Abstract
The pharmacokinetic profile of oral cocaine has not been fully characterized and prospective data on oral bioavailability are limited. A within-subject study was performed to characterize the bioavailability and pharmacokinetics of oral cocaine. Fourteen healthy inpatient participants (six males) with current histories of cocaine use were administered two oral doses (100 and 200 mg) and one intravenous (IV) dose (40 mg) of cocaine during three separate dosing sessions. Plasma samples were collected for up to 24 h after dosing and analyzed for cocaine and metabolites by gas chromatography-mass spectrometry. Pharmacokinetic parameters were calculated by non-compartmental analysis, and a two-factor model was used to assess for dose and sex differences. The mean ± SEM oral cocaine bioavailability was 0.32 ± 0.04 after 100 and 0.45 ± 0.06 after 200 mg oral cocaine. Volume of distribution (Vd) and clearance (CL) were both greatest after 100 mg oral (Vd = 4.2 L/kg; CL = 116.2 mL/[min kg]) compared to 200 mg oral (Vd = 2.9 L/kg; CL = 87.5 mL/[min kg]) and 40 mg IV (Vd = 1.3 L/kg; CL = 32.7 mL/[min kg]). Oral cocaine area-under-thecurve (AUC) and peak concentration increased in a dose-related manner. AUC metabolite-to-parent ratios of benzoylecgonine and ecgonine methyl ester were significantly higher after oral compared to IV administration and highest after the lower oral dose. In addition, minor metabolites were detected in higher concentrations after oral compared to IV cocaine. Oral cocaine produced a pharmacokinetic profile different from IV cocaine, which appears as a rightward and downward shift in the concentration–time profile. Cocaine bioavailability values were similar to previous estimates. Oral cocaine also produced a unique metabolic profile, with greater concentrations of major and minor metabolites.
Keywords: cocaine, oral, pharmacokinetic, bioavailability, benzoylecgonine, ecgonine methyl ester
Introduction
While cocaine is most commonly used by the smoked, intranasal and intravenous (IV) routes of administration, its oral use is widespread throughout South America. The coca leaves are chewed (either alone or with an activating alkaloid such as quinoa stalk ashes) and are used in various foods and beverages (e.g., cookies and teas). The few studies examining orally administered cocaine have demonstrated that orally and parenterally administered cocaine produce a qualitatively similar pharmacodynamic profile, including typical physiological (1) and stimulant-like subjective effects, although the time-to-onset and peak effects are delayed after oral compared to parenteral administration (2, 3).
Pharmacokinetic studies have shown that oral cocaine is absorbed well from the gastrointestinal tract, is detectable in plasma within 30 min of administration, and reaches peak plasma concentrations within 50–90 min (4, 5). Estimates of oral cocaine bioavailability, ranging from 0.20 to 0.60 (5, 6), have been calculated only retrospectively by comparing data across different groups of subjects who received acute doses of either oral or IV cocaine. To date, there have been no prospective studies in humans designed to determine the oral bioavailability of cocaine. In the context of efforts to develop a human laboratory model to study cocaine abuse and withdrawal, a series of studies (3, 7) using orally administered cocaine was conducted that allowed examination of the pharmacokinetic profile of oral cocaine.
Cocaine is largely metabolized through four pathways (8–10): (i) liver carboxylesterase 1 (hCE1) hydrolyzes the methyl ester linkage of cocaine to form benzoylecgonine (BZE), (ii) intestinal carboxylesterase (hCE2) hydrolyzes the benzoate linkage to form ecgonine methyl ester (EME), (iii) serum butyrylcholinesterase (BchE) also produces EME (though with low catalytic efficiency) (11) and (iv) CYP450 3A4 demethylates cocaine to form norcocaine (NCOC). Further oxidative metabolism produces several minor hydroxy (e.g., m- and p-HOCOC; m- and p-HOBZE) metabolites. It has also been reported that cocaine can spontaneously hydrolyze in vitro at physiological temperature and pH to form BZE and EME (12, 13) at a rate of 4.8% total cocaine/h (14), though this observation has not been verified in vivo. The metabolic disposition of oral cocaine administration has received little attention. A previous report (15) indicated that repeated oral cocaine administration produced a metabolic profile that was unlike that observed after parenteral administration; however, these data were obtained after repeated cocaine administration, and it was unclear whether the differences in metabolism were due to the chronicity of dosing, the oral route of administration, or a combination of the two. After acute parenteral administration, plasma area-under-the-curve (AUC) for BZE has been estimated to range from 1- to 7-fold that of cocaine (16), but repeated oral administration resulted in BZE AUC values ~20-fold higher than cocaine (15). Likewise, EME AUCs after parenteral cocaine administration are three-fourths that of cocaine (16) while EME AUCs after repeated oral administration were 5-fold higher than cocaine (15).
Under some circumstances, oral cocaine may be a useful route for human laboratory studies because (i) controlled dosing is easily achieved, (ii) the more gradual onset of effects, due to reduced systemic bioavailability and slower drug delivery to the brain and periphery, may present fewer medical risks than with parenteral administration and (iii) it obviates the exclusion of subjects with poor venous access and/or the need for approved smoking facilities. The present study is the first to characterize the bioavailability of oral cocaine prospectively and adds to the limited literature on the metabolic profile and pharmacokinetics of oral cocaine.
Methods
Chemicals and materials
Drug standards were obtained from the following sources: cocaine hydrochloride (Mallinckrodt, St. Louis, MO); BZE tetrahydrate, NCOC, benzoylnorecgonine (BNE), m-HOCOC, p-HOCOC, m-HOBZE and p-HOBZE (Research Biochemicals International, Natick, MA); and EME HCl, [d3]-cocaine HCl, [d3]-BZE tetrahydrate and [d3]-EME HCl (Sigma Chemical Company, St. Louis, MO). Methanol, methylene chloride, 2-propanol and acetonitrile were HPLC grade and all other chemicals were reagent grade. N,O-bis (trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) was purchased from Pierce Chemical Co. (Rockford, IL). Clean Screen® solid phase extraction columns (ZSDAU020) were purchased from United Chemical Technologies (Bristol, PA).
