Abstract
Background
The number of invasive group A Streptococcus (iGAS) infections due to hitherto extremely rare type emm74 strains has increased in several Canadian provinces since late 2015. We hypothesized that the cases recorded in the different provinces are linked and caused by strains of an emm74 clone that recently emerged and expanded explosively.
Methods
We analyzed both active and passive surveillance data for iGAS infections and used whole-genome sequencing to investigate the phylogenetic relationships of the emm74 strains responsible for these invasive infections country-wide.
Results
Genome analysis showed that highly clonal emm74 strains, genetically different from emm74 organisms previously circulating in Canada, were responsible for a country-wide epidemic of >160 invasive disease cases. The emerging clone belonged to multilocus sequence typing ST120. The analysis also revealed dissemination patterns of emm74 subclonal lineages across Canadian provinces. Clinical data analysis indicated that the emm74 epidemic disproportionally affected middle-aged or older male individuals. Homelessness, alcohol abuse, and intravenous drug usage were significantly associated with invasive emm74 infections.
Conclusions
In a period of 20 months, an emm74 GAS clone emerged and rapidly spread across several Canadian provinces located more than 4500 km apart, causing invasive infections primarily among disadvantaged persons.
Keywords: Canada, emerging strain genotype, epidemic, group A Streptococcus, homeless, invasive disease, outbreak, populations at risk
Group A Streptococcus (GAS) causes a wide variety of diseases, ranging from relatively mild pharyngitis and superficial skin infections to life-threatening invasive diseases such as necrotizing fasciitis and toxic shock syndrome [1]. Recovering patients may sometimes suffer from serious postinfection sequelae, such as rheumatic fever and glomerulonephritis [2]. GAS strains are differentiated into more than 240 types based on the DNA sequence of the hypervariable 5’ end of gene emm encoding M protein, a major virulence factor with antiphagocytic properties [3–5]. Despite this diversity, less than 30 emm types appear to be responsible for the majority of GAS disease worldwide [6]. Cyclical patterns of change in emm type distribution in a community— appearance, transient or more persistent dominance of particular emm types, and eventual replacement by other emm types—are common features of GAS epidemiology, as are unexpected emergence and spread of rare emm types, which can sometimes lead to increases in invasive GAS (iGAS) disease incidence [7–12]. A recent example was the sudden emergence and rapid dissemination across Canada and vast areas of the United States of a type emm59 clone that caused hundreds of iGAS infections in both countries in the period 2008–2015 [13–20].
In early 2016, an outbreak of iGAS infections was declared in a 543-bed shelter for homeless men in Toronto, Canada [21]. Most of the cases recorded during the outbreak were caused by strains of type emm74 GAS, hitherto exceedingly rare in Canada. Here, we report that emm74 iGAS disease cases have since dramatically increased across the country, with more than 160 emm74 iGAS cases recorded in several Canadian provinces by June 2017. To better understand emm74 iGAS emergence and spread across Canada, we analyzed available clinical data from patients identified by population-based surveillance for iGAS diseases, in combination with whole-genome sequencing analysis of the emm74 isolates responsible for these infections.
METHODS
Bacterial Isolate Collection and Case Definition
In Canada, laboratory surveillance of iGAS disease is primarily a passive system where isolates are forwarded by provincial public health laboratories to the National Microbiology Laboratory (NML), Public Health Agency of Canada. System limitations include variable regional standards, availability of bacterial isolates for testing, and over-representation of more invasive strains for which medical treatment was sought. However, data gathered by passive laboratory surveillance account for >90% of all iGAS cases reported in the National Notifiable Disease Surveillance System. In metropolitan Toronto and Peel region (henceforth the greater Toronto area), the Toronto Invasive Bacterial Diseases Network (TIBDN) operates an active surveillance program for iGAS disease (sensitivity estimated at 95%) that includes all hospitals and microbiology laboratories in the area. Our bacterial collection comprised 1 invasive isolate per each of the 168 patients with emm74 iGAS disease recorded nationally during the period May 2012 to June 2017. Isolates were from the provinces of Alberta (n = 25), British Columbia (n = 18), Quebec (n = 11), Saskatchewan (n = 2), and Ontario (n = 112, of which 54 were collected by passive laboratory surveillance, and 58 by TIBDN active surveillance; there was no overlap) (Supplementary Table 1). iGAS disease cases met the following criteria: (1) acute illness in association with isolation of GAS from a normally sterile site or (2) isolation of GAS from a nonsterile site (eg, skin, sputum) in the presence of confirmed or probable streptococcal toxic shock syndrome and/or soft tissue necrosis (including necrotizing fasciitis), meningitis, or death [22]. A temporally matched collection of 27 emm74 isolates recovered from infections that did not meet the criteria for iGAS disease (ie, superficial wounds or skin lesions) was also included (Supplementary Table 1). In addition, we included 6 historic emm74 iGAS isolates recovered in the 1990s and 2000s (Supplementary Table 1). Isolates were cultured on Columbia blood agar plates containing 5% sheep blood or in Todd-Hewitt broth supplemented with 0.2% yeast extract at 37°C with 5% CO2. DNA was prepared from overnight cultures using the QIAamp DNA minikit (Qiagen, Toronto, ON, Canada). emm typing was performed by polymerase chain reaction and DNA sequencing, as previously described [23]. Data were compared with sequences available at the US Centers for Disease Control and Prevention emm database (ftp://ftp.cdc.gov/pub/infectious_diseases/biotech/tsemm/).
Clinical Data Collection
Clinical data for the 168 emm74 iGAS cases were limited to patient age and sex, date of infection, and anatomical source of isolation of the strain, with the exception of clinical data collected by TIBDN (58 type emm74 iGAS cases and a temporally matched cohort of 54 emm1 iGAS cases), which also included disease presentation and outcomes, as well as underlying diseases or conditions that might have predisposed patients to invasive disease (eg, alcohol abuse, chronic underlying organ system disease, immunosuppression, homelessness, history of illicit drug use). Extended data collection and analysis were approved by the Research Ethics Boards of all participating TIBDN institutions.
