Abstract
This longitudinal qualitative study sought to understand how and why a livelihood intervention affected the health and health behaviors of HIV-infected Kenyan adults. The intervention included a microfinance loan, agricultural and financial training, and a human-powered water pump. In-depth interviews were conducted at two time points with intervention and control participants and program staff. We double coded interviews (n = 117) and used thematic content analysis of transcripts following an integrative inductive–deductive approach. Intervention participants described improvements in HIV health, including increased CD4 counts and energy, improved viral suppression, and fewer HIV-related symptoms. Better health was linked to improved clinic attendance and ART adherence through several mechanisms: (1) reductions in food insecurity and abject hunger; (2) improved financial stability; (3) improved productivity which enhanced social support; (4) better control over work situations; and, (5) renewed desire to prioritize their own health. Livelihood interventions may improve health by influencing upstream determinants of health behavior including food security and poverty.
Keywords: HIV/AIDS, Adherence, Food insecurity, Kenya, Qualitative research, Livelihood intervention
Introduction
Food insecurity, defined as having uncertain or limited availability of nutritionally adequate or safe food or the inability to acquire personally acceptable foods in socially acceptable ways [1], is a leading cause of morbidity and mortality worldwide and is inextricably linked with the HIV epidemic [2]. The prevalence of food insecurity is particularly high among people living with HIV/AIDS (PLHIV) in East Africa, where a majority of patients are moderately or severely food-insecure [3, 4]. Among PLHIV, food insecurity is associated with declines in physical health status, decreased viral suppression, worse immunologic status, increased incidence of serious illness, and increased mortality [3, 5-7].
Harmful healthcare behaviors—such as missed clinic visits and medication non-adherence—are key mechanisms by which food insecurity contributes to poor HIV-related health [2]. A recent systematic review confirmed a consistent association between food insecurity and antiretroviral therapy (ART) non-adherence across diverse settings and study designs [8]. In addition to problems with ART non-adherence, food insecure individuals also often miss scheduled clinic appointments and may delay ART initiation [9]. Since PLHIV often negotiate difficult tradeoffs, including giving up food in order to receive medical care and medications, there is a concern that the relatively high levels of adherence reported among ART-treated individuals in resource poor settings [10] may not be sustainable in the long-term unless improving food security becomes integrated as an essential component of HIV care programs.
Several studies have evaluated targeted food supplementation through clinic-based programs as a way to improve health outcomes, and have found that food supplementation may improve ART adherence and clinic attendance [11, 12]. Yet targeted food supplementation, while providing critical nutritional support, does not fully ameliorate food insecurity if individuals have ongoing anxiety about the food supply, or feel that it is socially unacceptable to rely on clinic-based or other programs to feed themselves or their families [13]. Additionally, direct food supplementation does not address issues of self-reliance and may be unsustainable [2]. Livelihood interventions, which address upstream causes of food insecurity, may be better able to address the numerous pathways through which food insecurity negatively impacts health [2, 14], and may be more empowering and sustainable. International health organizations have therefore long called for an integration of livelihood approaches into HIV care to maximize HIV health outcomes and reduce poverty [15, 16]. However, there are very limited data on the health impacts of livelihood interventions among HIV-infected populations [17]. There are also limited data on potential mechanisms through which food security or livelihood interventions may improve health, even though this is identified as an essential aspect of evaluating complex interventions [18].
We recently evaluated the health impacts of a multisectoral agricultural and finance intervention in rural Kenya called Shamba Maisha (meaning “farm-life” in Kiswahili). This intervention was tested among 140 participants in a randomized controlled trial, and we found statistically significant improvements in food security, diet quality, CD4 cell counts, and proportion virologically suppressed in the intervention group compared to the control group [19]. Here we report on results of a longitudinal qualitative study that was carried out simultaneously alongside the trial and aimed to understand how and why the intervention may have affected participant health.
Methods
The study was conducted in the Nyanza region of Kenya where HIV prevalence is 15.1 %-more than twice the national average [20], and the vast majority of individuals are food insecure [21]. Details of the Shamba Maisha pilot study design have been previously described [22]. In brief, we conducted a pilot randomized controlled trial in two communities to explore whether Shamba Maisha improved food security and HIV health outcomes among PLHIV.
The two sites selected for this study were randomly assigned to either intervention or control conditions by a biostatistician who was not involved in fieldwork. Participants were recruited through organized meetings held at each regional health facility and through announcements at patient support group sessions. Individuals who expressed interest were consented and screened for eligibility. Inclusion criteria for the trial and the current qualitative study included: HIV-infected and receiving ART, ages 18- to 49-years old, having access to farmland and surface water, being moderately or severely food insecure or malnourished (BMI < 18.5) at baseline or during the year preceding enrollment, and participating in or being willing to join a patient support group. Participants were enrolled starting in August 2012 and were followed for 1 year.
A cross-disciplinary team of experts on agriculture, development and HIV health designed the intervention, intended to sustainably improve food insecurity and HIV health. The Shamba Maisha intervention was comprised of three components, delivered as a package to intervention participants [22]: (1) “MoneyMaker” Hip Pump, a low-cost micro-irrigation water pump, which enabled farmers to irrigate their crops year-round, avoiding dependence on seasonal rainfall thus capitalizing on higher crop prices in the marketplace; (2) Training on sustainable farming practices and financial management delivered to groups of participants in eight didactic and practical demonstration sessions; and (3) Loan program of vouchers (worth ~$150 USD) to purchase the irrigation hip pump, seeds, fertilizers, and other farming implements. Study participants were expected to repay the loan in full by the end of two harvest seasons, but were not required to forfeit personal belongings (other than the hip pump) if they failed to repay the loan. Trainings and loan repayment occurred in the context of patient support groups.
Longitudinal Qualitative Study
In the quantitative analysis of our trial, the odds of viral suppression were more than seven-fold greater in intervention compared to control arm (odds ratio 7.6, 95 % confidence interval 2.2–26) [19]. We recognized a priori that the precise mechanisms for improved health and ART adherence would require additional exploration, since few structural interventions to date have unpacked how and why such interventions may impact adherence [23]. Hence we conducted a longitudinal qualitative study alongside the trial, aiming to unpack the mechanisms behind intervention impacts [19]. The design of the qualitative study was not informed by quantitative findings, since the qualitative and quantitative research were carried out simultaneously by two separate teams [24]. We hypothesized that improvements in health behavior, namely ART adherence and clinic attendance, would serve as key pathways towards improved health among participants in the intervention arm of the trial [22].
