Abstract
Balancing selection maintains advantageous diversity in populations through various mechanisms. Although extensively explored from a theoretical perspective, an empirical understanding of its prevalence and targets lags behind our knowledge of positive selection. Here, we describe the Non-central Deviation (NCD), a simple yet powerful statistic to detect long-term balancing selection (LTBS) that quantifies how close frequencies are to expectations under LTBS, and provides the basis for a neutrality test. NCD can be applied to a single locus or genomic data, and can be implemented considering only polymorphisms (NCD1) or also considering fixed differences with respect to an outgroup (NCD2) species. Incorporating fixed differences improves power, and NCD2 has higher power to detect LTBS in humans under different frequencies of the balanced allele(s) than other available methods. Applied to genome-wide data from African and European human populations, in both cases using chimpanzee as an outgroup, NCD2 shows that, albeit not prevalent, LTBS affects a sizable portion of the genome: ∼0.6% of analyzed genomic windows and 0.8% of analyzed positions. Significant windows (P < 0.0001) contain 1.6% of SNPs in the genome, which disproportionally fall within exons and change protein sequence, but are not enriched in putatively regulatory sites. These windows overlap ∼8% of the protein-coding genes, and these have larger number of transcripts than expected by chance even after controlling for gene length. Our catalog includes known targets of LTBS but a majority of them (90%) are novel. As expected, immune-related genes are among those with the strongest signatures, although most candidates are involved in other biological functions, suggesting that LTBS potentially influences diverse human phenotypes.
Keywords: natural selection, overdominance, site frequency spectrum, neutrality test, summary statistic, genome-wide scan
Introduction
Balancing selection refers to a class of selective mechanisms that maintains advantageous genetic diversity in populations. Decades of research have established HLA genes as a prime example of balancing selection (Meyer and Thomson 2001; Spurgin and Richardson 2010), with thousands of alleles segregating in humans, extensive support for functional effects of these polymorphisms (Prugnolle et al. 2005), and various well-documented cases of association between selected alleles and disease susceptibility (Raychaudhuri et al. 2012; Howell 2014). The catalog of well-understood non-HLA targets of balancing selection in humans remains small, but includes genes associated to phenotypes such as autoimmune diseases (Ferrer-Admetlla et al. 2008; Sironi and Clerici 2010), resistance to malaria (Malaria Genomic Epidemiology Network 2015), HIV infection (Biasin et al. 2007), or susceptibility to polycystic ovary syndrome (Day et al. 2015). Thus, besides historically influencing individual fitness, balanced polymorphisms shape current phenotypic diversity and susceptibility to disease.
Balancing selection encompasses several mechanisms (reviewed in Andrés 2011; Key, Teixeira, et al. 2014; Fijarczyk and Babik 2015). These include heterozygote advantage or overdominance, some cases of frequency-dependent selection (e.g., rare allele advantage), selective pressures that fluctuate in time (Bergland et al. 2014) or in space in panmitic populations (Charlesworth et al. 1997), and some cases of pleiotropy (Johnston et al. 2013). For overdominance, pleiotropy, and some instances of spatially variable selection, a stable equilibrium—at the frequency equilibrium, which maximizes fitness in the population—can be reached (Charlesworth and Charlesworth 2010). For other mechanisms, the frequency of the selected allele can change in time without reaching a stable equilibrium. Regardless of the mechanism, long-term balancing selection (LTBS) has the potential to leave identifiable signatures in genomic data. The first is an increase in the ratio of polymorphic to divergent sites: by reducing the probability of fixation of a variant, balancing selection increases the local time to the most recent common ancestor (Hudson and Kaplan 1988) and the density of polymorphisms. The HKA test is commonly used to detect this signature (Hudson et al. 1987). The second signature is an excess of alleles segregating at intermediate frequencies. In humans, the folded site frequency spectrum (SFS)—the frequency distribution of minor allele frequencies (MAF)—is typically L-shaped, showing an excess of low-frequency alleles when compared with expectations under neutrality and demographic equilibrium. Regions under LTBS, on the other hand, can show a markedly different SFS, with proportionally more alleles at intermediate frequency. Such a deviation in the SFS is identified by classical neutrality tests such as Tajima’s D (TajD) and newer statistics such as MWU-high (Nielsen et al. 2009). In some cases, very ancient balancing selection can maintain trans-species polymorphisms in sister species (Leffler et al. 2013; Teixeira et al. 2015). On the other hand, when balancing selection is very recent or transient (Sellis et al. 2011), signatures are difficult to distinguish from incomplete, recent selective sweeps (Key, Teixeira, et al. 2014).
Although balancing selection has been extensively explored from a theoretical perspective, an empirical understanding of its prevalence lags behind our knowledge of positive selection. This stems from technical difficulties in detecting balancing selection, as well as the perception that it may be a rare selective process. In fact, few methods have been developed to identify its targets, and only a handful of studies have sought to uncover them genome-wide in humans (Asthana et al. 2005; Bubb et al. 2006; Alonso et al. 2008; Andrés et al. 2009; Leffler et al. 2013; DeGiorgio et al. 2014; Rasmussen et al. 2014; Siewert and Voight 2017). Some studies have identified genes (Andrés et al. 2009) or genomic regions (DeGiorgio et al. 2014) with an excess of polymorphisms and intermediate frequency alleles, whereas others have identified trans-species polymorphisms between humans and their closest living relatives (chimpanzees and bonobos) (Leffler et al. 2013; Teixeira et al. 2015). Overall, these studies suggested that balancing selection may act on a small portion of the genome, although the limited extent of data used (e.g., exome data, Andrés et al. 2009; small sample size, DeGiorgio et al. 2014), and stringency of the criteria (e.g., trans-species polymorphisms predating human–chimpanzee divergence; Leffler et al. 2013; Teixeira et al. 2015) may underlie the paucity of detected regions.
In the tradition of neutrality tests analyzing the SFS directly (Nielsen et al. 2005, 2009; Williamson et al. 2007), we propose the “Non-central Deviation” (NCD) statistic, which measures the degree to which the local SFS deviates from expectations under balancing selection using only polymorphisms (NCD1) or also fixed differences to an outgroup (NCD2). We show, through simulations, that NCD2 outperforms existing methods under a realistic demographic scenario for human populations. We apply NCD2 to genome-wide data from four human populations to test the null hypothesis of neutral evolution, and use both outlier and simulation-based approaches to identify genomic regions bearing signatures of LTBS.
Methods
The NCD Statistic
Background
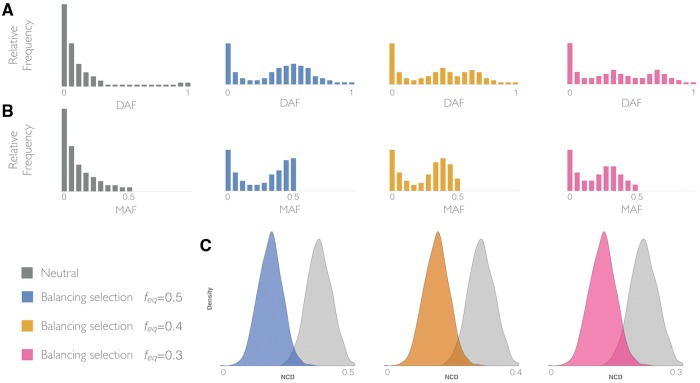
Owing to linkage, the signature of LTBS extends to the genetic neighborhood of the selected variant(s); therefore, patterns of polymorphism and divergence in a genomic region can be used to infer whether it evolved under LTBS (Charlesworth 2006; Andrés 2011). As mentioned, LTBS leaves two distinctive signatures in linked variation, when compared with neutral expectations: an increase in the ratio of polymorphic to divergent sites and an excess of alleles segregating at intermediate frequencies when compared with neutral expectations (fig. 1A and B). With heterozygote advantage, the frequency equilibrium (feq) depends on the relative fitness of each genotype (Charlesworth and Charlesworth 2010): when the two types of homozygotes have the same fitness (symmetric overdominance), feq = 0.5; when the fitness of the two homozygotes is different (asymmetric overdominance), feq ≠ 0.5. Under negative frequency-dependent selection and fluctuating selection, whereas an equilibrium may not be reached (supplementary information S1, Supplementary Material online), feq can be thought of as the frequency of the balanced polymorphism at the time of sampling. We focus on overdominance, as comparing different mechanisms of balancing selection falls outside of the scope of the paper.
Fig. 1.
—A schematic representation of site frequency spectra (SFS) under neutrality and selection, which motivates the NCD statistic. (A) Unfolded SFS (ranging from 0 to 1) of derived allele frequencies (DAF) for loci under neutrality (gray) or containing one site under balancing selection with frequency equilibrium (feq) of 0.5 (blue), 0.4 (orange), and 0.3 (pink). (B) Folded SFS (ranging from 0 to 0.5) for minor allele frequencies (MAF). Colors as in A. (C) Distribution of NCD expected under neutrality (gray) and under selection assuming tf = feq. Colors as in A. x axis shows minimum and maximum values that NCD can have for a given tf.
Under a model of balancing selection, we define tf (target frequency) as the expected frequency of a balanced allele, with the NCD statistic quantifying how far the sampled SNP frequencies are from it. Because biallelic loci have complementary allele frequencies, and there is no prior expectation regarding whether ancestral or derived alleles should be maintained at higher frequency, we use the folded SFS (fig. 1B). NCD is defined as:
| (1) |
where i is the i-th informative site in a locus, pi is the MAF for the i-th informative site, n is the number of informative sites, and tf is the target frequency with respect to which the deviations of the observed alleles frequencies are computed. Thus, NCD is a type of standard deviation (SD) that quantifies the dispersion of allelic frequencies from tf, rather than from the mean of the distribution. We note that tf is not an estimated quantity, but rather a hypothetical frequency expected under balancing selection, with respect to which we quantify deviations in genomic data. We consider the same tf for all SNPs in a given locus/window because we expect most small regions to have only one balanced allele, with the rest being shifted close to the tf (though often not reaching it) by linkage. Low NCD values reflect a low deviation of the SFS from a predefined tf (fig. 1C), as expected under LTBS (supplementary information S1 and supplementary fig. S1, Supplementary Material online). Of course, a priori feq is unknown, and thus the choice of a tf value may not be straightforward. We show that NCD values correlate strongly across tf values (supplementary fig. S9, Supplementary Material online), so while this is a key value in the statistic, it has limited influence in its applications. In any case, we propose below a practical approach to deal with the inherent feq uncertainty.
