Abstract
Objective
Propofol is an intravenously administered anesthetic that enhances γ-aminobutyric acid-mediated inhibition in the central nerve system. Other mechanisms may also be involved in general anesthesia. Propofol has been implicated in movement disorders. The cerebellum is important for motor coordination and motor learning. The aim of the present study was to investigate the propofol effect on excitatory synaptic transmissions in cerebellar cortex.
Methods
Excitatory postsynaptic currents by parallel fiber stimulation and complex spikes by climbing fiber stimulation were monitored in Purkinje cells of Wister rat cerebellar slice using whole-cell patch-clamp techniques.
Results
Decay time, rise time and amplitude of excitatory postsynaptic currents at parallel fiber Purkinje cell synapses and area of complex spikes at climbing fiber Purkinje cell synapses were significantly increased by propofol administration.
Conclusion
The detected changes of glutamatergic synaptic transmission in cerebellar Purkinje cell, which determine cerebellar motor output, could explain cerebellar mechanism of motor deficits induced by propofol.
Keywords: Anesthetics, Propofol, Cerebellum, Purkinje cells, Synaptic transmission
INTRODUCTION
Propofol (2,6-diisopropylphenol) is a short-acting intravenous anesthetic that is widely used for induction and maintenance of general anesthesia in clinics.1) The anesthetic activity of propofol primarily involves enhancement of γ-aminobutyric acid type A (GABAA) receptors.2) The effects of propofol on glutamatergic transmission have been studied.3)
Clinical use of propofol has been linked with movement disorders including dystonia and ataxia.4,5) Since, the cerebellum is essential for motor coordination and balance, cerebellar dysfunction could contribute to propofol-induced movement disorders.6,7)
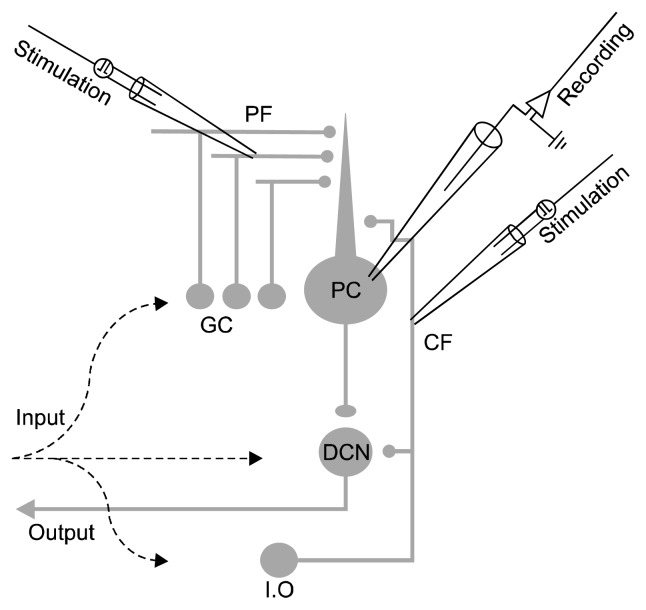
Purkinje cells (PCs) provide the signals required for motor planning, execution, and coordination in their neuronal activity.7,8) PCs occupy a central position in the cerebellar circuit and over 200,000 granule cell axons, known as parallel fibers (PFs), synapse onto a single PC (PF-PC) and a mature PC also receives excitatory input from the inferior olive via a single climbing fiber (CF, CF-PC).8) The changes of glutamatergic synaptic transmission in PCs might lead to alterations in ongoing motor activity and fine motor control (Fig. 1).9)
Fig. 1.
Circuits of the cerebellum that provide excitatory synaptic inputs to Purkinje cells. Two pathways provide excitatory input to Purkinje cells (PC), parallel fibers (PF) and climbing fibers (CF). These inputs arise from granule cells (GC) and cells in the inferior olive (I.O), respectively. PCs integrate these excitatory inputs and send GABAergic projections to the deep cerebellar nuclei (DCN). PFs were stimulated with electrodes positioned in the distal part of the molecular layer, and CF was stimulated with electrodes placed in the granule cell layer. All recordings were carried out in the presence of gamma-aminobutyric acid A receptor antagonist picrotoxin (100 μM).
