Abstract
Objective
To define the prevalence, time course, and associated factors of periodic limb movements during sleep (PLMS) in patients with ischemic stroke or TIA.
Methods
Patients enrolled in the prospective Sleep-Disordered Breathing in Transient Ischemia Attack (TIA)/Ischemic Stroke and Continuous Positive Airway Pressure (CPAP) Treatment Efficacy (SAS-CARE) study underwent a double polysomnographic investigation in the acute and chronic phases after stroke/TIA, together with a MRI brain scan and a 24-hour blood pressure evaluation. The prevalence of PLMS in patients was compared with that in a matched sample of randomly selected healthy controls from the HypnoLaus cohort. One hundred sixty-nine recordings were performed in the acute phase and 191 after 3 months (210 recordings were obtained from the same 105 patients in both phases) and were compared to those of 162 controls.
Results
The mean number of PLMS per hour and the percentage of participants with a PLMS index >10 and >15 per hour were similar between patients and controls. PLMS remained stable from the acute to the chronic phase after stroke. Factors positively associated with PLMS were age, body mass index, and history of hypertension. Blood pressure over 24 hours and the burden of cerebrovascular damage were similar between the groups with PLMS and without PLMS.
Conclusions
PLMS are equally frequent in patients with stroke/TIA and the general population. The absence of higher blood pressure values and of a greater vascular brain damage found in patients with PLMS compared to those without PLMS might be due to a greater use of antihypertensive medication among patients with PLMS, which corresponds to a higher prevalence of previous diagnosis of hypertension in these patients.
Periodic limb movements during sleep (PLMS) are repetitive leg jerks occurring in series with an interval between 5 and 90 seconds.1 Besides restless legs syndrome (RLS), PLMS also occur in other disorders and in healthy controls, with 28% of adults showing a PLMS index (PLMS per hour of sleep) >15.2 PLMS are associated with autonomic activations,3 with a magnitude of the rise in heart rate and blood pressure (BP) accompanying each limb movement comparable to that occurring in sleep apnea syndrome,4 which is a risk factor for cerebrovascular disease.5,6 Patients with PLMS are likely to have hundreds of events per night, potentially for many years.
In renal disease and in heart failure, PLMS predict mortality and stroke.7,8 A link between PLMS and hypertension, cardiovascular disease, cerebrovascular disease, peripheral vascular disease, left ventricular hypertrophy, and progression from transient to permanent atrial fibrillation has been observed but remains controversial.9–11
An association between PLMS and stroke has been reported in single cases (table e-1, links.lww.com/WNL/A417).12–20 PLMS in stroke might represent a preexisting favoring factor, the direct consequence of stroke, or simply an incidental finding.
Because PLMS can be abolished by dopamine agonists, it is relevant to assess their long-term effect. An investigation into PLMS in stroke might provide knowledge in this new area of preventive medicine.
The aims of this study were to explore whether PLMS are more prevalent in stroke patients than in controls without stroke, to assess the variability of PLMS from the acute to the chronic poststroke phase, and to compare sleep and cardiovascular parameters between stroke patients with and without PLMS.
Methods
Patients
This study is part of the prospective multicenter study (Sleep-Disordered Breathing in Transient Ischemia Attack [TIA]/Ischemic Stroke and Continuous Positive Airway Pressure [CPAP] Treatment Efficacy; SAS-CARE; NCT01097967) conducted in 4 sleep centers: Bern (Switzerland), Lugano (Switzerland), Munster (Germany), and Milan (Italy). Details regarding the design of the SAS-CARE study have been published previously.21 Briefly, the SAS-CARE cohort includes 35- to 75-year-old patients with a diagnosis of TIA or ischemic stroke who were admitted to the stroke unit within 2 days from the onset of symptoms. The exclusion criteria were unstable clinical situation (cardiorespiratory or life-threatening medical conditions), current CPAP treatment or during the last 3 months before stroke, nonischemic events (intracerebral/subarachnoid hemorrhage), and coma/stupor. Demographic and medical parameters from SAS-CARE were considered at admission and 3 months after stroke. All participants underwent a clinical examination, cerebral MRI, nocturnal polysomnography (PSG) during the acute (1–8 days after the cerebrovascular event) phase and/or 3 months after stroke or TIA, and 24-hour BP monitoring in the acute and chronic phases.
