Abstract
XBP1 (X-box binding protein 1), CD138 (Syndecan-1), and CS1 (SLAMF7) are highly expressed antigens in cancers including multiple myeloma (MM). Here we identify and characterize immunogenic HLA-A24 peptides derived from these antigens for potential vaccination therapy of HLA-A24+ patients with MM. The identified immunogenic HLA-A24-specific XBP1 unspliced (UN)185-193 (I S P W I L A V L), XBP1 spliced (SP)223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) and CS1240-248 (L F V L G L F L W) peptides induced antigen-specific CTL with anti-MM activity in an HLA-A24 restricted manner. Furthermore, a cocktail containing the four HLA-A24 peptides evoked MM-specific CTL with distinct phenotypic profiles (CD28, CD40L, 41BB, CD38, CD69) and anti-tumor activities, evidenced by perforin upregulation, CD107a degranulation (cytotoxicity) and Th1 type cytokines (IFN-γ/IL-2/TNF-α) production in response to HLA-A24+ MM cells. The multipeptide-specific CTL included antigen-specific memory CD8+ T cells expressing both T cell activation (CD38, CD69) and immune checkpoints antigens (CTLA, PD1, LAG3, TIM3). These results provide the framework for a multipeptide vaccination therapy to induce tumor-specific CTL in HLA-A24 positive patients with myeloma and other cancers expressing these antigens.
Keywords: Multiple Myeloma, Multipeptide Vaccine specific to HLA-A24+ Patients, XBP1, CD138, CS1
INTRODUCTION
Multiple myeloma (MM) is characterized by proliferation of plasma cells within the bone marrow, elevated serum and/or urine monoclonal protein, and clinical sequelae including hypercalcemia, renal dysfunction, anemia, bone disease, and immune suppression.1,2 Despite recent treatment advances using novel targeted immune agents, relapse is common and new treatment strategies are urgently needed.3 Immunotherapeutic approaches can provide durable immune responses in the MM patients; however, there are multiple obstacles to development of a successful immunotherapy for MM including tumor heterogeneity, ongoing DNA damage and clonal evolution, multiple tumor escape mechanisms, and compromised immune cell function in patients. Specifically, induction of dendritic cell dysfunction, defects in B-cell and T-cell immunity, decreased responsiveness to IL-2, reduced T-cell cytotoxic activity, and expansion of myeloid-derived suppressor cells is hallmarks of MM pathogenesis.4-7 These immune defects may account, at least in part, for the failure of recent immunotherapy trials.
Vaccine therapies in MM to date have most commonly been patient-specific, using dendritic cells pulsed with tumor-specific idiotype or tumor lysates.8,9, which are both costly and labor-intensive. To overcome this limitation, we and others have developed alternative approaches using tumor associated antigen-specific cancer vaccines. Among them, peptide-based vaccines have multiple advantages including broad applicability, ready detection of antigen-specific immune responses, feasibility of combining multiple target antigens, as well as ease and cost of manufacturing. Vaccination using immunogenic peptides with affinities to various MHC Class I molecules therefore may offer a more broadly applicable novel vaccine-based strategy.
In previous studies, we identified immunogenic peptides derived from the XBP1 (X-box binding protein 1), CD138 (Syndecan-1) and CS1 (SLAMF7), which are associated with MM pathogenesis and are highly expressed on the tumor cells, and reported on the generation and application of the antigen-specific CTL.10-12 We first identified HLA-A2 restricted peptides from these antigens, since HLA-A2 is the most dominant MHC Class I molecule, present in 50% of the patient population in North America. To expand the patient population and overcome the HLA-A2 restriction, we have developed a new immunotherapeutic option to treat patients expressing HLA-A24, which is the second most dominant MHC Class I in North America, as well as the most frequent MHC Class I in Asia. Here, we report on the identification of novel HLA-A24 specific peptides derived from the unspliced and spliced isoforms of XBP1, CD138 and CS1 antigens, and demonstrate their capacity to elicit CD3+CD8+ antigen-specific CTL as single or as a cocktail of four immunogenic peptides. These antigen-specific CTL displayed distinct phenotype profiles and anti-tumor activities against HLA-A24+ MM cells. Importantly the anti-MM activities were enhanced within the central memory and effector memory subsets of the ex vivo generated multipeptide-specific CTL, and could be further increased by treatment with immune modulators, checkpoint inhibitors or immune agonists. These studies provide the framework for use of a cocktail of four immunogenic HLA-A24 specific peptides as a cancer vaccine to treat HLA-A24+ patients with MM or other cancers.
METHODS
Synthetic Peptides
Identified HLA-A24 specific peptides derived from full-length XBP1 unspliced, XBP1 spliced, CD138 (Syndecan-1) and CS1 (SLAMF7) proteins were synthesized by standard fmoc (9-fluorenylmethyl-oxycarbonyl) chemistry, purified to > 95% using reverse-phase chromatography, and validated by mass-spectrometry for molecular weight (Biosynthesis, Lewisville, TX). HIV-1 envelope protein gp41583-591 (RYLKDQQLL) peptide was used as a control HLA-A24 specific peptide in the assays. Lyophilized peptides were dissolved in DMSO (Sigma), diluted in AIM-V medium (Gibco-Life Technologies), and stored at −140°C until needed.
Cell lines
Multiple myeloma cell lines (HLA-A24+ KMS11; HLA-A24− OPM1) were obtained from the ATCC (Manassas, VA). A T2 cell line transduced with HLA-A*2401 cDNA (T2-A24 cells) was provided by Dr. Y. Miyahara at Mie University School of Medicine (Mie, Japan) and Dr. E. Jaffee at the Johns Hopkins University School of Medicine (Baltimore, MD). LNCaP (HLA-A24+; XBP1un− XBP1sp− CS1−) and THP-1 (HLA-A24+; CD138−) obtained from ATCC were used as MHC HLA-A24 matched antigen-specific negative control cell lines. All cell lines were authenticated by STR profiling, tested for mycoplasma contamination and cultured in RPMI-1640 or DMEM medium (Gibco-Life Technologies, Rockville, MD) supplemented with 10% fetal calf serum (FCS; BioWhittaker, Walkersville, MD), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Gibco-Life Technologies).
