Abstract
Background
The 18-month efficacy of a single course of rituximab as compared with conventional immunosuppression with cyclophosphamide followed by azathioprine in patients with severe (organ-threatening) antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis is unknown.
Methods
In a multicenter, randomized, double-blind, double-dummy, noninferiority trial, we compared rituximab (375 mg per square meter of body-surface area administered once a week for 4 weeks) followed by placebo with cyclophosphamide administered for 3 to 6 months followed by azathioprine for 12 to 15 months. The primary outcome measure was complete remission of disease by 6 months, with the remission maintained through 18 months.
Results
A total of 197 patients were enrolled. As reported previously, 64% of the patients in the rituximab group, as compared with 53% of the patients in the cyclophosphamide–azathioprine group, had a complete remission by 6 months. At 12 and 18 months, 48% and 39%, respectively, of the patients in the rituximab group had maintained the complete remissions, as compared with 39% and 33%, respectively, in the comparison group. Rituximab met the prespecified criteria for noninferiority (P<0.001, with a noninferiority margin of 20%). There was no significant difference between the groups in any efficacy measure, including the duration of complete remission and the frequency or severity of relapses. Among the 101 patients who had relapsing disease at baseline, rituximab was superior to conventional immunosuppression at 6 months (P = 0.01) and at 12 months (P = 0.009) but not at 18 months (P = 0.06), at which time most patients in the rituximab group had reconstituted B cells. There was no significant between-group difference in adverse events.
Conclusions
In patients with severe ANCA-associated vasculitis, a single course of rituximab was as effective as continuous conventional immunosuppressive therapy for the induction and maintenance of remissions over the course of 18 months. (Funded by the National Institute of Allergy and Infectious Diseases and others; RAVE ClinicalTrials.gov number, NCT00104299.)
Granulomatosis with polyangiitis (previously termed Wegener’s granulomatosis) and microscopic polyangiitis are called antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides because they are frequently accompanied by autoantibodies against proteinase 3 or myeloperoxidase.1,2 For nearly four decades, cyclophosphamide and glucocorticoids have been the standard therapy for the induction of remission. However, the primary results of the Rituximab in ANCA-Associated Vasculitis (RAVE) trial3 and results from a European trial4 showed that rituximab was as effective as cyclophosphamide for the induction of remission in patients with severe disease. Moreover, the rituximab-based regimen was superior in patients who had relapsing disease at 6 months.3 Rituximab has been approved in many countries worldwide for the induction of remission in patients with severe ANCA-associated vasculitis.
Because most patients with ANCA-associated vasculitis eventually have a relapse, the duration of treatment-induced remissions, the severity of relapses, and cumulative treatment-related toxic effects are important factors in the choice of induction therapy.5–7 In the RAVE trial, patients in the rituximab group who completed the tapering of prednisone by 6 months and remained in remission received only placebo from month 6 through month 18.3 In contrast, the comparison group received a full 18 months of immunosuppressive therapy comprising cyclophosphamide followed by azathioprine. We report here the long-term results of the trial, with an emphasis on the efficacy and safety of the therapies and the factors that predicted relapses.
Methods
Study Oversight
The first and last authors designed the trial in collaboration with the clinical development team of the Immune Tolerance Network and clinical investigators of the research group (see the Supplementary Appendix, available with the full text of this article at NEJM.org). The data were collected by the site investigators and were analyzed by the study data committee, which consisted of the first and last authors and representatives of the Immune Tolerance Network, the National Institute of Allergy and Infectious Diseases, and a contract research organization (Rho), which served as the coordinating center. All drafts of the manuscript were written by the first and last authors, with input, as appropriate, from members of the study data committee and the clinical investigators. The study data committee made the decision to submit the manuscript for publication. All the authors vouch for the integrity and completeness of the data and the analyses reported and for the fidelity of the study to the protocol (which is available at NEJM.org). Genentech and Biogen Idec provided partial funding for the study and donated the study medication but had no determinative role in the study design, the analyses, or the preparation of the manuscript. ANCA enzyme-linked immunosorbent-assay (ELISA) kits were donated by EUROIMMUN, which had no role in any aspect of the study.
Patients
The details of the trial design have been reported previously.3,8 We enrolled patients with severe ANCA-associated vasculitis who had positive serum assays for proteinase 3–ANCA or myeloperoxidase-ANCA and Birmingham Vasculitis Activity Scores for Wegener’s Granulomatosis (BVAS/WG; with scores ranging from 0 to 63, and higher scores indicating more active disease) of 3 or more. Severe disease was defined as vital-organ involvement that posed an immediate threat to the function of that organ or the patient’s life (see the Supplementary Appendix).3,8 The same definition also applied to relapses.3,8 Patients were randomly assigned, in a 1:1 ratio, to rituximab or cyclophosphamide–azathioprine and were followed until the closeout date of the study (the date on which the last enrolled patient completed 18 months of study therapy). All patients provided written informed consent.
