SUMMARY
Head and neck (H&N) tumours are a heterogeneous group of neoplasms with 5-year relative survival ranging from about 25% for the hypopharynx to 60% for the larynx in Europe. To improve survival rates, along with therapeutic improvements, it is important to standardise and optimise care received by patients with H&N tumours across different healthcare providers. To reach this goal, it is necessary to evaluate adherence to standards of received care at a population level. Published guidelines can serve as the basis to develop indicators, which can be computed from administrative health databases, measuring the adherence to specific recommendations at the individual level in unselected H&N cancer patients, identified from a population cancer register. We developed a set of indicators and calculated them in a cohort of 2007-2012 incident cases of H&N tumours in the cancer register of the Milan province (n = 1441 cases). The study cohort was mainly composed of men (77%) and patients older than 50 years (89%). Surgery was the most frequently employed treatment (66%). Ten percent of patients had no recorded treatment. Timing between cyto-histological assessment and first therapy for those having a recorded microscopic verification procedure was ≤ 60 days for 90.4% of patients undergoing surgery, 86.3% of those undergoing radiotherapy, and 90.7% of patients receiving chemotherapy. Eighty-three percent of patients underwent cyto-histological assessment in the 180 days before the first treatment. Evaluation by a pain therapist, opioid therapy or hospitalisation for palliative therapy in the 90 days before death was performed in 51% of patients who eventually died of cancer. This is the first Italian study defining and calculating quality indicators to monitor adherence to standards of care received by H&N cancer patients at a population level.
KEY WORDS: Quality indicators, Head and neck neoplasms, Health care evaluation, Guideline adherence
RIASSUNTO
I tumori della testa e del collo (T&C) sono un gruppo eterogeneo di neoplasie che presentano ancora un elevato tasso di mortalità, con una sopravvivenza relativa a cinque anni che va da circa il 25% per l’ipofaringe al 60% per la laringe in Europa. Per incrementare ulteriormente i tassi di sopravvivenza, congiuntamente ai progressi diagnostici e terapeutici, è importante standardizzare e rendere aderente alle linee guida internazionali il percorso di diagnosi e cura a cui sono sottoposti i pazienti con tumore della T&C. Per raggiungere questo obiettivo, è necessario valutare il percorso di cura a livello dell’intera coorte dei pazienti con tumore della T&C di una determinata area geografica, determinata tramite un registro tumori di popolazione e quindi non selezionata. Le linee guida, nazionali ed internazionali, basate su evidenze scientifiche aggiornate possono servire da punto di partenza per sviluppare degli indicatori di aderenza calcolabili a partire dal registro tumori stesso e da database sanitari amministrativi correnti. Abbiamo sviluppato una serie di indicatori in grado di misurare, a livello individuale, l’adesione a raccomandazioni specifiche relative alla diagnosi ed al trattamento dei tumori della T&C. Li abbiamo poi calcolati nei pazienti con tumore della T&C individuati dal registro tumori della provincia di Milano (n = 1441 casi incidenti nel periodo 2007-2012). La coorte in studio è principalmente composta da uomini (77%) e pazienti di età superiore ai 50 anni (89%). Il dieci per cento dei pazienti non ha alcun trattamento registrato. Nei restanti pazienti, la chirurgia è stata il trattamento più frequentemente utilizzato (66%). Per quanto riguarda il tempo di attesa tra la valutazione cito-istologica e l’inizio della prima terapia, per i pazienti per cui è presente una verifica microscopica della neoplasia, esso è stato uguale o inferiore a 60 giorni per il 90,4% dei pazienti sottoposti come primo trattamento a chirurgia, per l’86,3% di quelli che hanno fatto radioterapia e per il 90,7% dei pazienti che hanno ricevuto chemioterapia. L’83% dei pazienti trattati ha effettuato una valutazione cito-istologica nei 180 giorni precedenti il primo trattamento. Tecniche di radioterapia ad intensità modulata (IMRT) sono state impiegate nel 37% dei pazienti trattati con radioterapia. Il 51% dei pazienti poi deceduti è stato valutato da un terapista del dolore, ha ricevuto oppiacei o è stato ricoverato nei 90 giorni precedenti l’exitus. Questo è il primo studio italiano che definisce e calcola un insieme di indicatori di aderenza, allo scopo di monitorare a livello di popolazione differenti aspetti del percorso di cura dei pazienti affetti da tumore T&C e potrebbe essere utilizzato come punto di partenza per monitorare l’aderenza alle linee guida a livello nazionale.
PAROLE CHIAVE: Indicatori di qualità del processo di cura, Neoplasie della testa e del collo, Valutazione delle cure, Aderenza alle Linee Guida
Introduction
Head and neck (H&N) tumours are a heterogeneous group of relatively infrequent neoplasms, with a worldwide age-standardised incidence rate of 14.3 per 100,000 in men and 4.4 per 100,000 in women 1. Mortality rates have been slowing decreasing in the last decades in western countries 1,2, presumptively due to advances in diagnosis and treatment 3-5, but also to the increase in the proportion of less aggressive HPV-related tumours 6. However, there is still a high local recurrence rate (40-60%) in patients with locally advanced tumours 5, i.e. stages III and IV according to American Joint Commission of Cancer – AJCC – classification 7th ed. 7, and 5-year relative survival across Europe ranges from about 25% for hypopharyngeal to 60% for laryngeal cancers 8. To further improve survival, it is important to deliver the best available care, optimising and standardising diagnosis and treatment received by patients with H&N tumours across different healthcare providers. An evaluation of adherence to standards of the care received, at a population level and in recent years, is necessary to detect deviations from international recommendations and take actions to improve the delivered care.