Neuroimaging
MRI stroke lesions were measured on strongly diffusion-weighted images (b = 1,000) with MRIcron (mccauslandcenter.sc.edu/mricro/mricron/). White matter lesions (WMLs) were assessed on the contralesional side on fluid-attenuated inversion recovery or T2 images. An expert-based qualitative analysis that used the Fazekas scale22 and a semiautomatic thresholding method that used MRIcron have been applied for the calculation of the WML load.
Polysomnography
All PSG recordings (titanium; Embla Flaga, Reykjavik, Iceland) included 6 EEG channels, submental EMG, electro-oculogram, nasal airflow, 2 channels of breathing effort and oximetry, and EMG of both tibialis anterior muscles. Recordings within the first 9 days (1–8 days) after stroke were performed in the stroke unit from 8 to 10 pm to 7 to 8 am. PSGs after 3 months were recorded in the sleep laboratory from 9 to 11 pm to 6 to 7.30 am. All recordings were scored manually according to the American Academy of Sleep Medicine standard criteria23 and then reviewed and corrected by a single expert (S.M.). PLMS were scored according to the official World Association of Sleep Medicine standards.24
Blood pressure
The 24-hour BP monitoring was performed with the calibrated oscillometric Cardioline (Milan, Italy) devices. The study began between 8 and 10 am. BP and heart rate were automatically measured at 30-minute intervals during the day and at 60-minute intervals at night for 24 hours. Nighttime was set for the period from 10 pm to 7 am. In the acute phase, 24-hour BP monitoring was performed in the in-patient setting. At the 3-month follow-up, patients were instructed to follow their usual daily activities. BP monitoring was performed on the nondominant side. Recordings were considered valid if at least 70% of daytime and 70% of nighttime measurements were available. The following parameters were analyzed: mean 24-hour, daytime, and nighttime systolic BPs and diastolic BPs. BP variability was assessed by the within-participant SD of all BP readings, by the coefficient variance, and by the difference between minimal and maximal BPs. Patients were considered affected by hypertension if they were treated with antihypertensive drugs (AHDs) at the time of their admission.
Healthy controls
PSGs belonging to patients and performed in the acute phase after stroke/TIA and those performed in the chronic phase were independently compared with those of sex- and age-matched healthy controls randomly selected from the HypnoLaus cohort.25 Briefly, the HypnoLaus Sleep Cohort study included 2,162 adults (51.2% women, mean age 58.4 ± 11.1 years) randomly selected from the population-based CoLaus/PsyCoLaus cohort who had a full PSG (titanium, Embla Flaga) at home between September 2009 and June 2013 in Lausanne, Switzerland.
Two trained sleep technicians manually scored the PSG recordings. Each recording was reviewed by an expert sleep physician, and a second sleep expert performed random quality checks. Sleep and related events were scored with the standard American Academy of Sleep Medicine criteria.23 PLMS were scored according to the official World Association of Sleep Medicine standards.24
Standard protocol approvals, registrations, and patient consents
We received approval from an ethics standards committee on human experimentation, and written informed consent was obtained from all participants of the study.
Statistical analysis
Data are presented as mean ± SD. The analysis of variance was performed to assess differences between controls and patients with stroke in the acute or chronic phases and to compare data on outcome variables among the 3 different patterns of PLMS distribution. The post hoc Tukey honest statistical difference test for unequal sample sizes was used to compare differences between groups. The χ2 test was used to compare the distribution of categorized variables. The paired t test was performed to establish changes between baseline and follow-up for all the indexes considered. The analysis of variance was applied to compare differences among the 3 groups of patients concerning stroke volume, WMLs, absolute BP values, BP variability, and dipping state in both acute and chronic phase. Logistic regression analysis was performed to identify the predictive factors for PLMS.
Data availability
The data that support the findings of this study are available from the corresponding authors (M.M. and C.B.) on reasonable request. The data are not publicly available because they contain information that could compromise research participant privacy/consent.