Disease-Assessment Instruments
The principal measure of disease activity was the BVAS/WG.9 The Cockcroft–Gault formula was used to estimate creatinine clearance.10 Other instruments used to assess outcomes included the Vasculitis Damage Index and the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36).11,12
Treatment Groups
The glucocorticoid-tapering regimen, which was identical for the two groups, called for complete cessation of prednisone after 5.5 months if the patient was in remission. The rituximab group received intravenous rituximab (375 mg per square meter of body-surface area once a week for 4 weeks) plus daily placebo cyclophosphamide.3,8 Patients in the rituximab group who completed the glucocorticoid tapering and had a sustained disease remission received no further active treatment.3,8 The standard-therapy group received placebo rituximab infusions plus daily cyclophosphamide (2 mg per kilogram of body weight, with the dose adjusted for renal insufficiency). If remission was achieved between month 3 and month 6, cyclophosphamide was discontinued and azathioprine (2 mg per kilogram) was initiated and was administered for the balance of the 18 months.3,8
Outcomes
The primary comparison at 12 and 18 months was the percentage of patients who had a score of 0 on the BVAS/WG, had completed the glucocorticoid-tapering regimen, and had not had a relapse or any other reason for treatment failure before the time point of interest (see the Supplementary Appendix). Adverse events were recorded for all patients from the time of enrollment through the closeout date of the study.13
Flow Cytometric and ANCA Measurements
At each visit, blood mononuclear cells were analyzed at a central location by means of five-color flow cytometry.8 B-cell depletion was defined as the presence of less than 10 CD19+ B cells per microliter, redetection was defined as at least 10 but less than 69 CD19+ B cells per microliter, and reconstitution was defined as 69 or more CD19+ B cells per microliter or a return to baseline levels. Serum samples were tested for proteinase 3–ANCA or myeloperoxidase-ANCA by means of an ELISA.14
Statistical Analysis
Comparisons of binary outcomes were performed with the use of a chi-square test for efficacy variables and Fisher’s exact test for safety variables. Wald 95% confidence intervals were calculated for differences in treatment. The noninferiority margin was a 20% difference in risk with rituximab as compared with cyclophosphamide–azathioprine at 6, 12, and 18 months. The protocol specified that an analysis for superiority should be performed in the event that the criterion for noninferiority was met; the criterion for superiority was a lower limit of the confidence interval calculated for the difference in treatment that was higher than 0. Relapse rates were compared by means of a Poisson regression model. Time-to-event comparisons were performed with the use of a log-rank test. Descriptive statistics were generated for analyses of time to event and were tested with the use of the Wilcoxon rank-sum test. Data sets for these analyses are accessible through TrialShare, a public Web site that was developed and is managed by the Immune Tolerance Network (https://www.itntrialshare.org/rave.html). Further details about TrialShare and the methods of the censoring and imputation of data and adjustments of the analysis for specific variables are provided in the Supplementary Appendix.
Results
Patients
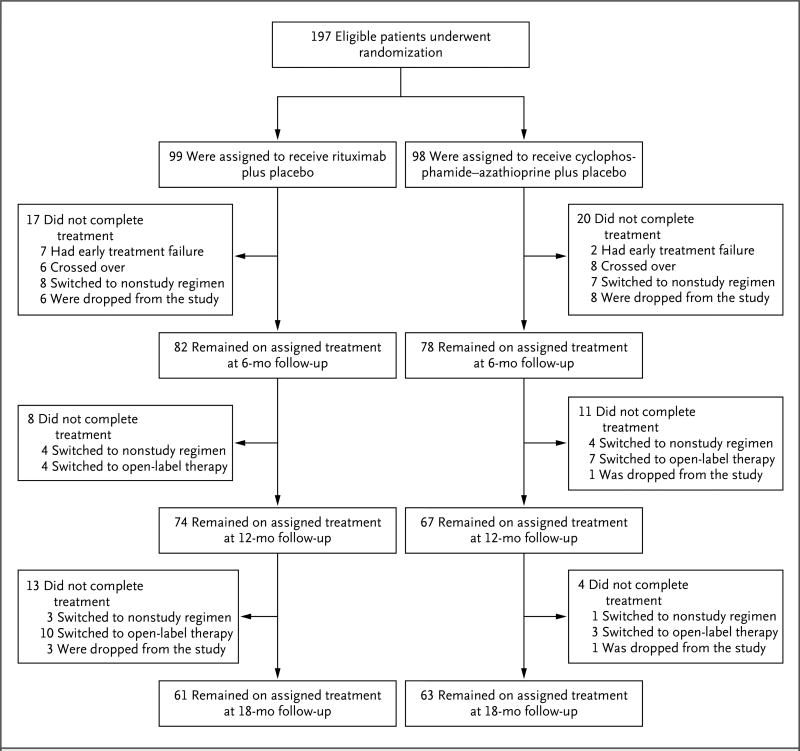
From December 2004 through June 2008, we enrolled 197 patients with severe ANCA-associated vasculitis. A total of 99 patients were randomly assigned to receive rituximab and 98 were randomly assigned to receive cyclophosphamide followed by azathioprine.3 The status of the patients with respect to the study treatment at 6, 12, and 18 months is shown in Figure 1. At 18 months, 61 patients (62%) who had been assigned to the rituximab group and 63 (64%) who had been assigned to the cyclophosphamide–azathioprine group remained in their original group since they did not have early treatment failure, did not cross over to the other regimen, had not been switched to another therapy that was considered, in the judgment of their physicians, to be a better therapy for them, and did not withdraw for other reasons.8
Figure 1. Randomization and Follow-up at 6, 12, and 18 Months.
