Abstract
Background
Paramyxoviruses include respiratory syncytial virus (RSV), parainfluenza virus (PIV), and human metapneumovirus (MPV), which may cause significant respiratory tract infectious disease (RTID) and mortality after allogeneic hematopoietic cell transplantation (HCT). However, clinical data regarding frequency and outcome are scarce.
Methods
We identified all paramyxovirus RTIDs in allogeneic HCT recipients diagnosed by multiplex polymerase chain reaction between 2010 and 2014. Baseline characteristics of patients, treatment, and outcome of each episode were analyzed; ie, moderate, severe, and very severe immunodeficiency (verySID) according to HCT ≤6 months, T- or B-cell depletion ≤3 months, graft-versus-host disease, neutropenia, lymphopenia, or hypo-gammaglobulinemia.
Results
One hundred three RTID episodes in 66 patients were identified (PIV 47% [48 of 103], RSV 32% [33 of 103], MPV 21% [22 of 103]). Episodes occurred in 85% (87 of 103) at >100 days post-HCT. Lower RTID accounted for 36% (37 of 103). Thirty-nine percent (40 of 103) of RTID episodes required hospitalization and more frequently affected patients with lower RTID. Six percent progressed from upper to lower RTID. Overall mortality was 6% and did not differ between paramyxoviruses. Sixty-one percent (63 of 103) of episodes occurred in patients with SID, and 20.2% (19 of 63) of episodes occurred in patients with verySID. Oral ribavirin plus intravenous immunoglobulin was administered in 38% (39 of 103) of RTIDs, preferably for RSV or MPV (P ≤ .001) and for SID patients (P = .001). Patients with verySID frequently progressed to lower RTID (P = .075), required intensive care unit transfer, and showed higher mortality.
Conclusion
Paramyxovirus RTID remains a major concern in allogeneic HCT patients fulfilling SID and verySID, emphasizing that efficacious and safe antiviral treatments are urgently needed.
Keywords: human metapneumovirus (MPV), IVIG, parainfluenza virus (PIV), respiratory syncytial virus (RSV), ribavirin
Respiratory tract infectious disease (RTID) caused by paramyxoviruses such as respiratory syncytial virus (RSV), parainfluenza virus (PIV), and human metapneumovirus (MPV) is an important cause of morbidity and mortality in patients after allogeneic hematopoietic cell transplantation (HCT). Mortality rates range from 5% to 80%, and the rate for RSV infections is the highest [1–12]. Although these viruses are closely related and show an intrinsic propensity for lower RTID, comparative data on course, management, and outcome are scarce in this patient group or focus on specific paramyxovirus members up to 100 days.
The reported incidence of paramyxovirus-RTID in patients after HCT ranges from 2% to 17% [4–7, 10, 12, 13]. However, comprehensive data are becoming available after the widespread availability of multiplex nucleic acid testing, not only for inpatients but also those attending outpatient clinics. Yet, progression from upper to lower RTID seems to be higher for RSV (up to 84%) compared with PIV and MPV [1, 3, 6, 8, 11, 12, 14, 15]. Risk factors for progression to lower RTID have been established for RSV and PIV [1, 7, 11, 13, 16–19], whereas those for MPV are less well defined [20].
Treatment recommendations as proposed by the European Conference on Infections in Leukaemia (ECIL)-4 guidelines suggest deferral of conditioning therapy, treatment with aerosolized ribavirin (RBV), or off-label use of systemic RBV and/or intravenous immunoglobulins (IVIG), in particular for patients at high risk of developing lower RTIDs [7]. Although the efficacy of RBV+IVIG has not been formally proven, some clinical experiences suggest that there might be a benefit if administered early and to patients at presumably highest risk for progression to lower RTID [10, 14]. In vitro and in vivo studies demonstrate efficacy of RBV and IVIG for PIV and MPV [21–24]. Some centers, including ours, consider RBV and IVIG treatment for PIV and MPV RTID in allogeneic HCT patients with clinical parameters suggesting severe immunodeficiency (SID), despite the lack of supporting studies [1, 3, 4, 16, 25–31]. In this study, we investigated patient characteristics, management, and outcome of RTIDs caused by paramyxoviruses in allogeneic HCT recipients at our institution.
METHODS
Patients and Definitions
In this single-center retrospective study at the University Hospital Basel, Switzerland, allogeneic HCT recipients were identified if they had symptoms or signs of RTID and the detection of RSV, PIV, or MPV by multiplex nucleic acid amplification testing (NAT) in nasopharyngeal swabs (NPS) or bronchoalveolar lavage between June 2010 and December 2014, and was followed up by pathogen-specific quantitative real-time NAT [32]. This study was approved by the ethical committee Nordwest- and Zentralschweiz (No. 2015-144).