Results
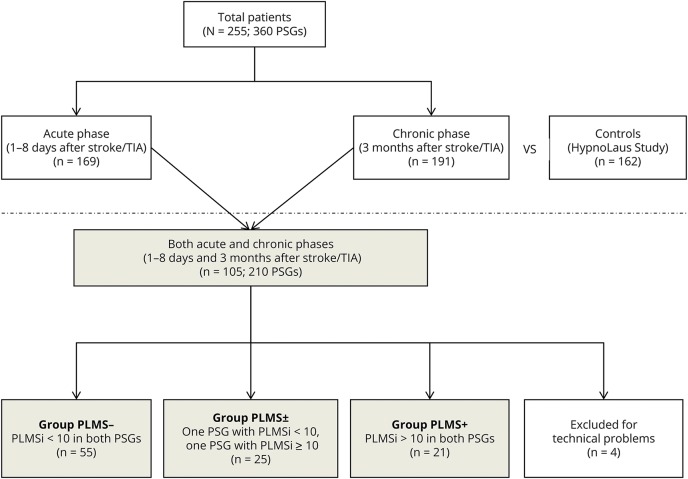
As shown in figure 1, a total of 360 PSG studies were available from 255 patients affected by ischemic stroke/TIA. One hundred sixty-nine patients (mean age 61.4 ± 9.4 years, 72.3% male) underwent a PSG in the acute phase of stroke/TIA; 191 patients (mean age 61.1 ± 9.3 years, 78.9% male) were recorded after 3 months (chronic phase). Among the above patients, 105 (mean age 61.1 ± 9.3 years, 78.9% male) were recorded in both acute and chronic phases. Four patients belonging to this group (105 patients) were excluded from the analysis because of technical PSG problems related to the tibialis anterior signal in 1 of the 2 recordings. These 101 patients with 2 high-quality recordings were then divided in 3 subgroups as follows: patients with a PLMS index <10 in both the acute and chronic PSGs were called PLMS−; those with a PLMS index ≥10 in both the acute and chronic PSGs were called PLMS+; and those with a PLMS index <10 in 1 of the 2 PSGs and ≥10 in the other PSG were called PLMS± (figure 1). A similar analysis was repeated considering a PLMS threshold of 15 and 30.
Figure 1. Flowchart.
Flow diagram of the study. PSG = polysomnography; PLMS = periodic limb movement during sleep; PLMSi = periodic limb movement index during sleep.
PSG data from the acute and chronic phase after stroke/TIA and those from 162 sex- and age-matched healthy controls (mean age 62.1 ± 8.4 years, 72.2% male) randomly selected from HypnoLaus Cohort are shown in table 1. Sleep quality of patients was significantly worse than that of controls, with patients showing a reduced mean total sleep time, sleep efficiency, and percentage of REM sleep; a longer wakefulness after sleep onset and sleep onset latency; and a higher percentage of N3 sleep. The apnea/hypopnea index (AHI) was similar in patients and controls. The mean limb movement index, mean PLMS index, and percentage of participants with a PLMS index ≥10 and ≥15 were not significantly different between the 2 groups. Table 1 also shows the comparison of the PSG parameters between patients in their chronic phase and the same group of healthy controls. Except for the limb movement index, which was higher in patients than in controls, all the other comparisons gave no significant results.
Table 1.
Comparison of PSG parameters of patients with stroke/TIA in the acute phase and controls and of patients with stroke/TIA in the chronic phase (3 months after stroke) and controls
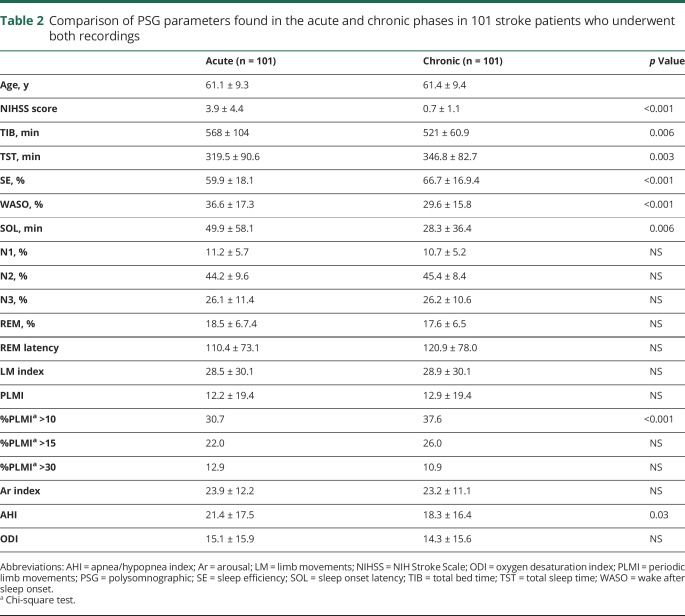
Table 2 shows the variation of PSG parameters from the acute to the chronic phase after stroke/TIA in the 101 patients who underwent both recordings. Herein, a general improvement of sleep was evident but remained worse than that of controls. The mean AHI decreased significantly after 3 months without a substantial change of the oxygen desaturation index (ODI). The mean PLMS index remained stable; however, the percentage of patients with a PLMS index ≥10 significantly increased from 30.7% in the acute to 36.7% in the chronic phase.