Recent studies have identified some alterations of cerebellar synaptic transmission in motor dysfunction animal models, especially ataxic mouse.9–11) However, the mechanism of action of propofol for PCs is poorly understood. We hypothesized that propofol modulates cerebellar function by altering the excitatory synaptic transmission provided by PF-PC and CF-PC synapses. Presently, we evaluate the actions of propofol on PF-PC and CF-PC synaptic transmission in the cerebellar cortex using whole-cell patch-clamp recordings.
METHODS
Cerebellar Slice Preparation
Experiments were performed using postnatal 17- to 28-day Wister rats. All animal procedures were carried out in accordance with the regulations of the Institutional Animal Care and Use Committee of Konyang University (Daejeon, Korea). The animals were decapitated after being anesthetized with ether, and their cerebellums were rapidly removed and placed in an ice-cold dissection solution containing (in mM) 220 sucrose, 2.5 KCl, 1 Na2HPO4, 2.5 MgCl2, 0.5 CaCl2, 25 NaHCO3, and 20 D-glucose, bubbled with 95% O2 and 5% CO2. Parasagittal slices (300 μm thick) from the cerebellar hemisphere were prepared using a VT 1000 M vibratome (Leica, Heidelberg, Germany). All experiments were performed at 30°C to 32°C (TC-324B; Warner Instrument, Hamden, CT, USA) after initial 1-hour incubation at 34°C.
Whole Cell Patch-clamp Recording
After incubation, the recording chamber was continuously perfused at a rate of 1.5 ml/min with oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM) 125 mM NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 25 D-glucose bubbled with 95% O2 and 5% CO2. Picrotoxin (100 μM; Sigma-Aldrich, St. Louis, MO, USA) was added to aCSF for all experiments. Recordings were made from PCs in lobules IV–VI, which were visually identified based on their location using a model BX50WI upright microscope (Olympus, Tokyo, Japan) with Nomarski optics and a 40× water-immersion objective. Recording pipettes (GC150T-7.5; Harvard Apparatus, St. Laurent, QC, Canada) were fabricated by pulling glass capillaries on a model PP-830 micro-electrode puller (Narishige Scientific Instruments, Tokyo, Japan). Patch pipettes had resistances of 3–5 MΩ. The standard internal solution was a K-based solution containing (in mM) 140 K-gluconate, 10 HEPES, 0.1 EGTA, 4 KCl, and 5 Mg-adenosine triphosphate (ATP) (pH adjusted to 7.3 with potassium hydroxide [KOH]).
All recordings of PF-excitatory postsynaptic currents (EPSCs) were performed in PCs voltage clamped at −70 mV in the whole-cell configuration. For PF stimulation, a standard patch pipette was filled with external aCSF and placed in the distal part of the molecular layer. Square pulses of 0.1-ms duration with amplitudes ranging from 10 to 100 μA were applied at 0.075 Hz. PF-EPSCs were identified by their characteristic features of graded response amplitude and paired-pulse facilitation (PPF).9) The paired-pulse ratio was calculated as the second EPSC amplitude over the first EPSC amplitude multiplied by 100.10)
CF-complex spikes (CSs) in current clamp recording were evoked with a stimulation electrode located in the granule cell layer, approximately 150 to 300 μm from the patched PC, using a stimulation intensity of 40 to 120 μA. CF responses were confirmed by all-or-none-response and presence of paired-pulse depression.12) During CS recordings, the baseline membrane potential was maintained at −70±1 mV via current injection. Propofol effects were studied by monitoring CSs every 60 seconds.
Experiments were performed using an EPC-10 amplifier; stimulation and data acquisition were controlled using PatchMaster software (HEKA Elektronik, Lambrecht, Germany). Signals were filtered at 3 kHz and digitized at 10 kHz. Recordings were discarded when input resistance values changed by >20%. Only recordings with an access resistance <30 MΩ were evaluated. Our control data were recorded in the presence of dimethyl sulfoxide (DMSO, Sigma-Aldrich; 0.01, 0.02, 0.04, 0.1%). Propofol (Sigma-Aldrich) was dissolved in DMSO to make a 250 mM stock solution, which was diluted into recording solution to the desired propofol concentration (25, 50, 100, 250 μM).
Statistical Analysis
Propofol application was evaluated using paired Student’s t test. Values in the text are expressed as mean±standard error of mean. Statistical analysis used Origin 8.0 software (Originlab, Northampton, MA, USA). The p values less than 0.05 were considered to indicate statistically significant differences.
