Abstract
Objective
To identify factors associated with treatment delays in pediatric patients with convulsive refractory status epilepticus (rSE).
Methods
This prospective, observational study was performed from June 2011 to March 2017 on pediatric patients (1 month to 21 years of age) with rSE. We evaluated potential factors associated with increased treatment delays in a Cox proportional hazards model.
Results
We studied 219 patients (53% males) with a median (25th–75th percentiles [p25–p75]) age of 3.9 (1.2–9.5) years in whom rSE started out of hospital (141 [64.4%]) or in hospital (78 [35.6%]). The median (p25–p75) time from seizure onset to treatment was 16 (5–45) minutes to first benzodiazepine (BZD), 63 (33–146) minutes to first non-BZD antiepileptic drug (AED), and 170 (107–539) minutes to first continuous infusion. Factors associated with more delays to administration of the first BZD were intermittent rSE (hazard ratio [HR] 1.54, 95% confidence interval [CI] 1.14–2.09; p = 0.0467) and out-of-hospital rSE onset (HR 1.5, 95% CI 1.11–2.04; p = 0.0467). Factors associated with more delays to administration of the first non-BZD AED were intermittent rSE (HR 1.78, 95% CI 1.32–2.4; p = 0.001) and out-of-hospital rSE onset (HR 2.25, 95% CI 1.67–3.02; p < 0.0001). None of the studied factors were associated with a delayed administration of continuous infusion.
Conclusion
Intermittent rSE and out-of-hospital rSE onset are independently associated with longer delays to administration of the first BZD and the first non-BZD AED in pediatric rSE. These factors identify potential targets for intervention to reduce time to treatment.
Status epilepticus (SE) is one of the most common neurologic emergencies, affecting approximately 5 to 20 children per 100,000 per year.1–3 Mortality in pediatric SE is approximately 0% to 3% in the short term1,4,5 and approximately 7% in the long term.6 Sequelae, such as neurologic, cognitive, and behavioral problems, are common.7 The main predictors of outcome are age, etiology, and duration of SE,5,7,8 but duration of SE is often most easily modified.
To minimize seizure duration, SE guidelines suggest timely treatment with rapid escalation of antiepileptic drugs (AEDs) if needed.9,10 Basic research and clinical studies suggest that delays in medication administration are associated with greater resistance to treatment and worse outcomes.11–16 However, time to treatment in SE is often longer than guidelines recommend in adults17,18 and children.18–20 In previous studies from the Pediatric Status Epilepticus Research Group (pSERG), we showed that time to the administration of the first AEDs was slower than guideline recommendations,21 and that treatment initiation delays were associated with worsened outcomes.21a However, there are limited data regarding factors associated with treatment delays. Previously, we showed that the time to AED administration was similar in patients with and without a prior diagnosis of epilepsy,22 but it is unknown whether there are other factors associated with treatment delays that might be amenable to intervention in efforts to reduce time to treatment in SE.
We aimed to address this gap in knowledge by identifying factors independently associated with delays to administration of AEDs in pediatric patients with convulsive refractory SE (rSE). We evaluated a series of factors potentially associated with delays to administration of treatment: type of rSE (continuous vs intermittent), site of rSE onset (in hospital vs out of hospital), time of day at onset (day vs night), period in the academic year (first half vs last half), race (white vs nonwhite), and increased awareness of the existence of marked delays in rSE treatment (no vs yes).
Methods
Study design
We performed a prospective, observational study within pSERG, an ongoing multicenter consortium that aims to generate data to inform clinical decision-making and improve outcomes in pediatric rSE.23 Detailed data on time to treatment,21 differences between patients with and without a prior diagnosis of epilepsy,22 and the association of treatment delays with outcomes21a have been previously reported.
Standard protocol approvals, registrations, and patient consents
The study was approved by the institutional review boards at each participating center.
Patients
This study involved patients with rSE. The inclusion criteria were as follows: (1) age from 1 month to 21 years; (2) admission between June 1, 2011, and March 1, 2017; and (3) focal or generalized convulsive seizures at onset that continued after administration of at least 2 AEDs, including at least one nonbenzodiazepine (non-BZD) AED or the use of a continuous infusion to treat rSE. Exclusion criteria were as follows: (1) nonconvulsive SE detected on EEG without convulsive seizures at onset, (2) nonconvulsive SE with motor manifestations limited to infrequent myoclonic jerks, and (3) insufficient basic demographic or clinical data. In summary, for the purposes of this study, rSE refers to a single convulsive seizure or a cluster of convulsive seizures without return to baseline that did not respond to administration of at least 2 AEDs, including at least one non-BZD AED or the use of a continuous infusion. If more than one rSE episode occurred during the study period, only the first episode was included in the statistical analysis.
