Abstract
Asthma is the most common chronic disease of childhood and the leading cause of childhood morbidity as measured by school absences, emergency department visits, and hospitalizations. Multiple factors play a role in the development, treatment and prevention of childhood asthma including racial/ethnic and socioeconomic disparities, both the home and school environments, and medication use. The goals of this review are to summarize these aspects of asthma in school-aged children and to present an updated review of medications as it relates to treatment strategies that will help in the care of these children. We conclude that phenotypic heterogeneity and appropriate environmental assessments and interventions are important considerations in the management of childhood asthma.
Keywords: asthma, racial/ethnic disparities, environment, medications, school, school-aged children, allergen, pollutant, inner-city, home, intervention, integrated pest management
Introduction
Asthma is the most common non-communicable childhood disease affecting approximately 14% of children globally and 8.6% of children in the United States (U.S.), with a rising prevalence worldwide (1, 2). Furthermore, asthma accounts for more than 14 million missed school days per year (3) and costs billions of dollars in health care utilization (4). Despite advancements in asthma management and the availability of effective medications, harmful environmental exposures and health disparities continue to exist (5).
Children of minority groups are more likely to have a higher prevalence of asthma, frequent emergency room visits and are at risk of having fragmented health care as a result of economic and non-economic factors (5). Moreover, environmental exposures are known to play a major role in the pathogenesis of asthma and its morbidity (6). Aeroallergen and airborne pollutant exposures in urban home and school environments have been implicated in worsening asthma symptoms in children (7). A Cochrane meta-analysis estimated that environmental control practices reduced the risk of current asthma by approximately 30–50% in children (8). Therefore, it is important that children with asthma take all the essential actions recommended to reduce their exposure to indoor environmental asthma triggers (9).
This review article will focus on current evidence supporting the clinical management of asthma among school-aged children in the context of existing racial/ethnic and socioeconomic disparities, environmental exposures, current guidelines-based treatments as well as promising novel therapeutics.
Racial/ethnic and socioeconomic disparities in childhood asthma management
Racial/ethnic minorities suffer a disproportionate rate of asthma morbidity in the U.S., with the highest rate of asthma found in Puerto Rican Americans (13.1%), followed by African Americans (9.5%), Caucasians (7.2%), and Mexican Americans (3.6%). Considerable asthma care disparities exist for African Americans compared to white youths with 4 times more emergency department visits, 3 times the hospitalization rate, and 7.6 times the death rate (10). Low socioeconomic status and low birth-weight, more common in African Americans, are risk factors for asthma and poses an increased risk of developing wheezing problems. Children from poor neighborhoods often spend more time indoors due to lack of parks, playgrounds, and recreational programs, resulting in an increased exposure to potential indoor allergens (11).
There are a number of psychological factors associated with economically disadvantaged neighborhoods that have been linked to higher rates of asthma incidence and worsening asthma morbidity in these communities (Table I) (12–14). These factors include but are not limited to stress, high rates of unemployment, increased crime and violence rates, substandard housing, and lack of social support. Kopel and colleagues illustrated that the primary caregivers’ perception of neighborhood safety is associated with childhood asthma morbidity among inner-city schoolchildren with asthma (13). They also demonstrated that inner-city caregiver stress is related to worsened asthma morbidity among children (14). Intervention studies to improve asthma outcomes in these vulnerable populations are needed.
Table I.
| Stress |
| Concentrated poverty |
| Widespread unemployment |
| Substandard housing |
| Inability to pay utilities |
| High crime and violence rates |
| Lack of social supports and control over their lives |
A patient’s cultural beliefs about healthcare can be a factor that impedes the quality of asthma care (15). The School Inner-City Asthma Study (SICAS) is a National Institute of Health (NIH) and National Institute of Allergy and Infectious Disease (NIAID) funded, comprehensive, prospective study of inner-city school and classroom-specific exposures and asthma morbidity among students in the Northeastern U.S., adjusting for home exposures (16). Gaffin et al. demonstrated in the SICAS cohort an association between Hispanic race and negative perceptions of asthma medications and further showed that this perception is connected to more asthma symptom days (17). They also revealed that caregivers of Hispanic children were 5 times more likely to be concerned that their child takes too much medication; these medication concerns were strongly and consistently associated with poor asthma control. In addition, the belief that their child received too much medication was independent of caregiver education, income, or Medicaid status suggesting racial/ethnic identification is the key influence on attitudes. Another study of Hispanic and African American parents of children with asthma found that 89% treated their children with some form of complementary medicine, and most did not inform the child’s physician. Of these, 59% believed the alternative therapy was at least as effective as the physician prescribed medications (18). McQuaid and colleagues showed that adherence to controller medications was lower among Latino children due to parental beliefs about medication necessity (19). Parental concerns regarding asthma medications might be a key modifiable risk factor for suboptimal controller mediation use, thereby, directly influencing adherence. Another critical issue associated with racial/ethnic disparities in asthma management is access to and quality of health care. It has been shown that racial/ethnic minority children are more likely to receive fragmented and episodic health care and are less likely to be given prescriptions for preventive medications (11).
Employing members of the underserved racial and ethnic communities as community health workers is one evidence-based strategy for providing culturally competent care. Community health workers have been legitimized for their cultural and linguistic proficiency, their ability to form trusting relationships with patients and their families and act as mediators between health care providers and their patients. This could be achieved through successful home visits that include asthma education, assessment of health literacy, medication adherence, environmental assessment of common asthma triggers, educational material for smoking cessation, and review of the child’s asthma action plan.
Environmental factors in the management of childhood asthma
The Indoor environment
The indoor environment contains numerous exposures that influence asthma development and morbidity (Table II) (20). Sensitization and exposure to indoor allergens including dust mites, rodents, cockroach, and pet allergens, worsen childhood asthma control and lung function due to greater airway inflammation and morbidity. More than 80% of school-aged children with asthma are sensitized to at least one indoor allergen, which is a strong predictor of disease persistence in later life (21). A recent study demonstrated that the timing of sensitization is an important factor and if it occurs at younger ages, it is associated with an increased risk of asthma in later childhood (22).
Table II.
Exposures in indoor environment that affect asthma [20]
Allergens
|
Pollutants
|
Likewise, exposure to common indoor pollutants, including secondhand smoke (SHS) and nitrogen dioxide (NO2), exacerbates asthma in children regardless of allergic sensitization (9). These exposures may be heightened in children due to the structure and mechanics of their airways as well as closer proximity to the floor which oftentimes is a reservoir of pollutants, chemicals, and/or allergens (23, 24).
Indoor Allergens
Dust mites
Der f 1 and Der p 2 allergens are from the two most common house dust mite species, Dermatophagoides farinae and Dermatophagoides pteronyssinus, respectively. They are microscopic members of the Arachnid class and require moisture to survive. About 30%–60% of children with persistent asthma are sensitized to dust mites (25). Since dust mites are microscopic, it is difficult to identify them as an allergic trigger. Therefore, when taking an environmental history, questions regarding climatic conditions and clutter which favor dust mite growth should be asked. A commercially available kit now available can test the dust mite content in a house dust sample (9).
Effective strategies to reduce dust mite exposures include: (1) frequent washing of all bed linens in hot water, (2) use of allergen-impermeable mattresses and pillow encasements, and (3) measures that target other dust mite allergen reservoirs such as vacuuming, removal of carpet and stuffed toys from the bed. Unfortunately, it is difficult to attain sustained reduction in indoor humidity, allowing for dust mite breeding and survival. Moreover, air filtration has not been shown to have a great effect on lessening dust mite exposure (26).