Upper and lower RTID was defined as described by the recent ECIL-4 guidelines [7]: upper RTID was defined as virus detection in upper respiratory secretions, together with symptoms involving the upper respiratory tract (nose and throat); lower RTID was defined as the presence of either hypoxia or compatible pulmonary infiltrates, together with virus detection in upper or lower respiratory secretions [7, 33, 34]. Duration of viral shedding was defined as the time from the first positive to the first negative virological result. Clearance was documented with 1 negative virological result using pathogen-specific NAT. Multiple episodes in 1 patient were only considered for inclusion if they occurred at least 1 week apart and were caused by different viruses or by the same virus with documentation of clinical response; clearance between both episodes indicated by a negative NAT result. Severe immunodeficiency was defined by one of the following: allogeneic HCT ≤6 months ago, acute graft-versus-host diseases (GvHD) grade ≥2 or bronchiolitis obliterans, leucopenia ≤1.0 × 109/liter or neutropenia <0.5 × 109/liter, lymphopenia ≤0.1 × 109/liter or hypogammaglobulinemia <4.5 g/L, or T-cell or B-cell depletion ≤3 months ago [10, 34]. VerySID was defined as having 2 or more of the SID criteria. In contrast, moderate immunodeficiency (MID) was defined as HCT >6 months ago, GvHD grade <2, absolute leucocyte count >1.0 × 109/liter, absolute lymphocyte count >0.1 × 109/liter, or absence of immunosuppressive drug treatment. Mortality attributable to viral infection was defined as death due to respiratory failure with no other cause identified except lower RTID [10].
Virology Procedures
Nasopharyngeal swabs and bronchoalveolar lavage (BAL) were analyzed by multiplex NAT using the RespiFinder-22 from 2010 to 2013 and the Luminex NxTAG Respiratory Pathogen Panel from 2013 to 2014 [32, 35–38]. Sensitive and quantitative pathogen-specific NAT was applied for specific follow-up until samples were negative [32].
Pulmonary Function Tests
The forced expiratory volume in 1 second (FEV1) and the vital capacity were expressed as a percentage of predicted normal values, calculated by using published equations for children and adults [28].
Management
Infection control procedures were performed as previously described [10]. Treatment decisions were based on the physician’s discretion and informed by our institutional guidelines recommending either systemic RBV, IVIG, or systemic RBV plus IVIG. Systemic RBV was usually administered orally with a loading dose of 10 mg/kg bodyweight followed by 200 mg three-times daily (tid) and was thereafter increased to 400 mg tid on day 2 and to 600 mg tid on day 3. Intravenous immunoglobulin was administered at 0.5 g/kg bodyweight 1 to 3 times per week. Treatment was discontinued if there was a clinical response, the respiratory viruses were no longer detectable, or in case of adverse events, eg, RBV-induced hemolysis [10].
Statistical Analysis
Patients were categorized according to virus type and degree of immunodeficiency. Comparisons between these 3 groups were performed by analysis of variance and Pearson χ2 test, with Fisher’s exact test, and Student t tests were used where appropriate, for continuous and categorical variables, respectively. Univariable and multivariable logistic regression models were performed to determine associations with the need for hospitalization. A 2-sided P value <.05 was considered to be significant. All statistical analyses were performed with STATA 14.0 (Stata Corp., College Station, TX).
RESULTS
Patient and Respiratory Tract Infectious Disease Characteristics
From June 2010 to December 2014, 103 paramyxovirus RTID episodes were identified in 66 patients, and causative pathogens were PIV (48 of 103, 47%), RSV (33 of 103, 32%), and MPV (22 of 103, 21%). Parainfluenza virus 3 (n = 25) was the leading agent followed by PIV4 (n = 12), PIV1 (n = 6), and PIV2 (n = 5). Table 1 shows the baseline characteristics of all paramyxovirus episodes. The most common underlying disease was acute myeloid leukemia (20 of 66, 30%). At RTID diagnosis, 82% (54 of 66) of patients were in complete hematological remission and 58% (38 of 66) of patients suffered from GvHD (grade ≥2 in 27%, 18 of 66). Patient’s baseline characteristics did not differ in respect to virus type.
Table 1.
Episode Characteristics
| Characteristics | All Episodes (n = 103) | PIV (n = 48) | RSV (n = 33) | MPV (n = 22) | P Value |