We propose two NCD implementations: NCD1 uses only polymorphic sites, and NCD2 also includes the number of fixed differences (FDs) relative to an outgroup species (i.e., all informative sites, ISs = SNPs + FDs, are used to compute the statistic). In NCD2, FDs are considered informative sites with MAF = 0; thus, the greater the number of FDs, the larger the NCD2 and the weaker the support for LTBS (supplementary fig. S2, Supplementary Material online). Because NCD2 includes also FDs, it uses more information and we focus on it. Nevertheless, NCD1 can be used in the absence of an outgroup, and we show its power statistics in detail in the supplements (supplementary information S1 and supplementary table S1, Supplementary Material online).
Simulations and Power Analyses
NCD’s power was evaluated by simulations (under neutrality and with selection) with MSMS (Ewing and Hermisson 2010) following the demographic model described in Gravel et al. (2011) for African, European, and East Asian human populations, and considering a generation time of 25 years, mutation rate of 2.5×10−8 per site, recombination rate of 1×10−8, and the human–chimpanzee split at 6.5 million years ago (Ma). For simulations with selection, a balanced polymorphism was added to the center of the simulated sequence and modeled to achieve a prespecified frequency equilibrium (feq = 0.3, 0.4, 0.5) following an overdominant model (supplementary information S1, Supplementary Material online). Simulations with and without selection were run for different sequence lengths (L = 3, 6, 12 kb) and times of onset of balancing selection (1, 3, 5 Ma). For each combination of parameter, 1,000 simulations were used to compare the relationship between true (TPR, the power of the statistic) and false (FPR) positive rates for the NCD statistics, with a FPR set to 0.05. When comparing performance under a given condition, power was averaged across demographic scenarios, L, and Tbs.
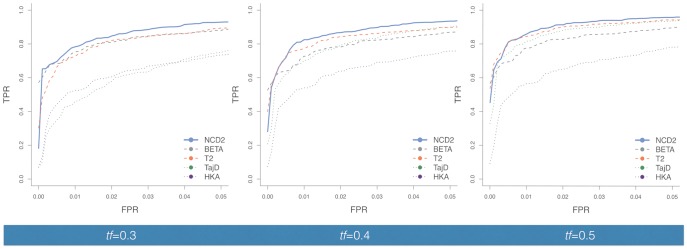
When comparing NCD performance to classical methods (TajD, HKA), we used NCD optimal conditions: 3 kb, Tbs = 5 Ma (see Results, fig. 2), which is also optimal for these other tests (supplementary table S1, Supplementary Material online). For the ß statistic (Siewert and Voight 2017) we used the folded version of the test and, for consistency, also of 3-kb windows (but we note that results do not change with 1-kb windows; supplementary table S1 and supplementary information S1, Supplementary Material online). For the T1 and T2 statistics, which require longer genomic regions to identify the signature of balancing selection, power was reported based on windows of 100 informative sites up and downstream of the target site, following BALLET’s original publication (DeGiorgio et al. 2014). Power values are for a sample size of 50 diploid individuals, but sample sizes of 30 and 10 individuals were also explored (supplementary information S1, Supplementary Material online).
Fig. 2.
—Power to detect balancing selection for NCD2(0.5) and other tests. The ROC curves summarize the true positive rate (TPR) as a function of the false positive rate (FPR) to detect LTBS for simulations where the balanced polymorphism was modeled to achieve feq of (A) 0.3, (B) 0.4, and (C) 0.5. Plotted values are for the African demography, Tbs = 5 Ma. L = 3 kb, except for T1 and T2 where L = 100 ISs (see Methods). BETA refers to the ß statistic (Siewert and Voight 2017). For NCD2 calculations, tf = feq. European demography yields similar results (supplementary fig. S10, Supplementary Material online). Power for NCD1, NCD1 + HKA, and T1 is provided in supplementary table S1, Supplementary Material online.
Identifying Signatures of LTBS in Human Genomes
Statistic
The implementation of the genome-wide analysis was informed by the power analyses (see Results). As our power results show, NCD2 has very high power. Although the feq of a putatively balanced allele—that is, the actual frequency of the balanced polymorphism at the time of sampling—is unknown, the simplicity of the NCD statistics makes it trivial to run for several tf values, allowing detection of balancing selection for a range of equilibrium frequencies. For each window in each population, we thus calculated NCD2 for three tf values (0.3, 0, 4, 0.5) although in neutral simulations these results are highly correlated (supplementary fig. S9, Supplementary Material online).
Data
We analyzed genome-wide data from two African (YRI: Yoruba in Ibadan, Nigeria; LWK: Luhya in Webuye, Kenya) and two European populations (GBR: British, England and Scotland; TSI: Toscani, Italy) from the 1000 Genomes (1000 G) Project phase I (Abecasis 2012), excluding SNPs only detected in the high coverage exome sequencing to avoid SNP density differences between coding and noncoding regions. We randomly sampled 50 unrelated individuals from each population (as in Key, Peter, et al. 2014) and did not consider Asian populations due to lower NCD performance for these populations according to our simulations (supplementary table S1 and figs. S7 and S8, Supplementary Material online). We only included in our analyses positions that passed mappability (50mer CRG, 2 mismatches; Derrien, Estellé, et al. 2012), segmental duplication (Cheng et al. 2005; Alkan et al. 2009) and tandem repeats filters (http://genome.ucsc.edu, last accessed September 2016), as well as the requirement of orthology to chimp (supplementary fig. S13, Supplementary Material online).
The analysis was performed on 3-kb sliding (step 1.5 kb) genomic windows. Windows were defined in physical distance since the presence of LTBS may affect the population-based estimates of recombination rate. In addition, we chose to define windows based on length rather than number of ISs because the density of SNPs is part of NCD2’s signature, and fixing the number of ISs may result in particularly short windows in regions under balancing selection, which is undesirable for NCD analyses (see Results). We excluded 3-kb windows with less than ten ISs in any population (supplementary information S2 and supplementary figs. S11 and S17, Supplementary Material online) or <500 bp with orthology in chimp (1.6%); the two criteria combined resulted in the exclusion of 2.2% of scanned windows. Finally, to show that undetected short duplications do not contribute substantially to our candidates, we analyzed an alternative modern human genome-wide data set (supplementary information S2, Supplementary Material Online), sequenced to an average coverage of 20×–30× per individual (Meyer et al. 2012; Prüfer et al. 2014). After all these filters, we analyzed the 1,657,989 windows that remained in all populations, covering 2,145,937,383 base pairs (supplementary fig. S13, Supplementary Material online).
Defining Significant and Outlier Windows
We defined two sets of windows with signatures of LTBS, which collectively we consider our candidate windows. Significant windows are defined as those for which the observed NCD2 value is lower than all values obtained from 10,000 neutral simulations with the same number of ISs. The matching by number of ISs aims to account for the higher variance in the statistic (in any SFS statistic) in windows with low numbers of informative sites (supplementary figs. S11 and S17, Supplementary Material online). Thus, all significant windows have the same P value (P < 0.0001).
In order to quantify how far the NCD2 value of each window is from neutral expectations, we quantified the number of SDs a window’s NCD2 value lies from the mean of the simulated distribution under neutrality (see supplementary information S2, Supplementary Material online). For that, we defined a standardized distance measure between the observed NCD2 (for a queried window) and the mean of the NCD2 values for the 10,000 simulations with the matching number of ISs:
| (2) |
where Ztf-ISis the standardized NCD2, conditional on the value of IS, NCD2tfis the NCD2 value with a given tf for the n-th empirical window, is the mean NCD2 for 10,000 neutral simulations for the corresponding value of IS, and sdtf-IS is the SD for 10,000 NCD2 values from simulations with matching ISs. Ztf-ISallows the ranking of windows for a given tf, while taking into account the residual effect of IS number on NCD2tf, as well as a comparison between the rankings of a window considering different tf values. Outlier windows are defined based on an empirical P value that is attributed to each window as a function of Ztf-IS values for each tf. The empirical P value is calculated by ranking the windows in terms of Ztf-ISvalues and dividing the ranking position by the number of scanned window (i.e., the lowest empirical P value is 1/1,657,989 = ). Windows with empirical P value ≤ 0.0005 (i.e., the 829 most extreme windows) were defined as outlier windows. With a few exceptions, outlier windows are a subset of significant windows. The coordinates for these windows are provided in supplementary table S5, Supplementary Material online. For simplicity, all candidate windows that were identified for multiple tf values had an assigned tf value, defined as the one that minimizes the empirical P value for a given window (supplementary information S2, Supplementary Material online).
Properties of Candidate Windows
Archaic Introgression and Ectopic Gene Conversion
We evaluated two potentially confounding biological factors. To assess ectopic gene conversion, we tested whether candidate genes have elevated number of paralogs in the same chromosome. To assess archaic introgression, we calculated the proportion of European SNPs in candidate windows that are potentially of archaic (Neandertal or Denisova) origin. Details are provided in supplementary information S3, Supplementary Material online.