Furry pets
Cat and dog are the most common indoor furry pets. Can f 1 and Fel d 1 are the major dog and cat allergens, respectively. About 25%–65% of children with persistent asthma are sensitized to cat or dog allergens (25). Allergen from furry animals adhere to clothing, walls, furniture and other upholstered surfaces, and are also carried on small particles (<10–20 μm) allowing them to remain airborne for long periods of time. Sensitization to pet allergens can occur either through direct or indirect exposure due to passive transfer of allergen from people who have furry pets into environments that do not contain them (27). Thus, the absence of an indoor furry pet does not exclude pet allergen exposure (9).
The first line strategy for reducing pet allergen exposure is removal of the pet, followed by cleaning to remove reservoirs. It is important to note that even after removal of a pet, it can take several months for significant reduction of allergen levels to occur (28). If a family is unwilling to remove their pet, other intervention strategies would include keeping pets out of the bedroom, encasing mattress and pillows, removing carpeting, and cat/dog immersion washing which must be frequent to be effective. There is no scientific evidence to support “hypoallergenic” pets. The utility of air filtration systems to reduce pet allergen levels is uncertain as several studies have shown its ineffectiveness in reducing airborne pet allergen levels (29).
Cockroach and rodents
In the late 1990’s, the National Cooperative Inner-City Asthma Study showed highly detectable levels of cockroach allergen in inner-city homes and demonstrated that children with asthma who were both sensitized and exposed to high levels of cockroach allergen had increased asthma morbidity (30). The major cockroach allergens include Bla g 1 and Bla g 2. Cockroach infestation is often associated with inner-cities, low socioeconomic status, and populated areas. In the same way, studies have shown that mouse allergen is prevalent in urban homes and its exposure is associated with increased childhood asthma morbidity (31, 32). Major mouse allergenic proteins are Mus m 1 and Mus m 2. These allergens can be found in mouse urine, dander, and hair follicles.
Control measures for both cockroach and rodents include home extermination of occupant and neighbors, but this is not effective alone. Integrated pest management (IPM), which is a multidisciplinary approach that uses a range of pest control methods, has been studied as an intervention strategy to reduce pest allergen levels. It includes four fundamental principles: 1) monitoring pest populations with sticky traps to find out where they are living and hiding (reservoirs); 2) blocking pest access and entryways; 3) eliminating food and water (facilitating factors); 4) selectively applying low-toxicity pesticides. IPM might be cost-prohibitive and difficult to implement in some situations. Also, removal of reservoirs such as carpeting, bedding, or other areas containing allergen is helpful.
Mold and Dampness
Approximately 12.2% of children with persistent asthma are sensitized to molds (33). There is a wide variety of indoor and outdoor molds and its allergenic proteins vary by mold species. Common indoor molds are Aspergillus and Penicillium species. Common outdoor molds are Cladosporium, Alternaria, and Epicoccum species. Molds depend on moisture for growth. Control measures include the use of air conditioner in the summer, placement of dehumidifier in the basement, repair of leaks, removal of water-damaged materials, running a vent in the bathroom and kitchen and cleaning moldy areas with a fungicide.
The most common species to which children are sensitized and exposed are Alternaria, Aspergillus, Cladosporium, and Penicillium (34). The National Survey of Lead and Allergens in Housing found that 56% of homes had mold levels above thresholds observed to be associated with asthma symptoms (35). Mold remediation has been shown to reduce asthma symptoms and the need for asthma medications, even in populations who are not mold sensitized (36).
Indoor air pollutants
Particulate Matter
Particulate Matter (PM) are airborne particles expressed as either PM2.5 (fine PM), with an aerodynamic diameter of 2.5 microns or less, or PM10 (coarse PM) with aerodynamic diameter more than 2.5 microns up to 10 microns. Indoor PM is formed mostly from indoor generated particles through smoking or other activities such as cooking and sweeping, and partly from outdoor generated particles. Indoor PM exposures are associated with worsening asthma symptoms in inner-city children with asthma (37) The use of high efficiency particulate arrestance (HEPA) air filters to reduce indoor PM is currently being studied as an effective long-term strategy to reduce indoor air pollution but more studies are needed (38).
Second-hand smoke (SHS)
Cigarette smoke is a major contributor to indoor pollutant particles in the U.S., where 30% of all children are exposed to indoor SHS (39) In a cross-sectional study, using NHANES data of 2,250 youths with asthma, 17.3% reported using tobacco smoke products, and of the non-smokers, 53.2% were exposed to SHS in their homes (40). Tobacco smoke contains solid particles, semi-volatile, and volatile organic compounds which function as respiratory irritants. SHS is known to exacerbate asthma.
There have been a few intervention trials targeting smoking cessation and reduction of SHS exposure in the home (41, 42). Evidence suggests that HEPA air filters might be useful, especially for children who are not able to avoid SHS. In a double-blind randomized control trial of children with asthma and known secondhand environmental tobacco smoke exposure, the use of HEPA air filters resulted in a reduction in the number of unscheduled asthma visits and fewer airborne nicotine particles in the intervention group when compared to the control group (42). Nevertheless, the most effective way to reduce or eliminate tobacco smoke exposure is smoking cessation by close family members and caregivers.
Several studies have noted a reduction in the harmful effects of SHS in children with asthma through the establishment of smoke-free public environments. A recent meta-analysis showed that implementation of the World Health Organization’s recommended smoke-free legislation (MPOWER) led to reductions in preterm birth, and hospital admissions for asthma exacerbations and respiratory tract infections in children (43). Ciaccio et al. demonstrated that indoor smoke-free legislation was associated with a 17% decrease in the incidence of childhood severe asthma emergency room visits (44). Another study reported a significant reduction in asthma-related emergency room visits in both children and adults after enactment of national smoke-free policies in public environments, including the workplace (45).
Air Pollution and Asthma
Traffic pollution is a major contributor of urban air pollution which can penetrate indoors and adversely affect the indoor air quality of schools (46, 47). This source of air pollution contains gaseous pollutants such as ozone, nitrogen dioxide (NO2), and sulfur dioxide (SO2) which can exacerbate asthma due to oxidative stress, resulting in lung injury and inflammation. Xiao and colleagues used multi-pollutant models to show that short term exposures to ozone, NO2, and SO2 is associated with an increase in asthma symptoms in children (48). This is important since schools are typically centrally located within a community in close proximity to highways, heavy traffic routes, and commercial and industrial buildings. School locations also serve as a site for drop-off/pick-up, idling of cars, bus stops, potentially contributing to an increase in ambient air pollution.
Ozone is produced by a photochemical reaction between sunlight and pollutant precursors like nitrogen oxide (49). Short term exposure to ozone is a known, evidence based cause of asthma exacerbations in both children and adults (50). Data from the National Cooperative Inner-City Asthma Study showed that ozone was associated with a decline in morning % peak expiratory flow rate and an increase in morning asthma in children with asthma (51).