In the qualitative study, we interviewed a purposive sample of intervention participants (n = 45) to achieve a representative sample in terms of gender and age, and also interviewed a subset of control participants (n = 9) to ascertain whether the impacts and mechanisms described by intervention participants were related to the intervention or to study participation. All participants approached for the qualitative sub-study agreed to participate. To triangulate our findings, we also interviewed 20 key informants, all of whom had detailed knowledge of and experience with the delivery of the intervention. A subset of participants were followed longitudinally (31 intervention participants and 12 key informants), with one interview early in the intervention (at 3–5 months after enrollment) and a second at intervention end (12 months after the start of the intervention), in order to better understand experiences with the intervention and in study implementation over time. Control participants were interviewed at the intervention end only. Overall, 117 in-depth interviews were conducted.
Interviews were conducted by four local researchers (two male, two female) who were fluent in local languages (Dholuo and Kiswahili) and English, and who held either bachelor’s or master’s degrees. Interviewers were trained extensively on qualitative research techniques, including interviewing in an exploratory, non-judgmental way and probing for rich descriptions of participants’ experiences [25, 26]. We carried out mock and pilot interviews to standardize techniques within and across interviewers. In addition to undergoing extensive training, study investigators (SLD, AMH, SDW) also reviewed early transcripts to provide feedback on interview and probing style.
Each interview took place in a private location of the participants’ choosing such as their home or the study office. Interviewers were gender-matched with participants. Interviews lasted between 45 minutes and 2.5 hours and were digitally recorded. Only the researcher and respondent were present during the interviews, and none of the interviewers were previously known to study participants. Researchers were hired specifically for qualitative research and were managed separately from the team delivering the Shamba Maisha intervention.
We used a semi-structured interview guide to explore participant experiences with the intervention and to understand their perceptions of whether and how Shamba Maisha impacted their health and health behaviors. The interview guide covered a broad range of topics related to food insecurity, diet quality, poverty, agricultural practices, mental health, health behavior, engagement in care, and HIV-related stigma. Among intervention participants, the guide contained specific probes on experiences with the Shamba Maisha intervention and perceptions of intervention components (microfinance, micro-irrigation pump, and agricultural trainings). Among control participants, we explored experiences with being a control participant and whether any changes in health or health behaviors occurred as a result of being followed in the trial. The interview guide was developed by six researchers (SDW, AMH, SK, EW, RLB, SLD) and underwent numerous iterations to fine-tune the questions.
Data Management and Analysis
Each interviewer led the transcription process for the interviews that they conducted. Interviews were transcribed and translated verbatim using emic words and phrases to retain local meanings that otherwise could not be translated accurately. In order to ensure accuracy of the translation, each transcript was then reviewed by at least one study investigator (AMH, SK, SDW) and returned to the interviewer with specific questions. Field notes were attached to individual transcripts, and transcripts were labeled with the location, timepoint, age, and gender of each participant.
Transcripts were managed using the qualitative analysis software Dedoose (SocioCultural Research Consultants, Manhattan Beach, CA, USA). Transcripts were double coded and analysed using content analysis methods [27]. First, four researchers (AMH, SK, SLD, SDW) developed a thematic coding framework based on topics covered in interview guides and themes that emerged during data collection. Next, three investigators (AMH, LLH, MN) coded the interviews within wide, thematic broad codes. Finally, three investigators (AMH, LLH, SDW) developed fine codes for sub-themes that emerged inductively. To establish inter-rater reliability, we double-coded a selection of transcripts, and then held a series of training phone calls amongst the coders and a senior supervising investigator (SDW) to establish consensus.
When coding was completed, we created analytic reports for each key theme, which synthesized the findings and included representative quotes as well as any divergent viewpoints. In our manuscript, we include exemplar quotes that are representative of the cohort or are divergent in important ways, demonstrating the range of qualitative findings [27]. Wherever possible, we also incorporate a longitudinal perspective by examining participant data at both timepoints, and also compare experiences and perceptions among intervention participants, control participants, and key informants.
Ethical Considerations
All interviews were conducted after receiving written informed consent. Participants were compensated for their time with 400 Ksh (~$4.70) for home-based interviews, consistent with ethical research procedures in Kenya. Prior to implementing this study, ethics approval was obtained from the Kenya Medical Research Institute (SSC #2178) and the University of California San Francisco (CHR #11-07435). This trial is registered at ClinicalTrials.gov (NCT01548599).
Results
Demographic Characteristics
The qualitative study participant cohort included 54 HIV-infected participants (45 intervention and 9 control) ranging from 23 to 56 years of age (see Table 1). The cohort was comprised of a similar proportion of women and men, most of whom (96 %) had at least two children. A majority of participants (60 %) were married, among whom 11 were living in polygamous marriages (one husband with more than one wife). Those who were not married were either living as widowed single persons or had been inherited by a family member of a deceased husband, a common practice in this setting. Most participants (86 %) had been on ART for at least 2 years prior to enrolling in the study. Many had experience as farmers prior to participating in the Shamba Maisha trial. The 20 key informants included research staff (study coordinators, research assistants) and stakeholders from KickStart International (agricultural partner), Adok Timo (microfinance partner) and FACES (clinical partner). There was a nearly even gender split among key informants, and 12 were followed at two timepoints.
Table 1.