Whole-Genome Sequencing, Closure of Reference Genome, Bioinformatics, and Phylogenetic Analysis
The genome of 1 randomly chosen emm74 isolate (strain NGAS979) was sequenced to closure using a combination of single-molecule real-time sequencing (Pacific Biosciences, Menlo Park, CA) and Illumina sequencing (Illumina, San Diego, CA). The genomes of 129 additional emm74 iGAS isolates, recovered between 2012 and 2017 (ie, 100% of the Quebec, Saskatchewan, and British Columbia isolates and 96% and 66% of the Alberta and Ontario iGAS isolates, respectively), were sequenced using Illumina technology. The genomes of the 6 abovementioned historic iGAS emm74 isolates and 11 of the 27 (41%) noninvasive emm74 isolates from Ontario and New Brunswick were also sequenced, for a total of 147 emm74 genomes (Supplementary Table 1). The A5 pipeline was used for de novo assembly of Illumina-generated sequences [24]. Single nucleotide polymorphisms (SNPs) and short insertions/deletions (indels) were identified relative to the genome of strain NGAS979 using VAAL [25] and/or Nucmer [26]. Whole-genome and/or core genome SNPs were used to construct neighbor-joining phylogenetic trees (1000 bootstrap replications) using SplitsTree4 [27]. Contigs were annotated with Prokka [28]. Recombination was evaluated using BRATNextGen [29]. Genome visualizations were created using BRIG [30]. Detailed bioinformatics methods are presented in the Supplementary Methods.
Statistical Analysis
Differences between groups were compared using the χ2 or Fisher exact test. The odds ratios (ORs) and 95% confidence intervals were calculated using SAS software, version 9.3 (SAS Institute). P values <.05 were considered statistically significant.
RESULTS
Dramatic Increase of Invasive emm74 Gas Disease in Canada Since 2015
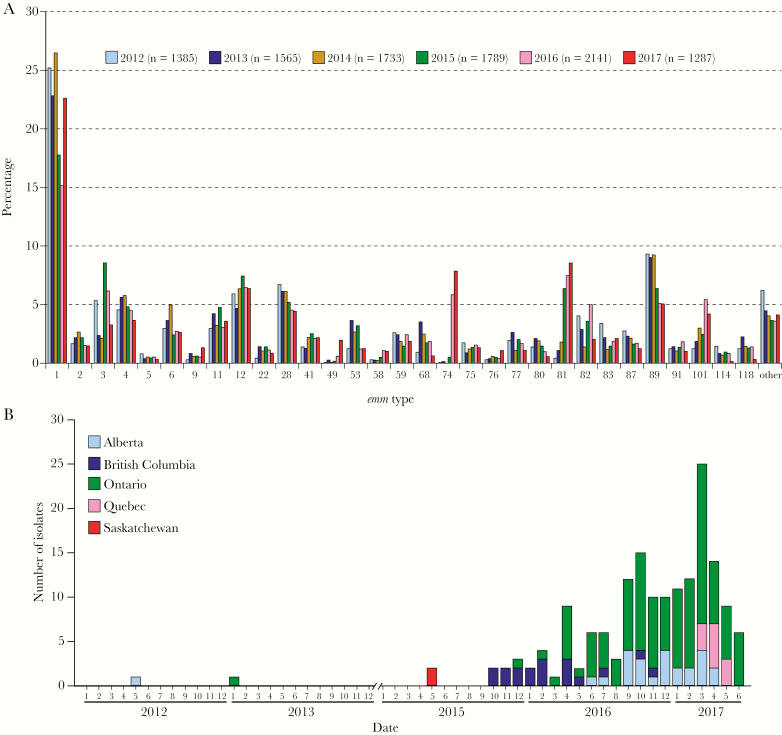
Analysis of national surveillance data identified only 2 emm74 isolates among the 4683 iGAS infections recorded in Canada between 2012 and 2014 (Figure 1A). Together with a recent investigation that found no emm74 isolates among a collection of 1454 iGAS isolates recovered in 2015 by the Active Bacterial Core Surveillance of the US Centers for Disease Control and Prevention [20], the data indicate that emm74 GAS has been very rare in North America. Surprisingly, in early 2016, an outbreak of GAS infections was declared in a 543-bed shelter for homeless men in Toronto that involved numerous type emm74 infections [21], including 6 invasive cases. Since the outbreak, both active (TIBDN) and passive (Public Health Ontario Laboratory, PHOL) surveillance systems in the province of Ontario began to identify increased numbers of emm74 iGAS isolates in the greater Toronto area and in other urban municipalities such as London and Peterborough, as well as in rural areas of Northwestern Ontario. The upsurge in emm74 iGAS isolations prompted us to retrospectively analyze national surveillance data. We identified 9 other emm74 iGAS cases that occurred in 2015 (ie, prior to the Toronto outbreak) in British Columbia (n = 6), Ontario (n = 1), and Saskatchewan (n = 2) (Figure 1B). During 2016, emm74 iGAS infections continued to be recorded in relatively high numbers in Ontario and British Columbia, and were noted in Alberta. By the end of 2016, emm74 organisms were the fifth most common cause of iGAS disease in the country (Figure 1A). In the first 6 months of 2017 covered by this investigation, numerous additional emm74 iGAS cases were recorded in Ontario and Alberta, and emm74 iGAS disease was first reported in Quebec (Figure 1B), with a local emm74 iGAS outbreak among homeless people declared in Montreal and adjacent municipalities. Thus, since 2015, emm74 iGAS disease has increasingly been reported across the country in geographical areas located more than 4500 km apart.