A recently published study has investigated the causes of deviation from guidelines in patients with H&N tumours, and found that patients not receiving standard treatment have a lower 3-year survival rate 9. Among factors associated to non-adherence to guidelines there are gender, age, socio-economic conditions and the presence of comorbidities 9. Concurrent chronic diseases are frequent in patients with H&N tumours, both because of advanced mean age at diagnosis and the high prevalence of tobacco smoking and alcohol consumption 9,10. In this group of tumours there are different entities, both in terms of aetiology (i.e. alcohol and tobacco vs. human papilloma virus) and site-related histology (i.e. salivary gland tumours vs. squamous cell carcinoma in the upper aerodigestive tract). According to this heterogeneity, recently developed guidelines such as those of the UK National Institute for Health and Care Excellence (NICE) and the Associazione Italiana di Oncologia Medica (AIOM) include recommendations common to all sites and aetiologies, and others that are specific for tumour site, stage and histology 11,12.
Indicators used in public health are measures, often proportions, meant to describe the quality of care for a group of patients. They can be compiled from either clinical or administrative data that has been recorded about particular aspects of care. They may evaluate structures, processes or outcomes of care, and are usually aimed at evaluating the quality of the delivered care to inform improvement activities 13,14.
Evidence-based guidelines can serve as the basis to develop process indicators, which can be computed from administrative health databases, measuring adherence to specific recommendations at the individual level in unselected H&N cancer patients, identified from a population cancer register. We decided to use NICE and AIOM guidelines 11,12 as they both cover the different aspects of care and provide documentation of the applied methodology, for selected indicators we also used additional guidelines (Appendix: Supplementary Table I). To implement a set of indicators capable of monitoring different aspects of care, we linked information included in the cancer register with administrative databases, stratifying for tumour site, age, gender and Charlson comorbidity index 15. Our aim is to describe the developed set of indicators and discuss their values as calculated in the cohort of the 2007-2012 incident cases of H&N tumours in the cancer register of the Milan province (Lombardy region, Italy).
Table. I.
Patient and tumour characteristics from the cohort of head and neck 2007-2012 incident cancers, non-metastatic at diagnosis (N = 1441), from the nationally accredited cancer register of the Milan province, Italy.
| No. | % | |
|---|---|---|
| Year of incidence | ||
| 2007 | 246 | 17.1 |
| 2008 | 266 | 18.5 |
| 2009 | 252 | 17.5 |
| 2010 | 195 | 13.5 |
| 2011 | 261 | 18.1 |
| 2012 | 221 | 15.3 |
| Age class | ||
| ≤ 40 years | 43 | 3.0 |
| 41-50 | 120 | 8.3 |
| 51-55 | 131 | 9.1 |
| 56-60 | 190 | 13.2 |
| 61-65 | 230 | 15.9 |
| 65-70 | 246 | 17.1 |
| 71-75 | 203 | 14.1 |
| > 75 | 278 | 19.3 |
| Gender | ||
| Male | 1110 | 77.0 |
| Female | 331 | 23.0 |
| Charlson index | ||
| 0 | 865 | 60.0 |
| 1-2 | 448 | 31.1 |
| ≥ 3 | 128 | 8.9 |
| Site | ||
| Oral cavity (C00-06) | 469 | 32.5 |
| Salivary glands (C07-08) | 79 | 5.5 |
| Oropharynx (C09-10) | 167 | 11.6 |
| Nasoharynx (C11) | 65 | 4.5 |
| Hypopharynx (C12-13) | 82 | 5.7 |
| Larynx (C32) | 567 | 39.4 |
| Other and ill-defined sites (C14) | 12 | 0.8 |
| Histology | ||
| Malignant tumour, not specified | 60 | 4.2 |
| Squamous cell carcinoma | 1245 | 86.4 |
| Adeno, muco, acinar, cystic carcinomas | 81 | 5.6 |
| Others* | 55 | 3.8 |
| First treatment | ||
| No recorded treatment | 142 | 9.9 |
| Surgery | 852 | 59.1 |
| Radiotherapy | 396 | 27.5 |
| CT alone | 51 | 3.5 |
| Total number of patients | 1441 |
*Including mixed and undifferentiated.
Abbreviations: RT = radiotherapy; CT = chemotherapy.
Materials and methods
Selection and description of the cohort
The cohort included all patients resident in the Milan province and registered with the regional health service developing an H&N cancer (ICDO-3 topographic codes 16: C00-06 oral cavity, C07-08 salivary glands, C09-13 pharynx, C32 larynx; C14 other and ill-defined sites in lip, oral cavity and pharynx) in the period 2007-2012. Nose and paranasal sinuses tumours were not included, as it is usual practice in tumour registry reports, because they are rare and often occupational cancers 17. The Milan province includes 14 municipalities around Milan in Northern Italy and had a population of 1,546,237 inhabitants on 1 Jan. 2013, 754,821 males and 791,416 females 18. The cancer register is nationally accredited and partially automated, using multiple sources of information (i.e. inpatient, histopathology and death certificate databases) and a record linkage algorithm to match all information at the individual level. The date of incidence was defined, according to international cancer registration rules 19, as the first available date among those of pathological examination, clinical diagnosis or death. Exclusion criteria were: previous malignant tumour (from 1996 to 3 months before diagnosis, excluding non-melanoma skin cancers), tumours identified only through death certificate and distant metastases at diagnosis. The latter were identified through the register and the inpatient database, searching in records of hospitalisation – occurred from 45 days prior to 180 days after H&N cancer diagnosis – ICD-9 codes 197 and 198 in any of the diagnosis fields.