SO2 is a by-product of energy production or industrial processes (46). Children are more susceptible to SO2 because they spend more time outdoors. Fortunately, SO2 exposure has decreased tremendously in developed counties due to the use of alternative energy sources such as coal combustion. However, SO2 exposure remains a struggle in developing countries. NO2 is a pollutant gas produced from high-temperature combustion from sources such as gas heating, appliances, unvented space heaters, fireplaces and old wood-burning stoves. Hansel et al. showed that high indoor NO2 exposure worsens inner-city childhood asthma (52). Similarly, Belanger and colleagues reported that children with asthma exposed to indoor NO2 levels well below the Environmental Protection Agency (EPA) outdoor standard (53 ppb), were at risk for increased frequency of wheeze, night symptoms, and use of rescue medication (53). An intervention strategy of replacing gas stoves with electric stoves resulted in a 40% to 50% reduction in indoor NO2 levels (54). Interestingly, data also suggests that overweight and obese individuals are more affected by air pollutant exposure with increased asthma symptoms (55).
Environmental interventions
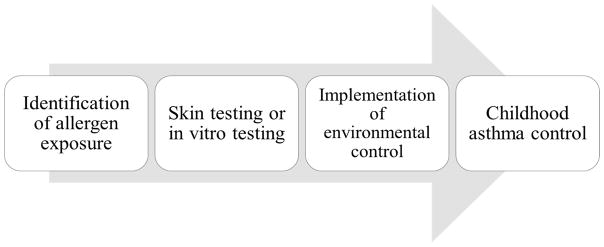
In the era of personalized medicine, the implementation of an individualized tailored environmental intervention strategy based on a child’s sensitization status and home exposures is ideal. A priority message from the National Asthma Education and Prevention Program (NAEPP) Expert Panel Report 3 guidelines for the management of asthma is that for any patient with persistent asthma, the clinician should: 1) identify allergen exposures; 2) use skin testing or in vitro testing to assess specific sensitivities to indoor allergens; and 3) implement environmental controls to reduce exposure to relevant allergens (56) (Fig 1). A thorough environmental history should be obtained to identify potential environmental exposures in the home that are known to trigger asthma symptoms and exacerbations, including allergens and pollutants. This would include questions regarding the presence of pets or pests, conditions favoring dust mite breeding, presence of smokers in the home, and the use of gas stoves.
Fig. 1.
NAEPP EPR-3 guidelines for management of childhood asthma [56]
A recent workshop report highlights the findings of several home environmental intervention trials and its influence on exposure reduction and asthma control (6). The term “multifaceted” has been used to describe interventions directed toward more than one asthma trigger or interventions with more than one component. In a landmark study, Morgan and colleagues revealed that among inner-city children with atopic asthma, an individualized, home-based, multifaceted environmental intervention decreased exposure to indoor allergens, including cockroach and dust-mite allergens, leading to decreased asthma morbidity (57). Rabito et al. demonstrated that a single low-cost intervention to reduce cockroach exposure, the strategic placement of insecticidal bait in the homes of children with asthma, resulted in significant cockroach reduction and improved asthma outcomes when compared to the no intervention control group (58). One randomized controlled trial evaluating the effect of individualized multifaceted allergen reduction in the home on the ability to reduce asthma controller therapy concluded that despite a significant reduction of measured allergen levels in the intervention group, it did not result in a reduction in asthma controller therapy (59). Another study compared a year-long IPM intervention plus pest management education vs pest management education alone among mouse-sensitized and exposed children; both groups had a reduction in mouse allergen levels but there was no significant difference in maximal asthma symptom days between the 2 groups (60).
The School Environment
An association between inner-city home environmental exposures and significant childhood asthma morbidity has been demonstrated through years of previous research (61). Many of these allergens and pollutants are also present in the inner-city school environment (62, 63). Although parents may attempt to reduce or eliminate indoor allergen exposures in the home, children spend a large of portion of their day in schools and daycare centers where environmental exposures are not as easily controlled. The environment outside of the home, especially in the U.S. is less well understood. As a consequence, successful home-based strategies currently serve as the prototype for school-based environmental interventions.
Prior studies have reported on the prevalence of various indoor allergens in school environments. Chew et al. showed cockroach and mouse allergens to be highly prevalent in urban schools (64). The SICAS results demonstrated substantial levels of mouse allergen in inner-city school classrooms compared to the same students’ homes (62), with exposure to mouse allergen in schools associated with increased asthma symptoms and decreased lung function (65). Interestingly, cat and dog allergen levels were lower in the SICAS inner-city schools when compared to European school-based studies showing higher levels, likely due to passive transfer from students who owned pets in their homes (66). High levels of mold in schools have also been reported in both the U.S. and Europe (67, 68).
There is a scarcity of thorough data on school-based environmental interventions and health outcomes. The few studies that have been published are small and not adequately powered to comprehensively assess asthma morbidity outcomes (69–73). Given the SICAS findings of high mouse allergen levels in school classrooms (62, 65), a NIH/NIAID School Inner-City Asthma Intervention Study (ClinicalTrials.gov NCT02291302), SICAS 2, using environmental control strategies modeled from successful home-based interventions, is underway with health outcomes results pending (74). This randomized, controlled trial is comprehensively evaluating the role of a school-based environmental intervention and is adequately powered to assess asthma outcomes, adjusting for home exposures. Pilot data from this study demonstrated that a classroom-based air cleaner intervention led to significant reductions in particulate matter with diameter of <2.5 μm and black carbon, compared to sham filters (38). The use of HEPA air filters to reduce indoor pollutant particles and its associated allergens is currently being studied in SICAS 2. Another pilot study showed that HEPA air filters reduced mold spore counts in daycare centers (75). If these results could be related to health effects in larger scale trials, implementation of multifaceted intervention strategies in school and daycare settings may be considered.
The relationship between air pollution and asthma morbidity in children is well established (76). Schools are usually located in close proximity to highways, heavy traffic routes, and commercial and industrial buildings within a community. Approximately 3.2 million (6.5%) children across the U.S. attend schools located within 100 meters of a major roadway as defined by the U.S. Census Bureau (77). Traffic pollution is an important source of PM, NO2 and black carbon which can penetrate and adversely affect the indoor air quality of schools (47). Other factors fueling the poor indoor air quality of schools are reduced ventilation (78, 79), inadequate building maintenance (80), and exposure to janitorial cleaning products and the by-products of heating and cooling systems.
The U.S. EPA created the Indoor Air Quality Tools for Schools Program (www.epa.gov/iaq-schools/indoor-air-quality-tools-schools-action-kit) with the aim of improving environmental conditions in schools such as keeping ventilation units in classrooms free of clutter, reducing the number of items made of cloth in the classroom, removing classroom pets that trigger asthma attacks in students and reporting maintenance problems in classrooms immediately. In addition, the School-Based Asthma Management Program (SAMPRO™) and the Centers for Disease Control and Prevention (CDC) Healthy Schools Program offer toolkits to schools showing how asthma-friendly schools provide appropriate school health services for students with asthma and a safe and healthy school environment to reduce asthma triggers (81).
A key component to effective care of a child with asthma is an education partnership between healthcare providers and patient/caregiver. Caregivers of children with asthma need to understand the disease, how and what medications are used to treat it, and steps that should be taken to treat an exacerbation. Healthcare providers also have a responsibility to ask about environmental exposures and to ensure that patients have the knowledge and resources to implement environmental control measures. Those with written asthma action plans are more likely to take actions to reduce exposures; however, less than half of children with asthma are actually given one (82). Current national asthma guidelines support provision of a written asthma action plan although recent literature suggests that adding a formal written asthma action plan does not lead to better outcomes beyond asthma education alone (83). In addition, monitoring peak expiratory flow (PEF) values is an option as part of asthma action plans and is recommended in emergency departments and hospitalized patients. However, many pediatric patients do not use peak flow meters correctly (84). Healthcare providers should take the time regularly observe patients using peak flow meters to help ensure correct use.