Socio-demographics (n = 74 participants in 117 interviews)
| Patients (n = 54) | Key informants (n = 20) | |
|---|---|---|
| Number (%) or median (IQR) | Number (%) or median (IQR) | |
| Participant group | ||
| Intervention (followed) | 31 (57.4) | – |
| Intervention (once-off) | 14 (25.9) | – |
| Control | 9 (16.7) | – |
| Key informant (followed) | – | 12 (60.0) |
| Key informant (once-off) | – | 8 (40.0) |
| Gender | ||
| Female | 26 (48.1) | 9 (45.0) |
| Male | 28 (51.9) | 11 (55.0) |
| Age | 38 (33–42) | – |
| Years on ART | 4 (2–6) | – |
| Marital status | ||
| Married (monogomous) | 20 (38.5) | – |
| Married (polygomous) | 11 (21.2) | – |
| Widow (single) | 15 (28.8) | – |
| Window (inherited) | 6 (11.5) | – |
| Children | 3 (2–4) | – |
| Years farming | 13 (5–20) | – |
| Key informant group | ||
| Intervention staff | – | 11 (55.0) |
| Agricultural | – | 4 (20.0) |
| Clinical | – | 4 (20.0) |
| Microfinance | – | 1 (5.0) |
Perceived Impacts of the Intervention on HIV-Related Health
During the interviews, a majority of intervention participants described improvements in HIV-related health outcomes. Improvements included increased CD4 counts, improved viral suppression, weight gain, greater energy and fewer symptoms of illness. Many participants proudly spoke of marked increases in CD4 counts and improved viral control. These improvements in physical health seemed to be similar across gender and age groups. For example, one widowed mother of four who had been on ART for 7 years noted that her CD4 count had jumped considerably:
In the house things are very good. In fact my CD4 has now gone up… Before I joined Shamba Maisha it was around 500, but now it’s around 900. (Female participant, intervention end, 44 years old)
A father of three who is the sole breadwinner for his family described improved viral suppression as a result of the intervention:
There is a difference because now at the clinic they test our viral load and tell us we are just fine…. We are not weak now, we have energy… It has really been encouraging us to put a lot of effort in our work… Currently I think [my viral load is] going back to zero. (Male participant, intervention end, 44 years old)
Similarly, a 35-year-old woman described changes in her HIV health as follows:
Then my CD4 used to fluctuate, but since Shamba Maisha came in I now have a very stable undetectable viral load. It has been tested twice and even my last viral load was undetectable I was told. (Female participant, intervention end, 35 years old)
Participants also enthusiastically discussed gaining weight, which provided a visual cue to others that they were doing well and were now in good health. A father of three who had “no steady source of income” before Shamba Maisha, described how neighbors noticed the visible increases in his weight:
I think I have put on some weight since it started! Because I have been eating better since we started…. In fact the other day a woman was telling me nowadays my face looks healthy and so on. And it’s only me who knows the secret to it. [Laughs.] It’s because I am surrounded by vegetables. So I think there is some positive change. (Male participant, early intervention, 42 years old)
Prior to the intervention, this participant’s family periodically lacked adequate food, sometimes eating only one meal per day. He describes that he was often perceived to be the inactive “weak one” by members of his community. At the end of the intervention he remarked that his “weight has shot up” and he had also seen an improvement in his CD4 count. A 28-year-old female participant echoed these sentiments at the intervention end: “Before people would ask me why I was growing thin but now they are asking why I am fat. Others even joke that I have been injected with drugs which make me grow fat.”
Increased strength and energy as a result of Shamba Maisha was another salient theme that emerged in interviews, along with a reduction in symptoms of debilitating illness. As described by one 36-year-old male participant: “The opportunistic infections that used to attack me because maybe I did excess work are no longer present. I also feel very energetic.”A29-year-oldwoman described a similar transformation in her health. Prior to the intervention, she frequently required hospitalization due to poor health, which negatively affected her farm:
I had a severe malarial attack and was admitted to the ward at night… My heart would start racing and I would even start shivering [so] that I would have to be rushed to the hospital… When you are sick and bedridden, a lot of things in the farm get spoilt. (Female participant, early intervention, 29 years old)
By the intervention end, she required fewer hospital visits and had increased stamina to work on the farm, which led to her children becoming healthier:
Yes, there is a difference. My kids are very healthy nowadays. Before they used to be weak and could fall sick more often, myself included. You know if you are not eating well then you are bound to always be weak. But now I am able to do my chores and work on the farm until even 1 PM without getting tired. (Female participant, intervention end, 29 years old)
Similarly, a man who had been on ART for 4 years at the start of the study and who used to suffer from frequent dizzy spells and infections noted an improvement in symptoms:
I am no longer bedridden as a result of illness.… I am also very energetic and able to just do my work throughout… You know not being able to get enough food may even bring some other illnesses, but in my case, that is no longer there… Before I used to feel dizzy but that is no longer the case. (Male participant, intervention end, 42 years old)
A clinical officer observed a reduction in hospitalizations among Shamba Maisha participants.
Participants are not admitted [to the hospital] anymore. They are coming to clinic. They are healthy. Those days, you see, they were unhealthy. They kept on being admitted… But these days I am off that, I no longer visit them in the wards. Actually, I think [for] one year I have not visited anyone in the ward… What I attribute this to is to the nature of food that these people take right now. I am comfortably attributing that to the food they eat, from that farm produce and the money they get from that farm produce. (Key informant: clinical officer, intervention end)
In contrast, while two control participants described increases in weight, none described improved viral load orCD4 cell counts. No control participant mentioned changes in energy levels or opportunistic infections. In the intervention group, only one participant expressed concern that his CD4 cell count had fallen, and pointed toward energy expenditure in farming as having been responsible for his low CD4 cell count.
Since I joined Shamba Maisha, my CD4 count has reduced due to the hard labor… When the rains stop, we will have to go back to the pump… We will therefore be forced to work more, and this is what contributed to the reduction of my CD4 count. (Male participant, early intervention, 34 years old)
This quote highlights a potential downside of the intervention for HIV health.
Improvements in Clinic Attendance and ART Adherence: Key Pathways Towards Health Impacts
Many participants attributed changes in HIV-related health to improvements in their ability to adhere to their HIV medications and attend clinic visits. These changes in HIVrelated health behaviors, in turn, appeared to occur via several interrelated pathways including: (1) reductions in food insecurity and abject hunger; (2) greater financial stability and income enabling participants to afford transport fees to clinic or pharmacy; (3) improved productivity which increased social support; (4) better control over work situations; and (5) an increased prioritization of health (See Fig. 1). Each of these mechanisms were perceived to contribute to both improved clinic attendance and adherence to HIV medications, which in turn led to better health outcomes. For many participants, changes in adherence and clinic attendance occurred early in the intervention, while several other participants noticed a lag in intervention effects, with more marked changes towards the end of the 1-year follow-up.
Fig. 1.