Patients were randomly assigned, in a 1:1 ratio, to the receive rituximab or cyclophosphamide–azathioprine. Shown are the numbers of patients who remained in the original treatment groups at 6, 12, and 18 months and the reasons that some patients discontinued the original treatment regimen before each time point. Patients could have had more than one protocol-defined reason for treatment change. Early treatment failure was defined as a lack of improvement or a worsening of disease activity before month 1. Patients crossed over to the opposite treatment group if they had a severe disease flare between month 1 and month 6. Patients were switched to a nonstudy therapy if it was deemed necessary according to best medical judgment. Patients were dropped from the study if they withdrew voluntarily or died. Open-label therapy, which was initiated in the case of a severe relapse, consisted of open-label rituximab and glucocorticoids.
Efficacy Assessments
Complete Remission at 6, 12, and 18 Months
A total of 64% of the patients in the rituximab group had a complete remission by 6 months, and 48% and 39% maintained the complete remission at 12 months and 18 months, respectively. The corresponding percentages in the cyclophosphamide–azathioprine group were 53%, 39%, and 33% (Table 1). Given these between-group differences in the treatment effect at 6, 12, and 18 months, rituximab met the criterion for noninferiority (i.e., <20% difference in risk) (P<0.001),3,8 but not the criterion for superiority.
Table 1.
Efficacy Outcomes.*
| Efficacy Measure | Rituximab (N = 99) |
Cyclophosphamide– Azathioprine (N = 98) |
Difference | P Value |
|---|---|---|---|---|
| number (percent) | percentage points (95% CI) | |||
|
| ||||
| Complete remission | ||||
|
| ||||
| 6 mo | 63 (64) | 52 (53) | 11 (−3 to 24) | 0.13 |
|
| ||||
| 12 mo | 47 (47) | 38 (39) | 9 (−5 to 22) | 0.22 |
|
| ||||
| 18 mo | 39 (39) | 32 (33) | 7 (−7 to 20) | 0.32 |
|
| ||||
| Remission and <10 mg/day of prednisone | ||||
|
| ||||
| 6 mo | 70 (71) | 60 (61) | 10 (−4 to 23) | 0.16 |
|
| ||||
| 12 mo | 59 (60) | 60 (61) | −2 (−15 to 12) | 0.82 |
|
| ||||
| 18 mo | 54 (55) | 52 (53) | 2 (−12 to 15) | 0.84 |
|
| ||||
| Complete remission at any time† | 76 (77) | 70 (71) | 0.15 | |
|
| ||||
| Remission and <10 mg/day of prednisone at any time‡ | 82 (83) | 84 (86) | 0.91 | |
|
| ||||
| Remission at any time‡ | 89 (90) | 89 (91) | 0.50 | |
|
| ||||
| Complete remission in patients with relapsing disease at baseline† | ||||
|
| ||||
| 6 mo | 34/51 (67) | 21/50 (42) | 25 (6 to 44) | 0.01 |
|
| ||||
| 12 mo | 25/51 (49) | 12/50 (24) | 25 (7 to 43) | 0.009 |
|
| ||||
| 18 mo | 19/51 (37) | 10/50 (20) | 17 (0 to 34) | 0.06 |
|
| ||||
| milliliters per minute | ||||
|
| ||||
| Estimated creatinine clearance | ||||
|
| ||||
| Total cohort | ||||
|
| ||||
| Baseline | 76.83±3.77 | 91.56±3.75 | −14.74 | 0.01 |
|
| ||||
| 6 mo | 78.59±3.75 | 93.14±3.73 | −14.55 | 0.01 |
|
| ||||
| 12 mo | 80.36±3.87 | 94.72±3.86 | −14.37 | 0.01 |
|
| ||||
| 18 mo§ | 82.12±4.12 | 96.30±4.12 | −14.18 | 0.02 |
|
| ||||
| Patients with major renal disease¶ | ||||
|
| ||||
| Baseline | 53.54±4.63 | 70.52±4.64 | −16.97 | 0.01 |
|
| ||||
| 6 mo | 57.06±4.59 | 73.71±4.60 | −16.65 | 0.01 |
|
| ||||
| 12 mo | 60.57±4.80 | 76.91±4.80 | −16.34 | 0.02 |
|
| ||||
| 18 mo§ | 64.08±5.21 | 80.10±5.10 | −16.02 | 0.03 |
Plus–minus values are means ±SE.