Table 2.
Comparison of PSG parameters found in the acute and chronic phases in 101 stroke patients who underwent both recordings
A multiple logistic regression model was built considering the presence of PLM ≥10 as the dependent variable and sex, age, body mass index (BMI), total sleep time and total non-REM duration (as percent of total sleep time), AHI, and ODI as independent variables. Only BMI was a statistically significant predictor in both the acute (odds ratio 1.4, 95% confidence interval 1.16–1.7, p < 0.001) and chronic (odds ratio 1.17, 95% confidence interval 1.02–1.33, p = 0.02) phases.
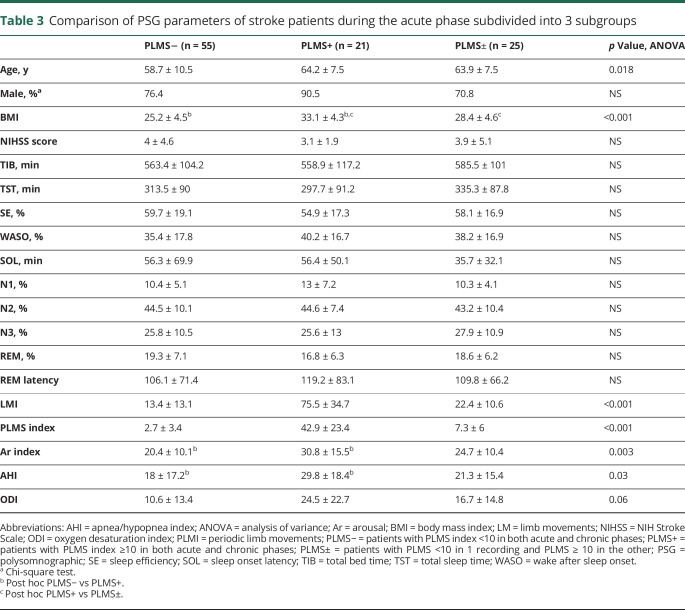
As shown in tables 3 and 4, more patients with PLMS (PLMS+ and PLMS±) were male; they also were significantly older and had a higher BMI than patients with PLMS−. The same patients also presented a higher mean arousal index, while all the other sleep parameters were similar. These results were confirmed in both the acute (table 3) and chronic (table 4) phases, except for the AHI and the ODI, which were significantly higher in the PLMS+ than the PLMS− group only in the acute (table 3), not in the chronic, phase (table 4).
Table 3.
Comparison of PSG parameters of stroke patients during the acute phase subdivided into 3 subgroups
Table 4.
Comparison of PSG parameters of stroke patients during the chronic phase subdivided into 3 subgroups
Neuroimaging parameters, in particular the mean punctate WML, deep WML, WML, and stroke volume, did not differ between the 3 groups in the acute phase (table e-2, links.lww.com/WNL/A417), considering a PLMS threshold of either 10 or 15.
The 24-hour BP parameters, including absolute values, variability, and dipping state, showed no differences among the 3 groups. Mean systolic BP values for the whole period ranged between 122.9 and 127.0 mm Hg in the acute phase and between 120.1 and 123.2 mm Hg in the chronic phase (table e-3, links.lww.com/WNL/A417). Nondipping state was common in all 3 groups, accounting for 60.5% in the PLMS−, 85.7% in the PLMS+, and 61.1% in the PLMS± group in the chronic phase.