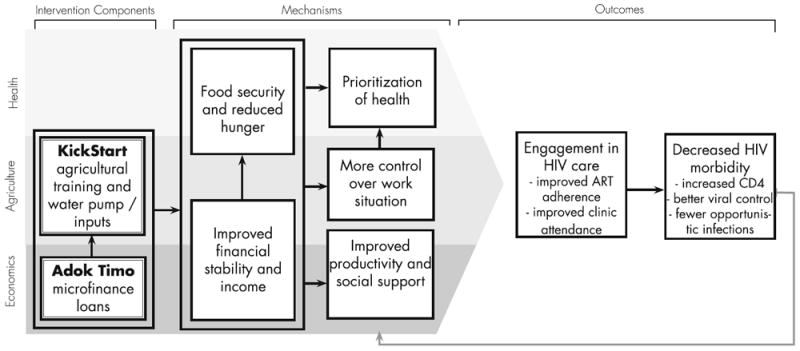
Mechanisms of improved adherence identified in qualitative findings
Importantly, these mechanisms were not described by control participants, who discussed ongoing struggles with adherence to treatment and care, particularly as a result of food insecurity and lack of disposable income. For example, one woman described having so little to eat that she would chew on unprocessed sugarcane, which is a common regional cash crop. This general hunger and weakness led not only to poor adherence but opportunistic infections and disease progression:
It was hard for me to take my medication as required when I was starving. At times when I was supposed to take the drugs and I had nothing to eat, I would just take something like sugarcane. Those days, I could not even go to the farm, and I found myself in a different stage and things like Cryptococcus also came. (Female control, study end, 35 years old)
Clinical officers also noted the lack of change in clinic attendance and ART adherence among Shamba Maisha control participants.
Pathway 1: Improved Food Security
Improved food security attenuated some obstacles that participants faced in adhering to ART or to scheduled clinic appointments. Many participants said that increases in their food supply from farming and the corresponding reduction in hunger resulted in fewer ART side effects. For example, a 34-year-old male participant explained, “I haven’t experienced body weakness, which I used to experience when I did not take food after taking my medication.” Many participants believed that they needed “full stomachs” to take ART without side effects. When they were unable to take their medications with food, they experienced significant side effects such as weakness, nausea, diarrhea, or dizziness. For some, these side effects lessened over the course of the trial due to improved availability of food:
Now, I eat well, hence I’m able to take the drugs as prescribed. I do not have any difficulty. If you do not eat well you can be very dizzy the next morning. You may even faint if you take the drugs on an empty stomach. Just ask someone how tenofovir works. It is very big and very strong… If you take it without food, it can make you fall, make you look like a drunkard. Therefore, regarding food, Shamba Maisha to me has helped me see a great change. I can now eat well. (Male participant, early intervention, 43 years old)
The change in this participant’s food insecurity and adherence occurred early in the intervention, which was similarly described by other participants who noticed improvements 1–2 months after the finance and agricultural training, or shortly after receipt of the water pump.
In addition to the alleviation of ART side effects with improved food security, many participants believed that ART would only “work” if they were eating enough food, eating regularly, or eating a diverse diet including food with certain nutrients such as “vitamins in vegetables.” Reflecting the experience of many participants, a 44-year-old widowed mother explained: “the project has helped since the drug can now find the required vitamins in my body, so when I take the drugs I feel like a normal healthy person.” Another participant who struggled with medication adherence before Shamba Maisha explained how having enough food in the intervention helped her consistently take ART:
You know these medications require that one eat well. If you try taking the drugs without eating well because there is no food, the drugs will not work well or you might get nauseated or even feel like fainting… So one could sometimes say that since they did not get anything to eat, they would not take the medication. But since I joined Shamba Maisha, there is no day that I have gone hungry because my vegetables have pushed me a lot. (Female participant, early intervention, 32 years old)
These changes continued though the end of follow-up, when the same woman said, “I have never missed taking my drugs since I joined Shamba Maisha.” This is a significant contrast to before the intervention, when she explained that “I could forget, or I could say I was tired of taking them.”
Improvements in food security from the intervention also contributed to better clinic attendance for many, in part because food was often needed to sustain participants during long clinic wait times. For example, one 29-year-old widowed mother of five explained that “at times you could be so hungry that you can’t afford to go to the clinic… because there is normally a long queue at the clinic so one might need to eat something.” The intervention had helped this woman address this barrier, and at the intervention end she stated: “Currently I go to the clinic very well without missing.”
Similarly, a male participant, who used to struggle with frequent illness before the intervention, noted that his extra energy and reduced fatigue from improved food intake in Shamba Maisha changed his ability and motivation to go to clinic, even early in the intervention.
One of the good things that it has brought is the vegetables that help us to have readily available food at all times… I have had a change in enthusiasm, that I now attend my clinic visits. I used to get fatigued and could take up to three days without doing any meaningful work, when I never attended my clinic visits regularly and missed my medication. Now I am able to do my daily chores without the initial fatigue. (Male participant, early intervention, 33 years old)
These changes were not reported by control participants. Reflective of the experiences of a number of individuals in the control group, one 46-year-old woman described that stress, financial burden, and food insecurity contributed to declines in her CD4 cell count and weight:
I was stressed because I needed school fees for my children… and when I went for the routine checkup my CD4 and weight was found to have reduced significantly… A lack of food brings that stress. (Female control participant, study end, 46 years old)
Another 40-year-old woman who was a participant in the control group lamented at study end that “this month I just couldn’t get food and people in my house slept hungry.”
Pathway 2: Financial Stability and Income
According to many participants, the increased income resulting from Shamba Maisha participation was an important contributor to improved engagement in HIV care and treatment. Intervention participants often used their income from selling vegetables to pay for transportation to the clinic or the pharmacy. A male participant who was frequently late to clinic appointments before the intervention explained this challenge of competing demands:
There are times when it is difficult… When there is no money, food is very little, and so sometimes you find that it is difficult getting from where you live to the clinic because you have to use transport. This transport money that you have, you can decide to leave it to the family to get some food and instead walk on foot. So sometimes you end up getting to the clinic late. (Male participant, early intervention, 37 years old)
By intervention end, this man regularly attended clinic “because now I am able to get money for my fare to the clinic from the farm produce.”
