Abstract
Mitochondria are the energy-producing organelles within a cell. Furthermore, mitochondria have a role in maintaining cellular homeostasis and proper calcium concentrations, building critical components of hormones and other signaling molecules, and controlling apoptosis. Structurally, mitochondria are unique because they have 2 membranes that allow for compartmentalization. The composition and molecular organization of these membranes are crucial to the maintenance and function of mitochondria. In this review, we first present a general overview of mitochondrial membrane biochemistry and biophysics followed by the role of different dietary saturated and unsaturated fatty acids in modulating mitochondrial membrane structure-function. We focus extensively on long-chain n–3 (ω-3) polyunsaturated fatty acids and their underlying mechanisms of action. Finally, we discuss implications of understanding molecular mechanisms by which dietary n–3 fatty acids target mitochondrial structure-function in metabolic diseases such as obesity, cardiac-ischemia reperfusion injury, obesity, type 2 diabetes, nonalcoholic fatty liver disease, and select cancers.
Keywords: cardiolipin, cancer, cardiac, diet, fatty acids, inner mitochondrial membrane, mitochondria, obesity, type 2 diabetes
Mitochondrial Membranes
Mitochondria are unique structures with 2 membrane bilayers. A general overview of mitochondrial outer and inner membrane structure-function is first presented followed by a brief overview of mitochondrial biophysics. We then present a summary of dietary FAs and the underlying biochemical and biophysical mechanisms by which they influence mitochondrial function.
The outer mitochondrial membrane
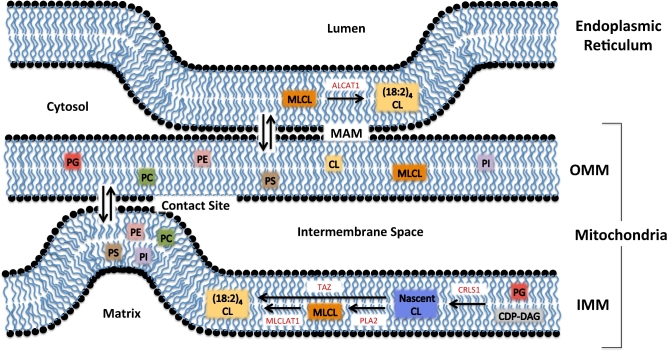
The outer mitochondrial membrane (OMM) encloses the entire mitochondrion and is in contact with the cytoplasm of the cell. The OMM and inner mitochondrial membrane (IMM) create a unique environment within the mitochondria, called the intermembrane space. The OMM is exceptionally porous and contains proteins called porins (e.g., voltage-dependent anionic channels) embedded into its structure that form channels and allow molecules ≤5 kDa in size to diffuse freely across the membrane and into the intermembrane space (1–3). The OMM also houses proteins that translocate larger proteins by recognizing an encoded mitochondria-targeting N-terminal sequence (4–6). The OMM, similar to the plasma membrane, has high concentrations of phosphatidylcholine and phosphatidylethanolamine and a 50:50 ratio of proteins to lipids (7). Cardiolipin, one of the hallmark phospholipids of the mitochondria, is only found at ∼4% of a normal functioning OMM (8–10) (Figure 1). However, during apoptosis, cardiolipin is released from the IMM and translocates to the OMM forming platforms for the Bax/Bid complex to bind and begin the apoptotic cascade (11–16). The OMM is associated with a multitude of functions in addition to protein transport and apoptosis, including the synthesis and elongation of FAs (17, 18). The OMM can also associate with the endoplasmic reticulum (ER) and create distinct mitochondria-associated membranes (MAMs) (Figure 2).
FIGURE 1.
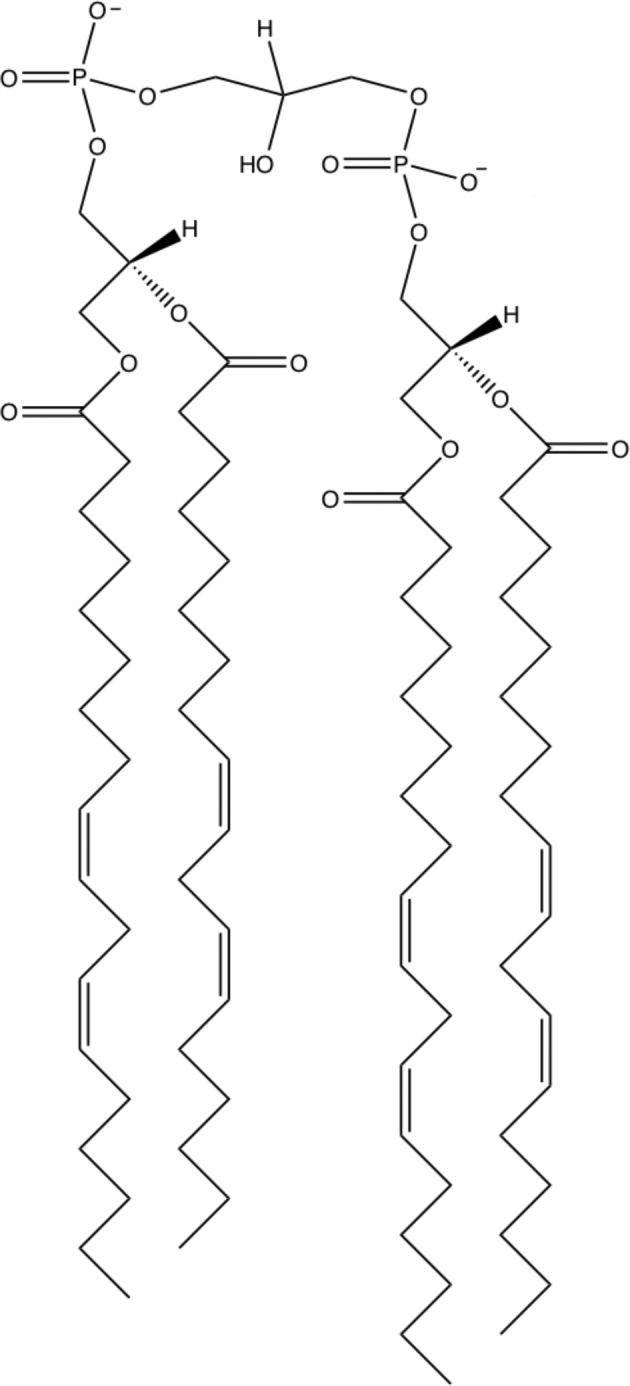
Structure of mature cardiolipin. Cardiolipin is a unique anionic phospholipid with 4 acyl chains and a small headgroup. Mature cardiac cardiolipin contains predominantly 4 linoleic acid (18:2) acyl chains under healthy conditions. Modifications to the acyl chains of cardiolipin are common in a range of diseases and can also be modified in response to dietary intake of differing FAs.
FIGURE 2.
Biosynthesis of mature CL. Mature CL is largely synthesized in the IMM. Nascent CL is synthesized from PG by using CDP-DAG. The acyl chains of nascent CL are then modified to mature CL containing 4 linoleic acid acyl chains (18:2) with the enzyme TAZ. Nascent CL can also undergo acyl chain cleavage with PLA2 to produce MLCL, which can serve as a substrate for MLCLAT1 to produce mature CL. A small fraction of mature CL is produced in the endoplasmic reticulum membrane through the use of ALCAT1. For simplicity, biosynthesis of other key phospholipids is not depicted. These phospholipids (PC, PE, PS, PI, PG, and PA) are found in differing concentrations across the ER, OMM, and IMM. ALCAT1, acyl-CoA:lysocardiolipin acyltransferase 1; CDP-DAG, cytidinediphosphate-diacylglycerol; CL, cardiolipin; CRLS1, cardiolipin synthase; ER, endoplasmic reticulum; IMM, inner mitochondrial membrane; MAM, mitochondria-associated membrane; MLCL, monolyso-cardiolipin; MLCLAT1, monolyso-cardiolipin acyltransferase 1; OMM, outer mitochondrial membrane; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PLA2, phospholipase A2; PS, phosphatidylserine; TAZ, taffazin; (18:2)4CL, tetralinoleoyl cardiolipin.
The MAMs
The OMM and the ER create MAMs that are critical for cellular physiology and homeostasis (19). MAMs have been observed with electron micrographs and fluorescence microscopy. They comprise ≤20% of the OMM and are held together via tethering complexes (20–24). Subcellular MAM fractions are enriched in enzymes that are important in lipid biosynthesis, phospholipid exchange, and calcium signaling (25–30). Due to constant fission and fusion, mitochondria require a well-regulated supply of phospholipids to maintain membrane integrity. MAMs allow for phospholipid flipping between the ER and OMM, independent of ATP (31–33). There are also phospholipid-remodeling enzymes associated with the MAM, including acyl-CoA:lysocardiolipin acyltransferase 1 (ALCAT1), which remodels cardiolipin with highly oxidizable acyl chains (34, 35) (Figure 2). MAMs also appear to be intermediate destinations in pathways that lead to VLDL assembly and secretion (20, 36–38). Thus, MAMs serve as a critical metabolic and trafficking hub in lipid metabolism. Finally, MAMs are also extremely important in calcium regulation. They create calcium microdomains at contact points that facilitate the efficient uptake of calcium, which sustains mitochondria and cellular homeostasis (28). Although more research is needed on MAMs, it is clear that these structures are critical in signaling, metabolism, and organelle physiology.
The IMM
The IMM encapsulates the matrix of the mitochondria and is surrounded by the intermembrane space. There are a multitude of IMM-associated proteins, most notably those involved in oxidative phosphorylation, ATP synthesis, and protein translocation (39–41). The IMM has a unique phospholipid composition closely resembling that of a bacterial membrane with a ratio of 4:1 proteins to lipids (7). The IMM is mainly composed of phosphatidylcholine (40%), phosphatidylethanolamine (30%), and cardiolipin (15–20%). The remaining 10% of the IMM phospholipidome consists of phosphatidylinositol (5%), phosphatidylserine (3–4%), and very small quantities of other lipids including phosphatidic acid and cholesterol (42). Cardiolipin has a unique structure with 4 acyl chains that promote the formation of non-bilayer phases and high curvature that can influence cristae formation and respiratory function (43).
Since the IMM's predominant role is energy production, it is only freely permeable to small metabolites, including oxygen and water (44–46). This lack of permeability separates the matrix from the cytosolic environment of the intermembrane space. This extreme compartmentalization is necessary for energy production because ATP synthesis relies on a proton gradient created across the IMM (47, 48). In fact, ATP is produced via multiple redox reactions that occur in 5 transmembrane enzyme complexes within the IMM—complex I [NADH: ubiquinone oxidoreductase], complex II (succinate dehydrogenase), complex III (cytochrome c reductase), complex IV (cytochrome c oxidase), and complex V (ATP synthase)—and 2 mobile electron carriers (the lipid-soluble coenzyme Q and the water-soluble cytochrome c). Initially, reducing equivalents such as NADH and FADH2 are oxidized by complex I and complex II, respectively. From there, electrons are passed through coenzyme Q to complex III and then from cytochrome c to complex IV. The final electron acceptor is oxygen, located in the matrix, which is reduced to water. Electron transport through the complexes is associated with proton pumping. Complexes I, III, and IV couple electron flow and proton pumping from the matrix to the intermembrane space to create and maintain an electrochemical gradient across the IMM. Complex V uses the electrochemical gradient to produce ATP by allowing protons to re-enter the matrix and providing the work needed for phosphorylation of ADP.
Overall, the aforementioned mitochondrial membranes play an important role in the organelle's functions and in maintaining cellular homeostasis. The composition of these membranes is very dynamic, especially the specialized IMM, and is tightly regulated but can be altered through the intake of dietary FAs. Alterations in the composition of membrane phospholipids will influence the biophysical properties of the membrane and thereby protein function (49).
