], [26]. IL-6 is involved in the pathologic process of retinopathy. Knockout of IL-6 attenuates retinal vascular inflammation, NADPH oxidase activity and VEGF expression [27]. NADPH oxidase NOX1 and NOX4 are main sources of ROS generation in retinal ECs [18], [28]. Increased expression of NOX4 in retinal ECs results in upregulation of VEGF and activation of VEGFR2/ERK signaling, which contribute to retinal vascular leakage in oxygen-induced retinopathy [29]. Importantly, NF-kB signaling appears to be a key regulator of IL-6, NADPH oxidase and VEGF, thereby controlling inflammation, oxidative stress and vascular dysfunction [29], [30]. The activity of NF-kB is primarily regulated by the IKK complex through the phosphorylation of IκBα with subsequent ubiquitination and degradation by the proteasome [31]. The PI3K/AKT pathway can activate IKK-mediated NF-kB signaling, while the phosphatase PTEN negatively regulates this process. Interestingly, in addition to IκBα, PTEN stability is ubiquitinated and degraded by the proteasome [21], [32]. However, whether LMP10 is involved in regulating PTEN and IκBα stability, and their downstream signals in retinopathy, remains unclear. Increasing evidence has indicated that the immunoproteasome regulates more efficiently immune signaling pathways, such as IKK/IkBα/NF-κB, than the standard proteasome [7]. As expected, our results indicated that Ang II-induced upregulation of LMP10 promotes PTEN degradation and AKT/IKKβ activation, which induced IkBα degradation, leading to activation of NF-kB and its target genes in the retinas (Figs. 3E and 6A), further demonstrating an important role for LMP10 in regulating PTEN/AKT/IKK/IkBα/NF-kB signaling in Ang II-induced retinopathy.
IKKβ is a key upstream kinase that is necessary for classical NF-κB activation. It has been implicated in diabetic retinopathy (DR), suggesting that IKKβ/NF-κB signaling may present a promising therapeutic target for this disease. Notably, local IKKβ/NF-kB inhibition within the eye also achieves a therapeutic effect while avoiding systemic toxicity [8]. The small molecule TPCA-1, a specific IKKβ inhibitor, efficiently inhibits NF-κB activation and attenuates laser-induced retinal vascular leakage and macrophage accumulation [33]. Administration of IMD-0354, a non–ATP-binding competitive selective IKKβ inhibitor, also significantly suppresses retinal inflammation and vascular damage in streptozotocin-induced diabetic mice [34]. However, little information about the effect of IMD-0354 on Ang II-induced hypertensive retinopathy has been reported. Here our results demonstrated that administration of IMD-0354 efficiently attenuated Ang II-induced retinal structural remodeling, vascular permeability, oxidative stress and inflammation in LMP10-injected mice (Fig. 4, Fig. 5). These effects were associated with increased IkBa stability and inhibition of NF-kB activity and its downstream targets including IL-1β, IL-6, NOX1, NOX4 and VEGF expression induced by Ang II (Figs. 4C–D, 5C, 5F and 6A). Thus, these results suggested that inhibition of IKKβ may represent a promising therapeutic target for preventing hypertensive retinopathy.
In conclusion, this study demonstrated for the first time that the expression and trypsin-like activity of LMP10 were significantly upregulated in Ang II-treated retinas and in the serum of patients with hypertensive retinopathy. Knockout of LMP10 markedly reduced central retinal thickness, vascular permeability, oxidative stress and inflammation. IKKβ inhibitor IMD-0354 had a similar beneficial effect. This study highlighted the importance of LMP10 as a driver for hypertensive retinopathy and possible target for future therapy. Further studies are needed to test the effect of LMP10 on retinopathy in other animal models and to develop novel LMP10 inhibitors for treating hypertensive retinopathy.
Acknowledgements
We thank Qi Cheng, Jun-qiang Liang and Jian-cong Wang from the Beijing Health Olight technology Co.,Ltd for their help with fluorescence angiography services.
Acknowledgments
Formatting of funding sources
Funding
This work was supported by grants from China National Natural Science Funds (81330003, 81630009), Dalian high level Talents Innovation and Entrepreneurship Projects (2015R019), and Chang Jiang Scholar Program (T2011160).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.02.022.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Fig. S1.
