Abstract
Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is a recently described auto-immune and paraneoplastic encephalitis with prominent neuropsychiatric manifestations affecting young adults with ovarian teratoma. The availability of a novel assay to measure these antibodies might suggest an etiology for this potentially life-threatening disease, which if early recognized can be treated promptly with surgery with chances of a good clinical outcome. Reported prognostic indicators for a good outcome depend on the presence of a tumor, prompt treatment and no admission to an intensive care unit. However, due to the rarity and unawareness of this disease, the diagnosis may be delayed as primary psychiatric disorders, and infective encephalitis is taken more into consideration and ruled out first. Here we report a case of anti-NMDAR encephalitis in a 22-year-old female prompted by an ovarian teratoma with a gradual and complete resolution of symptoms after surgical excision of the teratoma and immunomodulating therapies.
Keywords: Anti-N-Methyl-D-Aspartate Receptor Encephalitis; Teratoma, Ovarian
INTRODUCTION
Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is an auto-immune and paraneoplastic encephalitis with prominent neuropsychiatric manifestations. The N-methyl-D-aspartate receptor is located in the forebrain and hippocampus and plays a role in learning and memory. In this disease, the NR1 subunit of the NMDA receptors is targeted by antibodies resulting in internalization of NMDAR and progressive decline of NMDAR-associated synaptic functions resulting in neurological and psychiatric manifestations. 1 , 2 Anti-NMDAR encephalitis was first described in 1997 in two isolated case reports of young females with ovarian teratomas presenting with psychiatric manifestations and altered level of consciousness who subsequently showed substantial improvement in clinical symptoms after tumor removal. 3 , 4 In the following years, a handful of cases affecting both men and women have been reported in the literature with the majority of the cases (80%) linked with women. 5 The average age of onset of symptoms is 21 years, although cases have been described in patients ranging from 8 months to 85 years. 2 , 6 , 7 It was recently described that anti-NMDAR encephalitis is the more frequent cause of encephalitis in patients younger than 30 years as compared to viral encephalitis caused by herpes, West-Nile and Varicella-Zoster. 8 Outcomes differ from full recovery to 24-hour institutional care, to death after prolonged hospitalization. 9 - 11 Early surgical excision of the teratoma, with no need for intensive care admission, results in a better outcome with fewer relapses. 1 , 10 , 12 Nevertheless, a fourth of the patients have a grave clinical outcome with severe disability and death. Estimated mortality has been reported about 7%. 10
In this case report, we describe a 22-year-old previously healthy female presenting with a month history of mood lability, psychosis with progressive bradykinesia who found to have anti-NMDAR encephalitis associated with ovarian teratoma.
CASE REPORT
This patient is a 22-year-old female with no significant past medical history presented to the emergency room with the chief complaints of altered mental status, acute psychosis with suicidal ideation, decreased oral intake and reduced urine output. Her mother indicated that the patient has been having “mood swings”, anxiety, depression and progressing slow body movement for 3 weeks before admission. The mother contributed her behavior to her recent estranged relationship with her boyfriend, so they did not seek any medical advice at that time. On the day of admission, the patient was found to be agitated, and she attempted suicide by trying to jump out of the car on the freeway. She was then brought to our facility for medical care.
When the patient arrived at the hospital, she was placed on an involuntary hold by a psychiatrist. During that time patient was noted to be delirious, uncooperative, agitated, demanding, and impulsive and displayed aggressive behavior. Initial imaging did not show acute intracranial abnormalities. She was started on sodium valproate for a presumed bipolar disorder with psychotic features in addition to olanzapine and clonazepam. Following her ED visit, the patient was admitted by the Internal Medicine team to the Medical ICU. Neurologic exam was unable to be completed due to her agitation, confusion, and uncooperativeness. However cranial nerve II-XII strength and sensation appeared grossly intact. Her working diagnosis at this time was considered to be encephalopathy (including viral, fungal, bacterial, spirochetal, toxoplasmal, paraneoplastic antibodies, anti-NMDAR encephalitis), sepsis vs systemic inflammatory response syndrome (SIRS) from aspiration and acute hypoxic respiratory failure, due to her oxygen level desaturations. She had an extensive infectious, metabolic, autoimmune, and neurologic workup.