Mitochondrial Membrane Biophysics
Mitochondria are cellular organelles containing 2 structurally and functionally distinct membranes (50–52). The biophysical organization of the outer and inner membranes of the mitochondria is of great importance because both membranes regulate the trafficking of ions, metabolites, and many small molecules between the cellular cytosol and mitochondrial matrix. Any impairment in the biophysical organization of these membranes could severely affect mitochondrial bioenergetics and cellular homeostasis. Given that the IMM is the major site of oxidative phosphorylation and ATP synthesis, we focus on the biophysical organization of the IMM.
Biophysical organization of the IMM
The structure of the IMM is extensively folded and compartmentalized by invaginations of the inner membrane termed “cristae,” which provide a large amount of surface for biochemical reactions such as cellular respiration and ATP generation (53–55). Within the IMM, 2 distinct regions have been observed: the inner boundary membrane and the cristae membrane. The inner boundary membrane is adjacent to the OMM, whereas the cristae membrane represents invaginations that protrude into the mitochondrial matrix (54). These convoluted structures of the IMM were established by pioneering studies on mitochondrial ultrastructure. These studies led to the proposal of several hypothetical models describing the biophysical organization of the IMM. To date, 3 models of the IMM have been proposed, including the “baffle model,” the “septa model,” and the “crista junction” model (54). The baffle model was initially proposed in the early 1950s by Palade (56), who confirmed the existence of the outer and inner membranes of the mitochondria and originally described cristae as being invaginations with rather broad openings (50). An alternate interpretation of the IMM structure was proposed around the same time by Sjöstrand (51, 57), who termed the “septa model.” Sjöstrand suggested that sheets of the inner membrane are divided by septa, which separate the matrix into several distinct compartments.
More recent EM tomography studies have shown that cristae are attached to the inner boundary membrane by narrow tubular openings, which have been termed “crista junctions” (58–62). It is thought that crista junctions play a critical role in compartmentalizing the IMM, because they may limit the diffusion of metabolites, such as protons and ADP, between the intermembrane space and the intracristal space (59). Indeed, crista junctions could contribute to the regulation of oxidative phosphorylation (OXPHOS), and ultimately ATP production, because 1 of the key limiting metabolites is ADP. Although more structural details with regard to crista junctions have become available, the functional significance of these structures remains unknown. Therefore, investigating the mechanistic and functional relation between these various IMM structures and dietary fatty acids is critical for better understanding mitochondrial function in health and disease.
Cardiolipin structure
The biophysical organization of the IMM is highly regulated by cardiolipin, a unique anionic, polyunsaturated phospholipid consisting of 2 phosphatidic acid moieties linked by a central glycerol backbone (Figure 1). Under normal physiologic conditions, cardiolipin may only carry 1 negative charge at a time because the phosphates of cardiolipin are diastereotopically inequivalent, and thus ionize at 2 different pH levels (pK1 = ∼2.8, pK2 = ∼7.5–9.5) (63–66). This allows cardiolipin to trap protons within its headgroup and thereby localize the proton pool near the surface of the IMM. The bioenergetic importance of cardiolipin to function as a proton trap may be to supply a greater buffering capacity at the membrane-water interface (66). In theory, cardiolipin could bridge the gap between the proton donor and the proton acceptor, because highly mobile cardiolipin would laterally shuttle protons from OXPHOS protein complexes to the ATP synthase machinery (66).
Cardiolipin is predominantly found in membranes capable of generating an electrical potential, such as bacterial and mitochondrial membranes (67). In mitochondria, cardiolipin is almost exclusively localized to the inner membrane where it is synthesized and required for membrane structure, membrane fusion and fission, protein function, and apoptosis (43, 68–72). The functional importance of cardiolipin within the IMM arises from its ability to directly influence lipid molecular organization and thereby cluster proteins that require specific lipid microenvironments, including key enzymes involved in OXPHOS (43, 70, 71, 73–75). Previous studies have clearly shown the importance of cardiolipin within the IMM by showing that cardiolipin clusters respiratory enzymes in order to augment efficient electron channeling for optimal OXPHOS activity (66, 70, 71, 73, 75–77). However, due to cardiolipin's polyunsaturated nature, and its close association with OXPHOS, it is extremely susceptible to oxidative damage often arising from reactive oxygen species (ROS).
Many studies have suggested cardiolipin's acyl chain specificity to be critical for optimal mitochondrial function. In the mammalian heart, linoleic acid (LA; 18:2) constitutes 80–90% of total cardiolipin acyl chains, where tetralinoleoyl cardiolipin [(18:2)4CL] is the most abundant species (Figure 1) (78). The abundance of (18:2)4CL within the IMM suggests an important role in structure-function. Indeed, cardiolipin enriched in tetralinoleic acid (18:2)4 binds with high affinity to a multitude of proteins and enzymes within the IMM, including key respiratory chain enzymes (79–86).
The (18:2)4-rich composition of cardiolipin is achieved by an acyl chain remodeling process that occurs within the IMM. Details of the synthesis of mature cardiolipin are presented in Figure 2. Briefly, de novo cardiolipin biosynthesis occurs in a series of steps on the inner face of the inner membrane from phosphatidylglycerol (PG) and cytidinediphosphate-diacylglycerol (CDP-DAG) via cardiolipin synthase (87). Nascent cardiolipin must be remodeled into a composition enriched in LA by enzyme-dependent remodeling processes within the IMM. A small fraction of mature cardiolipin can also be synthesized in the ER membrane (Figure 2). Environmental and genetic factors, such as diet and mutations in essential remodeling enzymes, influence the bioavailability and abundance of (18:2)4CL within the IMM (78, 88, 89).
Cardiolipin microdomains
Membrane microdomains often form as a function of favorable physiochemical properties of lipids (90–94). This phenomenon is observed in the plasma membrane of cells where lipid rafts, which are enriched in cholesterol and sphingolipids, exist as highly ordered regions that serve to compartmentalize cellular processes (92, 94). However, the idea of “raft-like” mitochondrial microdomains remains debated because the concentration of lipids required for lipid rafts (i.e., cholesterol and sphingolipids) is very low (95). This does not rule out the notion that specific lipids (i.e., cardiolipin) may localize to concentrate proteins into confined signaling centers.
Due to cardiolipin's unique physicochemical properties, it can induce negative membrane curvature and form nonbilayer phases, such as the inverted-hexagonal phase (96–99). The ability of cardiolipin to organize into the inverted-hexagonal phase suggests that cardiolipin may promote specific localized structures within the IMM and implicate its potential role in the formation of mitochondrial microdomains. Indeed, some laboratories have suggested that cardiolipin-enriched microdomains exist within mitochondria as essential activating platforms for mitochondrial fusion and fission and apoptosis (15, 97, 100). Experiments that used lipid monolayers and bilayers have shown that specific mitochondrial proteins, such as mitochondrial creatine kinase and cytochrome c, tightly interact with cardiolipin and induce segregation into microdomains (101–104). These proteins are found at mitochondrial contact sites as well as the surface of the IMM, where the assembly of enriched domains of cardiolipin plays an important role in mitochondria-mediated apoptosis and OXPHOS. However, the notion that mitochondria contain localized subdomains enriched in cardiolipin is still in its infancy. Future studies need to address how these microdomains may cluster proteins, such as respiratory protein complexes, for optimal ATP production and enhanced mitochondrial signaling (70, 71, 73, 74, 105, 106).
Dietary FAs
SFAs
Dietary FAs will influence the composition of mitochondrial membranes and thereby function. The 2 most abundant SFA acyl chains within cellular membranes are palmitic (16:0) and stearic (18:0) acids (107, 108). Although the body can synthesize SFAs, they are consumed from a variety of substances, including butter, milk, palm oil, coconut oil, and ground beef or beef tallow. These foods also contain shorter SFAs, including lauric acid (12:0) acid and myristic acid (14:0).
SFAs play important roles in the membranes of a multitude of organelles. Due to their structure, SFAs can pack tightly together and increase the viscosity of membranes. They can also interact with cholesterol and create lipid raft domains within the plasma membrane, which are important in cellular signaling (109–112). In mitochondrial membranes, the abundance of SFAs is significantly lower than the amount of unsaturated FAs. However, an increase in dietary intake of SFAs can increase the amount of FAs such as palmitic and stearic acids within the mitochondrial membranes, leading to potential impairments (113). In one study, SFAs were increased in rat liver mitochondria, particularly in cardiolipin, which led to the impaired release of cytochrome c (114). Although significantly less studied than unsaturated FAs in relation to mitochondria, SFAs likely play an important role in regulating mitochondrial membrane structure and function. Elucidating how SFAs influence mitochondrial biophysical organization is an area for future investigation. One possibility is that SFAs may counter-regulate the effects of unsaturated FAs by perturbing the diffusion of protein complexes and thereby their ability to execute optimal respiratory function (Figure 3). This would be driven by the ability of SFAs to increase membrane microviscosity and potentially increase the thickness of the membrane bilayer.
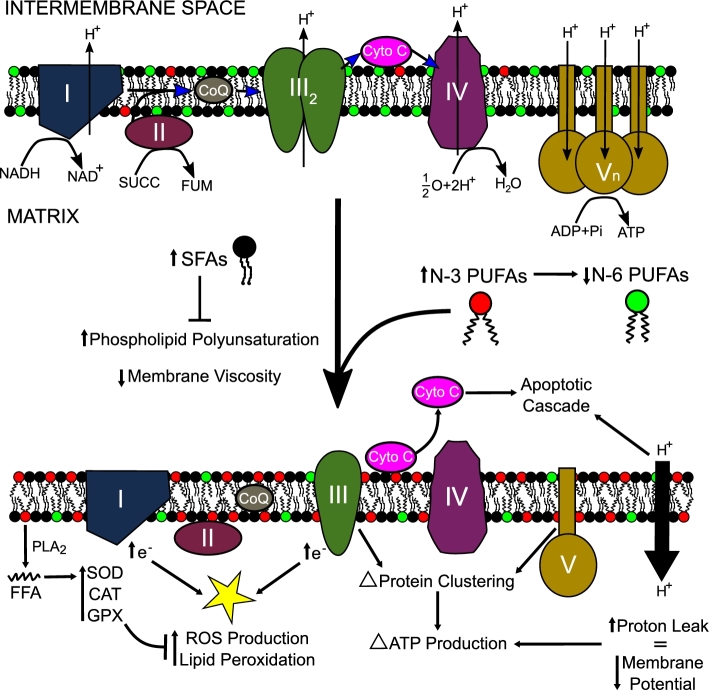
FIGURE 3.