|---|---|---|---|---|---|
| Age, median years (range) | 52.3 (19.9–70.6) | 51.6 (22.3–68.2) | 52.5 (19.9–70.6) | 53.6 (26.7–69.2) | .509 |
| Male, n (%) | 72 (69.9) | 34 (70.8) | 24 (72.7) | 14 (63.6) | .786 |
| Female, n (%) | 31 (30.1) | 14 (29.2) | 9 (27.3) | 8 (36.4) | |
| Time post-alloHCT, median (IQR) | .166 | ||||
| Underlying disease | 518 (212–1014) | 627.5 (234.5–1252.5) | 382 (162–709) | 503.5 (298.8–1066.8) | .859 |
| Acute lymphoid leukemia, n (%) | 17 (16.5) | 8 (16.7) | 5 (15.2) | 4 (18.2) | |
| Acute myeloid leukemia, n (%) | 31 (30.1) | 15 (31.3) | 9 (27.3) | 7 (31.8) | |
| Chronic myeloid leukemia, n (%) | 7 (6.8) | 2 (4.2) | 2 (6.1) | 3 (13.6) | |
| Chronic lymphoid leukemia, n (%) | 6 (5.8) | 2 (4.2) | 3 (9.1) | 1 (4.5) | |
| Myelodysplastic syndrome n (%) | 8 (7.8) | 4 (8.3) | 4 (12.1) | 0 | |
| Myeloproliferative syndrome, n (%) | 6 (5.8) | 2 (4.2) | 1 (3.0) | 3 (13.6) | |
| Multiple myeloma, n (%) | 13 (12.6) | 6 (12.5) | 4 (12.1) | 3 (13.6) | |
| Non-Hodgkin lymphoma, n (%) | 11 (10.7) | 7 (14.6) | 3 (9.1) | 1 (4.5) | |
| Primary immunodeficiencies, n (%) | 3 (2.9) | 2 (4.2) | 1 (3.0) | 0 | |
| Aplastic anemia, n (%) | 1 (1.0) | 0 | 0 | 1 (4.5) | |
| Type of transplant | .088 | ||||
| HLA-matched related, n (%) | 42 (40.8) | 24 (50.0) | 12 (36.4) | 6 (27.3) | |
| HLA-matched unrelated, n (%) | 36 (35.0) | 16 (33.3) | 9 (27.3) | 11 (50.0) | |
| HLA-mismatched related, n (%) | 5 (4.9) | 0 | 3 (9.1) | 2 (9.1) | |
| HLA-mismatched unrelated, n (%) | 20 (19.4) | 8 (16.7) | 9 (27.3) | 3 (13.6) | |
| Cytomegalovirus Status | .229 | ||||
| D/R−/−, n (%) | 39 (37.7) | 15 (31.3) | 12 (36.4) | 12 (54.5) | |
| D/R−/+, n (%) | 31 (30.1) | 17 (35.4) | 9 (27.3) | 5 (22.7) | |
| D/R+/−, n (%) | 5 (4.9) | 1 (2.1) | 4 (12.1) | 0 | |
| D/R+/+, n (%) | 28 (27.2) | 15 (31.3) | 8 (24.2) | 5 (22.7) | |
| Stem Cell Source | 1.000 | ||||
| Bone marrow, n (%) | 0 | 0 | 0 | 0 | |
| Peripheral blood, n (%) | 102 (99.0) | 47 (97.9) | 33 (100.0) | 22 (100.0) | |
| Umbilical cord blood, n (%) | 1 (1.0) | 1 (2.1) | 0 | 0 | |
| Conditioning Regimen | .840 | ||||
| Myeloablative, n (%) | 60 (58.3) | 27 (56.3) | 19 (57.6) | 14 (63.6) | |
| Non-myeloablative, n (%) | 43 (41.7) | 21 (43.8) | 14 (42.4) | 8 (36.4) | |
| Hematological Condition at RTID Diagnosis | |||||
| Complete remission | 87 (84.5) | 40 (83.3) | 28 (84.8) | 19 (86.4) | .422 |
| GvHD | 64 (62.1) | 29 (60.4) | 19 (57.6) | 16 (72.7) | .505 |
| GvHD grade ≥ 2 | 38 (36.9) | 17 (35.4) | 9 (27.3) | 12 (54.5) | .236 |
| Bronchiolitis obliterans, n (%) | 23 (22.3) | 13 (27.1) | 4 (12.1) | 6 (27.3) | .241 |
| Immunosuppressives | .139 | ||||
| Calcineurin inhibitors (TAC; CYA), n (%) | 53 (51.5) | 22 (45.8) | 19 (57.6) | 12 (54.5) | |
| Mycophenolate mofetil, n (%) | 3 (2.9) | 2 (4.2) | 1 (3.0) | 0 | |
| CNI + MMF, n (%) | 25 (24.3) | 11 (22.9) | 5 (15.2) | 9 (40.9) | |
| Steroids, n (%) | 49 (47.6) | 24 (50.0) | 14 (42.4) | 11 (50.0) | |
Abbreviations: alloHCT, allogeneic hematopoetic cell transplantation; CNI, calcineurin inhibitors; CYA, cyclosporine A; D/R, donor/recipient; GvHD, Graft-versus-host disease; HLA, human leucocyte antigen; IQR, interquartile range; MMF, mycophenolate mofetil; MPV, human metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RTID, respiratory tract infectious disease; TAC, tacrolimus.
Patients with more than 1 episode of RTID had similar baseline characteristics (Supplementary Table 1) compared with patients with only 1 RTID episode. Nineteen patients suffered from 2 or more different paramyxoviruses, whereas 9 patients were infected repeatedly with the same type. The median time of re-occurrence of patients infected with the same virus was 415 days (range, 87–1046).
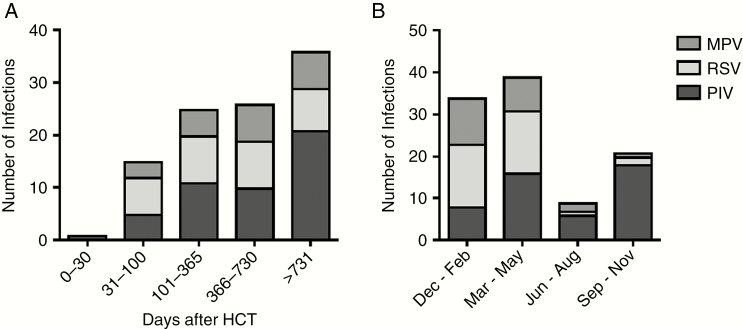
RTID episodes occurred more than 100 days post-HCT in 84.5% (87 of 103) of patients, at a median post-HCT time of 518 days (interquartile range [IQR], 212–1014). Respiratory syncytial virus was detected after a median of 382 days (IQR, 162–709), MPV was detected after a median of 503.5 days (IQR, 298.8–1066.8), and PIV was detected after a median of 627.5 days (IQR, 234.5–1252.5) post-HCT (Figure 1A). RSV and MPV infections occurred more frequently during the winter season, whereas PIV infections occurred more commonly in autumn compared with RSV and MPV infections (P ≤ .001). More importantly, the frequency of the different paramyxovirus infections was similar during spring (Figure 1B).
Figure 1.