Figure 1.
Canada-wide expansion of emm74 group A Streptococcus invasive disease. A, Isolation of invasive group A Streptococcus (iGAS) isolates in all Canadian provinces and territories from January 2012 to June 2017. Bars show emm type distribution as a percentage of the total number of isolates in each year (first 6 months for year 2017). The percentage of emm74 iGAS isolates, which were rarely seen in Canada, strikingly increased starting in 2015, spiked in 2016, and continued to be isolated in high numbers by June 2017, causing thus far more than 160 invasive cases in several Canadian provinces. B, Geographical origin and temporal distribution of 168 emm74 isolates recovered in Canada from individual patients with emm74 iGAS disease. For the period 2012 to 2014, only 2 emm74 iGAS cases were recorded in Canada, 1 in the province of Alberta (May 2012) and 1 in the province of Ontario (January 2013). No further emm74 invasive disease cases were observed until 2 years later, when 2 emm74 iGAS strains were isolated in Saskatchewan during the second quarter of 2015. Then, beginning in late 2015 and continuing to June 2017, increasing numbers of emm74 iGAS were isolated in the provinces of British Columbia, Ontario, Alberta, and Quebec.
Basic Demographic Characteristics of Patients With emm74 iGAS Disease in Canada Since 2012
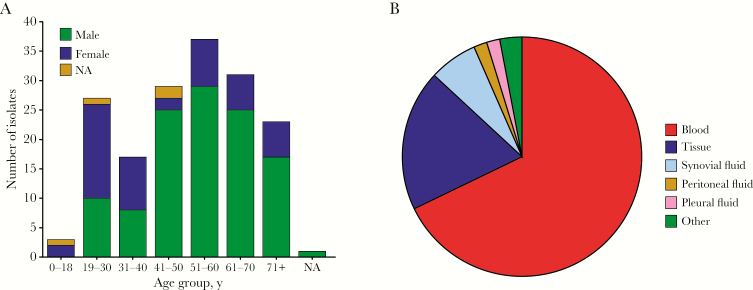
The median age of the 168 patients with emm74 iGAS disease (range) was 52 (11–93) years, and only 3 cases (2%) occurred in children under 18 years of age (Supplementary Table 1). A majority of cases (n = 115, 70.1%) occurred in men. The primary anatomical source from which emm74 iGAS isolates were recovered was blood (n = 114, 67.9%), followed by abscesses or soft tissue specimens (n = 32, 19%) and synovial fluid (n =11, 6.5%) (Figure 2B).
Figure 2.
Demographics of patients with type emm74 invasive disease in Canada and source of isolation of invasive emm74 group A Streptococcus invasive strains responsible for these infections. A, Age and sex of patients with emm74 iGAS disease in Canada for the period January 2012 to June 2017. Sex data were unavailable for 4 patients, and age was unavailable for 1 (indicated by NA). Overall, the distribution indicates that emm74 iGAS disease disproportionately affects adult middle-aged or older males. B, Anatomical source of isolation of the 168 emm74 iGAS isolates. Data are shown as percentage of the total number of isolates. The majority (n = 114, 67.9%) of isolates were recovered from blood, followed by abscesses and soft tissue (n = 32, 19%), synovial fluid (n = 11, 6.5%), peritoneal fluid (n = 3, 1.8%), and pleural fluid (n = 3, 1.8%). Five isolates (3%) were recovered from undetermined aspirates or from surgical specimens, collectively defined as “other” in the figure legend.
Emergence and Rapid Expansion of a Genetically Distinct emm74 Clone
We used genomics to test the hypothesis that the emm74 epidemic is due to the rapid expansion of a recently emerged emm74 genotype. We first sequenced to closure the genome of a randomly selected emm74 invasive organism (strain NGAS979, henceforth the reference strain, isolated in Ontario in 2016). The genome (GenBank accession number CP028140) was a circular chromosome of 1 790 938 bp (38.6% G+C content). A total of 1748 coding sequences, 8 prophage or prophage-like remnants, and 1 integrative conjugative element (ICE), some of which were integrated in well-described sites [31], were identified in the genome of the reference strain (Figure 3A; Supplementary Table 2). The organism was assigned to multilocus sequence typing ST120.
Figure 3.