RESULTS
Propofol Enhances PF-PC Synaptic Transmission
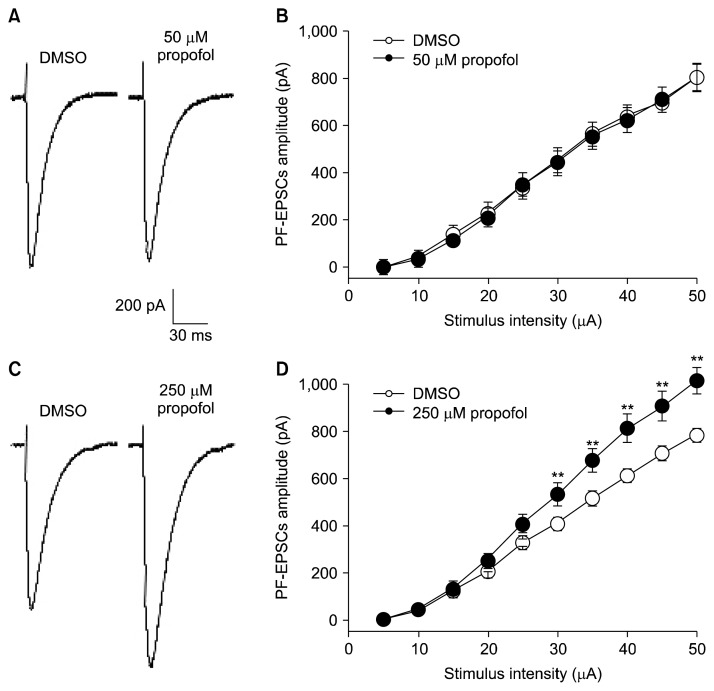
We first examined the effect of propofol on excitatory post-synaptic currents elicited by PF stimulation (PF-EPSC). PF-EPSCs were identified by amplitude enhancement in a graded manner with stimulus intensity and PPF. At concentrations of 25 to 100 μM (Table 1), 20-minute administration of propofol did not make a difference in the peak amplitudes of EPSCs compared with DMSO at all stimulus intensities (Fig. 2B; 5 to 50 μA, 50 μM, n=8). However, 250 μM propofol significantly enhanced the amplitude of PF-EPSCs by 128% of those of DMSO. The mean amplitude of PF-EPSCs evoked by 50 μA stimulation was 788.0±26.9 pA in DMSO and 1,014.8±56.4 pA in 250μM propofol (Fig. 2D). Analysis of PF-EPSC kinetics evoked by 50 μA stimulation revealed significant differences in both rise and decay times between DMSO (0.02, 0.04, 0.1%) and propofol (50, 100, 250 μM; Table 1).
Table 1.
Summary of time-course of synaptic currents at cerebellar parallel fiber (PF)–Purkinje cell (PC) synapses
| PF-EPSCs | 0.01% DMSO (n=8) | 25 μM propofol | 0.02% DMSO (n=8) | 50 μM propofol | 0.04% DMSO (n=10) | 100 μM propofol | 0.1% DMSO (n=8) | 250 μM propofol |
|---|---|---|---|---|---|---|---|---|
| Ipeak (pA) | 640.6±44.3 | 719.4±48.2 | 805.4±55.9 | 807.4±64.4 | 820.6±64.9 | 820.2±70.0 | 788.0±26.9 | 1,014.8±56.4** |
| 10–90% rise time (μs) | 1,875±106 | 2,125±168 | 1,928±112 | 2,193±95** | 1,810±108 | 2,000±101* | 2,484±81 | 2,991±150** |
| Decay time (ms) | 6.7±0.4 | 7.0±0.6 | 6.8±0.4 | 7.4±0.3* | 5.74±0.2 | 6.27±0.1* | 8.2±0.3 | 9.1±0.4** |
Values are number only or mean±standard error of mean; determined for evoked excitatory postsynaptic currents (EPSCs) at PF-PC synapses.
The 10–90% rise time and the decay time of PF-EPSCs evoked by 50 μA stimulation. The mean values determined for EPSCs exposed to dimethyl sulfoxide (DMSO) were compared to the corresponding values obtained using propofol.
Asterisks indicate the significant differences obtained from these comparisons (*p<0.05, **p<0.01; paired Student’s t test).