Gene Ontology and Tissue-Specific Expression Enrichment Analyses
We analyzed protein-coding genes overlapped by one or more candidate windows. GO and tissue of expression enrichment analyses were performed using GOWINDA (Kofler and Schlötterer 2012), which corrects for gene length-related biases and/or gene clustering. GO accession terms were downloaded from the GO Consortium website. We ran analyses in mode: gene (which assumes that all SNPs in a gene are completely linked) and performed 100,000 simulations for FDR (false discovery rate) estimation (significance at FDR ≤ 0.05). A minimum of three genes in the enriched category was required. For tissue-specific expression analysis, we used Illumina BodyMap 2.0 (Derrien, Johnson, et al. 2012) expression data for 16 tissues, and considered genes significantly highly expressed in a particular tissue when compared with the remaining 15 tissues using the DESeq package (Anders and Huber 2010), as done in Sankararaman et al. (2014). Details in supplementary information S4, Supplementary Material online.
SNP Annotations
Functional annotations for SNPs were obtained from ENSEMBL-based annotations on the 1000 G data and SNPs were categorized as intergenic, genic, exonic, regulatory, synonymous, and nonsynonymous. For each candidate window, we sum the number of SNPs in each category, and then across candidate windows. To compare with noncandidate windows, we performed 1,000 resamplings of the number of candidate windows from the set of all background (i.e., scanned) windows. Empirical P values were obtained by comparing proportions of SNPs in each category to those obtained in 1,000 resamplings (fig. 5 and supplementary table S5, Supplementary Material online). Because we considered the sum across windows, and counted each SNP only once, results should be insensitive to window length (as candidate windows were merged). Details in supplementary information S4, Supplementary Material online.
Fig. 5.
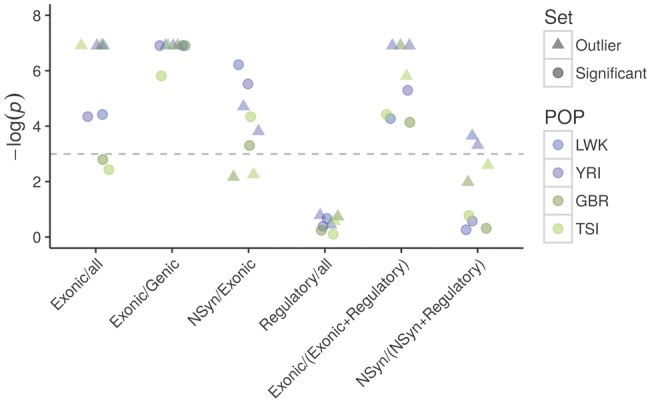
—Enrichment of classes of sites among candidate windows. Dashed lines mark the P = 0.975 (bottom) and P = 0.025 (top) thresholds for the one-tailed P values (hypothesis: enrichment). NSyn, nonsynonymous; all, Genic + Intergenic + Regulatory. The annotation is based on Ensembl variant predictor (supplementary information S4, Supplementary Material online). P< 0.001 was treated as 0.001 to avoid infinite values.
Splicing Variants, Mono-Allelic Expression, Immune-Related Genes
To test whether candidate genes have a larger number of transcripts than controls, while controlling for gene length, we: 1) divided candidate genes into quantiles of gene length; 2) sampled a set of genes (matching candidate genes in quantile bin; 3) calculated the mean and median number of transcripts for each set; 4) repeated this process 1,000 times and calculated the empirical one-tailed P value. The number of transcripts per gene was obtained from Ensembl Biomart. For this analysis, to reduce complexity, we used the set of candidate genes shared by populations within a continent (265 or 1594 for outlier and significant sets, respectively).
To test for enrichment for genes with mono-allelic expression (MAE), we quantified the proportions of candidate genes with MAE or biallelic expression in Savova et al. (2016) and compared these proportions to those in all genes analyzed (one-tailed Fisher’s test). The same procedure was adopted to test for enrichment of immune-related genes among our sets, using a list of 386 keywords from the Comprehensive List of Immune Related Genes (https://immport.niaid.nih.gov/immportWeb/queryref/immportgene/immportGeneList.do; last accessed July 2016).
Gene Cards was used to obtain basic functional information about genes. The GWAS catalog (Welter et al. 2014) was used to search for genome-wide phenotype associations included in the discussion (we only report “strong associations,” i.e., when there is at least one SNP with P < 10−8). All statistical analyses and figures were performed in R (Development Core Team R 2009) (scripts available on https://github.com/bbitarello/NCV_dir_package and NCD code available in https://github.com/bbitarello/NCD-Statistics).
Results
Power of NCD2 to Detect LTBS
We evaluated the sensitivity and specificity of NCD by benchmarking its performance using simulations under demographic scenarios inferred for African, European, and Asian human populations and under a neutral or an overdominant model (see Methods). We explored the influence of parameters that can affect the power of NCD: time since onset of LTBS (Tbs), frequency equilibrium defined by selection coefficients (feq), demographic history of the sampled population, tf used in NCD calculation and length of the genomic region analyzed (L). For simplicity, we averaged power estimates across African and European demographic models (Asian populations were not considered due to low power, see supplementary information S1, Supplementary Material online), L and Tbs; these averages reflect general changes in power driven by individual parameters. For simplicity, we initially discuss cases where tf = feq, and later relax this condition. We focus on NCD2 (table 1) power results and discuss some key points below. The complete set of power results, including NCD1, is presented in supplementary table S1, Supplementary Material online.
Table 1.
Power for Simulations under the African and European Demographic Models
| Tbs | feq | Africa |
Europe |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
NCD2 |
NCD1 |
NCD2 |
NCD1 |
||||||||||
|
tf |
tf |
tf |
tf |
||||||||||
| 0.5 | 0.4 | 0.3 | 0.5 | 0.4 | 0.3 | 0.5 | 0.4 | 0.3 | 0.5 | 0.4 | 0.3 | ||
| 5 | 0.5 | 0.96 | 0.94 | 0.84 | 0.93 | 0.91 | 0.39 | 0.97 | 0.95 | 0.84 | 0.92 | 0.85 | 0.20 |
| 5 | 0.4 | 0.94 | 0.94 | 0.89 | 0.89 | 0.89 | 0.67 | 0.95 | 0.94 | 0.91 | 0.85 | 0.83 | 0.60 |
| 5 | 0.3 | 0.90 | 0.91 | 0.93 | 0.72 | 0.80 | 0.84 | 0.84 | 0.86 | 0.89 | 0.47 | 0.57 | 0.74 |
| 3 | 0.5 | 0.91 | 0.88 | 0.68 | 0.86 | 0.80 | 0.24 | 0.93 | 0.89 | 0.68 | 0.81 | 0.69 | 0.15 |
| 3 | 0.4 | 0.88 | 0.86 | 0.76 | 0.78 | 0.78 | 0.56 | 0.89 | 0.88 | 0.79 | 0.74 | 0.71 | 0.46 |
| 3 | 0.3 | 0.75 | 0.77 | 0.81 | 0.56 | 0.64 | 0.71 | 0.73 | 0.76 | 0.80 | 0.39 | 0.48 | 0.63 |
Note.—Tbs, time in Ma since onset of balancing selection; feq, equilibrium frequency in the simulations. Power at false positive rate (FPR) = 5%. Simulations with L = 3 kb. Power on additional conditions is presented on supplementary table S1, Supplementary Material online.
Time since the Onset of Balancing Selection and Sequence Length
Signatures of LTBS are expected to be stronger for longer Tbs, because time to the most recent common ancestor is older and there will have been more time for linked mutations to accumulate and reach intermediate frequencies. Accordingly, power to detect LTBS is high with Tbs = 3 (0.91) and 5 (0.96) and low for 1 Ma (0.42, averaged across populations, L and feq) (supplementary figs. S3–S8 and table S1, Supplementary Material online). Further, in the absence of epistasis the long-term effects of recombination result in narrow signatures with long Tbs. Indeed, our simulations show that narrower windows yield highest power (on average, 13.6% higher for 3 than for 12 kb, when using NCD2(0.5), Tbs = 5 Ma; supplementary figs. S3–S8 and table S1, Supplementary Material online). Thus, we focus the remaining power discussion on old balancing selection (3 or 5 Ma) in regions of 3 kb.
Demography and Sample Size
Power is similar for samples simulated under African and European demographic histories for NCD2 (table 1) but considerably lower under the Asian one, possibly due to demography (supplementary table S1 and figs. S3–S8, Supplementary Material online). Although power estimates may be influenced by the particular demographic model used, we nevertheless focus on African and European populations, which by showing similar power allow fair comparisons between them. Although the power values discussed here are for samples of 100 chromosomes, NCD2 performs well even for sample sizes as small as 20 (supplementary table S1 and supplementary information S1, Supplementary Material online).
Simulated and Target Frequencies
So far, we have only discussed cases where tf =feq, which should favor the performance of NCD. Accordingly, under this condition NCD has very high power: 0.96, 0.94, and 0.89 for feq = 0.5, 0.4, and 0.3, respectively (averaged across Tbs and populations, 3 kb, table 1). However, in practice there is no prior knowledge about the feq of balanced polymorphisms, so we also evaluate the power of NCD when tf differs from the feq. When feq= 0.5, average power is high for tf = 0.5 and 0.4 (≥ 0.94), but lower for tf = 0.3 (0.83, table 1). Similar patterns are observed for other simulated feq (table 1). Therefore, NCD2 is overall well-powered both when feq is the same as tf and also when feq ≠ tf. In any case, the closer tf is to feq, the higher the power, so when possible it is desirable to perform tests across a range of tf.