Effect of LMP10 knockout on blood pressure and trypsin-like activity. A, Measurement of systolic blood pressure in wild-type (WT) mice and LMP10 knockout (KO) mice at 3 weeks after Ang II infusion by the tail-cuff method (n = 10 mice per group). B, Proteasome trypsin-like activity in the retinas from WT and LMP10 KO mice after saline or Ang II infusion (n = 10 mice per group). Data are the mean ± SEM. *P < 0.05, * **P < 0.001 versus WT mice infused with saline, ###P < 0.001 versus WT mice infused with Ang II.
Fig. S2.
Infection efficiency of rAAV2 and its effect on blood pressure. A, Infection efficiency of rAAV2-GFP and rAAV2-LMP10 in the retinas indicated by GFP fluorescence (green). Nuclei were counterstained with DAPI (blue). Scale bar, 50 µm. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. B, Immunoblotting analysis of LMP10 level in each group (upper). Quantitative analysis of protein intensity (lower, n = 4 mice per group). C, Measurement of systolic blood pressure in rAAV2-GFP- and rAAV2-LMP10-injected mice with or without IMD-0354 injection 3 weeks after Ang II infusion by the tail-cuff method (n = 10 mice per group). Data expressed as the mean ± SEM. *P < 0.05 vs rAAV2-GFP-injected mice infused with saline, #P < 0.05 vs rAAV2-GFP-injected mice infused with Ang II.
References
- 1.Katsi V., Marketou M., Vlachopoulos C. Impact of arterial hypertension on the eye. Curr. Hypertens. Rep. 2012;14:581–590. doi: 10.1007/s11906-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 2.Wong T.Y., McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br. Med. Bull. 2005;73–74:57–70. doi: 10.1093/bmb/ldh050. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson-Berka J.L. Angiotensin and diabetic retinopathy. Int. J. Biochem. Cell Biol. 2006;38:752–765. doi: 10.1016/j.biocel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Verma A., Shan Z., Lei B. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Mol. Ther. 2012;20:28–36. doi: 10.1038/mt.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louie J.L., Kapphahn R.J., Ferrington D.A. Proteasome function and protein oxidation in the aged retina. Exp. Eye Res. 2002;75:271–284. [PubMed] [Google Scholar]
- 6.Portbury A.L., Ronnebaum S.M., Zungu M. Back to your heart: ubiquitin proteasome system-regulated signal transduction. J. Mol. Cell. Cardiol. 2012;52:526–537. doi: 10.1016/j.yjmcc.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angeles A., Fung G., Luo H. Immune and non-immune functions of the immunoproteasome. Front. Biosci. (Landmark Ed.) 2012;17:1904–1916. doi: 10.2741/4027. [DOI] [PubMed] [Google Scholar]
- 8.Yan W., Bi H.L., Liu L.X. Knockout of immunoproteasome subunit beta2i ameliorates cardiac fibrosis and inflammation in DOCA/Salt hypertensive mice. Biochem. Biophys. Res. Commun. 2017;490:84–90. doi: 10.1016/j.bbrc.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Campello L., Esteve-Rudd J., Cuenca N. The ubiquitin-proteasome system in retinal health and disease. Mol. Neurobiol. 2013;47:790–810. doi: 10.1007/s12035-012-8391-5. [DOI] [PubMed] [Google Scholar]
- 10.Schuld N.J., Hussong S.A., Kapphahn R.J. Immunoproteasome deficiency protects in the retina after optic nerve crush. PLoS One. 2015;10:e0126768. doi: 10.1371/journal.pone.0126768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Li Y.L., Zhang C.C. Inhibition of Toll-like receptor 2 reduces cardiac fibrosis by attenuating macrophage-mediated inflammation. Cardiovasc. Res. 2014;101:383–392. doi: 10.1093/cvr/cvt258. [DOI] [PubMed] [Google Scholar]
- 12.Praddaude F., Cousins S.W., Pecher C. Angiotensin II-induced hypertension regulates AT1 receptor subtypes and extracellular matrix turnover in mouse retinal pigment epithelium. Exp. Eye Res. 2009;89:109–118. doi: 10.1016/j.exer.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lennikov A., Kitaichi N., Noda K. Amelioration of endotoxin-induced uveitis treated with an IkappaB kinase beta inhibitor in rats. Mol. Vis. 2012;18:2586–2597. [PMC free article] [PubMed] [Google Scholar]
- 14.Kinose Y., Sawada K., Makino H. IKKbeta regulates VEGF expression and is a potential therapeutic target for ovarian cancer as an antiangiogenic treatment. Mol. Cancer Ther. 2015;14:909–919. doi: 10.1158/1535-7163.MCT-14-0696. [DOI] [PubMed] [Google Scholar]