Fig. 2.
Propofol affects synaptic transmission at parallel fiber-Purkinje cell synapses. (A) Representative traces of parallel fiber evoked excitatory postsynaptic currents (PF-EPSCs) from 0.02% dimethyl sulfoxide (DMSO) and 50 μM propofol at 50μA. (B) Input-output relationship of PF-EPSCs from 0.02% DMSO (open circles, n=8) and 50 μM propofol (closed circles, n=8). (C) Representative traces of PF-EPSCs from 0.1% DMSO and 250 μM propofol at 50μA stimulation. (D) Mean peak amplitudes of PF-EPSCs in 250 μM propofol (closed circles, n=8) were significantly larger compared to 0.1% DMSO (open circles, n=8) over 25μA. Holding potential was −70 mV. Data shown represent the mean± standard error of mean. **p<0.01; paired Student’s t test for DMSO vs. propofol.
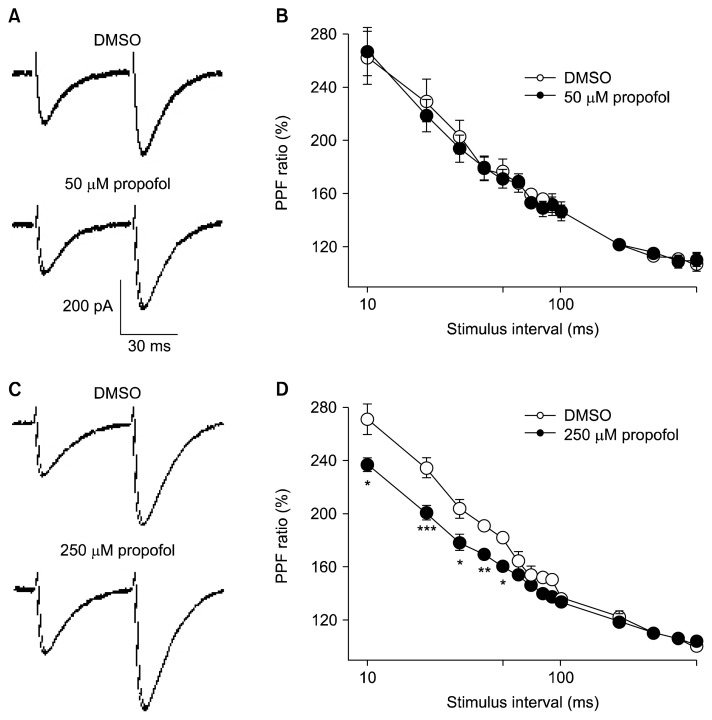
PF-EPSC enhancement suggested an increase in glutamatergic transmissions, which could be caused by facilitation of presynaptic glutamate release and/or increased postsynaptic sensitivity to glutamate. To check the existence of a presynaptic action, we evaluated the effect of propofol on PPF ratio at several intervals (10 to 500 ms). PPF is an increase in the second post-synaptic response when it is elicited shortly after the first, and it is a form of short-term plasticity widely considered to be of pre-synaptic activities in the central nervous system.13) PPF ratio in 50 μM propofol was equivalent to those in DMSO at all interstimulus intervals (Fig. 3B). However, 250 μM propofol significantly reduced the PPF ratio at interval of 50 ms, from 187.9±8.0% to 160.3±3.7% after propofol (Fig. 3D, p<0.01). These results suggest that 250 μM propofol increases glutamate release from PF and enhanced excitatory synaptic transmission in PCs.
Fig. 3.
Short-term plasticity is altered in propofol administration at parallel fiber-Purkinje cell synapses. (A) Representative traces of parallel fiber evoked excitatory postsynaptic currents (PF-EPSCs) evoked by paired-pulse stimulation (50 ms interval) in 0.02% dimethyl sulfoxide (DMSO) and 50 μM propofol. (B) Paired-pulse facilitation (PPF) ratios at different interstimulus intervals (10–500 ms) plotted for 0.02% DMSO (open circles, n=9) and 50 μM propofol (closed circles, n=9). (C) Representative traces of PF-EPSCs induced by paired-pulse stimulation (50 ms interval) in 0.1% DMSO and 250 μM propofol. (D) PPF ratios of 250 μM propofol (closed circles, n=8) were significantly smaller compared to 0.1% DMSO (open circles, n=8). The second response is expressed as a percentage of the response to the first pulse and plotted as a function of interstimulus intervals. Data shown represent the mean±standard error of mean. *p< 0.05; **p<0.01; ***p<0.001; paired Student’s t test for DMSO vs. propofol.