Lastly, school-based health centers (SHBCs) and their staff are uniquely positioned to help children with asthma maintain healthy indoor environments in both school and home by increasing awareness, facilitating an indoor air quality assessment, and implementing specific interventions to address mold, dust mites, pests, pets, and ventilation. They provide an ideal setting to incorporate environmental components into existing chronic disease programs. Research shows that SHBCs can lead to significant improvements in health outcomes for children with asthma including less missed school, less asthma-related restricted activity days, and reduced frequency of ER visits and/or hospitalizations (85–87).
Asthma Medications in School-Aged Children
Guideline-based Therapy
Asthma guidelines were developed through the years to increase asthma awareness among health professionals and to improve asthma management. The first international guidelines were formulated by the National Heart Lung and Blood Institute (NHLBI) in the U.S. in 1991 with the most recent version published in 2007 as the Expert Panel Report 3 (EPR-3) (56) of the NAEPP. Other guidelines followed including the PRACTALL Consensus Report published by the European Academy of Asthma and Allergy in 2008 (88) and most recently the 2017 updated version of the Global Initiative for Asthma (GINA) guidelines for the diagnosis and management of asthma in children (www.ginasthma.org) (89). The initial development of these guidelines to address the management of asthma in children was due in part to the lack of agreement on the diagnosis and management of asthma in young children; a paucity of randomized controlled clinical trials existed to examine different treatments for childhood asthma. Since then, pediatric clinical asthma trials have emerged to shed some light on this topic.
The theory has long been held that inhaled corticosteroid (ICS) use in young children can change the natural history of asthma and might prevent lung function decline. Asthma often has its origin in early childhood with evidence for reduced lung function growth during these early years persisting into adulthood (90). The Childhood Asthma Management Program (CAMP) study noted that 75% of children aged 5 to 12 years with mild to moderate asthma had abnormal patterns of lung growth (90). ICSs are recommended by asthma guidelines as the first line of treatment for school-aged children with persistent asthma (56, 89), both in prevention of exacerbations and reduction in symptoms. A 44 week, randomized, double-blind, placebo-controlled trial (TREXA) enrolled children/adolescents aged 5–18 years with mild persistent asthma and showed that daily ICS use is more effective than rescue albuterol alone in preventing exacerbations (91). Further, ICS as rescue medication with albuterol might be an effective step-down strategy for children with well controlled, mild asthma as it was shown to be more effective than rescue albuterol alone in reducing exacerbations (91). A 2015 Cochrane review showed that intermittent ICS treatment at the time of exacerbation in children with persistent asthma decreased the need for oral corticosteroid by half with no growth suppression noted in either pre-school or school-aged children (92).
For children with severe asthma, evidence-based guidelines recommend treatment with higher-dose ICS or oral corticosteroids combined with a second controller such as long-acting beta-agonists (LABAs), leukotriene receptor antagonists (LTRA), and theophylline (56) (93) (89). Lemanske et al. assessed the frequency of differential responses to three blinded step-up treatments (medium-dose ICS, low-dose ICS/LABA, or low-dose ICS/LTRA) in children 6 to 17 years of age who had uncontrolled asthma while receiving low-dose ICS (BADGER Trial) (94). Nearly all the children had a differential response to 1 of the 3 treatments, with 45% of children responding best to adding a LABA.
The effect of ICS on growth velocity and final adult height has been a subject of debate. Regular use of ICS at low or medium daily doses was associated with a mean reduction of 0.48 cm/year in linear growth velocity and a 0.61cm change from baseline in height during a one-year treatment period in children with mild to moderate persistent asthma (95). The CAMP trial, a prospective study starting in childhood with subjects followed through to adulthood, showed that a larger daily dose of ICS in the first 2 years was associated with a decrease in prepubertal height that persisted as a reduction in adult height (−0.1 cm for each microgram per kilogram of body weight; p = 0.007) in a non-progressive or cumulative manner (96). Despite these effects of ICS on linear growth, it is still important to remember that the safety profile of ICS preparations is much better than the alternative oral corticosteroids. Moreover, it is unknown if the reductions in growth represent a permanent effect or a temporary 1–2 year slowing in growth velocity. Physicians prescribing ICS to children should have knowledge of this possibility and carefully monitor linear growth.
Lastly, inhaled medications are only effective if used properly. Age and cooperation between the child and caregiver are important determinants of effective inhalation therapy. Particle size, device portability, and the availability of dose counters are other important factors (97). Offering appropriate education about its proper use is crucial to gain asthma control.
The Era of Biologics
Despite extensive studies and diverse regimens to treat children with asthma, a substantial portion of pediatric patients remain symptomatic and refractory to the aforementioned medications. Fortunately, with the advent of biologics, potential new therapies exist to treat these severe steroid-dependent asthmatics depending on their asthma phenotype (98, 99).
Omalizumab is the first biologic to be included in the asthma guidelines and up until recently the only one studied in the pediatric population. Omalizumab is a subcutaneous injectable recombinant humanized IgG1 monoclonal anti-IgE antibody that is administered every 2 to 4 weeks to patients with chronic allergic asthma and with sensitization to at least 1 perennial aeroallergen and an elevated serum IgE level (100). It is now FDA approved in children 6 years or older with moderate to severe asthma.
A randomized, double-blind, placebo controlled trial of 419 inner-city children with persistent allergic asthma as young as 6 years noted decreased exacerbations (48.8% vs 30.3%; P<0.001), decreased hospitalizations (6.3% vs 1.5%; P=0.02), and lower inhaled corticosteroid use (P<0.001) during a 60-week treatment period with omalizumab added to a regimen of guidelines-based therapy (101). Milgrom and colleagues showed in another randomized placebo controlled study that in children aged 6 to 12 years with moderate to severe asthma, omalizumab reduced the doses of ICS required (102). A multi-center clinical trial of urban children aged 6 to 17 years with allergic asthma and frequent asthma exacerbations demonstrated that adding omalizumab to ICSs 4 to 6 weeks before the return of school reduced fall asthma exacerbations, particularly in those children with severe asthma requiring step 5 treatment, as defined by guidelines-based asthma care (EPR-3) (103). Despite its limitations of IgE and weight requirements and its prohibitive cost, omalizumab seems to be effective in children who require high doses of ICS and suffer from frequent exacerbations.
Mepolizumab was recently FDA approved for use in children 12 years or older for the treatment of eosinophilic asthma. It is a subcutaneous injectable humanized monoclonal antibody directed against IL-5. In the DREAM Trial, mepolizumab treatment in patients as young as 12 years with severe eosinophilic asthma resulted in a significant reduction in asthma exacerbations when compared to placebo (104). Treatment with mepolizumab has also resulted in an increase in FEV1 from baseline, reduction in exacerbations necessitating emergency room visits and hospitalizations, and improvement in asthma symptom scores (105).
Other biologics are currently being studied in clinical trials for use in pediatric and adult asthma including reslizumab (anti-IL-5), dupilumab (anti-IL-4/13), and lebrikizumab (anti-IL-13), to name a few (106).
