Abstract
Rodent models have been invaluable for biomedical research. Preclinical investigations with rodents allow researchers to investigate diseases by using study designs that are not suitable for human subjects. The primary criticism of preclinical animal models is that results are not always translatable to humans. Some of this lack of translation is due to inherent differences between species. However, rodent models have been refined over time, and translatability to humans has improved. Transgenic animals have greatly aided our understanding of interactions between genes and disease and have narrowed the translation gap between humans and model animals. Despite the technological innovations of animal models through advances in genetics, relatively little attention has been given to animal diets. Namely, developing diets that replicate what humans eat will help make animal models more relevant to human populations. This review focuses on commonly used rodent diets that are used to emulate the Western dietary pattern in preclinical studies of obesity and type 2 diabetes, nonalcoholic liver disease, maternal nutrition, and colorectal cancer.
Keywords: laboratory rodent diets, diet-induced obesity, nonalcoholic fatty liver disease, colorectal cancer, total Western diet
Introduction
The primary goal of preclinical research is to make discoveries that can be translated from model organisms to humans. Animal models continue to be refined and improved through advances in biotechnology. For instance, genes can be modified to more closely replicate human physiology through genetic engineering, or immunocompromised mice can be humanized with engrafted human tissue. Recently, as the importance of the gut microbiome to chronic disease has become realized, germ-free mice are being humanized with human gut bacteria. Advances such as these have increased the translatability of preclinical studies to human populations. However, one variable of preclinical studies that has not changed appreciably in terms of increasing translatability during this same time frame is laboratory animal diets.
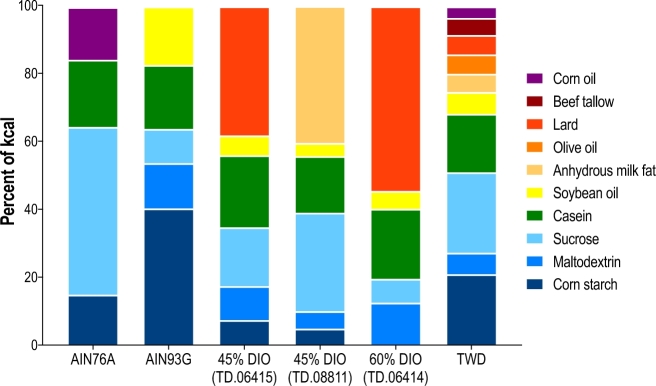
Until the NIH-7 open-source diet was developed by Knapka et al. (1), standardized nutrition in preclinical studies was not adequately considered. The NIH-7 diet, which is still in use today, contains a diverse array of commodity ingredients. The creation of open-source diets helped eliminate variation across experiments. However, because these diets contain commodity ingredients, variation can still be introduced in terms of differing dietary mineral content (2) and plant secondary compounds, such as phytoestrogens, that can influence reproductive endpoints (3, 4). Recognizing the need for a consistent, standardized rodent diet that also ensured animal health, the Council of the American Institute of Nutrition (AIN) commissioned the AIN76 rodent diet (5). The AIN76 diet was formulated with purified ingredients, including micronutrients provided at or near recommendations set by the NRC for rodents. To ensure consistency, the diet was formulated with purified ingredients, including sucrose, cornstarch, casein, corn oil, cellulose, and a vitamin and mineral supplement (Figure 1). In 1980, the AIN76 diet was slightly modified by increasing the vitamin K content and by inclusion of the antioxidant tert-butylhydroquinone (6). To address animal health concerns, in 1993, the AIN76A diet was modified to increase the n–3 PUFA content by changing the fat source from corn oil to soybean oil (7, 8). The resulting AIN93 growth and maintenance formulations (AIN93G and AIN93M, respectively) are now the standard basal diets for nutrition research (Figure 1).
FIGURE 1.
Ingredient profile of commonly used diets in preclinical chronic disease research. Example diets include the AIN76A and AIN93G diets; 45–60% of energy, high-fat DIO diets (Envigo); and the TWD. DIO, diet-induced obesity; TWD, Total Western Diet.
The creation of these standardized diets eliminated a significant source of experimental variation between different investigators. These low-fat diets result in a lean, healthy phenotype in rodents and are used as control diets for preclinical studies. Conversely, because diet is involved in the etiology of many chronic diseases, researchers who use chronic disease rodent models typically use “Western” diets to induce disease-specific phenotypes. Subsequently, the term “Western” diet has become a catch-all for any rodent diet that is higher in fat or manipulated in a way to induce chronic disease associated with the Western human dietary pattern. However, these diets typically have little resemblance to the human Western dietary pattern in terms of macro- and micronutrient content. According to NHANES, the typical American diet contains ∼49%, 35%, and 16% of energy from carbohydrates, fat, and protein, respectively (9), which is substantially different from frequently used high-fat “Western” diets used in chronic disease research. This review will examine several popular “Western” diets used to model chronic disease as well as the Total Western Diet (TWD), a novel rodent formulated to systematically emulate the American dietary pattern as defined by NHANES for both micro- and macronutrients (Figure 2).
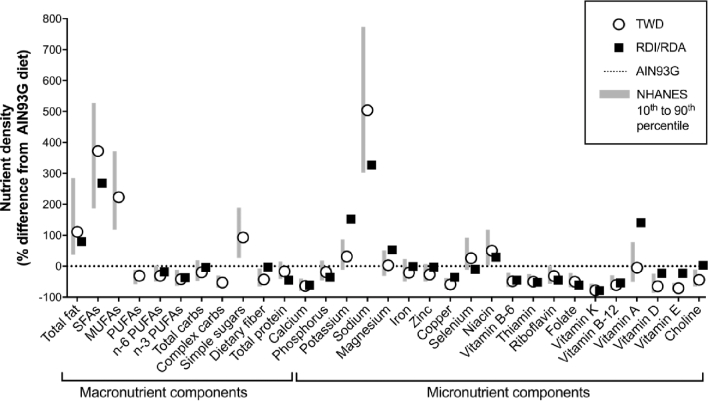
FIGURE 2.