Participants
To qualify for inclusion, individuals were required to report cocaine use of at least 6 months’ duration, including the use of smoked or IV cocaine at least twice weekly for the 6 weeks prior to admission. A urine drug screen prior to admission confirmed recent cocaine use. A negative pregnancy test was confirmed prior to enrollment and again prior to each drug administration. Participants underwent a physical examination, history, routine laboratory chemistries and psychiatric assessment. This protocol was approved by the local Institutional Review Board and complied with the Declaration of Helsinki. Participants provided written informed consent and were paid for participation.
Clinical protocol
This study was conducted on a closed inpatient research unit. Upon admission, participants began a 1-week washout phase to allow for elimination of cocaine resulting from prior illicit use. Following washout, three single dose sessions, separated by at least 48 h, were conducted to determine oral cocaine bioavailability and pharmacokinetics. Some individuals participated in another study separated by time and with separate washout periods; those data have been previously reported (15, 17). During each of three test sessions, participants received cocaine at 40 mg, IV, and 100 and 200 mg, oral. The IV dose was administered in a volume of 1 mL over a 1 min infusion. Oral cocaine was administered in double-encapsulated, hand-polished capsules. Participants were permitted to eat a standard single serving breakfast two h before the start of the session. Cigarette smoking was allowed ad libitum with the exception of 30 min before session until session completion. Participation was terminated if cardiovascular safety parameters were exceeded as follows: If heart rate > 130 or blood pressure > 165/100 within 4 min preceding a dose, of heart rate > (220 − subject age) × 0.85 at any time; or if blood pressure > 180/120 for 4 or more minutes.
Specimen collection and analysis
IV catheters were inserted into the antecubital vein into one or both arms; one for blood collection and the other for IV infusion when required. Blood samples were collected relative to IV dosing at the following times: −30, 1, 3, 5, 10, 15, 20, 30 and 45 min; and 1, 1.5, 2, 3, 4, 6, 12 and 24 h for the IV condition; and at −30, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 12 and 24 h relative to oral dosing. Blood (4 mL) was collected into 5 mL heparinized vacutainers containing 2% (w/v) sodium fluoride and acetic acid to prevent cocaine hydrolysis. These were centrifuged at 4°C for 10 min at 3,000 rpm. Plasma was separated and immediately frozen at −30°C until time of analysis.
Plasma specimens were analyzed for cocaine and metabolites by a previously published procedure (18) with minor modifications. Briefly, plasma samples were prepared for solid phase extraction by mixing with internal standard solution (d3cocaine, d3BZE and d3EME) and sodium acetate buffer (2 M; pH 4.0). Prepared samples were centrifuged (3,000 rpm for 10 min), and the supernatant extracted by solid phase extraction with mixed-mode columns. The eluent was evaporated under nitrogen in a 40°C water bath and reconstituted in 20 μL acetonitrile. The samples were then transferred to autosampler vials and combined with 20 μL of derivatizing reagent (BSTFA with 1% TMCS). The vials were sealed and incubated at 80°C for 30 min. The instrumentation included a Hewlett-Packard (Wilmington, DE) 5971 mass selective detector interfaced to a Hewlett-Packard 5890 A gas chromatograph with an autosampler (HP7673A). A 1 μL aliquot of the derivatized sample was injected in the splitless mode onto an HP-1 fused-silica capillary column (12 m × 0.2 mm I.D., 0.33 μm film thickness). The MS was operated in the selected ion-monitoring mode.
Duplicate calibration curves for each analyte were processed with each batch of samples. Curves were constructed in drug-free plasma across the concentration range of 1.25–1,000 ng/mL for cocaine, BZE, EME, BNE, NCOC, m-HOCOC, m-HOBZE, p-HOCOC and p-HOBZE. The limit of detection for all analytes was ~1 ng/mL. Control samples containing all analytes at concentrations of 100 and 500 ng/mL were processed in duplicate with each run. Accuracy of control measurements was within 20% for all analytes.
Pharmacokinetic and statistical analyses
Area-under-the-curve (AUC) and half-life (T1/2) were calculated following IV and oral cocaine administration by non-compartmental analysis with Win Nonlin software (Pharsight Corporation, Mountain View, CA). AUC measurements were then used for the calculation of CL and Vd. Other pharmacokinetic parameters were calculated with the following formulae:
To examine dose-proportionality across all three doses, AUC ratios for 100/40 mg, 200/40 mg and 200/100 mg were calculated for each individual and then averaged. Two-factor models (sex: two levels [male, female] and dose: three levels [oral 100 mg, oral 200 mg, IV 40 mg]) were used to test for differences between Cmax, Tmax, T1/2, AUC and AUC ratios of metabolites to cocaine. A separate 2-factor model (sex: two levels [male, female] and dose: two levels [oral 100 mg, oral 200 mg]) was used to compare oral bioavailability between males and females. Tukey t-tests were used to assess dose and sex post-hoc effects.
Results
Participants
Participants were healthy male (N = 6) and female (N = 8) cocaine users with a mean age of 33.9 years (range: 25–42). The mean weight of the males was 84.8 kg (range: 71.1–99.4) and mean weight of females was 69.9 kg (range: 50.9–99.2). Participants characteristics are summarized in Table I. In total, 15 individuals enrolled in the study; one was discharged due to poor venous access. No adverse effects were observed following drug administration for those who completed the study (n = 14).
Table I.
Subject demographic and drug use characteristics
| Age/race/sex | Weight (kg) | Lifetime cocaine usage (months) | Preferred/secondary route for cocaine | Other lifetime drug use history |
|---|---|---|---|---|
| 42/A/M | 97.1 | 36 | IV/SM | CA, OP |
| 34/A/M | 99.4 | 180 | SM/IV | CA, OP |
| 33/A/M | 75.7 | 60 | IV/SM | CA, OP |
| 34/A/M | 77.3 | 24 | SM/IV, IN | CA, OP |
| 31/A/M | 88.0 | 24 | SM/– | CA |
| 33/A/M | 71.1 | 36 | SM/– | CA |
| 25/A/F | 68.2 | 10 | IV/– | OP |
| 40/A/F | 99.2 | 60 | SM/– | CA |
| 33/A/F | 55.6 | 60 | SM/– | Sed, OP |
| 33/A/F | 94.2 | 12 | SM/– | – |
| 36/A/F | 83.5 | 24 | SM/– | Alc, CA, OP, ST |
| 34/A/F | 50.9 | 108 | SM/– | – |
| 34/C/F | 54.9 | NA | SM/– | Alc, CA |
| 32/A/F | 52.3 | 98 | SM/– | Alc, CA |
A = African-American C = Caucasian; M = male; F = female; IV = intravenous; SM = smoked; CA = cannabis; OP = opioids; Sed = sedatives; Alc = alcohol; ST = stimulants other than cocaine.