The initial panels have returned negative. However, her central nervous system fluid (CSF) differential showed a high white blood cell (WBC) count (14.4/mm3) with lymphocytic pleocytosis. Protein and glucose levels were normal. The preliminary HSV type 1 and type 2 PCR results were negative; the patient was started with acyclovir 500 IV q8h empirically. However, it was discontinued as soon as her final HSV type 1 and type 2 PCR report also came back negative.
Further investigation showed elevated titers of anti-NMDA antibodies evaluated by Enzyme-Linked Immunosorbent Assay. Anti-NMDA antibodies were detected in both serum (1:160) and CSF (1:80). Two brains images were done during patient’s hospitalization including a brain magnetic resonance imaging (MRI) (with and without contrast) and a brain computed tomography (CT) without contrast. Routine electroencephalogram (EEG) was done following admission followed by continued EEG monitoring throughout admission.
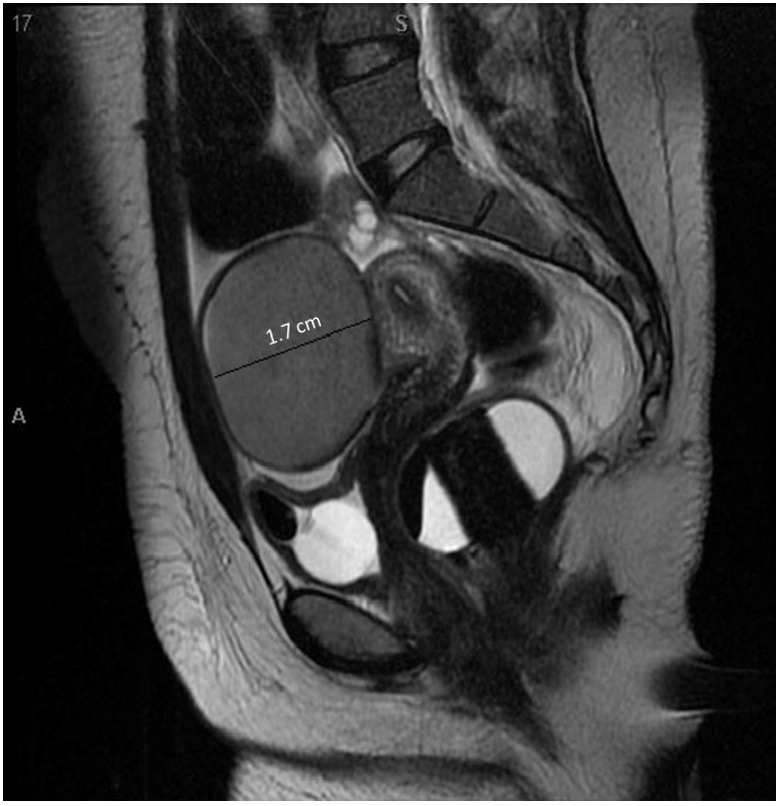
The patient was started on intravenous (IV) steroids, IV immunoglobulin (IVIG) and plasma exchange and gynecology was consulted for further workup as for possible associated ovarian teratoma. The pelvic MRI revealed bilateral ovarian cysts, which measured 1.7 cm on the right ovary and 6.4 cm on the left ovary ( Figure 1 ). The images appeared to be more consistent with a dermoid cyst or hemorrhagic cyst.
Figure 1. Pelvic MRI scan (T2 weighted image) in the sagittal plane showing the right.