Since participants routinely pick up medication at the clinic, money to pay for transport was also important for ART adherence. The intervention seemed to address financial barriers to adherence by changing the way that participants were able to access liquid assets. Multiple intervention participants spoke of “selling vegetables” on the way to the clinic in order to earn the pocket money required for public transportation. Illustrating this point, one participant described that before joining Shamba Maisha he and his three HIV-infected children would miss clinic visits, a problem that has improved as a result of the intervention:
I had some difficulties because of lack of transport… I could lack adequate fare, leaving me with lots of worries on how I will go to the clinic: “Today is my clinic day, what will I do? Who will I borrow some money from?” But nowadays, I do not need to borrow money because I have vegetables. When I sell them even for Ksh 200, I know that will be enough to take me to clinic. (Male participant, early intervention, 34 years old)
Similarly, a 32-year-old mother of four who once struggled with taking her medications as prescribed said Shamba Maisha helped her avoid treatment interruptions:
In the past I could even lack Ksh 20 for fare from my town to the clinic, so I could miss collecting drugs. Now, I need never miss it…So since I joined Shamba Maisha I am able to save money from my vegetable sales for my transport to the clinic. (Female participant, intervention end, 32 years old)
According to key informants, the district hospital itself became an unofficial market for produce, where intervention participants were able to sell to patients and providers. Despite the improvements in liquid assets for many participants, difficulties in clinic logistics or transit were sometimes described as persistent before and throughout the course of the study, suggesting that these factors were not altered for all participants; some intervention participants still could not regularly afford transport costs to the clinic throughout the study.
Pathway 3: Productivity and Social Support
ART and clinic adherence were commonly reported to improve alongside an increase in social support for intervention participants, largely from family, friends, and intimate partners. Social support improved in part through changes in experiences of stigma and discrimination related to HIV/AIDS, particularly as participants experienced a shift in social capital when personal productivity, food security and financial stability improved through participation in the intervention (Fig. 1). Improvements in their productivity, livelihood and social position contributed to improved interactions and relationships with immediate and extended family members, neighbors, support group members, and other patients at the clinic.
Encouragement to adhere took the form of reminders from family members (including children and spouses), friends, and support group members to take ART on time. This was reported by some intervention participants, but not by any control participants. A male participant shares how members of his support group co-taught each other the importance of ART adherence, and also discusses an example of family support of ART adherence:
In the groups we teach each other that when you are missing medication, you are just hurting yourself. This made me decide to change my medication time to 9 PM when I am at home. After eating even my youngest child would ask, “How come today you have not taken your medicine? Can I bring you water?” This is really giving me an easy time. (Male participant, early intervention, 33 years old)
Some described an improved dynamic with intimate partners due to improved financial and food security, which influenced adherence. For example, one female participant shared how her relationship with her husband changed, shifting from blame around HIV diagnosis to support around maintaining health over the course of Shamba Maisha participation:
Before I would have a challenge in taking my drugs because I was stressed. When I first tested positive… it was very hard for me to accept the results. I started blaming my husband for infecting me with HIV while on the other hand he was also blaming me so we had a lot of disagreements. We could even forget to take our medication. Since I joined Shamba Maisha there is a difference as we remind each other when it’s time for drugs… So our health is much better. In the past my husband used to fall sick often as a result of not taking his medication as required. I could bring him to the hospital every now and then but the case is different now… Now we are very keen on taking the drugs. (Female participant, intervention end, 28 years old)
Several intervention participants drew a connection between decreased HIV-related stigma and improved adherence to medical care. This occurred because participants were more open about their HIV status, clinic appointments and ART with family members, making it easier to adhere to medical recommendations. For example, one 40-year-old widow had not disclosed her status to her family prior to the intervention, which made it difficult for her to openly obtain ART. She recounted the experience of hiding her HIV status, and the impact that withholding her status had on her physical and mental health:
I used to be sickly but I was afraid to say what the problem was… It was very difficult to tell my children since I thought they would be stressed and think that I would die any time. So I did not tell them. (Female participant, early intervention, 40 years old)
After taking part in Shamba Maisha, this same woman said, “Now [I] am very free, I am so free that if I have to go to the clinic, I tell my children in advance that I will not be there and I give them directions on what to do.” By the intervention end, she had also disclosed to her children that she was taking ART, and her improved ART adherence had contributed to a rise in CD4 cell counts by 200 cells/mm3. Similarly, for a 37-year-old male participant at intervention end, decreased stigma and a connection to the support group helped give him confidence to attend his clinic appointments regularly. He explained: “Shamba Maisha has helped me in getting rid of stigma; now I do not fear doing anything or going anywhere.”
One important, unintended, pathway towards increased adherence across both intervention and control groups was regular visits by study researchers and the increased social support associated with these interactions.
Pathway 4: Greater Control Over Work Situation
A salient theme that emerged was a perceived link between improved control over one’s work situation and adherence to ART or clinic attendance. This occurred when intervention participants, unlike control participants, were able to work independently at their own farms rather than migrating to work at another location. For example, one participant had difficulty with ART adherence before Shamba Maisha because he needed to work at a distance to “get some money to help [his] family.” Yet, by the end of the intervention, he described an improved ability to attend clinic visits by decreasing the tension between his work and medical needs:
In the past I had some difficulties and I could miss going to clinic at times because I would find myself at work. I worked in Mfangano Island most of the time so I could spend a whole month there and miss coming for my medication. But being in Shamba Maisha, I am now able to get some income from it and I am here [at home] most of the time. So I am able to attend clinic well. (Male participant, intervention end, 33 years old)
Key informants reported similar changes in intervention participants’ ability to attend clinic. One clinician from a local hospital noted that her patients who participated in Shamba Maisha “are not relocating the way it used to be—they are based in one place,” which she linked to these patients also no longer defaulting on appointments. Another clinician observed that adherence to clinic visits improved among Shamba Maisha patients because they are better able to control their work:
Whenever you ask, “What makes you not able to concentrate on your treatment?” some tell you, “I am not able because of a lot of work.” But the Shamba Maisha people, they are able because whenever they have [clinic appointments] they are able to set their time while they are working on their shambas. So they are more advantaged compared to the others. They are able to adhere to their treatment, and even if you give them some more [appointment] days, they are able to come. (Medical Officer, intervention end)
Greater control over work situations was reported to help with ART adherence, even early in the intervention. One participant reflected on the greater ease of carrying drugs with him during the day when working on his own farm compared to working for others:
Before joining Shamba Maisha, there were occasions when I did not take the drug on time… because of our work here. I tried my level-best to take the drug on time, but the impact of that was that I had to sacrifice my work at times. However nowadays, after joining this program, I carry my drugs with safe drinking water even when going to the farm such that when the time to take them is due I just take them. The farm has made me no longer go look for other manual work. (Male participant, early intervention, 38 years old)
This change, which appeared early in the intervention, continued through follow-up for this participant. No control participant described a similar improvement in autonomy over their work situation.