(A) Occurrence of paramyxovirus respiratory tract diseases after allogeneic hematopoietic cell transplantation (HCT) and (B) seasonality of the paramyxoviruses. Median post-HCT time for respiratory syncytial virus (RSV) 382 days, for metapneumovirus (MPV) 504 days, and for parainfluenza virus (PIV) 628 days. Respiratory syncytial virus and MPV infections occur significantly more frequently during winter (P ≤ .001), whereas PIV infections appear significantly more often in autumn (P ≤ .001).
Moderate immunodeficiency criteria were fulfilled in 40 of 103 (38.8%) episodes, whereas 63 of 103 (61%) episodes occurred in patients with SID. In the SID group, 19 of 63 (30%) episodes had ≥2 SID criteria, thereby fulfilling the criteria of verySID (Supplementary Table 2).
Diagnosis and Clinical Presentation
Upper RTIs were identified in 58% (60 of 103) of patients, and lower RTIs were identified in 36% (37 of 103) of patients, including BAL for diagnostic NAT in 35% (36 of 103) (Table 2). Moderate immunodeficiency and SID patients had similar rates of upper and lower RTID episodes (lower RTID 14 of 40, 35.0% in MID patients and 23 of 63, 36.5% in SID patients, P = 1.000). Of note, the clinical presentation did not differ between the different paramyxoviruses. The majority of episodes presented without fever (83 of 103, 81%), but there was a trend of elevated median concentrations of C-reactive protein (P = .054). Serum-creatinine concentration (P = .942) or presence of anemia (P = 1.000) did not differ between the 3 viral infection groups (data not shown). Bacterial coinfections occurred in 9 episodes. No fungal coinfections were seen (data not shown). Viral coinfections were seen in 24 episodes and included cytomegalovirus, rhinovirus, influenza A, or coronavirus in 5 episodes, or adenovirus in 3, respectively. Only 1 dual paramyxovirus infection was detected with PIV and MPV.
Table 2.
Site of Infection at RTID Diagnosis
| Site of Infection | All Episodes (n = 103) | PIV (n = 48) | RSV (n = 33) | MPV (n = 22) |
|---|---|---|---|---|
| Unknown, n (%) | 6 (5.8) | 2 (4.2) | 3 (9.1) | 1 (4.5) |
| Upper RTI, n (%) | 60 (58.3) | 27 (56.3) | 22 (66.7) | 11 (50.0) |
| Lower RTI, n (%) | 37 (35.9) | 19 (39.6) | 8 (24.2) | 10 (45.5) |
Abbreviations: MPV, human metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RTID, respiratory tract infectious disease.
Intervention
Overall, 60 of 97 (61.9%) paramyxovirus RTIDs were treated with one of the following treatment regimens, ie, 34 of 60 (57%) upper RTID-episodes and in 26 of 37 (70%) of lower RTID-episodes. Treatment according to virus type is summarized in Table 3. Oral RBV+IVIG was administered in 38 of 97 (39.2%) episodes. Respiratory syncytial virus and MPV episodes were more frequently treated with RBV+IVIG than PIV episodes (P ≤ .001). Intravenous immunoglobulins alone was mainly administered for PIV infection with 40% compared with 9% in the RSV and MPV infection group, respectively. Palivizumab was administered to only 1 patient with verySID suffering from RSV in the early posttransplant period.
Table 3.
Intervention
| Intervention | All Episodes (n = 103) | PIV (n = 48) | RSV (n = 33) | MPV (n = 22) |
|---|---|---|---|---|
| Ribavirin+IVIGa, n (%) | 39 (37.9) | 3 (6.3) | 20 (60.6) | 16 (72.7) |
| IVIG, n (%) | 24 (23.3) | 19 (39.6) | 3 (9.1) | 2 (9.1) |
| None, n (%) | 40 (38.8) | 26 (54.2) | 10 (30.3) | 4 (18.2) |
Abbreviations: IVIG, intravenous immunoglobulin; MPV, human metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
aOne patient additionally received palivizumab, 2 patients did not receive IVIG.
Episodes among SID patients were more commonly treated than episodes with MID patients (47 of 63 vs 16 of 40; P = .001), and more episodes in SID patients were treated with RBV+IVIG, including 30 of 63 (47.6%) vs 9 of 40 (22.5%) in the MID group (P = .013). Hemolysis attributed to systemic RBV treatment was recorded in 25 of 39 (64%), leading to drug discontinuation.
Outcome
Sequential viral sampling was performed in 77 of 103 (74%) episodes. The median duration of viral shedding did not differ within the 3 viral RTID groups (P = .280) (Table 4) and was similar in the MID and SID patients (22, IQR 15–43 days and 19, IQR 14–36, respectively; P = .260).
Table 4.
Outcome
| Outcome Parameters | All Episodes (n = 103) | PIV (n = 48) | RSV (n = 33) | MPV (n = 22) | P Value |
|---|---|---|---|---|---|
| Duration of viral shedding, median (IQR) | 21 (15–38) | 17 (14–33) | 21 (14–35) | 32 (19.5–47.5) | .280 |
| Progression to lower RTID, n (%) | 6 (5.8) | 3 (6.3) | 1 (3.0) | 2 (9.1) | .570 |
| Hospitalization, n (%) | 40 (38.8) | 20 (41.7) | 10 (30.3) | 10 (45.5) | .473 |
| Admission to ICU, n (%) | 6 (5.8) | 3 (6.3) | 2 (6.1) | 1 (4.5) | 1.000 |
| Mechanic ventilation, n (%) | 2 (1.9) | 1 (2.1) | 1 (3.0) | 0 | |
| Mortality, n (%) | 6 (5.8) | 2 (4.2) | 2 (6.1) | 2 (9.1) | .761 |
| Viral infection attributable, n (%) | 4 (3.9) | 0 | 2 (6.1) | 2 (9.1) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; MPV, human metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RTID, respiratory tract infectious disease.