Variables
The primary outcome was time to administration of AEDs: first BZD, first non-BZD AED, and, for patients who received continuous infusion, time to first continuous infusion. The time to treatment was determined based on information provided by family and emergency medical services (EMS) for out-of-hospital onset and from provider information and hospital records once in the hospital. To minimize bias, we cross-referenced time information between all available sources of information. We evaluated the following dichotomous variables potentially associated with delays in treatment administration: type of rSE (continuous vs intermittent), site of rSE onset (in hospital vs out of hospital), time of day at onset (day vs night), period in the academic year (first half vs last half), race (white vs nonwhite), and increased awareness of the existence of marked delays in rSE treatment (no vs yes). Awareness of delays in treatment administration were dichotomized into the period 2011–2014 (when the existence of delays to treatment was a study question within pSERG) and the period 2015–2017 (when the existence of treatment delays was demonstrated21) as a rough surrogate marker of awareness. Time of day was dichotomized into day (8 am to 8 pm) and night (8 pm to 8 am) based on office working hours, although working schedules may vary among hospitals. The academic year was dichotomized into first half (July to December) and second half (January to June) to address the education and experience of new residents, although we acknowledge that the degree of reliance on residents may be different among institutions and at different times of the day within institutions. Racial inequalities in health and access to health care are well documented, and race is an indirect marker of access to health care.24,25 rSE was classified as continuous when it consisted of a single prolonged seizure and as intermittent when it consisted of repeated seizures without return to baseline, as based on all available information. We also controlled for potential confounders in the multivariable analysis: etiology (structural vs nonstructural vs unknown), prior diagnosis of epilepsy (no vs yes), prior episode of SE (no vs yes), and age (in years). Potential reasons for delay in time to treatment were considered based on prior knowledge.
Statistical analysis
Descriptive statistics summarized demographic and basic clinical features. We compared quantitative variables among 2 groups with the Wilcoxon rank sum test and among more than 2 groups with the Kruskal-Wallis test. Times to administration of AEDs were analyzed using Cox proportional hazard regression models. We evaluated the proportional hazard assumption with log-log and residuals graphs for each Cox model. There were no major departures from the proportional hazards assumptions. Because the location of rSE onset is a potential effect modifier, we supplemented our analysis of the entire population with a stratified analysis dividing patients by location of rSE onset: out of hospital vs in hospital. Within each population, a patient may have received treatment at various sites (home, by EMS, non-pSERG hospital, and pSERG hospital). Unless stated otherwise, quantitative variables are presented as median (25th–75th percentiles [p25–p75]) and categorical variables are presented as number and percentage. By convention, a 2-sided α level was set at 0.05 to denote statistical significance. We controlled for multiple comparisons with the Benjamini and Hochberg false discovery rate, with a threshold (q value) of 0.05.26 False discovery rate controls for expected proportion of false discoveries relative to total discoveries, in order to control for multiple comparisons across multiple tests.26 All analyses were performed using R: a language and environment for statistical computing (Vienna, Austria),27 RStudio,28 and the packages gmodels,29 lubridate,30 gdata,31 car,32 and survival.33
Data availability
All statistical analyses and results are available in supplemental data (links.lww.com/WNL/A432). The original data are available on request.
Results
Demographic and clinical features
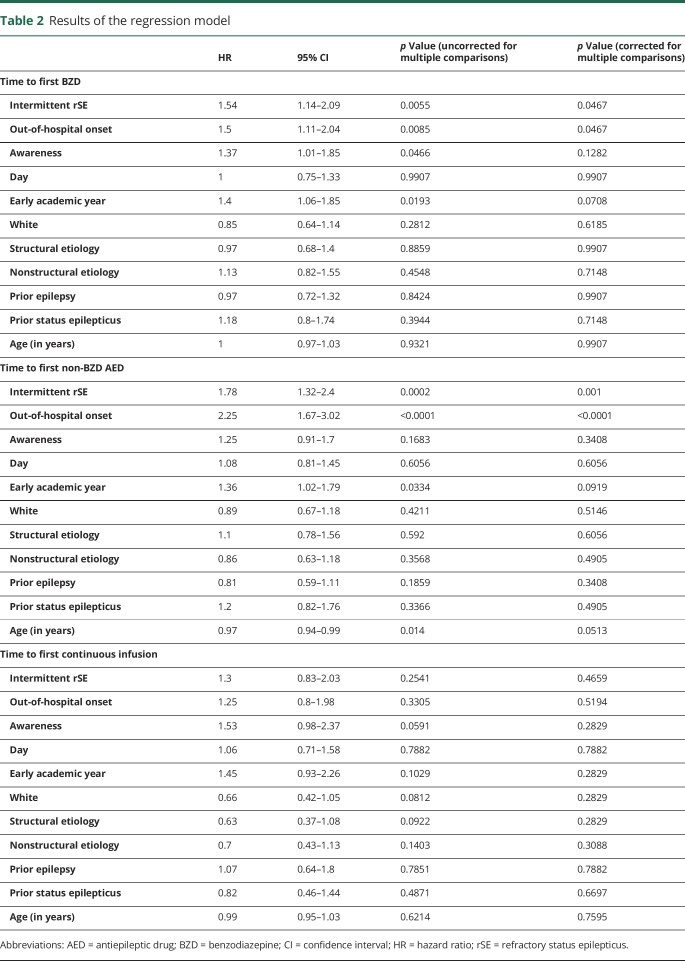
We enrolled 219 patients (53% males) with a median (p25–p75) age of 3.9 (1.2–9.5) years (table 1; table e-1, links.lww.com/WNL/A431). rSE episodes started out of the hospital in 141 patients and in the hospital in 78 patients. The median (p25–p75) time to first BZD administration was 16 (5–45) minutes (figure 1A). The first BZD was administered at home in 35 (16%) patients, by the EMS in 47 (21.5%) patients, in a non-pSERG hospital in 49 (22.4%) patients, and in a pSERG hospital in 88 (40.2%) patients. The median (p25–p75) time to first BZD varied by site of administration: at home 5 (4–14) minutes, by EMS 20 (11–24.5) minutes, in a non-pSERG hospital 45 (15–88) minutes, and in a pSERG hospital 13.5 (5–54.3) minutes (p < 0.0001). The median (p25–p75) time to first non-BZD AED was 63 (33–146) minutes (figure 1B). The first non-BZD AED was administered by the EMS in 10 (4.6%) patients, in a non-pSERG hospital in 82 (38%) patients, and at a pSERG hospital in 124 (57.4%) patients. The median (p25–p75) time to first non-BZD AED did not vary by site of administration: by EMS 35.5 (19.8–128.8) minutes, in a non-pSERG hospital 60 (38.5–100) minutes, and in a pSERG hospital 66.5 (29.8–162) minutes (p = 0.336). Among 107 patients who received at least one continuous infusion, the median (p25–p75) time to first continuous infusion was 170 (107–539) minutes (figure 1C).
