Abstract
Erythropoietin (EPO) plays an important role in the development and maturation of the gastrointestinal tract. Recombinant EPO (rEPO) has been used to prevent anemia of prematurity. The gastrointestinal trophic effects of EPO may reduce feeding intolerance and necrotizing enterocolitis (NEC) in preterm neonates. The aim of this systematic review of randomized controlled trials (RCTs) was to evaluate the effects of rEPO on clinical outcomes such as feeding intolerance, stage II or higher NEC, any stage NEC, sepsis, retinopathy of prematurity, and bronchopulmonary dysplasia in preterm neonates. Twenty-five RCTs (intravenous: 13; subcutaneous: 10; enteral: 2; n = 4025) were eligible for inclusion. Meta-analysis of data from 17 RCTs (rEPO compared with placebo) with the use of a fixed-effects model showed no significant effect of rEPO on stage II or higher NEC (RR: 0.87; 95% CI: 0.64, 1.19; P = 0.39). Meta-analysis of data from 25 RCTs (rEPO compared with placebo) showed that rEPO significantly decreased the risk of any stage NEC [cases/total sample: 120/2058 (5.83%) compared with 146/1967 (7.42%); RR: 0.77; 95% CI: 0.61, 0.97; P = 0.03]. Only one RCT reported on time to full feedings. Meta-analysis of data from 15 RCTs showed a significant reduction in late-onset sepsis after rEPO administration (RR: 0.81; 95% CI: 0.71, 0.94; P = 0.004). Meta-analysis of 13 RCTs showed no significant effect of rEPO on mortality, retinopathy of prematurity, and bronchopulmonary dysplasia. Prophylactic rEPO had no effect on stage II or higher NEC, but it reduced any stage NEC, probably by reducing feeding intolerance, which is often labeled as stage I NEC. Adequately powered RCTs are required to confirm these findings.
Keywords: infants, erythropoietin, feed intolerance, necrotizing enterocolitis, preterm
Introduction
The survival of extremely preterm infants has improved significantly following the advances in neonatal intensive care. Optimization of nutrition is a priority in this population, considering its short- as well as long-term benefits on growth and neurodevelopment. Feeding intolerance (e.g., abdominal distension, bile-stained gastric residuals) due to gastrointestinal immaturity is common in the first 2–3 wk of life in extremely preterm neonates born at <28 wk of gestational age (1). This population is also at high risk of necrotizing enterocolitis (NEC)—an illness with significant mortality and morbidity, including long-term neurodevelopmental impairment (1–3). In the absence of a reliable marker, feeding intolerance is often confused with early (stage I) NEC. The frequent stoppage of feeding due to the fear of NEC is associated with suboptimal nutrition in the early postnatal life of extremely preterm infants (3).
Despite implementing strategies such as antenatal glucocorticoids (4, 5), early preferential use of breast milk (6), and standardized feeding regimens (7–9), the burden of definite NEC (stage II or higher) continues to be high, especially in extremely preterm neonates. Probiotics reduce the risk of NEC and improve feeding tolerance, but issues such as the optimal strain or strains, dose, probiotic sepsis, and long-term safety need to be addressed (10–14). The safety and efficacy of lactoferrin in preventing NEC need to be confirmed in further studies (15, 16). Considering the importance of early optimal nutrition, interventions that will improve the gastrointestinal maturity and function, thereby reducing feed intolerance and NEC, are needed for preterm, especially extremely preterm, infants.
Erythropoietin (EPO) is a glycoprotein hormone that regulates erythropoiesis (17). Halperin et al. (18) first reported the use of recombinant EPO (rEPO) in anemia of prematurity. Subsequently, many clinical studies have shown that rEPO stimulates erythropoiesis and reduces the need for transfusion for anemia of prematurity (19, 20). In addition to its erythropoietic effects, EPO exerts mitogenic, vasodilatory, and angiogenic effects in nonerythropoietic tissues, including the gastrointestinal tract, endothelial cells, neurons, splenic cells, and cardiomyocytes (21–26). EPO receptors are present on the luminal side of villi in human fetal and neonatal intestine, suggesting a physiologic role of EPO in the developing gut (23, 26). EPO treatment promotes cell division, stimulates enterocyte migration, and reduces enterocyte apoptosis (27). Investigators have reported that rEPO exerts a trophic effect on small bowel villi, stimulates the growth of mucosal cells, and increases the villus surface area, length, and density (28). EPO reduces experimental NEC-induced NO production (29) and promotes vasculogenesis, thereby increasing the nutrient supply to the bowel (30). Overall, these data support the hypothesis that the trophic effects of EPO on the gastrointestinal tract may benefit preterm neonates.
A systematic review of randomized controlled trials (RCTs) studying the effects of rEPO in preterm neonates was therefore conducted to report the outcomes reflecting its effects on gastrointestinal maturity and function (eg., feeding intolerence, stage II or higher NEC, sepsis). The minimum effective concentration for the action of EPO is more important than the routes of administration (31, 32). Results of an RCT showed that oral and subcutaneous EPO have similar effects in preterm low-birth-weight infants (33). Hence, it was hypothesized that the effects of EPO will be similar, irrespective of its route of administration.
Methods
The Cochrane methodology was used for the conduct of this systematic review. RCTs comparing rEPO with placebo or standard treatment without rEPO supplementation were included. Primary outcomes were incidence of stage II or higher NEC (34) and “any stage” NEC. Secondary outcomes were as follows: 1) feeding intolerance as defined by authors of the RCTs, 2) time to reach full feedings, 3) late-onset sepsis (LOS: blood culture–positive infection 48 h after birth), 4), bronchopulmonary dysplasia (BPD), and 5) mortality. LOS was included as a secondary outcome because optimization of feeding is associated with a reduced risk of this outcome in preterm neonates. Given the concern that rEPO may be associated with increased risk of retinopathy of prematurity (ROP), it was included as a safety outcome. The details of the data sources that were searched, study selection process, data extraction, assessment of risk of bias (ROB), data synthesis, assessment of publication bias, and GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) evidence (35) are given in SupplementalMethods.