Model depicting how dietary SFA, n–6 PUFA, and n–3 PUFA acyl chains target IMM structure-function. An increase in n–3 PUFA acyl chains within the IMM will replace the n–6 PUFA acyl chains and thereby influence microviscosity. This may cause the membrane to become “leaky” and allow for more proton leak back into the matrix. In addition, an increase in SFAs will decrease polyunsaturation and increase viscosity. n–3 PUFA incorporation into the membrane may also alter protein clustering and enzyme activity, which may alter the amount of ATP produced. Respiratory enzymes are influenced by the increase in n–3 PUFAs and may allow more electrons to escape during oxidative phosphorylation, which will lead to an increase in ROS production and peroxidation. However, n–3 PUFAs can also be cleaved from the membrane via PLA2 and increase the antioxidant capacity of the mitochondria. In addition, an increase in n–3 PUFAs may release cytochrome c, starting the apoptotic cascade as seen in some cancer models. Overall, FAs through the diet likely have a wide range of different roles within the IMM that require further investigation in both healthy and diseased states. CAT, catalase; CoQ, coenzyme Q; Cyto C, cytochrome c; FUM, fumarate; GPX, glutathione peroxidase; IMM, inner mitochondrial membrane; Pi, inorganic phosphate; PLA2, phospholipase A2; SOD, superoxide dismutase; SUCC, succinate.
n–6 PUFAs
n–6 PUFAs play an essential role in membrane structure and function. Generally, due to their multiple double bonds, n–6 PUFAs decrease the viscosity of the membrane (115). They do not pack as tightly as SFAs and will not form liquid ordered microdomains (116). LA (18:2) is the predominant n–6 PUFA in the Western diet. LA is obtained through the diet from vegetable oils, such as sunflower, soybean, corn, and canola oils, as well as walnuts and seeds. Dietary n–6 PUFAs will incorporate into mitochondrial phospholipids. Without LA incorporation into cardiolipin's acyl chains, the function of respiratory and other membrane-associated proteins may be diminished (117–119). In many diseases, including cardiovascular diseases, diabetes, and Barth syndrome, the loss of LA in cardiolipin is detrimental to the overall function of the mitochondria (120, 121). However, one research group, by using a yeast model concluded that unremodeled cardiolipin (i.e., containing a significant amount of saturated acyl chains) is functionally equivalent to fully remodeled cardiolipin (containing predominately PUFA acyl chains that are 18:2) (122). In addition, another study that used a yeast model determined that a decrease in the cardiolipin-to– monolyso-cardiolipin ratio caused deficiencies in respiration (123). Although innumerable studies have been conducted on the role of cardiolipin remodeling, its role in mitochondrial bioenergetics still requires further investigation.
Dietary arachidonic acid (AA; 20:4) is mainly obtained through the consumption of chicken, eggs, and beef. AA will also incorporate into mitochondrial membranes, although it is controversial if it is beneficial to the function of the mitochondria (124, 125). For example, rats were supplemented with an AA-enriched diet for 10 wk, which led to the incorporation of AA at the expense of LA acyl chains, particularly in cardiolipin. This replacement delayed the opening of the mitochondrial permeability transition pore (MPTP) and reduced the risk of apoptosis but did not alter mitochondrial respiration (125). Conversely, a study showed that AA induced the opening of the MPTP but did not cause depolarization or respiratory inhibition. When AA was added to MH1C1 rat hepatoma cells, it rapidly opened the MPTP and induced cytochrome c release and apoptosis (126). In addition, in studies in which the FFA form of AA is incorporated into the mitochondria, there are uncoupling effects during state 4 respiration and an overall inhibition in uncoupled respiration. There is also a decrease in complex I and complex III activity as well as an increase in ROS production and membrane permeability (124). Overall, n–6 PUFAs have differing effects on mitochondrial membranes. LA is the most abundant acyl chain in mitochondrial membranes and is required for optimal function and AA is also found in mitochondrial membrane phospholipids but to a lesser extent. The role of AA in mitochondrial membranes is still unclear and requires further scientific exploration, particularly in the context of n–3 PUFAs, which lower n–6 PUFAs (127, 128).
n–3 PUFAs
The most notable n–3 PUFAs are α-linolenic acid (ALA; 18:3), EPA (20:5), and DHA (22:6). ALA is a short-chain n–3 PUFA and an essential FA that is obtained through the consumption of flaxseed, canola, and walnut oils. ALA can undergo elongation and desaturation to produce long-chain n–3 PUFAs such as EPA and DHA (129). Elongation and desaturation from ALA are highly inefficient, so most long-chain n–3 PUFAs come from dietary sources such as marine oils or as dietary supplements.
Long-chain n–3 PUFAs play an important role in the structure and function of cellular membranes (130). Due to their extreme chain length and multiple double bonds, they dramatically decrease membrane viscosity (130). EPA and DHA can incorporate into the plasma membrane of a multitude of cell types, particularly immune cells, and disrupt plasma membrane raft domains and cellular signaling (131–136). Presumably, similar mechanisms exist by which EPA and DHA target the organization of mitochondrial membranes. Indeed, there is evidence that n–3 PUFAs incorporate into the cardiac mitochondrial phospholipidome (137).
Effects of Dietary FAs on Mitochondrial Function Independent of Changes in Mitochondrial Membrane Organization
This review focused on how dietary FAs regulate mitochondrial membrane structure-function. However, it is essential to recognize that dietary FAs can influence mitochondrial function independent of changes in the composition of the OMM and IMM. Here we briefly describe how dietary FAs directly influence mitochondrial function through metabolic gene regulation. Long-chain SFAs and unsaturated FAs act as ligands for PPARs, a superfamily of nuclear transcription factors responsible for upregulating genes that influence mitochondria and peroxisome function. These nuclear transcription factors (PPARα, PPARβ/δ, and PPARγ) heterodimerize with retinoid X-receptor (RXR), promoting target gene transcription through DNA binding of the peroxisome proliferator response element (PPRE) in the nucleus (138, 139).
Endogenous ligands for PPARs, such as PUFAs, eicosanoids, and other lipid metabolites, can also activate nuclear transcription factors. Furthermore, PPAR-induced gene transcription differs by subtype and tissue distribution, with PPARα and PPARβ/δ primarily responsible for regulating β-oxidation genes in hepatocytes, cardiomyocytes, myocytes, and enterocytes (140, 141), and PPARγ is responsible for adipocyte differentiation and immune cell metabolism, differentiation, and function, respectively (142, 143). Within the mitochondria, activation of PPARs by long-chain FAs induces gene expression of several key mitochondrial enzymes, including carnitine palmitoyltransferase (CPT) enzyme I and II, located on the OMM and IMM, respectively; acetyl-CoA synthetase (ACS); and numerous other β-oxidation enzymes (144). Thus, long-chain FA activation of PPARs induces their own metabolism through this transcriptionally regulated pathway, directly influencing mitochondrial function by increasing FA oxidation.
The consumption of high-fat diets and resultant elevated endogenous FFAs have previously been shown to increase mitochondrial biogenesis through a PPARδ-mediated mechanism involving increased PPARγ co-activator 1 (PGC-1) in rat muscle. However, the increase in mitochondrial biogenesis did not result in improved insulin resistance caused by consuming a high-fat diet (145). Compared with low-fat diets, the prolonged consumption of a high-fat diet in Wistar rats resulted in obesity, dyslipidemia, insulin resistance, and skeletal muscle mitochondrial dysfunction, with notable significant differences in phosphorylation efficiency and mitochondrial respiration (146). These findings support numerous investigations in rodent models that show that high-fat diet consumption results in greater β-oxidation, likely mediated by PPAR activation, concomitant with greater free radical and oxidant production (147). These direct mitochondrial effects of high-fat diets likely contribute to mitochondrial, cellular, and systemic metabolic dysfunction (147). Taken together, it is essential to recognize that dietary FAs can target mitochondrial function independent of changes in structural membrane organization.
Effects of Long-Chain n–3 PUFAs on Mitochondrial Membrane Structure-Function
The mitochondrial phospholipidome is remodeled with the consumption of n–3 PUFAs
As mentioned previously, the composition of the IMM is highly regulated due to its unique structure and function in maintaining the electrochemical gradient required for OXPHOS. However, the composition of the IMM is subject to change in response to dietary FA consumption (88). Many studies have addressed how n–3 PUFAs target mitochondrial membrane properties in health and disease (125, 137, 148–156). In Table 1, we present an overview of key studies showing the effects of n–3 PUFAs on mitochondrial membrane organization and function (i.e., membrane potential, respiration, individual complex activities, and ROS production). Although most of the studies differ in methodology, the results on membrane structure are similar. An increase in n–3 PUFA concentrations leads to an increase in EPA and/or DHA incorporation into mitochondrial membranes at the expense of n–6 PUFAs (Figure 3). Incorporation of n–3 PUFAs into mitochondrial membranes also alters the biophysical organization by decreasing membrane viscosity and potentially changing lipid-lipid and lipid-protein interactions (Figure 3).
TABLE 1.
Summary of key studies on n–3 PUFAs and mitochondrial membrane structure-function1
| Supplementation | Organism/tissue | Membrane alterations | Functional endpoints | Reference |
|---|---|---|---|---|
| Diet containing DHA at 2.5% total caloric intake for 10 wk | Male Wistar rats/cardiac mitochondria | ↑ Amount of DHA and displaced AA and LA | ↓ In (18:2)4CL, no effect on respiration | (125) |
| 100 μM n–3 PUFA treatment for 72 h | H9c2 cardiac myoblasts | ↑ Highly unsaturated CL containing n–3 PUFAs | ↑ High-molecular-weight CL and ↓ low-molecular-weight CL | (137) |
| Fusion of mitochondria with 18:0/22:6 PC vesicles | 4-wk-old male mice/liver mitochondria | ↓ In DHA and viscosity | ↓ RCI and membrane potential and ↑ proton movement | (148) |
| 10% Menhaden oil for 3 wk | 2-mo or 21-mo CBA/ca female mice/liver mitochondria | ↑ In EPA and DHA and ↓ in AA; DHA was primarily associated with PE and PC | ↑ Age-related mitochondrial dysfunction | (148) |
| DHA ethyl esters (2% of energy) | C57BL/6 obese mice | ↓ Formation of membrane domains | ↓ In enzyme activity | (150) |
| n–3 Complete: 2 g EPA and 1 g DHA/d for 12 wk | Recreationally active men aged 22.7 ± 0.8 y/subsarcolemmal and intermyofibrillar mitochondria | ↑ In EPA/DHA in PC and PE but not in CL | ↑ Sensitivity to ADP and ROS but not in oxidant products | (151) |
| Diet containing DHA or EPA at 2.5% total caloric intake for 8 wk | Male Wistar rats/cardiac mitochondria | ↑ EPA/DHA and ↓ AA | ↓ Ca2+-induced opening of the MPTP and swelling | (152) |
| 11.5 g Menhaden fish oil/100 g for 2 wk | Male Sprague-Dawley rats/colonic crypt mitochondria | ↑ In unsaturation index of CL, PE, and PC | ↑ ROS- initiated apoptotic cascade | (153) |
| 20% wt:vol Menhaden fish oil for 4 wk | Male Sprague-Dawley rats/renal cortical mitochondria | ↓ In OA, LA, and AA and ↑ in EPA and DHA | ↑ In PLA2 activity and mitochondrial damage via ROS; ↓ in state 3 respiration and complex I | (154) |
| 15% (wt:wt) fish-oil or EPA and DHA pure ethyl ester diet for 2 wk | Male Sprague-Dawley rats/colonic crypt mitochondria | ↑ In EPA and DHA at the expense of AA and LA | ↓ In membrane potential and ↑ in caspase 3 activity | (155) |
| 20% Fish-oil diet–12% tuna oil and 8% sardine oil for 12 wk | Wistar male rats/liver mitochondria | ↓ In viscosity | ↑ Membrane potential, respiration, and complex V activity; ↓ complex III and IV activity | (156) |
AA, arachidonic acid (20:4); CL, cardiolipin; LA, linoleic acid (18:2); MPTP, mitochondrial permeability transition pore; OA, oleic acid (18:1); PC, phosphatidylcholine; PE, phosphatidylethanolamine; PLA2, phospholipase A2; RCI, Respiratory Control Index; ROS, reactive oxygen species; (18:2)4CL, tetralinoleoyl cardiolipin; ↑, increase/increased; ↓, decrease/decreased.
Although some studies show that n–3 PUFAs influence membrane viscosity, the field has not moved beyond to establish how EPA and DHA acyl chains are targeting the formation of mitochondrial microdomains, protein-phospholipid binding, and protein-protein clustering. Our laboratory has very recently shown that DHA, upon incorporation into cardiolipin, can prevent formation of mitochondrial microdomains and binding of cardiolipin to complex IV (150). However, more work is needed in this area.