Propofol Affects CS Area at CF-PC Synapse
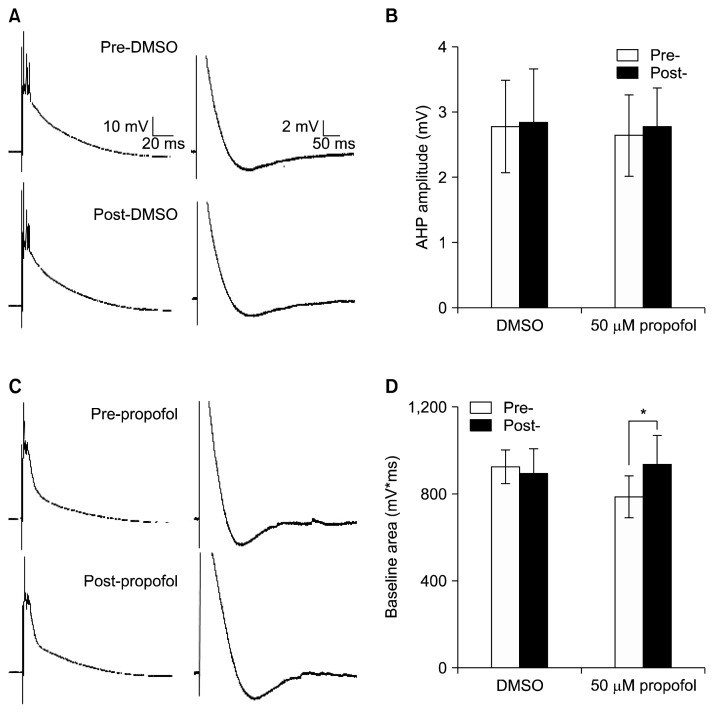
Another distinct excitatory pathway involving CF innervates PC (Fig. 1). This pathway provides powerful input that release glutamate at the proximal portion of the dendritic tree of PC.14) CF activation elicits a unique response, known as the complex spike (CF-CS) consisting of a fast Na+ spike followed by several secondary spikelets and an afterhyperpolarization (AHP; Fig. 4A).14,15)
Fig. 4.
Complex spikes were changed by 50 μM propofol. (A) Representative traces of complex spikes induced by climbing fiber (CF) stimulation before dimethyl sulfoxide (DMSO) and after DMSO administration. The section trace of CF-complex spike (CS) area (left) and CS afterhyperpolization (right) from a single whole trace. (B) Summary of results for the CS afterhyperpolization. (C) Sample traces of CSs before 50 μM propofol and after propofol admistration. (D) Summary of results for the CS area. Data shown represent the mean±standard error of mean. *p<0.05; paired Student’s t test.
The first Na+ spike amplitudes were 59.4±2.56 mV before DMSO and 58.0±2.47 mV after DMSO administration (n=4). Propofol at 50 μM did not affect the Na+ spike amplitudes (58.3±8.35 mV/57.2±7.40 mV; pre-/post-propofol; n=4). In analysis in baseline area of CS (Fig. 4D), DMSO administration did not make a difference in CS area, measured from the onset of the event until the start of the AHP (n=4).12) However, 50 μM propofol significantly increased in CS area by 120% of the value before propofol administration (Fig. 4D, n=4). These findings suggest that propofol administration could alter CF-PC synaptic transmission.
DISCUSSION
Motor dysfunctions are often associated with cerebellar dysfunction. The present electrophysiological study was undertaken to describe changes of synaptic response in cerebellar PCs induced by popofol. The results reported here show that the motor deficits after propofol administration may be associated with changed cerebellar circuit, including input-output relationships and decay time of EPSC at PF-PC synapses and CS area at CF-PC synapses.