Identification of the set of indicators and calculation at patient level
Referring mainly to the comprehensive NICE and AIOM guidelines 11,12, but also to specific guidelines reported in Supplementary Table I, a group of epidemiologists and a multidisciplinary team of surgeons, medical and radiation oncologists, which are members of the Associazione Italiana di Oncologia Cervico-Cefalica, Associazione Italiana di Radioterapia Oncologica, Associazione Italiana di Oncologia Medica, and Società Italiana di Otorinolaringologia e Chirurgia Cervico Facciale, developed a set of process indicators. All indicators, each measuring adherence to a selected guideline, had to be computable from the available administrative data. In order to calculate the indicators at the patient level, we used all available computerised sources of health information of the Lombardy region from January 2006 to December 2014. These sources included: inpatient database (SDO), prescription databases, database of outpatient diagnostic and therapeutic procedures. The aim was to trace different facets of care, from organisational aspects, to diagnosis and treatment, and to use multiple independent sources of information to improve the reliability of the indicators.
Vital status was updated at 30 Dec. 2015, using the database of the people registered with the Regional Health Service (Nuova Anagrafe Regionale), where an update is performed every 6 months covering at least 95% of deaths. We derived gender and age at diagnosis from the register. On the available administrative databases, both in and outpatients, we also calculated the Charlson comorbidity index 15,20. From the administrative databases, we also derived type and date of the first administered therapy and hospital where it was administered. First therapy, as used in the calculation of the indicators, does not consider combined therapies and was defined as following: we identified all therapeutic events i.e. surgery, radiotherapy (RT) or chemotherapy (CT) in the 180 days preceding and following the date of diagnosis recorded in the register. We then assigned the patient to the surgery category if he/she had undergone, in the defined time-window, any of the following procedures (ICD-9 codes) irrespective of RT or CT: oral cavity, 24.31, 25.1-25.4, 27.3x, 27.42, 27.43, 27.49, 27.72; salivary glands, 26.29 26.3x; pharynx, 28.2-28.6, 29.3x; larynx, 30.1-30.4, 30.09; facial bones: 76.2x-76.6x, 76.9x; soft tissues 83.49. If the patient had not received surgery but RT (from inpatient database: V58.0, 92.2; from outpatient treatment database: 92.23.1 to 92.27.5, 92.29), we assigned him/her to the RT category regardless of having performed also CT. If the patient had not received surgery nor RT but CT only, we assigned him/her to CT (from inpatient database: V58.1, 99.25; from outpatient treatment database: 99.25, MAC01, MAC02, MAC04; from database of prescription ‘file F’, ATC code: LO1).
To assess the cyto-histological confirmation of the tumour, we searched for the codes reported in Table II, footnote *. In a sample of revised medical records, we noticed that the biopsy performed during an endoscopy was not always coded. Consequently, we calculated two versions of the indicators involving cyto-histological evaluation (S1, S2, S4, D1): one using biopsy codes only (Table II, footnote *) and the other assuming that endoscopies of the pharynx and larynx (ICD-9 codes 29.11 and 31.42) were concomitant with a non-coded cyto-histological assessment. The true value of the S1, S2, S4, and D1 indicators is expected to be between the two versions of the indicator. Two indicators, T1 and T2, are technically outcome indicators by they are commonly used as quality indicators for surgery 21,22, even if they need to be interpreted with caution. Deterministic record linkage on a unique key was used to match all information at the patient level. Record linkage was performed at the local health authority of Milan, according to the national legislation on sensitive data 23.
Table. II.
Definition and values of the indicators evaluating time between diagnosis and treatment, and between treatments.
| Indicator code | Description Proportion of patients having an: | No. of eligible pts | Value (%) |
|---|---|---|---|
| S1 | Interval between cyto-histological assessment* and surgery as the first treatment ≤ 60 days | 665† | 90.4 |
| S2 | Interval between cyto-histological assessment* and RT as the first treatment ≤ 60 days | 357† | 86.3 |
| S3 | Interval between discharge from primary surgery and postoperative RT ≤ 60 days | 302 | 69.9 |
| S4 | Interval between cyto-histological assessment* and CT as the first treatment ≤ 60 days | 43† | 90.7 |
*ICD-9 codes. Biopsies: cranial nerves, 04.11, 04.12, 05.11; oral cavity, 24.11, 25.01, 25.02, 27.21-27.24; salivary glands, 26.11, 26.12; pharynx, 28.11, 29.12; larynx, 31.43, 31.45; facial bones, 76.11; soft tissues, 83.21; lymph-nodes, 40.11. Microscopic examination of specimen from: ear, nose, throat, and larynx, 90.3; lymph-nodes, 90.7. Pharyngoscopy: 29.11; Laryngoscopy: 31.42.
†The eligible patients where those having a procedure of those included in note * in the 180 days before the first treatment, i.e. patients for whom there was no record of a cyto-histological assessment were not included. Also the 9 patients undergoing surgery after adjuvant RT or CT were excluded from the denominator of S1.
Abbreviations: RT = radiotherapy; CT = chemotherapy.