Six of 103 episodes (6%) progressed from upper to lower RTID. Five of 6 (83%) episodes with progression to lower RTID belonged to the SID group and were treated with RBV+IVIG during the phase of upper RTID. One episode of PIV infection in an MID patient progressed to lower RTID without administration of any antiviral drug. Progression to lower RTID did not differ between patients with MID and SID (1 of 40, 2.5% and 2 of 44, 4.6%; P = 1.000). By contrast, episodes in verySID patients tended to have a higher progression rate (3 of 19, 15.8%; P = .075) compared with patients with MID and SID taken together (3 of 84, 3.6%).
Pulmonary function tests (PFT) were performed in 87 episodes. In 15 episodes, 3 sequential PFTs were available. Overall, a reduction of FEV1 from baseline PFT to RTID diagnosis was documented for RTIDs caused by RSV and PIV. The PFTs 4 to 6 months after resolution of RTID showed comparable values for FEV1 as documented at baseline in each virus group (Supplementary Table 3). The DLCOc/VA was measured in 75 episodes (86.2%) and was only marginally influenced by the RTID (data not shown). With respect to patients with previous diagnosis of bronchiolitis obliterans, the PFT remained stable after resolution of RTID.
Forty of 103 (38.8%) RTID episodes required hospitalization. Lower RTID, absence of hematological complete remission, HCT ≤6 months, as well as the presence of verySID criteria (P < .001) were significantly associated with a higher risk of hospitalization in univariate analysis (Table 5). In multivariable analysis, presence of lower RTID and verySID remained significant predictors for hospitalization.
Table 5.
Risk Factors for Hospitalization
| Risk Factors for Hospitalization | Univariable | Multivariablea | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Baseline Characteristics | ||||
| Age at RTI diagnosis | 1.01 (0.98–1.04) | .359 | ||
| Gender (male vs female) | 0.83 (0.35–1.96) | .672 | ||
| Acute myeloid leukemia | 1.46 (0.62–3.43) | .388 | ||
| Type of transplant, HLA-matched related | 0.67 (0.30–1.52) | .343 | ||
| Peripheral blood as stem cell source | - | - | ||
| Myeloablative conditioning regimen | 0.49 (0.22–1.09) | .080 | ||
| Hematological complete remission at RTI diagnosis | 0.32 (0.10–0.95) | .041 | 0.62 (0.13–2.85) | .539 |
| SID Criteria | ||||
| One SIDb criteria | 1.31 (0.57–2.97) | .525 | ||
| VerySID | 13.33 (3.56–49.96) | <.001 | 12.08 (1.59–91.54) | .016 |
| alloHCT ≤6 months | 5.84 (1.88–18.13) | .002 | 2.35 (0.39–14.14) | .351 |
| Leucocyte ≤1.0 × 109/liter; neutrocyte <0.5 × 109/liter | - | - | ||
| Lymphocyte ≤0.1 × 109/liter | - | - | ||
| T-cell or B-cell depletion ≤3 months | 1.62 (0.31–8.46) | .566 | ||
| Hypogammaglobulinemia <4.5 g/L | 2.46 (0.72–8.37) | .150 | ||
| Graft-versus-host-disease | 0.86 (0.38–1.95) | .722 | ||
| Graft-versus-host-disease grade ≥2 | 0.61 (0.26–1.42) | .250 | ||
| Bronchiolitis obliterans | 0.62 (0.23–1.68) | .351 | ||
| Immunosuppressive Treatment | ||||
| Calcineurin inhibitors | 1.07 (0.48–2.37) | .866 | ||
| Mycophenolate mofetil | 3.26 (0.29–37.22) | .341 | ||
| Calcineurin inhibitors + mycophenolate mofetil | 0.53 (0.20–1.42) | .205 | ||
| Steroids | 1.63 (0.73–3.62) | .230 | ||
| Factors Related to RTID | ||||
| Virus diagnosis (RSV vs all others) | 0.58 (0.24–1.40) | .225 | ||
| Lower RTID | 7.08 (2.89–17.38) | <.001 | 12.74 (3.96–40.99) | <.001 |
| Progression to lower RTID | 8.71 (0.98–77.62) | .052 | ||
Abbreviations: alloHCT, allogeneic hematopoetic cell transplantation; CI, confidence interval; HLA, human leucocyte antigen; OR, odds ratio; RSV, respiratory syncytial virus; RTID, respiratory tract infectious disease; SID, severe immunodeficiency.
aThe model includes the following: lower RTID, alloHCT ≤6 months, hematological complete remission, and corrects for episodes among equal patients. Hosmer-Lemeshow χ2 = 0.15, P = .985.
bSID criteria were defined as alloHCT ≤6 months ago, graft-versus-host-disease grade ≥2, leucopenia ≤1.0 × 109/liter or neutropenia <0.5 × 109/liter, lymphopenia ≤0.1 × 109/liter or hypogammaglobulinemia <4.5 g/liter, or T-cell or B-cell depletion ≤3 months ago.