Reagents
Fluorochrome conjugated mouse anti-human CD3, CD4, CD8, CD45RO, CCR7, CD28, CD38, CD40L, CD45RO, CD69, CD107a, Granzyme B, Perforin, IFN-γ, TNF-α, 41BB (CD137), CTLA-4, PD-1, TIM-3, LAG-3, VISTA and HLA-A24 monoclonal antibodies were purchased from Becton Dickinson (BD) Pharmingen or BD Biosciences (San Diego, CA). Recombinant human GM-CSF was obtained from Immunex (Seattle, WA), and recombinant human IL-2, IL-4, IFN-α, and TNF-α were purchased from R&D Systems (Minneapolis, MN).
HLA-A24 peptide affinity assay
HLA-A24 specific binding for each peptide was evaluated by flow cytometry using the T2-A24 cell line. In the assay, T2-A24 cells were washed in serum-free AIM-V medium and resuspended to a final concentration of 1 × 106 cells/ml. The cells were pulsed with various concentrations (0 – 200 μg/ml) of respective unspliced XBP1, spliced XBP1, CD138 or CS1 peptide, or with a control HIV-1 envelope protein gp41583-591 peptide (50 μg/ml) plus human β2-microglobulin (3 μg/ml) (Sigma), and then incubated at 26~28°C in 5% CO2 humidified air. Following overnight incubation, the cells were washed in PBS (Gibco-BRL) containing 5% FCS, stained with mouse anti-human HLA-A24-PE mAb, and analyzed using a FACSCanto™ flow cytometer. HLA-A24 peptide binding affinity was determined by measuring the level of up-regulation of HLA-A24 molecules on the T2-A24 cells induced by specific and stable peptide binding. HLA-A24 peptide binding affinity is reported as the median fluorescence intensity (MFI) of HLA-A24 expression. and the results are shown as the mean MFI ± SE. We have tested N=5 for testing the HLA-A24-specific affinity of each peptide.
Induction of HLA-A24 individual peptide-specific CTL or multipeptide-specific CTL
HLA-A24 specific XBP1 unspliced (UN) or XBP1 spliced (SP) peptide-specific CTL (XBP1 UN-CTL or XBP1 SP-CTL), CD138 peptide-specific CTL (CD138-CTL), CS1 peptide-specific CTL (CS1-CTL) or the multipeptide-specific CTL (MP-CTL) were generated ex vivo by repeated stimulation of enriched CD3+ T lymphocytes obtained from HLA-A24+ normal donors (N=5) with the respective HLA-A24 specific peptide or a cocktail of the four HLA-A24 peptides. Antigen-presenting cells (APC), either autologous mature dendritic cells or T2-A24 cells, were pulsed overnight at 26~28°C in 5% CO2 humidified air. The peptide(s) pulsed APC were washed, irradiated (200 Gy), and used to prime CD3+ T cells at a 1:20 APC/peptide-to-CD3+ T cell ratio in AIM-V medium supplemented with 10% human AB serum (BioWhittaker). IL-2 (50 U/ml) was added to the cultures two days after the second stimulation. The CTL cultures were restimulated weekly with peptide-pulsed APC for a total of 4 or 5 cycles. We have evaluated the immunogenicity of each HLA-A24-specific peptide in the CTL generated in phenotype and functional assays (N=5).
Phenotypic characterization of HLA-A24 peptide specific-CTL
HLA-A24 antigen-specific CD3+CD8+ CTL were evaluated for their Naïve:Memory T cell subsets by expression (% positive cells, MFI) of cell surface markers (CD28, CD38, CD69, CD40L, 41BB) and checkpoint molecules (CTLA-4, PD-1, TIM-3, LAG-3, VISTA). After staining, cells were washed, fixed in 2% paraformaldehyde, acquired using a LSRII Fortessa™ flow cytometer, and analyzed by DIVAä v8.0 (BD) or FlowJo v10.0.7 (Tree star, Ashland, OR) software. For analyses, CD3+CD8+ T cells were gated and analyzed for Naïve:Memory T cell subsets, as well as cell surface markers and checkpoint molecules within different CTL memory subsets.
HLA-A24 peptide-specific CTL proliferation and functional activities in response to myeloma cell lines
To evaluate HLA-A24-restricted CTL proliferation, CFSE (Molecular Probes) labeled HLA-A24 peptide-specific CTL were co-incubated with irradiated (20 Gy) HLA-A A24+ or HLA-A24− myeloma cell lines. On days 5 and 7, the cultures were harvested, stained with fluorochrome conjugated anti-CD3 and anti-CD8 mAbs, and analyzed by flow cytometry to determine specific CD3+CD8+ CTL proliferation to MM cells. The functional anti-tumor activities of each respective HLA-A24 peptide-specific CTL or HLA-A24 multipeptide-specific CTL was assessed and analyzed (N=5) by CD107a degranulation, granzyme B and perforin production, and IFN-γ/IL-2/TNF-α cytokine production in response to myeloma cells. Antigen-specific CTL were co-incubated with myeloma cells in the presence of anti-CD107a mAb. After 1 hour co-culture of effector and target cells, a cocktail of Brefeldin A and Monensin (BD was added, and the cultures were incubated an additional 5 hours. Cells were then harvested, washed, and stained with mAbs specific to cell surface antigens including CD3, CD8, CD45RO, CCR7, and 41BB. The cells were washed, fixed and permeabilized, and stained with mAb specific to granzyme B, perforin, IFN-γ, IL-2 or TNF-α. Cells were acquired and analyzed using a LSRII Fortessa™ flow cytometer and DIVAä v8.0 software.