Table 1.
Demographic and clinical features in our population (n = 219)
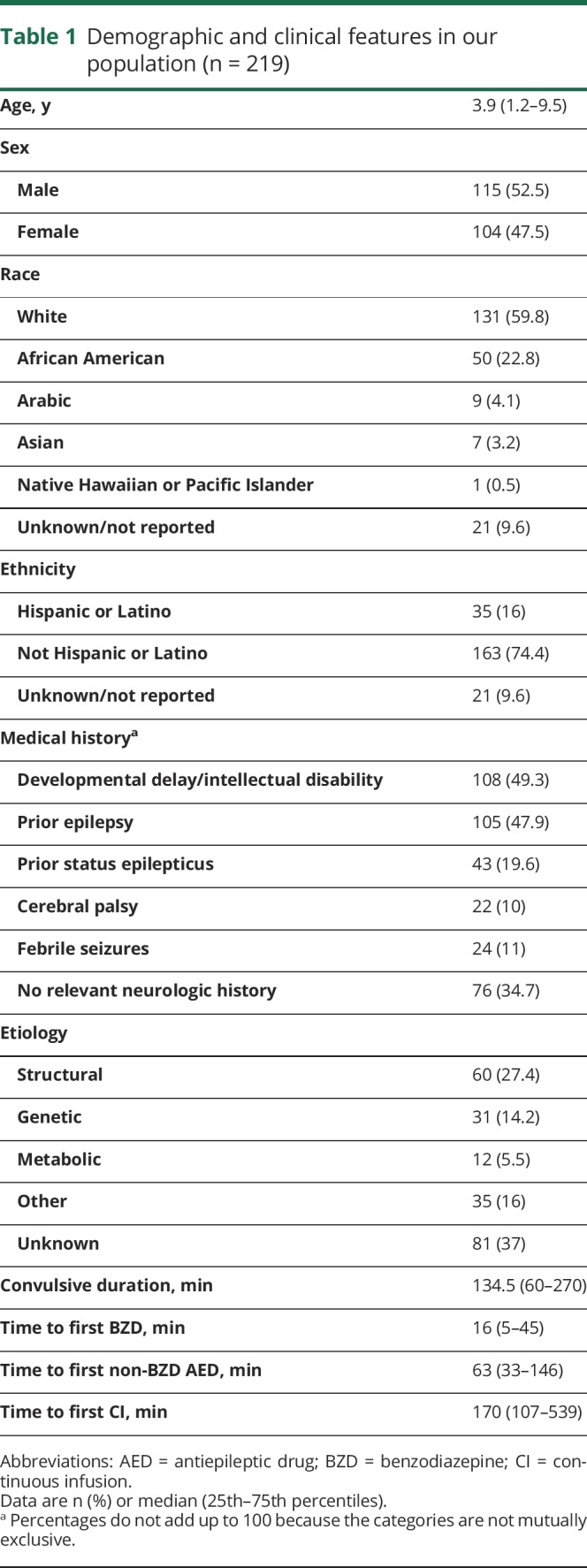
Figure 1. Time to treatment in the whole population.
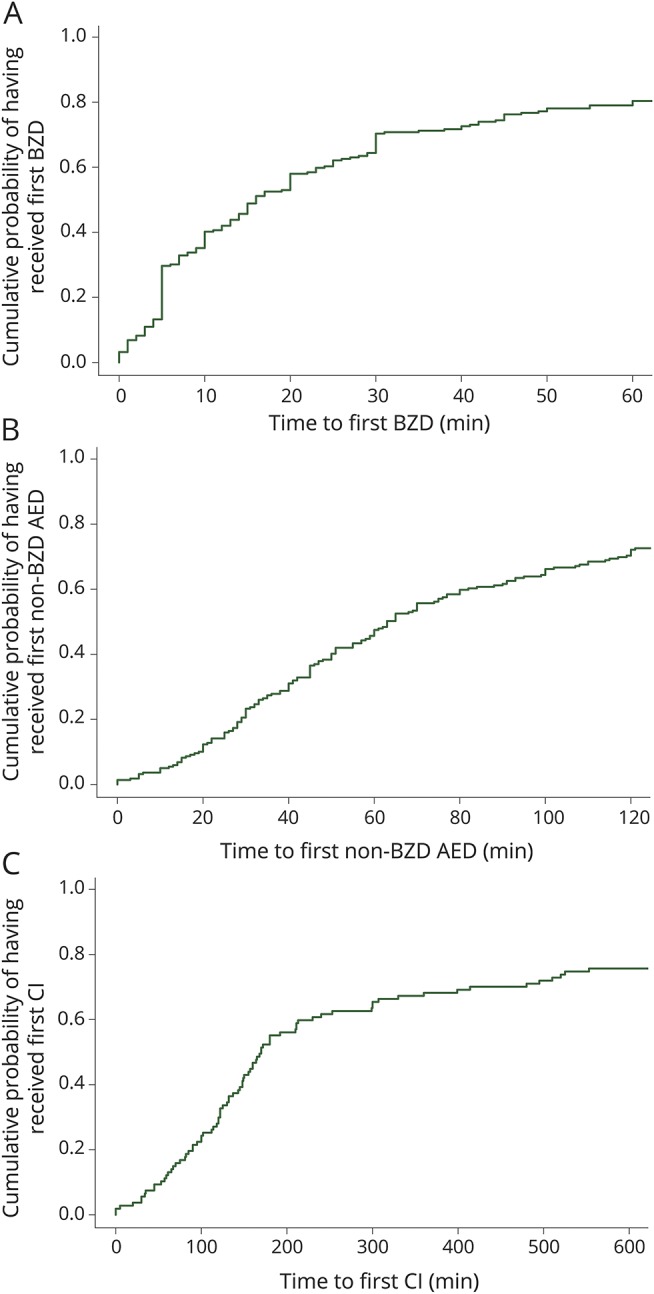
(A) Time to receive the first BZD. The x-axis is truncated at 60 minutes for clarity. (B) Time to receive the first non-BZD AED. The x-axis is truncated at 120 minutes for clarity. (C) Time to receive the first continuous infusion. The x-axis is truncated at 600 minutes for clarity. AED = antiepileptic drug; BZD = benzodiazepine; CI = continuous infusion.