Six of 103 (6%) episodes were admitted to the intensive care unit and 2 required mechanical ventilation (Table 4). Episodes in patients with verySID more commonly required intensive care (Table 6).
Table 6.
Outcome Regarding Immunodeficiency
| Outcome Parameters | MID (n = 40) | SID (n = 44) | VerySID (n = 19) | P Valuea |
|---|---|---|---|---|
| Duration of viral shedding, median (IQR) | 19 (14–36) | 21 (14.5–39.5) | 31 (20–44) | .140 |
| Progression to Iower RTID, n (%) | 1 (2.5) | 2 (4.5) | 3 (15.8) | .075 |
| Hospitalization, n (%) | 14 (35) | 10 (22.7) | 16 (84.2) | <.001 |
| Admission to ICU, n (%) | 0 | 1 (2.3) | 5 (26.3) | <.001 |
| Mechanic ventilation, n (%) | 0 | 0 | 2 (10.5) | |
| Mortality, n (%) | 0 | 0 | 6 (31.6) | <.001 |
| Viral infection attributable, n (%) | 0 | 0 | 4 (21.1) |
Abbreviations: ICU, intensive care unit; ID, immunodeficiency; IQR, interquartile range; MID, moderate immunodeficiency; RTID, respiratory tract infectious disease; SID, severe immunodeficiency.
aComparisons between patients with verySID and patients with MID or SID.
The overall mortality was 6% (6 of 103 RTID episodes). All deaths occurred in patients with verySID (Table 6). Respiratory tract infectious disease-attributable mortality occurred in 4 episodes, 2 of which were caused by MPV and 2 by RSV (Table 4).
DISCUSSION
In this study, we investigated 103 episodes of paramyxovirus RTID identified by multiplex NAT in the current era of allogeneic HCT. Unlike previous studies reporting follow-ups for the first 100 days posttransplant, paramyxovirus RTID occurred at >100 days posttransplant with a median time close to 1 year. It is notable that lower RTID accounted for 36% of episodes, and most of these patients required admission. Thus, paramyxovirus RTID after allogeneic HCT continues to pose a significant clinical burden. Overall mortality was low, and indeed lower than compared with previous reports, and typically occurred in verySID patients having 2 and more SID criteria. Thus, patient susceptibility appears to be a key determinant of outcome even 1 year after allogeneic HCT, pointing to an immunologically still vulnerable time period.
The reasons for the later presentation of paramyxovirus RTID compared with other reports is presently unknown. However, they may reflect differences in the study design of earlier studies that were limited to 100 days posttransplant as well as to changes in management of community-acquired respiratory virus infections such as deferral of HCT, consistent use of multiplex NAT for all symptomatic patients including outpatients, close follow-up, and the overall longer survival after HCT. A recent study also demonstrated that RTIDs continue to occur at high frequency in the late post-HCT phase after a median of 4 months [39]. Given that our patients were, for the most part, in hematological remission, return to a normal, less protected life with social contacts appears to be a plausible explaination for exposure to respiratory viruses within the first year after allogeneic HCT. Thus, awareness of the extended time of vulnerability emerging from this study as well as others may be an important observation for future antiviral and vaccine developments. This is also reflected in the recently completed trial of the RSV fusion inhibitor presatovir, in which patients presented with upper or lower RTID at 278 days (9.3 months) and 451 days (15 months) posttransplantation [40].
Most of the RTID episodes in our center were managed without hospitalization, and patients had uncomplicated courses with low progression rates to lower RTID, absence of persisting airflow obstruction, and low mortality. This may be explained by a better, general clinical condition and partial immune reconstitution expected later post-HCT and is reflected by the lower number of patients with neutropenia and lymphopenia. In a recent study, Kim et al [19] identified that an absolute lymphocyte count >1.0 × 109/liter at the time of upper RTID onset is protective regarding progression to lower RTID. This is in agreement with our data illustrating that only 1 of 54 patients with upper RTID and absolute lymphocyte count >1.0 × 109/liter progressed to lower RTID. In contrast, a threshold of <0.1 × 109/liter has been reported as a risk factor for progression [28, 41] and was adopted by our and earlier studies [10, 34], whereas others proposed the closely related cutoff of 0.2 × 109/liter [14]. Thus, low or very low lymphocyte counts are an important determinant of progression and outcome.
The impact of treatment with RBV and/or IVIG on outcome cannot be ascertained in our study due to the lack of an appropriately sized, preferably randomized control group. However, such studies are difficult to conduct given ethical concerns and an operative protocol. This is also reflected in a recent study for presatovir, in which systemic RBV was not excluded, but rather patients were stratified according to its use. As first reported in 2008 [10], systemic RBV and IVIG were consistently available at the discretion of the treating physician in our center together with internal guidance regarding their administration, supporting data only existing for RSV-RTID [14]. Accordingly, 61% of paramyxovirus RTID episodes were treated, and RBV+IVIG was mainly administered in RSV- and MPV-RTIDs, whereas IVIG alone was preferred for PIV RTID. In our study, the use of RBV was associated with hemolysis, leading to discontinuation of the drug typically after 3 to 5 days of therapy.