Statistical analysis
The characteristics of the included patients and tumours are described using percentages. For all indicators, we calculated the proportion of patients who received the procedure, in the defined time window, among those eligible. Indicators of diagnosis and treatment are presented overall and stratified for tumour site. All indicators were also stratified by: gender, age (< 60, 60-70 and > 70 years), and comorbidities (Charlson index 0 vs ≥ 1). Overall survival was estimated using the Kaplan-Meier method 24. To investigate the association between respecting each guideline and survival, we fitted a separate Cox model for each indicator (indicators measuring proportion of death were excluded), first without covariates and then adjusting for gender, age, Charlson index and tumour site. This analysis does not aim to establish causal relationships, as important confounders as stage at diagnosis and socioeconomic status are not accounted for. Results are presented as hazard ratio (HR) of death for not fulfilling vs. fulfilling the guideline with its 95% confidence interval (CI). All analyses were performed with SAS software (v.9.4, SAS Institute, Cary NC).
Results
Population
From 1st January 2007 to 31st December 2012 there were 1,650 incident cases of invasive H&N cancers in the register (Fig. 1). After exclusions (DCO, n = 7; multiple primary cancers n = 108; patients with distant metastasis at diagnosis, n = 94), the analysed cohort included 1,441 cases, whose characteristics are reported in Table I.
Fig. 1.
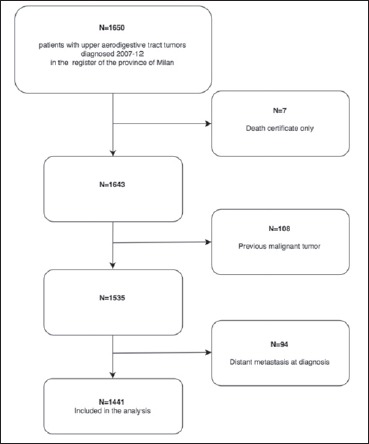
Sample selection flow-chart.
The study cohort was mainly composed of men (77.0%). Eighty-nine percent of patients were 51 years or older and 40% had at least one comorbidity. Concerning site, the most frequent were laryngeal (39.4%) and oral (32.5%) cancers. By far the most frequent histological type was squamous cell carcinoma (86.4%). Ten percent of patients (n = 142) had no recorded treatment. However, this figure includes those patients having a clinically believed benign tumour, which then resulted malignant at pathological examination, and was completely excised during the diagnostic procedure and consequently required no further treatment. Among treated patients, surgery was the most frequently employed treatment (65.6%).
Considering the hospitals delivering surgery, the number of any head and neck therapeutic surgical procedures in H&N patients per provider in a year, calculated on 2015, varied from to 1 to 78. RT treatments were performed in centers with an annual volume of procedures for all cancers varying from 7,580 to 64,198 (measured for 2016).
Identified set of indicators
The identified indicators were organised into three groups: 1) indicators measuring the time between diagnosis and relevant procedures or treatments (Table II), 2) indicators evaluating diagnostic procedures (Table IIIa) and 3) indicators evaluating therapeutic procedures (Table IIIb). No follow-up indicators were calculated because of the absence of high level evidence on the type and timing of follow-up 11.
Table IIIa.
Definition and values of the indicators evaluating diagnostic procedures, overall and across tumour types.
| Indicator code | Description Proportion of patients: | No. eligible overall | Value (%) | ||||
|---|---|---|---|---|---|---|---|
| Oral cavity N = 469 |
Salivary glands N = 79 |
Pharynx N = 314 |
Larynx N = 567 |
Overall | |||
| D1 | Having performed a cyto-histological assessment* of the primary tumour in the 180 days before the first treatment | 1299 | 77.7 | 77.1 | 90.6 | 82.7 | 82.6 |
| D2 | With distant metastasis risk tumour type† undergoing systemic staging with PET-CT or whole body computed tomography | 249 | 41.0 | 41.0 | |||
*ICD-9 codes. Biopsies: cranial nerves, 04.11, 04.12, 05.11; oral cavity, 24.11, 25.01, 25.02, 27.21-27.24; salivary glands, 26.11, 26.12; pharynx, 28.11, 29.12; larynx, 31.43, 31.45; facial bones, 76.11; soft tissues, 83.21; lymph-nodes, 40.11. Microscopic examination of specimen from: ear, nose, throat, and larynx, 90.3; lymph-nodes, 90.7. Pharyngoscopy: 29.11; Laryngoscopy: 31.42.
† rhino and hypo-pharynx.
Table IIIb.
Definition and values of the indicators evaluating treatment procedures, overall and across tumour types.
| Indicator code | Description Proportion of patients: |
No. eligible overall | Value (%) | ||||
|---|---|---|---|---|---|---|---|
| Oral cavity N = 469 |
Salivary glands N = 79 |
Pharynx N = 314 |
Larynx N = 567 |
Overall | |||
| T1 | Deceased in perioperative period (≤15 days from surgery) | 852 | 1.6 | 1.1 | 0.3 | 0.8 | |
| T2 | With a second hospital access ≤ 30 days from discharge for primary surgery | 852 | 15.1 | 10.2 | 19.1 | 27.1 | 20.8 |
| T3 | Treated with IMRT* | 706 | 32.4 | 37.2 | 51.1 | 26.4 | 36.8 |
| T4 | Deceased and evaluated by pain therapist, under opioids or hospitalised for palliation in the 90 days before† | 606 | 56.5 | 67.9 | 53.3 | 40.9 | 51.0 |
*IMRT codes 92.24.6, 92.29K e 92.29L.
†ICD9 codes 89.01.1,89.70.1, 92.28.6 in the outpatient procedures, ATC code N02A* in prescription database, V66.7 in inpatient database.
Abbreviations: PET-CT = Positron Emission Tomography-Computed Tomography, IMRT = Intensity Modulated Radiotherapy, CT = chemotherapy.