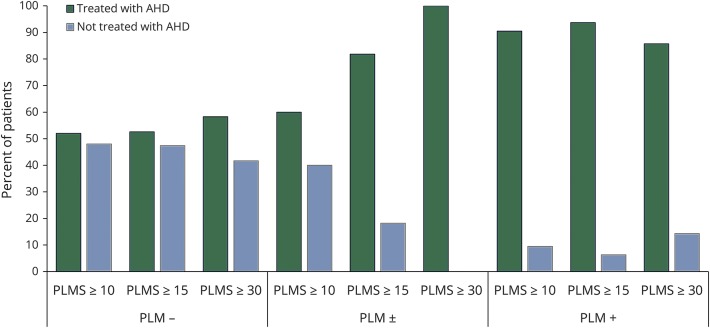
As shown in figure 2, the percentage of patients treated with AHDs, which corresponded to a previous diagnosis of hypertension, was much higher in the PLMS+ than in the PLMS− group, with the PLMS± group showing an intermediate percentage (89.5%, 54.5%, and 61.9%, respectively; χ2 = 7.1, p = 0.02). This difference in use of AHDs between the groups increased further when the cutoff threshold value of the PLMS index was increased from 10 to 15, with a presence of AHDs in the PLMS+ group of 93.8%, in the PLMS− group of 52.6%, and in the PLMS± group of 81.8% (χ2 = 10.9, p = 0.004). Again, similar results were found using a cutoff of 30 (χ2 = 6.4, p = 0.04, figure 2). Moreover, considering only patients under treatment, the number of AHDs taken by the PLMS+ and PLMS± groups was significantly higher than that in the PLMS− group (table e-4, links.lww.com/WNL/A417). Although less evident, a similar tendency of an association between PLMS and AHDs was also found in the HypnoLaus cohort. The percentage of controls treated with AHDs was as follows: 31.1% in those with a PLMS index <10, 37.5% in those with a PLMS index ≥10, 42.3% in those with a PLMS index ≥15, and 41.6% in those with a PLMS index ≥30.
Figure 2. Antihypertensive medication.
Percentage of patients in treatment with AHDs (corresponding to a previous diagnosis of hypertension) in the 3 different groups of patients with stroke/TIA (PLM+, PLM±, PLM−) for 3 different calculated thresholds of PLMSi (PLMS 10, PLMS 15, PLMS 30). AHD = antihypertensive drug; PLMS = periodic limb movements during sleep; PLMSi = periodic limb movement index during sleep.
Discussion
This large-scale prospective instrumental investigation in patients with ischemic stroke/TIA with a specific focus on PLMS demonstrated a strong relationship between PLMS and diagnosis of hypertension.
PLMS were equally represented in patients and healthy controls, considering both the acute and chronic phases of stroke/TIA. This result cannot support the hypothesis that PLMS per se represent a significant risk factor for ischemic stroke/TIA. Results of our study indicates that single cases or small series of patients with PLMS and stroke previously described in the literature need to be re-evaluated, considering the possibility that PLMS existed before stroke.12–20
Compared to the pathologic breathing events, which, as expected, significantly decreased from the acute to the subacute/chronic phase after stroke, PLMS remained quite stable when reassessed after 3 months after stroke/TIA. This consolidates the idea that stroke per se has a limited effect on PLMS. However, the percentage of patients with a PLMS >10 increased from the acute to the chronic phase, together with an expected improvement of sleep quality. This might be due to a consolidation in sleep continuity after the acute phase, resulting in having more chances to produce a series of limb movements >4 in a row. Alternatively, this might be due to a masking effect of the breathing events in the acute phase in which hypopneas/apneas were more numerous than in the chronic phase. Otherwise, this might suggest that, despite being limited, stroke/TIA has an effect on PLMS. In addition, we cannot exclude that stroke/TIA might influence PLMS in terms of organization of periodicity (time structure) rather than their quantitative aspect (PLMS index).26,27
As expected, patients with PLMS were more often male, were older, and had a higher BMI and AHI than those without PLMS. The increase in PLMS index across life is already well known.28 Despite the fact that RLS is more prevalent in women than in men,29 previous investigations have shown that PLMS are more frequent in men than in women.10,30 The association of PLMS with sleep apnea is already known and might explain the link between PLMS and BMI. However, in our cohort, the association between PLMS and AHI was mild in the acute phase and even lost in the chronic phase, while the association between PLMS and BMI remained strong, with BMI being an independent risk factor for PLMS. A positive association between PLMS and BMI in the general population and between RLS and BMI in stroke patients was also described in previous large studies.2,31