RESULTS
High binding affinities of XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) and CS1240-248 (L F V L G L F L W) peptides to HLA-A24 molecules
The full length amino acid sequences of unspliced or spliced XBP1, CD138, and CS1 proteins were examined using the search software NetMHC, SYFPEITHI, IEDB and MHCBN databases, followed by the BIMAS and NetCTL programs to select potential high affinity HLA-A24 nanomer peptides with extended half-time disassociation rates, proteasomal C terminal cleavage, and TAP transport. A total of seven peptides from unspliced XBP1 protein, four peptides from spliced XBP1, seven peptides from CD138 protein, and six peptides from CS1 protein were selected for synthesis and evaluation of HLA-A24 affinity and stability (Supplemental Table). Individual peptides were initially screened at a high peptide concentration (200 μg/ml) to select those with an HLA-A24 binding affinity greater than or similar to the HLA-A24 specific HIV-1 envelope protein gp41583-591 (RYLKDQQLL) control peptide (Supplemental Figure 1). Among those screened, a total of eight peptides were subsequently selected for further evaluation at lower peptide concentrations (12.5 μg/ml, 25 μg/ml, 50 μg/ml, 100 μg/ml) as follows; XBP1 UN12-20 (D G T P K V L L L), XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F), CD13821-29 (L P Q I V A T N L), CD13813-21 (L A L S L Q P A L), CS1321-329 (T M P D T P R L F) and CS1240-248 (L F V L G L F L W). At lower peptide concentrations, XBP1 UN12-20 (D G T P K V L L L), XBP1 UN185-193 (I S P W I L A V L) and XBP1 SP223-231 (V Y P E G P S S L) peptides consistently showed higher HLA-A24 affinity (*p < 0.05), as compared to unpulsed control (N=3; Figure 1A). Among the XBP1 unspliced peptides, XBP1 UN185-193 (I S P W I L A V L) showed a higher affinity than XBP1 UN12-20 (D G T P K V L L L) peptide. In addition, CD138265-273 (I F A V C L V G F) peptide had a greater HLA-A24 affinity (*p < 0.05) than either the CD13821-29 (L P Q I V A T N L) or CD13813-21 (L A L S L Q P A L) peptide at all of the peptide concentrations (N=3; Figure 1B). Finally, the CS1240-248 (L F V L G L F L W) peptide showed a higher level of HLA-A24 affinity (*p < 0.05) at each of the low peptide concentrations and a slightly higher level of HLA-A24 affinity than CS1321-329 (T M P D T P R L F) peptide (N=3; Figure 1C). Based on these results, we chose the peptides with the highest HLA-A24 specific binding affinity, XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) and CS1240-248 (L F V L G L F L W), for further evaluation of their immunogenicity.
Figure 1. Identification of HLA-A24 specific XBP1, CD138 and CS1 peptides.
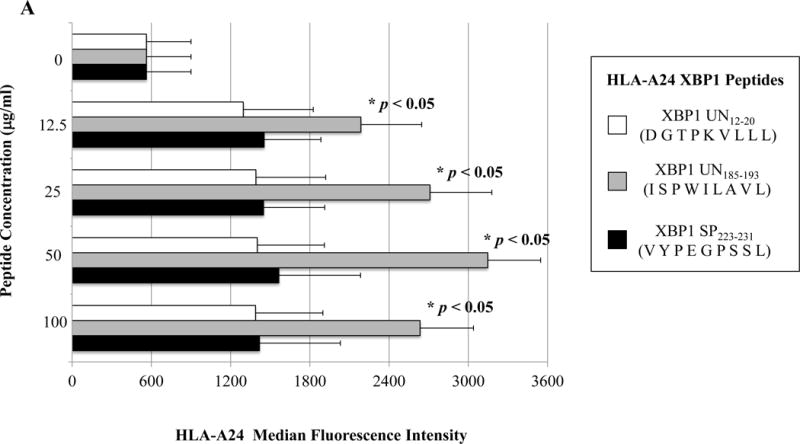
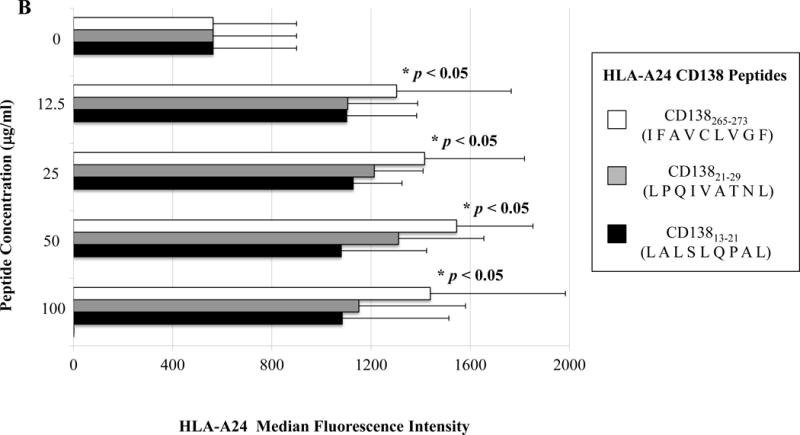
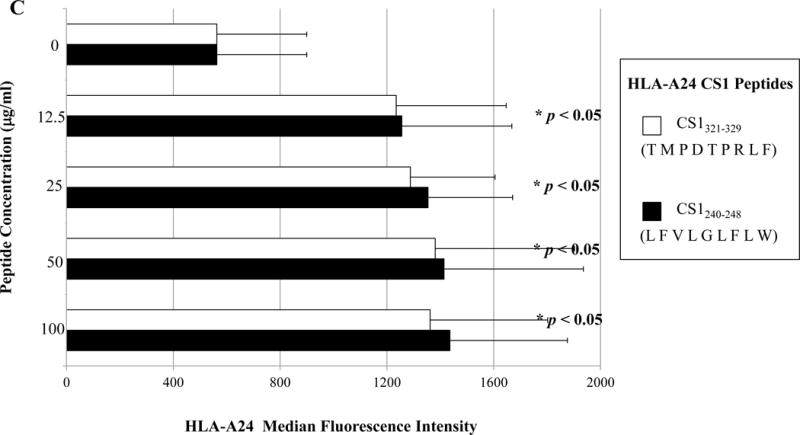
A, B, and C. HLA-A24 binding affinity of the XBP1, CD138 and CS1 peptides at low peptide concentrations.