Concerning timing between diagnosis and surgery as the first treatment (Table II), 90.4% of patients having a cyto- or histological examination record (n = 665) had an interval between the two events shorter than or equal to 60 days (S1, when calculated not including fibroscopy only codes: 73.8%), and 68.9% shorter than or equal to 30 days (when calculated not including fibroscopy only codes: 55.3%). For patients receiving radiotherapy as the first treatment, 86.3% of patients having a cyto or histological examination record (n = 357) had an interval between the two events shorter than or equal to 60 days (S2, when calculated not including fibroscopy codes: 78.7%), and 48.7% shorter than or equal to 30 days (when not including fibroscopy codes: 37.0%). When RT was performed after surgery (n = 302), the interval between discharge after the surgical intervention and RT was lower or equal than 60 days in 69.9% of patients (S3). For those undergoing CT, 90.7% of patients having a cyto- or histological examination record (n = 43) had an interval between the two events shorter than or equal to 60 days (S4, when calculated not including fibroscopy codes: 83.7%), and 32.6% shorter than or equal to 30 days (when calculated not including fibroscopy codes: 25.6%). Variations across gender, age and comorbidity level (Table IV) were negligible for the interval between cyto-histological assessment and surgery (S1). For RT (S2), the proportion was lower for patients with comorbidities (80.6% for Charlson index ≥ 1 vs. 90.9% for patients with 0). Concerning CT (S4), the proportion was lower for older patients (92.3% for over 70 year olds vs 100% for 60 year olds or younger).
Table. IV.
Values of the indicators stratified for gender, age group and Charlson index.
| Indicator code |
Value (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gender | Age group | Charlson index | ||||||
| Male N = 1110 |
Female N = 331 |
≤ 60 N = 484 |
61-70 N = 476 |
> 70 N = 481 |
0 N = 865 |
≥ 1 N = 576 |
||
| S1 | Interval between cyto-histological assessment and surgery as the first treatment ≤ 60 days | 91.2 | 87.4 | 87.9 | 94.6 | 88.6 | 90.8 | 89.8 |
| S2 | Interval between cyto-histological assessment and RT as the first treatment ≤ 60 days | 85.2 | 90.4 | 87.6 | 86.7 | 84.3 | 90.9 | 80.6 |
| S3 | Interval between discharge from primary surgery and postoperative RT ≤ 60 days | 69.8 | 70.0 | 69.6 | 67.8 | 72.9 | 70.6 | 68.8 |
| S4 | Interval between cyto-histological assessment and CT as the first treatment ≤ 60 days | 87.5 | 100.0 | 100.0 | 81.3 | 92.3 | 91.3 | 90.0 |
| D1 | Having performed a cyto-histological assessment of the primary tumour in the 180 days before the first treatment | 82.9 | 81.5 | 83.5 | 83.1 | 81.1 | 81.3 | 84.6 |
| D2 | With distant metastasis risk tumour type undergoing systemic staging with PET-CT or whole body computed tomography | 40.6 | 41.9 | 41.8 | 50.6 | 29.3 | 35.2 | 49.0 |
| T1 | Deceased in perioperative period (≤ 15 days from surgery) | 0.6 | 1.5 | 0.4 | 0.4 | 1.7 | 0.8 | 0.9 |
| T2 | With a second hospital access ≤ 30 days from discharge for primary surgery | 22.5 | 14.9 | 19.8 | 22.4 | 20.1 | 19.7 | 22.5 |
| T3 | Treated with IMRT |
36.2 | 39.1 | 47.4 | 36.3 | 24.9 | 34.3 | 40.2 |
| T4 | Deceased and evaluated by pain therapist, under opioids or hospitalised for palliation in the 90 days before | 50.7 | 51.9 | 61.6 | 52.1 | 44.2 | 55.4 | 46.5 |
Regarding indicators measuring diagnostic procedures (Table IIIa), 82.6% of patients underwent cyto-histological assessment in the 180 days before first treatment (D1, when calculated not including fibroscopy codes: 71.8%): 78.9% of those undergoing surgery, 90.1% of those receiving RT, and 84.3% of patients submitted to CT. The percentage varied from 77.1% for salivary glands to 90.6% for pharyngeal tumours, and it was fairly stable across gender, age and Charlson index (Table IV).
Forty-one percent of patients with a tumour site at high risk of distant metastasis (naso- and hypo-pharyngeal cancers) underwent systemic staging (D2) before treatment, less frequently (29.3%) patients over 70 years and more frequently those with comorbidities (49.0%, Table IV).
Concerning treatment indicators (Table IIIb), 0.8% of patients died in the perioperative period (≤ 15 days from curative surgery, T1); 28.8% of patients had a second hospital access within 30 days from discharge after surgery as the first treatment (T2), excluding hospitalisations for RT and CT, plastic surgery, prosthesis positioning, stoma checking and palliative care. Women had a second hospital access less frequently (14.9%, Table IV). In the cohort of patients that underwent RT, intensity modulated RT (IMRT) techniques were used in 36.8% of patients undergoing RT (T3). Fifty-one percent of deceased patients were evaluated by a pain therapist, under opioids or hospitalised for palliation in the 90 days before death (T4), less frequently if they had comorbidities (46.5%) or were over 70 years (44.2%, Table IV).
Five-year overall survival was 60.6% (95% CI, 57.9-63.1%). Median follow-up time was 6.1 years (95% CI, 5.8-6.2 years). An interval between RT and cyto-histological assessment of the primary tumour longer than 60 days (S2) was significantly associated with a higher risk of death only in the unadjusted model (HR, 1.57; 95% CI, 1.05-2.34, Table V).
Table. V.