ROS production and antioxidant capacity are mechanistic targets of n–3 PUFAs
The mitochondria are an important source of ROS within the cell. ROS occur when electrons “escape” or leak from the enzyme complexes associated with OXPHOS. The main contributors to electron leakage are complexes I and III (157). Once these electrons escape, they interact with oxygen to produce a superoxide. In normal-functioning mitochondria, these superoxides interact with redox enzymes within the matrix of the mitochondria, reducing the superoxide to water. However, superoxides can still damage the mitochondria, by peroxidizing phospholipid acyl chains, mitochondrial DNA, and proteins (158). The peroxidation of phospholipids within the IMM can lead to a disorganization of the membrane, an accumulation of toxic products, and increased levels of free radicals, enhancing mitochondrial damage.
Acyl chains such as EPA and DHA are highly susceptible to peroxidation via ROS due to their tremendous level of unsaturation (Figure 3). Dietary supplementation of EPA or DHA increases the levels of n–3 PUFA acyl chains within mitochondrial membranes, which leads to membrane disorganization and potentially increased electron leakage. Several studies show that an increase in the polyunsaturation of phospholipids, particularly cardiolipin, increases the production of ROS (153, 159). When ROS production overwhelms the antioxidant capacity of the mitochondria, phospholipase A2 is activated and enhances the release of peroxidized fatty acyl chains from membrane phospholipids, resulting in membrane disorganization and mitochondrial damage (160). This mechanism of increased oxidative stress, resulting in mitochondrial dysfunction and thereby apoptosis, is implicated in the anticancer properties of n–3 PUFAs, as discussed below.
Although, the presence of n–3 PUFA acyl chains within the IMM can increase ROS production, this does not always translate to increased mitochondrial dysfunction. Figure 3 shows how n–3 PUFAs mechanistically can increase antioxidant potential and increase ROS production within the mitochondrial matrix. Several literature reports show that supplementation with n–3 PUFAs increases the antioxidant capacity of the cell through a variety of mechanisms (161–165). For instance, n–3 PUFA supplementation increased the concentrations of oxidative stress products, including lipid hydroperoxides and malondialdehyde. However, superoxide dismutase activity was upregulated, which increased mitochondrial antioxidant capacity and thereby maintaining protein function (161). In addition, in HepG2 cells supplemented with DHA, there was no increase in lipid peroxidation but an increase in glutathione-related antioxidant enzyme activities and superoxide dismutase activity (162). DHA has also been implicated in several studies for inhibiting free radical generators, including NADPH oxidases (165). The role of n–3 PUFAs and their antioxidant capacity is relevant in a multitude of disease states and warrants more extensive research (164).
Consumption of n–3 PUFAs influences mitochondrial protein activity
The IMM houses a multitude of proteins that perform a variety of functions, most notably of those involved in OXPHOS. The IMM is a sea of proteins with imbedded phospholipids with a protein to phospholipid ratio of 4:1 (7). Despite a high concentration of proteins within the IMM, phospholipids play a crucial role in maintaining protein organization and function.
Cardiolipin binds to a multitude of IMM proteins and aides in their biophysical organization and function. This includes binding to individual electron transport chain (ETC) enzymes as well as promoting the formation of supercomplexes. For example, there are 9 binding sites for cardiolipin in bovine complex I (166). Complex III has 6 cardiolipin binding sites that have functional and structural importance. Structural studies suggest that cardiolipin binds to sites on complex III and aids in proton uptake (81, 167). Cardiolipin is also functionally required for complex IV activity. There are 4 cardiolipin binding sites in complex IV: 2 are “high affinity” and 2 are “low affinity.” The high-affinity cardiolipin binding sites are associated with the regulation of electron activity and the low affinity sites are important in structural integrity of the complex in its dimer form (80, 168–171). In addition, complex V dimers require cardiolipin for assembly into larger oligomeric structures (79, 172, 173). As well as binding to individual complexes, cardiolipin is also considered to function as a “glue” by holding respiratory supercomplexes together (174).
Cardiolipin undergoes remodeling in the mitochondria from its nascent form to a highly regulated species that is in the cardiac tissue ∼90% tetra-linoleic or (18:2)4 (Figure 2). It is well documented that reductions in the concentration of (18:2)4CL in the IMM will lead to supercomplex dissociation, loss of ATP production, and structural abnormalities, most notably seen in Barth syndrome (120, 121, 175). Along with a loss of content, alterations in acyl chain species of cardiolipin may also result in a disorganization of the membrane and thereby decreased respiratory enzyme activity and supercomplex formation. For example, in Barth syndrome, respiratory supercomplexes are destabilized due to cardiolipin acyl chain remodeling and decreased cardiolipin concentrations (176). However, the importance of cardiolipin concentration compared with cardiolipin acyl chain remodeling in supercomplex destabilization has yet to be definitively determined.
The consumption of n–3 PUFAs also targets enzymatic activity, which could be through a direct effect on respiratory enzymes, formation of supercomplexes, or both. In 1 study, rats were fed a 30% fish-oil diet for 12 wk, which resulted in a decrease in complex II+III and complex IV activities (156). Several other studies showed that an increase in n–3 PUFA consumption reduced ATP production and various states of respiration (148, 152). Other laboratories reported no loss of respiratory function with n–3 PUFA supplementation (125, 151). A recent study from our group showed that DHA ethyl esters lowered respiratory enzyme activities of complexes I, IV, V, and I+III. The underlying mechanism was driven by replacement of LA by DHA in the mitochondrial phospholipidome (150). Although an increase in n–3 PUFAs disorganizes the mitochondrial membrane and can potentially alter protein function, studies are overall inconsistent. More standardized experiments need to be conducted on the role of n–3 PUFA acyl chains and their effects on mitochondrial enzyme functions.
Effects of Long-Chain n–3 PUFAs on Diseased Mitochondrial Membranes and Function
Ischemia reperfusion injury
Cardiac ischemia reperfusion (IR) injury is characterized by an acute loss of blood flow followed by the reintroduction of blood and oxygen. The pathogenesis of IR injury is complex and involves multiple pathways, including ROS and inflammation (177, 178). In the mitochondria, IR is characterized by a distinct loss in cardiolipin, energy dysregulation, and overabundance of ROS. Several studies suggest that treatment with n–3 PUFAs improves myocardial resistance to IR injury and reduces infarct size, although the mechanisms remain elusive (179–182).
n–3 PUFAs could improve IR injury through differing mechanisms, either before ischemia or during the reperfusion phase. Several laboratories report that chronic n–3 PUFA supplementation reduces the severity of IR injury and decreases myocardial infarct size. These studies also showed that n–3 PUFA treatment upregulates mitochondrial antioxidant activity, including superoxide dismutase, catalase, and glutathione peroxidase, which combat lipid peroxidation during IR injury (161, 180, 183, 184). Chronic n–3 PUFA treatment may be associated with a reinforced antioxidant defense system that protects the mitochondria from ROS produced during the reperfusion phase of IR injury. Acute treatment with n–3 PUFAs during the reperfusion phase of IR injury has also been explored. One study gave a bolus of n–3 PUFA TGs to rats after ischemia but before reperfusion. The results indicated that acute treatment with an EPA-to-DHA ratio of 6:1 improved the outcome of IR injury by reducing vascular inflammation and oxidative stress (185). Another laboratory found that infusion with DHA diminished cardiac damage and increased antioxidant protection (186).
Although a multitude of studies have measured the effects of n–3 PUFAs before and during IR treatment, little is known about their mechanisms of action in relation to the IMM. Several reports suggest that n–3 PUFAs, particularly DHA, incorporate into the membrane and regulate Ca2+ channels and delay the Ca2+-dependent MPTP opening. The MPTP opening is associated with cardiovascular diseases, including IR injury, and interventions to target its opening are of significant clinical relevance (152, 187). However, some laboratories suggest that supplementation with EPA or DHA has no effect on MPTP opening in diseased hearts, only in healthy heart tissue (188). Likewise, as mentioned above, supplementation with EPA or DHA can potentially alter enzymatic activities, which could increase the adverse effects of IR injury. More research is needed on n–3 PUFAs and IR injury in addition to other cardiovascular diseases, such as heart failure (78). This is highly relevant given the debate about n–3 PUFA efficacy for cardiovascular diseases (78, 189).
Obesity and type 2 diabetes
Several literature reports suggest that the consumption of n–3 PUFAs can have beneficial effects for obesity and type 2 diabetes (190). The mechanisms for improvements consist of improving insulin signaling, preventing alterations in glucose metabolism, and reversing dyslipidemia via targeting of transcription factors and gene expression (191, 192). n–3 PUFAs also modulate inflammation by decreasing the n–6-to-n–3 PUFA ratio, which reduces the burden of these diseases through n–6-derived lipid mediators (127, 128). Although much is known about the role of n–3 PUFAs in regulating gene expression in these diseases, less is known about how n–3 PUFAs modulate mitochondrial membranes during obesity and type 2 diabetes. One set of experiments showed that an increase in DHA intake partially prevented mitochondrial dysfunction induced by insulin resistance in cardiac mitochondria (193).
Some studies suggest that an increase in n–3 PUFAs within mitochondrial membranes will modulate proton conductance and enhance proton uncoupling, leading to limited weight gain or increased weight loss (194, 195). However, evidence that n–3 PUFAs affect weight loss is lacking and at times contradictory (196, 197). Paradoxically, some studies showed that pathological remodeling of cardiolipin will increase oxidative damage that contributes to mitochondrial dysfunction in obesity and type 2 diabetes. For instance, Li et al. (35) showed that DHA concentrations in C2C12 cells were elevated upon upregulation of ALCAT1 and increased oxidative stress. ALCAT1 is relevant because a murine ALCAT1 knockout model was shown to protect against diet-induced obesity and insulin resistance (35). In addition, shotgun lipidomic studies showed a significant decrease in cardiolipin abundance and a profound remodeling of the remaining cardiolipin species to include DHA acyl chains in rat diabetic myocardium (198, 199). These studies suggest that an increase in n–3 PUFA acyl chains within mitochondrial membranes could be detrimental for mitochondrial function.
The mechanisms by which n–3 PUFAs regulate mitochondrial membranes and protein function are still vastly unknown in obesity and type 2 diabetes. However, an increase in acyl chain unsaturation may result in membrane disorganization, which would lead to altered lipid-lipid interactions and protein-lipid interactions and potentially mitochondrial dysfunction (Figure 3). It is likely that there is a fine balance between detrimental and beneficial effects of n–3 PUFAs, which will require extensive experimentation as a function of dose of EPA and DHA.
Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is characterized by an increase in the accumulation of lipids in the liver and can lead to fibrosis and cirrhosis. NAFLD is also associated with insulin resistance, oxidative stress, inflammation, and altered lipid metabolism (200). NAFLD impairs mitochondrial dysfunction, which can occur in a number of ways, including lipid and mitochondrial DNA peroxidation, ultrastructure abnormalities, reduced ATP stores, and alterations in respiratory chain enzyme activities (201–207). Several studies and meta-analyses suggest that an increase in n–3 PUFA intake can reduce the effects of NAFLD by regulating gene transcription, reducing inflammation markers, and increasing β-oxidation (208–217). In fact, recent clinical data suggest that supplementation with n–3 PUFAs positively affects plasma lipid profiles, improves liver histology, and decreases inflammatory markers in patients with NAFLD (218, 219).
Although much is known on the role of n–3 PUFAs in abating the effects of NAFLD, little is known on their role in mitochondrial membranes during the development of this disease. For example, incubating HepG2 cells (a steatotic hepatocyte model) with 50 μmol EPA or DHA/L rescued the reduction in mitofusin 2, a multifunctional protein that participates in proliferation and metabolism, which caused an increase in the length of mitochondrial tubules (220). This study also showed that n–3 PUFAs increased ATP production and decreased ROS production (220). The role of n–3 PUFA incorporation into mitochondrial membranes during NAFLD warrants more scientific exploration.