Subgroup analysis
Subgroup analysis was planned on the basis of the route of rEPO administration.
Sensitivity analysis
Considering the importance of random-sequence generation and allocation concealment in RCTs, sensitivity analyses were planned by excluding studies with a high ROB in these 2 domains separately (36). A sensitivity analysis was planned by excluding studies in which NEC was not the primary outcome.
Analysis of data on very-low-birth-weight and extremely low-birth-weight neonates
We aimed to conduct separate analyses of studies by exclusively enrolling 1) very-low-birth-weight (VLBW; birth weight <1500 g) or 2) extremely low-birth-weight (ELBW; birth weight <1000 g) neonates considering that they are at high risk of NEC.
Results
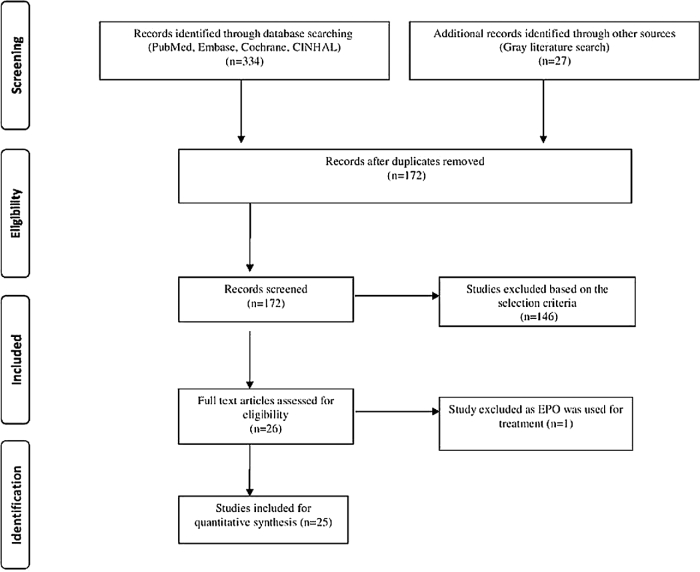
The literature search retrieved 361 potentially relevant citations. After carefully reviewing the abstracts, 189 duplicate studies were excluded. A total of 146 studies were excluded due to nonfulfillment of the inclusion criteria. A total of 26 studies were assessed for eligibility. One study was excluded becaise rEPO was used for treatment rather than for prevention of NEC (37). Finally, 25 RCTs (17, 38–60) were included in the review. The flow diagram of the study selection process is given in Figure 1, and the characteristics of the included studies are shown in Supplemental Table 1.
FIGURE 1.
Flowchart showing study selection process. EPO, erythropoietin.
Clinical studies
Among the included 25 RCTs (n = 4025; EPO compared with control: 2058 compared with 1967), 13 studied the effect of intravenous rEPO, 10 studied the effect of subcutaneous rEPO, and 2 studied the effect of enteral rEPO on stage II or higher NEC in preterm infants. The sample size of the included RCTs ranged from 15 to 377. The rEPO dose varied depending on the route of administration (enteral: 88 U/kg; subcutaneous: 100–3000 U/kg; intravenous: 200–3000 U/kg). NEC was the primary outcome in only 2 of 25 RCTs. The detailed characteristics of the included studies, including the route, dose, and duration of EPO supplementation, are given in Supplemental Table 1.
ROB of included studies
All of the included RCTs (n = 25) had some methodologic weakness. Of the 25 RCTs, 15 (60%) were considered to have low ROB for the domain “random-sequence generation” and 17 (68%) were judged to have low ROB for “allocation concealment.” Random-sequence generation was unclear in 10 RCTs (42, 44, 44, 49, 51, 55–58, 60), allocation concealment was unclear in 7 (17, 45, 48, 50, 55, 58, 60), blinding of participants and personnel was unclear in 1 (45), and the risk of performance bias was high in 7 RCTs (48, 51, 53, 55–57, 59). Blinding of outcome assessment and detection bias was high in 7 RCTS (48, 51, 53, 55–57, 59). The risk of attrition bias was high in 6 RCTs (38, 43, 45, 46, 49, 59) and unclear in 1 RCT (56). The information on reporting bias and other bias was unclear in 20 RCTs (17, 38–40, 42–45, 48–51, 53–58, 60) and 7 RCTs (40, 43, 45–47, 53, 57), respectively. The ROB summary of the included RCTs is shown in Figure 2.
FIGURE 2.