Cocaine pharmacokinetics
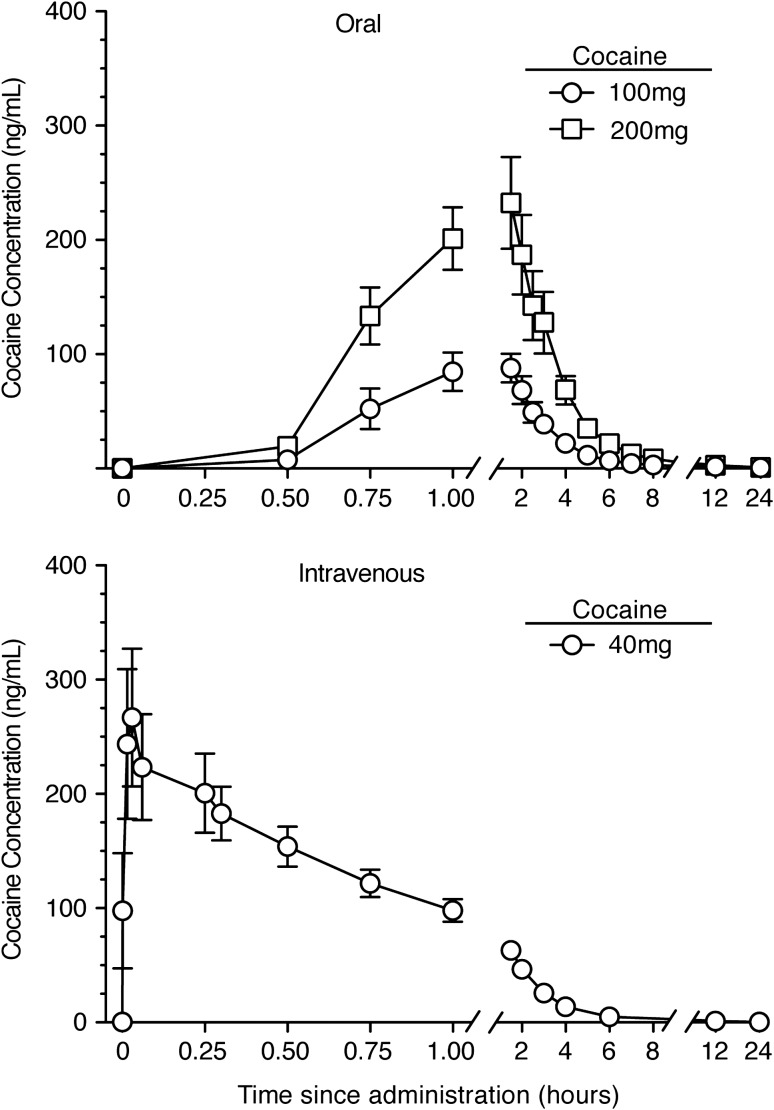
Mean plasma cocaine concentration–time plots after IV and oral cocaine administration are shown in Figure 1. For most participants, a biphasic distribution and elimination curve, characterized by a slower distribution phase followed by an elimination phase, was observed after IV cocaine. Two types of concentration–time profiles were evident: those with the greatest plasma concentrations tended to reach Cmax more quickly. The seven individuals with the greatest Cmax values (above the median) had a mean Tmax of 6.3 min while the seven with the lowest Cmax values (below the median) had a mean Tmax of 14.3 min. These time-concentration patterns were not observed with oral dosing. After oral administration, cocaine was rapidly absorbed and detected in plasma within 30 min in all participants. Concentration–time plots for oral cocaine showed an absorption phase followed by an elimination phase, but a distribution phase was not evident.
Figure 1.
Mean ± SEM (N = 14) plasma cocaine concentrations for 24 h following administration of 100 and 200 mg oral cocaine (top panel) and 40 mg IV cocaine (bottom panel). Cocaine dosing occurred at time 0.
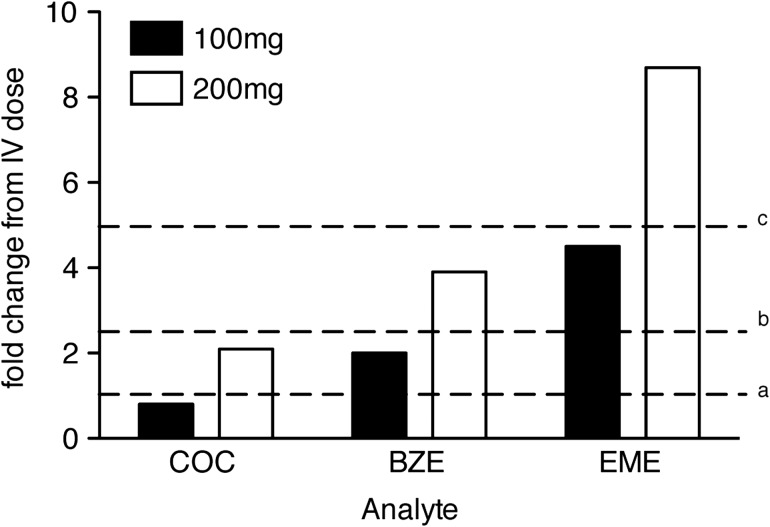
The mean oral cocaine bioavailability of the 200 mg dose (0.45 ± 0.06) was significantly higher than the 100 mg dose (0.32 ± 0.04), F(1, 12) = 11.37, P = 0.006. While females had higher mean bioavailability than males for both doses (females [100:34.3 ± 6.2 and 200:47.9 ± 8.9] and males [100:29.9 ± 3.8 and 200:40.1 ± 6.0]), the groups did not significantly differ, perhaps because of the greater variability observed for females than males. Pharmacokinetic parameters that differed as a function of dose and/or route of cocaine administration in the initial two-factor (sex × dose) model were Cmax, Tmax, T1/2, AUC, Vd and CL, P < 0.05 for all (Table II). The oral cocaine AUC ratio (200 mg AUC/100 mg AUC) was 2.86 (range: 0.94–4.84), indicating near-dose proportionality for the oral doses; however, the AUC ratios for oral compared to IV doses were significantly lower than expected if cocaine AUC was dose-proportional independent of route of administration (Figure 2).