Potential Treatments
Vitamin D has been studied extensively as a potential immunomodulator and treatment for asthma given a significant body of epidemiologic and cross-sectional associations between vitamin D insufficiency and increased incidence and severity of childhood asthma (107–109). A recent systematic review and meta-analysis of individual participant data (955 participants) showed that vitamin D supplementation reduced the overall rate of asthma exacerbations requiring treatment with systemic corticosteroids; there was no definitive evidence that the effects of vitamin D differed across subgroups of patients (110). Further randomized controlled trials are required to determine if school-aged children with asthma might benefit from Vitamin D supplementation.
Macrolide therapy is also being studied as a potential treatment for asthma, particularly in asthmatics with a neutrophil predominant phenotype (111). Due to their immunomodulatory effect on neutrophil-based airway inflammation and its antibacterial effect, macrolides have become an area of interest in asthma. Several small studies showed that its use in school-aged children with asthma increased the number of symptom-free days, shortened the duration of an exacerbation, improved bronchial hyper-responsiveness, and reduced airway neutrophils (112, 113). A multicenter randomized, double-blind, placebo-controlled, parallel-group trial demonstrated that the use of azithromycin early during a respiratory tract illness (RTI) reduced the likelihood of severe lower RTI in young preschool children (114). Additional randomized controlled trials in school-aged children with asthma are warranted to confirm its effects in this age group.
Conclusion
Management of asthma in school-aged children requires an encompassing environmental approach. Regular assessment and monitoring, control of factors that contribute to or aggravate symptoms, guidelines-based pharmacologic therapy and education of children and their caregivers are of paramount importance. However, understanding how racial/ethnic and socioeconomic disparities along with the surrounding environment affects asthma outcomes in children is just as crucial to clinical management. Future research should continue to examine intervention strategies to improve childhood asthma as it pertains to disparities and harmful environmental exposures. Furthermore, pediatric randomized trials are needed if novel therapeutics is to be considered in school-aged children.
Acknowledgments
Funding and Support: This work was supported by grants K24 AI 106822, R01 HL 137192, U01 AI 110397, and U01 AI 126614 (PI: Dr. Phipatanakul), and K23 AI 123517 (PI: Dr. Permaul) from the National Institutes of Health. This work was also supported in part from the Allergy and Asthma Awareness Initiative, Inc.
Abbreviations
- IgE
Immunoglobulin E
- HEPA
high efficiency particulate arrestance
- IPM
integrated pest management
- NAEPP
National Asthma Education and Prevention Program
- SICAS
School Inner-City Asthma Study
- NIH
National Institutes of Health
- NIAID
National Institute of Allergy and Infectious Disease
- PM
particulate matter
- NO2
nitrogen dioxide
- EPA
Environmental Protection Agency
- SAMPRO
School-Based Asthma Management Program
- CDC
Centers for Disease Control and Prevention
- SBHC
school-based health centers
- SHS
secondhand smoke
- ICS
inhaled corticosteroid
- CAMP
Childhood Asthma Management Program
- GINA
Global Initiative for Asthma
- EPR-3
Expert Panel Report 3
- LABA
long-acting beta agonist
- LTRA
leukotriene receptor antagonist
Footnotes
Disclosure Statement: The authors declare they have no conflicts to disclose, regarding to or outside this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zar HJ, Ferkol TW. The global burden of respiratory disease—impact on child health. Pediatric pulmonology. 2014;49(5):430–4. doi: 10.1002/ppul.23030. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control Asthma D, Statistics and Surveillance. Most Recent Asthma Data. 2014 Available from: http://www.cdc.gov/asthma/most_recent_data.htm.
- 3.Centers for Disease Control Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morbidity and mortality weekly report. 2011;60(17):547. [PubMed] [Google Scholar]
- 4.Hasegawa K, Tsugawa Y, Brown DF, Camargo CA. Childhood asthma hospitalizations in the United States, 2000–2009. The Journal of pediatrics. 2013;163(4):1127–33e3. doi: 10.1016/j.jpeds.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleason M, Cicutto L, Haas-Howard C, Raleigh BM, Szefler SJ. Leveraging Partnerships: Families, Schools, and Providers Working Together to Improve Asthma Management. Current allergy and asthma reports. 2016;16(10):74. doi: 10.1007/s11882-016-0655-0. [DOI] [PubMed] [Google Scholar]
- 6.Gold DR, Adamkiewicz G, Arshad SH, Celedón JC, Chapman MD, Chew GL, et al. NIAID, NIEHS, NHLBI, and MCAN Workshop Report: The indoor environment and childhood asthma—implications for home environmental intervention in asthma prevention and management. Journal of Allergy and Clinical Immunology. 2017 doi: 10.1016/j.jaci.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauptman M, Phipatanakul W. The school environment and asthma in childhood. Asthma Res Pract. 2015:1. doi: 10.1186/s40733-015-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maas T, Kaper J, Sheikh A, Knottnerus JA, Wesseling G, Dompeling E, et al. Mono and multifaceted inhalant and/or food allergen reduction interventions for preventing asthma in children at high risk of developing asthma. The Cochrane Library. 2009 doi: 10.1002/14651858.CD006480.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Matsui EC, Abramson SL, Sandel MT. Indoor environmental control practices and asthma management. Pediatrics. 2016;138(5):e20162589. doi: 10.1542/peds.2016-2589. [DOI] [PubMed] [Google Scholar]
- 10.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Supplement 3):S131–S45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 11.Price JH, Khubchandani J, McKinney M, Braun R. Racial/ethnic disparities in chronic diseases of youths and access to health care in the United States. BioMed Research International. 2013;2013 doi: 10.1155/2013/787616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopel LS, Phipatanakul W, Gaffin JM. Social disadvantage and asthma control in children. Paediatric respiratory reviews. 2014;15(3):256–62. doi: 10.1016/j.prrv.2014.04.017. quiz 62–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopel LS, Gaffin JM, Ozonoff A, Rao DR, Sheehan WJ, Friedlander JL, et al. Perceived neighborhood safety and asthma morbidity in the school inner-city asthma study. Pediatr Pulmonol. 2015;50(1):17–24. doi: 10.1002/ppul.22986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopel LS, Petty CR, Gaffin JM, Sheehan WJ, Baxi SN, Kanchongkittiphon W, et al. Caregiver stress among inner-city school children with asthma. The journal of allergy and clinical immunology In practice. 2017;5(4):1132–4e3. doi: 10.1016/j.jaip.2017.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansour ME, Lanphear BP, DeWitt TG. Barriers to asthma care in urban children: parent perspectives. Pediatrics. 2000;106(3):512–9. doi: 10.1542/peds.106.3.512. [DOI] [PubMed] [Google Scholar]
- 16.Phipatanakul W, Bailey A, Hoffman EB, Sheehan WJ, Lane JP, Baxi S, et al. The school inner-city asthma study: design, methods, and lessons learned. Journal of Asthma. 2011;48(10):1007–14. doi: 10.3109/02770903.2011.624235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaffin JM, Landrum A, Petty CR, Baxi S, Sheehan WJ, Phipatanakul W. Black and Hispanic perceptions of asthma medication in the School Inner City Asthma Study. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2015;114(6):533. doi: 10.1016/j.anai.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph CL, Williams LK, Ownby DR, Saltzgaber J, Johnson CC. Applying epidemiologic concepts of primary, secondary, and tertiary prevention to the elimination of racial disparities in asthma. Journal of Allergy and Clinical Immunology. 2006;117(2):233–40. doi: 10.1016/j.jaci.2005.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McQuaid EL, Everhart RS, Seifer R, Kopel SJ, Mitchell DK, Klein RB, et al. Medication adherence among Latino and non-Latino white children with asthma. Pediatrics. 2012;129(6):e1404–e10. doi: 10.1542/peds.2011-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diette GB, McCormack MC, Hansel NN, Breysse PN, Matsui EC. Environmental issues in managing asthma. Respiratory care. 2008;53(5):602–17. [PMC free article] [PubMed] [Google Scholar]