NCD2 in Comparison to Other Methods
We compared NCD2 to two statistics commonly used to detect balancing selection (TajD and HKA), and two recently proposed high-powered statistics: the composite likelihood-based measure T2 (DeGiorgio et al. 2014) and ß (Siewert and Voight 2017). The T2 statistic, similarly to NCD2, considers both the SFS and FDs, whereas ß only considers the SFS. When feq = 0.5, NCD2(0.5) has the highest power: for example, for the African demographic scenario (using Tbs = 5 Ma, and 3-kb windows) NCD2(0.5) has a power of 0.96 (the highest among other tests is 0.94, for T2) but the difference in power is highest when feq departs from 0.5. For feq = 0.4, NCD2(0.4) power is 0.93 (compared with 0.90 for TajD and T2, and lower for the other tests). For feq = 0.3, NCD2(0.3) power is 0.93 (compared with 0.90 for ß, 0.89 for T2 and lower for the other tests). These patterns are consistent in the African and European simulations (fig. 2 and supplementary fig. S10, Supplementary Material online), where NCD2 has greater or comparable power than other available methods.
NCD2 is simple to implement and fast to run, and performs slightly or substantially better than all other methods tested (fig. 2 and supplementary fig. S10, Supplementary Material online). Altogether, the advantage of NCD2 over classic neutrality tests and ß is its higher power, especially when feq departs from 0.5; the main advantage over T2 is simplicity and speed of implementation. We note that NCD2 can be computed for particular loci, even in the absence of genome-wide data (which, if present, allows an empirical approach to define significance) or a demographic model (which, if present, allows simulations to define significance). In addition, NCD2 outperforms NCD1 (see supplementary information S1, Supplementary Material online), illustrating the gain in power by incorporating FDs in the NCD statistic, which is also more powerful than combining NCD1 and HKA (supplementary table S1 and supplementary information S1, Supplementary Material online). Because NCD2 outperforms NCD1, we used it for our scan of human populations. However, NCD1 also outperforms several of the existing methods (supplementary table S1, Supplementary Material online) and is a good choice when outgroup data are lacking.
Identifying Signatures of LTBS in Human Genomes
The ideal window size will vary depending on species demographic properties and sampling. Informed by our power analyses, we computed the highly powered NCD2 in sliding windows of 3 kb. These were defined as sliding windows in a genome-wide framework to ensure overlap with the narrow signatures of balancing selection. We note though that alternative approaches to define the windows, such as windows centered on each IS, are also possible. After filtering (see Methods), we analyzed ∼81% of the autosomal genome (supplementary fig. S13, Supplementary Material online), overlapping 18,633 protein-coding genes. Between 6,226 and 6,854 (0.37–0.41%) of the scanned windows in each population have a lower NCD2(0.5) value than any of the 10,000 neutral simulations (P < 0.0001), placing them in the significant category (table 2). The proportions are similar for NCD2(0.4) and NCD2(0.3) (table 2). In each population, the union of significant windows considering all tf values spans, on average, 0.6% of the windows (table 2) and 0.77% of the base pairs.
Table 2.
Candidate Windows and Protein-Coding Genes across Populations
| Population |
LWK |
YRI |
GBR |
TSI |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tf | 0.3 | 0.4 | 0.5 | U | 0.3 | 0.4 | 0.5 | U | 0.3 | 0.4 | 0.5 | U | 0.3 | 0.4 | 0.5 | U |
| Significant windows | 7,364 | 6,841 | 6,226 | 10,072 | 8,098 | 7,420 | 6,854 | 10,997 | 6,173 | 6,858 | 6,519 | 9,521 | 5,998 | 6,661 | 6,403 | 9,282 |
| Outlier windows | 829 | 829 | 829 | 1,248 | 829 | 829 | 829 | 1,230 | 829 | 829 | 829 | 1,251 | 829 | 829 | 829 | 1,270 |
| Significant genes | 1,182 | 1,099 | 1,026 | 1,457 | 1,326 | 1,187 | 1,110 | 1,616 | 1,026 | 1,094 | 1,051 | 1,414 | 1,039 | 1,091 | 1,056 | 1,414 |
| Outlier genes | 151 | 158 | 167 | 249 | 142 | 151 | 157 | 232 | 140 | 157 | 159 | 222 | 143 | 157 | 166 | 231 |
Note.—Significant and outlier genes and windows, see main text. Total number of queried windows per population is 1,657,989. Union of all candidate genes is 2,348 (significant) and 402 (outlier). U, union of windows considering three tf values.
Next, we defined as outlier windows for each tf those with the most extreme signatures of LTBS (in the 0.05% lower tail of the respective Ztf-ISdistribution, see Methods). This more conservative set contains 829 outlier windows for each population and tf value (table 2), which cover only ∼ 0.09% of the base pairs analyzed and are largely included in the set of significant windows. Significant and outlier windows are collectively referred to as candidate windows.
Reliability of Candidate Windows
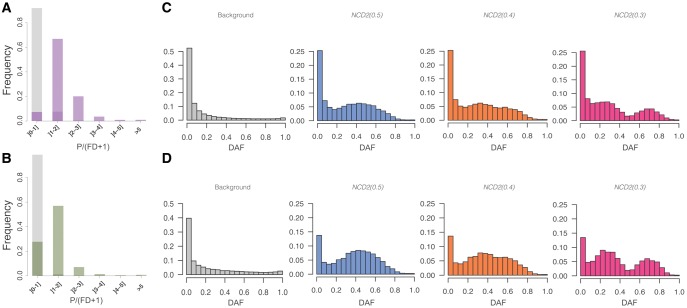
Significant windows are enriched both for polymorphic sites (fig. 3A and B) and intermediate-frequency alleles, and the SFS shape reflects the tf for which they are significant (fig. 3C and D). Although expected, because these were the patterns used to identify these windows, this shows that significant windows are unusual in both signatures. In addition, as expected with LTBS, the significant windows are largely shared across populations (see below). The striking differences of significant windows with respect to the background distribution, combined with the fact that neutral simulations do not have NCD2 values as low as those of the significant windows, precludes relaxation of selective constraint as a an alternative explanation to their signatures (Andrés et al. 2009). Relaxation of purifying selection can shift slightly the SFS (mostly for the functional sites, e.g., nonsynonymous), but it cannot explain our significant windows because their SFS is strikingly different from the genome-wide, background distribution (which represents mostly neutral regions) and from simulations run under neutrality.
Fig. 3.
—Polymorphism-to-divergence and SFS. (A and B) SNPs/(FDs + 1) for LWK (A) and GBR (B) populations. SNPs/(FDs + 1) measures the proportion of polymorphic-to-divergent sites for the union of significant windows for all tf (purple, green) compared with all scanned windows (gray). (C and D) SFS in LWK (C) and GBR (D) of all scanned windows in chr1 (gray), significant windows for NCD2(0.5) (blue), NCD2(0.4) (orange), NCD2(0.3) (pink). DAF, derived allele frequency.
Windows with low NCD2 values have similar coverage to the rest of the genome, that is, they are not enriched in unannotated, polymorphic duplications. In fact, candidate windows are not only not enriched in high-coverage regions: they show a depletion of them (supplementary fig. S14, Supplementary Material online) (Mann–Whitney U two-tail test; P < 0.02 for tf = 0.5 and tf = 0.4 for GBR and TSI). We also examined whether these signatures could be driven by two biological mechanisms other than LTBS: archaic introgression into modern humans and ectopic gene conversion (among paralogs). These mechanisms can increase the number of polymorphic sites and (in some cases) shift the SFS toward intermediate frequency alleles. We find introgression is an unlikely confounding mechanism, since candidate windows are depleted in SNPs introgressed from Neandertals (supplementary fig. S16, supplementary table S4 and supplementary information S3, Supplementary Material online). In addition, genes overlapped by significant windows are not predicted to be affected by ectopic gene conversion with neighboring paralogs to an unusually high degree, with the exception of olfactory receptor genes (supplementary fig. S15 and supplementary information S3, Supplementary Material online).
The candidate windows are enriched in heterozygous positions in the genomes of archaic humans (Neandertal and Denisova, all P values < 0.001). This shows that, as expected, the polymorphisms in identified candidate windows tend to be older than those in neutral regions of the genome, predating the split between humans and archaic hominins (supplementary information S6 and supplementary fig. S22, Supplementary Material online). Thus, candidate windows represent a catalog of strong candidate targets of LTBS in human populations.
Assigned tf Values
Many windows were significant for more than one tf. For these cases, we used the Ztf-IS statistic (Equation 2) to identify which tf provides the strongest support for LTBS (i.e., for which tf the departure from neutral expectations was greatest). In this way, we could assign a tf to each significant window. On average, ∼53% of the candidate windows are assigned to tf = 0.3, 27% to tf = 0.4 and 20% to tf = 0.5 (supplementary table S3 and supplementary information S2, Supplementary Material online).
Nonrandom Distribution across Chromosomes
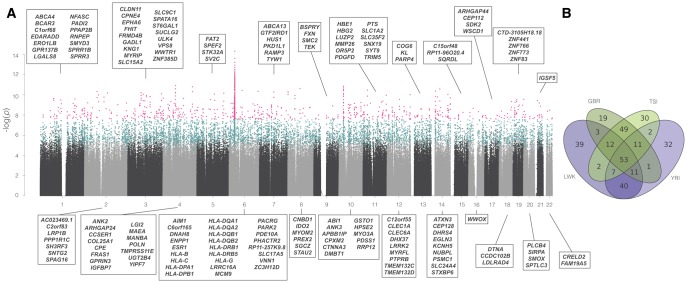
Candidate windows are not randomly distributed across the genome. Chromosome 6 is the most enriched for signatures of LTBS, contributing, for example, 10.2% of significant and 25% of outlier windows genome-wide for LWK while having only 6.4% of analyzed windows (supplementary fig. S12, Supplementary Material online). This is explained by the MHC region (fig. 4A), rich in genes with well-supported evidence for LTBS. Specifically, ten HLA genes are among the strongest candidates for balancing selection in all four populations, most of which have prior evidence of balancing selection (supplementary table S4 and supplementary information S4 and S5, Supplementary Material online).
Fig. 4.