Energy density–normalized macro- and micronutrient comparisons of the TWD with the AIN93G diet in relation to intakes reported in NHANES (10) and RDI/RDA (11) values. The relation between the TWD and the range of intakes for the typical American diet (10th−90th percentiles) is shown for each diet component compared with AIN93G (dotted line). Also shown are the normalized RDI or RDA values (individuals aged >2 y). carbs, carbohydrates; RDI, Reference Daily Intake; TWD, Total Western Diet.
Current Status of Knowledge
Diets used to induce obesity and type 2 diabetes
Increasingly, obesity and related chronic diseases, such as type 2 diabetes (T2D), have become a worldwide health concern. As a result, preclinical models of obesity and T2D are in great demand. There are several ways to induce obesity in laboratory animals, including the use of genetic animal models. Commonly used genetic models harbor mutations associated with satiety, such as the ob (leptin) mouse or the db (leptin receptor) mouse and zucker rat (12–14). Although these models are very effective at achieving an obese phenotype and have been invaluable to our understanding of obesity and related diseases, these single mutation models poorly emulate the etiology of obesity and T2D in humans. Therefore, dietary induction of obesity [diet-induced obesity (DIO)] in polygenetically susceptible animals, such as the C5BL/6J mouse, has become a very common approach in preclinical studies. In this review, low-, medium-, and high-fat diets are defined as <20%, 20–35%, and >35% of total energy, respectively.
Some of the earliest DIO modeling involved the use of extremely high-fat diets fed long term to rats, including diets that contained ≤82% of calories from fat and caused aberrations in metabolism (15–21). Over the years, these protocols were refined by Surwit et al. (22) to induce obesity-related hyperglycemia and hyperinsulemia with the use of inbred mice. In their initial study, male A/J or C57BL/6J mice were fed either low-fat rodent nonpurified diet or a high-fat, high-sugar diet that contained ∼59% of energy from lard and 26% of energy from sucrose. Both A/J and C57BL6/J mice gained more weight than those in nonpurified diet–fed cohorts. However, the high fat–fed C57BL6/J mice gained significantly more weight than did their A/J counterparts. The same group went on to show in a series of studies that the obesogenic properties of these high-fat diets were specific for the C57BL6/J strain (23), with specific differences between obesity-resistant A/J mice and C57BL6/J mice in terms of fat cell number, mesenteric fat mass, and lipoprotein lipase activity (24). These strain-by-diet interactions of increased adiposity were largely explained by increased feed efficiency of C57BL6/J compared with A/J mice when fed the 60%-fat diets. However, the strain-specific increase in feed efficiency was not observed when mice were fed low-fat control diets. The resulting obese phenotype in the high fat–fed C57BL6/J mice also resulted in a T2D phenotype because the C57BL6/J mice had significantly higher fasted glucose and insulin than did C57BL6/J mice fed the low-fat control diet and A/J mice fed either the high- or low-fat diets. It is interesting to note that the investigators also tested effects of high or low dietary sucrose (13% compared with 0% of energy) in this study and found that sucrose did not significantly affect T2D endpoints but did decrease feed efficiency (25).
This early work is the basis for the very commonly used C57BL6/J DIO T2D model. Typically, male mice are fed a diet that contains 60% or 45% of energy from fat, which is supplied as lard and soybean oil (usually a 9:1 ratio, respectively) (Figure 1). A common experimental protocol is to feed 4- to 5-wk-old male C57BL6/J mice high-fat diets for 12–20 wk, with the primary study endpoints being body composition, fasted glucose, fasted insulin, oral glucose tolerance, insulin resistance (using HOMA-IR), and measurement of inflammatory cytokines and adipokines. Commercial diet companies sell open-source formulations of these high-fat diets as well as matched low-fat control diets. However, these diets are not representative of the Western dietary pattern with respect to the total amount of dietary fat and the FA composition (10). Importantly, similar to the AIN diets, the micronutrient content of these high-fat diets is formulated to promote animal health, which is also inconsistent with the Western dietary pattern (10).
Diets used to induce fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is the result of metabolic dysregulation and is characterized by a hepatic TG content >5.56% (26). It is considered the hepatic manifestation of metabolic syndrome (27). NAFLD includes simple steatosis, nonalcoholic steatohepatitis (NASH), hepatic fibrosis, and cirrhosis (28). There are estimates that ≤30% of those in Western countries have hepatic steatosis and between 20% and 30% of these individuals will subsequently develop NASH (29). In overweight individuals, NAFLD occurrence may be ≤58% and may be ≤98% in obese subjects without diabetes (27). Although the initiation and development of NAFLD are highly correlated with markers of metabolic syndrome, the mechanism or mechanisms driving disease progression of NAFLD to NASH are unknown (30).
Several review articles have been published that evaluate rodent diets that cause hepatosteatosis and, to some extent, the progression to NASH (31–35). A simple and effective way to induce NAFLD in rodents via diet is to restrict essential nutrients, such as choline and methionine, which are necessary for proper hepatic lipid metabolism. Mechanisms of liver damage caused by choline restriction have been elucidated and include effects on phospholipid synthesis, lipoprotein secretion, and oxidative and endoplasmic reticulum stress (36). In rats, diets devoid of choline induce NAFLD in 10 wk, which includes steatosis, inflammation, and fibrosis (37). Choline-deficient diets also impair the respiratory function of mitochondria (38), including decreased respiratory efficiency and increased amounts of protein oxidation products (39). Diets that are deficient in both methionine and choline (MCD) are also used to investigate NAFLD and produce a more severe phenotype in a shorter period of time (35). These diets typically contain >40% sucrose and 10% fat as corn oil (33), which stresses hepatic lipid trafficking by promoting de novo lipogenesis. MCD diets are valuable for the study of the progression of NAFLD to NASH, because inflammation, hepatocyte apoptosis, and fibrosis are more likely to develop than when mice are fed a high-fat diet (35). Despite their value in modeling the liver injury in NASH, MCD diets do not accurately recapitulate the metabolic dysregulation in humans associated with NAFLD because MCD-fed rodents lose weight due to lower calorie intake (33).