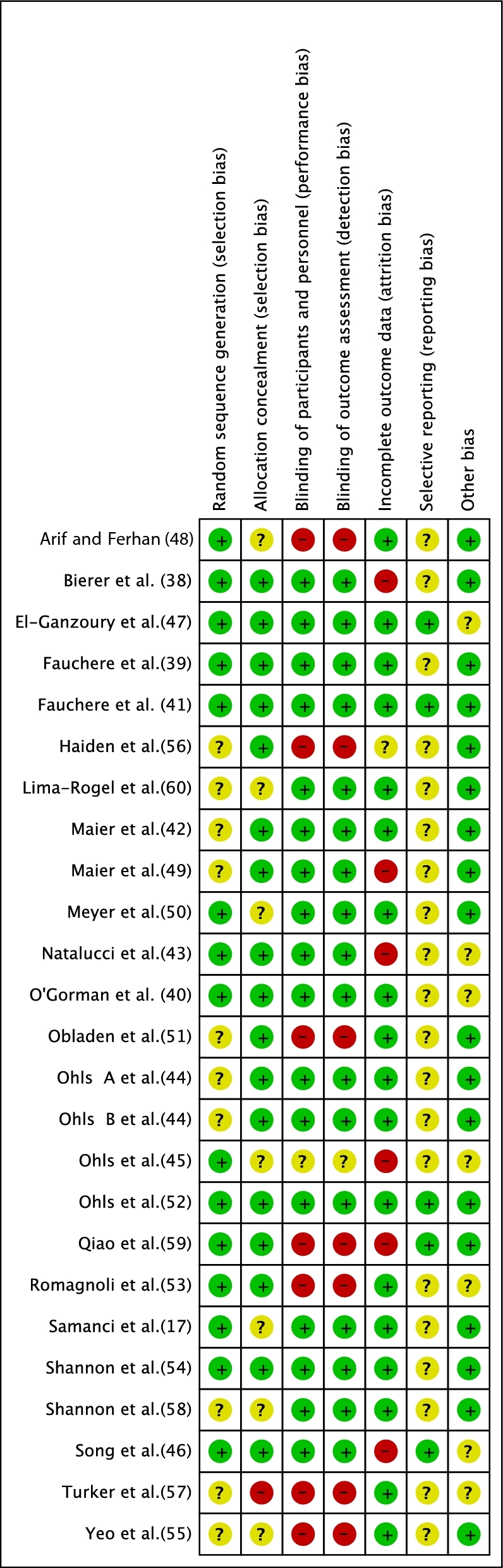
Risk-of-bias summary of the included RCTs. RCT, randomized controlled trial.
Outcomes
Primary outcomes
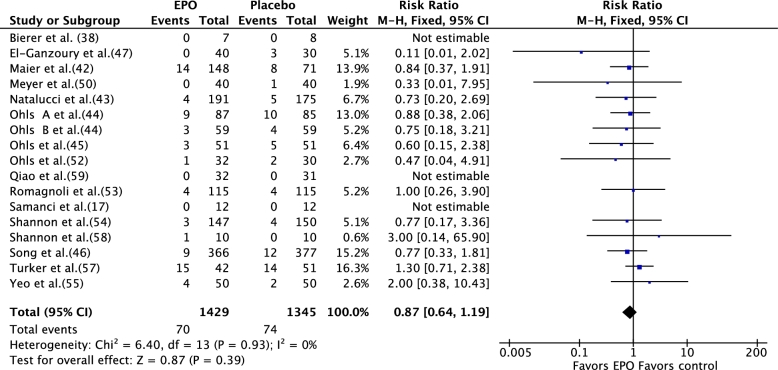
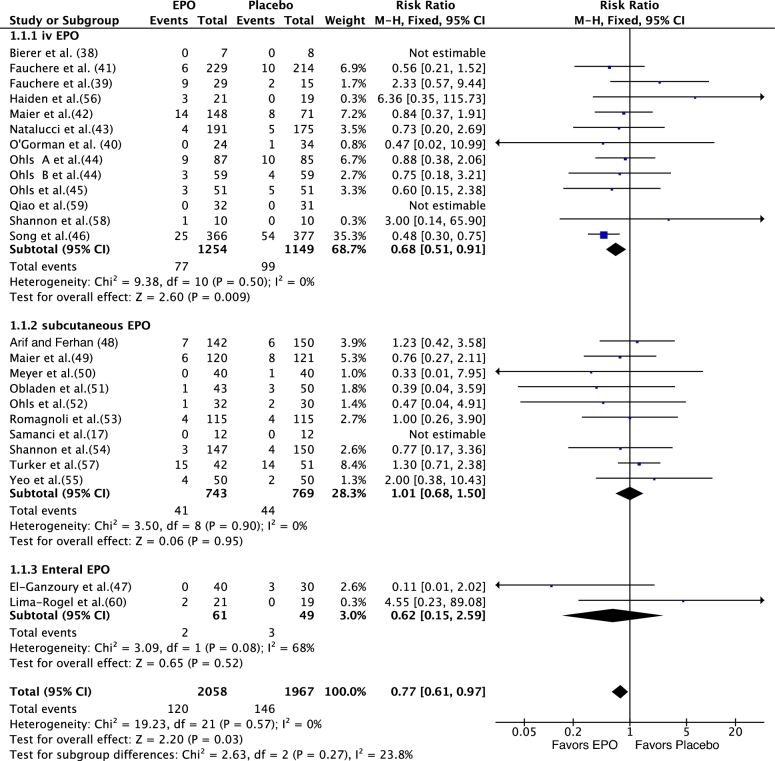
Meta-analysis [fixed-effects model (FEM)] of data from the 17 RCTs (rEPO compared with placebo) showed no significant effect of rEPO on stage II or higher NEC (RR: 087; 95% CI: 0.64, 1.19; P = 0.39) (Figure 3). Meta-analysis of data from 25 RCTs (n = 4025) that compared rEPO with placebo or no rEPO showed that rEPO significantly decreased the incidence of any stage NEC [120/2058 (cases/total sample) (6.14%) compared with 146/1967 (7.42%); RR: 0.77; 95% CI: 0.61, 0.97; P = 0.03; I2 = 0; number needed to treat = 59] (Figure 4). The results of meta-analysis that used a random-effects model (REM) were close to those based on FEM (RR: 0.79; 95% CI: 0.62, 1.00; P = 0.05; I2 = 0; level of evidence: high] (Supplemental Figure 1).Visual inspection of the funnel plot suggested that publication bias was unlikely (Figure 5).
FIGURE 3.
Effect of rEPO on definite necrotizing enterocolitis (stage II or higher) in preterm neonates (fixed-effects model). EPO, erythropoietin; M-H, Mantel-Haenszel method; rEPO, recombinant erythropoietin.
FIGURE 4.