Propofol increased decay time regardless of enhancing amplitude of PF-EPSC (Table 1). PF-EPSC is an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated EPSC, because cerebellar PCs are devoid of NMDA receptors. Recent studies reported strong immunoreactivity of GluR1/AMPA receptor subunit in P14 and P21 Wistar rat cerebellar cortex, and described that propofol slowed the channel kinetics of the AMPA receptor by elevating phosphorylation in GluR1.16–18)
We hypothesize that the slowing in decay time of PF-EPSC could be related to cerebellar dysfunction caused by propofol. Slowing of the EPSC decay may contribute to slower rising EPSPs and increased duration of the EPSP plateau, and should also worsen the temporal precision of spike generation, which will block fine motor control in the cerebellar circuits.19–21)
One of the proper functions of an anesthetic is immobility. In humans, immobility is defined as suppression of movement in response to surgical stimulation.22) Recent reviews have suggested that one mechanism cannot totally account for anesthetic-induced immobility and that another; nonspecific mechanism could induce suppression of movement.23) In this study, propofol administered at relatively low concentrations (25 to 100 μM) did not affect the peak amplitude of PF-EPSCs. In contrast, at concentrations of 250 μM, the amplitudes of PF-EPSCs were strongly increased compared with those recorded under control conditions. This result suggests a possible mechanism by which propofol produces immobility. One possibility is that increased amplitude of PF-EPSCs depresses the excitability of deep cerebellar nuclei with inhibitory signal and leads to suppressed movement by acting on the higher center of the cerebellum, rather than on the spinal cord, which is the site of anesthetic action.24,25) Since PCs are the sole output of the computational circuitry of the cerebellar cortex and principally project GABAergic signals to the deep cerebellar nuclei, which projects to motor centers, augmentation in the strengths of PF-PC synapses could block appropriate motor outputs.7,8,26)
The decreased PPF ratio observed in this study suggests that the increased amplitude of PF-EPSCs by propofol may be related to the fact that an increment change in pre-synaptic calcium entry augments neurotransmitter release at the PF-PC synapses.13) It is also possible that some effects other than calcium entry resulting from the propofol administration could contribute to the observed changes in cerebellar synaptic transmission.
The concentrations of propofol in this study were higher than a clinically-relevant range. This discrepancy might originate from the differences between physiological conditions in the brain and various brain slice recording systems. Compared to other cellular preparation, the relatively higher concentrations of propofol are required to produce effects in brain slice preparation.17,27,28)
At present, despite many plausible candidates, there is neither single channels gated by ligand/voltage to explain the immobility induced by general anesthetics, nor any combination of effects to explain immobilization.23) There has been a great deal of attention regarding the inhibitory GABAA receptor as the site of action of general anesthetics.29) On the other hand, the glutamate receptor, which is the cardinal class of excitatory neurotransmitter-gated receptor channels, have received relatively less attention as to whether they are a molecular target for anesthetics in the central nervous system.18) Although propofol enhances GABAergic synaptic transmission, the changes of the glutamatergic synaptic transmissions by propofol shown in the current study indicate that immobility and motor dysfunction induced by propofol could not be entirely mediated by activation of the GABAergic system.2)
In drug interaction, additivity has a common site of action, and synergy has different sites of action.23) The anesthetic interaction of between propofol and midazolam—two drugs that act on GABAA receptors—might be expected to be additive. However, a synergistic interaction that produces immobility has been described.30) Moreover, while propofol produces immobility, midazolam alone cannot suppress movement to noxious stimulation.31,32) These reports indicate that GABAA receptor does not seem to be sufficient to explain immobility and that propofol may have another site of action to suppress movement besides the GABAergic system.23) Thus, we suggest that enhanced PF-EPSCs change the glutamatergic synaptic transmission in cerebellar circuit, and could be a mechanism of immobility, at least in propofol anesthesia.
Although the physiological relevance of changes in PC firing or synaptic response triggered by the CS and the channels that mediate the CS are not fully understood, it has been shown that CSs play a central role in the induction of long-term depression (LTD) at PF-PC synapses, in which motor adaptation and learning allow appropriate motor activity to occur.6,33) Assuming that the first spike of the CS is generated by voltage-dependent Na+ channels, and the secondary spikelets superimposed on slow depolarization plateau are mediated by noninactivating and resurgent Na+ currents, voltage dependent Ca2+ channels, Kv3 K+ channels, and small-conductance Ca2+ activated K+ channels are candidate mediators of the AHP.14,15,34) We previously reported that 50 μM propofol induce LTD impairment35) and presently show that 50 μM propofol changes the CS area of the slow phase without affecting the fast Na+ spike and AHP of CS. The findings are in general agreement with the idea that alterations in CF-CSs could contribute to the motor dysfunction.11,36) Future studies should determine which current is responsible to slow CS components and the precise propofol targets.