—Manhattan plot and population sharing. (A) Manhattan plot of all scanned windows, for one analysis (NCD2(0.5) for LWK). y-axis, P value (log-scale) based on Ztf-IS. x-axis, ordered location of analyzed windows on the genome. Each point is a scanned (gray and black), significant (blue), or outlier (pink) window. Names of outlier protein-coding genes are provided, sorted by name. Significant windows were defined based of simulations, not on Ztf-IS. (Ztf-IS is used to rank even those with P < 0.0001) (B) Venn diagram showing the overlap in signatures of the 167 outlier genes annotated in (A) with other populations.
Biological Pathways Influenced by LTBS
To gain insight on the biological pathways influenced by LTBS, we focused on protein-coding genes containing at least one candidate window (222–249 outlier and 1,404–1,616 significant genes per population), and investigated their annotations (all results are presented in supplementary table S2, Supplementary Material online). Regarding functional categories, significant genes are overrepresented in 28 GO categories, 24 of which are shared by at least two populations and 18 by four populations (supplementary table S2, Supplementary Material online). Thirteen categories are immune-related according to a list of 386 immune-related keywords from ImmPort (Methods). The more stringent sets of outlier genes are enriched for 28 GO categories (21 shared by all four populations), 18 of which are immune-related. Furthermore, in both sets several of the remaining enriched categories are directly related to antigen presentation although not classified as immune-related (e.g., “ER to golgi transport vesicle membrane,” “integral to membrane”). Among the non immune-related categories are “sarcolemma,” “epidermis development,” “keratin filament,” and “negative regulation of blood coagulation.”
When classical HLA genes are removed from the analyses (supplementary information S4, Supplementary Material online), only two categories remain enriched: “sarcolemma” (in YRI) and “epidermis development” (GBR), but the small set of genes per population hampers power. For the significant windows, “antigen processing and presentation of endogenous peptide antigen via MHC class I” remains significantly enriched (driven by TAP1, TAP2, ERAP1, and ERAP2; supplementary table S2, Supplementary Material online). Significant windows remain enriched in categories related to the extracellular space—“extracellular regions,” “integral to membrane”—as in other studies (Andrés et al. 2009; DeGiorgio et al. 2014; Key, Teixeira, et al. 2014) and “keratin filament.” These categories are not immune-related per se, but they represent physical barriers to the invasion by pathogens. This indicates that LTBS maintains advantageous diversity in numerous defense-related genes other than classical HLA genes.
Overall, 33% of the outlier (and 31% of the significant) genes have at least one immune-related associated GO category, compared with only 24% of scanned genes (see Methods). These results collectively suggest that immunity and defense are frequent targets of LTBS, although a large fraction of the candidates for LTBS have non immune related functions or indirect connections with immunity hitherto unknown.
Functional Annotation of SNPs in Candidate Windows
We next tested whether LTBS preferentially targets SNPs at particular types of functional sites. To do so, we investigated the overlap of candidate windows with different classes of functional annotations in the human genome (Methods and supplementary information S4, Supplementary Material online), and tested the hypothesis of enrichment of certain classes of sites within our sets of candidate windows, when compared with sets of randomly sampled windows from the genome.
SNPs in outlier windows are disproportionally represented in protein-coding exons in all the populations (P ≤ 0.001, one-tail test; fig. 5 and supplementary table S5, Supplementary Material online). The protein-coding enrichment is even stronger when considering only SNPs within genes, which both in outlier (P < 0.001) and significant windows (P ≤ 0.003) are strongly enriched in protein-coding exons (fig. 5). Within the protein-coding exons, outlier windows in Africa (P ≤ 0.022) and significant windows in all populations (P ≤ 0.037) are enriched for nonsynonymous SNPs (fig. 5). These observations show that our candidate targets of LTBS tend to be enriched in exonic and potentially functional (amino-acid altering) SNPs.
Conversely, outlier and significant windows have no excess of SNPs annotated as regulatory (P ≥ 0.458 in all populations, fig. 5). When we explicitly compared protein-coding exons versus regulatory sites by restricting our analysis to sites in these two categories, outlier windows have an excess of exonic SNPs (P ≤ 0.003). The same is true for significant windows (P ≤ 0.016; fig. 5). When only nonsynonymous and regulatory sites are considered, we see enrichment for LWK and YRI for the outlier windows (P ≤ 0.036, fig. 5) but not for the significant windows (P ≥ 0.458 for all populations, fig. 5), although the two analyses that consider nonsynonymous SNPs are likely underpowered due to low SNP counts (supplementary table S5, Supplementary Material online).
Finally, results using more detailed RegulomeDB annotations generally agree with the observation of lack of enrichment of regulatory sites in our candidate windows (P ≥ 0.121 for a one-tail test for enrichment for RegulomeDB1 + 3 for SNPs with MAF ≥ 0.2) (supplementary table S5, Supplementary Material online). Although perhaps limited by the quality of the annotation of regulatory sites and the low power associated to small SNP counts for nonsynonymous variants, we do not have strong evidence that LTBS in human populations has preferentially shaped variation at sites with a role in gene expression regulation.
Overlap across Populations
On average, 86% of outlier windows in a given population are shared with another population (79% for significant windows), and 77% with another population within the same continent (66% for significant ones) (supplementary fig. S18, Supplementary Material online). The sharing is similar when tf are considered separately (supplementary figs. S19 and S20, Supplementary Material online). Therefore, there is also considerable overlap of candidate protein-coding genes across populations: for example, in LWK (tf = 0.5), 76.6% of outlier genes are shared with at least another population, and 66% are shared with YRI (89% and 77% for significant genes; fig. 4B). In fact, on average, 44% of outlier genes for a given population are shared across all populations and 78.7% are shared by a same-continent population (50% and 77% for significant genes; supplementary fig. S21, Supplementary Material online).
Candidate Genes in More than One Population
Instances where signatures of LTBS are not shared between populations may result from changes in selective pressure, which may be important during fast, local adaptation (de Filippo et al. 2016). On the other hand, loci with signatures of LTBS across human populations are more likely to represent stable selection. We considered as “shared” those candidate protein-coding genes (from the union of candidate windows for all tf) that are shared by all populations (supplementary table S4, Supplementary Material online). We considered as “African” those shared between YRI and LWK (but neither or only one European population), and “European” those shared between GBR and TSI (but neither or only one African population). We note that these designations do not imply that genes referred to as “African” or “European” are putative targets of LTBS for only one continent (partially because there are some power differences between Africa and Europe, table 1). The 79 African, 84 European, and 102 shared outlier genes add up to 265 genes in total (∼1.4% of all queried genes) and the 458 African, 400 European, and 736 shared significant genes add up to 1,594 (∼8.5% of all queried genes; supplementary table S4, Supplementary Material online). Several of them have been detected in other studies, but the vast majority are novel candidates (supplementary information S4 and S5, Supplementary Material online).
Expression of Candidate Genes
Candidate genes are disproportionally expressed in a number of tissues: lung, adipose tissue, adrenal tissue, kidney, and prostate (FDR ≤ 0.05, supplementary table S2, Supplementary Material online). In addition, candidate genes have an unusually large number of different transcripts: outlier genes shared by at least two populations have on average, 8.69 transcripts (compared with 7 in the controls), with a similar pattern holding for significant genes (8.73 compared with 7.1 in controls). These are both significantly higher than controls even after controlling for gene length (P < 0.001 in both cases for a one-tail test).
Genes with MAE—that is, the random and mitotically stable choice of an active allele for a given locus—were enriched among the small set of genes previously reported to be under LTBS (Savova et al. 2016). Our observations are in agreement with these findings, with 64% and 62% of the outlier and significant genes shared by at least two populations having MAE status (Savova et al. 2016), compared with only 41% for genes without signatures of LTBS (P < 1.12−6 Fisher’s exact test, one-sided).
Discussion
Limitations of NCD Statistics
LTBS produces signatures that are unexpected under neutrality. Still, all tests for balancing selection are affected by demographic history, in particular strong population substructure, which can result in an excess of polymorphism that, in certain cases (e.g., with similar contribution of the two subpopulations, or with positive selection) may be at intermediate frequencies. We benefit from the extensive prior work on the 1000 G populations showing absence of substructure and providing a parameter-rich demographic model, which can be used for hypothesis testing. Some types of migration and introgression can also result in genome-wide or local excess of diversity and, under positive selection, also intermediate frequencies. We show that Neandertal introgression has a minimal effect on our inferences of LTBS. However, as with any neutrality test, applying NCD to other species requires consideration of the demographic history of the population to weight the possibility of alternative explanations. If the demographic history is unknown, a purely empirical approach allows the identification of putative targets of balancing selection. Nevertheless, substructure, migration, and introgression should to be investigated as alternative explanations, using additional methods (e.g., LD-based).
Certain selective regimes can also generate signatures that are similar to those of LTBS. For example, incomplete or soft sweeps can produce an excess of intermediate frequency variants (Hermisson and Pennings 2017) that could be mistaken as evidence for balancing selection when only polymorphisms are used. Still, because none of these types of selection increase the time to the most recent common ancestor and density of polymorphisms, they do not confound NCD2. Recent balancing selection, on the other hand, is difficult to distinguish from those two selective scenarios, so we focused our analyses and interpretations on LTBS. Of note, by definition NCD requires predefining a tf, which in practice is unknown, but this can be easily addressed by running the test with different tf (whose results correlate strongly) and combining results. So, overall, and despite these limitations, NCD is a powerful, simple, and fast method to identify the signatures of LTBS in polymorphism data, which we expect will be used in model and nonmodel organisms.