- 15.Kilkenny C., Browne W.J., Cuthill I.C. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrs-Silva H., Dinculescu A., Li Q. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol. Ther. 2011;19:293–301. doi: 10.1038/mt.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tawfik A., Markand S., Al-Shabrawey M. Alterations of retinal vasculature in cystathionine-beta-synthase heterozygous mice: a model of mild to moderate hyperhomocysteinemia. Am. J. Pathol. 2014;184:2573–2585. doi: 10.1016/j.ajpath.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson-Berka J.L., Deliyanti D., Rana I. NADPH oxidase, NOX1, mediates vascular injury in ischemic retinopathy. Antioxid. Redox Signal. 2014;20:2726–2740. doi: 10.1089/ars.2013.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGill T.J., Prusky G.T., Luna G. Optomotor and immunohistochemical changes in the juvenile S334ter rat. Exp. Eye Res. 2012;104:65–73. doi: 10.1016/j.exer.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hacker H., Karin M. Regulation and function of IKK and IKK-related kinases. Sci. STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 21.Madrid L.V., Mayo M.W., Reuther J.Y. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 22.Kim J.H., Lee G., Cho Y.L. Desmethylanhydroicaritin inhibits NF-kappaB-regulated inflammatory gene expression by modulating the redox-sensitive PI3K/PTEN/Akt pathway. Eur. J. Pharmacol. 2009;602:422–431. doi: 10.1016/j.ejphar.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 23.Ferrington D.A., Hussong S.A., Roehrich H. Immunoproteasome responds to injury in the retina and brain. J. Neurochem. 2008;106:158–169. doi: 10.1111/j.1471-4159.2008.05345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussong S.A., Kapphahn R.J., Phillips S.L. Immunoproteasome deficiency alters retinal proteasome's response to stress. J. Neurochem. 2010;113:1481–1490. doi: 10.1111/j.1471-4159.2010.06688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussong S.A., Roehrich H., Kapphahn R.J. A novel role for the immunoproteasome in retinal function. Investig. Ophthalmol. Vis. Sci. 2011;52:714–723. doi: 10.1167/iovs.10-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H., Hartnett M.E. Roles of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in angiogenesis: isoform-specific effects. Antioxidants (Basel) 2017;6 doi: 10.3390/antiox6020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojas M., Zhang W., Lee D.L. Role of IL-6 in angiotensin II-induced retinal vascular inflammation. Investig. Ophthalmol. Vis. Sci. 2010;51:1709–1718. doi: 10.1167/iovs.09-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J., Wang J.J., Yu Q. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59:1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J., Wang J.J., Zhang S.X. NADPH oxidase 4-derived H2O2 promotes aberrant retinal neovascularization via activation of VEGF receptor 2 pathway in oxygen-induced retinopathy. J. Diabetes Res. 2015;2015:963289. doi: 10.1155/2015/963289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cervia D., Catalani E., Dal Monte M. Vascular endothelial growth factor in the ischemic retina and its regulation by somatostatin. J. Neurochem. 2012;120:818–829. doi: 10.1111/j.1471-4159.2011.07622.x. [DOI] [PubMed] [Google Scholar]
- 31.Gao G., Dudley S.C., Jr. Redox regulation, NF-kappaB, and atrial fibrillation. Antioxid. Redox Signal. 2009;11:2265–2277. doi: 10.1089/ars.2009.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu W., Wang X., Zhang W. Zinc-induced PTEN protein degradation through the proteasome pathway in human airway epithelial cells. J. Biol. Chem. 2003;278:28258–28263. doi: 10.1074/jbc.M303318200. [DOI] [PubMed] [Google Scholar]
- 33.Gaddipati S., Lu Q., Kasetti R.B. IKK2 inhibition using TPCA-1-loaded PLGA microparticles attenuates laser-induced choroidal neovascularization and macrophage recruitment. PLoS One. 2015;10:e0121185. doi: 10.1371/journal.pone.0121185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lennikov A., Hiraoka M., Abe A. IkappaB kinase-beta inhibitor IMD-0354 beneficially suppresses retinal vascular permeability in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2014;55:6365–6373. doi: 10.1167/iovs.14-14671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material