Ovarian Teratoma
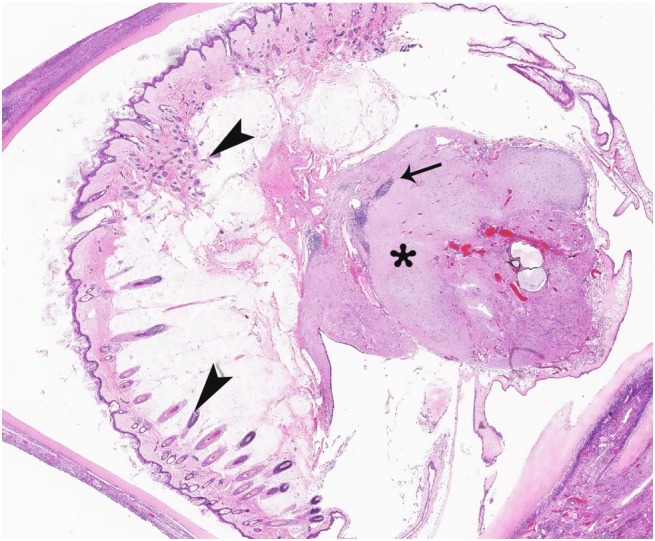
She underwent a right salpingo-oophorectomy and left ovarian cystectomy. The left ovarian cyst measured 8.0 cm in its greatest dimension with tan brown smooth cyst wall containing dark reddish brown adherent blood clot that histologically revealed an endometroid cyst. The right ovary measured 4.2 cm in its greatest dimension with multiple unilocular cysts ranging from 0.2 to 0.6 cm. There was also a tan-gray 1.4 cm solid area within the ovary, which has a fleshy cut surface with attached dark brown hair. On the histology, the mass in the right ovary revealed a mature cystic teratoma ( Figure 2 ).
Figure 2. Tissue section of right ovary showing mature neural and epithelial elements intermixed with mature lymphocytes (H&E, 10X). Note epithelial elements (arrowheads), lymphocytes (arrow) and neural elements (asterisk).
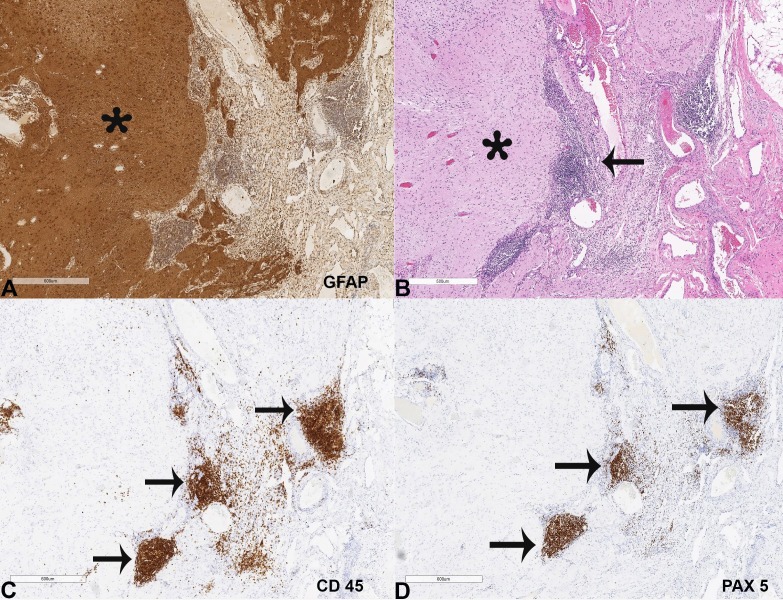
The teratoma contained neural elements resembling white matter. The immunohistochemical stain confirmed that the neural component strongly expressed glial fibrillary acidic protein (GFAP) ( Figure 3A ) and S100. Aggregates of mature-appearing lymphocytes were noted adjacent to the neural tissue ( Figure 3B ), stained positive with CD45 ( Figure 3C ). Approximately 80% of these lymphocytes expressed PAX-5, and therefore these cells were of B-cell lineage ( Figure 3D ).
Figure 3. A – Tissue section from the right ovarian teratoma showing neural tissue strongly expressing the glial fibrillary acidic protein (GFAP, 50X) (asterisk); B – aggregates of mature-appearing lymphocytes adjacent to neural tissue (H&E, 50X) (arrow); C – The lymphocytes were positive for CD 45 in (50X) (arrows); and D – The lymphocytes were also positive for PAX5 (50X) (arrows).
The patient was started on high dose steroids (dexamethasone 10 mg three times a day IV) with gradual taper in the next three months post-surgery, intravenous immunoglobulins (IVIG) 2 rounds (with one month interval) of 10% IVIG 17.5 g, and repeated cycles of plasma exchange with gradual improvement in symptoms three weeks’ post-surgery as evidenced by restoration of sleep-wake cycles, increased the frequency of alertness, ability to more consistently follow commands, decreased the frequency of fevers, improvement of blood pressure and tachycardia. Finally, after two and half month’s post-surgery she was discharged with full return of function. She returned for follow up six months after surgery, before returning to college, this time she reported feeling that her memory and movement had improved and she felt more like her normal self. Her neurological and psychiatric workup was completely within normal, and it was considered that she did not need future treatment or follow-up as she was completely recovered.