Effect of rEPO on necrotizing enterocolitis in preterm neonates (fixed-effects model). EPO, erythropoietin; M-H, Mantel-Haenszel method; rEPO, recombinant erythropoietin.
FIGURE 5.
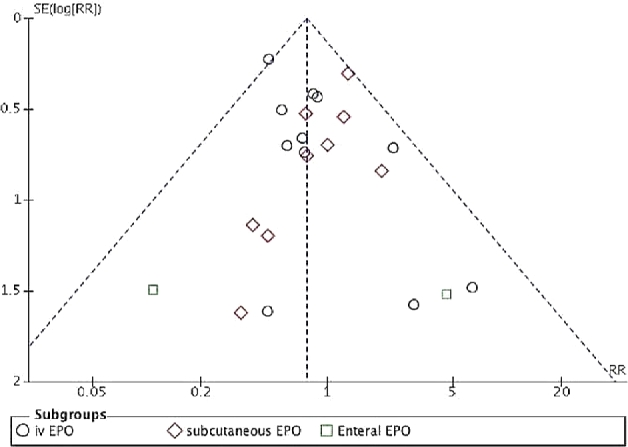
Funnel plot of the included RCTs for assessing publication bias. EPO, erythropoietin; RCT, randomized controlled trial.
Subgroup analysis
Results of our prestated subgroup (by route of administration) analysis showed that only intravenous rEPO significantly reduced any stage NEC [77/1254 (6.14%) compared with 99/1149 (8.61%); RR: 0.68; 95% CI: 0.51, 0.91; P = 0.009 by FEM] (Figure 4). The results remained significant when an REM was used (RR: 0.67; 95% CI: 0.49, 0.90; P = 0.008) (Supplemental Figure 1).
Sensitivity analysis
The beneficial effects of rEPO on any stage NEC were observed in studies with low ROB for random sequence generation and for allocation concealment (Supplemental Table 2). Only 2 of 20 RCTs reported NEC as the primary outcome, and pooled data did not show beneficial effects.
ELBW and VLBW infants
Preplanned separate analyses in this review showed that rEPO significantly reduced any stage NEC in studies exclusively enrolling VLBW [79/1723 (4.58%) compared with 111/1724 (6.43%); RR: 0.70; 95% CI: 0.53, 0.93; P = 0.01] but not ELBW [39/325 (12%) compared with 42/335 (12.53%); RR: 0.98; 95% CI: 0.66, 1.46; P = 0.94] infants.
Summary of findings
The overall evidence as per GRADE guidelines is provided in Supplemental Table 3.
Publication bias
The funnel plot showed symmetrical distribution, suggesting that publication bias was less likely (Figure 5).
Secondary outcomes
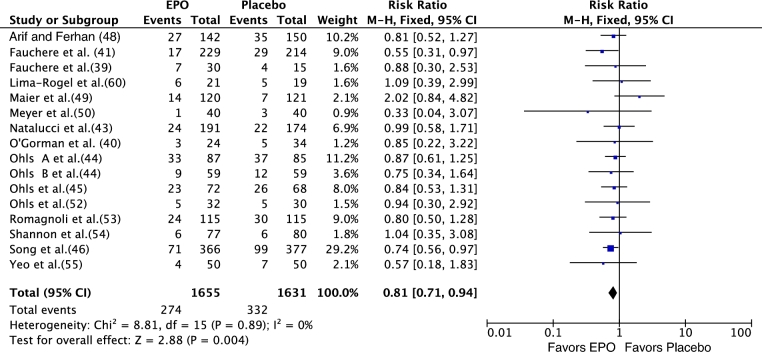
Time to full feedings and feeding intolerance were secondary outcomes in El-Ganzoury et al. (47) and Shannon et al. (58), respectively. El-Ganzoury et al. (47) reported a significant decrease in the time to full feedings (mean ± SD) in the enteral EPO group infants (EPO compared with placebo: 13.4 ± 4.9 compared with 16.3 ± 5.3 d; P = 0.032). Shannon et al. (58) reported feeding intolerance in 7 of the 147 (4.76%) compared with 7 of the 150 (4.66%) EPO infants compared with placebo-group infants; this was not significant. For mortality, in 13 of 25 RCTs that reported the outcome, meta-analysis showed no significant effect of rEPO on mortality [66/995 (6.63%) compared with 81/1010 (8.01%); RR: 0.78; 95% CI: 0.58; 1.04; P = 0.09]. Some of the included trials (39–41, 43–46, 48–50, 52–55, 60) reported a significant effect of rEPO on LOS [274/1655 (16.55%) compared with 332/1631 (20.35%); RR: 0.81; 95% CI: 0.71,0.94; P = 0.004] (Figure 6, Supplemental Figure 2).
FIGURE 6.
Effect of rEPO on sepsis in preterm neonates (fixed-effects model). EPO, erythropoietin; M-H, Mantel-Haenszel method; rEPO, recombinant erythropoietin.
Safety
None of the included RCTs reported adverse effects related to the administration of rEPO. The incidence of ROP (38, 40–46, 48, 49, 52–57) and BPD (38–46, 48, 51–53, 55–57) reported in various included RCTs was comparable in rEPO and control-group neonates (Supplemental Figures 3 and 4).
Discussion
This systematic review of 25 RCTs showed that rEPO had no effect on stage II or higher NEC, but it significantly decreased the incidence of any stage NEC in preterm neonates. The prestated subgroup analysis indicated that intravenous rEPO reduced the risk of any stage NEC. Improvement only in stage II or higher (i.e., definite) NEC is usually considered as a significant outcome in such studies. However, improvement in any stage NEC (stage I or higher) is important in the context of the gastrointestinal trophic effects of rEPO that are expected to reduce “feeding intolerance”—an entity that is often confused or reported as stage I NEC. The findings that LOS was reduced significantly and the risk of BPD, ROP, and mortality was not higher after EPO administration support the safety of rEPO in preterm neonates. To our knowledge, this is the first systematic review and meta-analysis of RCTs of rEPO in preterm neonates that reported outcomes (e.g., NEC and feeding intolerance) related to improvement in gastrointestinal maturity and function after the intervention.