- 21.Sheehan WJ, Phipatanakul W. Indoor allergen exposure and asthma outcomes. Current opinion in pediatrics. 2016;28(6):772. doi: 10.1097/MOP.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. Journal of Allergy and Clinical Immunology. 2017;139(2):501–7. doi: 10.1016/j.jaci.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foos B, Marty M, Schwartz J, Bennett W, Moya J, Jarabek AM, et al. Focusing on children’s inhalation dosimetry and health effects for risk assessment: an introduction. Journal of Toxicology and Environmental Health, Part A. 2007;71(3):149–65. doi: 10.1080/15287390701597871. [DOI] [PubMed] [Google Scholar]
- 24.Miller MD, Marty MA, Arcus A, Brown J, Morry D, Sandy M. Differences between children and adults: implications for risk assessment at California EPA. International journal of toxicology. 2002;21(5):403–18. doi: 10.1080/10915810290096630. [DOI] [PubMed] [Google Scholar]
- 25.Weiss ST, Horner A, Shapiro G, Sternberg AL Group CAMPR. The prevalence of environmental exposure to perceived asthma triggers in children with mild-to-moderate asthma: data from the Childhood Asthma Management Program (CAMP) Journal of allergy and clinical immunology. 2001;107(4):634–40. doi: 10.1067/mai.2001.113869. [DOI] [PubMed] [Google Scholar]
- 26.Portnoy J, Miller JD, Williams PB, Chew GL, Miller JD, Zaitoun F, et al. Environmental assessment and exposure control of dust mites: a practice parameter. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2013;111(6):465. doi: 10.1016/j.anai.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almqvist C, Wickman M, Perfetti L, Berglind N, Renstrom A, Hedren M, et al. Worsening of asthma in children allergic to cats, after indirect exposure to cat at school. American journal of respiratory and critical care medicine. 2001;163(3):694–8. doi: 10.1164/ajrccm.163.3.2006114. [DOI] [PubMed] [Google Scholar]
- 28.Wood RA, Chapman MD, Adkinson NF, Jr, Eggleston PA. The effect of cat removal on allergen content in household-dust samples. J Allergy Clin Immunol. 1989;83(4):730–4. doi: 10.1016/0091-6749(89)90006-7. [DOI] [PubMed] [Google Scholar]
- 29.Sulser C, Schulz G, Wagner P, Sommerfeld C, Keil T, Reich A, et al. Can the use of HEPA cleaners in homes of asthmatic children and adolescents sensitized to cat and dog allergens decrease bronchial hyperresponsiveness and allergen contents in solid dust? International archives of allergy and immunology. 2009;148(1):23–30. doi: 10.1159/000151502. [DOI] [PubMed] [Google Scholar]
- 30.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. New England Journal of Medicine. 1997;336(19):1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 31.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. Journal of allergy and clinical immunology. 2000;106(6):1070–4. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 32.Ahluwalia SK, Peng RD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. Journal of Allergy and Clinical Immunology. 2013;132(4):830–5e2. doi: 10.1016/j.jaci.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byeon JH, Ri S, Amarsaikhan O, Kim E, Ahn SH, Choi IS, et al. Association Between Sensitization to Mold and Impaired Pulmonary Function in Children With Asthma. Allergy, Asthma & Immunology Research. 2017;9(6):509–16. doi: 10.4168/aair.2017.9.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pongracic JA, O’connor GT, Muilenberg ML, Vaughn B, Gold DR, Kattan M, et al. Differential effects of outdoor versus indoor fungal spores on asthma morbidity in inner-city children. Journal of Allergy and Clinical Immunology. 2010;125(3):593–9. doi: 10.1016/j.jaci.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salo PM, Arbes SJ, Crockett PW, Thorne PS, Cohn RD, Zeldin DC. Exposure to multiple indoor allergens in US homes and its relationship to asthma. Journal of Allergy and Clinical Immunology. 2008;121(3):678–84e2. doi: 10.1016/j.jaci.2007.12.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burr ML, Matthews IP, Arthur RA, Watson HL, Gregory CJ, Dunstan FDJ, et al. Effects on patients with asthma of eradicating visible indoor mould: a randomised controlled trial. Thorax. 2007;62(9):767–72. doi: 10.1136/thx.2006.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams DA, Curtin-Brosnan J, et al. In-home particle concentrations and childhood asthma morbidity. Environmental health perspectives. 2009;117(2):294. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jhun I, Gaffin JM, Coull BA, Huffaker MF, Petty CR, Sheehan WJ, et al. School environmental intervention to reduce particulate pollutant exposures for children with asthma. The Journal of Allergy and Clinical Immunology: In Practice. 2017;5(1):154–9e3. doi: 10.1016/j.jaip.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC. Indoor air pollution and asthma in children. Proceedings of the American Thoracic Society. 2010;7(2):102–6. doi: 10.1513/pats.200908-083RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kit BK, Simon AE, Brody DJ, Akinbami LJ. US prevalence and trends in tobacco smoke exposure among children and adolescents with asthma. Pediatrics. 2013;131(3):407–14. doi: 10.1542/peds.2012-2328. [DOI] [PubMed] [Google Scholar]
- 41.Butz AM, Matsui EC, Breysse P, Curtin-Brosnan J, Eggleston P, Diette G, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Archives of pediatrics & adolescent medicine. 2011;165(8):741–8. doi: 10.1001/archpediatrics.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanphear BP, Hornung RW, Khoury J, Yolton K, Lierl M, Kalkbrenner A. Effects of HEPA air cleaners on unscheduled asthma visits and asthma symptoms for children exposed to secondhand tobacco smoke. Pediatrics. 2011;127(1):93–101. doi: 10.1542/peds.2009-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faber T, Kumar A, Mackenbach JP, Millett C, Basu S, Sheikh A, et al. Effect of tobacco control policies on perinatal and child health: a systematic review and meta-analysis. The Lancet Public Health. 2017;2(9):e420–e37. doi: 10.1016/S2468-2667(17)30144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciaccio CE, Gurley-Calvez T, Shireman TI. Indoor tobacco legislation is associated with fewer emergency department visits for asthma exacerbation in children. Ann Allergy Asthma Immunol. 2016;117(6):641–5. doi: 10.1016/j.anai.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Croghan IT, Ebbert JO, Hays JT, Schroeder DR, Chamberlain AM, Roger VL, et al. Impact of a countywide smoke-free workplace law on emergency department visits for respiratory diseases: a retrospective cohort study. BMC pulmonary medicine. 2015;15:6. doi: 10.1186/1471-2466-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet (London, England) 2014;383(9928):1581–92. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brandt EB, Myers J, Ryan PH, Hershey G. Air pollution and allergic diseases. Current opinion in pediatrics. 2015;27(6):724–35. doi: 10.1097/MOP.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Q, Liu Y, Mulholland JA, Russell AG, Darrow LA, Tolbert PE, et al. Pediatric emergency department visits and ambient Air pollution in the US State of Georgia: a case-crossover study. Environmental Health. 2016;15(1):115. doi: 10.1186/s12940-016-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.US EPA O, National Center for Environmental Assessment, NCEA-RTP. Integrated Science Assessment (ISA) of Ozone and Related Photochemical Oxidants. 2013 Feb; Available from: https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=247492.