The Targets of LTSB in the Human Genome
Using simulation-based and outlier approaches, we uncovered windows with signatures of LTBS in humans. We showed that these windows are unlikely to be affected by technical artifacts or biological processes other than LTBS, such as introgression from archaic hominins. On average, across populations, 0.6% of the windows in a population meet our criterion of significance based on neutral simulations. These windows contain on average 0.77% of the base-pairs and 1.6% of the SNPs in the analyzed genome per population, and although they amount to a low proportion of the genome, on average, 7.9% of the protein-coding genes contain at least one significant window (considering UTRs, introns, and protein coding exons). For the outlier windows (those in the 0.05% lower tail of the NCD2 genomic distribution), on average, 1.2% of genes show some evidence of selection. These proportions are similar when requiring that a significant or outlier window be shared by at least two same-continent populations (8.5% and 1.4%, respectively). We note that although these sets probably include some false positives, our method is only sensitive to very old balancing selection, so, in practice, we are likely underestimating the total influence of balancing selection in human genomes. For instance, a more widespread influence of younger instances of balancing selection, which cannot be detected by our methods, could in principle be detected by methods able to distinguish the signatures of recent balancing selection from those of incomplete sweeps or selection on standing variation. Alternatively, recent balancing selection targeting particular pathways or gene networks could in principle be detected by gene set enrichment analyses as described in Daub et al. (2013) in the context of polygenic adaptation.
We identified a number of previously known targets of LTBS, but also many new ones: almost 70% of the outlier genes shared at least by another same-continent population (and 90% of the significant ones) are novel. Many of the candidate genes show strongest evidence for LTBS at tf values different from 0.5. This is expected, for instance, under asymmetric overdominance, and highlights the importance of considering selective regimes with different frequencies of the balanced polymorphism.
Functional Properties of SNPs in Candidate Windows
In this study, we confirm cases where protein-coding regions are the likely targets of selection, such as HLA-B and HLA-C (Hughes and Nei 1988), as well as cases where regulatory regions are probably targeted, such as UGT2B4 and TRIM5 (Cagliani, Fumagalli, et al. 2010; Sun et al. 2011). Although LTBS has been proposed to play an important role in maintaining genetic diversity that affects gene expression (Leffler et al. 2013; Savova et al. 2016), we find that regulatory SNPs are underrepresented within the candidate regions. This does not imply that there are no regulatory SNPs under LTBS, but rather that with existing annotations (which are less precise for regulatory than protein-coding sites) they are not enriched within candidate windows. Overall, we found an enrichment of exonic and of nonsynonymous SNPs in the candidate windows, suggesting an overrepresentation of potentially functional SNPs within selected regions. This is compatible with two scenarios: 1) direct selection on protein-coding sites or 2) accumulation of functional (including slightly deleterious) variants as a biproduct of LTBS.
Overlap with Previous Studies
Whereas the various scans for positive selection show a remarkably low overlap with respect to the genes they identify, 34% of our outlier genes (11% of significant ones) had evidence of LTBS in at least one previous study (Andrés et al. 2009; Leffler et al. 2013; DeGiorgio et al. 2014). Remarkably, 47% of the shared outliers across all four populations (17% of the shared significant ones) have been detected in at least one previous study, and the proportions are similar even when classical HLA genes are removed (39% and 16% overlap, respectively). This is a high degree of overlap, considering the differences in methods and data sets across studies.
Properties of Candidate Genes
Below, we discuss some of the candidate genes (supplementary table S4, Supplementary Material online), highlighting the variety of biological functions and known genetic associations potentially shaped by LTBS in humans.
Gene Expression
Candidate genes have on average a larger number of transcripts than other genes. As examples, CLDN11, ROBO2, ESR1, or PRKCE (discussed below) have 10–15 different transcripts, a higher number than at least 75% of scanned genes. It is possible that these genes benefit from high levels of diversity, contributed both by genetic diversity and transcript diversity. In addition, in agreement with previous findings, we find a significant excess of MAE genes among targets of LTBS. This excess is not driven by HLA genes and supports the claim for a biological link between MAE and balancing selection (Savova et al. 2016). Heterozygosity in a MAE gene could lead to cell-to-cell heterogeneity within same-cell clusters, which could be potentially advantageous (Savova et al. 2016), particularly in the case of cell-surface proteins. Some of these MAE genes found in our study, and not previously detected in scans for LTBS, are involved in immunity/defense barriers (e.g., IL1RL1-IL18R1, FAM114A1, EDARADD, SIRPA, TAS2R14), oxygen transport and hypoxia (e.g., PRKCE, HBE1, HBG2, EGLN3), or reproduction (e.g., CLDN11).
Oxygen Transport and Response to Hypoxia
Among the outlier genes with MAE, we find EGLN3, which is significantly upregulated under hypoxia (Escribese et al. 2012) and plays a role in skeletal muscle differentiation (Fu et al. 2007). The encoded protein hydroxylates the product of EPAS1, a gene shown to harbor variants responsible for human adaptation to high altitude in Tibet (Yi et al. 2013). We also find members of the ß-globin cluster (HBE1-HBG2) that are involved in oxygen transport and have strong associations to hemoglobin levels and ß-thalassemia (Danjou et al. 2015), and for which there is also evidence for recent positive selection in Andean (HBE1, HBG2) or Tibetan (HBG2) populations (Bigham et al. 2010; Rottgardt et al. 2010; Yi et al. 2010). It is plausible that these genes have been under LTBS and undergone a shift in selective pressures in high-altitude populations, but further analyses are required to confirm this possibility. Another outlier gene, PRKCE, is also associated to hemoglobin levels and red blood cell traits (Astle et al. 2016).
Immunological Function and Defense Barriers
It has long been argued that genes of immune function are prime candidates for LTBS. As expected, we detect several classical HLA with known signatures of LTBS. However, many non-HLA candidates from our set of outlier genes have immunological functions. We confirm signatures of LTBS in the ABO locus (supplementary information S5, Supplementary Material online), a well-known case of LTBS in humans (Ségurel et al. 2012), and TRIM5, a gene with antiviral function (Cagliani, Fumagalli, et al. 2010). Among novel candidates, we find several genes involved in autoimmune disease. For example, IL1RL1-IL18R1 have strong associations atopic dermatitis, an autoimmune disease (Hirota et al. 2012). HLA-DQB2 mediates superantigen activation of T cells and is associated both to infectious (hepatitis B) and autoimmune diseases (Lee et al. 2012; Jiang et al. 2015). Two other significant genes for which there is prior evidence for LTBS, ERAP1 and ERAP2 (Andrés et al. 2010; Cagliani, Riva, et al. 2010), are associated with ankylosing spondylitis and psoriasis (Strange et al. 2010; Evans et al. 2011; Robinson et al. 2015). Finally, there are several associations to autoimmune disease and susceptibility to infections in the classical HLA genes that we identify. In brief, our results are consistent with the hypothesis that autoimmune disease is linked to natural selection favoring effective immune response against pathogens (Corona et al. 2010; Sironi and Clerici 2010).
Another important aspect of defense is the avoidance of poisonous substances. As suggested previously by studies on polymorphism in PTC receptors (Wooding et al. 2004), avoidance of bitterness might have been adaptive throughout human evolutionary history because several potentially harmful substances are bitter. The TAS2R14 gene encodes for a bitter taste receptor, and in humans it has strong associations to taste perception of quinine and caffeine (Ledda et al. 2014), is considered a promiscuous receptor (Meyerhof et al. 2010; Karaman et al. 2016), and is one of the few bitter taste receptors that binds a vast array of compounds, and for which no common structure has been found (Behrens et al. 2004; Meyerhof et al. 2010). This entails diversity in the antigen binding portions of the receptors, which may be enhanced by balancing selection. Indeed, an elevated dN/dS ratio was reported for a cluster of bitter taste receptors which includes TAS2R14 (Kosiol et al. 2008). To our knowledge, our study is the first in detecting signatures of LTBS in this gene.
Cognition
Several candidate genes are involved in cognitive abilities, or their variation is associated with diversity in related phenotypes. KL (life extension factor klotho) is a gene that has been associated to human longevity (Arking et al. 2002) and for which signatures of LTBS have been previously reported (DeGiorgio et al. 2014). In mice, decreased levels of klotho shorten lifespan (Welberg 2014). In humans, heterozygotes for the KL-VS variant show higher levels of serum klotho and enhanced cognition, independent of sex and age, than wild-type homozygotes. On the other hand, KL-VS homozygotes show decreased lifespan and reduced cognition (Dubal et al. 2014). If higher cognition is advantageous, overdominance for this phenotype can explain the signatures of balancing selection we observe, although the effect in lifespan itself could also influence the selective regime.
PDGFD encodes a growth factor that plays an essential role in wound healing and angiogenesis. PDGFD-induced signaling is crucial for human (but not mouse) proliferation of the neocortex due to neural stem-cell proliferation (Lui et al. 2014), a trait that underlies human cognition capacities. This gene has strong associations to coronary artery disease and myocardial infarction, which are related to aging.
In addition, among our outliers is a gene with a cognitive-related genetic association, ROBO2, which encodes a transmembrane receptor involved in axon guidance. Associations with vocabulary growth have been reported for variants in its vicinity (St Pourcain et al. 2014). ROBO2 has signatures of ancient selective sweeps in modern humans after the split with Neandertals and Denisova (Peyrégne et al. 2017) on a portion of the gene (chr3: 77027850–77034264) almost 40 kb apart from the one for which we identified a signature of LTBS (chr3: 76985072–76988072). The occurrence of both these signatures highlights the complex evolutionary history of this gene.
Associations of candidate genes with cognition are also exemplified by case–control and cohort studies linking polymorphisms in the estrogen receptor alpha (ER-α) gene, ESR1, to dementia and cognitive decline. Links between ER-α variants and anxiety and depression in women have been proposed but lack confirmation (reviewed in Sundermann et al. 2010). Interestingly, three other candidate genes (PDLIM1, GRIP1, SMYD3) interact with ER-α at the protein level (Szklarczyk et al. 2015), and two (PDLIM1, GRIP1) have strong association with suicide risk (Perlis et al. 2010; Mullins et al. 2014).