Factors associated with delayed treatment
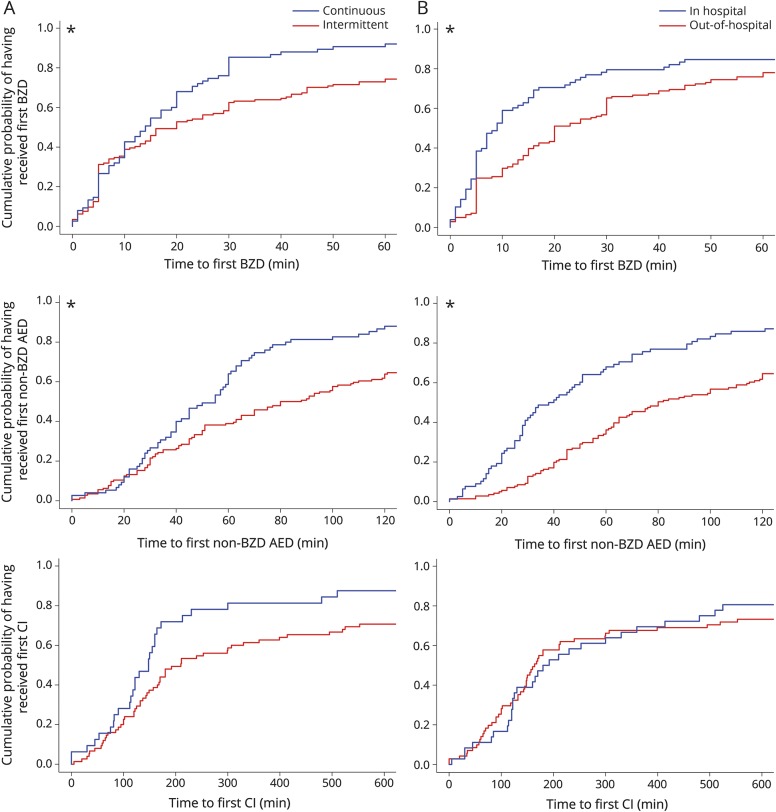
Factors associated with more delays to administration of the first BZD were intermittent rSE (hazard ratio [HR] 1.54, 95% confidence interval [CI] 1.14–2.09; p = 0.0467) and out-of-hospital rSE onset (HR 1.5, 95% CI 1.11–2.04; p = 0.0467) (table 2, figure 2). Factors associated with more delays to administration of the first non-BZD AED were intermittent rSE (HR 1.78, 95% CI 1.32–2.4; p = 0.001) and out-of-hospital rSE onset (HR 2.25, 95% CI 1.67–3.02; p < 0.0001) (table 2, figure 2). None of the studied factors were associated with a delayed administration of continuous infusion.
Table 2.
Results of the regression model
Figure 2. Comparison of the time to treatment between subgroups among the full cohort. Only the factors that were different are displayed in the figure.
(A) Comparison between continuous (blue) and intermittent (red) rSE. (B) Comparison between patients with onset of rSE in hospital (blue) and out of hospital (red). The first row contains time to first BZD (x-axis truncated at 60 minutes for clarity), the second row time to first non-BZD AED (x-axis truncated at 120 minutes for clarity), and the third row time to first CIs (x-axis truncated at 600 minutes for clarity). *Statistically significantly different. AED = antiepileptic drug; BZD = benzodiazepine; CI = continuous infusion; rSE = refractory status epilepticus.