Table II.
Pharmacokinetic parameters for cocaine and metabolites after oral and intravenous cocaine administration*
| Analyte | C max (ng/mL) | T max (h) | T 1/2 (h) | AUC (ng h/mL) | V d (L/kg) | Clearance (mL/[min kg]) | AUC ratio metabolite: cocaine |
|---|---|---|---|---|---|---|---|
| Dose | Mean (SEM) range | Mean (SEM) range | Mean (SEM) range | Mean (SEM) range | Mean (SEM) range | Mean (SEM) range | Mean (SEM) range |
| COC | |||||||
| IV 40 mg | 301 (61.6)a | 0.17 (0.03)a | 1.1 (0.1)a | 306 (32.2)a | 1.3 (0.2)a | 32.7 (2.7)a | NA |
| 111–925 | 0.05–0.33 | 0.8–1.5 | 182–656 | 0.3–2.5 | 10.8–53.3 | ||
| Oral 100 mg | 115 (14.7)b | 1.3 (0.1)b | 1.2 (0.1)b | 235 (30.0)a | 4.2 (0.5)b | 116.2 (13.1)b | NA |
| 45.1–201 | 0.8–2.0 | 0.8–1.4 | 115–448 | 1.6–7.5 | 51.5–190.2 | ||
| Oral 200 mg | 268 (33.2)a | 1.3 (0.2)b | 1.3 (0.1)c | 654 (90.0)b | 2.9 (0.5)c | 87.5 (12.9)b | NA |
| 67.0–570 | 0.8–3.0 | 1.0–2.0 | 151–1,510 | 1.2–7.6 | 38.9–221.4 | ||
| BZE | |||||||
| IV 40 mg | 274 (29)a | 2.0 (0.3)a | 6.0 (0.3)a | 2,886 (204)a | – | – | 10.1 (0.8)a |
| 172–614 | 0.8–4.0 | 4.7–8.2 | 2,051–4,604 | 5.5–16.2 | |||
| Oral 100 mg | 587 (37)b | 2.0 (0.2)a | 5.8 (0.3)b | 5,782 (441)b | – | – | 27.1 (1.9)b |
| 353–826 | 1.0–3.0 | 4.5–8.2 | 3,785–9,351 | 16.2–38.2 | |||
| Oral 200 mg | 951 (56)c | 3.0 (0.2)b | 6.2 (0.3)c | 11,132 (786)c | – | – | 20.1 (2.4)c |
| 586–1,296 | 1.5–4.0 | 4.8–9.1 | 6,892–16,453 | 8.8–45.9 | |||
| EME | |||||||
| IV 40 mg | 24 (3)a | 1.9 (0.2) | 5.4 (0.7)a | 226 (32)a | – | – | 0.8 (0.1)a |
| 7–52 | 1.0–3.0 | 2.9–11.7 | 77–507.1 | 0.3–1.3 | |||
| Oral 100 mg | 179 (15)b | 1.9 (0.2) | 3.2 (0.2)b | 1,019 (68.2)b | – | – | 5.2 (0.7)b |
| 122–277 | 1.0–3.0 | 2.3–4.5 | 680.5–1,524 | 1.8–9.3 | |||
| Oral 200 mg | 301 (18)c | 2.2 (0.2)c | 3.4 (0.2)b | 1,976 (119.5)c | – | – | 4.3 (1.1)b |
| 227–408 | 1.0–4.0 | 2.6–4.2 | 1,176–2,930 | 1.3–17.4 | |||
*Data are typically means of n = 14. There was a dose effect for all listed parameters (except for EME Tmax), with P values ranging from <0.0001 to 0.027. Values within each analyte that do not share a letter are significantly different.
COC = Cocaine; BZE = Benzoylecgonine; EME = Ecgonine methyl ester; NA = Not applicable.
Figure 2.
Area-under-the-curve (AUC) oral 100 mg:IV 40 mg and oral 200 mg:IV 40 mg ratios for cocaine (COC) and major metabolites, benzoylecgonine (BZE) and ecgonine methyl ester (EME). AUC ratios for each oral/IV dose were calculated for each participant and averaged to determine dose-proportionality of cocaine and metabolite profile across the three dosing conditions. aAUCs for cocaine and metabolites after 40 mg IV cocaine = 1, bdose-adjusted AUC estimate for 100 mg dose = 2.5-fold higher than IV 40 mg dose, cdose-adjusted AUC estimate for 200-mg dose = 5-fold higher than IV 40 mg dose. Both Cocaine and BZE AUCs were lower than the dose-proportional estimated values. Cocaine AUCs were 0.8 and 2.1 for oral 100 and 200 mg relative to IV 40 mg, and BZE AUCs were 2- and 3.9-fold higher than the IV dose. EME AUCs after oral cocaine exceeded the dose-proportional estimated values, with AUCs after the 100 and 200 mg dose 5.6- and 10.9-fold higher than the IV dose.