T2-A24 cells were pulsed overnight at lower concentrations (12.5 μg/ml, 25 μg/ml, 50 μg/ml, 100 μg/ml) of respective peptide in serum-free AIM-V medium. Following incubation, the cells were harvested, washed, and stained with HLA-A24-PE mAb for flow cytometric analyses. XBP1 UN185-193 (I S P W I L A V L) (Figure 1A), XBP1 SP223-231 (V Y P E G P S S L) (Figure 1A), CD138265-273 (I F A V C L V G F) (Figure 1B), and CS1240-248 (L F V L G L F L W) (Figure 1C) displayed the highest levels of HLA-A24 binding affinity at all the concentrations tested. Peptide affinity to HLA-A24 is shown as an increase in the specific HLA-A24-PE mean fluorescence intensity (MFI) in the peptide binding assays (Average ± SE; N=5).
Generation of CD3+CD8+ CTL by repeated stimulation of T cells with HLA-A24 specific XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) or CS1240-248 (L F V L G L F L W) peptide
Individual antigen-specific CTL were generated by repeated stimulation of HLA-A24+ normal donors’ enriched CD3+ T lymphocytes (N=5) with the selected XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) or CS1240-248 (L F V L G L F L W) peptide-pulsed APC. The CD3+CD8+ T cell subset showed a gradual increase above baseline levels after each round of individual HLA-A24 specific peptide stimulation; XBP1 UN185-193, XBP1 SP223-231, CD138265-273 or CS1240-248 (Table 1, Average ± standard deviation).
Table 1.
Increased frequency of CD3+CD8+ CTL stimulated with HLA-A24 XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) or CS1240-248 (L F V L G L F L W) peptide after each round of peptide stimulation
| Number of Peptide Stimulation | XBP1 UN185-193 (ISPWILAVL) | XBP1 SP223-231 (VYPEGPSSL) | CD138265-273 (IFAVCLVGF) | CS1240-248 (LFVLGLFLW) |
|---|---|---|---|---|
| 1 | 15 ± 1 | 16 ± 2 | 17 ± 3 | 16 ± 2 |
| 2 | 32 ± 3 | 36 ± 19 | 49 ± 10 | 26 ± 5 |
| 3 | 53 ± 2 | 50 ± 16 | 55 ± 12 | 35 ± 4 |
| 4 | 72 ± 14 | 59 ± 7 | 59 ± 8 | 62 ± 15 |
IFN-γ production by CD3+CD8+ antigen-specific CTL, generated by individual HLA-A24+ peptides, in response to multiple myeloma cells
One week after the fourth stimulation, individual HLA-A24 peptide-specific CTL were analyzed for their immune functional activities against the HLA-A24+ multiple myeloma cell line KMS11. Each of the HLA-A24 peptide specific-CTL, XBP1 UN185-193 (Figure 2A), XBP1 SP223-231 (Supplemental Figure 2), CD138265-273 (Figure 2B) or CS1240-248 (Figure 2C) produced high levels of IFN-γ in response to HLA-A24+ KMS11 myeloma cells. These results demonstrate the immunogenicity of each individual HLA-A24 specific peptide to generate effector CD3+CD8+ antigen-specific CTL with Th1 cytokine production in response to multiple myeloma cells.
Figure 2. Individual HLA-A24 antigen-specific CTL demonstrate functional IFN-γ production in response to HLA-A24+ multiple myeloma cells.