The results of this review are in contrast to previous meta-analyses that focused on rEPO for reducing the need for transfusion for anemia of prematurity (19, 20). They did not report significant benefits of early (11 RCTs; n = 1347) (19) or late (6 RCTs; n = 656) (20) administration of rEPO on NEC (any stage or stage II or higher NEC) in preterm neonates. The likely reason for this difference is the inclusion of the large RCT (n = 743) by Song et al. (46) in this systematic review, which had 2465 more preterm neonates than the 2 previous reviews, increasing its power to detect a significant effect of EPO on NEC (19, 20).
RCTs with high ROB are known to overestimate the effect size, leading to spuriously optimistic results (36). It was reassuring to note that the results of the sensitivity analyses were significant after excluding studies with high ROB on random-sequence generation and allocation concealment separately. Subgroup analysis of 18 studies (n = 3447) including more mature preterm (born at <33 wk) VLBW neonates showed that rEPO reduced the incidence of any stage NEC (RR: 0.70; 95% CI: 0.53, 0·93; P = 0.01).The lack of benefits in ELBW neonates may relate to the small sample size of this subgroup (n = 660).
The effect of rEPO is based on the duration for which its critical blood concentration is maintained and this is unrelated to the route of administration (31). Subgroup analysis in this review showed a significant benefit of rEPO on any stage NEC only with the intravenous route. The lack of beneficial effect of subcutaneous EPO on NEC is difficult to explain, knowing that subcutaneous EPO has been shown to be beneficial in stimulating erythropoiesis in preterm infants (48, 61–64).
The strength of this review includes its comprehensive nature and robust methodology. Furthermore, the value of I2, a marker of statistical heterogeneity, was zero, and REM results were close to FEM results, increasing their validity. The benefits of intravenous rEPO in reducing any stage NEC are valid considering the total sample size, consistency of results, narrow CIs, and very small P values. The limitations include variations in the rEPO protocol in the included trials, the fact that NEC was not the primary outcome in majority of the trials, and data on NEC were not available from 40 of 57 RCTs of EPO for anemia of prematurity (19, 20).The use of Bell staging for classifying NEC is a limitation, but a universally agreed-on alternative classification is not yet available (65). Due to the lack of relevant data and small sample size, the effect of dose or AUC relation could not be explored in this review.
The neuroprotective effects of EPO are important in the context of our results (66, 67). Fischer et al. (68) reported a systematic review and meta-analysis of data from RCTs (n = 4; 1133 participants) (43, 45, 46, 52), assessing if prophylactic rEPO improves neurodevelopmental outcomes in very preterm infants (68, 65). Prophylactic rhEPO significantly reduced the incidence of children with a Mental Developmental Index <70 on the Bayley Scales of Infant Development assessment (primary outcome) at 18–24 mo of corrected age (OR: 0.51; 95% CI: 0.31, 0.81; P < 0.005; number needed to treat: 14). EPO had no effect on the secondary outcomes of Psychomotor Development Index <70, cerebral palsy, visual impairment, and hearing impairment (68). Lowe et al. (69) assessed the effect of erythropoiesis-stimulating agent (ESAs) exposure during the early postnatal period and family demographic factors on early childhood behavior. Preterm infants (birth weight: 500–1250 g) who had participated in an RCT of ESAs (n = 35) compared with placebo (n = 14) and term healthy infants (controls: n = 22) were followed at 3.5–4 y of age. The Socioeconomic Composite scores on behavioral symptoms (P = 0.04) and externalizing scales (P = 0.04) were significantly better in the ESA group than in the placebo group. An interaction was observed between study group and Socioeconomic Composite score (P = 0.001) (66, 69).
In summary, the results in this review indicate that rEPO, particularly intravenous rEPO, has the potential to prevent NEC in preterm infants. A definitive RCT is required to confirm our findings. Because the definition of stage II or higher NEC is more precise than “any stage NEC,” the primary outcome interest for such an RCT could be “stage II or higher NEC.” Another reason for choosing “stage II or higher NEC” as the primary outcome is the enormous burden associated with this condition in preterm infants (1, 2). The sample size for such a trial would be ∼3000 (1500/arm) to detect a significant reduction of 30% in the risk of stage II or higher NEC (baseline incidence: 10%) in ELBW infants with 80% power and a significance of P < 0.05. Providing separate data for different stages of NEC and assessment of other enteral nutrition–related outcomes and long-term neurodevelopmental follow-up will be important aspects of such a trial.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SP: was responsible for the concept and design, rechecked the data and analysis, and gave input for revision of the initial and final versions of the manuscript; AA and HB: performed the independent literature search, study selection, data extraction, and analysis, and wrote the first and final draft of the manuscript with guidance from SR and SP; SR: rechecked the methodology and interpretation; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: AA, HB, SR, and SP, no conflicts of interest.