- 50.Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182(3):307–16. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mortimer K, Neas L, Dockery D, Redline S, Tager I. The effect of air pollution on inner-city children with asthma. European respiratory journal. 2002;19(4):699–705. doi: 10.1183/09031936.02.00247102. [DOI] [PubMed] [Google Scholar]
- 52.Hansel NN, Breysse PN, McCormack MC, Matsui EC, Curtin-Brosnan J, Williams DAL, et al. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner-city children with asthma. Environmental health perspectives. 2008;116(10):1428. doi: 10.1289/ehp.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belanger K, Holford TR, Gent JF, Hill ME, Kezik JM, Leaderer BP. Household levels of nitrogen dioxide and pediatric asthma severity. Epidemiology (Cambridge, Mass) 2013;24(2):320. doi: 10.1097/EDE.0b013e318280e2ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paulin LM, Diette G, Scott M, McCormack M, Matsui E, Curtin-Brosnan J, et al. Home interventions are effective at decreasing indoor nitrogen dioxide concentrations. Indoor Air. 2014;24(4):416–24. doi: 10.1111/ina.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu KD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, D’ann LW, et al. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. Journal of Allergy and Clinical Immunology. 2013;131(4):1017–23e3. doi: 10.1016/j.jaci.2012.12.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National AE, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. The Journal of allergy and clinical immunology. 2007;120(5 Suppl):S94. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 57.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, III, et al. Results of a home-based environmental intervention among urban children with asthma. New England Journal of Medicine. 2004;351(11):1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 58.Rabito FA, Carlson JC, He H, Werthmann D, Schal C. A single intervention for cockroach control reduces cockroach exposure and asthma morbidity in children. Journal of Allergy and Clinical Immunology. 2017 doi: 10.1016/j.jaci.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 59.DiMango E, Serebrisky D, Narula S, Shim C, Keating C, Sheares B, et al. Individualized household allergen intervention lowers allergen level but not asthma medication use: a randomized controlled trial. The Journal of Allergy and Clinical Immunology: In Practice. 2016;4(4):671–9e4. doi: 10.1016/j.jaip.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsui EC, Perzanowski M, Peng RD, Wise RA, Balcer-Whaley S, Newman M, et al. Effect of an integrated pest management intervention on asthma symptoms among mouse-sensitized children and adolescents with asthma: a randomized clinical trial. Jama. 2017;317(10):1027–36. doi: 10.1001/jama.2016.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crain EF, Walter M, O’Connor TG, Mitchell H, Gruchalla RS, Kattan M, et al. Home and allergic characteristics of children with asthma in seven US urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environmental health perspectives. 2002;110(9):939. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Permaul P, Hoffman E, Fu C, Sheehan W, Baxi S, Gaffin J, et al. Allergens in urban schools and homes of children with asthma. Pediatric Allergy and Immunology. 2012;23(6):543–9. doi: 10.1111/j.1399-3038.2012.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salo PM, Sever ML, Zeldin DC. Indoor allergens in school and day care environments. Journal of Allergy and Clinical Immunology. 2009;124(2):185–92e9. doi: 10.1016/j.jaci.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chew G, Correa J, Perzanowski M. Mouse and cockroach allergens in the dust and air in northeastern United States inner-city public high schools. Indoor air. 2005;15(4):228–34. doi: 10.1111/j.1600-0668.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 65.Sheehan WJ, Permaul P, Petty CR, Coull BA, Baxi SN, Gaffin JM, et al. Association between allergen exposure in inner-city schools and asthma morbidity among students. JAMA pediatrics. 2017;171(1):31–8. doi: 10.1001/jamapediatrics.2016.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perzanowski MS, Rönmark E, Nold B, Lundbäck B, Platts-Mills TA. Relevance of allergens from cats and dogs to asthma in the northernmost province of Sweden: schools as a major site of exposure. Journal of allergy and clinical immunology. 1999;103(6):1018–24. doi: 10.1016/s0091-6749(99)70173-9. [DOI] [PubMed] [Google Scholar]
- 67.Borràs-Santos A, Jacobs JH, Täubel M, Haverinen-Shaughnessy U, Krop EJ, Huttunen K, et al. Dampness and mould in schools and respiratory symptoms in children: the HITEA study. Occup Environ Med. 2013 doi: 10.1136/oemed-2012-101286. oemed-2012-101286. [DOI] [PubMed] [Google Scholar]
- 68.Baxi SN, Muilenberg ML, Rogers CA, Sheehan WJ, Gaffin J, Permaul P, et al. Exposures to molds in school classrooms of children with asthma. Pediatric Allergy and Immunology. 2013;24(7):697–703. doi: 10.1111/pai.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pilotto LS, Nitschke M, Smith BJ, Pisaniello D, Ruffin RE, McElroy HJ, et al. Randomized controlled trial of unflued gas heater replacement on respiratory health of asthmatic schoolchildren. International journal of epidemiology. 2004;33(1):208–11. doi: 10.1093/ije/dyh018. [DOI] [PubMed] [Google Scholar]
- 70.Lignell U, Meklin T, Putus T, Rintala H, Vepsäläinen A, Kalliokoski P, et al. Effects of moisture damage and renovation on microbial conditions and pupils’ health in two schools—a longitudinal analysis of five years. Journal of Environmental Monitoring. 2007;9(3):225–33. doi: 10.1039/b615459j. [DOI] [PubMed] [Google Scholar]
- 71.Meklin T, Potus T, Pekkanen J, Hyvärinen A, Hirvonen MR, Nevalainen A. Effects of moisture-damage repairs on microbial exposure and symptoms in schoolchildren. Indoor air. 2005;15(s10):40–7. doi: 10.1111/j.1600-0668.2005.00357.x. [DOI] [PubMed] [Google Scholar]
- 72.Karlsson A-S, Andersson B, Renström A, Svedmyr J, Larsson K, Borres MP. Airborne cat allergen reduction in classrooms that use special school clothing or ban pet ownership. Journal of allergy and clinical immunology. 2004;113(6):1172–7. doi: 10.1016/j.jaci.2003.12.590. [DOI] [PubMed] [Google Scholar]
- 73.Karlsson AS, Renström A, Hedren M, Larsson K. Allergen avoidance does not alter airborne cat allergen levels in classrooms. Allergy. 2004;59(6):661–7. doi: 10.1111/j.1398-9995.2004.00519.x. [DOI] [PubMed] [Google Scholar]
- 74.Phipatanakul W, Koutrakis P, Coull BA, Kang C-M, Wolfson JM, Ferguson ST, et al. The School Inner-City Asthma Intervention Study: Design, rationale, methods, and lessons learned. Contemporary Clinical Trials. 2017;60:14–23. doi: 10.1016/j.cct.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernstein J, Levin L, Crandall M, Perez A, Lanphear B. A pilot study to investigate the effects of combined dehumidification and HEPA filtration on dew point and airborne mold spore counts in day care centers. Indoor air. 2005;15(6):402–7. doi: 10.1111/j.1600-0668.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 76.Zheng X-y, Ding H, Jiang L-n, Chen S-w, Zheng J-p, Qiu M, et al. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: a systematic review and meta-analysis. PLoS One. 2015;10(9):e0138146. doi: 10.1371/journal.pone.0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kingsley SL, Eliot MN, Carlson L, Finn J, MacIntosh DL, Suh HH, et al. Proximity of US schools to major roadways: a nationwide assessment. Journal of Exposure Science and Environmental Epidemiology. 2014;24(3):253–9. doi: 10.1038/jes.