Metabolite profiles following IV and oral cocaine administration
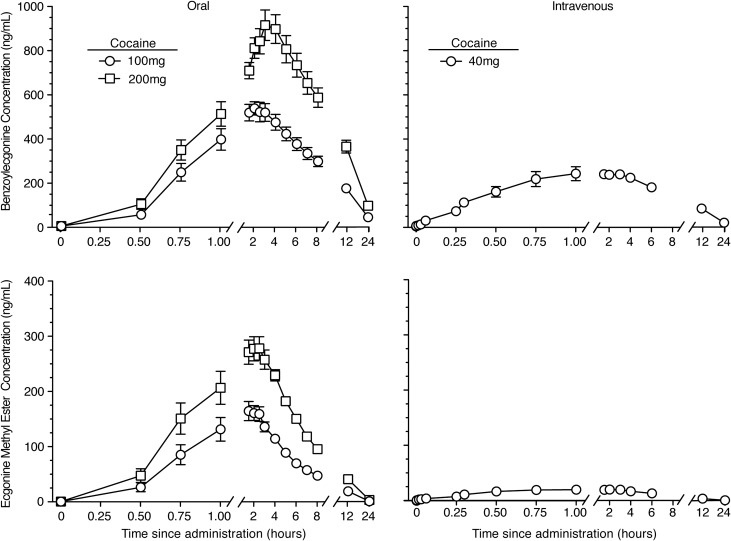
Benzoylecgonine (BZE) and ecgonine methyl ester (EME) were the primary metabolites detected in plasma following IV and oral cocaine and were generally detected within 30–60 min. BZE was detected in plasma for at least 24 h, and EME was detected in plasma for ~12 h. Mean plasma cocaine concentration–time plots for BZE and EME after IV and oral cocaine administration are shown in Figure 3. Metabolite pharmacokinetic parameters that differed as a function of dose and/or route of cocaine administration were Cmax, Tmax, T1/2 and AUC, P < 0.05 (Table II). Peak concentrations for BZE and EME were generally reached within 3 h for all conditions, but BZE Tmax was significantly delayed after 200 mg oral cocaine compared to the other two doses (HSD P = 0.006 for both comparisons). Both BZE and EME were detected at significantly greater concentrations after oral compared to IV cocaine administration, and the AUCs increased in a dose-related fashion for the two oral doses. The relative magnitude of AUC for cocaine, BZE and EME was BZE > cocaine > EME for IV administration and BZE > EME > cocaine for oral administration.
Figure 3.
Mean ± SEM (N = 14) plasma benzoylecgonine (top panels) and ecgonine methyl ester (bottom panels) concentrations for 24 h after administration of 100 and 200 mg oral cocaine (left panels) and 40 mg IV cocaine (right panels). Cocaine dosing occurred at time 0.
The AUCs of BZE exceeded those of cocaine by a factor of 10–27 (Table II; AUC ratio BZE:Cocaine). A comparison of the BZE:Cocaine AUC ratios by route of administration (i.e., oral 100 and 200 mg doses vs. IV 40 mg dose), revealed that less than a dose-proportional amount of BZE was formed after oral cocaine administration compared to IV cocaine administration (Figure 2), as would be expected due to a large first-pass effect.
AUCs of EME exceeded cocaine by a factor of 4–5 after oral cocaine administration only; the AUC ratio of EME to cocaine was close to 1, indicating that an equal concentration of the two analytes was present over the 24-h measurement period (Table II; AUC ratio EME:Cocaine). EME was present after oral cocaine administration in concentrations exceeding what would be expected based on dose-proportional pharmacokinetics as demonstrated by 5–10-fold higher AUC ratios of EME:cocaine after 100 and 200 mg oral cocaine than after 40 mg IV cocaine administration (Figure 2).
Minor metabolites were detected in higher concentrations after oral, compared to IV, cocaine. After all dosing conditions, p-HOBZE was detected at the highest concentration of any of the minor metabolites (average Cmax 28.5 and 64 ng/mL for oral 100 and 200 mg and 6.3 ng/mL for IV 40 mg), while m-HOCOC and p-HOCOC were consistently detected in the smallest concentrations (average Cmax < 10 ng/mL for both oral doses and < 1 ng/mL for the IV dose). The relative magnitude of Cmax after oral cocaine was: p-HOBZE > BNE > m-HOBZE ≅ NCOC > p-HOCOC > m-HOCOC. In contrast, the relative magnitude of Cmax after oral cocaine was: p-HOBZE > m-HOBZE > BNE > m-HOCOC > p-HOCOC. While NCOC was present in plasma after oral cocaine administration (average Cmax 4.1 and 23.1 ng/mL after 100 and 200 mg), it was only identified in plasma from one participant after IV dosing (Cmax 86.8 ng/mL at 3 h post dose). Notably, this was the individual with the highest cocaine, BZE and EME concentrations after each cocaine dose.
Sex comparisons
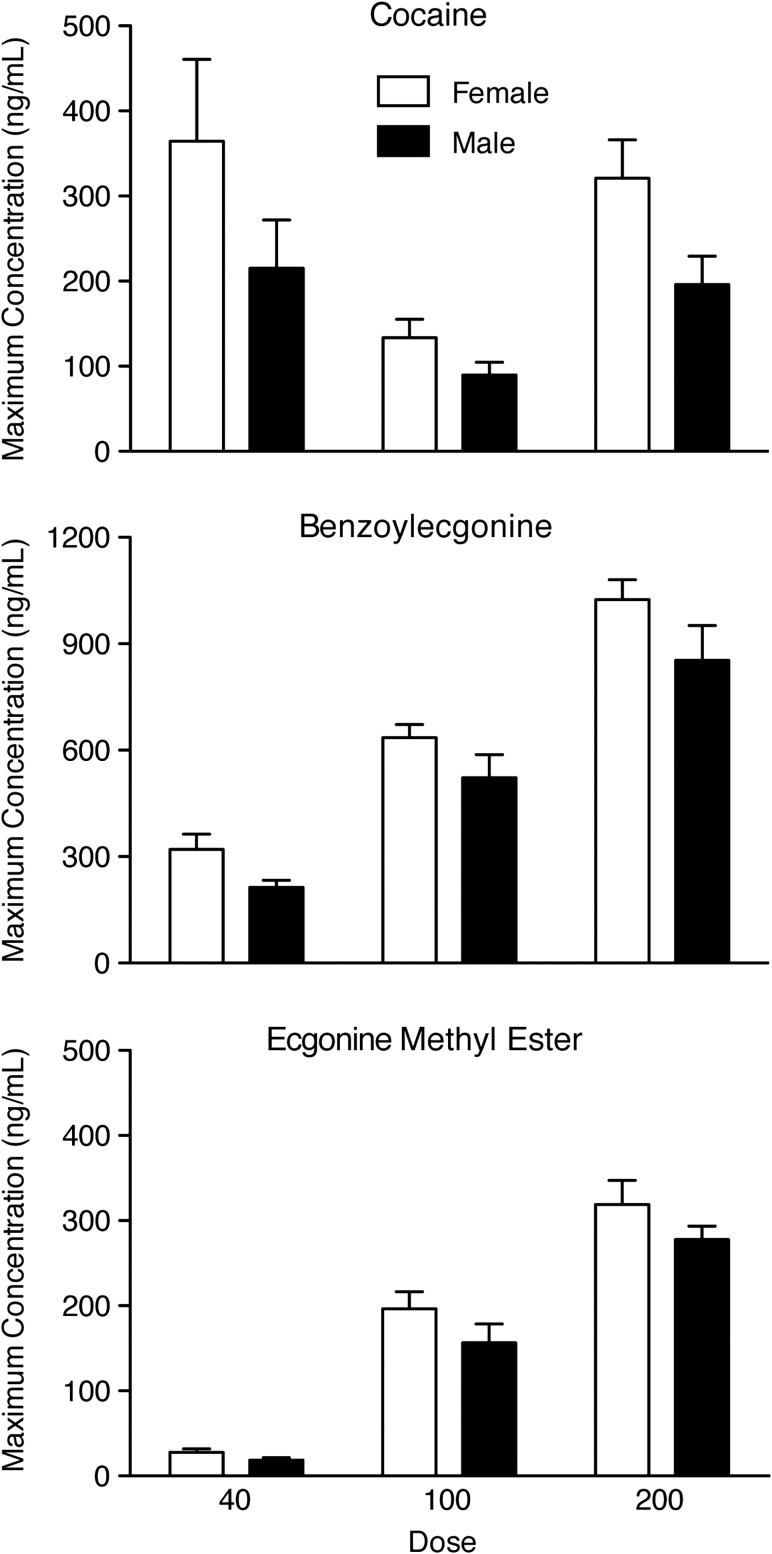
C max was the only pharmacokinetic parameter that varied as a function of sex, with higher Cmax values observed for females than males when collapsing across doses (Figure 4); however, individual sex comparisons at each dose were not significant. Females also had higher BZE and EME Cmax values than males after each cocaine dose (Figure 4), but these were not significant. When values were adjusted for body weight, cocaine, BZE and EME Cmax were all significantly different between sexes, P < 0.05.
Figure 4.
Maximum plasma concentration of cocaine (top), benzoylecgonine (BZE) (middle), and ecgonine methyl ester (EME) (bottom) for females (open bars) and males (solid bars). Top panel: There was a significant effect of sex for cocaine Cmax [F(1,12) = 4.81, P = 0.049], but Tukey comparisons were non-significant when comparing females and males within each of the three dosing conditions, P > 0.05. While sex was not a significant factor, females also displayed higher Cmax values than males for BZE (middle panel) and EME (bottom panel).
Discussion
Cocaine pharmacokinetics and bioavailability