Supplemental Methods, Supplemental Tables 1–3, Supplemental Figures 1–4, and Supplemental References are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used:
- BPD
bronchopulmonary dysplasia
- ELBW
extremely low birth weight
- EPO
erythropoietin
- ESA
erythropoiesis-stimulating agent
- FEM
fixed-effects model
- LOS
late-onset sepsis
- NEC
necrotizing enterocolitis
- RCT
randomized controlled trial
- REM
random-effects model
- rEPO
recombinant erythropoietin
- ROB
risk of bias
- ROP
retinopathy of prematurity
- VLBW
very low birth weight
References
- 1. Henry MC, Moss RL. Neonatal necrotizing enterocolitis. Semin Pediatr Surg 2008;17:98–109. [DOI] [PubMed] [Google Scholar]
- 2. Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol 2006;20:498–506. [DOI] [PubMed] [Google Scholar]
- 3. Li YF, Lin HC, Torrazza RM, Parker L, Talaga E, Neu J. Gastric residual evaluation in preterm neonates: a useful monitoring technique or a hindrance? Pediatr Neonatol 2014;55:335–40. [DOI] [PubMed] [Google Scholar]
- 4. Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017;3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ofek Shlomai N, Deshpande G, Rao S, Patole S. Probiotics for preterm neonates: what will it take to change clinical practice? Neonatology 2014;105:64–70. [DOI] [PubMed] [Google Scholar]
- 6. Maffei D, Schanler RJ. Human milk is the feeding strategy to prevent necrotizing enterocolitis! Semin Perinatol 2017;41:36–40. [DOI] [PubMed] [Google Scholar]
- 7. Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 2016;13:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christensen RD, Gordon PV, Besner GE. Can we cut the incidence of necrotizing enterocolitis in half—today? Fetal Pediatr Pathol 2010;29:185–98. [DOI] [PubMed] [Google Scholar]
- 9. Jasani B, Patole S. Standardized feeding regimen for reducing necrotizing enterocolitis in preterm infants: an updated systematic review. J Perinatol 2017;37(7):827–33. [DOI] [PubMed] [Google Scholar]
- 10. Kolacek S, Hojsak I, Canani RB, Guarino A, Indrio F, Orel R, Pot B, Shamir R, Szajewska H, Vandenplas Y. et al. Commercial probiotic products: a call for improved quality control. A position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr 2017Apr 11 (Epub ahead of print; DOI: 10.1097/MPG.0000000000001603). [DOI] [PubMed] [Google Scholar]
- 11. Szajewska H, van Goudoever JB. To give or not to give probiotics to preterm infants. Am J Clin Nutr 2014;100:1411–2. [DOI] [PubMed] [Google Scholar]
- 12. Dermyshi E, Wang Y, Yan C, Hong W, Qiu G, Gong X, Zhang T. The “Golden Age” of probiotics: a systematic review and meta-analysis of randomized and observational studies in preterm infants. Neonatology 2017;112:9–23. [DOI] [PubMed] [Google Scholar]
- 13. Modi N. Probiotics and necrotising enterocolitis: the devil (as always) is in the detail. (Commentary on N. Ofek Shlomai et al.: Probiotics for preterm neonates: what will it take to change clinical practice?) Neonatology 2014;105:71–3. [DOI] [PubMed] [Google Scholar]
- 14. Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 2016;387:649–60. [DOI] [PubMed] [Google Scholar]
- 15. Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2015;2:CD007137. [DOI] [PubMed] [Google Scholar]
- 16. Sharma D, Shastri S, Sharma P. Role of lactoferrin in neonatal care: a systematic review. J Matern Fetal Neonatal Med 2016;30:1920–32. [DOI] [PubMed] [Google Scholar]
- 17. Samanci N, Ovali F, Dagoglu T.. Effects of recombinant human erythropoietin in infants with very low birth weights. J Int Med Res 1996;24:190–8. [DOI] [PubMed] [Google Scholar]
- 18. Halperin DS, Wacker P, Lacourt G, Felix M, Babel JF, Aapro M, Wyss M. Effects of recombinant human erythropoietin in infants with the anemia of prematurity: a pilot study. J Pediatr 1990;116:779–86. [DOI] [PubMed] [Google Scholar]
- 19. Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2014;4:CD004863. [DOI] [PubMed] [Google Scholar]
- 20. Aher SM, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2014;4:CD004868. [DOI] [PubMed] [Google Scholar]
- 21. Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci USA 1994;91:3974–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masuda S, Nagao M, Takahata K, Konishi Y, Gallyas F Jr., Tabira T, Sasaki R. Functional erythropoietin receptor of the cells with neural characteristics: comparison with receptor properties of erythroid cells. J Biol Chem 1993;268:11208–16. [PubMed] [Google Scholar]
- 23. Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev 1998;52:235–49. [DOI] [PubMed] [Google Scholar]
- 24. Wald MR, Borda ES, Sterin-Borda L. Mitogenic effect of erythropoietin on neonatal rat cardiomyocytes: signal transduction pathways. J Cell Physiol 1996;167:461–8. [DOI] [PubMed] [Google Scholar]
- 25. Okada A, Kinoshita Y, Maekawa T, Hassan MS, Kawanami C, Asahara M, Matsushima Y, Kishi K, Nakata H, Naribayashi Y. et al. Erythropoietin stimulates proliferation of rat-cultured gastric mucosal cells. Digestion 1996;57:328–32. [DOI] [PubMed] [Google Scholar]
- 26. Juul SE, Joyce AE, Zhao Y, Ledbetter DJ. Why is erythropoietin present in human milk? Studies of erythropoietin receptors on enterocytes of human and rat neonates. Pediatr Res 1999;46:263–8. [DOI] [PubMed] [Google Scholar]