Overall, we found a low complication rate of all paramyxoviruses regarding a number of important parameters such as progression from upper to lower RTID, need for hospitalization, fixed airflow obstruction after RTID resolution, as well as overall and virus-attributable mortality. Thus, the question arises whether SID and verySID patients, in particular, would have fared worse without treatment. A recent meta-analysis demonstrated the efficacy of aerosolized and systemic RBV, with or without immunomodulators, for RSV infections in patients after allogeneic HCT [9, 10, 14, 18, 42]. By contrast, the impact of systemic RBV for MPV- and PIV-RTIDs is undefined. In our study, treatment was more often provided in the setting of MPV infections than PIV infections. Although we did not observe a difference in progression to lower RTID or mortality, it seemed that the viral shedding was shorter for RBV-treated MPV infections, but this trend was not found for PIV.
In view of the unmet clinical need of paramyxovirus RTID after HCT and the undocumented efficacy, the following key questions remain: who should be treated, and should all paramyxovirus RTID be treated with RBV+IVIG? Until better, more specific, and more potent antiviral therapies become available, we conclude from our study that SID patients, particularly verySID patients fulfilling 2 or more SID criteria, can be treated safely with systemic RBV+IVIG, whereas IVIG alone can be offered to such patients with PIV-RTID. Shah et al [43] recently identified risk factors for poor outcome of RSV-RTIDs after allogeneic HCT and proposed a scoring index from 1 to 10 to classify patients with low (score 0–2), moderate (score 3–6), and high risk (7–10) for progression to identify those who would benefit most from (inhaled RBV-based) antiviral therapy. Their proposed criteria are similar to ours but also introduced age ≥40 years, myeloablative conditioning regimen, and use of corticosteroids within the prior 30 days [43]. In our center, previously reported criteria have been reported some time ago and were available for use since then. As can be seen from the direct comparison, there is a substantial overlap (Supplementary Table 4). Unfortunately, the score by Shah et al [43] is awaiting independent validation. Until further validation in a prospective setting is done, we will apply our simpler criteria of SID for respiratory virus RTID with essentially similar conclusions. Taken together, the data support the view that host immunodeficiency remains a major determinant of outcome, not only for RSV but also for MPV and PIV. Thus, there is a need to further validate such risk strata in prospective trials to balance treatment versus overtreatment and adverse events, particularly for MID HCT patients.
Our study has several limitations. First, the use of a comprehensive, specific, and sensitive diagnostic tests such as multiplex NAT facilitates diagnosis and initiation of timely treatment, and no screening is advocated in our center, which largely restrict diagnosis to symptomatic patients, and potentially late presentations, for which treatment may not readily modify outcome, as shown for influenza. Second, although we have standardized guidelines on therapeutic regimens for these infections in our hospital, its use was based on the discretion of the treating physician. Thus, more severe presentation may have been favored for treatment. Third, our study lacks a randomized control group to evaluate our treatment approach. The prospects of conducting such a trial for RBV have to await the results on currently ongoing trials testing more specifically designed new antivirals for RSV and hopefully other paramyxoviruses [44]. Finally, although we included a large cohort of hematologic patients, this was a single-center study and therefore our results might not be transferable to other centers.
CONCLUSIONS
In conclusion, we report that paramyxovirus RTID are frequent >100 days after allogeneic HCT and clinically more severe in SID patients despite preferential antiviral treatment. Although no definite conclusions about efficacy can be made, current mortality was reduced compared with our earlier studies. The data suggest that timely diagnosis and early treatment with effective pan-paramyxovirus antivirals will have a major impact on morbidity and mortality of SID HCT patients. Well designed randomized studies are needed to further validate risk strata to balance treatment versus overtreatment and adverse events.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Kathrin Ullrich for the data management.
Disclaimer. The funding institutions had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was funded by the Swiss National Foundation (grant PZ00P3_142403; to N. K.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Chemaly RF, Ghosh S, Bodey GP, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006; 85:278–87. [DOI] [PubMed] [Google Scholar]
- 2. Nichols WG, Corey L, Gooley T, et al. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood 2001; 98:573–8. [DOI] [PubMed] [Google Scholar]
- 3. Peck AJ, Englund JA, Kuypers J, et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood 2007; 110:1681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant 2005; 11:781–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raboni SM, Nogueira MB, Tsuchiya LR, et al. Respiratory tract viral infections in bone marrow transplant patients. Transplantation 2003; 76:142–6. [DOI] [PubMed] [Google Scholar]
- 6. Whimbey E, Champlin RE, Couch RB, et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis 1996; 22:778–82. [DOI] [PubMed] [Google Scholar]
- 7. Hirsch HH, Martino R, Ward KN, et al. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013; 56:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ljungman P. Respiratory virus infections in stem cell transplant patients: the European experience. Biol Blood Marrow Transplant 2001; 7:5S–7S. [DOI] [PubMed] [Google Scholar]
- 9. Avetisyan G, Mattsson J, Sparrelid E, Ljungman P. Respiratory syncytial virus infection in recipients of allogeneic stem-cell transplantation: a retrospective study of the incidence, clinical features, and outcome. Transplantation 2009; 88:1222–6. [DOI] [PubMed] [Google Scholar]
- 10. Khanna N, Widmer AF, Decker M, et al. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis 2008; 46:402–12. [DOI] [PubMed] [Google Scholar]