Pathway 5: Prioritization of Health
Intervention participants spoke about a renewed commitment to attend clinic appointments and adhere to ART because they saw notable changes in their own health. For example, a male participant who was frequently bedridden before Shamba Maisha explained: “Before Shamba Maisha, I was not adhering to medication and I did not care, but now should I think of missing my medication, I just feel like I am hurting myself.” He explained that with his renewed commitment to health, he was able to encourage his wife to take her medications as prescribed. He added: “Since then we do not get the many opportunistic infections that we used to get. Ever since I joined Shamba Maisha I have no longer been unable to do my work because of sickness.”
This ‘positive feedback loop’ allowed participants to better keep track of their health-related progress, and provided a new outlook and hope for the future, sometimes described as “leading a new life.” For example, a young widowed mother was frequently non-adherent to ART before taking part in Shamba Maisha, but after participation, she explained that adherence was a personal commitment that she was now able to meet. By intervention end, she articulated how her own health had become a priority, and she seemed to take pride in contributing to the wellbeing of her entire family:
It is my life!… I am the one who wants to succeed in the things that I do. My family’s life and my daily chores depend on my health, which is pegged on the ARVs. Hence I am motivated to take them in the right way to get more energy and live to see my successes. (Female participant, intervention end, 26 years old)
Discussion
In this longitudinal qualitative study, we found that participants enrolled in the intervention arm of a multisectoral livelihood intervention in Kenya described substantial improvements in HIV-related health that were not noted by control participants. Health changes were attributed to better clinic attendance and enhanced ART adherence, which in turn were related to improved food security, financial security, social support, work autonomy, and ability to prioritize their health. Findings from this qualitative study clarify the mechanisms behind our previously published quantitative findings which showed improved food security and HIV-related health outcomes among intervention participants compared to control participants [19].
Our findings support previous literature that has shown that ameliorating food insecurity can improve ART adherence among patients. For example, clinic-based studies in sub-Saharan Africa, Haiti, and Honduras have found that food supplementation delivered as part of HIV care can lead to better treatment adherence and improved clinic attendance [11-13, 28, 29]. Yet there has been little previous research on more sustainable approaches that address root causes of food insecurity and poverty. Our study provides a unique contribution to the literature by evaluating a livelihood intervention as a novel strategy to improve ART adherence and health. We found that a relatively simple agricultural and finance intervention can be transformative for the health and wellbeing of HIV-infected patients in a low-income setting.
While our findings build upon previous research on how food insecurity and poverty interfere with ART adherence, this study provides a novel contribution by unpacking the mechanisms underlying how and why a sustainable food security and poverty alleviation intervention may reverse these impacts, improving clinic attendance and ART adherence. For example, previous qualitative research has found transportation costs to be an important barrier to ART adherence [30-32]. We found that this livelihood intervention was able to overcome this barrier, improving adherence in part via an improved ability to afford transport fees due to increased income from produce sales. Similarly, previous studies have reported that food insecurity compromises ART adherence due to worsened ART side effects and competing demands [9, 33]. Our research showed that improved food security from Shamba Maisha addressed this barrier by reducing ART side effects and decreasing competing demands between food and other resources, facilitating improved adherence to ART and clinic attendance.
Beyond reducing hunger and increasing income, we identified several novel mechanisms to explain why agriculture and livelihood interventions may be particularly effective at improving health compared with traditional food or income supplementation programs. Our intervention led to improved work and personal productivity, which reduced HIV stigma and decreased stress, in turn contributing to improved social support from partners and neighbors. While previous research has established social support as an important determinant of ART adherence [34], our findings extend this work by suggesting that social support is an important mediator for how a livelihood intervention may improve ART adherence. Another important mechanism was that participants in our study hadmore control over theirwork situations as farmers on their own lands, allowing them to avoid economic migration, a well-known contributor to treatment interruptions and poor engagement in care [35-37]. Finally, intervention participants increasingly prioritized their own health, and reported a virtuous cycle of feeling better, eating a higher quality diet, taking HIV medications, and working harder on their farms. This finding supports previous literature on the critical role of health empowerment as a means to improve health outcomes in HIV [38].
Our findings have numerous implications for HIV policy and programs. Many adherence interventions have focused on changing individual-level predictors of non-adherence, such as reducing pill burden, directly observing pill-taking, adherence education, and sending text message reminders [39, 40]. While these are important tools for improving adherence, these approaches are not always effective [41, 42], and scholars have criticized such approaches for focusing too narrowly on drug-taking behaviors rather than on the social and structural determinants of poor health [41, 43, 44]. As elaborated in our conceptual framework, our research shows that a livelihood intervention that addresses underlying structural causes of non-adherence can improve health and health behaviors through multiple mutually reinforcing pathways. Importantly, the Shamba Maisha intervention has the potential to be sustainable, which is significant given the increasing emphasis on economic efficiency in the context of structural interventions to address food insecurity and HIV health. This study supports calls from leading international health organizations to better integrate economic, agricultural and health programs, which have historically been highly compartmentalized and poorly coordinated [45-47]. By contributing to dialogue among those involved in international agricultural policy, nutrition, development, and ART delivery in sub-Saharan Africa, the proposed study can inform these sectors, facilitating effective utilization of limited resources. Finally, our findings have significant implications not only for PLHIV, but also for individuals living with other chronic illnesses who may similarly benefit from these upstream approaches.
Limitations
There were several important limitations to our study. The intervention was limited to PLHIV already on ART, though in view of new WHO guidelines, this will soon comprise the majority of HIV-infected individuals [48]. Intervention participants may have looked back upon the intervention more favorably upon recollection. We tried to address this by conducting longitudinal interviews, by triangulating the findings with our quantitative study, and by providing comparisons with several control participants and stakeholders [49]. Social desirability bias may have influenced the data presented here. To minimize this bias, we hired local researchers who were not involved in intervention delivery, and trained them extensively on open-ended interview techniques, including avoiding leading questions [27]. We did not collect baseline qualitative data before participants initiated the intervention, and hence participant’s reports of their experiences before Shamba Maisha were provided retrospectively, which may have introduced recall bias. Finally, we did not interview a large number of control participants, which may have made it difficult to detect changes in health and adherence among the control group. However, we found the data related to health, adherence and clinic attendance did reach saturation among the control group, and these qualitative findings are consistent with previously published quantitative findings [19].