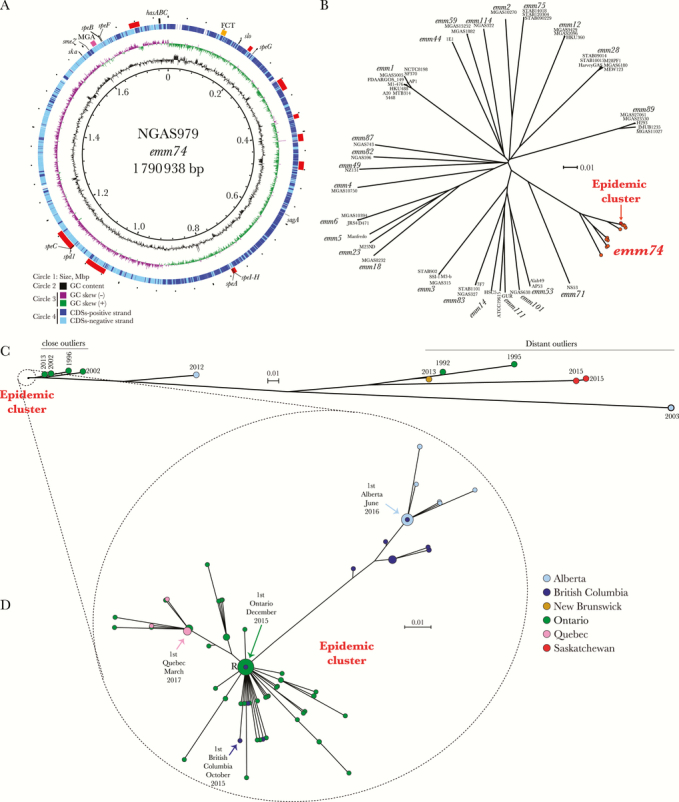
Genomic features and phylogenetic relationships of emm74 invasive group A Streptococcus isolates. A, Genome atlas of emm74 group A Streptococcus (GAS) reference strain NGAS979. Data from innermost to outermost circles in the atlas are described in the figure legend, with the exception of the outermost circle, which depicts genome landmarks such as mobile genetic element (prophages, prophage remnants, and integrative-conjugative elements, indicated by red boxes) and virulence genes, including those encoding superantigens. The hasABC locus is indicated by a black box, the FCT locus by a yellow box, and the mga regulon by a dark pink box. GC skew -or (G-C)/(G+C)- is averaged over a moving window of 10 000 bp. B, Inferred phylogenetic relationships between emm74 GAS strains and 55 strains of 25 other emm types, for which complete genome sequences are available in GenBank. A neighbor-joining phylogenetic tree was constructed using 76 311 nonredundant biallelic single nucleotide polymorphism (SNP) loci identified in the genomes of the strains relative to the core genome of the reference emm74 strain NGAS979. Strain names and emm types for non-emm74 strains are indicated at the tip of the branches. The reference emm74 strain and all 136 emm74 invasive and noninvasive isolates recovered since October 2015 in Canada clustered tightly in a discrete epidemic cluster (indicated in red). All other emm74 isolates from Canada are depicted in orange. C, Inferred phylogenetic relationship among 147 emm74 GAS strains from Canada. A neighbor-joining phylogenetic tree was constructed using 10 224 nonredundant biallelic SNP loci identified in the genomes of the emm74 isolates relative to the core genome of the reference emm74 strain NGAS979. The 136 emm74 GAS isolates recovered in Canada since October 2015 for which we generated genome data (the epidemic cluster) are monoclonal and genetically distinct from previously isolated emm74 strain SNPs. We arbitrarily divided these nonepidemic emm74 isolates into 3 groups: a first group of “close outliers,” comprising 4 isolates from Ontario in 1996, 2002, and 2013, a more distantly related single isolate from Alberta isolated in 2012, a group of 5 highly divergent “distant outlier” emm74 isolates recovered in Alberta, Saskatchewan, and Ontario from invasive infections, and 1 noninvasive isolate from New Brunswick recovered in 2013. Provinces are indicated by different colors, as per the caption in (D). D, Diversification of the emm74 GAS epidemic clone. The neighbor-joining phylogenetic tree was constructed using 71 nonredundant SNP loci identified among the 136 epidemic emm74 isolates relative to the genome of the reference strain NGAS979. The circles are colored to indicate province of isolation, as per the figure caption, and their sizes are proportional to the number of isolates with identical genotypes. The location in the phylogenetic tree of the first emm74 invasive isolate in each province is indicated by an arrow. Abbreviations: CDS, coding DNA sequence; GC, Guanine-cytosine.
We next sequenced the genomes of 146 additional emm74 strains (129 from iGAS infections and 11 from noninvasive infections) recorded from 2012 to June 2017 in Canada, as well as the genomes of 6 historic emm74 iGAS isolates from the 1990s and 2000s recovered in Ontario and Alberta (Supplementary Table 1 lists Sequence-Read Archive [SRA] accession numbers). Whole-genome SNP-based phylogenetic analysis of newly sequenced emm74 genomes and all 55 complete GAS genomes available in GenBank (as of October 2017, representing 25 other emm types) indicated that emm74 organisms are a discrete GAS subpopulation and that they are more closely related to an emm71 isolate associated with skin colonization [32] than to any other emm type (Figure 3B). As a population, emm74 strains were relatively genetically diverse. However, consistent with our hypothesis, all analyzed emm74 iGAS strains isolated in Canada since October 2015 (n = 136) were highly clonal and clustered tightly in a phylogenetic tree (Figure 3B, in red, and C). In addition to this cluster (henceforth “the epidemic clone”), minor emm74 subpopulations were identified. These included “close outlier” strains from Ontario (n = 4, isolated in 1996, 2002, and 2013), 1 isolate from Alberta recovered in 2012, and 6 “distant outlier” strains from Ontario (n = 2, isolated in 1992 and 1995), Saskatchewan (n = 2, isolated in 2015), Alberta (n = 1, isolated in 2003), and the 2013 noninvasive emm74 strain from New Brunswick (Figure 3C).
The lack of sequence identity with other GAS emm types (Figure 3B) suggests that the different emm74 populations, and the epidemic clone in particular, have not arisen through emm type switching. Bayesian analysis of polymorphism distribution did not identify obvious signals of acquisition of foreign genetic material by the epidemic clone by means of recombination (Supplementary Figure 1). All emm74 strains possessed the genes speA, speG, speH, speI, and smeZ, encoding exotoxins (Table 1). In addition, the epidemic clone and “close and distant outlier” strains from Ontario possessed the exotoxin-encoding gene speC and the DNase-encoding gene spd1. However, speC and sdp1 were carried by different prophages in the different groups (Supplementary Figure 2). In comparison with “close outlier” strains, the epidemic clone had 1 SNP in the surface protein–encoding epf gene predicted to result in a D929E amino acid substitution in the predicted translated sequence of this protein factor involved in keratinocyte adhesion and internalization [33]. Additionally, “close outliers” and epidemic strains differed by the presence of nonsynonymous SNPs in the 2 component regulator–encoding gene vicR, in sagD, a gene involved in streptolysin production, and in the global regulator–encoding gene ropB. Additional work will be needed to evaluate whether these or other additional SNPs (Supplementary Table 3) play a role in the virulence, fitness, or other biological traits of the epidemic clone.