Stratification by site of rSE onset
Out-of-hospital onset
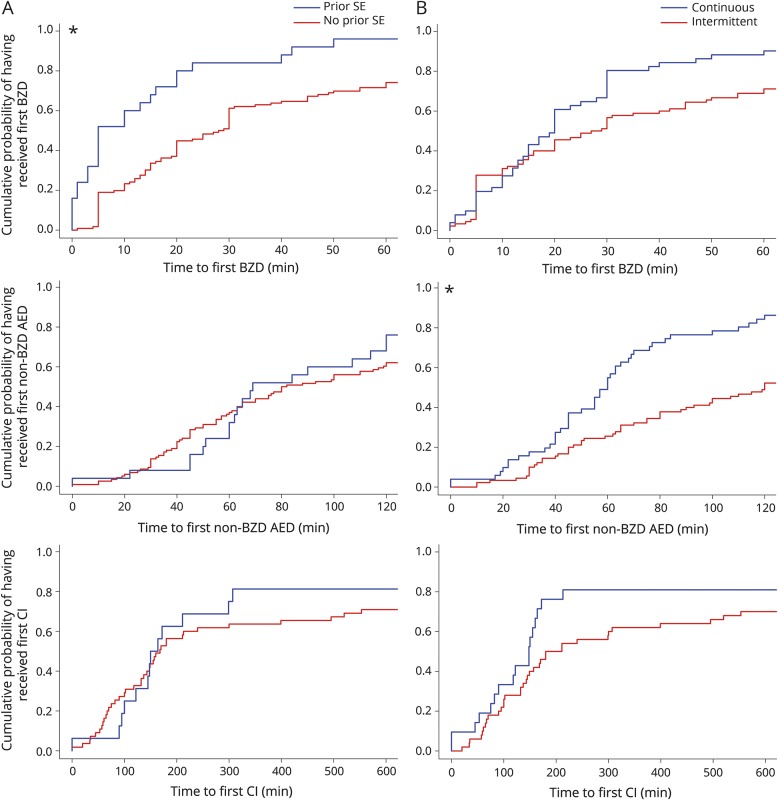
A total of 141 patients had out-of-hospital rSE onset (tables e-2 and e-3, links.lww.com/WNL/A431). The median (p25–p75) time to first BZD administration was 20 (8–55) minutes (figure e-1A, links.lww.com/WNL/A430). Among 113 patients with available information on time to hospital arrival, 44 (38.9%) patients did not receive any medication before hospital arrival. The first BZD was administered at home in 34 (24.1%) patients, by the EMS in 46 (32.6%) patients, in a non-pSERG hospital in 35 (24.8%) patients, and at a pSERG hospital in 26 (18.4%) patients. The median (p25–p75) time to first BZD varied by site of administration: at home 5 (3.5–12.8) minutes, by EMS 18.5 (10.5–24.5) minutes, in a non-pSERG hospital 55 (30–90) minutes, and in a pSERG hospital 50 (30–181.8) minutes (p < 0.0001). The median (p25–p75) time to first non-BZD AED administration was 80 (45–165) minutes (figure e-1B). The first non-BZD AED was administered by the EMS in 9 (6.5%) patients, in a non-pSERG hospital in 67 (48.6%) patients, and at a pSERG hospital in 62 (44.9%) patients. The median (p25–p75) time to first non-BZD AED varied by site of administration: by EMS 35 (19–150) minutes, in a non-pSERG hospital 65 (45–118.5) minutes, and in a pSERG hospital 119.5 (63–261.8) minutes (p = 0.0036). Among 71 patients who received at least one continuous infusion, the median (p25–p75) time to first continuous infusion was 164 (97.5–641) minutes (figure e-1C). In the population with out-of-hospital rSE onset, the factor associated with more delay to the first BZD was no prior SE (HR 2.32, 95% CI 1.58–3.42; p = 0.0053). The factor associated with more delay to the first non-BZD AED was intermittent rSE (HR 2.33, 95% CI 1.58–3.42; p = 0.0002). None of the studied factors were associated with a delayed administration of continuous infusions (table e-4, figure 3).
Figure 3. Comparison of the time to treatment between subgroups for the out-of-hospital–onset subgroup.
(A) Comparison between history of SE (blue) and no history of SE (red). (B) Comparison between continuous (blue) and intermittent (red) refractory SE. The first row contains time to first BZD (x-axis truncated at 60 minutes for clarity), the second row time to first non-BZD AED (x-axis truncated at 120 minutes for clarity), and the third row time to first CIs (x-axis truncated at 600 minutes for clarity). *Statistically significantly different. AED = antiepileptic drug; BZD = benzodiazepine; CI = continuous infusion; SE = status epilepticus.
In-hospital onset
A total of 78 patients had in-hospital rSE onset (tables e-5 and e-6, links.lww.com/WNL/A431). The median (p25–p75) time to first BZD administration was 9 (5–24.8) minutes (figure e-2A, links.lww.com/WNL/A430). The first BZD was administered in a non-pSERG hospital in 14 (18.4%) patients and at a pSERG hospital in 62 (81.6%) patients. The median (p25–p75) time to first BZD did not vary by site of administration: in a non-pSERG hospital 8.5 (2.8–15.8) minutes and in a pSERG hospital 8.5 (5–25.8) minutes (p = 0.4976). The median (p25–p75) time to first non-BZD AED administration was 39 (21.3–73) minutes (figure e-2B). The first non-BZD AED was administered in a non-pSERG hospital in 15 (19.5%) patients and at a pSERG hospital in 62 (80.5%) patients. The median (p25–p75) time to first non-BZD AED did not vary by site of administration: in a non-pSERG hospital 31 (20–41.5) minutes and in a pSERG hospital 44 (21.3–91) minutes (p = 0.1339). Among 36 patients who received at least one continuous infusion, time to first continuous infusion was 186 (120–487.5) minutes (figure e-2C). None of the studied factors were associated with a delayed administration of the first BZD, first non-BZD AED, or first continuous infusion (tables 2 and e-7).
Additional information on our study population is presented in supplemental results (links.lww.com/WNL/A433).
Discussion
Intermittent rSE and out-of-hospital rSE onset are independently associated with longer delays to administration of the first BZD and the first non-BZD AED in pediatric rSE. Among patients with out-of-hospital onset, no prior SE episode was independently associated with longer delays to administration of the first BZD, and intermittent rSE was independently associated with longer delays to administration of the first non-BZD AED. These factors identify potential targets for intervention to reduce time to treatment.