Complete remission was defined as a score of 0 on the Birmingham Vasculitis Activity Scores for Wegener’s Granulomatosis (BVAS/WG; with scores ranging from 0 to 63, and higher scores indicating more active disease), no prednisone therapy, and no other reason to be considered as having had treatment failure. Data were analyzed according to the intention-to-treat principle with worst-case imputation. Only patients who had a complete remission at 6 months were included in the 12-month analysis, and only those who maintained the complete remission at 12 months were included in the 18-month analysis.
Remission was defined as a score of 0 on the BVAS/WG.
The two groups did not differ significantly with respect to improvement in estimated creatinine clearance levels between baseline and 18 months (P = 0.90 for the total cohort and P = 0.80 for the subgroup of patients with major renal disease).
A total of 51 patients in each of the groups had major renal disease, which was defined as the presence of at least one factor designated as a major abnormality in the renal category of the BVAS/WG (urinary red-cell casts, biopsy-proven glomerulonephritis, or an increase of >30% in baseline serum creatinine concentration).
There was no significant difference between the two groups in any efficacy outcome measure, including the percentage of patients with remission; the duration of remission; the number, rate and severity of relapses; the area under the BVAS/WG curve; or scores on the Vasculitis Damage Index and SF-36 at 12 or 18 months (Table 1, and the Supplementary Appendix).
Duration of Complete Remission
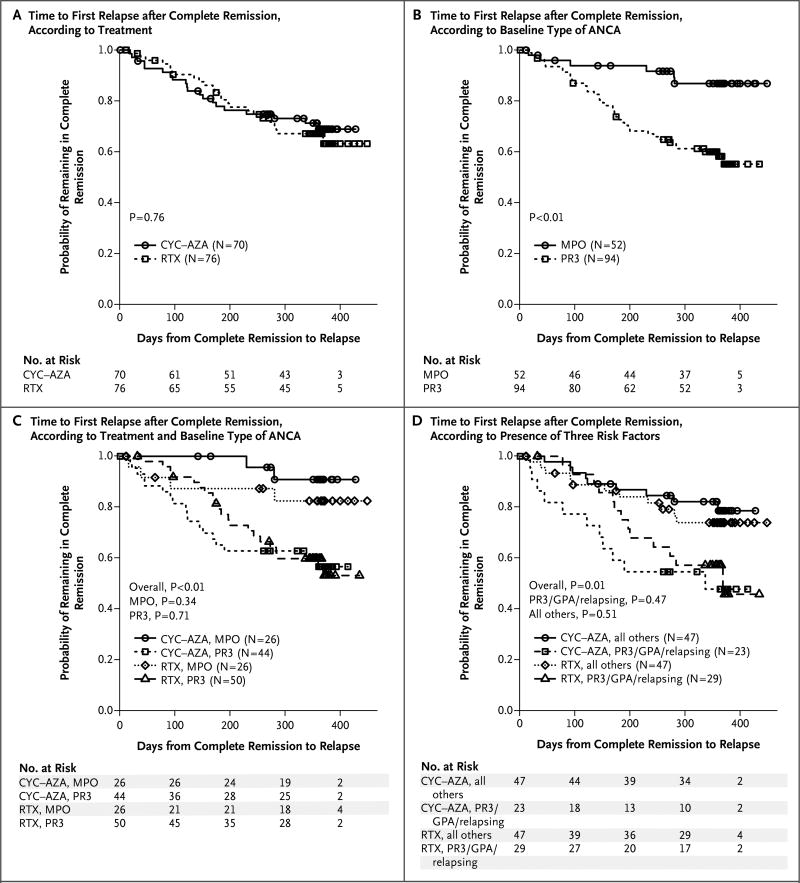
A total of 146 patients (74%), including 76 (77%) in the rituximab group and 70 (71%) in the cyclophosphamide–azathioprine group, had a complete remission after remission-induction treatment at some time during the first 18 months. The mean duration of complete remission was similar in the two treatment groups (P = 0.76) (Fig. 2A). Among the 76 patients in the rituximab group who had a complete remission, 24 (32%) had a relapse before the 18-month time point, with a mean (±SD) time to relapse of 176±91.2 days. Among the 70 patients in the cyclophosphamide–azathioprine group who had a complete remission, 20 (29%) had a relapse before 18 months, with a mean time to relapse of 142±99.2 days (P = 0.16 for the between-group comparison).
Figure 2. Kaplan–Meier Plots of the Risk of Disease Relapse after Complete Remission.