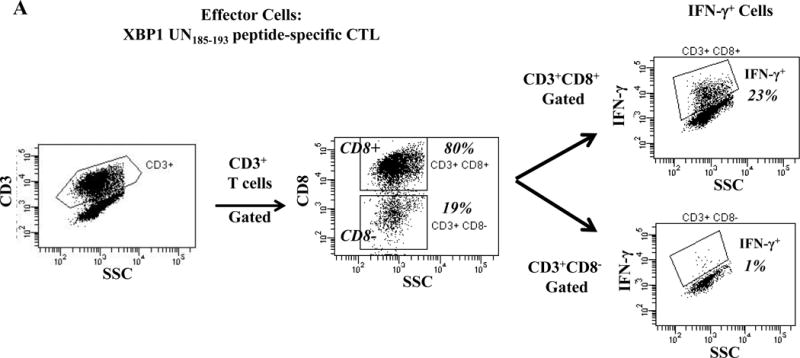
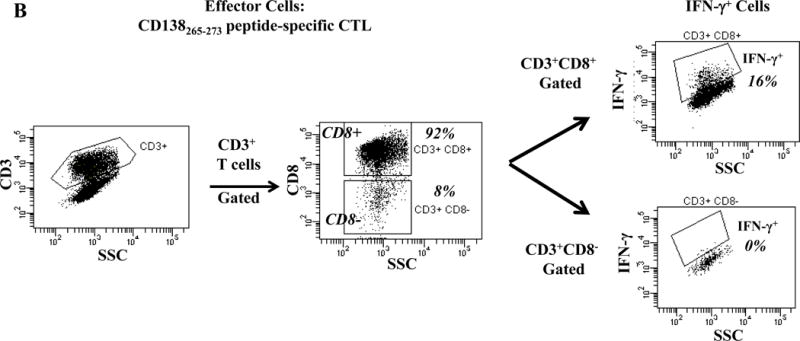
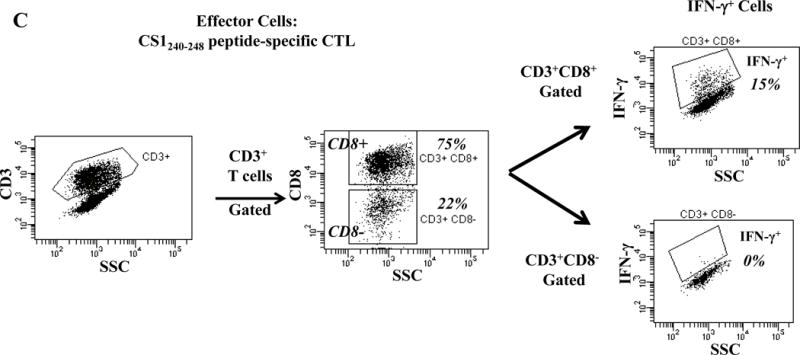
The immune functional activity of each peptide-specific CTL was evaluated in response to HLA-A24+ KMS11 myeloma cells by flow cytometry. CD3+CD8+ CTL generated with individual HLA-A24 specific XBP1 UN185-193 (Figure 2A), XBP1 SP223-231 (Supplemental Figure 2), CD138265-273 (Figure 2B) or CS1240-248 peptide (Figure 2C) display increased IFN-γ production in response to HLA-A24+ KMS 11 myeloma cells
Proliferation of antigen-specific CTL generated by individual HLA-A24+ peptides upon recognition of multiple myeloma cells in a HLA-A24-restricted manner
Specific CTL proliferation was identified as the percent decrease in CFSE expression (CFSE-low) within the CD3+CD8+ T cell population. A significantly higher level of CTL proliferation (shown as % CFSE-low) was seen in response to HLA-A24+ MM cells than MHC-mismatched HLA-A24− MM cells (XBP1+/CD138+/CS1+) or antigen-mismatched HLA-A24+ tumor cells (LnCaP; XBP1−, THP-1; CD138−, LnCaP; CS1−) for XBP1 