Table 1.
Superantigen- and Other Virulence Factor–Encoding Genes Found in the Different emm74 Genotypes Circulating in Canada
| Genotype | Strain | Superantigen-Encoding Gene | Virulence Factor-Encoding Gene | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| speA | speC | speG | speH | speI | speJ | speK | speL | speM | smeZ | ssa | slaA | spd1 | spd3 | speB | ||
| Epidemic clone (n = 136) | NGAS979 and 135 others | + | + | + | + | + | - | - | - | - | + | - | - | + | -a | + |
| Ontario close outliers (n = 4) | 8198, 22173, 22351, NGAS664 | + | + | + | + | + | - | - | - | - | + | - | - | + | - | + |
| Ontario distant outliers (n = 2) | 1018, 5870 | + | + | + | + | + | - | - | - | - | + | - | - | + | - | + |
| Alberta 2012 (n = 1) | SC173172 | + | - | + | + | + | - | + | - | - | + | - | + | - | - | + |
| Saskatchewan distant outliers (n = 2) | SC152081, SC152083 | + | - | + | + | + | - | + | - | - | + | - | + | - | - | + |
| New Brunswick distant outlier (n = 1) | SC132849 | + | - | + | + | + | - | - | - | - | + | - | - | - | + | + |
| Alberta 2003 (n = 1) | SC173171 | + | - | + | + | + | - | - | - | - | + | - | - | - | + | + |
aOne epidemic isolate was positive for spd3.
Transmission Patterns of the Epidemic emm74 Clone
As a population, the invasive and noninvasive emm74 epidemic isolates recovered from October 2015 to June 2017 for which we generated genome data had only 92 nonredundant polymorphic loci relative to the core genome of the reference strain (Supplementary Table 4). Indels accounted for 21 of these loci. The vast majority of indels (n = 19, 90%) were isolate specific, and although they ranged in size from 1 to 126 bp, most (n = 15, 71%) were a single nucleotide event. The remaining 71 polymorphisms were biallelic SNPs, of which 12 occurred in noncoding regions. Predicted coding sequences accounted for 85.6% of the NGAS979 core genome, and a relatively similar percentage (83.1%) of the core SNPs occurred in coding sequences. Nonsynonymous SNPs accounted for 59% (n = 35/59 coding SNPs), while synonymous SNPs accounted for 41% (n = 24/59). Most of the SNPs were strain specific, and only a small percentage the SNP loci (n = 18, 25.4% of total SNP loci) were present in 2 or more of the epidemic strains and therefore were phylogenetically informative (Supplementary Table 4). Despite the minimal genetic differences, when analyzed together with temporal data, the inferred phylogenetic relationships permitted us to conclude that (1) older (late 2015 and early 2016) epidemic strains causing disease in British Columbia and Ontario constitute a single emm74 subclone; (2) Alberta isolates are likely derived from a second emm74 GAS subclone previously circulating in British Columbia; and (3) the Quebec isolates are a genetic emm74 sublineage whose origin can be traced back to strains circulating in Ontario (Figure 3D).
Risk Factors for Epidemic emm74 Disease
Centralized GAS typing at the provincial and national levels permits efficient tracking of iGAS epidemiology in Canada. However, capture of comprehensive patient clinical data for iGAS infections enabling enhanced investigation of epidemics is limited to select areas. Here, we used data from TIBDN active surveillance to assess emm74 iGAS disease characteristics and patient risk factors. While the geographical area covered by TIBDN is limited to the metropolitan Toronto-Peel region, TIBDN isolates represented almost 35% (n = 58/168) of the total number of epidemic invasive cases recorded in the country since October 2015. Initial inspection of the data revealed that most emm74 iGAS cases presented primarily as soft tissue infections, followed by arthritis and bacteremia without focus (Table 2). We next compared the subset of patients with emm74 iGAS disease with a matched cohort comprising all patients with emm1 iGAS disease (n = 54) in the same population area during the same time period (December 2015 to June 2017). The analysis showed that compared with emm1 iGAS disease, emm74 iGAS disease was significantly more associated with non-necrotizing fasciitis soft tissue infections (P = .012) and significantly less associated with respiratory tract infections (P < .0001) (Table 2). Proportionally fewer patients with emm74 iGAS disease had streptococcal toxic shock syndrome, and significantly fewer required intensive care unit admission (P = .05) (Table 2). Homelessness, alcohol abuse, and intravenous drug use were significantly associated with emm74 iGAS disease (Table 2).
Table 2.