DISCUSSION
First described in 1997 by Dalmau et al. in two isolated case reports, anti-NDMAR encephalitis is a potentially life-threatening condition with a wide spectrum of symptoms. 1 The initial prodromal phase is a nonspecific viral-like symptom disease with headache, nausea, vomiting, fever, and fatigue, which later on progresses rapidly into two stages of neuropsychiatric abnormalities. 2 The early stage symptoms include confusion, memory loss, paranoia, hallucinations, mood disturbances, anxiety, self-harming behaviors, seizures and dyskinesia such as facial twitching and choreoathetosis. Due to the predominance of the psychiatric symptoms at this stage, the majority of these patients consult psychiatrists initially, 5 and many of them are misdiagnosed with new-onset psychiatric disorders and are started on anti-psychotics. However, these patients do not respond to these drugs and rapidly progress to late-stage symptoms of decreased responsiveness, hypoventilation, and autonomic instability manifesting as hypotension or hypertension, bradycardia or tachycardia, hyperthermia, and urinary incontinence.
Although cases have been reported in patients ranging from 8 months to 85 years old, 2 , 6 the disease mostly affects young adults with a median age of 21 years. Most of the patients are between the ages 12 and 45 years old with Asian or African American descent, and present with an associated teratoma. 2 , 6 Only 9% of the patients younger than 14 years had accompanying teratomas. 7 Most commonly, the tumors located in the ovary; however, rarely mediastinal teratomas have also been implicated. 9 Reports have also suggested that anti-NMDAR encephalitis can also be related to other germ-cell and rarely non-germ cell tumors. 2 , 6 , 9
Previous studies conjectured that the syndrome was a paraneoplastic process due to an antibody to an unidentified antigen expressed in the hippocampus. 13 In 2007, the associated antibody was discovered to be the anti-NMDA-receptor. 9 In this disease process, antibodies are generated in response to the neural elements within the teratoma. The newly formed autoantibodies react with the NMDA Receptor 1 (NR1) subunit of the ligand-gated cation channels NMDA receptor that is primarily expressed in the hippocampus and forebrain, which are implicated in memory and learning. 14
Occasionally this syndrome has been associated to patients with no detectable underlying neoplasms where it is theorized that the syndrome may be triggered by microscopic germ cell tumors undetectable by imaging diagnostic methods. 5 This hypothesis is supported by the findings that these group of patients established clinical responses to immunomodulatory regimens, including steroids, intravenous immunoglobulin, and plasmapheresis. 7 , 10 Moreover, it has been reported that ovarian teratomas were detected years after the presentation of anti-NMDAR encephalitis symptoms. 5
Here we present a case of a young female who presented with typical neuropsychiatric symptoms and was diagnosed with paraneoplastic anti-NMDA receptor encephalitis. Our patient was diagnosed within 24 hours after admission, and her ovarian teratoma was removed approximately 6 weeks after the onset of symptoms. Despite this rapid intervention, she did not start to improve until 3 weeks later. Finally, after two and half months of hospital course with immunosuppressive drugs, plasma exchanges, she was discharged with full return of function. In our case as well as in other reported cases, the symptoms of anti-NMDAR encephalitis evidently improved within a month of tumor removal and immunosuppressive treatment. However, recovery can continue for up to 2 years. 2 , 6 Gradual improvement with periods of relapse seemed to be a distinctive of anti-NMDA encephalitis, likely because of the failure of common immunomodulatory therapies to provide persistent control of the immune response. 1
Diagnosis of encephalitis should be considered in patients with acute onset of neuropsychiatric symptoms unresponsive to antipsychotic medications. Serum and CSF studies for viral and autoimmune causes of encephalitis, MRI, and EEG should be performed to obtain a specific diagnosis. It is important to note that although brain MRI may be within normal limits in 67% of patients, 90% of these patients commonly show EEG abnormalities. 6 The final diagnosis is made with the detection of anti-NMDAR antibodies in the blood or CSF. 2 , 6 The conclusive diagnosis of anti-NMDAR encephalitis should prompt further imaging studies such as pelvic ultrasound, MRI, CT, and positron emission tomography to evaluate for an underlying teratoma. In rapidly decompensating patients with a clinical suspicion of anti-NMDAR encephalitis, imaging and removal of any detected neoplasms should be considered without delay, as in this case. Any delay in treatment may result in deterioration of the autoimmune process with progression to autonomic instability, catatonia, status epilepticus, or coma and even death. 6 The majority of the patients who undergo surgical excision of the tumor and immunosuppressive treatment are found to have a significant improvement in the neurologic status 2 and a decrease in both serum and cerebrospinal fluid levels of the pathologic autoantibody. 11 The main immunosuppressive therapies commonly used include intravenous steroids and IVIG or plasmapheresis as the first line with rituximab and cyclophosphamide as the second line. 2 , 6
It is clinically relevant to note that NMDAR-antibodies can be produced after post-herpetic encephalitis. 15 According to a recently published report in 2017, 16 about one-fourth (26%) of patients with newly diagnosed Herpes simplex encephalitis went on to develop post-herpetic encephalitis and amongst them, a majority of the patients (86%) showed antibodies to neuronal antigens and the most commonly detected antibodies were anti-NMDAR antibodies (71%).