UN185-193 peptide-specific CTL (Figure 3A), XBP1 SP223-231 peptide-specific CTL (Supplemental Figure 3), CD138265-273 peptide-specific CTL (Figure 3B) or CS1240-248 peptide-specific CTL (Figure 3C).
Figure 3. Individual HLA-A24 antigen-specific CTL demonstrate proliferative response to HLA-A24+ multiple myeloma cells.
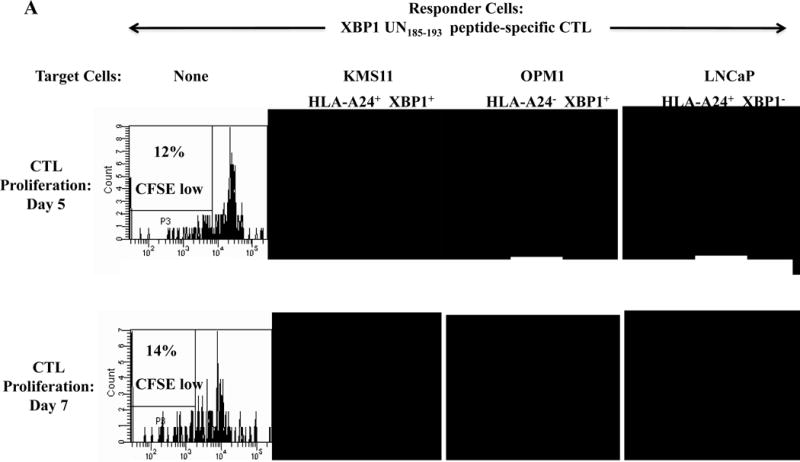
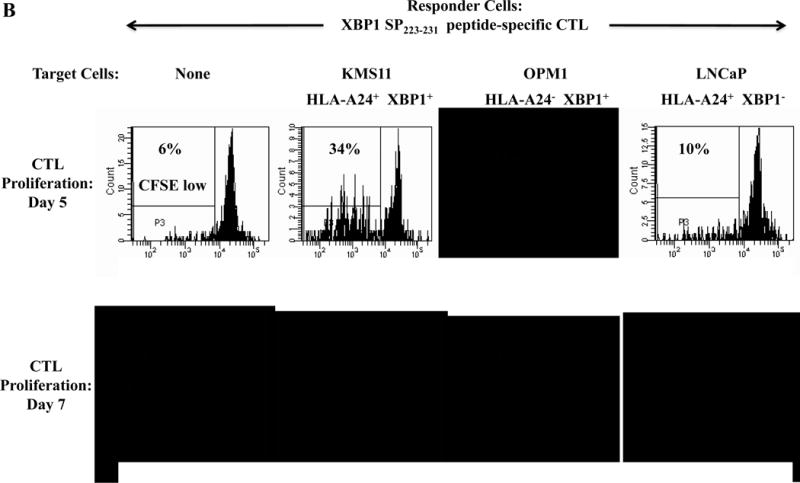
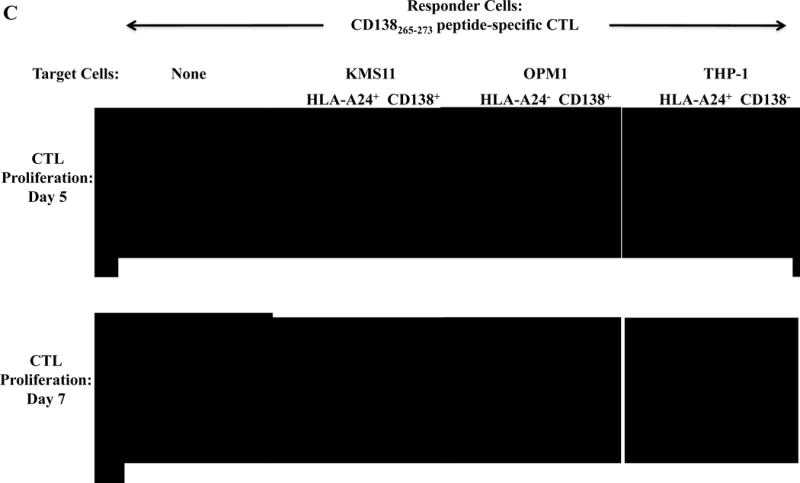
The proliferation of antigen-specific CTL was measured in response to HLA-A24+ or HLA-A24− myeloma cells in CFSE proliferation assays on day 5 or day 7 of culture by flow cytometry. The CTL generated by stimulation with an individual HLA-A24 peptide including XBP1 UN185-193 (Figure 3A), XBP1 SP223-231 (Supplemental Figure 3), CD138265-273 (Figure 3B) or CS1240-248 (Figure 3C) demonstrated time-dependent cell proliferation in response to HLA-A24+ KMS11 myeloma cells, but not to MHC mismatched HLA-A24− OPM1 myeloma cells. Background proliferation was determined using CFSE labeled individual antigen-specific CTL cultured in media alone.