Pharmacokinetic parameters for IV cocaine in the present study are in good agreement with what has been observed previously in controlled laboratory studies (2, 3, 19–22), with one exception. Tmax was reached at ~11 min in the current study, which is 2–4-fold longer than what has been previously reported (3, 22). As expected, oral cocaine administration resulted in a different concentration–time profile compared to IV administration, (i.e., delayed time-to-detection of cocaine and Tmax), also consistent with the few previously reported studies of oral cocaine administration (4, 5). Females reached greater maximum plasma concentrations than males, but no other significant sex differences were observed. The small sample size in this study may have been insufficient to detect sex effects, if they are present. This study is the first to report the pharmacokinetic parameters of CL and Vd for oral cocaine. CL for the 100 and 200 mg oral cocaine doses was 3.6- and 2.7-fold greater than for IV cocaine. Vd for the 100 and 200 mg oral cocaine doses was 3.2- and 2.2-fold greater than for IV cocaine. The relative magnitude of both CL and Vd values relative to cocaine dose administered was 100 mg oral > 200 mg oral > 40 mg IV. Logically, CL and Vd were lowest after IV dosing because the cocaine was more constrained to the vascular compartment, but it is less clear why the lower oral cocaine dose would produce the highest CL and Vd values. It is possible that exposure to greater concentrations of cocaine during absorption may have saturated hepatic/intestinal metabolism, allowing more unchanged cocaine to reach systemic circulation after 200 mg. Though previous reports of oral cocaine bioavailability (0.20–0.60) were calculated with data obtained from a combination of oral and IV data obtained from different subjects and/or across different studies (5, 6), qualitatively similar values were obtained for bioavailability in the current study (range: 0.15–0.93). Also of interest (and supporting the existence of hepatic/intestinal metabolic saturation at high oral cocaine doses) is that the mean bioavailability of the 200 mg dose (44.6 ± 5.6) was significantly higher than the 100 mg dose (32.4 ± 3.8).
Cocaine behaves as a high-extraction drug (23) which undergoes first-pass metabolism in the intestines and liver, and this may explain the non-dose-proportional AUC values comparing IV and oral dosing (40 mg IV: 306 ± 32.2; 100 mg oral: 235 ± 30.0; 200 mg oral: 655 ± 90.0). Cocaine is absorbed from the gut and metabolized before reaching systemic circulation. If AUC was dose-proportional regardless of route of administration (as would be expected for high-extraction drugs primarily metabolized hepatically), cocaine 100 and 200 mg should have produced AUC values 2.5 and 5-fold higher than 40 mg, but AUCs were actually 0.77 and 2.14 for oral 100 and 200 mg relative to IV 40 mg.
Metabolite pharmacokinetics
Benzoylecgonine (BZE) and ecgonine methyl ester (EME) were the primary metabolites detected in plasma following IV and oral cocaine administration, but minor metabolites including BNE, p-HOBZE and NCOC were also detected (though in lower concentrations). In general, females had greater maximum plasma concentrations of BZE and EME than males. All metabolites were detected in greater concentrations after oral compared to IV cocaine. Only one study has reported plasma metabolite concentrations after controlled oral cocaine administration (15) and consistent with that report (i.e., AUC ratios for: BZE/cocaine = 20; EME/cocaine = 5), the AUC ratios of BZE and EME to cocaine observed here (i.e., AUC ratios for: BZE/cocaine = 20–27; EME/cocaine = 4.3–5.2) were significantly greater after oral than IV cocaine. This finding indicated that the rates of elimination were slower than the rates of formation for BZE after oral and IV cocaine administration and EME after oral cocaine administration. As the previous study administered ascending doses of cocaine every day for 16 days, the unique metabolic profile could have been attributed to chronicity of dosing and/or the oral route; the present data suggest that the oral route of administration is responsible for the unique metabolic profile. Interestingly, the AUC ratios of BZE and EME to cocaine were highest after the low oral cocaine dose; perhaps due to saturation of one or more metabolic pathways (e.g., hCE1/hCE2 conversion of cocaine to BZE). This reduction in the BZE/cocaine ratio at higher oral cocaine doses was previously reported (15). A saturable metabolic pathway could also explain the delayed BZE Tmax after 200 mg compared to 100 mg oral cocaine.