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daisey JM, Angell WJ, Apte MG. Indoor air quality, ventilation and health symptoms in schools: an analysis of existing information. Indoor air. 2003;13(1):53–64. doi: 10.1034/j.1600-0668.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- 79.Godwin C, Batterman S. Indoor air quality in Michigan schools. Indoor air. 2007;17(2):109–21. doi: 10.1111/j.1600-0668.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 80.Mendell MJ, Heath GA. Do indoor pollutants and thermal conditions in schools influence student performance? A critical review of the literature. Indoor air. 2005;15(1):27–52. doi: 10.1111/j.1600-0668.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- 81.Lemanske RF, Kakumanu S, Shanovich K, Antos N, Cloutier MM, Mazyck D, et al. Creation and implementation of SAMPRO™: A school-based asthma management program. Journal of Allergy and Clinical Immunology. 2016;138(3):711–23. doi: 10.1016/j.jaci.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simon AE, Akinbami LJ. Asthma action plan receipt among children with asthma 2–17 years of age, United States, 2002–2013. The Journal of pediatrics. 2016;171:283–9e1. doi: 10.1016/j.jpeds.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelso JM. Do written asthma action plans improve outcomes? Pediatric allergy, immunology, and pulmonology. 2016;29(1):2–5. doi: 10.1089/ped.2016.0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Self TH, George CM, Wallace JL, Patterson SJ, Finch CK. Incorrect use of peak flow meters: are you observing your patients? Journal of Asthma. 2014;51(6):566–72. doi: 10.3109/02770903.2014.914218. [DOI] [PubMed] [Google Scholar]
- 85.Webber MP, Carpiniello KE, Oruwariye T, Lo Y, Burton WB, Appel DK. Burden of asthma in inner-city elementary schoolchildren: do school-based health centers make a difference? Archives of pediatrics & adolescent medicine. 2003;157(2):125–9. doi: 10.1001/archpedi.157.2.125. [DOI] [PubMed] [Google Scholar]
- 86.Mansour ME, Rose B, Toole K, Luzader CP, Atherton HD. Pursuing perfection: An asthma quality improvement initiative in school-based health centers with community partners. Public health reports. 2008;123(6):717–30. doi: 10.1177/003335490812300608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo J, Jang R, Keller K, McCracken A, Pan W, Cluxton R. Impact of school-based health centers on children with asthma. Journal of Adolescent Health. 2005;37(4):266–74. doi: 10.1016/j.jadohealth.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Bacharier LB, Guilbert TW, Zeiger RS, Strunk RC, Morgan WJ, Lemanske RF, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. Journal of Allergy and Clinical Immunology. 2009;123(5):1077–82e5. doi: 10.1016/j.jaci.2008.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Global Initiative for Asthma. Global strategy for asthma management and prevention. updated 2017. Available from: www.ginasthma.org.
- 90.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. New England Journal of Medicine. 2016;374(19):1842–52. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. The Lancet. 2011;377(9766):650–7. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chong J, Haran C, Asher I. Intermittent inhaled corticosteroid therapy versus placebo for persistent asthma in children and adults. The Cochrane Library. 2014 doi: 10.1002/14651858.CD011032.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. European Respiratory Journal. 2013 doi: 10.1183/09031936.00202013. erj02020-2013. [DOI] [PubMed] [Google Scholar]
- 94.Lemanske RF, Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. New England Journal of Medicine. 2010;362(11):975–85. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang L, Pruteanu AI, Prietsch SO, Chauhan BF, Ducharme FM. Cochrane in context: Inhaled corticosteroids in children with persistent asthma: effects on growth and dose–response effects on growth. Evidence-Based Child Health: A Cochrane Review Journal. 2014;9(4):1047–51. doi: 10.1002/ebch.1984. [DOI] [PubMed] [Google Scholar]
- 96.Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367(10):904–12. doi: 10.1056/NEJMoa1203229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hossny E, Rosario N, Lee BW, Singh M, El-Ghoneimy D, Soh JY, et al. The use of inhaled corticosteroids in pediatric asthma: update. World Allergy Organization Journal. 2016;9(1):26. doi: 10.1186/s40413-016-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huffaker MF, Phipatanakul W, Huffaker M, Phipatanakul W. Pediatric asthma: guidelines-based care, omalizumab, and other potential biologic agents. Immunology and allergy clinics of North America. 2015;35(1):129. doi: 10.1016/j.iac.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bousquet J, Brusselle G, Buhl R, Busse WW, Cruz AA, Djukanovic R, et al. Care pathways for the selection of a biologic in severe asthma. The European respiratory journal. 2017;50(6) doi: 10.1183/13993003.01782-2017. [DOI] [PubMed] [Google Scholar]
- 100.Darveaux J, Busse WW. Biologics in asthma—the next step toward personalized treatment. The Journal of Allergy and Clinical Immunology: In Practice. 2015;3(2):152–60. doi: 10.1016/j.jaip.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. New England Journal of Medicine. 2011;364(11):1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108(2):E36. doi: 10.1542/peds.108.2.e36. [DOI] [PubMed] [Google Scholar]
- 103.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. Journal of Allergy and Clinical Immunology. 2015;136(6):1476–85. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. The Lancet. 2012;380(9842):651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 105.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. New England Journal of Medicine. 2014;371(13):1198–207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 106.Wright LS, Phipatanakul W. Treatment of moderate to severe pediatric asthma Omalizumab and potential future use of monoclonal antibodies. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2016;117(1):17. doi: 10.1016/j.anai.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pfeffer PE, Hawrylowicz CM. Vitamin D in Asthma: Mechanisms of Action and Considerations for Clinical Trials. Chest. 2017 doi: 10.1016/j.chest.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 108.van Oeffelen AA, Bekkers MB, Smit HA, Kerkhof M, Koppelman GH, Haveman-Nies A, et al. Serum micronutrient concentrations and childhood asthma: the PIAMA birth cohort study. Pediatr Allergy Immunol. 2011;22(8):784–93. doi: 10.1111/j.1399-3038.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 109.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA, Jr, Kerley CP, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. The Lancet Respiratory medicine. 2017 doi: 10.1016/S2213-2600(17)30306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rollins DR, Good JT, Jr, Martin RJ. The role of atypical infections and macrolide therapy in patients with asthma. The journal of allergy and clinical immunology In practice. 2014;2(5):511–7. doi: 10.1016/j.jaip.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 112.Koutsoubari I, Papaevangelou V, Konstantinou GN, Makrinioti H, Xepapadaki P, Kafetzis D, et al. Effect of clarithromycin on acute asthma exacerbations in children: an open randomized study. Pediatric Allergy and Immunology. 2012;23(4):385–90. doi: 10.1111/j.1399-3038.2012.01280.x. [DOI] [PubMed] [Google Scholar]
- 113.Piacentini GL, Peroni DG, Bodini A, Pigozzi R, Costella S, Loiacono A, et al. Allergy and asthma proceedings. OceanSide Publications, Inc; 2007. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary report. [DOI] [PubMed] [Google Scholar]
- 114.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA. 2015;314(19):2034–44. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]