Clinical Features of Invasive Group A Streptococcal Disease due to emm74 and emm1 in Residents of Metropolitan Toronto and Peel Region, Canada, 12/2015–5/2017
| No. (%) of Isolates | P Value | Odds Ratio (95% CL) | ||
|---|---|---|---|---|
| emm74 (N = 58) | emm1 (N = 54) | |||
| Age group, y | ||||
| 0–18 | 0 | 11 (20) | .0002 | |
| 19–40 | 13 (22) | 11 (20) | .8 | |
| 41–60 | 22 (38) | 9 (17) | .02 | |
| ≥61 | 23 (40) | 23 (43) | .9 | |
| Male sex | 38 (66) | 30 (56) | .3 | |
| Risk factor for infection | ||||
| Alcohol abuse | 12 (21) | 2 (3.7) | .009 | 6.8 (1.4–32) |
| Homeless | 17 (29) | 0 (0) | <.0001 | N/A |
| IV drug user | 13 (22) | 1 (1.9) | .001 | 15 (1.9–122) |
| Underlying chronic disease | ||||
| Any | 53 (91) | 40 (74) | .02 | 3.7 (1.2–11) |
| Cardiac disease | 9 (16) | 10 (19) | .8 | |
| Diabetes mellitus | 11 (19) | 6 (11) | .3 | |
| Malignancy (last 2 y) | 5 (8.6) | 5 (9.3) | 1.0 | |
| HIV infection | 3 (5.2) | 0 (0) | .2 | |
| Clinical presentationa | ||||
| Soft tissue infection (all) | 31 (53) | 19 (35) | .06 | |
| Non-necrotizing fasciitis | 29 (50) | 14 (26) | .01 | 2.9 (1.3–6.3) |
| Necrotizing fasciitis | 2 (3.4) | 5 (9.3) | .3 | |
| Respiratory tract infection | 4 (6.9) | 22 (41) | .0001 | 0.11 (0.03–0.34) |
| Bacteremia without focus | 9 (16) | 9 (17) | 1.0 | |
| Arthritis | 10 (17) | 7 (13) | .6 | |
| Otherb | 6 (10) | 5 (9.3) | 1.0 | |
| Streptococcal toxic shock syndrome | 8 (14) | 12 (22) | .3 | |
| Outcomes | ||||
| ICU admission | 17 (29) | 26 (48) | .05 | 0.45 (0.20–0.97) |
| Death | 5 (8.6) | 6 (11) | .8 | |
Abbreviations: CL, confidence limit; ICU, intensive care unit; IV, intravenous.
aTotals are greater than the number of cases because patients may present with more than 1 site of infection.
bOther includes 4 cases of osteomyelitis (2 each emm74 and emm1); 2 of peritonitis (1 each emm74 and emm1), and 1 case each of endocarditis (emm74), mastoiditis/meningitis (emm1), peripartum infection (emm1), other gynecologic infection (emm74), and urinary tract infection (emm74).
DISCUSSION
Emergence and rapid spread of a novel emm74 clone has caused more than 160 iGAS disease episodes in Canada since October 2015. Available data showed that cases were primarily associated with soft tissue infections and arthritis and that the disease disproportionally affected older middle-aged males and the homeless population. Alcohol abuse and intravenous drug use were common among patients with emm74 iGAS disease. Overall, similar disease manifestations and patient risk factors were identified in a previous emm59 GAS epidemic that occurred Canada-wide in 2006–2010 [17], suggesting that a disadvantaged population continues to be at higher risk for GAS soft tissue infections.
Recent reports have described GAS outbreaks affecting homeless populations that were caused by strains of emm types 82, 83, 87, 101, and 114 in Canada [7], 44 in France [34], and 32 and 66 in Great Britain [35, 36]. One common theme between the current emm74 and prior emm59 epidemics, as well as in these other reports, is that the offending GAS strains belong to emm types of pattern D (skin tropism) or E (generalist, ie, both throat and skin tropism) [37, 38]. However, while strain genetics is expected to play a role in the observed overabundance of skin and soft tissue infections among homeless patients, it is unlikely to be the only factor. For example, a recent outbreak among the homeless population in Alaska that was also characterized by a high incidence of soft tissue infections was caused by emm26 GAS strains (ie, a pattern A-C emm type with throat tropism) [12]. Thus, the poor environmental and hygienic conditions commonly associated with homelessness and homeless shelters (crowding, poor sanitation, frequent skin breakdown), the weak immune response to GAS in the skin [39], and/or the underlying alcohol and intravenous drug use are undoubtedly major contributors to the overabundance of soft tissue infections among homeless patients.
Our genomic data unambiguously show that the current epidemic has been caused by emm74 GAS organisms that are genetically homogeneous, with epidemic strains differing from one another on average by fewer than 5 genetic polymorphisms genome-wide. In addition to the main clinical outcomes, another key similarity between the current emm74 and previous emm59 [13, 18, 19] Canadian epidemics is the fact that both were caused by highly clonal strains that spread very rapidly across vast geographic areas. During the first 5 years of the emm59 iGAS disease epidemic, emm59-specific pharyngitis was rarely observed, leading to the notion that emm59 organisms had a restricted population size [18]. Similarly, emm74 organisms appear to be also very rarely associated with pharyngitis [40]. Thus, a restricted emm74 population size may be 1 reason for the minimal strain genetic variation reported here. However, compared with the emm59 epidemic reports [13, 18, 19], less time has elapsed between the inception of the emm74 epidemic and our analysis (22 months). Thus, the relatively short time involved in the current epidemic is also likely a factor explaining the very modest genetic diversity observed among emm74 strains. Although minimal, genetic differences among strains revealed by whole-genome-based phylogenetic analysis permitted us to identify clear patterns of interprovincial emm74 GAS subclone dissemination.
Genomic investigations can provide definite proof that introduction of novel GAS clones within a naïve population can lead to a rapid increase in iGAS incidence [9, 41, 42]. Genomics identified that a single epidemic clone was responsible for the prior Canada-wide emm59 epidemic, and as no cases of emm59 iGAS disease had occurred in the country in the approximately 20 years preceding the epidemic [18, 19], this clone was most likely introduced from abroad. Similarly, it can be hypothesized that the epidemic emm74 clone has recently been introduced to Canada from elsewhere. Although rare in North America and Europe, emm74 iGAS disease, including clusters of emm74 infections, has been described in Africa, India, New Zealand, New Caledonia, and Hawaii [8, 20, 40, 43–46]. We were unable to obtain isolates or genomic data for emm74 organisms isolated in these other geographic locations to test this hypothesis. However, in contrast to the emm59 epidemic, other emm74 genotypes were circulating in Canada prior to the sudden emergence of the emm74 epidemic clone. It might be possible that the epidemic emm74 clone originates from one of these prior, relatively closely related genotypes, which by acquisition of additional genetic material now possess enhanced ability to spread and/or to cause disease. Despite extended comparisons of the genome sequences of the epidemic clone and other emm74 genotypes, we were unable to identify obvious gains of genetic regions or single genes encoding known or putative virulence factors by the epidemic clone. However, the epidemic clone and older emm74 genotypes from Ontario possessed the virulence factor–encoding genes speC and spd1, carried on different prophages (Supplementary Figure 2). It might be possible that speC and spd1 are differentially regulated in the epidemic clone and that this results in epidemic emm74 strains that have enhanced ability to spread. Recently, an emm3 GAS lineage that acquired a prophage carrying the speC and spd1 genes was found to be responsible for an upsurge in iGAS infections in the United Kingdom [47]. Further experiments are needed to test this hypothesis.