If first-pass metabolism to BZE occurs primarily in the liver, the dose-adjusted AUC for BZE would be similar regardless of the route of administration (i.e., cocaine 100 mg and 200 mg would have produced BZE AUC values 2.5 and 5-fold higher than 40 mg). This parent-to-metabolite relationship has been demonstrated for other high-extraction drugs (e.g., morphine) (24). BZE AUCs for the two oral doses were only 2- and 3.9-fold higher than the IV dose, indicating that an extrahepatic pathway may be partially responsible for pre-systemic metabolism of cocaine. The fact that EME AUCs were higher than would be expected for the two oral cocaine doses (4.5- and 8.7-fold higher than the IV dose) suggests that hCE2 metabolism in the gut and small intestine has an important role in oral cocaine metabolism.
Minor metabolites were detected at greater concentrations after oral compared to IV cocaine. Animal studies with mice, rats and guinea pigs have indicated that m- and p-HOCOC are formed by hepatic microsomes (25); therefore, it is logical that a high-extraction drug like cocaine would produce more of these metabolites after oral compared to IV administration. NCOC is the only active metabolite of cocaine, has been shown to contribute to overall effects of IV and oral cocaine in rodents (26) and non-human primates (27), and is the precursor of other oxidative hepatotoxic metabolites (28, 29). NCOC is typically detected in very low concentrations (i.e., > 10 ng/mL) after parenteral and smoked cocaine administration (16, 30); however, following repeated oral cocaine administration in humans, NCOC was detected in plasma at concentrations exceeding 100 ng/mL (15). In the present study, maximum plasma concentrations of NCOC were 4.1 and 23.1 ng/mL after 100 and 200 mg oral cocaine, suggesting that the repeated dosing in the earlier report (15) contributed to the high NCOC concentrations more than the oral route of administration.
Conclusion
The present study characterized the pharmacokinetics and bioavailability of two single oral doses (100 and 200 mg) compared to an IV dose (40 mg) of cocaine in 14 healthy participants. As expected, oral cocaine produced a pharmacokinetic profile different from IV cocaine, which appears as a rightward and downward shift in the concentration–time profile. Cocaine AUC values were not dose-proportional when comparing IV to oral doses, as oral cocaine AUCs were lower than dose-adjustments would predict. Clearance and volume of distribution were highest after 100 mg oral cocaine, perhaps due to initial saturation of hepatic/intestinal metabolism after the 200 mg dose. Both BZE and EME were detected in greater concentrations after oral cocaine than what is observed after IV dosing. The less-than-dose-proportional AUCs for BZE after oral IV and oral cocaine suggest that a metabolic pathway (hCE1) is saturable, while the greater-than-dose-proportional AUCs for EME suggests that the pre-systemic metabolism by hCE2 plays an important role in oral cocaine metabolism. Finally, a unique metabolic profile was observed after oral cocaine that resulted in the production of substantial amounts of minor metabolites, some of which have not been detected in plasma after IV cocaine administration.
Acknowledgments
The authors would like to acknowledge Shirley Podgurski for nursing support, David Darwin and Lisa Notes for technical support and Bonnie Koeppl and Paul Nuzzo for assistance with statistical analyses.
Funding
This study was funded, in part, by a grant from the National Institute on Drug Abuse (DA10029 to S.L.W.).
References
- 1. Rowbotham M.C., Jones R.T., Benowitz N.L., Jacob P. III (1984) Trazodone-oral cocaine interactions. Archives of General Psychiatry, 41, 895–899. [DOI] [PubMed] [Google Scholar]
- 2. Chow M.J., Ambre J.J., Ruo T.I., Atkinson A.J. Jr, Bowsher D.J., Fischman M.W. (1985) Kinetics of cocaine distribution, elimination, and chronotropic effects. Clinical Pharmacology & Therapeutics, 38, 318–324. [DOI] [PubMed] [Google Scholar]
- 3. Cone E.J. (1995) Pharmacokinetics and pharmacodynamics of cocaine. Journal of Analytical Toxicology, 19, 459–478. [DOI] [PubMed] [Google Scholar]
- 4. Van Dyke C., Jatlow P., Ungerer J., Barash P.G., Byck R. (1978) Oral cocaine: plasma concentrations and central effects. Science, 200, 211–213. [DOI] [PubMed] [Google Scholar]
- 5. Wilkinson P., Van Dyke C., Jatlow P., Barash P., Byck R. (1980) Intranasal and oral cocaine kinetics. Clinical Pharmacology & Therapeutics, 27, 386–394. [DOI] [PubMed] [Google Scholar]
- 6. Mayersohn M., Perrier D. (1978) Kinetics of pharmacologic response to cocaine. Research Communications in Chemical Pathology and Pharmacology, 22, 465–474. [PubMed] [Google Scholar]
- 7. Jufer R.A., Wstadik A., Walsh S.L., Levine B.S., Cone E.J. (2000) Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. Journal of Analytical Toxicology, 24, 467–477. [DOI] [PubMed] [Google Scholar]
- 8. Bencharit S., Morton C.L., Xue Y., Potter P.M., Redinbo M.R. (2003) Structural basis of heroin and cocaine metabolism by a promiscuous human drug-processing enzyme. Nature Structural Biology, 10, 349–356. [DOI] [PubMed] [Google Scholar]