Cancers
There are data to suggest that the consumption of n–3 PUFAs decreases the risk of various cancers, including breast cancer, colon cancer, prostate cancer, and leukemias (221–224). The mechanisms by which n–3 PUFAs destroy cancer cells are multifaceted. These mechanisms include suppression of AA-derived eicosanoids by decreasing the n–6-to-n–3 PUFA ratio, influencing transcription factors and gene expression, increasing oxidative stress, and altering cellular membrane organization (132, 225, 226).
Supplementation with n–3 PUFAs increases the polyunsaturation index and decreases the membrane potential of the IMM, thereby priming the cell to undergo apoptosis (153, 227–229). In HL-60 (promyelocytic leukemia) cells, supplementation with n–3 PUFAs led to induction of the apoptosis cascade by activating various caspase enzymes and releasing cytochrome c from the IMM (223). In addition, n–3 PUFA supplementation also decreased the membrane potential effectively depolarizing the mitochondria (223). As another example, DHA, with the addition of butyrate, induced apoptosis in colonocytes, which protected against tumorigenesis (221). Apoptosis is mediated through Ca2+-mediated pathways, enhancing phospholipid oxidation, and increasing proton leak across the IMM (221, 222). n–3 PUFAs may also have a role in improving outcomes in cancer linked to obesity through anti-inflammatory and pro-resolving mechanisms (230, 231).
Conversely, it has been suggested that n–3 PUFAs increase the antioxidant capacity of the cell by upregulating antioxidant enzyme production, thereby suppressing inflammation, ROS production, and carcinogenesis (224). These pathways are potentially contradictory and further research needs to be done to determine the primary role of n–3 PUFAs in cancer suppression. One likely mechanism is DHA influencing mitochondrial membrane dynamics and thereby interactions with cytochrome c.
Conclusions and Future Directions
Mitochondria have a central role in cellular function across a range of tissues in health and disease. The underlying mechanisms by which SFAs and unsaturated FAs influence mitochondrial membrane structure are in their infancy. However, there are clear indications that dietary FAs remodel the mitochondrial phospholipidome, particularly in dysfunctional mitochondria, which influences a range of mitochondrial responses. More research is needed in FA structure and membrane biophysics to elucidate how FAs go beyond disrupting membrane microviscosity to regulate a range of mechanisms, including MAM formation, microdomain distribution, phospholipid-protein binding, protein activity, antioxidant capacity, apoptosis, and respiration.
Acknowledgments
All authors read and approved the final manuscript.
Notes
Supported by NIH R01HL123647 (SRS, DAB), R01AT008375 (SRS), and NIH P30DK056350 (SRS, MAB).
Author disclosures: EMS, ERP, WDG, MAB, DAB, and SRS, no conflicts of interest.
Abbreviations used:
- AA
arachidonic acid
- ALA
α-linolenic acid
- ALCAT1
acyl-CoA:lysocardiolipin acyltransferase 1
- ER
endoplasmic reticulum
- IMM
inner mitochondrial membrane
- IR
ischemia reperfusion
- LA
linoleic acid
- MAM
mitochondria-associated membrane
- MPTP
mitochondrial permeability transition pore
- NAFLD
nonalcoholic fatty liver disease
- OMM
outer mitochondrial membrane
- OXPHOS
oxidative phosphorylation
- ROS
reactive oxygen species
- (18:2)4CL
tetralinoleoyl cardiolipin
References
- 1. Nikaido H. Proteins forming large channels from bacterial and mitochondrial outer membranes: porins and phage lambda receptor protein. Methods Enzymol 1983;97:85–100. [DOI] [PubMed] [Google Scholar]
- 2. Mihara K, Sato R. Molecular cloning and sequencing of cDNA for yeast porin, an outer mitochondrial membrane protein: a search for targeting signal in the primary structure. EMBO J 1985;4:769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci USA 2000;97:4666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mayer A, Neupert W, Lill R. Mitochondrial protein import: reversible binding of the presequence at the trans side of the outer membrane drives partial translocation and unfolding. Cell 1995;80:127–37. [DOI] [PubMed] [Google Scholar]
- 5. Meisinger C, Ryan MT, Hill K, Model K, Lim JH, Sickmann A, Müller H, Meyer HE, Wagner R, Pfanner N. Protein import channel of the outer mitochondrial membrane: a highly stable Tom40-Tom22 core structure differentially interacts with preproteins, small tom proteins, and import receptors. Mol Cell Biol 2001;21:2337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Künkele K, Heins S, Dembowski M, Nargang FE, Benz R, Thieffry M, Walz J, Lill R, Nussberger S, Neupert W. The preprotein translocation channel of the outer membrane of mitochondria. Cell 1998;93:1009–19. [DOI] [PubMed] [Google Scholar]
- 7. Krauss S. Mitochondria: structure and role in respiration. eLS 2001. doi: 10.1002/9780470015902.a0001380. [DOI] [Google Scholar]
- 8. de Kroon AIPM, Dolis D, Mayer A, Lill R, de Kruijff B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa: is cardiolipin present in the mitochondrial outer membrane? Biochim Biophys Acta 1997;1325:108–16. [DOI] [PubMed] [Google Scholar]
- 9. Comte J, Maǐsterrena B, Gautheron DC. Lipid composition and protein profiles of outer and inner membranes from pig heart mitochondria: comparison with microsomes. Biochim Biophys Acta 1976;419:271–84. [DOI] [PubMed] [Google Scholar]
- 10. Sperka-Gottlieb CDM, Hermetter A, Paltauf F, Daum G. Lipid topology and physical properties of the outer mitochondrial membrane of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta 1988;946:227–34. [DOI] [PubMed] [Google Scholar]
- 11. Ott M, Zhivotovsky B, Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ 2007;14:1243–7. [DOI] [PubMed] [Google Scholar]
- 12. Petrosillo G, Ruggiero FM, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J 2003;17:2202–8. [DOI] [PubMed] [Google Scholar]
- 13. Fernandez MG, Troiano L, Moretti L, Nasi M, Pinti M, Salvioli S, Dobrucki J, Cossarizza A. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ 2002;13:449–55. [PubMed] [Google Scholar]
- 14. Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signaling platform to launch apoptosis. Biochim Biophys Acta 2009;1788:2022–31. [DOI] [PubMed] [Google Scholar]
- 15. Sorice M, Manganelli V, Matarrese P, Tinari A, Misasi R, Malorni W, Garofalo T. Cardiolipin-enriched raft-like microdomains are essential activating platforms for apoptotic signals on mitochondria. FEBS Lett 2009;583:2447–50. [DOI] [PubMed] [Google Scholar]
- 16. Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJA, Petit PX, Vaz FM, Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol 2008;183:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colli W, Hinkle PC, Pullman ME. Characterization of the fatty acid elongation system in soluble extracts and membrane preparations of rat liver mitochondria. J Biol Chem 1969;244:6432–43. [PubMed] [Google Scholar]
- 18. Howard CF. Synthesis of fatty acids in outer and inner membranes of mitochondria. J Biol Chem 1970;245:462–8. [PubMed] [Google Scholar]
- 19. Raturi A, Simmen T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim Biophys Acta 2013;1833:213–24. [DOI] [PubMed] [Google Scholar]
- 20. Rusiñol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J Biol Chem 1994;269:27494–502. [PubMed] [Google Scholar]
- 21. Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem 2000;275:34534–40. [DOI] [PubMed] [Google Scholar]
- 22. Vance JE. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta 2014;1841:595–609. [DOI] [PubMed] [Google Scholar]
- 23. de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008;456:605–10. [DOI] [PubMed] [Google Scholar]
- 24. Kornmann B. The molecular hug between the ER and the mitochondria. Curr Opin Cell Biol 2013;25:443–8. [DOI] [PubMed] [Google Scholar]
- 25. Patergnani S, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Giorgi C, Marchi S, Missiroli S, Poletti F. et al. Calcium signaling around mitochondria associated membranes (MAMs). Cell Commun Signal 2011;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Vliet AR, Verfaillie T, Agostinis P. New functions of mitochondria associated membranes in cellular signaling. Biochim Biophys Acta 2014;1843:2253–62. [DOI] [PubMed] [Google Scholar]
- 27. Hayashi T, Rizzuto R, Hajnoczky G, Su T. MAM: more than just a housekeeper. Trends Cell Biol 2017;19:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci STKE 2004;2004:re1. [DOI] [PubMed] [Google Scholar]
- 29. Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 1990;265:7248–56. [PubMed] [Google Scholar]
- 30. Shiao Y, Lupo G, Vance J. Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. J Biol Chem 1995;270:11190–8. [DOI] [PubMed] [Google Scholar]
- 31. Holthuis JCM, Levine TP. Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol 2005;6:209–20. [DOI] [PubMed] [Google Scholar]
- 32. Achleitner G, Gaigg B, Krasser A, Kainersdorfer E, Kohlwein SD, Perktold A, Zellnig G, Daum G. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur J Biochem 1999;264:545–53. [DOI] [PubMed] [Google Scholar]
- 33. Tatsuta T, Scharwey M, Langer T. Mitochondrial lipid trafficking. Trends Cell Biol 2017;24:44–52. [DOI] [PubMed] [Google Scholar]
- 34. Cao J, Liu Y, Lockwood J, Burn P, Shi Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J Biol Chem 2004;279:31727–34. [DOI] [PubMed] [Google Scholar]
- 35. Li J, Romestaing C, Han X, Li Y, Hao X, Wu Y, Sun C, Liu X, Jefferson LS, Xiong J. et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab 2010;12:154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murphy DJ, Vance J. Mechanisms of lipid-body formation. Trends Biochem Sci 1999;24:109–15. [DOI] [PubMed] [Google Scholar]
- 37. Yao Z, Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol 2012;23:206–12. [DOI] [PubMed] [Google Scholar]
- 38. Nishimaki-Mogami T, Yao Z, Fujimori K. Inhibition of phosphatidylcholine synthesis via the phosphatidylethanolamine methylation pathway impairs incorporation of bulk lipids into VLDL in cultured rat hepatocytes. J Lipid Res 2002;43:1035–45. [DOI] [PubMed] [Google Scholar]
- 39. Fernie AR, Carrari F, Sweetlove LJ. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol 2004;7:254–61. [DOI] [PubMed] [Google Scholar]
- 40. Strauss M, Hofhaus G, Schröder RR, Kühlbrandt W. Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J 2008;27:1154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vonck J, Schäfer E. Supramolecular organization of protein complexes in the mitochondrial inner membrane. Biochim Biophys Acta 2009;1793:117–24. [DOI] [PubMed] [Google Scholar]
- 42. Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res 2013;52:590–614. [DOI] [PubMed] [Google Scholar]
- 43. Schlame M, Ren M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim Biophys Acta 2009;1788:2080–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verkman AS. Solute and macromolecule diffusion in cellular aqueous compartments. Trends Biochem Sci 2002;27:27–33. [DOI] [PubMed] [Google Scholar]
- 45. Calamita G, Ferri D, Gena P, Liquori GE, Cavalier A, Thomas D, Svelto M. The inner mitochondrial membrane has aquaporin-8 water channels and is highly permeable to water. J Biol Chem 2005;280:17149–53. [DOI] [PubMed] [Google Scholar]
- 46. Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol 1998;201:1129. [DOI] [PubMed] [Google Scholar]
- 47. Nicholls DG. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem 1974;50:305–15. [DOI] [PubMed] [Google Scholar]
- 48. Saraste M. Oxidative phosphorylation at the fin de siecle .Science 1999;283:1488. [DOI] [PubMed] [Google Scholar]