Panel A shows the time to the first disease relapse after complete remission according to treatment group (rituximab [RTX] or cyclophosphamide–azathioprine [CYC–AZA]). Panel B shows the time to the first disease relapse after complete remission according to baseline type of antineutrophil cytoplasmic antibody (ANCA) (proteinase 3–ANCA [PR3] or myeloperoxidase-ANCA [MPO]). Panel C shows the time to the first disease relapse after complete remission according to baseline type of ANCA in each treatment group. Panel D shows the time to the first disease relapse after complete remission among patients with a diagnosis of granulomatosis with polyangiitis (GPA) who were also positive for proteinase 3–ANCA and had a severe relapse at baseline, as compared with all other patients in each treatment group. In Panels C and D, the overall P values are for the comparison of the four patient groups, whereas the other P values are for the comparisons between the two treatment groups within each defined patient subgroup. For additional details, see https://www.itntrialshare.org/RAVE18mos/Fig2.html.
Patients with proteinase 3–ANCA were more likely to have a relapse by 18 months than were those with myeloperoxidase-ANCA (P<0.001) (Fig. 2B), patients with granulomatosis with polyangiitis were more likely to have a relapse than were those with microscopic polyangiitis, and patients who entered the trial with relapsing disease were more likely to have a relapse than were those with newly diagnosed disease (see the Supplementary Appendix). Patients with all three of these factors were at the highest risk for relapse (see the Supplementary Appendix). We found no significant difference between the treatment groups with respect to these clinical factors, and the likelihood of relapse was not affected by the presence of major renal disease at baseline (Fig. 2C and 2D, and the Supplementary Appendix).
Relapsing Disease versus Newly Diagnosed Disease
Among the 101 patients with relapsing disease at entry, 67% in the rituximab group had a complete remission at 6 months, and 49% and 37% maintained the complete remission at 12 months and 18 months, respectively; the corresponding numbers in the cyclophosphamide–azathioprine group were 42%, 24%, and 20% (Table 1). Given these differences, rituximab met the criterion for superiority at 6 months (P = 0.01) and 12 months (P = 0.009) but not at 18 months (P = 0.06). Patients with relapsing disease at entry who were treated with rituximab also had fewer severe relapses than did those who were treated with cyclophosphamide–azathioprine, both at 6 months (1 vs. 9; rate, 0.004 per participant-month vs. 0.034 per participant-month; P = 0.02) and at 12 months (7 vs. 15; rate, 0.014 per participant-month vs. 0.033 per participant-month; P = 0.03). However, by month 18 — when B cells had been reconstituted in most rituximab-treated patients — the difference was no longer significant (13 vs. 17; rate, 0.018 per participant-month vs. 0.027 per participant-month; P = 0.19).
Renal Outcomes at 12 and 18 Months
A total of 102 patients had major renal disease at baseline, which was defined as the presence of at least one factor designated as a major abnormality in the renal category of the BVAS/WG (urinary red-cell casts, biopsy-confirmed glomerulonephritis [which was present in 49 patients], or an increase of >30% in baseline serum creatinine concentration). A total of 51 patients in each treatment group met this prespecified criterion for major renal disease at baseline. Of these, 51% in the rituximab group and 41% in the cyclophosphamide–azathioprine group had estimated creatinine clearance levels that were lower than 50 ml per minute, and 20% and 16%, respectively, had levels that were lower than 30 ml per minute. A total of 38 patients (75%) with major renal disease who received rituximab and 39 (76%) who received cyclophosphamide–azathioprine had a complete remission at some time before 18 months. The magnitude of improvement in mean estimated creatinine clearance levels over time was similar in the two treatment groups (P = 0.80), even though the mean baseline estimated creatinine clearance level was significantly lower among patients with major renal disease who were assigned to rituximab than among those who were assigned to cyclophosphamide–azathioprine (54 ml per minute vs. 71 ml per minute, P = 0.01) (Table 1).
Disease Relapses as Related to B-Cell detectability and ANCA
Of the 76 patients in the rituximab group and the 70 patients in the cyclophosphamide–azathioprine group who had a complete remission, 24 in the rituximab group (32%) and 20 in the cyclophosphamide–azathioprine group (29%) had a relapse between month 6 and month 18. In the rituximab group, the first relapse was severe in 9 patients and limited in 15. In the cyclophosphamide–azathioprine group, the first relapse was severe in 10 patients and limited in 10.
B cells could be detected in 21 of the 24 patients (88%) in the rituximab group who had a relapse, with a mean time between the redetection of B cells and relapse of 80.2±85.1 days (range, 1 to 286). In the other 3 patients, limited relapses occurred in the absence of B cells. In contrast, B cells could be detected in 11 of 20 patients (55%) in the cyclophosphamide–azathioprine group who had a relapse, with a mean time between the redetection of B cells and relapse of 101.8±98.2 days (range, 0 to 259). In that group, relapses occurred in 6 patients who did not have detectable B cells; two of these relapses were severe. The other 3 patients with a relapse never met the criteria for B-cell depletion.