A second way to induce NAFLD in rodents is to add something to the diet in excess, such as fat, cholesterol, or sucrose. High-fat diets are routinely used that contain between 45% and 75% of calories as fat, the majority being lard. Rodents fed high-fat diets develop both obesity and steatosis and become insulin resistant (35), and hepatic insulin resistance has been shown to precede fat deposition in peripheral tissues (40). In addition, high-fat feeding in mice was associated with glucose intolerance, increased leptin, and dysregulated lipid metabolism, yet compared with mice fed MCD diets, the degree of steatosis and liver injury was less severe (41). Adding cholesterol or cholate has also been used in models of NAFLD (31, 33, 35, 41, 42). In mice, the addition of 1.25% cholesterol and 0.5% cholate promotes steatosis, inflammation, and fibrosis over 24 wk. Although the mice develop hepatic insulin resistance, they also lose weight and maintain systemic insulin sensitivity. Similarly, in rats, the addition of cholesterol at >1.25% has been shown to promote steatosis, inflammation, and fibrosis (43), but the phenotype does not recapitulate the metabolic effects associated with NAFLD in humans.
Rodent diets with a sucrose content >25% causes steatosis (44). Interestingly, the average American diet contains ∼20% of energy from sugar (45), approximately one-half of which is fructose. Diets with >60% fructose have been shown to induce macrovesicular steatosis and inflammation, but the pattern of fat deposition does not match that in human NAFLD (32). Increasingly, fructose is used in combination with other stressors such as high fat and cholesterol. A common strategy has been to add fructose, or high-fructose corn syrup, to the drinking water of rodents. In general, this strategy appears to promote steatosis and the progression to NASH (32, 33, 35).
It is generally accepted that there is no perfect dietary model for NAFLD, which is likely due to different goals of investigators working in this area. An intractable aspect of modeling NAFLD, and ways to understand its progression, is the low percentage of cases that progress to NASH. To induce NASH consistently, and thus to characterize molecular mechanisms, diets are used that lack essential nutrients and thus are not physiologically relevant to humans. On the other hand, diets that more closely model those consumed by humans may either not induce NAFLD or do so in a time frame that is economically (in time and money) unrealistic. Diets that combine nutrient manipulations from various rodent dietary NAFLD models reviewed here, such as diets with moderately elevated fat and moderately high fructose, low choline amounts, and some dietary cholesterol, suggest that diets may be developed that can both accurately model human diets and also cause NAFLD.
Prenatal rodent diets and phenotypic outcomes in the offspring
Preclinical studies investigating the impact of maternal diet on offspring health have used diets that varied greatly in fat content, from 20% to 60% of energy, and often had reduced carbohydrate and protein content to accommodate the increase in FAs (46). Two recent meta-analyses (47, 48) showed that maternal high-fat intake is associated with greater offspring body weight at weaning and adulthood, along with elevated adiposity, systolic blood pressure, and concentrations of insulin, leptin, TGs, and cholesterol in males and females. The largest effect sizes were observed for obese phenotypes and immune activation in male offspring and hyperglycemia in female offspring (47, 49). This sex-specific response to maternal diet may arise due to different epigenetic regulation in the placenta (50) and adaptation to environmental vulnerabilities (51). Moreover, the maternal diet composition underlies different programming effects, whereby the cafeteria diet paradigm that allows free access to a wide range of energy-dense foods resulted in rapid weight gain, whereas diets that exchanged carbohydrate for fat disrupted lipid and insulin metabolism in the offspring (47).
In contrast to the carbohydrate content being predictive of weaning weight in males, the ratio of fat content did not correlate with metabolic disturbances in the offspring (47). This lack of relation may be due to the current limitations in study design that do not directly measure or standardize FA composition of the diet. Animal models of maternal diet commonly use different sources of fat consisting of either animal fat (lard) or hydrogenated vegetable oil (shortening). Lard has high amounts of linoleic acid and vitamin D, which have been suggested to influence metabolic outcomes (52–54). An accentuated increase in weight gain and insulin resistance with a lard-based diet compared with a hydrogenated vegetable-shortening diet indicates that standardizing FA composition and type of fat used in determining outcomes of consuming high-fat diets is critical (55). Potential mechanisms underlying the programming effects of FA composition involve hypothalamic inflammation and epigenetic programming, including DNA methylation (56) and microRNA expression (57), that lead to alterations in the neuroendocrine functions (58).
Metabolic perturbations in the offspring appear to also depend on the timing of diet exposure. Studies that used cross-fostering indicate that high-fat diet exposure during lactation was more influential for programming greater body weight and adiposity and altered appetite regulatory systems toward obesity compared with exposure during pregnancy (47, 48, 59). The postweaning period can additionally affect the offspring phenotype because matching the fat content between the maternal and pup diet prevented metabolic disturbances and impairment of acetylcholine-induced endothelium-dependent relaxation (60).
Although species or strain and maternal weight gain did not account for interstudy heterogeneity (47), these differences may modulate the relation between maternal diet and offspring outcomes. Mouse strains showed greater metabolic changes after maternal high-fat exposure compared with rats, with overall more metabolic changes and of greater magnitude (47). In addition, the effects of maternal high-fat diet on glucose and TG concentrations are suggested to depend on maternal obesity (47, 48). Variations in the maternal diet composition appear to produce differences in the metabolic response of the dams, which may be contributing to differences in the phenotypic outcomes in the offspring. Thus, future investigations should focus on standardization of the diets that consistently define maternal background characteristics, which will clarify directionality between the prenatal diet and offspring phenotype.