Conclusions
Upstream intervention strategies are required if HIV care and treatment programs are to reach UNAIDS goals of 90 % of HIV-infected persons on sustained ART and achieving viral suppression [50]. Shamba Maisha is among the first interventions to evaluate health impacts and mechanisms of a theory-based multisectoral livelihood intervention aimed to improve food security and HIV-related health. Our results indicate that livelihood interventions such as Shamba Maisha that address underlying structural drivers of non-adherence and poor health in resource limited settings are promising and may be selfsustaining. These findings suggest that livelihood interventions can improve health and health behaviors by addressing financial, nutritional, and social barriers to engagement in care. Our study supports growing global interest in integrating agriculture, nutrition, and health programs to improve outcomes in these sectors simultaneously. Such multisectoral collaborations are well positioned to contribute toward sustainable public health solutions to improve the intersecting problems of food insecurity, poverty, and HIV/AIDS morbidity and mortality in sub-Saharan Africa.
Acknowledgments
We gratefully acknowledge the important support of the Kenyan Medical Research Institute (KEMRI) and Family AIDS Care & Educational Services (FACES). We also thank the women and men who generously gave their time to participate in the study. We gratefully acknowledge the Director of KEMRI and the Director of KEMRI’s Centre for Microbiology for their support in conducting this research. We also thank the Kevin Kadede, Priscah Owato, Pamela Kimwele, Gina Clark, Kyle Pusateri, and Nandy Nittur for their important contributions to this research.
Funding This study was funded by National Institute of Mental Health (grant R34MH094215). Additional funding was provided by the World Food Programme and the Burke Family foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Radimer KL, Olson CM, Greene JC, Campbell CC, Habicht JP. Understanding hunger and developing indicators to assess it in women and children. J Nutr Educ. 1992;24(Suppl 1):36S–45S. [Google Scholar]
- 2.Weiser SD, Young SL, Cohen CR, Kushel MB, Tsai AC, Tien PC, et al. Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am J Clin Nutr. 2011;94(suppl):1729S–39S. doi: 10.3945/ajcn.111.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiser SD, Yuan C, Guzman D, Frongillo EA, Riley ED, Bangsberg DR, et al. Food insecurity and HIV clinical outcomes in a longitudinal study of urban homeless and marginally housed HIV-infected individuals. AIDS. 2013;27(18):2953–8. doi: 10.1097/01.aids.0000432538.70088.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagata JM, Cohen CR, Young SL, Wamuyu C, Armes MN, Otieno BO, et al. Descriptive characteristics and health outcomes of the food by prescription nutrition supplementation program for adults living with HIV in Nyanza Province, Kenya. PLoS ONE. 2014;9(3):e91403. doi: 10.1371/journal.pone.0091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalichman SC, Cherry C, Amaral C, White D, Kalichman MO, Pope H, et al. Health and treatment implications of food insufficiency among people living with HIV/AIDS, Atlanta, Georgia. J Urban Health. 2010;87(4):631–41. doi: 10.1007/s11524-010-9446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiser SD, Tsai AC, Gupta R, Frongillo EA, Kawuma A, Senkungu J, et al. Food insecurity is associated with morbidity and patterns of healthcare utilization among HIV-infected individuals in a resource-poor setting. Aids. 2012;26(1):67–75. doi: 10.1097/QAD.0b013e32834cad37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiser SD, Fernandes KA, Brandson EK, Lima VD, Anema A, Bangsberg DR, et al. The association between food insecurity and mortality among HIV-infected individuals on HAART. J Acquir Immune Defic Syndr. 2009;52(3):342–9. doi: 10.1097/QAI.0b013e3181b627c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer AW, Weiser SD, McCoy SI. Does food insecurity undermine adherence to antiretroviral therapy? A systematic review. AIDS Behav. 2015;19(8):1510–26. doi: 10.1007/s10461-014-0873-1. [DOI] [PubMed] [Google Scholar]
- 9.Weiser SD, Tuller DM, Frongillo EA, Senkungu J, Mukiibi N, Bangsberg DR. Food insecurity as a barrier to sustained antiretroviral therapy adherence in Uganda. PLoS ONE. 2010;5(4):e10340. doi: 10.1371/journal.pone.0010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296(6):679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 11.Martinez H, Palar K, Linnemayr S, Smith A, Derose KP, Ramirez B, et al. Tailored nutrition education and food assistance improve adherence to HIV antiretroviral therapy: evidence from Honduras. AIDS Behav. 2014;18(Suppl 5):566–77. doi: 10.1007/s10461-014-0786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantrell RA, Sinkala M, Megazinni K, Lawson-Marriott S, Washington S, Chi BH, et al. A pilot study of food supplementation to improve adherence to antiretroviral therapy among foodinsecure adults in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;49(2):190–5. doi: 10.1097/QAI.0b013e31818455d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byron E, Gillespie S, Nangami M. Integrating nutrition security with treatment of people living with HIV: lessons from Kenya. Food Nutr Bull. 2008;29(2):87–97. doi: 10.1177/156482650802900202. [DOI] [PubMed] [Google Scholar]
- 14.Tsai AC, Bangsberg DR, Weiser SD. Harnessing poverty alleviation to reduce the stigma of HIV in Sub-Saharan Africa. PLoS Med. 2013;10(11):e1001557. doi: 10.1371/journal.pmed.1001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartlander B, Stover J, Hallett T, Atun R, Avila C, Gouws E, et al. Towards an improved investment approach for an effective response to HIV/AIDS. Lancet. 2011;377(9782):2031–41. doi: 10.1016/S0140-6736(11)60702-2. [DOI] [PubMed] [Google Scholar]
- 16.UNAIDS. UNAIDS outcome framework 2009–2011. 2010 http://www.unaids.org/en/media/unaids/contentassets/dataimport/pub/basedocument/2010/jc1713_joint_action_en.pdf.