- 9. Brzezinski M.R., Abraham T.L., Stone C.L., Dean R.A., Bosron W.F. (1994) Purification and characterization of a human liver cocaine carboxylesterase that catalyzes the production of benzoylecgonine and the formation of cocaethylene from alcohol and cocaine. Biochemical Pharmacology, 48, 1747–1755. [DOI] [PubMed] [Google Scholar]
- 10. Stewart D.J., Inaba T., Tang B.K., Kalow W. (1977) Hydrolysis of cocaine in human plasma by cholinesterase. Life Sciences, 20, 1557–1563. [DOI] [PubMed] [Google Scholar]
- 11. Carmona G.N., Jufer R.A., Goldberg S.R., Gorelick D.A., Greig N.H., Yu Q.S., et al. (2000) Butyrylcholinesterase accelerates cocaine metabolism: in vitro and in vivo effects in nonhuman primates and humans. Drug Metabolism & Disposition, 28, 367–371. [PubMed] [Google Scholar]
- 12. Baselt R.C. (1983) Stability of cocaine in biological fluids. Journal of Chromatography, 268, 502–505. [DOI] [PubMed] [Google Scholar]
- 13. Skopp G., Klingmann A., Potsch L., Mattern R. (2001) In vitro stability of cocaine in whole blood and plasma including ecgonine as a target analyte. Therapeutic Drug Monitoring, 23, 174–181. [DOI] [PubMed] [Google Scholar]
- 14. Warner A., Norman A.B. (2000) Mechanisms of cocaine hydrolysis and metabolism in vitro and in vivo: a clarification. Ther Drug Monit, 22, 266–270. [DOI] [PubMed] [Google Scholar]
- 15. Jufer R.A., Walsh S.L., Cone E.J. (1998) Cocaine and metabolite concentrations in plasma during repeated oral administration: development of a human laboratory model of chronic cocaine use. Journal of Analytical Toxicology, 22, 435–444. [DOI] [PubMed] [Google Scholar]
- 16. Kolbrich E.A., Barnes A.J., Gorelick D.A., Boyd S.J., Cone E.J., Huestis M.A. (2006) Major and minor metabolites of cocaine in human plasma following controlled subcutaneous cocaine administration. Journal of Analytical Toxicology, 30, 501–510. [DOI] [PubMed] [Google Scholar]
- 17. Walsh S.L., Stoops W.W., Moody D.E., Lin S.N., Bigelow G.E. (2009) Repeated dosing with oral cocaine in humans: assessment of direct effects, withdrawal, and pharmacokinetics. Experimental and Clinical Psychopharmacology, 17, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cone E.J., Hillsgrove M., Darwin W.D. (1994) Simultaneous measurement of cocaine, cocaethylene, their metabolites, and “crack” pyrolysis products by gas chromatography-mass spectrometry. Clinical Chemistry, 40, 1299–1305. [PubMed] [Google Scholar]
- 19. Javaid J.I., Fischman M.W., Schuster C.R., Dekirmenjian H., Davis J.M. (1978) Cocaine plasma concentration: relation to physiological and subjective effects in humans. Science, 202, 227–228. [DOI] [PubMed] [Google Scholar]
- 20. Javaid J.I., Musa M.N., Fischman M., Schuster C.R., Davis J.M. (1983) Kinetics of cocaine in humans after intravenous and intranasal administration. Biopharmaceutics & Drug Disposposition, 4, 9–18. [DOI] [PubMed] [Google Scholar]
- 21. Cone E.J., Kumor K., Thompson L.K., Sherer M. (1988) Correlation of saliva cocaine levels with plasma levels and with pharmacologic effects after intravenous cocaine administration in human subjects. Journal of Analytical Toxicology, 12, 200–206. [DOI] [PubMed] [Google Scholar]
- 22. Barnett G., Hawks R., Resnick R. (1981) Cocaine pharmacokinetics in humans. Journal of Ethnopharmacology, 3, 353–366. [DOI] [PubMed] [Google Scholar]
- 23. Parker R.B., Laizure S.C. (2010) The effect of ethanol on oral cocaine pharmacokinetics reveals an unrecognized class of ethanol-mediated drug interactions. Drug Metabolism & Disposition, 38, 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osborne R., Joel S., Trew D., Slevin M. (1990) Morphine and metabolite behavior after different routes of morphine administration: demonstration of the importance of the active metabolite morphine-6-glucuronide. Clinical Pharmacology & Therapeutics, 47, 12–19. [DOI] [PubMed] [Google Scholar]
- 25. Watanabe K., Hida Y., Matsunaga T., Yamamoto I., Yoshimura H. (1993) Formation of p-hydroxycocaine from cocaine by hepatic microsomes of animals and its pharmacological effects in mice. Biological and Pharmaceutical Bulletin, 16, 1041–1043. [DOI] [PubMed] [Google Scholar]
- 26. Wang Q., Simpao A., Sun L., Falk J.L., Lau C.E. (2001) Contribution of the active metabolite, norcocaine, to cocaine’s effects after intravenous and oral administration in rats: pharmacodynamics. Psychopharmacology (Berlin), 153, 341–352. [DOI] [PubMed] [Google Scholar]
- 27. Bedford J.A., Borne R.F., Wilson M.C. (1980) Comparative behavioral profile of cocaine and norcocaine in rats and monkeys. Pharmacology, Biochemistry and Behavior, 13, 69–75. [DOI] [PubMed] [Google Scholar]
- 28. Pellinen P., Stenback F., Raunio H., Pelkonen O., Pasanen M. (1994) Modification of hepatic cytochrome P450 profile by cocaine-induced hepatotoxicity in DBA/2 mouse. European Journal of Pharmacology, 292, 57–65. [DOI] [PubMed] [Google Scholar]
- 29. Boelsterli U.A., Goldlin C. (1991) Biomechanisms of cocaine-induced hepatocyte injury mediated by the formation of reactive metabolites. Archives of Toxicology, 65, 351–360. [DOI] [PubMed] [Google Scholar]
- 30. Paul B.D., Lalani S., Bosy T., Jacobs A.J., Huestis M.A. (2005) Concentration profiles of cocaine, pyrolytic methyl ecgonidine and thirteen metabolites in human blood and urine: determination by gas chromatography-mass spectrometry. Biomedical Chromatography, 19, 677–688. [DOI] [PubMed] [Google Scholar]