Increases in ANCA titer did not predict relapses in either treatment group (Table S2 in the Supplementary Appendix). However, relapses were rare in the absence of both B cells and ANCA, occurring in only one patient in the rituximab group (limited relapse) and in two patients (one with a limited relapse and one with a severe relapse) in the cyclophosphamide–azathioprine group.
Adverse Events
When adverse events were analyzed on an intention-to-treat basis through the closeout date of the study, there were no significant differences between the treatment groups in the numbers or rates of total adverse events, serious adverse events, or non–disease-related adverse events at 18 months (Table S3 in the Supplementary Appendix). There was also no significant between-group difference in the number of participants who had at least one serious or non–disease-related adverse event. There were four deaths, two in each group (see the Supplementary Appendix).
To compare the safety of rituximab with that of cyclophosphamide–azathioprine, we analyzed the adverse events in patients while they were receiving their originally assigned treatment (Table 2). There were fewer episodes of leukopenia of grade 2 or higher (a white-cell count of <3000 per cubic millimeter) in the rituximab group than in the cyclophosphamide–azathioprine group (5 vs. 23, P<0.001). There was no significant difference between the treatment groups in the number or rate of infections of grade 3 or higher. However, there were 4 episodes of pneumonia in 3 patients in the rituximab group, as compared with 11 episodes in 11 different patients in the cyclophosphamide–azathioprine group (P = 0.03); not all of these patients required hospitalization or intravenous antibiotic therapy. Leukopenia led to more dose adjustments in the cyclophosphamide–azathioprine group than in the rituximab group but was not clearly associated with infections or pneumonias. Serum levels of IgG, IgA, and IgM, as well as the number of patients with low levels of these immunoglobulins, did not differ significantly between the two groups at baseline, 6 months, or 18 months (Table S4 in the Supplementary Appendix), and there was no significant association between low IgG levels and severe infections (data not shown). No additional cancers were observed in the trial after the primary end-point report3 up to the closeout date of the trial (see the Supplementary Appendix).
Table 2.
Adverse Events through 18 Months.*
| Variable | Rituximab (N = 99) |
Cyclophosphamide– Azathioprine (N = 98) |
Total (N = 197) |
P Value |
|---|---|---|---|---|
| Total no. of participant-months | 1371.5 | 1331.9 | 2703.4 | |
| Adverse events | ||||
| Total no. of events | 1399 | 1420 | 2819 | |
| Participants with ≥1 event — no. (%) | 98 (99) | 98 (100) | 196 (99) | >0.99 |
| Events/participant-mo | 1.02 | 1.07 | 1.04 | 0.24 |
| Serious adverse events | ||||
| Total no. of events | 59 | 63 | 122 | |
| Participants with ≥1 event — no. (%) | 42 (42) | 37 (38) | 79 (40) | 0.50 |
| Events/participant-mo | 0.04 | 0.05 | 0.05 | 0.63 |
| Deaths — no. (%)† | 2 (2) | 2 (2) | 4 (2) | |
| Participants with ≥1 episode of leukopenia of grade 2 or higher — no. (%) | 5 (5) | 23 (23) | 28 (14) | <0.001 |
| Participants with ≥1 episode of infection of grade 3 or higher — no. (%) | 12 (12) | 11 (11) | 23 (12) | >0.99 |
| Pneumonia-related adverse events | ||||
| Total no. of events | 4 | 11 | 15 | |
| Participants with ≥1 episode of pneumonia — no. (%) | 3 (3) | 11 (11) | 14 (7) | 0.03 |
| Pneumonia-related adverse events/participant-mo | 0.0029 | 0.0083 | 0.0055 | 0.08 |
Data were censored at time of crossover, initiation of open-label treatment for severe relapse, or a change in treatment according to best medical judgment.
Deaths that occurred after the date on which data were censored are included when the death was attributable to an adverse event that started before that date (see the Supplementary Appendix for details about deaths).
Discussion
The 18-month results of this trial help inform the treatment of ANCA-associated vasculitis. The efficacy outcomes in the rituximab group were consistently as good as those in the cyclophosphamide–azathioprine group over the course of 18 months, despite the fact that patients in the rituximab group who had a complete remission by 6 months received no additional immunosuppression for more than 1 year. Thus, a single course of rituximab, at a dose of 375 mg per square meter once a week for 4 weeks, followed by placebo, is as effective in the treatment of severe ANCA-associated vasculitis as is conventional immunosuppressive therapy with cyclophosphamide and azathioprine administered for 18 months. Our data also show that neither B-cell counts nor ANCA titers alone predict relapses, but as long as both B cells and ANCA remain undetectable, the risk of relapse is low. Finally, no differences in overall adverse events were observed between the treatment groups, with the exception of fewer cases of leukopenia and pneumonia in the rituximab group.