Dietary models of colorectal cancer
On the basis of the strong epidemiologic evidence supporting a link between obesity and increased risk of colorectal cancer (CRC) in humans, researchers have long investigated a potential link between obesogenic diets and CRC in animal models of the disease. The link between the consumption of a high-fat diet and cancer has been widely studied, particularly with respect to development of CRC in rodent models, with >400 reports on the topic in PubMed as of this review. Early studies from the 1980s pointed to a role of dietary fat in promoting colon tumorigenesis, although at the time there was substantial debate on the role of specific dietary fats, with some reports suggesting that the type of fat was inconsequential (61, 62) and others suggesting that specific fats had differential effects on tumor development in the colon (63, 64). Yet, other groups had contrary findings, with no observed effects of dietary fat on colon tumorigenesis (65, 66). Toward the end of the decade, the research community had concluded that dietary fat was a critical factor in the etiology of CRC, yet acknowledged that specific fat types were likely responsible for the cancer-promoting effect of high-fat diets, including corn oil, beef fat, safflower oil, and lard (67, 68).
In the following 30 y, the general thinking about high-fat diets and CRC has remained fairly consistent. Researchers continue to use various commercial high-fat diets, including the DIO diets, to probe mechanisms of colorectal carcinogenesis and to investigate the contribution of systemic inflammation resulting from DIO on tumor development. One should note, however, that the vast majority of such studies used diet formulations consisting of 40–60% of energy as fat, typically as soybean oil and lard (Figure 1). These commercial diet formulas contrast with the fat content of a typical American diet, with a median fat intake of only ∼34% and a wide diversity of fat sources consumed (10). Thus, although these reports do show a link between fat consumption and cancer development and provide insights on mechanisms by which dietary fat promotes cancer, their usefulness as dietary models of human nutrition in preclinical studies intended to evaluate disease risk is not as clear. In recognition of these limitations, some researchers are using diet formulas that attempt to more closely emulate the diversity of fat sources consumed by Americans. For example, it was reported that the consumption of a high-fat diet that was similar to an American diet with respect to the percentage of dietary fat altered inflammatory signaling in adipose tissue and the tumor microenvironment in a manner consistent with the promotion of intestinal tumors (69). Also recently, it was reported that human CRC xenografts grew at an accelerated rate when transplanted subcutaneously and orthotopically into immunodeficient mice that had acquired an obese phenotype via consumption of a high-fat diet (40% of energy consisting of equal parts vegetable shortening, milk fat, and lard) compared with their lean counterparts fed a low-fat (12.4%) diet. However, this study design did not allow the authors to conclusively dissect the potential impact of an obesity phenotype on tumor development independent of dietary intakes, or vice versa (70).
With respect to CRC, a significant flaw in the aforementioned strategies to investigate the impact of a Western-type diet on CRC risk was the lack of appropriate consideration of the contribution of micronutrients to tumor development. Indeed, the vast majority of studies that used DIO-type diets or custom high-fat diets used a standard micronutrient formulation modeled after the AIN76 or AIN93 diets. In a series of studies over the past 3 decades, Newmark et al. (71–73) used a selective approach in modeling a Western diet, wherein specific components of the diet were modified to emulate typical US intakes. Their first study used a “stress” diet, which was quite low in calcium and vitamin D, and modestly reduced in phosphate compared with the reference diet, AIN76A. In addition, the stress diet contained 20% fat as corn oil (40% of energy) compared with only 5% (12% of energy) in the reference diet (71). A subsequent study extended this stress diet to incorporate dietary components necessary for the generation of methyl donors (folic acid, methionine, choline, and vitamin B-12) and determined that this new diet also enhanced spontaneous tumor development in aged C57BL/6J mice, an effect that was reversed when calcium and vitamin D were added back to the stress diet (72, 73). Although this series of studies convincingly showed a role for dietary calcium and vitamin D in modulating spontaneous colon carcinogenesis in mice, the scope of the diet remained limited in that it did not consider the possible contribution of the dietary fat source, carbohydrates, or proteins and did not reflect typical human nutrition patterns for other key micronutrients, such as sodium, selenium, or vitamins A or E.
Modeling animal diets based on human intakes: the TWD
Our understanding of chronic disease has been greatly advanced through preclinical studies that model such diseases. As reviewed earlier, commonly used proxies for the Western diets are effective for generating disease phenotypes. In nutrition studies that use these models, nonessential nutrients or botanical extracts are often added to the disease-generating basal diets to investigate protective effects or, conversely, amounts of individual macronutrients or micronutrients are altered to determine their role in health. Although this strategy has led to significant findings, a basal rodent diet that is more representative of the diet consumed by at-risk populations may be necessary to appropriately evaluate effects of dietary components. Some investigators have sought to address this issue by using “cafeteria”-style diets (animals are free to select from a variety of tasty, processed foods) in an attempt to emulate typical Western dietary patterns for rodent models. However, the cafeteria diet has limited value as an experimental model because it is poorly defined with respect to micronutrient composition and unlikely to provide robust experimental replication (74, 75).