- 27. McPherson RJ, Juul SE. High-dose erythropoietin inhibits apoptosis and stimulates proliferation in neonatal rat intestine. Growth Horm IGF Res 2007;17:424–30. [DOI] [PubMed] [Google Scholar]
- 28. Maheshwari A. Role of cytokines in human intestinal villous development. Clin Perinatol 2004;31:143–55. [DOI] [PubMed] [Google Scholar]
- 29. Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 2000;92:71–7. [DOI] [PubMed] [Google Scholar]
- 30. Ashley RA, Dubuque SH, Dvorak B, Woodward SS, Williams SK, Kling PJ. Erythropoietin stimulates vasculogenesis in neonatal rat mesenteric microvascular endothelial cells. Pediatr Res 2002;51:472–8. [DOI] [PubMed] [Google Scholar]
- 31. Doshi S, Krzyzanski W, Yue S, Elliott S, Chow A, Perez-Ruixo JJ. Clinical pharmacokinetics and pharmacodynamics of erythropoiesis-stimulating agents. Clin Pharmacokinet 2013;52:1063–83. [DOI] [PubMed] [Google Scholar]
- 32. Kato M, Okano K, Sakamoto Y, Miura K, Uchimura T, Saito K. Pharmacokinetics and pharmacodynamics of recombinant human erythropoietin in rats. Arzneimittelforschung 2001;51:91–5. [DOI] [PubMed] [Google Scholar]
- 33. Molavi M Mirjalili R, Nazemi A, Saadat H, Goodarzi R, Hamedi Y, Safa M. Comparison of oral and injection erythropoietin on level of hemoglobin concentration in premature neonates: a randomized controlled trial. Asian J Med Pharm Res 2012;2:64–8. [Google Scholar]
- 34. Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 1987;17:213–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, Brozek J, Norris S, Meerpohl J, Djulbegovic B. et al. GRADE guidelines: 12. Preparing summary of findings tables—binary outcomes. J Clin Epidemiol 2013;66:158–72. [DOI] [PubMed] [Google Scholar]
- 36. Egger M, Ebrahim S, Smith GD. Where now for meta-analysis? Int J Epidemiol 2002;31:1–5. [DOI] [PubMed] [Google Scholar]
- 37. Qi W, Shen Q, Zhang L, Han LP, Wang S. Study on the inflammatory intervention of erythropoietin on NEC. Exp Ther Med 2016;11:2221–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics 2006;118:e635–40. [DOI] [PubMed] [Google Scholar]
- 39. Fauchere JC, Dame C, Vonthein R, Koller B, Arri S, Wolf M, Bucher HU. An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics 2008;122:375–82. [DOI] [PubMed] [Google Scholar]
- 40. O'Gorman RL, Bucher HU, Held U, Koller BM, Huppi PS, Hagmann CF. Tract-based spatial statistics to assess the neuroprotective effect of early erythropoietin on white matter development in preterm infants. Brain 2015;138(Part 2):388–97. [DOI] [PubMed] [Google Scholar]
- 41. Fauchere JC, Koller BM, Tschopp A, Dame C, Ruegger C, Bucher HU. Safety of early high-dose recombinant erythropoietin for neuroprotection in very preterm infants. J Pediatr 2015;167:52–7, e1–3. [DOI] [PubMed] [Google Scholar]
- 42. Maier RF, Obladen M, Muller-Hansen I, Kattner E, Merz U, Arlettaz R, Groneck P, Hammer H, Kossel H, Verellen G. et al. Early treatment with erythropoietin beta ameliorates anemia and reduces transfusion requirements in infants with birth weights below 1000 g. J Pediatr 2002;141:8–15. [DOI] [PubMed] [Google Scholar]
- 43. Natalucci G, Latal B, Koller B, Ruegger C, Sick B, Held L, Bucher HU, Fauchere JC. Effect of early prophylactic high-dose recombinant human erythropoietin in very preterm infants on neurodevelopmental outcome at 2 years: a randomized clinical trial. JAMA 2016;315:2079–85. [DOI] [PubMed] [Google Scholar]
- 44. Ohls RK, Ehrenkranz RA, Wright LL, Lemons JA, Korones SB, Stoll BJ, Stark AR, Shankaran S, Donovan EF, Close NC. et al. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birth weight: a multicenter, randomized, controlled trial. Pediatrics 2001;108:934–42. [DOI] [PubMed] [Google Scholar]
- 45. Ohls RK, Ehrenkranz RA, Das A, Dusick AM, Yolton K, Romano E, Delaney-Black V, Papile LA, Simon NP, Steichen JJ. et al. Neurodevelopmental outcome and growth at 18 to 22 months' corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics 2004;114:1287–91. [DOI] [PubMed] [Google Scholar]
- 46. Song J, Sun H, Xu F, Kang W, Gao L, Guo J, Zhang Y, Xia L, Wang X, Zhu C. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann Neurol 2016;80:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. El-Ganzoury MM, Awad HA, El-Farrash RA, El-Gammasy TM, Ismail EA, Mohamed HE, Suliman SM. Enteral granulocyte-colony stimulating factor and erythropoietin early in life improves feeding tolerance in preterm infants: a randomized controlled trial. J Pediatr 2014;165:1140–5, e1. [DOI] [PubMed] [Google Scholar]
- 48. Arif B, Ferhan K. Recombinant human erythropoietin therapy in low-birthweight preterm infants: a prospective controlled study. Pediatr Int 2005;47:67–71. [DOI] [PubMed] [Google Scholar]
- 49. Maier RF, Obladen M, Scigalla P, Linderkamp O, Duc G, Hieronimi G, Halliday HL, Versmold HT, Moriette G, Jorch G. et al. European Multicentre Erythropoietin Study Group. The effect of epoetin beta (recombinant human erythropoietin) on the need for transfusion in very-low-birth-weight infants. N Engl J Med 1994;330:1173–8. [DOI] [PubMed] [Google Scholar]