The identification of patients who are at risk for relapse remains a challenge in the treatment of ANCA-associated vasculitis. Our results showed that patients with granulomatosis with polyangiitis, proteinase 3–ANCA positivity, and relapsing disease at baseline had the highest risk of relapse. These findings are consistent with previous observations.7,15–19 Although our trial was not designed to evaluate the efficacy of azathioprine as maintenance therapy, findings in this subgroup of high-risk patients showed that relapse occurred frequently after remission induced by cyclophosphamide and maintained with azathioprine therapy. When remission was induced by rituximab, the risk of relapse increased when B cells became detectable again. This finding raises questions about the most effective approach to minimizing relapses in this high-risk subgroup. Retreatment with rituximab has recently been shown to maintain complete remission in patients who are positive for proteinase 3–ANCA and who have had relapsing disease.20,21 Whether conventional remission-maintenance therapy or repeated B-cell depletion with rituximab is more effective in preventing relapses after initial induction of remission with rituximab deserves further study.
Rituximab effectively induced B-cell depletion in all patients, but nearly all the patients showed reconstitution of peripheral-blood B cells by 18 months. Previous studies have characterized the effects of cyclophosphamide on the numbers and functions of B cells in the blood.22–29 Surprising findings in this trial, however, were that the majority of patients treated with cyclophosphamide also met the criteria for B-cell depletion and that this depletion was often sustained during azathioprine maintenance therapy: 71% of the patients treated with cyclophosphamide–azathioprine did not have reconstituted B cells by 18 months, and 29% still had undetectable B cells. Although B cells were profoundly reduced in both treatment groups, this variable alone did not predict the risk of relapse for individual patients. Changes in ANCA titers were also not associated with the occurrence of relapse. However, among the 44 patients who had a relapse between month 6 and month 18, only 3 had the relapse when their B cells were undetectable and their ANCA assays were negative.
Most patients with severe ANCA-associated vasculitis receive immunosuppressive therapy for a minimum of 18 months — typically 4 to 6 months of cyclophosphamide therapy for the induction of remission followed by maintenance therapy with azathioprine.17,30,31 The patients who were randomly assigned to rituximab received only a single course by design,8 but the effects of rituximab on the immune system beyond 6 months are poorly understood and probably vary among patients. These differences between treatment regimens have implications for adverse effects. Leukopenia, for example, which is an independent risk factor for death,32 occurred more frequently in the cyclophosphamide–azathioprine group than in the rituximab group, despite our efforts to minimize this risk by monitoring complete blood counts every 2 weeks and requiring appropriate dose adjustments of these medications. As anticipated, treatment interruptions and dose adjustments were more frequent in the cyclophosphamide–azathioprine group than in the rituximab group.
Our trial has several strengths. Blinding was maintained throughout the observation period, patients with relapsing disease were eligible for enrollment, and glucocorticoids were withdrawn completely once remission was achieved.3,8 These design features are in contrast to those in most vasculitis trials, which have been randomized but unblinded trials, have enrolled only newly diagnosed patients, and have not included the discontinuation of glucocorticoids as part of the study design.4,17,33–36 Both the inclusion of patients with relapsing disease and the discontinuation of glucocorticoids contributed to the high rate of relapse, but the relapse rate was equally high in the conventional-treatment group despite continued immunosuppressive therapy during follow-up. The data from this trial, in which rituximab was not read-ministered, reflect the reality of this disease, which is that relapses are common when the effects of immunosuppressive therapy wear off. The data also point the way to more effective, long-term disease control through intermittent retreatment. In addition, our trial reflects the broad spectrum of organ-threatening disease activity in ANCA-associated vasculitis because it included 95 patients who did not have organ-threatening renal disease at baseline but who had severe manifestations in other organ systems that would have warranted treatment with cyclophosphamide under the standard of care that existed when the trial began.
The trial also has some limitations. Patients were excluded from the trial if they had nonsevere ANCA-associated vasculitides, were ANCA-negative, had alveolar hemorrhage severe enough to require ventilatory support, or had advanced renal dysfunction (serum creatinine level, >4.0 mg per deciliter [354 µmol per liter]).3,8 Thus, the comparative efficacy of the two treatment regimens for such patients remains uncertain.
In conclusion, our results indicate that for the treatment of severe ANCA-associated vasculitis, a single course of rituximab, at a dose of 375 mg per square meter once a week for 4 weeks, combined with glucocorticoids is not inferior to 18 months of the conventional regimen of glucocorticoids and cyclophosphamide followed by maintenance therapy with azathioprine.
Supplementary Material
Acknowledgments
This research was performed as a project of the Immune Tolerance Network (NIH contract N01-AI-15416; protocol number ITN021AI), an international clinical research consortium headquartered at the University of California, San Francisco.