In summary, we describe here a large epidemic of invasive GAS disease affecting primarily a specific group of disadvantaged patients. The epidemic is caused by strains of a single emm74 GAS clone that expanded across 4 Canadian provinces in a very short period of time. Our unpublished surveillance data indicate that the epidemic is still ongoing, and we have very recently become aware of additional emm74 iGAS cases in other Canadian jurisdictions. Based on the similarities with a previous emm59 epidemic [13, 18, 19], further spread of the emm74 epidemic clone into additional areas of Canada and/or into the United States is likely. Continued monitoring for invasive emm74 GAS infections is warranted.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the staff at the different diagnostic microbiology laboratories across Canada for their continuous efforts in identifying and forwarding iGAS isolates to provincial public health laboratories. We are grateful to Chin-Yu (Amanda) Hong, Jeffrey Li, and Wallis Rudnick (TIBDN), and to Alex Marchand-Austin, Kirby Cronin, and Vithusha Ravirajan (PHOL) for their help with isolate database curation. We also thank Averil Griffith, Karla Montes, and Ravinder Singh (NML, Public Health Agency of Canada, Winnipeg) for technical support. We are grateful to the staff at PHOL Genome Core and NML Genomics Core for Illumina genome sequencing of the emm74 isolates and to the staff of the Alberta Provincial Laboratory for Public Health for emm typing of GAS isolates from Alberta.
Financial support. This work was supported in part by Public Health Ontario through internal grant RRB-18-008 to N.F. and by internal funding from the Public Health Agency of Canada.
Potential conflicts of interest. The authors declare that they do not have conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Olsen RJ, Musser JM. Molecular pathogenesis of necrotizing fasciitis. Annu Rev Pathol 2010; 5:1–31. [DOI] [PubMed] [Google Scholar]
- 2. Soderholm AT, Barnett TC, Sweet MJ, Walker MJ. Group A streptococcal pharyngitis: immune responses involved in bacterial clearance and GAS-associated immunopathologies. J Leukoc Biol. 2018; 103:193–213. [DOI] [PubMed] [Google Scholar]
- 3. Lancefield RC. The antigenic complex of Streptococcus haemolyticus: I. Demonstration of a type-specific substance in extracts of Streptococcus haemolyticus. J Exp Med 1928; 47:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scott JR, Pulliam WM, Hollingshead SK, Fischetti VA. Relationship of M protein genes in group A streptococci. Proc Natl Acad Sci U S A 1985; 82:1822–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manjula BN, Acharya AS, Fairwell T, Fischetti VA. Antigenic domains of the streptococcal Pep M5 protein. Localization of epitopes crossreactive with type 6 M protein and identification of a hypervariable region of the M molecule. J Exp Med 1986; 163:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steer AC, Law I, Matatolu L, et al. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis 2009; 9:611–6. [DOI] [PubMed] [Google Scholar]
- 7. Athey TB, Teatero S, Sieswerda LE, et al. High incidence of invasive group A Streptococcus disease caused by strains of uncommon emm types in Thunder Bay, Ontario, Canada. J Clin Microbiol 2016; 54:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Erdem G, Abe L, Kanenaka RY, et al. Characterization of a community cluster of group a streptococcal invasive disease in Maui, Hawaii. Pediatr Infect Dis J 2004; 23:677–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner CE, Abbott J, Lamagni T, et al. Emergence of a new highly successful acapsular group A Streptococcus clade of genotype emm89 in the United Kingdom. MBio 2015; 6:e00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu L, Olsen RJ, Nasser W, et al. A molecular trigger for intercontinental epidemics of group A Streptococcus. J Clin Invest 2015; 125:3545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sumby P, Porcella SF, Madrigal AG, et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events. J Infect Dis 2005; 192:771–82. [DOI] [PubMed] [Google Scholar]
- 12. Mosites E, Frick A, Gounder P, et al. Outbreak of invasive infections from subtype emm26.3 group A Streptococcus among homeless adults-Anchorage, Alaska, 2016–2017. Clin Infect Dis. 2018; 66:1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fittipaldi N, Olsen RJ, Beres SB, et al. Genomic analysis of emm59 group A Streptococcus invasive strains, United States. Emerg Infect Dis 2012; 18:650–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown CC, Olsen RJ, Fittipaldi N, et al. Spread of virulent group A Streptococcus type emm59 from Montana to Wyoming, USA. Emerg Infect Dis 2014; 20:679–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olsen RJ, Fittipaldi N, Kachroo P, et al. Clinical laboratory response to a mock outbreak of invasive bacterial infections: a preparedness study. J Clin Microbiol 2014; 52:4210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Engelthaler DM, Valentine M, Bowers J, et al. Hypervirulent emm59 clone in invasive group A Streptococcus outbreak, Southwestern United States. Emerg Infect Dis 2016; 22:734–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tyrrell GJ, Lovgren M, St Jean T, et al. Epidemic of group A Streptococcus M/emm59 causing invasive disease in Canada. Clin Infect Dis 2010; 51:1290–7. [DOI] [PubMed] [Google Scholar]
- 18. Fittipaldi N, Beres SB, Olsen RJ, et al. Full-genome dissection of an epidemic of severe invasive disease caused by a hypervirulent, recently emerged clone of group A Streptococcus. Am J Pathol 2012; 180:1522–34. [DOI] [PubMed] [Google Scholar]