Hazard ratio (HR) of death for indicator not fulfilled vs. fulfilled. The adjusted model includes gender, age, Charlson index and tumour site as covariates.
| Indicator | Unadjusted model | Adjusted model | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| S1 | Interval between cyto-histological assessment and surgery as the first treatment ≤ 60 days | 0.99 | 0.64 | 1.54 | 0.97 | 0.62 | 1.51 |
| S2 | Interval between cyto-histological assessment and RT as the first treatment ≤ 60 days | 1.57 | 1.05 | 2.34 | 1.25 | 0.81 | 1.92 |
| S3 | Interval between discharge from primary surgery and postoperative RT ≤ 60 days | 0.81 | 0.55 | 1.19 | 0.79 | 0.53 | 1.18 |
| S4 | Interval between cyto-histological assessment and CT as the first treatment ≤ 60 days | 1.01 | 0.31 | 3.36 | 0.70 | 0.20 | 2.43 |
| D1 | Having performed a cyto-histological assessment of the primary tumour in the 180 days before the first treatment | 1.01 | 0.80 | 1.26 | 1.00 | 0.80 | 1.27 |
| D2* | With distant metastasis risk tumour type undergoing systemic staging with PET-CT or whole body computed tomography | 0.97 | 0.68 | 1.38 | 0.93 | 0.65 | 1.33 |
| T2 | With a second hospital access ≤ 30 days from discharge for primary surgery | 1.01 | 0.76 | 1.34 | 0.99 | 0.74 | 1.32 |
| T3 | Treated with IMRT | 1.26 | 0.99 | 1.59 | 1.20 | 0.94 | 1.54 |
*not adjusted for tumour site.
Abbreviations: HR = hazard ratio, CI: = confidence interval.
Discussion
In the last decade there has been much work intended to evaluate the quality of care delivered to oncologic patients using administrative databases 13,25, although H&N cancers have seldom been considered 26. This is the first study aiming at identifying quality indicators for H&N cancer from routinely available administrative health data and a cancer registry in Italy. Those indicators have been designed and interpreted together by epidemiologists and health professionals directly involved in the care of patients, and may serve as the basis to define quality standards both in Lombardy region and Italy. The relative rarity and heterogeneity of H&N tumours creates additional problems in determining guidelines common to all sites and consequently in developing indicators to evaluate adherence to guidelines. We implemented a set of indicators to collectively monitor adherence to important diagnostic and therapeutic guidelines for H&N tumours, using data included in a cancer register and administrative health databases, and tested it on the cohort of 2007-2012 incident cases of the Milan province.
In this cohort, 54% of treated patients received a single treatment modality, which was surgical intervention in 40% of cases. The remaining 46% of patients received a multimodal treatment. This remarks the importance of a multidisciplinary approach, especially in patients with more advanced stages 27,28. Multidisciplinary evaluation is recommended in guidelines 11,12 and is an important quality indicator to measure. However, for the analysed years, it could not be monitored reliably using administrative data. Surgery was characterised by a short waiting time, lower than 30 days from diagnosis for almost 70% of patients receiving it as first treatment. The indicator measuring a second hospital access within 30 days from discharge after surgery (21%) needs to be interpreted with caution, as it does not distinguish between unplanned, i.e. radicalisations or complications, and planned readmissions such as a programmed second surgical procedure as a part of the primary intervention e.g. neck dissection after transoral glossectomy, second-look cordectomy after laser microresection of glottic carcinoma involving the anterior commissure.
When RT was the first therapy, only 49% of patients started it within 30 days from diagnosis. A multidisciplinary approach could also lower this waiting time, because patients are almost always referred to RT by other specialists (e.g. surgeon, medical oncologists) and this implies a delay between diagnosis and the start of RT 27-30. Similar considerations apply when interpreting that about 70% of patients started the postoperative RT within 60 days from discharge after surgery. According to international guidelines 11, RT should start as soon as possible ideally within 6 weeks from surgery. However, some clinical factors such as postoperative complications as well as logistic challenges (e.g. limited number of RT unit, arrangements for concomitant CT) could explain our finding. Regarding the type of radiation treatment, considering the period analysed in this study (i.e. from 2007 to 2012) it is not surprising that only 39% of patients were treated with more recent and advanced approaches like IMRT, which have diffused in recent years 31. Almost 20% of patients, even assuming that pharyngoscopy and laryngoscopy close to the diagnosis included a non-coded cyto-histological assessment, had no recorded microscopic verification procedure before treatment. These cases would need a more detailed ascertainment of clinical records, as there may be patients undergoing a single diagnostic-therapeutic procedure, i.e. very small lesions on which a biopsy would produce macroscopic changes, tumours in sites that would require general anaesthesia for both biopsy and treatment, such as glottis erythroleukoplakia. Also, as in all studies using administrative databases, there could be a minimal loss of information due to private healthcare providers.
We are well aware of the limitations of our study that derive from the use of the administrative data. Not all the indicators judged to be important to monitor the care process can be calculated because of lack of information in the routinely collected health data of a particular health system. In this case, the availability of stage at diagnosis would have allowed to calculate additional stage-specific indicators, to better monitor the therapeutic process. In addition, the relatively small number of cases does not allow to investigate predictors of adherence to guidelines in a multivariate model in order to evaluate the impact of the hospital case volume, year of diagnosis and other factors that most probably are associated with treatment outcome.
This type of approach on a larger cohort of patients could also allow evaluation of the relationship between time from diagnosis to treatment, or among different treatments, and outcome.