- 49. Shaikh SR, Brown DA. Models of plasma membrane organization can be applied to mitochondrial membranes to target human health and disease with polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 2012;88:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Palade GE. The fine structure of mitochondria. Anat Rec 1952;114:427–51. [DOI] [PubMed] [Google Scholar]
- 51. Sjostrand FS. Electron microscopy of mitochondria and cytoplasmic double membranes: ultra-structure of rod-shaped mitochondria. Nature 1953;171:30–1. [DOI] [PubMed] [Google Scholar]
- 52. Rasmussen N. Mitochondrial structure and the practice of cell biology in the 1950s. J Hist Biol 1995;28:381–429. [DOI] [PubMed] [Google Scholar]
- 53. Mannella CA. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta 2006;1763:542–8. [DOI] [PubMed] [Google Scholar]
- 54. Zick M, Rabl R, Reichert AS. Cristae formation—linking ultrastructure and function of mitochondria. Biochim Biophys Acta 2009;1793:5–19. [DOI] [PubMed] [Google Scholar]
- 55. Cogliati S, Enriquez JA, Scorrano L. Mitochondrial cristae: where beauty meets functionality. Trends Biochem Sci 2016;41:261–73. [DOI] [PubMed] [Google Scholar]
- 56. Palade GE. An electron microscope study of the mitochondrial structure. J Histochem Cytochem 1953;1:188–211. [DOI] [PubMed] [Google Scholar]
- 57. Sjöstrand FS. The ultrastructure of cells as revealed by the electron microscope. Int Rev Cytol 1956;5:455–533. [Google Scholar]
- 58. Mannella CA, Marko M, Penczek P, Barnard D, Frank J. The internal compartmentation of rat-liver mitochondria: tomographic study using the high-voltage transmission electron microscope. Microsc Res Tech 1994;27:278–83. [DOI] [PubMed] [Google Scholar]
- 59. Mannella CA, Marko M, Buttle K. Reconsidering mitochondrial structure: new views of an old organelle. Trends Biochem Sci 2017;22:37–8. [DOI] [PubMed] [Google Scholar]
- 60. Frey TG, Mannella CA. The internal structure of mitochondria. Trends Biochem Sci 2000;25:319–24. [DOI] [PubMed] [Google Scholar]
- 61. Mannella CA, Pfeiffer DR, Bradshaw PC, Moraru II, Slepchenko B, Loew LM, Hsieh CE, Buttle K, Marko M. Topology of the mitochondrial inner membrane: dynamics and bioenergetic implications. IUBMB Life 2001;52:93–100. [DOI] [PubMed] [Google Scholar]
- 62. Frey TG, Renken CW, Perkins GA. Insight into mitochondrial structure and function from electron tomography. Biochim Biophys Acta 2002;1555:196–203. [DOI] [PubMed] [Google Scholar]
- 63. Olofsson G, Sparr E. Ionization constants pKa of cardiolipin. PLoS One 2013;8:e73040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kates M, Syz J, Gosser D, Haines TH. pH-dissociation characteristics of cardiolipin and its 2'-deoxy analogue. Lipids 1993;28:877–82. [DOI] [PubMed] [Google Scholar]
- 65. Lewis RNAH, McElhaney RN. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim Biophys Acta 2009;1788:2069–79. [DOI] [PubMed] [Google Scholar]
- 66. Haines TH, Dencher NA. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Lett 2002;528:35–9. [DOI] [PubMed] [Google Scholar]
- 67. Mileykovskaya E, Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta 2009;1788:2084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Cardiolipin and mitochondrial function in health and disease. Antioxid Redox Signal 2014;20:1925–53. [DOI] [PubMed] [Google Scholar]
- 69. Claypool SM, Koehler CM. The complexity of cardiolipin in health and disease. Trends Biochem Sci 2016;37:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 2014;1837:408–17. [DOI] [PubMed] [Google Scholar]
- 71. Klingenberg M. Cardiolipin and mitochondrial carriers. Biochim Biophys Acta 2009;1788:2048–58. [DOI] [PubMed] [Google Scholar]
- 72. Shen Z, Ye C, McCain K, Greenberg ML. The role of cardiolipin in cardiovascular health. BioMed Res Int 2015;2015:891707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brown DA, Sabbah HN, Shaikh SR. Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion injury. Pharmacol Ther 2013;140:258–66. [DOI] [PubMed] [Google Scholar]
- 74. Genova ML, Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochim Biophys Acta 2014;1837:427–43. [DOI] [PubMed] [Google Scholar]
- 75. Mileykovskaya E, Dowhan W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem Phys Lipids 2014;179:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schwall CT, Greenwood VL, Alder NN. The stability and activity of respiratory complex II is cardiolipin-dependent. Biochim Biophys Acta 2012;1817:1588–96. [DOI] [PubMed] [Google Scholar]
- 77. Bazán S, Mileykovskaya E, Mallampalli VKPS, Heacock P, Sparagna GC, Dowhan W. Cardiolipin-dependent reconstitution of respiratory supercomplexes from purified saccharomyces cerevisiae complexes III and IV. J Biol Chem 2013;288:401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Phys Cell Phys 2007;292:C33–44. [DOI] [PubMed] [Google Scholar]
- 79. Eble KS, Coleman WB, Hantgan RR, Cunningham CC. Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J Biol Chem 1990;265:19434–40. [PubMed] [Google Scholar]
- 80. Arnarez C, Marrink SJ, Periole X. Identification of cardiolipin binding sites on cytochrome c oxidase at the entrance of proton channels. Sci Rep 2013;3:1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Arnarez C, Mazat J, Elezgaray J, Marrink S, Periole X. Evidence for cardiolipin binding sites on the membrane-exposed surface of the cytochrome bc1. J Am Chem Soc 2013;135:3112–20. [DOI] [PubMed] [Google Scholar]
- 82. Planas-Iglesias J, Dwarakanath H, Mohammadyani D, Yanamala N, Kagan V, Klein-Seetharaman J. Cardiolipin interactions with proteins. Biophys J 2015;109:1282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol 2014;20:14205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Iverson SL, Orrenius S. The cardiolipin–cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys 2004;423:37–46. [DOI] [PubMed] [Google Scholar]
- 85. Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together: cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem 2002;277:43553–6. [DOI] [PubMed] [Google Scholar]
- 86. Zhang M, Mileykovskaya E, Dowhan W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J Biol Chem 2005;280:29403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mejia EM, Nguyen H, Hatch GM. Mammalian cardiolipin biosynthesis. Chem Phys Lipids 2014;179:11–6. [DOI] [PubMed] [Google Scholar]
- 88. Sullivan EM, Fix A, Crouch MJ, Sparagna GC, Zeczycki TN, Brown DA, Shaikh SR. Murine diet-induced obesity remodels cardiac and liver mitochondrial phospholipid acyl chains with differential effects on respiratory enzyme activity. J Nutr Biochem 2017;45:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett 2006;580:5450–5. [DOI] [PubMed] [Google Scholar]
- 90. Simons K, Vaz WLC. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct 2004;33:269–95. [DOI] [PubMed] [Google Scholar]
- 91. Soula H, Coulon A, Beslon G. Membrane microdomains emergence through non-homogeneous diffusion. BMC Biophys 2012;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science 2010;327:46–50. [DOI] [PubMed] [Google Scholar]
- 93. Jessup W. Membrane microdomain signaling: lipid rafts in biology and medicine. Immunol Cell Biol 2005;83:449. [Google Scholar]
- 94. Fan J, Sammalkorpi M, Haataja M. Formation and regulation of lipid microdomains in cell membranes: theory, modeling, and speculation. FEBS Lett 2010;584:1678–84. [DOI] [PubMed] [Google Scholar]
- 95. Zheng YZ, Berg KB, Foster LJ. Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J Lipid Res 2009;50:988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Osman C, Voelker DR, Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol 2011;192:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Renner LD, Weibel DB. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc Natl Acad Sci USA 2011;108:6264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jouhet J. Importance of the hexagonal lipid phase in biological membrane organization. Front Plant Sci 2013;4:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. de Kruijff B, Verkleij AJ, van Echteld CJA, Gerritsen WJ, Noordam PC, Mombers C, Rietveld A, de Gier J, Cullis PR, Hope MJ. et al. Non-bilayer lipids and the inner mitochondrial membrane. Cell Biol Int 1981:559–71. [Google Scholar]
- 100. Stepanyants N, Macdonald PJ, Francy CA, Mears JA, Qi X, Ramachandran R. Cardiolipin's propensity for phase transition and its reorganization by dynamin-related protein 1 form a basis for mitochondrial membrane fission. Mol Biol Cell 2015;26:3104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Beales PA, Bergstrom CL, Geerts N, Groves JT, Vanderlick TK. Single vesicle observations of the cardiolipin-cytochrome c interaction: induction of membrane morphology changes. Langmuir 2011;27:6107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Maniti O, Lecompte M, Marcillat O, Desbat B, Buchet R, Vial C, Granjon T. Mitochondrial creatine kinase binding to phospholipid monolayers induces cardiolipin segregation. Biophys J 2008;96:2428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Epand RF, Tokarska-Schlattner M, Schlattner U, Wallimann T, Epand RM. Cardiolipin clusters and membrane domain formation induced by mitochondrial proteins. J Mol Biol 2007;365:968–80. [DOI] [PubMed] [Google Scholar]
- 104. Trusova VM, Gorbenko GP, Molotkovsky JG, Kinnunen PKJ. Cytochrome c-lipid interactions: new insights from resonance energy transfer. Biophys J 2010;99:1754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bogdanov M, Mileykovskaya E, Dowhan W. Lipids in the assembly of membrane proteins and organization of protein supercomplexes: implications for lipid-linked disorders. Subcell Biochem 2008;49:197–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kuhlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol 2015;13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Andersson A, Nälsén C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr 2002;76:1222–9. [DOI] [PubMed] [Google Scholar]
- 108. Schumann J, Leichtle A, Thiery J, Fuhrmann H. Fatty acid and peptide profiles in plasma membrane and membrane rafts of PUFA supplemented RAW264.7 macrophages. PLoS One 2011;6:e24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore K, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res 2012;53:2002–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Almeida PFF, Pokorny A, Hinderliter A. Thermodynamics of membrane domains. Biochem Biophys Acta 2005;1720:1–13. [DOI] [PubMed] [Google Scholar]
- 111. Pitman MC, Suits F, MacKerell AD, Feller SE. Molecular-level organization of saturated and polyunsaturated fatty acids in a phosphatidylcholine bilayer containing cholesterol. Biochemistry 2004;43:15318–28. [DOI] [PubMed] [Google Scholar]
- 112. Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 2010;11:688–99. [DOI] [PubMed] [Google Scholar]
- 113. Crescenzo R, Bianco F, Mazzoli A, Giacco A, Cancelliere R, di Fabio G, Zarrelli A, Liverini G, Iossa S. Fat quality influences the obesogenic effect of high fat diets. Nutrients 2015;7:9475–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ruiz-Ramirez A, Barrios-Maya M, Lopez-Acosta O, Molina-Ortiz D, El-Hafidi M. Cytochrome c release from rat liver mitochondria is compromised by increased saturated cardiolipin species induced by sucrose feeding. Am J Physiol Endocrinol Metab 2015;309:E777. [DOI] [PubMed] [Google Scholar]
- 115. Rajamoorthi K, Petrache HI, McIntosh TJ, Brown MF. Packing and viscoelasticity of polyunsaturated ω-3 and ω-6 lipid bilayers as seen by 2H NMR and X-ray diffraction. J Am Chem Soc 2005;127:1576–88. [DOI] [PubMed] [Google Scholar]