The hypothesis that patients with PLMS have a preexisting higher cerebrovascular load compared to those without PLMS was not confirmed by our analysis (taking into consideration the unaffected hemisphere). Only 1 study in the literature has reported that patients affected by a sufficiently long history of RLS have a higher small vessel cerebrovascular damage than controls without RLS; however, this investigation focused on RLS (not PLMS) and on patients without risk factors for stroke.32
In the present study, all the BP parameters from the 24-hour analysis were similar between patients with and without PLMS. On the other hand, the presence of an AHD treatment, which realistically corresponds to a previous diagnosis of hypertension, was notably higher in the PLMS+ group, with a positive relationship with the severity of PLMS. In addition, patients with PLMS need more AHDs to control their pressure levels. Although less solid, a relationship between PLMS and AHD was also found in the control group (part of the HypnoLaus cohort) of the present study, and an association between hypertension and PLMS was confirmed in the large instrumental epidemiologic HypnoLaus study, in which this link was observed in the general population at the comparative level but not confirmed at the logistic regression analysis, while the association with AHDs (β-blockers) was a significant predictor of PLMS.2 It is possible that the presence of AHD masks the difference in BP measurements in our patients with PLMS. While the general population is never screened for PLMS, hypertension is usually checked and rapidly corrected by general practitioners. Evidence has indicated that the supposed cardiovascular risk associated with RLS/PLMS can be mediated by hypertension, which might be necessary to translate the sympathetic hypertonia accompanying RLS/PLMS into brain damage.33 This might also explain why in our cohort the vascular lesion load was slightly higher in PLMS+ vs PLMS− patients, but the difference did not reach statistical significance. The confounding value of a preexisting AHD should be carefully evaluated in the available large epidemiologic studies focused on the relationship between RLS and hypertension and might, at least partially, explain their contradictory results.34–39 A further confounding factor might be the possible direct effect of some AHDs such as clonidine on PLMS. A common mechanism behind both RLS/PLM and hypertension might be a hypertonic sympathetic activity due to a dysfunction of the inhibitory descending hypothalamospinal pathway that from the A11 region projects to the intermediolateral columns where the preganglion sympathetic neurons are located.40
The present study has some limitations: (1) patients and controls belong to 2 different cohorts, but each study was corrected by 1 single scorer who used the same scoring criteria; (2) PSGs in the acute and the chronic phases after stroke were carried out in 2 different locations (stroke unit and sleep laboratories); and (3) our investigation did not consider hemorrhagic events, which represent almost 20% of all strokes and have a more direct correlation with hypertension than ischemic events.
In the present study, ischemic stroke/TIA was not associated with a higher prevalence of PLMS compared to the general population, and stroke per se in its acute phase affected sleep severely but did not significantly affect PLMS. Despite the similar results in BP monitoring between patients with and without PLMS, the relationship between PLMS and hypertension was strong, with a much higher use of AHDs among PLMS-positive patients. Further large investigations are warranted to challenge our results and to better explain both the basis of the association between PLMS and BMI and the role of BMI as a linking factor between PLMS, hypertension, and stroke.
Acknowledgments
The authors thank all investigators, study nurses, and other technical and laboratory personnel involved in study conduct for their efforts: for Bellinzona: Dr. M. Pfefferle; for Bern: Miriam Heldner, Marianne Kormann, Rudolf Lüdi, Dr. Corinne Roth, Andrea Surtmann Monika Wüthrich, and Daniela Wyss; and for Lugano: J. Frangi, V. Pifferini, Dr. C. Zunzunegui, and Dr. D. Kuen.