We found more delays to treatment administration when rSE was intermittent. A potential explanation for treatment delay may include the perception that the episode is spontaneously resolving if clinical seizures wax and wane rather than occurring continuously. This result identifies a potential target for intervention with education on the need for emergent treatment even if seizures are not occurring continuously but intermittently. Most delays in treatment occur out of the hospital and, as in other series,12,21 most SE and rSE episodes started out of the hospital. Hospitals currently have limited ability to start managing and treating patients with rSE continuously in the prehospital setting. Furthermore, in some states, rescue treatment via the EMS crew is restricted. Families and EMS may not be aware of the importance of a timely treatment. Therefore, delays in treatment administration may be attributable to complexities of a fragmented health care system and operational capacity (e.g., prompt availability of AEDs, pharmacy processing times) more than to physician willingness to promptly administer treatment. Among patients with out-of-hospital onset, those who received their first BZD at home or by EMS had much shorter time to first BZD than patients who received their first BZD at the hospital. Ensuring prompt initial treatment at home or by EMS might greatly reduce overall time to initial treatment. Because delays in treatment administration occur even in patients with a diagnosis of epilepsy,22 educational programs that emphasize the need for an emergent treatment of prolonged seizures out of the hospital may greatly reduce overall time to treatment. Despite most delays occurring out of the hospital, there is also room for improvement in the time to treatment for patients with in-hospital rSE onset. An active educational and interventional campaign focusing on the risk factors for delays and establishing acute treatment pathways similar to that of stroke or myocardial infarction care may reduce time to treatment.
The longer the seizures last, the more resistant they become to the initial SE treatments. The time-dependent pharmacoresistance to BZDs has been found in animal models,34–36 and clinical studies have shown that as seizures last longer, they often become self-sustained and progressively more resistant to treatment.11–13 In a series of 66 patients with generalized tonic-clonic SE, one of the factors associated with death was time to treatment administration.37 Within the pSERG series, AED administration delays are associated with higher mortality and poorer outcomes.21a
Despite the association of time to treatment with outcomes, delays in treatment administration still occur. In a series of 542 pediatric patients, the median time from arrival at the hospital to administration of a non-BZD AED was 24 minutes.19 In a study of 625 adults and 264 children with SE, approximately 60% of patients received their first AED after 30 minutes and approximately 25% after 60 minutes.18 Within the pSERG series, we previously showed that the median time to first, second, and third AED was 28 minutes, 40 minutes, and 59 minutes, respectively,21 and treatment delays occurred even in patients already diagnosed with epilepsy.22 The current analysis with 219 patients establishes that intermittent rSE and rSE onset out of hospital are independently associated with more delays to treatment administration.
The following factors were not independently associated with more delays in time to treatment: time of day or night, the first (2011–2014) vs the second (2015–2017) part of the cohort period, first half vs last half of the academic year, and white vs nonwhite race. Dichotomizing variables leads to some loss of information, but the number of predictors in the regression models had to be limited to prevent overfitting.
Our study population may or may not be representative of all children with SE. The pSERG consortium focuses on convulsive rSE, and our results are therefore not necessarily generalizable to patients with nonrefractory SE or with nonconvulsive SE. Times and the classification of rSE as continuous or intermittent were evaluated based on information provided by family and EMS for out-of-hospital onset and from provider information and hospital records once in the hospital. Because this method of data acquisition is subject to information and recall bias, we cross-referenced information with families, EMS records, nurses, and medication administration records when available to reduce bias. There are limitless potential factors accounting for time to treatment administration. We selected the most relevant potential predictors and potential confounders based on prior knowledge. Including more variables in the model might have resulted in overfitting and unstable results. In contrast, our results were stable in the whole population, the in-hospital and out-of-hospital subgroups, and robust to sensitivity analyses. Our results were also robust to correction for multiple testing.
Intermittent rSE and out-of-hospital rSE onset are independently associated with longer delays to administration of the first BZD and the first non-BZD AED in pediatric rSE.
Glossary
- AED
antiepileptic drug
- BZD
benzodiazepine
- CI
confidence interval
- EMS
emergency medical services
- HR
hazard ratio
- p25–p75
25th–75th percentiles
- pSERG
Pediatric Status Epilepticus Research Group
- rSE
refractory status epilepticus
- SE
status epilepticus
Footnotes
Podcast: NPub.org/so1tnv
Contributor Information
Collaborators: Pediatric Status Epilepticus Research Group (pSERG), Seema Bansal, Sarah Kelley, Carl Stafstrom, Eric Kossoff, Christa Habela, Dalila Lewis, Iván Sánchez Fernández, Marina Gaínza-Lein, Alejandra Vasquez, Marta Amengual-Gual, Michele Jackson, Justice Clark, Arnold Sansevere, Tobias Loddenkemper, Kush Kapur, J. Nicholas Brenton, Howard P. Goodkin, Mark Wainwright, Joshua Goldstein, Robert Faist, Katrina Peariso, Ravindra Arya, Tracy A. Glauser, Adam Ostendorf, Kevin E. Chapman, Amanda Weber, Asri Yuliati, Aimee Luat, Azara Singh, Dmrity Tchapyjnikov, Ashley Helseth, David Turner, Mohamad Mikati, Anne Anderson, Anuranjita Nayak, James J. Riviello, Jr, Kumar Sannagowdara, Kurt Hecox, Raquel Farias-Moeller, David Goldstein, Erin Heinzen Cox, Colin Malone, Tiffani L McDonough, Zachary Grinspan, Nicholas Abend, Alexis Topjian, Angus Wilfong, Korwyn Williams, Brian Appavu, Juan Piantino, Eric Payne, Lindsey Morgan, Edward Novotny, Rejéan Guerriero, Cecil Hahn, Linda Huh, Jessica Carpenter, Nathan Dean, and William Gaillard
Author contributions
I.S.F. participated in drafting and revising the manuscript for content, including medical writing, in study concept and design, data acquisition, analysis and interpretation of data, statistical analysis, and study supervision or coordination. M.G.-L. participated in drafting and revising the manuscript for content, including medical writing, in study concept and design, data acquisition, analysis and interpretation of data, and study supervision or coordination. N.S.A. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. A.E.A. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. R.A. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. J.N.B. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. J.L.C. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. K.E.C. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. J.C. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. W.D.G. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. T.A.G. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. J.L.G. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. H.P.G. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. A.R.H. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. M.C.J. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. K.K. participated in revising the manuscript for content, including medical writing, in study concept and design, analysis and interpretation of data, statistical analysis, and study supervision or coordination. Y.-C.L. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. T.L.M. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. M.A.M. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. A.N. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. K.P. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. J.J.R. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. R.C.T. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. D.T. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. A.A.T. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. M.S.W. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. A.W. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. K.W. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination. T.L. participated in revising the manuscript for content, including medical writing, in study concept and design, data acquisition, and study supervision or coordination.