- 19. Fittipaldi N, Tyrrell GJ, Low DE, et al. Integrated whole-genome sequencing and temporospatial analysis of a continuing group A Streptococcus epidemic. Emerg Microbes Infect 2013; 2:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chochua S, Metcalf BJ, Li Z, et al. Population and whole genome sequence based characterization of invasive group A Streptococci recovered in the United States during 2015. MBio 2017; 8:e01422-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finkelstein M, McGeer A, Sachdeva H, et al. Outbreak of group A Streptococcus (GAS) in a shelter for homeless men. Paper presented at: AMMI Canada-CACMID 2017 Annual Conference; May 3–6, 2017; Toronto, ON, Canada. [Google Scholar]
- 22. Davies HD, McGeer A, Schwartz B, et al. Invasive group A streptococcal infections in Ontario, Canada. Ontario group A Streptococcal Study Group. N Engl J Med 1996; 335:547–54. [DOI] [PubMed] [Google Scholar]
- 23. Beall B, Gherardi G, Lovgren M, et al. emm and sof gene sequence variation in relation to serological typing of opacity-factor-positive group A streptococci. Microbiology 2000; 146:1195–209. [DOI] [PubMed] [Google Scholar]
- 24. Tritt A, Eisen JA, Facciotti MT, Darling AE. An integrated pipeline for de novo assembly of microbial genomes. PLoS One 2012; 7:e42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nusbaum C, Ohsumi TK, Gomez J, et al. Sensitive, specific polymorphism discovery in bacteria using massively parallel sequencing. Nat Methods 2009; 6:67–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delcher AL, Kasif S, Fleischmann RD, et al. Alignment of whole genomes. Nucleic Acids Res 1999; 27:2369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 2006; 23:254–67. [DOI] [PubMed] [Google Scholar]
- 28. Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014; 30:2068–9. [DOI] [PubMed] [Google Scholar]
- 29. Marttinen P, Hanage WP, Croucher NJ, et al. Detection of recombination events in bacterial genomes from large population samples. Nucleic Acids Res 2012; 40:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 2011; 12:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beres SB, Musser JM. Contribution of exogenous genetic elements to the group A Streptococcus metagenome. PLoS One 2007; 2:e800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bao YJ, Li Y, Liang Z, et al. Comparative pathogenomic characterization of a non-invasive serotype M71 strain Streptococcus pyogenes NS53 reveals incongruent phenotypic implications from distinct genotypic markers. Pathog Dis 2017; 75:ftx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Linke C, Siemens N, Oehmcke S, et al. The extracellular protein factor Epf from Streptococcus pyogenes is a cell surface adhesin that binds to cells through an N-terminal domain containing a carbohydrate-binding module. J Biol Chem 2012; 287:38178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cady A, Plainvert C, Donnio P-, et al. Clonal spread of Streptococcus pyogenesemm44 among homeless persons, Rennes, France. Emerg Infect Dis 2011; 17:315–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cornick JE, Kiran AM, Vivancos R, et al. Epidemiological and molecular characterization of an invasive group A Streptococcus emm32.2 outbreak. J Clin Microbiol 2017; 55:1837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bundle N, Bubba L, Coelho J, et al. Ongoing outbreak of invasive and non-invasive disease due to group A Streptococcus (GAS) type emm66 among homeless and people who inject drugs in England and Wales, January to December 2016. Euro Surveill 2017; 22:pii=30446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bessen DE, Lizano S. Tissue tropisms in group A streptococcal infections. Fut Microbiol 2010; 5:623–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bessen DE, Kumar N, Hall GS, et al. Whole-genome association study on tissue tropism phenotypes in group A Streptococcus. J Bacteriol 2011; 193:6651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sabharwal H, Michon F, Nelson D, et al. Group A Streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J Infect Dis 2006; 193:129–35. [DOI] [PubMed] [Google Scholar]
- 40. Williamson DA, Smeesters PR, Steer AC, et al. M-protein analysis of Streptococcus pyogenes isolates associated with acute rheumatic fever in New Zealand. J Clin Microbiol 2015; 53:3618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beres SB, Sylva GL, Sturdevant DE, et al. Genome-wide molecular dissection of serotype M3 group A Streptococcus strains causing two epidemics of invasive infections. Proc Natl Acad Sci U S A 2004; 101:11833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nasser W, Beres SB, Olsen RJ, et al. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3615 genome sequences. Proc Natl Acad Sci U S A 2014; 111:E1768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dey N, McMillan DJ, Yarwood PJ, et al. High diversity of group A Streptococcal emm types in an Indian community: the need to tailor multivalent vaccines. Clin Infect Dis 2005; 40:46–51. [DOI] [PubMed] [Google Scholar]
- 44. Menon T, Lloyd C, Malathy B, et al. emm type diversity of beta-haemolytic streptococci recovered in Chennai, India. J Med Microbiol 2008; 57:540–2. [DOI] [PubMed] [Google Scholar]
- 45. Le Hello S, Doloy A, Baumann F, et al. Clinical and microbial characteristics of invasive Streptococcus pyogenes disease in New Caledonia, a region in Oceania with a high incidence of acute rheumatic fever. J Clin Microbiol 2010; 48:526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haggar A, Nerlich A, Kumar R, et al. Clinical and microbiologic characteristics of invasive Streptococcus pyogenes infections in north and south India. J Clin Microbiol 2012; 50:1626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Al-Shahib A, Underwood A, Afshar B, et al. Emergence of a novel lineage containing a prophage in emm/M3 group A Streptococcus associated with upsurge in invasive disease in the UK. Microb Genom 2016; 2:e000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.