Glossary
- AHD
antihypertensive drug
- AHI
apnea/hypopnea index
- BMI
body mass index
- BP
blood pressure
- ODI
oxygen desaturation index
- PLMS
periodic limb movements during sleep
- PSG
polysomnography
- RLS
restless legs syndrome
- SAS-CARE
Sleep-Disordered Breathing in Transient Ischemia Attack (TIA)/Ischemic Stroke and Continuous Positive Airway Pressure (CPAP) Treatment Efficacy
- WML
white matter lesion
Author contributions
M.M.: conception and design of the study, acquisition and analysis of data, and drafting a significant portion of the manuscript. F.F. and R.F.: acquisition and analysis of data and drafting a significant portion of the manuscript. S.M., J.H.-R., and R.H.: acquisition and analysis of data. T.H.: acquisition and analysis of data and drafting a significant portion of the manuscript. P.P.: acquisition and analysis of data. P.Y. and G.M.: drafting a significant portion of the manuscript. A.S.: acquisition and analysis of data and drafting a significant portion of the manuscript. C.C. and L.N.: acquisition and analysis of data. R.W.: acquisition and analysis of data and drafting a significant portion of the manuscript. S.R.O.: acquisition and analysis of data. C.L.B.: conception and design of the study, acquisition and analysis of data and drafting a significant portion of the manuscript.
Study funding
This study was supported by a grant from the Swiss National Science Foundation (NCT01097967-SNF-320000-122301). The HypnoLaus, CoLaus, and PsyCoLaus studies were supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of the University of Lausanne, the Swiss National Science Foundation (grants 3200B0-105993, 3200B0-118308, 33CSCO-122661, 33CS30-139468, and 33CS30-148401), the Leenaards Foundation, and the Vaud Pulmonary League (Ligue Pulmonaire Vaudoise).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Haba-Rubio J, Marti-Soler H, Marques-Vidal P, et al. Prevalence and determinants of periodic limb movements in the general population. Ann Neurol 2016;79:464–474. [DOI] [PubMed] [Google Scholar]
- 3.Manconi M, Ferri R, Zucconi M, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol 2012;71:834–844. [DOI] [PubMed] [Google Scholar]
- 4.Pennestri MH, Montplaisir J, Colombo R, et al. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology 2007;68:1213–1218. [DOI] [PubMed] [Google Scholar]
- 5.Penzel T, Wessel N, Riedl M, et al. Cardiovascular and respiratory dynamics in patients with sleep apnea. Conf Proc IEEE Eng Med Biol Soc 2010;2010:276–279. [DOI] [PubMed] [Google Scholar]
- 6.Reishtein JL. Obstructive sleep apnea: a risk factor for cardiovascular disease. J Cardiovasc Nurs 2011;26:106–116. [DOI] [PubMed] [Google Scholar]
- 7.Benz RL, Pressman MR, Hovick ET, et al. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis 2000;35:1052–1060. [DOI] [PubMed] [Google Scholar]
- 8.Yatsu S, Kasai T, Suda S, et al. Impact on clinical outcomes of periodic leg movements during sleep in hospitalized patients following acute decompensated heart failure. Circ J 2017;81:495–500. [DOI] [PubMed] [Google Scholar]
- 9.Koo BB, Sillau S, Dean DA, et al. Periodic limb movements during sleep and prevalent hypertension in the multi-ethnic study of atherosclerosis. Hypertension 2015;65:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scofield H, Roth T, Drake C. Periodic limb movements during sleep: population prevalence, clinical correlates, and racial differences. Sleep 2008;31:1221–1227. [PMC free article] [PubMed] [Google Scholar]
- 11.Mirza M, Shen WK, Sofi A, et al. Frequent periodic leg movement during sleep is associated with left ventricular hypertrophy and adverse cardiovascular outcomes. J Am Soc Echocardiogr 2013;26:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JS, Lee SB, Park SK, et al. Periodic limb movement during sleep developed after pontine lesion. Mov Disord 2003;18:1403–1405. [DOI] [PubMed] [Google Scholar]
- 13.Kang SY, Sohn YH, Lee IK, et al. Unilateral periodic limb movement in sleep after supratentorial cerebral infarction. Parkinsonism Relat Disord 2004;10:429–431. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Lee PH, Huh K. Periodic limb movements in sleep after a small deep subcortical infarct. Mov Disord 2005;20:260–261. [DOI] [PubMed] [Google Scholar]
- 15.Unrath A, Kassubek J. Symptomatic restless leg syndrome after lacunar stroke: a lesion study. Mov Disord 2006;21:2027–2028. [DOI] [PubMed] [Google Scholar]