- 17.Bateganya MH, Dong M, Oguntomilade J, Suraratdecha C. The impact of social services interventions in developing countries: a review of the evidence of impact on clinical outcomes in people living with HIV. J Acquir Immune Defic Syndr. 2015;68(Suppl 3):S357–67. doi: 10.1097/QAI.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Bmj. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiser SD, Bukusi EA, Steinfeld RL, Frongillo EA, Weke E, Dworkin SL, et al. Shamba Maisha: randomized controlled trial of an agricultural and finance intervention to improve HIV health outcomes. AIDS. 2015;29(14):1889–94. doi: 10.1097/QAD.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenya National Bureau of Statistics and ICF Macro. Kenya Demographic and Health Survey 2008-09. Calverton: Kenya National Bureau of Statistics and ICF Macro; 2010. [Google Scholar]
- 21.Kenya National Bureau of Statistics. Food insecurity assessment in Kenya. Nairobi: Government of Kenya; 2008. [Google Scholar]
- 22.Cohen CR, Steinfeld RL, Weke E, Bukusi EA, Hatcher AM, Shiboski S, et al. Shamba Maisha: Pilot agricultural intervention for food security and HIV health outcomes in Kenya: design, methods, baseline results and process evaluation of a clusterrandomized controlled trial. SpringerPlus. 2015;4:122. doi: 10.1186/s40064-015-0886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatcher AM, Bonell CP. High time to unpack the ‘how’ and ‘why’ of adherence interventions. AIDS. 2016;30(8):1301–4. doi: 10.1097/QAD.0000000000001071. [DOI] [PubMed] [Google Scholar]
- 24.Creswell JW, Clark VLP. Designing and conducting mixed methods research. New Jersey: Wiley Online Library; 2007. [Google Scholar]
- 25.Patton M. Qualitative research and evaluation methods. 3. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- 26.Bernard H. Research methods in anthropology: qualitative and quantitative approaches. 4. Lanham: AltaMira Press; 2006. [Google Scholar]
- 27.Miles MB, Huberman AM, Saldaña J. Qualitative data analysis: a methods sourcebook. Thousand Oaks: SAGE Publications; 2013. [Google Scholar]
- 28.Nyamathi A, Sinha S, Ganguly KK, Ramakrishna P, Suresh P, Carpenter CL. Impact of protein supplementation and care and support on body composition and CD4 count among HIV-infected women living in rural India: results from a randomized pilot clinical trial. AIDS Behav. 2013;17(6):2011–21. doi: 10.1007/s10461-013-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivers LC, Chang Y, Jerome JG, Freedberg KA. Food assistance is associated with improved body mass index, food security and attendance at clinic in an HIV program in central Haiti: a prospective observational cohort study. AIDS Res Ther. 2010;7:33. doi: 10.1186/1742-6405-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardon AP, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerrits T, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19(5):658–65. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- 31.Kagee A, Remien RH, Berkman A, Hoffman S, Campos L, Swartz L. Structural barriers to ART adherence in Southern Africa: challenges and potential ways forward. Glob Public Health. 2011;6(1):83–97. doi: 10.1080/17441691003796387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav. 2010;14(4):778–84. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young S, Wheeler AC, McCoy SI, Weiser SD. A review of the role of food insecurity in adherence to care and treatment among adult and pediatric populations living with HIV and AIDS. AIDS Behav. 2014;18(Suppl 5):S505–15. doi: 10.1007/s10461-013-0547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ware NC, Idoko J, Kaaya S, Biraro IA, Wyatt MA, Agbaji O, et al. Explaining adherence success in sub-Saharan Africa: an ethnographic study. PLoS Med. 2009;6(1):e11. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanser F, Barnighausen T, Vandormael A, Dobra A. HIV treatment cascade in migrants and mobile populations. Curr Opin HIV AIDS. 2015;10(6):430–8. doi: 10.1097/COH.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 36.Taylor BS, Reyes E, Levine EA, Khan SZ, Garduno LS, Donastorg Y, et al. Patterns of geographic mobility predict barriers to engagement in HIV care and antiretroviral treatment adherence. AIDS Patient Care STDS. 2014;28(6):284–95. doi: 10.1089/apc.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lima V, Fernandes K, Rachlis B, Druyts E, Montaner J, Hogg R. Migration adversely affects antiretroviral adherence in a population-based cohort of HIV/AIDS patients. Soc Sci Med. 2009;68(6):1044–9. doi: 10.1016/j.socscimed.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 38.Johnson MO, Rose CD, Dilworth SE, Neilands TB. Advances in the conceptualization and measurement of Health Care Empowerment: development and validation of the Health Care Empowerment inventory. PLoS ONE. 2012;7(9):e45692. doi: 10.1371/journal.pone.0045692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS ONE. 2014;9(2):e88166. doi: 10.1371/journal.pone.0088166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Barnighausen T. Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. AIDS. 2014;28(Suppl 2):S187–204. doi: 10.1097/QAD.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 41.Ford N, Nachega JB, Engel ME, Mills EJ. Directly observed antiretroviral therapy: a systematic review and meta-analysis of randomised clinical trials. Lancet. 2009;374(9707):2064–71. doi: 10.1016/S0140-6736(09)61671-8. [DOI] [PubMed] [Google Scholar]
- 42.Ramjan R, Calmy A, Vitoria M, Mills EJ, Hill A, Cooke G, et al. Systematic review and meta-analysis: patient and programme impact of fixed-dose combination antiretroviral therapy. Trop Med Int Health. 2014;19(5):501–13. doi: 10.1111/tmi.12297. [DOI] [PubMed] [Google Scholar]
- 43.Bangsberg DR, Mills EJ. Long-term adherence to antiretroviral therapy in resource-limited settings: a bitter pill to swallow. Antivir Ther. 2013;18(1):25–8. doi: 10.3851/IMP2536. [DOI] [PubMed] [Google Scholar]
- 44.Wouters E, Masquillier C, Ponnet K, le Roux Booysen F. A peer adherence support intervention to improve the antiretroviral treatment outcomes of HIV patients in South Africa: the moderating role of family dynamics. Soc Sci Med. 2014;113:145–53. doi: 10.1016/j.socscimed.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Weigers E. The role of the agricultural sector in mitigating the impact of HIV/AIDS in sub-Saharan Africa. NJAS Wagening J Life Sci. 2008;56(3):155–66. [Google Scholar]
- 46.Economic Commission for Africa. Mitigating the impact of HIV/AIDS on smallholder agriculture, food security and rural livelihoods in Southern Africa: Challenges and Action Plan. Addis Ababa: Economic Commission for Africa; 2006. [Google Scholar]
- 47.UNAIDS. Report on the global AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2008. [Google Scholar]
- 48.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 49.Creswell JW, Miller DL. Determining validity in qualitative inquiry. Theory into practice. 2000;39(3):124–30. [Google Scholar]
- 50.UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Joint United Nations Programme on HIV/AIDS; Geneva: 2014. [Google Scholar]