In genes like KL, where heterozygotes show higher cognitive abilities than homozygotes, cognition may be a driving selective force. This is a possible scenario in other genes, too. Still, given the complexity of brain development and function, it is also possible that cognitive effects of this variation are a byproduct of diversity maintained for other phenotypes. For example, MHC proteins and other immune effectors are believed to affect connectivity and function of the brain (reviewed in Shatz 2009; Needleman and McAllister 2012), with certain alleles being clearly associated with autism disorder.
Reproduction
Among candidate genes, there is an enrichment for preferential expression in the prostate. There are also a number of outlier genes involved in the formation of the sperm. For example, CLDN11 encodes a protein expressed in several tissues and crucial for spermatogenesis. Knockout mice for the murine homologue show neurological and reproductive impairment (Gow et al. 1999; Wu et al. 2012) and some variants in humans are strongly associated to prostate cancer.
ESR1, mentioned earlier, encodes an estrogen-activated transcription factor and leads to abnormal secondary sexual characteristics in females when defective (Quaynor et al. 2013). ER-α interacts directly with the product of BRCA1 and has strong associations to breast cancer (Michailidou et al. 2013), breast size (Eriksson et al. 2012) and age of menarche. In males, it is involved in gonadal development and differentiation, and lack of estrogen and/or ER-α can lead to poor sperm viability (Lazari et al. 2009). Strikingly, this gene also has SNPs strongly associated to a diverse array of phenotypes, including height, bone mineral density (spine and hip), and sudden cardiac arrest (Rivadeneira et al. 2009; Aouizerat et al. 2011; Wood et al. 2014). Two other genes among our candidates are also part of the estrogen signaling pathway: PLCB4 and ADCY5 (which is strongly associated to birth weight). Estrogens are not only involved in reproductive functions (both in male and females) but also in several processes of neural (see above), muscular or immune nature, and the ER-α–estrogen complex can act directly on promoter regions of other genes. In this case, balancing selection could be explained by the high level of pleiotropy, including the function in male and female reproduction (if different alleles are beneficial in males than females).
Conclusions
We present two new summary statistics, NCD1 and NCD2, which are both simple and fast to implement on large data sets to identify genomic regions with signatures of LTBS. They have a high degree of sensitivity for different equilibrium frequencies of the balanced polymorphism and, unlike classical statistics such as Tajima’s D or the Mann–Whitney U (Andrés et al. 2009; Nielsen et al. 2009), allow an exploration of the most likely frequencies at which balancing selection maintains the polymorphisms. This property is shared with the ß and the likelihood-based T2 tests (DeGiorgio et al. 2014; Siewert and Voight 2017). We show that NCD is well-powered to detect LTBS within a complex demographic scenario, such as that of human populations. NCD can be applied to either single loci or the whole-genome, in species with or without detailed demographic information, and both in the presence and absence of an appropriate outgroup (NCD2 and NCD1, respectively).
More than 85% of our outlier windows are shared across populations, raising the possibility that long-term selective pressures have been maintained after human populations colonized new areas of the globe. Still, ∼15% of outlier windows show signatures exclusively in one sampled population, and 16 of these show opposing signatures (OAS1, C15orf48, OR11A1, GSTO1, DHRS4, PABPC1, MYOZ3, OR6K5, KRTAP10-7, GRTP1, MINOS1, GANC, FBXO15, TIMM21, ZNF780A, MAPT, supplementary table S4, Supplementary Material online) of selective regimes between human groups. They are of particular relevance to understand how recent human demography might impact loci evolving under LTBS for millions of years or subsequent local adaptations through selective pressure shifts (de Filippo et al. 2016).
Our analyses indicate that LTBS in humans may be shaping variation in <2% of variable genomic positions, but that these on average overlap with ∼8% of all protein-coding genes. Importantly, almost 70% of the candidate genes shared by at least same-continent populations cannot be ascribed to immune-related functions, suggesting that diverse biological functions, and the corresponding phenotypes, contain advantageous genetic diversity.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
We would like to dedicate this manuscript to Scott Williamson, in memoriam, for playing a fundamental role in the conception of NCD. We also thank Warren Kretszchmar for analyses on the properties of related statistics not included here, and Eric Green for his support of that work. We thank Michael DeGiorgio for assistance with BALLET, Felix Key with 1000 Genomes data sets, Michael Dannemann with the implementation of expression analyses, Stéphane Peyrégne for comments on the manuscript, David Reher, members of the Evolutionary Genetics Group (São Paulo), Alex Cagan and Svante Päabo for helpful comments. We also thank two reviewers for helping improve this manuscript. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (grant numbers 11/12500-2 and 12/19563-2 to B.D.B. and 12/18010-0 to D.M.) and the Max Planck Society (A.M.A.).
Literature Cited
- Abecasis GR. 2012. An integrated map of genetic variation from 1,092 human genomes. Nature 491(7422):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan C, et al. , 2009. Personalized copy number and segmental duplication maps using next-generation sequencing. Nat Genet. 41(10):1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso S, Lopez S, Izagirre N, de la Rua C.. 2008. Overdominance in the human genome and olfactory receptor activity. Mol Biol Evol. 25(5):997–1001. [DOI] [PubMed] [Google Scholar]
- Anders S, Huber W.. 2010. Differential expression analysis for sequence count data. Genome Biol. 11(10):R106.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés AM. 2011. Balancing selection in the human genome. In Encyclopedia of Life Sciences Wiley. eLS 1–8. [Google Scholar]
- Andrés AM, et al. , 2009. Targets of balancing selection in the human genome. Mol Biol Evol. 26(12):2755–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés AM, et al. , 2010. Balancing selection maintains a form of ERAP2 that undergoes nonsense-mediated decay and affects antigen presentation. PLoS Genet. 6(10):e1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouizerat BE, et al. , 2011. GWAS for discovery and replication of genetic loci associated with sudden cardiac arrest in patients with coronary artery disease. BMC Cardiovasc Disord. 11:29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, et al. , 2002. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A. 99(2):856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthana S, Schmidt S, Sunyaev SR.. 2005. A limited role for balancing selection. Trends Genet. 21(1):30–32. [DOI] [PubMed] [Google Scholar]
- Astle WJ, et al. , 2016. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell 167(5):1415–1429.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M, et al. , 2004. The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. Biochem Biophys Res Commun. 319(2):479–485. [DOI] [PubMed] [Google Scholar]
- Bergland AO, Behrman EL, O’Brien KR, Schmidt PS, Petrov DA.. 2014. Genomic evidence of rapid and stable adaptive oscillations over seasonal time scales in Drosophila. PLoS Genet. 10(11):e1004775.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasin M, et al. , 2007. Apolipoprotein B mRNA—editing enzyme, catalytic polypeptide—like 3G: a possible role in the resistance to HIV of HIV-exposed seronegative individuals. J Infect Dis. 195(7):960–964. [DOI] [PubMed] [Google Scholar]
- Bigham A, et al. , 2010. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 6(9):e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb KL, et al. , 2006. Scan of human genome reveals no new loci under ancient balancing selection. Genetics 173(4):2165–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliani R, Fumagalli M et al. , 2010. Long-term balancing selection maintains trans-specific polymorphisms in the human TRIM5 gene. Hum Genet. 128(6):577–588. [DOI] [PubMed] [Google Scholar]
- Cagliani R, Riva S et al. , 2010. Genetic diversity at endoplasmic reticulum aminopeptidases is maintained by balancing selection and is associated with natural resistance to HIV-1 infection. Hum Mol Genet. 19(23):4705–4714. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D.. 2010. Elements of evolutionary genetics. 1st ed.Greenwood Village (Colorado): Roberts and Company Publishers. [Google Scholar]
- Charlesworth B, Nordborg M, Charlesworth D.. 1997. The effects of local selection, balanced polymorphism and background selection on equilibrium patterns of genetic diversity in subdivided population. Genet Res. 70(2):155–174. [DOI] [PubMed] [Google Scholar]
- Charlesworth D. 2006. Balancing selection and its effects on sequences in nearby genome regions. PLoS Genet. 2(4):e64–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, et al. , 2005. A genome-wide comparison of recent chimpanzee and human segmental duplications. Nature 437(7055):88–93. [DOI] [PubMed] [Google Scholar]
- Corona E, Dudley JT, Butte AJ.. 2010. Extreme evolutionary disparities seen in positive selection across seven complex diseases. PLoS One 5(8):e12236–e12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjou F, et al. , 2015. Genome-wide association analyses based on whole-genome sequencing in Sardinia provide insights into regulation of hemoglobin levels. Nat Genet. 47(11):1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day FR, et al. , 2015. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 6:8464.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub JT, et al. , 2013. Evidence for polygenic adaptation to pathogens in the human genome. Mol Biol Evol 30(7):1544–1558. [DOI] [PubMed] [Google Scholar]
- de Filippo C, et al. , 2016. Recent selection changes in human genes under long-term balancing selection. Mol Biol Evol. 33(6):1435–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio M, Lohmueller KE, Nielsen R.. 2014. A model-based approach for identifying signatures of ancient balancing selection in genetic data. PLoS Genet. 10(8):e1004561.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T, Estellé J et al. , 2012. Fast computation and applications of genome mappability. PLoS One 7(1):e30377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T, Johnson R et al. , 2012. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 22(9):1775–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Development Core Team R. 2009. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Dubal DB, et al. , 2014. Life extension factor klotho enhances cognition. Cell Rep. 7(4):1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson N, et al. , 2012. Genetic variants associated with breast size also influence breast cancer risk. BMC Med Genet. 13:53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribese M, et al. , 2012. The prolyl hydroxylase PHD3 identifies proinflammatory macrophages and its expression is regulated by activin A. J Immunol. 189(4):1946–1954. [DOI] [PubMed] [Google Scholar]