- 16.Coelho FM, Georgsson H, Narayansingh M, et al. Higher prevalence of periodic limb movements of sleep in patients with history of stroke. J Clin Sleep Med 2010;6:428–430. [PMC free article] [PubMed] [Google Scholar]
- 17.Sechi G, Agnetti V, Galistu P, et al. Restless legs syndrome and periodic limb movements after ischemic stroke in the right lenticulostriate region. Parkinsonism Relat Disord 2008;14:157–160. [DOI] [PubMed] [Google Scholar]
- 18.Kizilay F, Ozkaynak S, Hatipoglu E, et al. Periodic limb movement during sleep following cerebellar infarct. Acta Neurol Belg 2010;110:284–286. [PubMed] [Google Scholar]
- 19.Ruppert E, Kilic-Huck U, Wolff V, et al. Restless legs syndrome as a first manifestation of a cerebral infarct. J Clin Sleep Med 2014;10:1037–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han SH, Park KY, Youn YC, et al. Restless legs syndrome and akathisia as manifestations of acute pontine infarction. J Clin Neurosci 2014;21:354–355. [DOI] [PubMed] [Google Scholar]
- 21.Cereda CW, Petrini L, Azzola A, et al. Sleep-disordered breathing in acute ischemic stroke and transient ischemic attack: effects on short- and long-term outcome and efficacy of treatment with continuous positive airways pressure: rationale and design of the SAS CARE study. Int J Stroke 2012;7:597–603. [DOI] [PubMed] [Google Scholar]
- 22.Fazekas, et al. Am J Roentgenol 1987. [Google Scholar]
- 23.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events: deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med 2006;7:175–183. [DOI] [PubMed] [Google Scholar]
- 25.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferri R, Zucconi M, Manconi M, et al. Computer-assisted detection of nocturnal leg motor activity in patients with restless legs syndrome and periodic leg movements during sleep. Sleep 2005;28:998–1004. [DOI] [PubMed] [Google Scholar]
- 27.Ferri R, Zucconi M, Manconi M, et al. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep 2006;29:759–769. [PubMed] [Google Scholar]
- 28.Ferri R, Manconi M, Lanuzza B, et al. Age-related changes in periodic leg movements during sleep in patients with restless legs syndrome. Sleep Med 2008;9:790–798. [DOI] [PubMed] [Google Scholar]
- 29.Berger K, Luedemann J, Trenkwalder C, et al. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med 2004;164:196–202. [DOI] [PubMed] [Google Scholar]
- 30.Moore H, Winkelmann J, Lin L, et al. Periodic leg movements during sleep are associated with polymorphisms in BTBD9, TOX3/BC034767, MEIS1, MAP2K5/SKOR1, and PTPRD. Sleep 2014;37:1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlesinger I, Erikh I, Nassar M, et al. Restless legs syndrome in stroke patients. Sleep Med 2015;16:1006–1010. [DOI] [PubMed] [Google Scholar]
- 32.Ferri R, Cosentino FI, Moussouttas M, et al. Silent cerebral small vessel disease in restless legs syndrome. Sleep 2016;39:1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferini-Strambi L, Walters AS, Sica D. The relationship among restless legs syndrome (Willis-Ekbom disease), hypertension, cardiovascular disease, and cerebrovascular disease. J Neurol 2014;261:1051–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wali SO, Abaalkhail B. Prevalence of restless legs syndrome and associated risk factors among middle-aged Saudi population. Ann Thorac Med 2015;10:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Den Eeden SK, Albers KB, Davidson JE, et al. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a retrospective cohort study from Kaiser Permanente Northern California. Sleep 2015;38:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De VK, Li Y, Batool-Anwar S, et al. Prospective study of obesity, hypertension, high cholesterol, and risk of restless legs syndrome. Mov Disord 2014;29:1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giannini G, Zanigni S, Melotti R, et al. Association between restless legs syndrome and hypertension: a preliminary population-based study in South Tyrol, Italy. Eur J Neurol 2014;21:72–78. [DOI] [PubMed] [Google Scholar]
- 38.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res 2002;53:547–554. [DOI] [PubMed] [Google Scholar]
- 39.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med 2006;7:545–552. [DOI] [PubMed] [Google Scholar]
- 40.Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology 2006;67:125–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors (M.M. and C.B.) on reasonable request. The data are not publicly available because they contain information that could compromise research participant privacy/consent.