Collectively, our findings suggest that the motor deficits effects of propofol are, at least partly a consequence of cerebellar function alterations, and that propofol exposure could adversely affect PF-PC and CF-PC synaptic transmissions in the cerebellar cortex, as well as a number of complex brain functions.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03036479) and the Konyang University Myunggok Research fund of 2017. The authors declare no conflicts of interest.
REFERENCES
- 1.Peduto VA, Concas A, Santoro G, Biggio G, Gessa GL. Biochemical and electrophysiologic evidence that propofol enhances GABAergic transmission in the rat brain. Anesthesiology. 1991;75:1000–1009. doi: 10.1097/00000542-199112000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Vanlersberghe C, Camu F. Propofol Handb Exp Pharmacol. 2008;(182):227–252. doi: 10.1007/978-3-540-74806-9_11. [DOI] [PubMed] [Google Scholar]
- 3.Orser BA, Bertlik M, Wang LY, MacDonald JF. Inhibition by propofol (2,6 di-isopropylphenol) of the N-methyl-D-aspartate subtype of glutamate receptor in cultured hippocampal neurones. Br J Pharmacol. 1995;116:1761–1768. doi: 10.1111/j.1476-5381.1995.tb16660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks DE. Propofol-induced movement disorders. Ann Emerg Med. 2008;51:111–112. doi: 10.1016/j.annemergmed.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Bendiksen A, Larsen LM. Convulsions, ataxia and hallucinations following propofol. Acta Anaesthesiol Scand. 1998;42:739–741. doi: 10.1111/j.1399-6576.1998.tb05312.x. [DOI] [PubMed] [Google Scholar]
- 6.Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 7.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 8.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Matsushita K, Wakamori M, Rhyu IJ, Arii T, Oda S, Mori Y, et al. Bidirectional alterations in cerebellar synaptic transmission of tottering and rolling Ca2+ channel mutant mice. J Neurosci. 2002;22:4388–4398. doi: 10.1523/JNEUROSCI.22-11-04388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KY, Kim JS, Kim SH, Park HS, Jeong YG, Lee NS, et al. Altered Purkinje cell responses and calmodulin expression in the spontaneously ataxic mouse, Pogo. Eur J Neurosci. 2011;33:1493–1503. doi: 10.1111/j.1460-9568.2011.07641.x. [DOI] [PubMed] [Google Scholar]
- 11.Barnes JA, Ebner BA, Duvick LA, Gao W, Chen G, Orr HT, et al. Abnormalities in the climbing fiber-Purkinje cell circuitry contribute to neuronal dysfunction in ATXN1[82Q] mice. J Neurosci. 2011;31:12778–12789. doi: 10.1523/JNEUROSCI.2579-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamudio-Bulcock PA, Morton RA, Valenzuela CF. Third trimester-equivalent ethanol exposure does not alter complex spikes and climbing fiber long-term depression in cerebellar Purkinje neurons from juvenile rats. Alcohol Clin Exp Res. 2014;38:1293–1300. doi: 10.1111/acer.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 14.Schmolesky MT, Weber JT, De Zeeuw CI, Hansel C. The making of a complex spike: ionic composition and plasticity. Ann N Y Acad Sci. 2002;978:359–390. doi: 10.1111/j.1749-6632.2002.tb07581.x. [DOI] [PubMed] [Google Scholar]
- 15.Zagha E, Lang EJ, Rudy B. Kv3.3 channels at the Purkinje cell soma are necessary for generation of the classical complex spike waveform. J Neurosci. 2008;28:1291–1300. doi: 10.1523/JNEUROSCI.4358-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castejón OJ, Dailey ME. Immunohistochemistry of GluR1 subunits of AMPA receptors of rat cerebellar nerve cells. Biocell. 2009;33:71–80. [PubMed] [Google Scholar]
- 17.Krampfl K, Cordes AL, Schlesinger F, Wolfes H, Bufler J. Effects of propofol on recombinant AMPA receptor channels. Eur J Pharmacol. 2005;511:1–7. doi: 10.1016/j.ejphar.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Haines M, Mao LM, Yang L, Arora A, Fibuch EE, Wang JQ. Modulation of AMPA receptor GluR1 subunit phosphorylation in neurons by the intravenous anaesthetic propofol. Br J Anaesth. 2008;100:676–682. doi: 10.1093/bja/aen051. [DOI] [PubMed] [Google Scholar]