- 11. Chemaly RF, Hanmod SS, Rathod DB, et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood 2012; 119:2738–45; quiz 969. [DOI] [PubMed] [Google Scholar]
- 12. Renaud C, Campbell AP. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr Opin Infect Dis 2011; 24:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schiffer JT, Kirby K, Sandmaier B, et al. Timing and severity of community acquired respiratory virus infections after myeloablative versus non-myeloablative hematopoietic stem cell transplantation. Haematologica 2009; 94:1101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011; 117:2755–63. [DOI] [PubMed] [Google Scholar]
- 15. Boeckh M, Englund J, Li Y, et al. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin Infect Dis 2007; 44:245–9. [DOI] [PubMed] [Google Scholar]
- 16. Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant 2001; 7:11–5S. [DOI] [PubMed] [Google Scholar]
- 17. Torres HA, Aguilera EA, Mattiuzzi GN, et al. Characteristics and outcome of respiratory syncytial virus infection in patients with leukemia. Haematologica 2007; 92:1216–23. [DOI] [PubMed] [Google Scholar]
- 18. Shah DP, Ghantoji SS, Shah JN, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother 2013; 68:1872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim YJ, Guthrie KA, Waghmare A, et al. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis 2014; 209:1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2014; 59(Suppl 5):S344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006; 144:344–9. [DOI] [PubMed] [Google Scholar]
- 22. Wyde PR, Chetty SN, Jewell AM, et al. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Res 2003; 60:51–9. [DOI] [PubMed] [Google Scholar]
- 23. Hamelin ME, Prince GA, Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob Agents Chemother 2006; 50:774–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilbert BE, Knight V. Biochemistry and clinical applications of ribavirin. Antimicrob Agents Chemother 1986; 30:201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell AP, Chien JW, Kuypers J, et al. Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT. J Infect Dis 2010; 201:1404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Machado AF, Sallum MA, Vilas Boas LS, et al. Molecular characterization of strains of respiratory syncytial virus identified in a hematopoietic stem cell transplant outpatient unit over 2 years: community or nosocomial infection?Biol Blood Marrow Transplant 2008; 14:1348–55. [DOI] [PubMed] [Google Scholar]
- 27. Williams JV, Martino R, Rabella N, et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis 2005; 192:1061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erard V, Chien JW, Kim HW, et al. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis 2006; 193:1619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chakrabarti S, Collingham KE, Holder K, et al. Parainfluenza virus type 3 infections in hematopoetic stem cell transplant recipients: response to ribavirin therapy. Clin Infect Dis 2000; 31:1516–8. [DOI] [PubMed] [Google Scholar]
- 30. Shima T, Yoshimoto G, Nonami A, et al. Successful treatment of parainfluenza virus 3 pneumonia with oral ribavirin and methylprednisolone in a bone marrow transplant recipient. Int J Hematol 2008; 88:336–40. [DOI] [PubMed] [Google Scholar]
- 31. Piralla A, Percivalle E, Di Cesare-Merlone A, et al. Multicluster nosocomial outbreak of parainfluenza virus type 3 infection in a pediatric oncohematology unit: a phylogenetic study. Haematologica 2009; 94:833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beckmann C, Hirsch HH. Comparing luminex NxTAG-respiratory pathogen panel and RespiFinder-22 for multiplex detection of respiratory pathogens. J Med Virol 2016 Aug; 88(8):1319–24. [Epub 2016 Feb 18]. doi: 10.1002/jmv.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ljungman P, Ward KN, Crooks BN, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2001; 28:479–84. [DOI] [PubMed] [Google Scholar]
- 34. Khanna N, Steffen I, Studt JD, et al. Outcome of influenza infections in outpatients after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2009; 11:100–5. [DOI] [PubMed] [Google Scholar]
- 35. Beckmann C, Hirsch HH. Diagnostic performance of near-patient testing for influenza. J Clin Virol 2015 Jun; 67:43–6. [Epub 2015 Mar 31]. doi: 10.1016/ j.jcv.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dumoulin A, Widmer AF, Hirsch HH. Comprehensive diagnostics for respiratory virus infections after transplantation or after potential exposure to swine flu A/H1N1: what else is out there? Transpl Infect Dis 2009 Aug; 11(4):287–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Egli A, Bucher C, Dumoulin A, et al. Human metapneumovirus infection after allogeneic hematopoietic stem cell transplantation. Infection 2012; 40:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peghin M, Hirsch HH, Len Ó, et al. Epidemiology and immediate indirect effects of respiratory viruses in lung transplant recipients: a 5-year prospective study. Am J Transplant 2017; 17:1304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wolfromm A, Porcher R, Legoff J, et al. Viral respiratory infections diagnosed by multiplex PCR after allogeneic hematopoietic stem cell transplantation: long-term incidence and outcome. Biol Blood Marrow Transplant 2014; 20:1238–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boeckh M, Bergeron A, Ljungman P, et al. A phase 2B, randomized, double-blind, placebo-controlled trial of presatovir (GS-5806) for treatment of hematopoietic-cell transplantation patients with an respiratory syncitial virus upper respiratory tract infection. EBMT European Societey for Blood and Marrow Transplantation Annual Meeting 2018. 2018; OS6-3. [Google Scholar]
- 41. Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003; 168:208–14. [DOI] [PubMed] [Google Scholar]
- 42. Whimbey E, Champlin RE, Englund JA, et al. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant 1995; 16:393–9. [PubMed] [Google Scholar]
- 43. Shah DP, Ghantoji SS, Ariza-Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood 2014; 123:3263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKimm-Breschkin JL, Jiang S, Hui DS, et al. Prevention and treatment of respiratory viral infections: presentations on antivirals, traditional therapies and host-directed interventions at the 5th ISIRV Antiviral Group conference. Antiviral Res 2018; 149:118–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.