To conclude, awareness and diagnosis of anti-NMDAR encephalitis are of extreme importance to pediatricians, and gynecologists as well as to the general practitioner and psychiatrist as complete recovery after surgical resection is possible despite the severity of symptoms. Young patients presenting with neuropsychiatric symptoms are occasionally misdiagnosed with psychiatric illness or viral encephalitis. A simple yet reliable measure of autoantibody titers, as well as clinical suspicion of and ovarian teratoma, would benefit an increasing number of patients from prompt diagnosis and treatment of this novel difficult-to-treat clinical entity.
The manuscript is in accordance with the Institutional Ethics committee requirements.
Footnotes
How to cite: Datta Mitra A, Afify A. Ovarian teratoma associated Anti-N-methyl-D-aspartate receptor encephalitis: a difficult diagnosis with a favorable prognosis. Autops Case Rep [Internet]. 2018;8(2):e2018019. http://dx.doi.org/10.4322/acr.2018.019
Financial support: None
REFERENCES
- 1. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-8. 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74. 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nokura K, Yamamoto H, Okawara Y, Koga H, Osawa H, Sakai K. Reversible limbic encephalitis caused by ovarian teratoma. Acta Neurol Scand. 1997;95(6):367-73. 10.1111/j.1600-0404.1997.tb00227.x . [DOI] [PubMed] [Google Scholar]
- 4. Okamura H, Oomori N, Uchitomi Y. An acutely confused 15-year-old girl. Lancet. 1997;350(9076):488. 10.1016/S0140-6736(97)06208-9. [DOI] [PubMed] [Google Scholar]
- 5. Mann A, Lukas R, Grebenciucova E. Anti-N-methyl-D-aspartate-receptor encephalitis: diagnosis, optimal management, and challenges. Ther Clin Risk Manag. 2014;10:517-25. 10.2147/TCRM.S61967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-65. 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66(1):11-8. 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Nephrol Dial Transplant. 2012;54(7):899-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36. 10.1002/ana.21050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ishiura H, Matsuda S, Higashihara M, et al. Response of anti-NMDA receptor encephalitis without tumor to immunotherapy including rituximab. Neurology. 2008;71(23):1921-3. 10.1212/01.wnl.0000336648.43562.59. [DOI] [PubMed] [Google Scholar]
- 11. Seki M, Suzuki S, Iizuka T, et al. Neurological response to early removal of ovarian teratoma in anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry. 2008;79(3):324-6. 10.1136/jnnp.2007.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayes-Jordan A. Surgical management of the incidentally identified ovarian mass. Semin Pediatr Surg. 2005;14(2):106-10. 10.1053/j.sempedsurg.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 13. Vitaliani R, Mason W, Ances B, Zwerdling T, Jiang Z, Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58(4):594-604. 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11(1):37-49. 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- 15. Armangue T, Moris G, Cantarín-Extremera V, et al. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology. 2015;85(20):1736-43. 10.1212/WNL.0000000000002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armangue T, Martínez-Hernández E, Graus F, et al. Brain autoimmunity following herpes simplex encephalitis (HSE): 100 cases. Neurology. 2017;88(16):1. [Google Scholar]