Conclusions
This is the first study in Italy defining and calculating from a cancer register and administrative health data indicators of adherence to guidelines in H&N cancer patients, and could be the starting point to propose indicators to inform health policies at the national level.
Acknowledgements
Supported by grant RF-2011-02348959 from the Italian Ministry of Health.
APPENDIX: Supplementary Table I.
Evidence of the recommendations evaluated by the developed indicators.
| Indicator code |
Description Proportion of patients having an: |
Recommendation | Evidence type and strength (grade) |
|---|---|---|---|
| S1 | Interval between cyto-histological assessment and surgery as the first treatment ≤ 60 days | Cancer care needs to be timel 1 | Meta-analysis 2 Moderate |
| S4 | Interval between cyto-histological assessment and CT as the first treatment ≤ 60 days | ||
| S2 | Interval between cyto-histological assessment and RT as the first treatment ≤ 60 days | Cancer care needs to be timely 1 | Meta-analysis 3 Strong |
| S3 | Interval between discharge from primary surgery and postoperative RT ≤ 60 days | Time between surgery and start of radiotherapy should be less than 6 weeks 4 | Observational studies 5,6 Week |
| D1 | Having performed a cyto-histological assessment of the primary tumor in the 180 days before the first treatment |
A clinically suspected diagnosis of malignancy should be confirmed by biopsy or cytology before operation 7 | No specific studies Week |
| D2 | With distant metastasis risk tumor type undergoing systemic staging with PET-CT or whole body computed tomography | Offer systemic staging to all people with cancer of the upper aerodigestive tract except those with T1N0 or T2N0 disease. Offer FDG PET-CT to people with T4 cancer of the hypopharynx or nasopharynx. Offer FDG PET-CT to people with N3 cancer of the upper aerodigestive tract 8 |
RCTs and observationals 8 High |
| T1 | Deceased in perioperative period (≤ 15 days from surgery) | Used as a proxy for adequate pre-surgical evaluation and quality of surgery 9 |
Expert opinion 10 Week |
| T2 | With a second hospital access ≤ 3 days from discharge for primary surgery | Used as a proxy for adequate pre-surgical evaluation and quality of surgery 11,12 |
Observational studies 13 Week |
| T3 | Treated with IMRT | IMRT therapy is recommended for pharyngeal cancer 4 |
Observational studies 14,15 Week |
| T4 | Deceased and evaluated by pain therapist, under opioids or hospitalized for palliation in the 90 days before | Patients with advanced cancer, whether patient or outpatient, should receive dedicated palliative care services, early in the disease course, concurrent with active treatment 16 |
Systematic review 17 Strong |
References
- 1. Institute of Medicine Committee on Quality of Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington (DC): National Academies Press. [Google Scholar]
- 2. Seoane J, Takkouche B, Varela-Centelles P, et al. Impact of delay in diagnosis on survival to head and neck carcinomas: a systematic review with meta-analysis. Clin Otolaryngol Off J ENT-UK Off J Neth Soc Oto-Rhino-Laryngol Cervico-Facial Surg 2012;37:99-106. [DOI] [PubMed] [Google Scholar]
- 3. Chen Z, King W, Pearcey R, et al. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol 2008;87:3-16. [DOI] [PubMed] [Google Scholar]
- 4. Associazione Italiana di Oncologia Medica. Tumori della testa e del collo. 2016. [Google Scholar]
- 5. Vikram B. Importance of the time interval between surgery postoperative radiation therapy in the combined management of head & neck cancer. Int J Radiat Oncol Biol Phys 1979;5:1837-40. [DOI] [PubMed] [Google Scholar]
- 6. Parsons JT, Mendenhall WM, Stringer SP, et al. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys 1997;39:137-48. [DOI] [PubMed] [Google Scholar]
- 7. Helliwell TR, Giles TE. Pathological aspects of the assessment of head and neck cancers: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol 2016;130:S59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence. Cancer of the upper aerodigestive tract: assessment and management in people aged 16 and over NICE Guideline 36 Full guideline. [PubMed] [Google Scholar]
- 9. AHRQ Quality Indicators. Available from: http://www.qualityindicators.ahrq.gov/Modules/iqi_resources.aspx. [Google Scholar]
- 10. Watters DA, Hollands MJ, Gruen RL, et al. Perioperative mortality rate (POMR): a global indicator of access to safe surgery and anaesthesia. World J Surg 2015;39:856-64. [DOI] [PubMed] [Google Scholar]
- 11. NRD. Overview. Available from: https://www.hcup-us.ahrq.gov/nrdoverview.jsp. [Google Scholar]
- 12. Tsai TC, Joynt KE, Orav EJ, et al. Variation in surgical-readmission rates and quality of hospital care. N Engl J Med 2013;369:1134-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer C, Lingsma HF, Marang-van DM, et al. Is the readmission rate a valid quality indicator? A review of the evidence. PLoS ONE 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pacholke HD, Amdur RJ, Morris CG, et al. Late xerostomia after intensity-modulated radiation therapy versus conventional radiotherapy. Am J Clin Oncol 2005;28:351-8. [DOI] [PubMed] [Google Scholar]
- 15. Vergeer MR, Doornaert PAH, Rietveld DHF, et al. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys 2009;74:1-8. [DOI] [PubMed] [Google Scholar]
- 16. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2016;35:96-112. [DOI] [PubMed] [Google Scholar]
- 17. Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med 2008;148:147. [DOI] [PubMed] [Google Scholar]
References