- Evans DM, et al. , 2011. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 43(8):761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing G, Hermisson J.. 2010. MSMS: a coalescent simulation program including recombination, demographic structure and selection at a single locus. Bioinformatics 26(16):2064–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Admetlla A, et al. , 2008. Balancing selection is the main force shaping the evolution of innate immunity genes. J Immunol. 181(2):1315–1322. [DOI] [PubMed] [Google Scholar]
- Fijarczyk A, Babik W.. 2015. Detecting balancing selection in genomes: limits and prospects. Mol Ecol. 24(14):3529–3545. [DOI] [PubMed] [Google Scholar]
- Fu J, Menzies K, Freeman RS, Taubman MB.. 2007. EGLN3 prolyl hydroxylase regulates skeletal muscle differentiation and myogenin protein stability. J Biol Chem. 282(17):12410–12418. [DOI] [PubMed] [Google Scholar]
- Gow A, et al. , 1999. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 99(6):649–659. [DOI] [PubMed] [Google Scholar]
- Gravel S, et al. , 2011. Demographic history and rare allele sharing among human populations. Proc Natl Acad Sci U S A. 108(29):11983–11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermisson J, Pennings PS.. 2017. Soft sweeps and beyond: understanding the patterns and probabilities of selection footprints under rapid adaptation. Methods Ecol Evol. 8(6):700–716. [Google Scholar]
- Hirota T, et al. , 2012. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat Genet. 44: 1222–1226. [DOI] [PubMed] [Google Scholar]
- Howell WM. 2014. HLA and disease: guilt by association. Int J Immunogenet. 41(1):1–12. [DOI] [PubMed] [Google Scholar]
- Hudson RR, Kaplan NL.. 1988. The coalescent process in models with selection and recombination. Genetics 120(3):831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson RR, Kreitman M, Aguade M.. 1987. A test of neutral molecular evolution based on nucleotide data. Genetics 116:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AL, Nei M.. 1988. Pattern of nucleotide substitution at major histocompatibility class I loci reveals overdominant selection. Lett Nat. 335(6186):167–170. [DOI] [PubMed] [Google Scholar]
- Jiang D-K, et al. , 2015. Genetic variants in five novel loci including CFB and CD40 predispose to chronic hepatitis B. Hepatology 62(1):118–128. [DOI] [PubMed] [Google Scholar]
- Johnston SE, et al. , 2013. Life history trade-offs at a single locus maintain sexually selected genetic variation. Nature 502(7469):93–95. [DOI] [PubMed] [Google Scholar]
- Karaman R, et al. , 2016. Probing the binding pocket of the broadly tuned human bitter taste receptor TAS2R14 by chemical modification of cognate agonists. Chem Biol Drug Des. 88(1):66–75. [DOI] [PubMed] [Google Scholar]
- Key FM, Peter B et al. , 2014. Selection on a variant associated with improved viral clearance drives local, adaptive pseudogenization of interferon lambda 4 (IFNL4). PLoS Genet. 10(10):e1004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key FM, Teixeira JC, de Filippo C, Andrés AM.. 2014. Advantageous diversity maintained by balancing selection in humans. Curr Opin Genet Dev. 29:45–51. [DOI] [PubMed] [Google Scholar]
- Kofler R, Schlötterer C.. 2012. Gowinda: unbiased analysis of gene set enrichment for genome-wide association studies. Bioinformatics 28(15):2084–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosiol C, et al. , 2008. Patterns of positive selection in six Mammalian genomes. PLoS Genet. 4(8):e1000144–e1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazari MFM, et al. , 2009. Estrogen receptors and function in the male reproductive system. Arq Bras Endocrinol Metabol. 53(8):923–933. [DOI] [PubMed] [Google Scholar]
- Ledda M, et al. , 2014. GWAS of human bitter taste perception identifies new loci and reveals additional complexity of bitter taste genetics. Hum Mol Genet. 23(1):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Bae S-C, Choi SJ, Ji JD, Song GG.. 2012. Genome-wide pathway analysis of genome-wide association studies on systemic lupus erythematosus and rheumatoid arthritis. Mol Biol Rep. 39(12):10627–10635. [DOI] [PubMed] [Google Scholar]
- Leffler EM, et al. , 2013. Multiple instances of ancient balancing selection shared between humans and chimpanzees. Science 339(6127):1578–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, et al. , 2014. Radial glia require PDGFD–PDGFRβ signalling in human but not mouse neocortex. Nature 515(7526):264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaria Genomic Epidemiology Network. 2015. A novel locus of resistance to severe malaria in a region of ancient balancing selection. Nature 526:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Thomson G.. 2001. How selection shapes variation of the human major histocompatibility complex: a review. Ann Hum Genet. 65(Pt 1):1–26. [DOI] [PubMed] [Google Scholar]
- Meyer M, et al. , 2012. A high-coverage genome sequence from an archaic denisovan individual. Science 338(6104):222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W, et al. , 2010. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 35(2):157–170. [DOI] [PubMed] [Google Scholar]
- Michailidou K, et al. , 2013. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 45(4):353–361, 361–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins N, et al. , 2014. Genetic relationships between suicide attempts, suicidal ideation and major psychiatric disorders: a genome-wide association and polygenic scoring study. Am J Med Genet B Neuropsychiatr Genet. 165B(5):428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman LA, McAllister AK.. 2012. The major histocompatibility complex and autism spectrum disorder. Dev Neurobiol. 72(10):1288–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R, et al. , 2005. A scan for positively selected genes in the genomes of humans and chimpanzees. PLoS Biol. 3(6):e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R, et al. , 2009. Darwinian and demographic forces affecting human protein coding genes. Genome Res. 19(5):838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, et al. , 2010. Genome-wide association study of suicide attempts in mood disorder patients. Am J Psychiatry 167(12):1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrégne S, Boyle MJ, Dannemann M, Prüfer K.. 2017. Detecting ancient positive selection in humans using extended lineage sorting. Genome Res. 27(9):1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüfer K, et al. , 2014. The complete genome sequence of a Neanderthal from the Altai mountains. Nature 505(7481):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prugnolle F, et al. , 2005. Pathogen-driven selection and worldwide HLA class I diversity. Curr Biol. 15(11):1022–1027. [DOI] [PubMed] [Google Scholar]
- Quaynor SD, et al. , 2013. Delayed puberty and estrogen resistance in a woman with estrogen receptor α variant. N Engl J Med. 369(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MD, Hubisz MJ, Gronau I, Siepel A.. 2014. Genome-wide inference of ancestral recombination graphs. PLoS Genet. 10(5):e1004342.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S, et al. , 2012. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. 44(3):291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivadeneira F, et al. , 2009. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 41(11):1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PC, et al. , 2015. ERAP2 is associated with ankylosing spondylitis in HLA-B27-positive and HLA-B27-negative patients. Ann Rheum Dis. 74(8):1627–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottgardt I, Rothhammer F, Dittmar M.. 2010. Native highland and lowland populations differ in γ-globin gene promoter polymorphisms related to altered fetal hemoglobin levels and delayed fetal to adult globin switch after birth. Anthropol Sci. 118(1):41–48. [Google Scholar]
- Sankararaman S, et al. , 2014. The genomic landscape of Neanderthal ancestry in present-day humans. Nature 507(7492):354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savova V, et al. , 2016. Genes with monoallelic expression contribute disproportionately to genetic diversity in humans. Nat Genet. 48(3):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségurel L, et al. , 2012. The ABO blood group is a trans-species polymorphism in primates. Proc Natl Acad Sci U S A. 109(45):18493–18498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellis D, Callahan BJ, Petrov D. a, Messer PW.. 2011. Heterozygote advantage as a natural consequence of adaptation in diploids. Proc Natl Acad Sci U S A. 108(51):20666–20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. 2009. MHC class I: an unexpected role in neuronal plasticity. Neuron 64(1):40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewert KM, Voight BF.. 2017. Detecting long-term balancing selection using allele frequency correlation. Mol Biol Evol. 34(11):2996–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi M, Clerici M.. 2010. The hygiene hypothesis: an evolutionary perspective. Microbes Infect. 12(6):421–427. [DOI] [PubMed] [Google Scholar]
- Spurgin LG, Richardson DS.. 2010. How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc Biol Sci. 277(1684):979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pourcain B, et al. , 2014. Common variation near ROBO2 is associated with expressive vocabulary in infancy. Nat Commun. 5:4831.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange A, et al. , 2010. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 42(11):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, et al. , 2011. A signature of balancing selection in the region upstream to the human UGT2B4 gene and implications for breast cancer risk. Hum Genet. 130(6):767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Maki PM, Bishop JR.. 2010. A review of estrogen receptor α gene (ESR1) polymorphisms, mood, and cognition. Menopause 17(4):874–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, et al. , 2015. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43(D1):D447–D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira JC, et al. , 2015. Long-term balancing selection in LAD1 maintains a missense trans-species polymorphism in humans, chimpanzees, and bonobos. Mol Biol Evol. 32(5):1186–1196. [DOI] [PubMed] [Google Scholar]
- Welberg L. 2014. Cognition: klotho spins cognitive fate. Nat Rev Neurosci. 15(7):425.. [DOI] [PubMed] [Google Scholar]
- Welter D, et al. , 2014. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 42(D1):D1001–D1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson SH, et al. , 2007. Localizing recent adaptive evolution in the human genome. PLoS Genet. 3(6):e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AR, et al. , 2014. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 46(11):1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding S, et al. , 2004. Natural selection and molecular evolution in PTC, a bitter-taste receptor gene. Am J Hum Genet. 74(4):637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, et al. , 2012. Transgene-mediated rescue of spermatogenesis in Cldn11-null mice1. Biol Reprod. 86(5):139, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, et al. , 2010. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, et al. , 2013. Sequencing of fifty human exomes reveals adaptation to high altitude. Science 329:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.