- 116. Rockett BD, Franklin A, Harris M, Teague H, Rockett A, Shaikh SR. Membrane raft organization is more sensitive to disruption by (n-3) PUFA than nonraft organization in EL4 and B cells. J Nutr 2011;141:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J Cell Biol 2008;182:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, Greenberg ML. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J Biol Chem 2000;275:22387–94. [DOI] [PubMed] [Google Scholar]
- 119. Gohil VM, Hayes P, Matsuyama S, Schägger H, Schlame M, Greenberg ML. Cardiolipin biosynthesis and mitochondrial respiratory chain function are interdependent. J Biol Chem 2004;279:42612–8. [DOI] [PubMed] [Google Scholar]
- 120. Dudek J, Cheng I, Balleininger M, Vaz FM, Streckfuss-Bömeke K, Hübscher D, Vukotic M, Wanders RJA, Rehling P, Guan K. Cardiolipin deficiency affects respiratory chain function and organization in an induced pluripotent stem cell model of Barth syndrome. Stem Cell Res 2013;11:806–19. [DOI] [PubMed] [Google Scholar]
- 121. Kiebish MA, Yang K, Liu X, Mancuso DJ, Guan S, Zhao Z, Sims HF, Cerqua R, Cade WT, Han X, Gross RW. Dysfunctional cardiac mitochondrial bioenergetic, lipidomic, and signaling in a murine model of Barth syndrome. J Lipid Res 2013;54:1312–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Baile MG, Sathappa M, Lu Y, Pryce E, Whited K, McCaffery JM, Han X, Alder NN, Claypool SM. Unremodeled and remodeled cardiolipin are functionally indistinguishable in yeast. J Biol Chem 2014;289:1768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ye C, Shen Z, Greenberg ML. Cardiolipin remodeling: a regulatory hub for modulating cardiolipin metabolism and function. J Bioenerg Biomembr 2016;48:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cocco T, Di M, Papa P, Lorusso M. Arachidonic acid interaction with the mitochondrial electron transport chain promotes reactive oxygen species generation. Free Radic Biol Med 1999;27:51–9. [DOI] [PubMed] [Google Scholar]
- 125. Khairallah RJ, Kim J, O'Shea KM, O'Connell KA, Brown BH, Galvao T, Daneault C, Des Rosiers C, Polster BM, Hoppel CL. et al. Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids. PLoS One 2012;7:e34402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition: implications for tumor necrosis factor-a apoptotic signaling. J Biol Chem 2001;276:12035–40. [DOI] [PubMed] [Google Scholar]
- 127. Simopoulos PA. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016;8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Liu H, Qiu Y, Mu Y, Zhang X, Liu L, Hou XH, Zhang L, Xu XN, Ji AL, Cao R. et al. A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats. Nutr Res 2013;33:849–58. [DOI] [PubMed] [Google Scholar]
- 129. Russo GL. Dietary n−6 and n−3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol 2009;77:937–46. [DOI] [PubMed] [Google Scholar]
- 130. Shaikh SR, Kinnun JJ, Leng X, Williams JA, Wassall SR. How polyunsaturated fatty acids modify molecular organization in membranes: insight from NMR studies of model systems. Biochem Biophys Acta 2015;1848(1, Part B):211–9. [DOI] [PubMed] [Google Scholar]
- 131. Stulnig TM, Huber J, Leitinger N, Imre E, Angelisová P, Nowotny P, Waldhäusl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem 2001;276:37335–40. [DOI] [PubMed] [Google Scholar]
- 132. Schley PD, Brindley DN, Field CJ. (n-3) PUFAs alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr 2007;137:548–53. [DOI] [PubMed] [Google Scholar]
- 133. Shaikh SR. Biophysical and biochemical mechanisms by which dietary n-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J Nutr Biochem 2012;23:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kucerka N, Marquardt D, Harroun TA, Nieh M, Wassall SR, de Jong DH, Schäfer LV, Marrink SJ, Katsaras J. Cholesterol in bilayers with PUFA chains: doping with DMPC or POPC results in sterol reorientation and membrane-domain formation. Biochemistry 2010;49:7485–93. [DOI] [PubMed] [Google Scholar]
- 135. Li Q, Wang M, Tan L, Wang C, Ma J, Li N, Li Y, Xu G, Li J. Docosahexaenoic acid changes lipid composition and interleukin-2 receptor signaling in membrane rafts. J Lipid Res 2005;46:1904–13. [DOI] [PubMed] [Google Scholar]
- 136. Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 2013;88:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ting H, Chao Y, Hsu YH. Polyunsaturated fatty acids incorporation into cardiolipin in H9c2 cardiac myoblast. J Nutr Biochem 2015;26:769–75. [DOI] [PubMed] [Google Scholar]
- 138. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 1997;94:4312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 2011;1812:1007–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K. et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA 2003;100:15924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 1996;137: 354–66. [DOI] [PubMed] [Google Scholar]
- 142. Brun RP, Tontonoz P, Forman BM, Ellis R, Chen J, Evans RM, Spiegelman BM. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev 1996;10:974–84. [DOI] [PubMed] [Google Scholar]
- 143. Széles L, Töröcsik D, Nagy L. PPARγ in immunity and inflammation: cell types and diseases. Biochim Biophys Acta 2007;1771:1014–30. [DOI] [PubMed] [Google Scholar]
- 144. Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci 2004;61:393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hancock CR, Han D, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 2008;105:7815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Jorgensen T, Grunnet N, Quistorff B. One-year high fat diet affects muscle-but not brain mitochondria. J Cereb Blood Flow Metab 2015;35:943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kakimoto PA, Kowaltowski AJ. Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol 2016;8:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Stillwell W, Jenski LJ, Thomas Crump F, Ehringer W. Effect of docosahexaenoic acid on mouse mitochondrial membrane properties. Lipids 1997;32:497–506. [DOI] [PubMed] [Google Scholar]
- 149. Pennington ER, Fix A, Sullivan EM, Brown DA, Kennedy A, Shaikh SR. Distinct membrane properties are differentially influenced by cardiolipin content and acyl chain composition in biomimetic membranes. Biochim Biophys Acta 2017;1859:257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sullivan EM, Pennington ER, Sparagna GC, Torres MJ, Neufer PD, Harris M, Washington J, Anderson EJ, Zeczycki TN, Brown DA. et al. Docosahexaenoic acid lowers cardiac mitochondrial enzyme activity by replacing linoleic acid in the phospholipidome. J Biol Chem 2018;293:466–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Herbst EAF, Paglialunga S, Gerling C, Whitfield J, Mukai K, Chabowski A, Heigenhauser GJF, Spriet LL, Holloway GP. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J Physiol 2014;592:1341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Khairallah RJ, Sparagna GC, Khanna N, O'Shea KM, Hecker PA, Kristian T, Fiskum G, Des Rosiers C, Polster BM, Stanley WC. Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochim Biophys Acta 2010;1797:1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, Sanders LM, Fan YY, Davidson LA, Murphy ME. et al. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis 2002;23:1919–26. [DOI] [PubMed] [Google Scholar]
- 154. Malis CD, Weber PC, Leaf A, Bonventre JV. Incorporation of marine lipids into mitochondrial membranes increases susceptibility to damage by calcium and reactive oxygen species: evidence for enhanced activation of phospholipase A2 in mitochondria enriched with n-3 fatty acids. Proc Natl Acad Sci USA 1990;87:8845–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chapkin RS, Young Hong M, Fan Y, Davidson LA, Sanders LM, Henderson CE, Barhoumi R, Burghardt RC, Turner ND, Lupton JR. Dietary n-3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids 2002;37:193. [DOI] [PubMed] [Google Scholar]
- 156. Aoun M, Feillet-Coudray C, Fouret G, Chabi B, Crouzier D, Ferreri C, Chatgilialoglu C, Wrutniak-Cabello C, Cristol, Carbonneau MA. et al. Rat liver mitochondrial membrane characteristics and mitochondrial functions are more profoundly altered by dietary lipid quantity than by dietary lipid quality: effect of different nutritional lipid patterns. Br J Nutr 2012;107:647–59. [DOI] [PubMed] [Google Scholar]
- 157. Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 2003;278:36027–31. [DOI] [PubMed] [Google Scholar]
- 158. Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 2004;37:755–67. [DOI] [PubMed] [Google Scholar]
- 159. Watkins SM, Carter LC, German JB. Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J Lipid Res 1998;39:1583–8. [PubMed] [Google Scholar]
- 160. McLean LR, Hagaman KA, Davidson WS. Role of lipid structure in the activation of phospholipase A2 by peroxidized phospholipids. Lipids 1993;28:505–9. [DOI] [PubMed] [Google Scholar]
- 161. Abdukeyum GG, Owen JA, Larkin AT, McLennan LP. Up-regulation of mitochondrial antioxidant superoxide dismutase underpins persistent cardiac nutritional-preconditioning by long chain n-3 polyunsaturated fatty acids in the rat. J Clin Med 2016;5. doi: 10.3390/jcm5030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Di Nunzio M, Valli V, Bordoni A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot Essent Fatty Acids 2011;85:121–7. [DOI] [PubMed] [Google Scholar]
- 163. Anderson E, Thayne K, Harris M, Carraway K, Shaikh S. Aldehyde stress and up-regulation of Nrf2-mediated antioxidant systems accompany functional adaptations in cardiac mitochondria from mice fed n-3 polyunsaturated fatty acids. Biochem J 2011;441:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Giordano E, Visioli F. Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions. Prostaglandins Leukot Essent Fatty Acids 2014;90:1–4. [DOI] [PubMed] [Google Scholar]
- 165. Richard D, Wolf C, Barbe U, Kefi K, Bausero P, Visioli F. Docosahexaenoic acid down-regulates endothelial Nox 4 through a sPLA2 signalling pathway. Biochem Biophys Res Commun 2009;389:516–22. [DOI] [PubMed] [Google Scholar]
- 166. Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem 1981;256:1874–80. [PubMed] [Google Scholar]
- 167. Hunte C, Palsdottir H, Trumpower BL. Protonmotive pathways and mechanisms in the cytochrome bc1 complex. FEBS Lett 2003;545:39–46. [DOI] [PubMed] [Google Scholar]
- 168. Robinson NC. Specificity and binding affinity of phospholipids to the high-affinity cardiolipin sites of beef heart cytochrome c oxidase. Biochemistry 1982;21:184–8. [DOI] [PubMed] [Google Scholar]
- 169. Robinson NC. Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr 1993;25:153–63. [DOI] [PubMed] [Google Scholar]
- 170. Sedlak E, Robinson NC. Phospholipase A2 digestion of cardiolipin bound to bovine cytochrome c oxidase alters both activity and quaternary structure. Biochemistry 1999;38:14966–72. [DOI] [PubMed] [Google Scholar]
- 171. Sedlak E, Panda M, Dale MP, Weintraub ST, Robinson NC. Photolabeling of cardiolipin binding subunits within bovine heart cytochrome c oxidase. Biochemistry 2006;45:746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Paumard P, Vaillier J, Coulary B, Schaeffer J, Soubannier V, Mueller DM, Brèthes D, di Rago JP, Velours J. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J 2002;21:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Acehan D, Malhotra A, Xu Y, Ren M, Stokes D, Schlame M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J 2011;100:2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Milenkovic D, Blaza JN, Larsson N, Hirst J. The enigma of the respiratory chain supercomplex. Cell Metab 2017;25:765–76. [DOI] [PubMed] [Google Scholar]