- 50. Meyer MP, Meyer JH, Commerford A, Hann FM, Sive AA, Moller G, Jacobs P, Malan AF. Recombinant human erythropoietin in the treatment of the anemia of prematurity: results of a double-blind, placebo-controlled study. Pediatrics 1994;93:918–23. [PubMed] [Google Scholar]
- 51. Obladen M, Maier R, Segerer H, Grauel EL, Holland BM, Stewart G, Jorch G, Rabe H, Linderkamp O, Hoffmann HG. et al. Efficacy and safety of recombinant human erythropoietin to prevent the anaemias of prematurity. European Randomized Multicenter Trial. Contrib Nephrol 1991;88:314–26. [DOI] [PubMed] [Google Scholar]
- 52. Ohls RK, Christensen RD, Kamath-Rayne BD, Rosenberg A, Wiedmeier SE, Roohi M, Lacy CB, Lambert DK, Burnett JJ, Pruckler B. et al. A randomized, masked, placebo-controlled study of darbepoetin alfa in preterm infants. Pediatrics 2013;132:e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Romagnoli C, Zecca E, Gallini F, Girlando P, Zuppa AA. Do recombinant human erythropoietin and iron supplementation increase the risk of retinopathy of prematurity? Eur J Pediatr 2000;159:627–8. [DOI] [PubMed] [Google Scholar]
- 54. Shannon KM, Keith JF III, Mentzer WC, Ehrenkranz RA, Brown MS, Widness JA, Gleason CA, Bifano EM, Millard DD, Davis CB. et al. Recombinant human erythropoietin stimulates erythropoiesis and reduces erythrocyte transfusions in very low birth weight preterm infants. Pediatrics 1995;95:1–8. [PubMed] [Google Scholar]
- 55. Yeo CL, Choo S, Ho LY. Effect of recombinant human erythropoietin on transfusion needs in preterm infants. J Paediatr Child Health 2001;37:352–8. [DOI] [PubMed] [Google Scholar]
- 56. Haiden N, Cardona F, Schwindt J, Berger A, Kuhle S, Homoncik M, Jilma-Stohlawetz P, Pollak A, Jilma B. Changes in thrombopoiesis and platelet reactivity in extremely low birth weight infants undergoing erythropoietin therapy for treatment of anaemia of prematurity. Thromb Haemost 2005;93:118–23. [DOI] [PubMed] [Google Scholar]
- 57. Turker G, Sarper N, Gokalp AS, Usluer H. The effect of early recombinant erythropoietin and enteral iron supplementation on blood transfusion in preterm infants. Am J Perinatol 2005;22:449–55. [DOI] [PubMed] [Google Scholar]
- 58. Shannon KM, Mentzer WC, Abels RI, Freeman P, Newton N, Thompson D, Sniderman S, Ballard R, Phibbs RH. Recombinant human erythropoietin in the anemia of prematurity: results of a placebo-controlled pilot study. J Pediatr 1991;118:949–55. [DOI] [PubMed] [Google Scholar]
- 59. Qiao L, Tang Q, Zhu W, Zhang H, Zhu Y, Wang H. Effects of early parenteral iron combined erythropoietin in preterm infants: a randomized controlled trial. Medicine (Baltimore) 2017;96:e5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lima-Rogel V, Torres-Montes A, Espinosa Griesse S, Villegas Alvarez C, Hernandez-Sierra F, Bissett Mandeville P, Correa Gonzalez C. [Efficacy of early erythropoietin use in critically ill, very-low-birthweight premature newborn infants: controlled clinical trial.] Sangre (Barc) 1998;43:191–5(in Spanish). [PubMed] [Google Scholar]
- 61. Soubasi V, Kremenopoulos G, Diamanti E, Tsantali C, Sarafidis K, Tsakiris D. Follow-up of very low birth weight infants after erythropoietin treatment to prevent anemia of prematurity. J Pediatr 1995;127:291–7. [DOI] [PubMed] [Google Scholar]
- 62. Soubasi V, Kremenopoulos G, Diamandi E, Tsantali C, Tsakiris D. In which neonates does early recombinant human erythropoietin treatment prevent anemia of prematurity? Results of a randomized, controlled study. Pediatr Res 1993;34:675–9. [DOI] [PubMed] [Google Scholar]
- 63. Salvado A, Ramolfo P, Escobar M, Nunez A, Aguayo I, Standen J, Sanchez L, Cabello A. Early erythropoietin use for the prevention of anemia in infant premature. Rev Med Chil 2000;128:1313–7. [PubMed] [Google Scholar]
- 64. Avent M, Cory BJ, Galpin J, Ballot DE, Cooper PA, Sherman G, Davies VA. A comparison of high versus low dose recombinant human erythropoietin versus blood transfusion in the management of anaemia of prematurity in a developing country. J Trop Pediatr 2002;48:227–33. [DOI] [PubMed] [Google Scholar]
- 65. Juhl SM, Hansen ML, Fonnest G, Gormsen M, Lambaek ID, Greisen G. Poor validity of the routine diagnosis of necrotising enterocolitis in preterm infants at discharge. Acta Paediatr 2017;106:394–8. [DOI] [PubMed] [Google Scholar]
- 66. Juul SE, Pet GC. Erythropoietin and neonatal neuroprotection. Clin Perinatol 2015;42:469–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang J, Wang Q, Xiang H, Xin Y, Chang M, Lu H. Neuroprotection with erythropoietin in preterm and/or low birth weight infants. J Clin Neurosci 2014;21:1283–7. [DOI] [PubMed] [Google Scholar]
- 68. Fischer HS, Reibel NJ, Bührer C, Dame C. Prophylactic early erythropoietin for neuroprotection in preterm infants: a meta-analysis. Pediatrics 2017;139 (Epub 2017 Apr 7; DOI: 10.1542/peds.2016-4317). [DOI] [PubMed] [Google Scholar]
- 69. Lowe JR, Rieger RE, Moss NC, Yeo RA, Winter S, Patel S, Phillips J, Campbell R, Baker S, Gonzales S. et al. Impact of erythropoiesis-stimulating agents on behavioral measures in children born preterm. J Pediatr 2017;184:75–80, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.