- 19.Wall MJ, Robert A, Howe JR, Usowicz MM. The speeding of EPSC kinetics during maturation of a central synapse. Eur J Neurosci. 2002;15:785–797. doi: 10.1046/j.1460-9568.2002.01910.x. [DOI] [PubMed] [Google Scholar]
- 20.Walter JT, Alviña K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–397. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- 21.Cathala L, Brickley S, Cull-Candy S, Farrant M. Maturation of EPSCs and intrinsic membrane properties enhances precision at a cerebellar synapse. J Neurosci. 2003;23:6074–6085. doi: 10.1523/JNEUROSCI.23-14-06074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eger EI, 2nd, Halsey MJ, Harris RA, Koblin DD, Pohorille A, Sewell JC, et al. Hypothesis: volatile anesthetics produce immobility by acting on two sites approximately five carbon atoms apart. Anesth Analg. 1999;88:1395–1400. doi: 10.1213/00000539-199906000-00036. [DOI] [PubMed] [Google Scholar]
- 23.Eger EI, 2nd, Raines DE, Shafer SL, Hemmings HC, Jr, Sonner JM. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg. 2008;107:832–848. doi: 10.1213/ane.0b013e318182aedb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–1709. doi: 10.1152/jn.1999.82.4.1697. [DOI] [PubMed] [Google Scholar]
- 25.Shakkottai VG, Chou CH, Oddo S, Sailer CA, Knaus HG, Gutman GA, et al. Enhanced neuronal excitability in the absence of neurodegeneration induces cerebellar ataxia. J Clin Invest. 2004;113:582–590. doi: 10.1172/JCI200420216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartmann J, Konnerth A. Determinants of postsynaptic Ca2+ signaling in Purkinje neurons. Cell Calcium. 2005;37:459–466. doi: 10.1016/j.ceca.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Shirasaka T, Yoshimura Y, Qiu DL, Takasaki M. The effects of propofol on hypothalamic paraventricular nucleus neurons in the rat. Anesth Analg. 2004;98:1017–1023. doi: 10.1213/01.ANE.0000107960.89818.35. table of contents. [DOI] [PubMed] [Google Scholar]
- 28.Miyawaki I, Nakamura K, Yokubol B, Kitamura R, Mori K. Suppression of cyclic guanosine monophosphate formation in rat cerebellar slices by propofol, ketamine and midazolam. Can J Anaesth. 1997;44:1301–1307. doi: 10.1007/BF03012780. [DOI] [PubMed] [Google Scholar]
- 29.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 30.Short TG, Chui PT. Propofol and midazolam act synergistically in combination. Br J Anaesth. 1991;67:539–545. doi: 10.1093/bja/67.5.539. [DOI] [PubMed] [Google Scholar]
- 31.Vuyk J, Engbers FH, Burm AG, Vletter AA, Griever GE, Olofsen E, et al. Pharmacodynamic interaction between propofol and alfentanil when given for induction of anesthesia. Anesthesiology. 1996;84:288–299. doi: 10.1097/00000542-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Hall RI, Schwieger IM, Hug CC., Jr The anesthetic efficacy of midazolam in the enflurane-anesthetized dog. Anesthesiology. 1988;68:862–866. doi: 10.1097/00000542-198806000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Hansel C, Linden DJ, D’Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- 34.Hurlock EC, McMahon A, Joho RH. Purkinje-cell-restricted restoration of Kv3.3 function restores complex spikes and rescues motor coordination in Kcnc3 mutants. J Neurosci. 2008;28:4640–4648. doi: 10.1523/JNEUROSCI.5486-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KY, Kim YI, Kim SH, Park HS, Park YJ, Ha MS, et al. Propofol effects on cerebellar long-term depression. Neurosci Lett. 2015;609:18–22. doi: 10.1016/j.neulet.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 36.Kitazawa S, Kimura T, Yin PB. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature. 1998;392:494–497. doi: 10.1038/33141. [DOI] [PubMed] [Google Scholar]