To more closely model human intakes, we developed the TWD for rodents with energy and nutrient profiles that emulate a typical Western diet with the use of NHANES data. The TWD was formulated by using a nutrient density approach, described in detail elsewhere (45). Briefly, the amount of each macro- and micronutrient in the AIN93G basal diet, a diet routinely used in cancer studies today, was adjusted to match 50th-percentile intakes for Americans, as reported in NHANES data. These mass amounts were then normalized to caloric intake (mass of nutrient per kilocalories). The TWD has fewer calories from protein and carbohydrate sources and twice that from fat than does the AIN93G diet. The TWD contains more saturated and monounsaturated fats, less polyunsaturated fat, more complex carbohydrates, and twice the amount of simple sugars. The TWD also contains a much more diverse dietary fat portfolio, with the exception of long-chain n–6 and n–3 PUFAs, than conventional high-fat diets and the AIN93 diet (Figure 1). Compared with the AIN93 diet, the TWD contains less calcium, copper, folate, thiamine, and vitamins B-6, B-12, D, and E but much more sodium. Overall, the TWD is not necessarily extreme in the amount of any given nutrient, but rather reflects the overall US dietary pattern (Figure 2). Our research team and others have shown that this diet affects feeding behavior, metabolism, and response to CRC (76–78).
The TWD as a dietary model for obesity, metabolism, and NAFLD
To determine if feeding the TWD produced similar metabolic perturbations as a traditional 45%-fat DIO diet and to disseminate the role that micro- and macronutrients play in producing the obese phenotype and on various health variables, including weight gain, insulin resistance, and systemic inflammation, male C57BL/6J mice were fed the following diets: 1) an AIN-93G low-fat control diet, 2) a TWD, 3) a 45%-fat DIO diet, 4) an AIN93G diet modified with TWD macronutrients [macronutrient-modified diet (MM)], or 5) an AIN93G diet modified with TWD micronutrients (vitamin- and mineral-modified diet) (76). Compared with the DIO treatment, mice fed the TWD gained less weight and generally had a metabolic phenotype closer to the AIN93G-fed mice despite being fed a moderately high-fat diet. However, when mice were fed the MM diet, which was identical to the TWD in terms of macronutrients but contained the same amounts of micronutrients as the AIN93G diet, mice had a similar phenotype to the DIO-fed mice. Compared with the TWD treatment, the MM- and DIO-fed mice consumed more energy, had increased feed efficiency, had increased body weight gain and fat mass percentage, had increased subcutaneous and visceral fat, and were more insulin resistant. These data suggest that, in the context of the TWD, suboptimal vitamin and mineral intakes in mice specifically inhibit the hyperphagia and the resulting increased weight gain associated with the higher fat content of the TWD. In addition, it is important to note that the micronutrient profile of the TWD did not limit lean mass accretion, suggesting that the mice were not stunted. Although results of this study were counter to our original hypothesis, these findings are important in that they show a role of dietary micronutrients in moderating the hyperphagic behavior shown by C57BL/6J mice fed a moderately high-fat diet.
In this study, we predicted that mice fed a TWD might develop an NAFLD phenotype. This premise was based on the low choline content of the TWD. The Adequate Intake for choline is between 450 and 550 mg/d (11), which translates to 180–220 µg/kcal on a nutrient-density basis. The TWD contains 113 µg/kcal, which is only 62% of the Adequate Intake. As a reference, choline nutrient density is 228 µg/kcal for the AIN93 diet. For the common DIO diets, the values are 136 µg/kcal for the 60%-kcal-from-fat and 151 µg/kcal for the 45%-kcal-from-fat diet. In addition, the TWD contains ∼20% sucrose by mass and derives ∼20% of the calories from sucrose, amounts that have been shown to promote steatosis in rodents (44). However, mice fed the TWD did not have higher liver TGs relative to the AIN93G control diet.
The TWD as a dietary model for CRC
As outlined above, many researchers use standard AIN76 or AIN93 diets in preclinical cancer studies, including cancer prevention studies that use dietary bioactives. One of the key questions our group wished to address with the use of the TWD was whether the consumption of this more representative Western diet would influence the efficacy of a well-known anticancer bioactive, specifically green tea polyphenols. To determine if there was an interaction between the TWD basal and green tea extract (GTE) on azoxymethane-induced CRC, lipid metabolism, and SCFA metabolism, A/J mice were fed either the TWD or the AIN93G diet with or without GTE added to the water in a 2 × 2 factorial design (78). There were significant interactions between the basal diet and GTE on several experimental endpoints. For instance, GTE reduced body weight but only in mice fed the TWD. Fasting glucose was reduced by GTE treatment in mice fed the TWD but not the AIN93G diet. Cecal SCFAs were reduced by GTE, but only in mice fed the TWD. Conversely, GTE decreased liver TGs but only in mice fed the AIN93G diet. Importantly, mice fed the TWD had increased aberrant crypt foci multiplicity compared with AIN93G-fed mice, suggesting that the TWD as a basal diet promotes CRC. Notably, GTE reduced aberrant crypt foci only in mice fed the TWD but not the AIN93G diet. In an additional CRC study from another laboratory, Nakanishi et al. (77) found that the inclusion of walnuts suppressed tumor development in mice fed the TWD but not the AIN76A diet. These results suggest that standardized basal diets, such as the AIN93G, may underestimate or fail to show the efficacy of bioactives such as GTE in CRC preclinical models.
Conclusions
Basal diet is an important consideration in preclinical studies to model human disease. Diet models, such as the high-fat DIO diet or the MCD protocol, have greatly aided our understanding of nutrition-related chronic diseases, and these protocols will continue to be useful tools to generate appropriate phenotypes. However, investigators must be cautious not to confuse these diets with the Western dietary pattern, because they do not recapitulate many features of this dietary pattern (e.g., diverse fat sources, amounts of micronutrients). Thus, these diets should be considered as tools to generate disease, not models of “Western” nutrition.
Making animal diets more relevant to at-risk human populations, such as the TWD, is a step forward in improving the translational fidelity of preclinical mouse models. For instance, our data suggest that, compared with AIN93G, the TWD may be a more suitable basal diet to model CRC. This matches well with epidemiologic data that show a link between the Western dietary pattern and CRC. For example, Americans of African descent have a CRC rate 65:100,000 compared with <5:100,000 in rural Africans, and this difference is thought to be caused primarily by the American dietary pattern (79).