Supported by the National Institute of Allergy and Infectious Diseases, the Juvenile Diabetes Research Foundation, Genentech, and Biogen Idec. At the Mayo Clinic, the trial was supported by a Clinical and Translational Science Award (CTSA) grant (UL1 RR024150-01) from the National Center for Research Resources (NCRR). At Johns Hopkins, the trial was supported by a CTSA grant (UL1 RR025005) from the NCRR and by grants (K24 AR049185, to Dr. Stone; and K23 AR052820, to Dr. Seo) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). At Boston University, the trial was supported by CTSA grants (UL1 RR025771 and NIH M01 RR00533) from the NCRR and a grant (K24 AR02224) from the NIAMS (all to Dr. Merkel) and an Arthritis Foundation Investigator Award (to Dr. Monach).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 2.Finkielman JD, Lee AS, Hummel AM, et al. ANCA are detectable in nearly all patients with active severe Wegener’s granulomatosis. Am J Med. 2007;120(7):e9–e14. doi: 10.1016/j.amjmed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RB, Tervaert JWC, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211–20. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 6.Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63:257–66. doi: 10.1002/art.27763. [DOI] [PubMed] [Google Scholar]
- 7.Walsh M, Flossmann O, Berden A, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64:542–8. doi: 10.1002/art.33361. [DOI] [PubMed] [Google Scholar]
- 8.Specks U, Merkel PA, Hoffman GS, et al. Design of the Rituximab in ANCA-Associated Vasculitis (RAVE) Trial. Open Arthritis J. 2011;4:1–18. [Google Scholar]
- 9.Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum. 2001;44:912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–80. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE, Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–12. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v.3. doi: 10.1200/JOP.2015.006106. ( http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf) [DOI] [PMC free article] [PubMed]
- 14.Damoiseaux J, Dähnrich C, Rosemann A, et al. A novel enzyme-linked immunosorbent assay using a mixture of human native and recombinant proteinase-3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibody-associated vasculitis. Ann Rheum Dis. 2009;68:228–33. doi: 10.1136/ard.2007.086579. [DOI] [PubMed] [Google Scholar]
- 15.Nachman PH, Hogan SL, Jennette JC, Falk RJ. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:33–9. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 16.Franssen C, Gans R, Kallenberg C, Hageluken C, Hoorntje S. Disease spectrum of patients with antineutrophil cytoplasmic autoantibodies of defined specificity: distinct differences between patients with anti-proteinase 3 and anti-myeloperoxidase autoantibodies. J Intern Med. 1998;244:209–16. doi: 10.1046/j.1365-2796.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 17.Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 18.Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41:776–84. doi: 10.1016/s0272-6386(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 19.Lionaki S, Blyth ER, Hogan SL, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64:3452–62. doi: 10.1002/art.34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cartin-Ceba R, Golbin JM, Keogh KA, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): ten-year experience at a single center. Arthritis Rheum. 2012;64:3770–8. doi: 10.1002/art.34584. [DOI] [PubMed] [Google Scholar]
- 21.Smith RM, Jones RB, Guerry MJ, et al. Rituximab for remission maintenance in relapsing antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64:3760–9. doi: 10.1002/art.34583. [DOI] [PubMed] [Google Scholar]
- 22.Hurd ER, Giuliano VJ. The effect of cyclophosphamide on B and T lymphocytes in patients with connective tissue diseases. Arthritis Rheum. 1975;18:67–75. doi: 10.1002/art.1780180113. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson HC, Fauci AS. Activation of human B lymphocytes. XII. Differential effects of in vitro cyclophosphamide on human lymphocyte subpopulations involved in B-cell activation. Immunology. 1980;39:391–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol. 1982;128:2453–7. [PubMed] [Google Scholar]
- 25.Zhu LP, Cupps TR, Whalen G, Fauci AS. Selective effects of cyclophosphamide therapy on activation, proliferation, and differentiation of human B cells. J Clin Invest. 1987;79:1082–90. doi: 10.1172/JCI112922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol. 1999;103:885–94. doi: 10.1016/s0091-6749(99)70434-3. [DOI] [PubMed] [Google Scholar]
- 27.Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum. 2001;44:2836–40. doi: 10.1002/1529-0131(200112)44:12<2836::aid-art471>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:262–8. doi: 10.1002/art.20718. [DOI] [PubMed] [Google Scholar]
- 29.Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial. Am J Respir Crit Care Med. 2006;173:180–7. doi: 10.1164/rccm.200507-1144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langford CA, Talar-Williams C, Barron KS, Sneller MC. A staged approach to the treatment of Wegener’s granulomatosis: induction of remission with glucocorticoids and daily cyclophosphamide switching to methotrexate for remission maintenance. Arthritis Rheum. 1999;42:2666–73. doi: 10.1002/1529-0131(199912)42:12<2666::AID-ANR24>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 31.The Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352:351–61. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 32.Little MA, Nightingale P, Verburgh CA, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis. 2010;69:1036–43. doi: 10.1136/ard.2009.109389. [DOI] [PubMed] [Google Scholar]
- 33.De Groot K, Rasmussen N, Bacon PA, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 34.Pagnoux C, Mahr A, Hamidou MA, et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med. 2008;359:2790–803. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 35.de Groot K, Harper L, Jayne DR, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 36.Hiemstra TF, Walsh M, Mahr A, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010;304:2381–8. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.