Study funding
This study and consortium were funded by the Epilepsy Foundation of America (EF-213583, Targeted Initiative for Health Outcomes), by the American Epilepsy Society/Epilepsy Foundation of America Infrastructure Award, by the Pediatric Epilepsy Research Foundation, and the Epilepsy Research Fund.
Disclosure
I. Sánchez Fernández was funded by Fundación Alfonso Martín Escudero and the HHV6 Foundation. M. Gaínza-Lein, N. Abend, A. Anderson, R. Arya, J. Brenton, J. Carpenter, K. Chapman, and J. Clark report no disclosures relevant to the manuscript. W. Gaillard is an editor for Epilepsia and Epilepsy Research. T. Glauser is funded by NIH grants 2U01-NS045911, U10-NS077311, R01-NS053998, R01-NS062756, R01-NS043209, R01-LM011124, and R01-NS065840. He has received consulting fees from Supernus, Sunovion, Eisai, and UCB. He also serves as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medico-legal cases. He receives royalties from a patent license. J. Goldstein, H. Goodkin, A. Helseth, M. Jackson, K. Kapur, Y. Lai, T. McDonough, M. Mikati, A. Nayak, and K. Peariso report no disclosures relevant to the manuscript. J. Riviello is a member of a data safety monitoring board for GW Pharmaceuticals. His spouse is an editor for UpToDate. R. Tasker, D. Tchapyjnikov, and A. Topjian report no disclosures relevant to the manuscript. M. Wainwright serves as a scientific consultant and on the clinical advisory board for Sage Pharmaceuticals. A. Wilfong receives research funding from Novartis, Eisai, Pfizer, UCB, Acorda, Lundbeck, GW Pharma, Upsher-Smith, and Zogenix and receives publication royalties from UpToDate. K. Williams reports no disclosures relevant to the manuscript. T. Loddenkemper serves on the Laboratory Accreditation Board for Long Term (Epilepsy and Intensive Care Unit) Monitoring, on the Council (and as Vice President) of the American Clinical Neurophysiology Society, on the American Board of Clinical Neurophysiology, as an associate editor for Seizure, as contributing editor for Epilepsy Currents, and as an associate editor for Wyllie's Treatment of Epilepsy, 6th edition. He is part of pending patent applications to detect and predict seizures and to diagnose epilepsy. He receives research support from the Epilepsy Research Fund, the American Epilepsy Society, the Epilepsy Foundation of America, the Epilepsy Therapy Project, PCORI, the Pediatric Epilepsy Research Foundation, CURE, HHV-6 Foundation, and received research grants from Lundbeck, Eisai, Upsher-Smith, Acorda, and Pfizer. He serves as a consultant for Zogenix, Upsher-Smith, Eisai, Engage, Sunovion, and Lundbeck. He performs video-EEG long-term and ICU monitoring, EEGs, and other electrophysiologic studies at Boston Children's Hospital and affiliated hospitals and bills for these procedures, and he evaluates pediatric neurology patients and bills for clinical care. He has received speaker honorariums from national societies, including the AAN, AES, and ACNS, and for grand rounds at various academic centers. His wife, Dr. Karen Stannard, is a pediatric neurologist and she performs video-EEG long-term and ICU monitoring, EEGs, and other electrophysiologic studies and bills for these procedures, and she evaluates pediatric neurology patients and bills for clinical care. Go to Neurology.org/N for full disclosures.