Future work to increase the translatability of animal diets to human diets should extend beyond macro- and micronutrients. Although they may complicate study design and interpretation, other variables that contribute to human diets should also be considered in translational preclinical models when possible, such as the complex food matrix, cooking oxidation products, plant secondary compounds, food additives, and diverse sources of fiber. Although rodent nonpurified diets provide some of these variables, they lack conformity in their preparation and introduce variation between experiments. Standardizing a rodent diet that addresses these many variables may be a daunting task, but such an endeavor would undoubtedly improve the translatability between animal and human studies.
Acknowledgments
We thank Stephany Monsanto, a former graduate student involved in the early TWD studies discussed in this review. All authors read and approved the final manuscript.
Notes
Supported by the Utah Agricultural Experiment Station.
Author disclosures: KJH, ADB, CEC, and REW, no conflicts of interest.
Abbreviations used:
- db
leptin receptor
- CRC
colorectal cancer
- DIO
diet-induced obesity
- GTE
green tea extract
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- MCD
methionine choline–deficient diet
- MM
macronutrient-modified diet
- ob
leptin
- T2D
type 2 diabetes
- TWD
Total Western Diet
References
- 1. Knapka JJ, Smith KP, Judge FJ. Effect of open and closed formula rations on the performance of three strains of laboratory mice. Lab Anim Sci 1974;24(3):480–7. [PubMed] [Google Scholar]
- 2. Finley JW. Selenium accumulation in plant foods. Nutr Rev 2005;63(6 Part 1):196–202. [DOI] [PubMed] [Google Scholar]
- 3. Degen GH, Janning P, Diel P, Bolt HM. Estrogenic isoflavones in rodent diets. Toxicol Lett 2002;128(1–3):145–57. [DOI] [PubMed] [Google Scholar]
- 4. Thigpen JE, Setchell KD, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci 1999;49(5):530–6. [PubMed] [Google Scholar]
- 5. Report of the American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies. J Nutr 1977;107(7):1340–8. [DOI] [PubMed] [Google Scholar]
- 6. Bieri JG. Second report of the Ad Hoc Committee on Standards for Nutritional Studies. J Nutr 1980;110:1726. [DOI] [PubMed] [Google Scholar]
- 7. Reeves PG. AIN-76 diet: should we change the formulation? J Nutr 1989;119(8):1081–2. [DOI] [PubMed] [Google Scholar]
- 8. Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr 1993;123(11):1923–31. [DOI] [PubMed] [Google Scholar]
- 9. USDA, Agricultural Research Service. Nutrient intakes from food and beverages: mean amounts consumed per individual, by gender and age, What We Eat in America, NHANES 2013–2014. Beltsville (MD): United States Department of Agriculture, Agriculture Research Service; 2016. [Google Scholar]
- 10. USDA, Agricultural Research Service; Beltsville Human Nutrition Research Center; Food Surveys Research Group; US Department of Health and Human Services; CDC; National Center for Health Statistics. What we eat in America, NHANES 2013–2014. Beltsville (MD): United States Department of Agriculture, Agriculture Research Service; 2014. [Google Scholar]
- 11. Institute of Medicine. Dietary Reference Intakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 12. Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE. et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996;84(3):491–5. [DOI] [PubMed] [Google Scholar]
- 13. Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Phenotype-linked amino acid alteration in leptin receptor cDNA from Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun 1996;222(1):19–26. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372(6505):425–32. [DOI] [PubMed] [Google Scholar]
- 15. Lemonnier D. Obesity due to a high-fat diet in the rat and mouse. Nutr Dieta Eur Rev Nutr Diet 1967;9(1):27–42. [PubMed] [Google Scholar]
- 16. Lemonnier D, Tremolieres J. Experimental obesity induced by high-fat diets. Probl Actuels Endocrinol Nutr 1963;7:77–85. [PubMed] [Google Scholar]
- 17. Peckham SC, Entenman C, Carroll HW. The influence of a hypercaloric diet on gross body and adipose tissue composition in the rat. J Nutr 1962;77:187–97. [DOI] [PubMed] [Google Scholar]
- 18. Lavau M, Susini C. [U-14C]glucose metabolism in vivo in rats rendered obese by a high fat diet. J Lipid Res 1975;16(2):134–42. [PubMed] [Google Scholar]
- 19. Susini C, Lavau M. In-vitro and in-vivo responsiveness of muscle and adipose tissue to insulin in rats rendered obese by a high-fat diet. Diabetes 1978;27(2):114–20. [DOI] [PubMed] [Google Scholar]
- 20. Lavau M, Fried SK, Susini C, Freychet P. Mechanism of insulin resistance in adipocytes of rats fed a high-fat diet. J Lipid Res 1979;20(1):8–16. [PubMed] [Google Scholar]
- 21. Susini C, Lavau M, Herzog J. Adrenaline responsiveness of glucose metabolism in insulin-resistant adipose tissue of rats fed a high-fat diet. Biochem J 1979;180(2):431–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988;37(9):1163–7. [DOI] [PubMed] [Google Scholar]
- 23. Surwit RS, Seldin MF, Kuhn CM, Cochrane C, Feinglos MN. Control of expression of insulin resistance and hyperglycemia by different genetic factors in diabetic C57BL/6J mice. Diabetes 1991;40(1):82–7. [DOI] [PubMed] [Google Scholar]
- 24. Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism 1993;42(11):1405–9. [DOI] [PubMed] [Google Scholar]
- 25. Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 1995;44(5):645–51. [DOI] [PubMed] [Google Scholar]