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon, France. Int. Agency Res. Cancer 2013. Available from: http://globocan.iarc.fr [Google Scholar]
- 2. Associazione Italiana Registri Tumori. Incidence and mortality data of the Cancer Registries, 1998-2002. 2006. Available from: http://www.registri-tumori.it/cms/?q=Indice2006 [Google Scholar]
- 3. D’Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 2015;373:521-9. [DOI] [PubMed] [Google Scholar]
- 4. Forastiere AA, Weber RS, Trotti A. Organ preservation for advanced larynx cancer: issues and outcomes. J Clin Oncol Off J Am Soc Clin Oncol 2015;33:3262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Machiels J-P, Lambrecht M, Hanin F-X, et al. Advances in the management of squamous cell carcinoma of the head and neck. F1000prime Rep 2014;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc 2016;91:386-96. [DOI] [PubMed] [Google Scholar]
- 7. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol 2010;17:1471-4. [DOI] [PubMed] [Google Scholar]
- 8. Gatta G, Botta L, Sánchez MJ, et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur J Cancer 2015;51:2130-43. [DOI] [PubMed] [Google Scholar]
- 9. Dronkers EAC, Mes SW, Wieringa MH, et al. Noncompliance to guidelines in head and neck cancer treatment; associated factors for both patient and physician. BMC Cancer 2015;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bøje CR, Dalton SO, Primdahl H, et al. Evaluation of comorbidity in 9388 head and neck cancer patients: a national cohort study from the DAHANCA database. Radiother Oncol J Eur Soc Ther Radiol Oncol 2014;110:91-7. [DOI] [PubMed] [Google Scholar]
- 11. National Institute for Health and Care Excellence. Cancer of the upper aerodigestive tract: assessment and management in people aged 16 and over NICE Guideline 36 Full guideline. Available from: https://www.nice.org.uk/guidance/ng36 [PubMed] [Google Scholar]
- 12. Associazione Italiana di Oncologia Medica. Tumori della testa e del collo. 2016. Available from: http://www.aiom.it/professionisti/documenti-scientifici/linee-guida/testa-collo/1,711,1 [Google Scholar]
- 13. Albert JM, Das P. Quality assessment in oncology. Int J Radiat Oncol Biol Phys 2012;83:773-81. [DOI] [PubMed] [Google Scholar]
- 14. Freeman T. Using performance indicators to improve health care quality in the public sector: a review of the literature. Health Serv Manage Res 2002;15:126-37. [DOI] [PubMed] [Google Scholar]
- 15. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. [DOI] [PubMed] [Google Scholar]
- 16. International Classification of Diseases for Oncology. Third Edition, First Revision. Geneva: World Health Organization. [Google Scholar]
- 17. Luce D, Gérin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer 1993;53:224-31. [DOI] [PubMed] [Google Scholar]
- 18. Istituto Nazionale di Statistica. Demo-Geodemo. Mappe, Popolazione, Statistiche Demografiche dell’ISTAT. 2013. Available from: http://demo.istat.it/archivio.html [Google Scholar]
- 19. Jensen OM International Agency for Research on Cancer, World Health Organization, International Association of Cancer Registries, editors. Cancer registration: principles and methods. Lyon, France: New York: International Agency for Research on Cancer; Distributed in the USA by Oxford University Press. [Google Scholar]
- 20. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676-82. [DOI] [PubMed] [Google Scholar]
- 21. Agency for Healthcare Research and Quality. Inpatient quality indicators. Available from: http://www.qualityindicators.ahrq.gov/Modules/iqi_resources.aspx [Google Scholar]
- 22. Watters DA, Hollands MJ, Gruen RL, et al. Perioperative mortality rate (POMR): a global indicator of access to safe surgery and anaesthesia. World J Surg 2015;39:856-64. [DOI] [PubMed] [Google Scholar]
- 23. Il garante per la protezione dei dati personali. Autorizzazione n. 2/2012 al trattamento dei dati idonei a rivelare lo stato di salute e la vita sessuale. 2012. Available from: http://www.garanteprivacy.it/web/guest/home/docweb/-/docweb-display/docweb/2158850 [Google Scholar]
- 24. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457. [Google Scholar]
- 25. Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: the Quality Oncology Practice Initiative. J Clin Oncol 2005;23:6233-9. [DOI] [PubMed] [Google Scholar]
- 26. Eskander A, Monteiro E, Irish J, et al. Adherence to guideline-recommended process measures for squamous cell carcinoma of the head and neck in Ontario: impact of surgeon and hospital volume. Head Neck 2016;38(Suppl 1):E1987-1992. [DOI] [PubMed] [Google Scholar]
- 27. Kelly SL, Jackson JE, Hickey BE, et al. Multidisciplinary clinic care improves adherence to best practice in head and neck cancer. Am J Otolaryngol 2013;34:57-60. [DOI] [PubMed] [Google Scholar]
- 28. Starmer H, Sanguineti G, Marur S, et al. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope 2011;121:2131-5. [DOI] [PubMed] [Google Scholar]
- 29. Murphy CT, Galloway TJ, Handorf EA, et al. Increasing time to treatment initiation for head and neck cancer: an analysis of the National Cancer Database. Cancer 2015;121:1204-13. [DOI] [PubMed] [Google Scholar]
- 30. van Harten MC, Hoebers FJP, Kross KW, et al. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol 2015;51:272-8. [DOI] [PubMed] [Google Scholar]
- 31. Palazzi M, Alterio D, Tonoli S, et al. Patterns of postoperative radiotherapy for head and neck cancer in Italy: a prospective, observational study by the head and neck group of the Italian Association for Radiation Oncology (AIRO). Tumori 2011;97:170-6. [DOI] [PubMed] [Google Scholar]