References
- 1.Chin RF, Neville BG, Peckham C, et al. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet 2006;368:222–229. [DOI] [PubMed] [Google Scholar]
- 2.Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French-speaking Switzerland: (EPISTAR). Neurology 2000;55:693–697. [DOI] [PubMed] [Google Scholar]
- 3.Wu YW, Shek DW, Garcia PA, Zhao S, Johnston SC. Incidence and mortality of generalized convulsive status epilepticus in California. Neurology 2002;58:1070–1076. [DOI] [PubMed] [Google Scholar]
- 4.Loddenkemper T, Syed TU, Ramgopal S, et al. Risk factors associated with death in in-hospital pediatric convulsive status epilepticus. PLoS One 2012;7:e47474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maytal J, Shinnar S, Moshé SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics 1989;83:323–331. [PubMed] [Google Scholar]
- 6.Chin RF, Neville BG, Scott RC. A systematic review of the epidemiology of status epilepticus. Eur J Neurol 2004;11:800–810. [DOI] [PubMed] [Google Scholar]
- 7.Raspall-Chaure M, Chin RF, Neville BG, Scott RC. Outcome of paediatric convulsive status epilepticus: a systematic review. Lancet Neurol 2006;5:769–779. [DOI] [PubMed] [Google Scholar]
- 8.Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Hauser WA. Long-term mortality after a first episode of status epilepticus. Neurology 2002;58:537–541. [DOI] [PubMed] [Google Scholar]
- 9.Abend NS, Loddenkemper T. Pediatric status epilepticus management. Curr Opin Pediatr 2014;26:668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 11.Alldredge BK, Wall DB, Ferriero DM. Effect of prehospital treatment on the outcome of status epilepticus in children. Pediatr Neurol 1995;12:213–216. [DOI] [PubMed] [Google Scholar]
- 12.Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol 2008;7:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson K, Metsaranta P, Huhtala H, Auvinen A, Kuusela AL, Koivikko M. Treatment delay and the risk of prolonged status epilepticus. Neurology 2005;65:1316–1318. [DOI] [PubMed] [Google Scholar]
- 14.Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci 2005;25:5511–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maegaki Y, Kurozawa Y, Tamasaki A, et al. Early predictors of status epilepticus-associated mortality and morbidity in children. Brain Dev 2015;37:478–486. [DOI] [PubMed] [Google Scholar]
- 16.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 2005;25:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kämppi L, Mustonen H, Soinila S. Analysis of the delay components in the treatment of status epilepticus. Neurocrit Care 2013;19:10–18. [DOI] [PubMed] [Google Scholar]
- 18.Pellock JM, Marmarou A, DeLorenzo R. Time to treatment in prolonged seizure episodes. Epilepsy Behav 2004;5:192–196. [DOI] [PubMed] [Google Scholar]
- 19.Lewena S, Pennington V, Acworth J, et al. Emergency management of pediatric convulsive status epilepticus: a multicenter study of 542 patients. Pediatr Emerg Care 2009;25:83–87. [DOI] [PubMed] [Google Scholar]
- 20.Seinfeld S, Shinnar S, Sun S, et al. Emergency management of febrile status epilepticus: results of the FEBSTAT study. Epilepsia 2014;55:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez Fernández I, Abend NS, Agadi S, et al. Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology 2015;84:2304–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Gaínza-Lein M, Sánchez Fernández I, Jackson M, et al. Association of time to treatment with short-term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol 2018;75:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez Fernández I, Abend NS, Agadi S, et al. Gaps and opportunities in refractory status epilepticus research in children: a multi-center approach by the Pediatric Status Epilepticus Research Group (pSERG). Seizure 2014;23:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez Fernández I, Jackson MC, Abend NS, et al. Refractory status epilepticus in children with and without prior epilepsy or status epilepticus. Neurology 2017;88:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA 2000;283:2579–2584. [DOI] [PubMed] [Google Scholar]
- 25.Williams DR. Miles to go before we sleep: racial inequities in health. J Health Soc Behav 2012;53:279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 2014;67:850–857. [DOI] [PubMed] [Google Scholar]
- 27.R: A Language and Environment for Statistical Computing. Version 3.4.1. [software]. 2015. Vienna: R Foundation for Statistical Computing. Available at: R-project.org/. [Google Scholar]
- 28.RStudio: Integrated Development Environment for R [software]. 2015. Boston: RStudio. Available at: rstudio.com/. [Google Scholar]
- 29.gmodels: Various R Programming Tools for Model Fitting. Version 2.16.2 [software]. 2015. Available at: CRAN.R-project.org/package=gmodels. [Google Scholar]
- 30.Lubridate: Dates and Times Made Easy with Lubridate [software]. J Stat Softw 2011;40:1–25. Available at: jstatsoft.org/v40/i03/. [Google Scholar]
- 31.Gdata: Various R Programming Tools for Data Manipulation. Version 2.18.0. [software]. 2015. Available at: CRAN.R-project.org/package=gdata.
- 32.Car: An R Companion to Applied Regression [software]. 2011. Available at: socserv.socsci.mcmaster.ca/jfox/Books/Companion.
- 33.Survival: A Package for Survival Analysis in S. Version 2.38 [software]. 2015. Available at: CRAN.R-project.org/package=survival.
- 34.Goodkin HP, Kapur J. The impact of diazepam's discovery on the treatment and understanding of status epilepticus. Epilepsia 2009;50:2011–2018. [DOI] [PubMed] [Google Scholar]
- 35.Goodkin HP, Liu X, Holmes GL. Diazepam terminates brief but not prolonged seizures in young, naive rats. Epilepsia 2003;44:1109–1112. [DOI] [PubMed] [Google Scholar]
- 36.Jones DM, Esmaeil N, Maren S, Macdonald RL. Characterization of pharmacoresistance to benzodiazepines in the rat Li-pilocarpine model of status epilepticus. Epilepsy Res 2002;50:301–312. [DOI] [PubMed] [Google Scholar]
- 37.Sagduyu A, Tarlaci S, Sirin H. Generalized tonic-clonic status epilepticus: causes, treatment, complications and predictors of case fatality. J Neurol 1998;245:640–646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All statistical analyses and results are available in supplemental data (links.lww.com/WNL/A432). The original data are available on request.