- 26. Koch M, Nothlings U, Lieb W. Dietary patterns and fatty liver disease. Curr Opin Lipidol 2015;26(1):35–41. [DOI] [PubMed] [Google Scholar]
- 27. Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol 2014;7:221–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Banini BA, Sanyal AJ. Nonalcoholic fatty liver disease: epidemiology, pathogenesis, natural history, diagnosis, and current treatment options. Clin Med Insights Ther 2016;8:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reccia I, Kumar J, Akladios C, Virdis F, Pai M, Habib N, Spalding D. Non-alcoholic fatty liver disease: a sign of systemic disease. Metabolism 2017;72:94–108. [DOI] [PubMed] [Google Scholar]
- 30. Abenavoli L, Milic N, Peta V, Alfieri F, De Lorenzo A, Bellentani S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J Gastroenterol 2014;20(45):16831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol 2006;87(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2012;18(19):2300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ibrahim SH, Hirsova P, Malhi H, Gores GJ. Animal models of nonalcoholic steatohepatitis: eat, delete, and inflame. Dig Dis Sci 2016;61(5):1325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hansen HH, Feigh M, Veidal SS, Rigbolt KT, Vrang N, Fosgerau K. Mouse models of nonalcoholic steatohepatitis in preclinical drug development. Drug Discov Today 2017;22(11):1707–18. [DOI] [PubMed] [Google Scholar]
- 35. Lau JK, Zhang X, Yu J. Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J Pathol 2017;241(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol 2012;28(2):159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujita K, Nozaki Y, Yoneda M, Wada K, Takahashi H, Kirikoshi H, Inamori M, Saito S, Iwasaki T, Terauchi Y. et al. Nitric oxide plays a crucial role in the development/progression of nonalcoholic steatohepatitis in the choline-deficient, l-amino acid-defined diet-fed rat model. Alcohol Clin Exp Res 2010;34(Suppl 1):S18–24. [DOI] [PubMed] [Google Scholar]
- 38. Hensley K, Kotake Y, Sang H, Pye QN, Wallis GL, Kolker LM, Tabatabaie T, Stewart CA, Konishi Y, Nakae D. et al. Dietary choline restriction causes complex I dysfunction and increased H(2)O(2) generation in liver mitochondria. Carcinogenesis 2000;21(5):983–9. [DOI] [PubMed] [Google Scholar]
- 39. Teodoro JS, Rolo AP, Duarte FV, Simoes AM, Palmeira CM. Differential alterations in mitochondrial function induced by a choline-deficient diet: understanding fatty liver disease progression. Mitochondrion 2008;8(5–6):367–76. [DOI] [PubMed] [Google Scholar]
- 40. Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 2004;279(31):32345–53. [DOI] [PubMed] [Google Scholar]
- 41. Schattenberg JM, Galle PR. Animal models of non-alcoholic steatohepatitis: of mice and man. Dig Dis 2010;28(1):247–54. [DOI] [PubMed] [Google Scholar]
- 42. Rosso N, Chavez-Tapia NC, Tiribelli C, Bellentani S. Translational approaches: from fatty liver to non-alcoholic steatohepatitis. World J Gastroenterol 2014;20(27):9038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ichimura M, Kawase M, Masuzumi M, Sakaki M, Nagata Y, Tanaka K, Suruga K, Tamaru S, Kato S, Tsuneyama K. et al. High-fat and high-cholesterol diet rapidly induces non-alcoholic steatohepatitis with advanced fibrosis in Sprague-Dawley rats. Hepatol Res 2015;45(4):458–69. [DOI] [PubMed] [Google Scholar]
- 44. Zhou AL, Hintze KJ, Jimenez-Flores R, Ward RE. Dietary fat composition influences tissue lipid profile and gene expression in Fischer-344 rats. Lipids 2012;47(12):1119–30. [DOI] [PubMed] [Google Scholar]
- 45. Hintze KJ, Benninghoff AD, Ward RE. Formulation of the Total Western Diet (TWD) as a basal diet for rodent cancer studies. J Agric Food Chem 2012;60(27):6736–42. [DOI] [PubMed] [Google Scholar]
- 46. Kereliuk SM, Brawerman GM, Dolinsky VW. Maternal macronutrient consumption and the developmental origins of metabolic disease in the offspring. Int J Mol Sci 2017;18(7):1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ribaroff GA, Wastnedge E, Drake AJ, Sharpe RM, Chambers TJG. Animal models of maternal high fat diet exposure and effects on metabolism in offspring: a meta-regression analysis. Obes Rev 2017;18(6):673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tellechea ML, Mensegue MF, Pirola CJ. The association between high fat diet around gestation and metabolic syndrome-related phenotypes in rats: a systematic review and meta-analysis. Sci Rep 2017;7(1):5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dudele A, Hougaard KS, Kjolby M, Hokland M, Winther G, Elfving B, Wegener G, Nielsen AL, Larsen A, Nohr MK. et al. Chronic maternal inflammation or high-fat-feeding programs offspring obesity in a sex-dependent manner. Int J Obes (Lond) 2017;41(9):1420–6. [DOI] [PubMed] [Google Scholar]
- 50. Gabory A, Ferry L, Fajardy I, Jouneau L, Gothie JD, Vige A, Fleur C, Mayeur S, Gallou-Kabani C, Gross MS. et al. Maternal diets trigger sex-specific divergent trajectories of gene expression and epigenetic systems in mouse placenta. PLoS One 2012;7(11):e47986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dahlhoff M, Pfister S, Blutke A, Rozman J, Klingenspor M, Deutsch MJ, Rathkolb B, Fink B, Gimpfl M, Hrabe de Angelis M. et al. Peri-conceptional obesogenic exposure induces sex-specific programming of disease susceptibilities in adult mouse offspring. Biochim Biophys Acta 2014;1842(2):304–17. [DOI] [PubMed] [Google Scholar]