Poly-functional immune responses of CD3+CD8+ antigen-specific CTL generated by individual HLA-A24+ peptides upon recognition of multiple myeloma cells in a HLA-A24-restricted manner
One week after the fourth peptide stimulation, each peptide-specific CTL (N=3) were analyzed and we detected poly-functional immune responses against HLA-A24+ KMS cells by the XBP1 UN185-193 peptide-specific CTL (IFN-γ+ cells: 14.5%, IL-2+ cells: 6.0%, CD107a+ IFN-γ+ cells: 16.0%) (Figure 4A), XBP1 SP223-231 peptide-specific CTL (IFN- γ+ cells: 4.6%, IL-2+ cells: 25.4%, CD107a+ IFN- γ+ cells: 3.8%) (Supplemental Figure 4), CD138265-273 peptide-specific CTL (IFN- γ+ cells: 8.1%, IL-2+ cells: 21.7%, CD107a+ IFN- γ+ cells: 7.6%) (Figure 4B) or CS1240-248 peptide-specific CTL (IFN- γ+ cells: 8.0%, IL-2+ cells: 13.4%, CD107a+ IFN- γ+ cells: 10.0%) (Figure 4C). The respective peptide-specific CTL did not recognize nor respond to the MHC mismatched HLA-A24− OPM1 myeloma cell line (Figures 4A, 4B, 4C, Supplemental Figure 4). These results offer additional evidence on the immunogenicity of each novel HLA-A24 peptide to induce poly-functional CTL with HLA-A24 restricted anti-MM activities.
Figure 4. HLA-A24-restricted anti-myeloma activities (CD107a degranulation, IL-2/IFN-γ cytokines production) of antigen-specific CTL generated by XBP1 UN185-193, XBP1 SP223-231, CD138265-273 or CS1240-248 peptide.
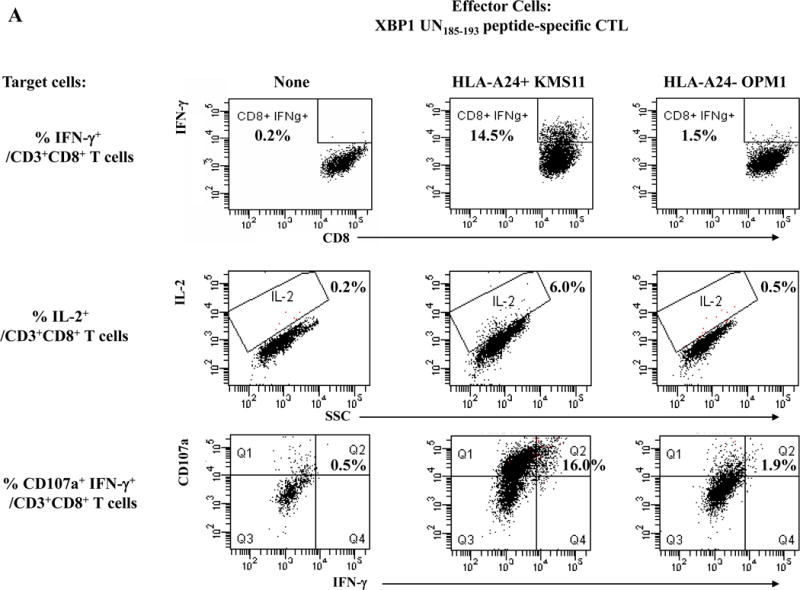
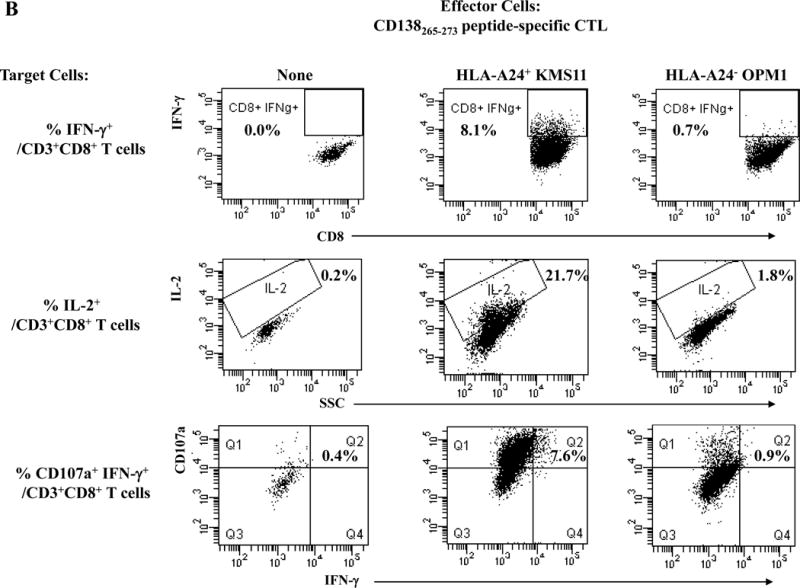
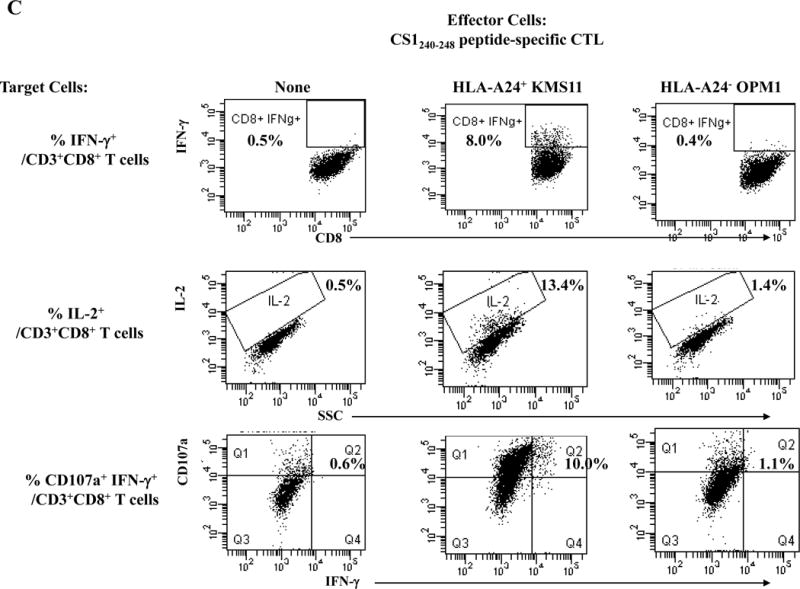
The individual XBP1 UN185-193 (Figure 4A), XBP1 SP223-231 (Supplemental Figure 4), CD138265-273 (Figure 4B) or CS1240-248 (Figure 4C) antigen-specific CTL display functional anti-myeloma activities (IFN-γ production, IL-2 production, CD107a degranulation/IFN-γ production) in response to MHC-matched HLA-A24+ KMS11 myeloma cells, but not to MHC-mismatched HLA-A24− OPM1 myeloma cells.
Phenotypic characterization of multipeptide-specific CTL generated with a cocktail of HLA-A24 specific XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) and CS1240-248 (L F V L G L F L W) peptides
We next evaluated the ability of a cocktail of these four HLA-A24 peptides to induce multipeptide-specific CTL with anti-myeloma effects. The multipeptide-specific CTL displayed increased expression of critical T cell markers including CD28, CD40L, 41BB, CD38 and CD69 (Figure 5A). We also observed increased checkpoint molecule expression on the multipeptide-specific CTL, as compared to the control T cells without multipeptide stimulation. Specifically, we observed high expression levels of PD1, TIM3 and LAG3 on the multipeptide-specific CTL (Figure 5B). In contrast, CTLA-4 or VISTA expression was lower on the multipeptide-specific CTL. Next, we evaluated the functional activities of the multipeptide-specific CTL against myeloma cells. The multipeptide-specific CTL consistently demonstrated increased perforin, IFN-γ, IL-2 and TNF-α production in response to HLA-A24+ KMS11 myeloma cells (Figure 5C), supporting the use of a cocktail of the four HLA-A24 peptides to evoke multipeptide-specific CTL with poly-functional activities against myeloma cells.
Figure 5. Phenotypic and functional characterization of HLA-A24 multipeptide-specific CTL generated with a cocktail of XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) and CS1240-248 (L F V L G L F L W) peptides.