- 175. Gonzalvez F, D'Aurelio M, Boutant M, Moustapha A, Puech J, Landes T, Arnauné-Pelloquin L, Vial G, Taleux N, Slomianny C. et al. Barth syndrome: cellular compensation of mitochondrial dysfunction and apoptosis inhibition due to changes in cardiolipin remodeling linked to tafazzin (TAZ) gene mutation. Biochim Biophys Acta 2013;1832:1194–206. [DOI] [PubMed] [Google Scholar]
- 176. McKenzie M, Lazarou M, Thorburn DR, Ryan MT. Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. J Mol Biol 2006;361:462–9. [DOI] [PubMed] [Google Scholar]
- 177. Luo X, Cai H, Ni J, Bhindi R, Lowe HC, Chesterman CN, Khachigian LM. c-Jun DNAzymes inhibit myocardial inflammation, ROS generation, infarct size, and improve cardiac function after ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 2009;29:1836. [DOI] [PubMed] [Google Scholar]
- 178. Hu H, Zhai C, Qian G, Gu A, Liu J, Ying F, Xu W, Jin D, Wang H, Hu H. et al. Protective effects of tanshinone IIA on myocardial ischemia reperfusion injury by reducing oxidative stress, HMGB1 expression, and inflammatory reaction. Pharm Biol 2015;53:1752–8. [DOI] [PubMed] [Google Scholar]
- 179. Asada D, Itoi T, Nakamura A, Hamaoka K. Tolerance to ischemia reperfusion injury in a congenital heart disease model. Pediatr Int 2016;58:1266–73. [DOI] [PubMed] [Google Scholar]
- 180. Farias JG, Carrasco-Pozo C, Carrasco Loza R, Sepulveda N, Alvarez P, Quezada M, Quiñones J, Molina V, Castillo RL. Polyunsaturated fatty acid induces cardioprotection against ischemia-reperfusion through the inhibition of NF-kappaB and induction of Nrf2. Exp Biol Med 2017;242:1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Madingou N, Gilbert K, Tomaro L, Prud'homme Touchette C, Trudeau F, Fortin S, Rousseau G. Comparison of the effects of EPA and DHA alone or in combination in a murine model of myocardial infarction. Prostaglandins Leukot Essent Fatty Acids 2016;111:11–6. [DOI] [PubMed] [Google Scholar]
- 182. McMillin JB, Bick RJ, Benedict CR. Influence of dietary fish oil on mitochondrial function and response to ischemia. Am J Physiol Heart Circ Physiol 1992;263:H1479. [DOI] [PubMed] [Google Scholar]
- 183. Castillo RL, Arias C, Farías JG. Omega 3 chronic supplementation attenuates myocardial ischaemia-reperfusion injury through reinforcement of antioxidant defense system in rats. Cell Biochem Funct 2014;32:274–81. [DOI] [PubMed] [Google Scholar]
- 184. Gao K, Chen L, Yang M, Han L, Yiguang S, Zhao H, Chen X, Hu W, Liang H, Luo J, Ma J. Marine n-3 PUFA protects hearts from I/R injury via restoration of mitochondrial function. Scand Cardiovasc J 2015;49:264–9. [DOI] [PubMed] [Google Scholar]
- 185. Burban M, Meyer G, Olland A, Severac F, Yver B, Toti F, Schini-Kerth V, Meziani F, Boisramé-Helms J. An intravenous bolus of EPA:DHA 6:1 protects against myocardial ischemia-reperfusion-induced shock. Shock 2016;46:549–56. [DOI] [PubMed] [Google Scholar]
- 186. Richard D, Oszust F, Guillaume C, Millart H, Laurent-Maquin D, Brou C, Bausero P, Visioli F. Infusion of docosahexaenoic acid protects against myocardial infarction. Prostaglandins Leukot Essent Fatty Acids 2014;90:139–43. [DOI] [PubMed] [Google Scholar]
- 187. Stanley WC, Khairallah RJ, Dabkowski ER. Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care 2012;15:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. O'Shea KM, Khairallah RJ, Sparagna GC, Xu W, Hecker PA, Robillard-Frayne I, Des Rosiers C, Kristian T, Murphy RC, Fiskum G. et al. Dietary ω-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol 2009;47:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Michas G, Micha R, Zampelas A. Dietary fats and cardiovascular disease: putting together the pieces of a complicated puzzle. Atherosclerosis 2014;234:320–8. [DOI] [PubMed] [Google Scholar]
- 190. Pinel A, Pitois E, Rigaudiere J, Jouve C, De Saint-Vincent S, Laillet B, Montaurier C, Huertas A, Morio B, Capel F. EPA prevents fat mass expansion and metabolic disturbances in mice fed with a Western diet. J Lipid Res 2016;57:1382–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Pinel A, Morio-Liondore B, Capel F. n-3 Polyunsaturated fatty acids modulate metabolism of insulin-sensitive tissues: implication for the prevention of type 2 diabetes. J Physiol Biochem 2014;70:647–58. [DOI] [PubMed] [Google Scholar]
- 192. Lorente-Cebrian S, Costa AG, Navas-Carretero S, Zabala M, Martinez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J Physiol Biochem 2013;69:633–51. [DOI] [PubMed] [Google Scholar]
- 193. Ovide-Bordeaux S, Grynberg A. Docosahexaenoic acid affects insulin deficiency- and insulin resistance-induced alterations in cardiac mitochondria. Am J Physiol Regul Integr Comp Physiol 2004;286:R519. [DOI] [PubMed] [Google Scholar]
- 194. Janovská P, Flachs P, Kazdová L, Kopecký J. Anti-obesity effect of n-3 polyunsaturated fatty acids in mice fed high-fat diet is independent of cold-induced thermogenesis. Physiol Res 2013;62:153–61. [DOI] [PubMed] [Google Scholar]
- 195. Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Vecka M, Tvrzicka E, Bryhn M, Kopecky J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004;39:1177–85. [DOI] [PubMed] [Google Scholar]
- 196. Damsgaard CT, Stark KD, Hjorth MF, Biltoft-Jensen A, Astrup A, Michaelsen KF, Lauritzen L. n-3 PUFA status in school children is associated with beneficial lipid profile, reduced physical activity and increased blood pressure in boys. Br J Nutr 2013;110:1304–12. [DOI] [PubMed] [Google Scholar]
- 197. Rockett BD, Harris M, Shaikh SR. High dose of an n-3 polyunsaturated fatty acid diet lowers activity of C57BL/6 mice. Prostaglandins Leukot Essent Fatty Acids 2012;86:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Han X, Yang J, Cheng H, Yang K, Abendschein DR, Gross RW. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry 2005;44:16684–94. [DOI] [PubMed] [Google Scholar]
- 199. Han X, Yang J, Yang K, Zhao Z, Abendschein DR, Gross RW. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry 2007;46:6417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. J Am Med Assoc 2015;313:2263–73. [DOI] [PubMed] [Google Scholar]
- 201. Nair S, P. Chacko V, Arnold C, Diehl AM. Hepatic ATP reserve and efficiency of replenishing. Am J Gastroenterol 2003;98:466–70. [DOI] [PubMed] [Google Scholar]
- 202. Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2007;22:S20–7. [DOI] [PubMed] [Google Scholar]
- 203. Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW. et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 2010;52:727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001;120:1183–92. [DOI] [PubMed] [Google Scholar]
- 205. Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol 2002;37:56–62. [DOI] [PubMed] [Google Scholar]
- 206. Pérez-Carreras M, Del Hoyo P, Martín MA, Rubio JC, Martín A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 2003;38:999–1007. [DOI] [PubMed] [Google Scholar]
- 207. Fromenty B, Robin M, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab 2004;30:121–38. [DOI] [PubMed] [Google Scholar]
- 208. Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bedogni G, Svegliati-Baroni G, Sofi F, Milani S, Abbate R. et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther 2006;23:1143–51. [DOI] [PubMed] [Google Scholar]
- 209. de Castro GS, Calder PC. Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids. Clin Nutr 2018;37(1):37–55. [DOI] [PubMed] [Google Scholar]
- 210. Di Minno MND, Russolillo A, Lupoli R, Ambrosino P, Di Minno A, Tarantino G. Omega-3 fatty acids for the treatment of non-alcoholic fatty liver disease. World J Gastroenterol 2012;18:5839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Dongiovanni P, Lanti C, Riso P, Valenti L. Nutritional therapy for nonalcoholic fatty liver disease. J Nutr Biochem 2016;29:1–11. [DOI] [PubMed] [Google Scholar]
- 212. Masterton GS, Plevris JN, Hayes PC. Review article: omega-3 fatty acids—a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2010;31:679–92. [DOI] [PubMed] [Google Scholar]
- 213. Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr 2013;33:231–48. [DOI] [PubMed] [Google Scholar]
- 214. Shapiro H, Tehilla M, Attal-Singer J, Bruck R, Luzzatti R, Singer P. The therapeutic potential of long-chain omega-3 fatty acids in nonalcoholic fatty liver disease. Clin Nutr 2011;30:6–19. [DOI] [PubMed] [Google Scholar]
- 215. Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, Papa G, Rabuazzo AM, Purrello F. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis 2008;40:194–9. [DOI] [PubMed] [Google Scholar]
- 216. Tapia G, Valenzuela R, Espinosa A, Romanque P, Dossi C, Gonzalez-Mañán D, Videla LA, D'Espessailles A. n-3 Long-chain PUFA supplementation prevents high fat diet induced mouse liver steatosis and inflammation in relation to PPAR-α upregulation and NF-κB DNA binding abrogation. Mol Nutr Food Res 2014;58:1333–41. [DOI] [PubMed] [Google Scholar]
- 217. Valenzuela R, Videla LA. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct 2011;2:644–8. [DOI] [PubMed] [Google Scholar]
- 218. Nogueira MA, Oliveira CP, Ferreira Alves VA, Stefano JT, Rodrigues LS, Torrinhas RS, Cogliati B, Barbeiro H, Carrilho FJ, Waitzberg DL. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2016;35:578–86. [DOI] [PubMed] [Google Scholar]
- 219. Kelley NS. Treatment of nonalcoholic fatty liver disease with long-chain n-3 polyunsaturated fatty acids in humans. Metab Syndr Relat Disord 2016;14:417–30. [DOI] [PubMed] [Google Scholar]
- 220. Zhang Y, Jiang L, Hu W, Zheng Q, Xiang W. Mitochondrial dysfunction during in vitro hepatocyte steatosis is reversed by omega-3 fatty acid–induced up-regulation of mitofusin 2. Metabolism 2011;60:767–75. [DOI] [PubMed] [Google Scholar]
- 221. Kolar SSN, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res 2007;67:5561. [DOI] [PubMed] [Google Scholar]
- 222. Fan Y, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr 2003;133:1913–20. [DOI] [PubMed] [Google Scholar]
- 223. Arita K, Kobuchi H, Utsumi T, Takehara Y, Akiyama J, Horton AA, Utsumi K. Mechanism of apoptosis in HL-60 cells induced by n-3 and n-6 polyunsaturated fatty acids. Biochem Pharmacol 2001;62:821–8. [DOI] [PubMed] [Google Scholar]
- 224. Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr 2004;79:935–45. [DOI] [PubMed] [Google Scholar]
- 225. Harris M, Kinnun JJ, Kosaraju R, Leng X, Wassall SR, Shaikh SR. Membrane disordering by eicosapentaenoic acid in B lymphomas is reduced by elongation to docosapentaenoic acid as revealed with solid-state nuclear magnetic resonance spectroscopy of model membranes. J Nutr 2016;146:1283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Davidson LA, Nguyen DV, Hokanson RM, Callaway ES, Isett RB, Turner ND, Dougherty ER, Wang N, Lupton JR, Carroll RJ. et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res 2004;64:6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Schley PD, Jijon HB, Robinson LE, Field CJ. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat 2005;92:187–95. [DOI] [PubMed] [Google Scholar]
- 228. Ng Y, Barhoumi R, Tjalkens RB, Fan Y, Kolar S, Wang N, Lupton JR, Chapkin RS. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis 2005;26:1914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Giros A, Grzybowski M, Sohn VR, Pons E, Fernandez-Morales J, Xicola RM, Sethi P, Grzybowski J, Goel A, Boland CR. et al. Regulation of colorectal cancer cell apoptosis by the n-3 polyunsaturated fatty acids docosahexaenoic and eicosapentaenoic. Cancer Prev Res 2009;2:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Khatib SA, Rossi EL, Bowers LW, Hursting SD. Reducing the burden of obesity-associated cancers with anti-inflammatory long-chain omega-3 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat 2016;125:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol 2015;27:200–15. [DOI] [PMC free article] [PubMed] [Google Scholar]