- 52. Alvheim AR, Malde MK, Osei-Hyiaman D, Lin YH, Pawlosky RJ, Madsen L, Kristiansen K, Froyland L, Hibbeln JR. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring) 2012;20(10):1984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri JM, Guesnet P. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res 2006;45(3):203–36. [DOI] [PubMed] [Google Scholar]
- 54. Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK. et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 2013;10(2):e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kubant R, Poon AN, Sanchez-Hernandez D, Domenichiello AF, Huot PS, Pannia E, Cho CE, Hunschede S, Bazinet RP, Anderson GH. A comparison of effects of lard and hydrogenated vegetable shortening on the development of high-fat diet-induced obesity in rats. Nutr Diabetes 2015;5:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cesar HC, Pisani LP. Fatty-acid-mediated hypothalamic inflammation and epigenetic programming. J Nutr Biochem 2017;42:1–6. [DOI] [PubMed] [Google Scholar]
- 57. Wilson RA, Deasy W, Hayes A, Cooke MB. High fat diet and associated changes in the expression of micro-RNAs in tissue: lessons learned from animal studies. Mol Nutr Food Res 2017;61(6):1600943. [DOI] [PubMed] [Google Scholar]
- 58. Sullivan EL, Riper KM, Lockard R, Valleau JC. Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm Behav 2015;76:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gugusheff JR, Ong ZY, Muhlhausler BS. The early origins of food preferences: targeting the critical windows of development. FASEB J 2015;29(2):365–73. [DOI] [PubMed] [Google Scholar]
- 60. Khan I, Dekou V, Hanson M, Poston L, Taylor P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation 2004;110(9):1097–102. [DOI] [PubMed] [Google Scholar]
- 61. Reddy BS, Narisawa T, Weisburger JH. Effect of a diet with high levels of protein and fat on colon carcinogenesis in F344 rats treated with 1,2-dimethylhydrazine. J Natl Cancer Inst 1976;57(3):567–9. [DOI] [PubMed] [Google Scholar]
- 62. Reddy BS. Dietary fat and its relationship to large bowel cancer. Cancer Res 1981;41(9 Part 2):3700–5. [PubMed] [Google Scholar]
- 63. El-Khatib SM, Cora EM. Role of high-fat diet in tumorigenesis in C57BL/1 mice. J Natl Cancer Inst 1981;66(2):297–301. [PubMed] [Google Scholar]
- 64. Reddy BS, Maruyama H. Effect of dietary fish oil on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res 1986;46(7):3367–70. [PubMed] [Google Scholar]
- 65. Schmahl D, Habs M, Habs H. Influence of a non-synthetic diet with a high fat content on the local occurrence of colonic carcinomas induced by N-nitroso-acetoxymethylmethylamine (AMMN) in Sprague-Dawley rats. Hepatogastroenterology 1983;30(1):30–2. [PubMed] [Google Scholar]
- 66. Nauss KM, Locniskar M, Newberne PM. Effect of alterations in the quality and quantity of dietary fat on 1,2-dimethylhydrazine-induced colon tumorigenesis in rats. Cancer Res 1983;43(9):4083–90. [PubMed] [Google Scholar]
- 67. Carroll KK. Summation: which fat/how much fat—animals. Prev Med 1987;16(4):510–5. [DOI] [PubMed] [Google Scholar]
- 68. Reddy BS. Dietary fat and colon cancer: animal models. Prev Med 1987;16(4):460–7. [DOI] [PubMed] [Google Scholar]
- 69. Day SD, Enos RT, McClellan JL, Steiner JL, Velazquez KT, Murphy EA. Linking inflammation to tumorigenesis in a mouse model of high-fat-diet-enhanced colon cancer. Cytokine 2013;64(1):454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O'Neill AM, Burrington CM, Gillaspie EA, Lynch DT, Horsman MJ, Greene MW. High-fat Western diet-induced obesity contributes to increased tumor growth in mouse models of human colon cancer. Nutr Res 2016;36(12):1325–34. [DOI] [PubMed] [Google Scholar]
- 71. Newmark HL, Lipkin M, Maheshwari N. Colonic hyperplasia and hyperproliferation induced by a nutritional stress diet with four components of western-style diet. J Natl Cancer Inst 1990;82(6):491–6. [DOI] [PubMed] [Google Scholar]
- 72. Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis 2001;22(11):1871–5. [DOI] [PubMed] [Google Scholar]
- 73. Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis 2009;30(1):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moore BJ. The cafeteria diet—an inappropriate tool for studies of thermogenesis. J Nutr 1987;117(2):227–31. [DOI] [PubMed] [Google Scholar]
- 75. Rothwell NJ, Stock MJ. The cafeteria diet as a tool for studies of thermogenesis. J Nutr 1988;118(8):925–8. [DOI] [PubMed] [Google Scholar]
- 76. Monsanto SP, Hintze KJ, Ward RE, Larson DP, Lefevre M, Benninghoff AD. The new total Western diet for rodents does not induce an overweight phenotype or alter parameters of metabolic syndrome in mice. Nutr Res 2016;36(9):1031–44. [DOI] [PubMed] [Google Scholar]
- 77. Nakanishi M, Chen Y, Qendro V, Miyamoto S, Weinstock E, Weinstock GM, Rosenberg DW. Effects of walnut consumption on colon carcinogenesis and microbial community structure. Cancer Prev Res (Phila) 2016;9(8):692–703. [DOI] [PubMed] [Google Scholar]
- 78. Ward RE, Benninghoff AD, Healy BJ, Li M, Vagu B, Hintze KJ. Consumption of the Total Western Diet differentially affects the response to green tea in rodent models of chronic disease compared to the AIN93G diet. Mol Nutr Food Res 2017;61:1600720. [DOI] [PubMed] [Google Scholar]
- 79. O'Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E. et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun 2015;6:6342. [DOI] [PMC free article] [PubMed] [Google Scholar]