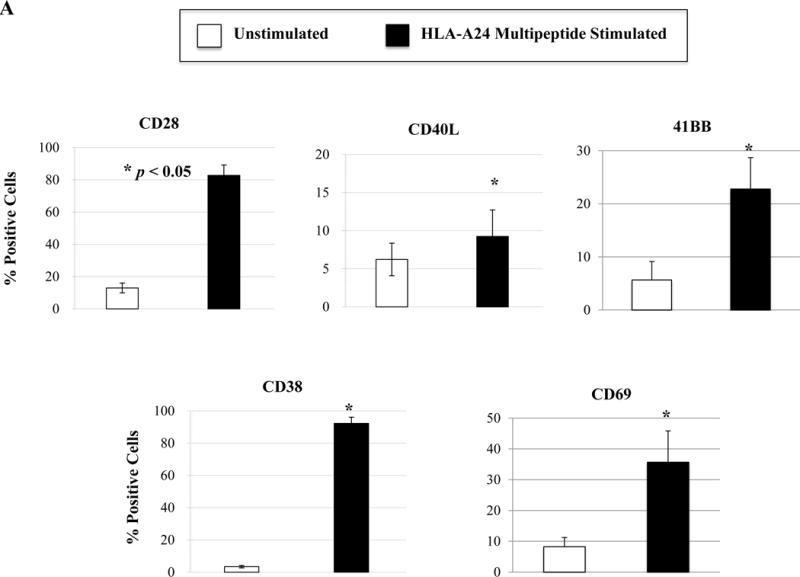
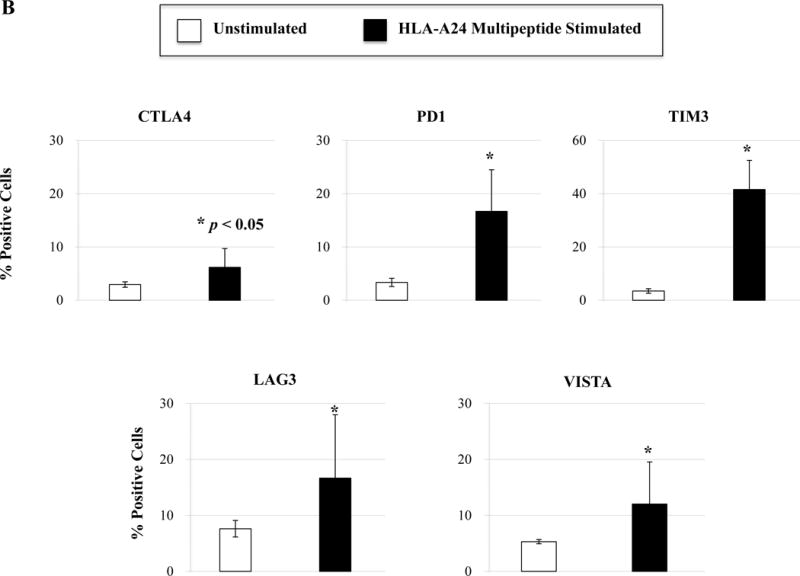
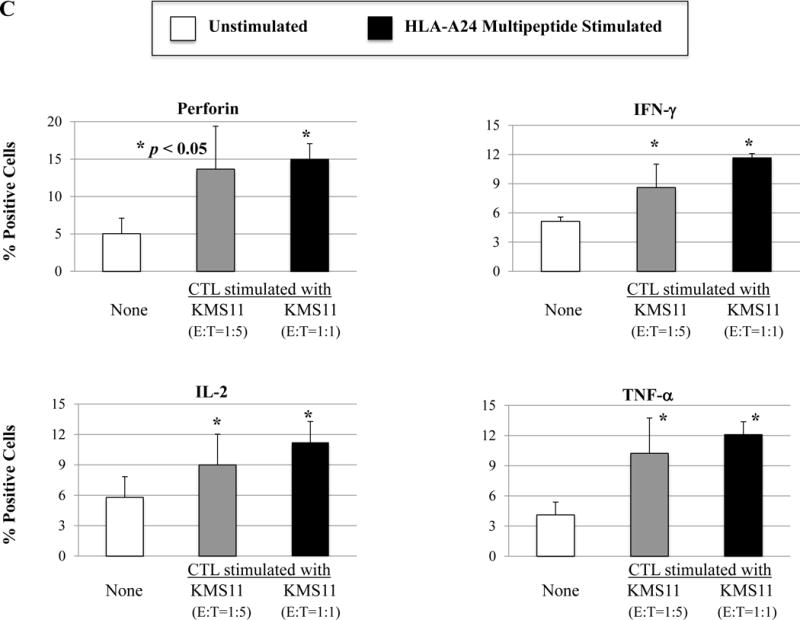
Phenotypic and immune functional characterization of multipeptide-specific CTL was performed one week after the fourth stimulation with a cocktail of the four HLA-A24 peptides (XBP1 UN185-193, XBP1 SP223-231, CD138265-273, CS1240-248). The multipeptide-specific CTL displayed increased CD3+CD8+ T cells expressing co-stimulatory molecules (CD28, CD40L, 41BB) and activation molecules (CD38, CD69) (Figure 5A) (n=3, mean ± SE). The CTL also expressed immune checkpoint antigens including CTLA4, PD1, TIM3, LAG3 and VISTA (Figure 5B) (n=3, mean ± SE). In addition, the multipeptide-specific CTL demonstrated functional anti-tumor activities including perforin and cytokines (IFN-γ, IL-2, TNF-α) production in response to HLA-A24+ KMS11 myeloma cells at both Effector:Target ratios tested (1:5, 1:1) (Figure 5C) (Average ± SE; N=5).
Characterization of central memory and effector memory CD8+ CTL generated by HLA-A24 XBP1/CD138/CS1 multipeptide
Following HLA-A24 multipeptide stimulation, the multipeptide-specific CTL (N=5) were evaluated for their Naïve:Memory T cell subset profiles and anti-myeloma activities. Representative flow cytometric analyses of memory cell development from two HLA-A24+ individuals’ after each round of multipeptide stimulation is shown in Figure 6A. We detected an increase in the central memory (CM; CD45RO+ CCR7+) CTL population following 2 cycles of multipeptide stimulation (CM – Donor 1: 44%, Donor 2: 39%) and effector memory (EM; CD45RO+ CCR7−) population following 3 cycles of stimulation (EM – Donor 1: 24%, Donor 2: 18%) (Figure 6B). With each additional cycle of HLA-A24+ XBP1/CD138/CS1 multipeptide stimulation, the specific CTL showed further differentiation into EM CTL, with more than 80% of the multipeptide-specific CTL (n=3) having the EM phenotype upon an additional cycle of peptides stimulation (Figure 6B). The Naïve:Memory cell subsets of multipeptide-specific CTL were also evaluated for their expression of specific activation or co-stimulatory markers after 4 cycles of multipeptide stimulation. We observed a high expression (frequency and MFI) of CD38+ cells in the CM, EM and TE subsets (Figure 6C). High expression level of CD69, an early cell activation marker, was distinctly detected within the CM CTL subset, as were co-stimulatory CD40L and 41BB.
Figure 6. Differentiation and development of memory CD3+CD8+ subsets upon repeated stimulation with a cocktail of XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) and CS1240-248 (L F V L G L F L W) peptides.
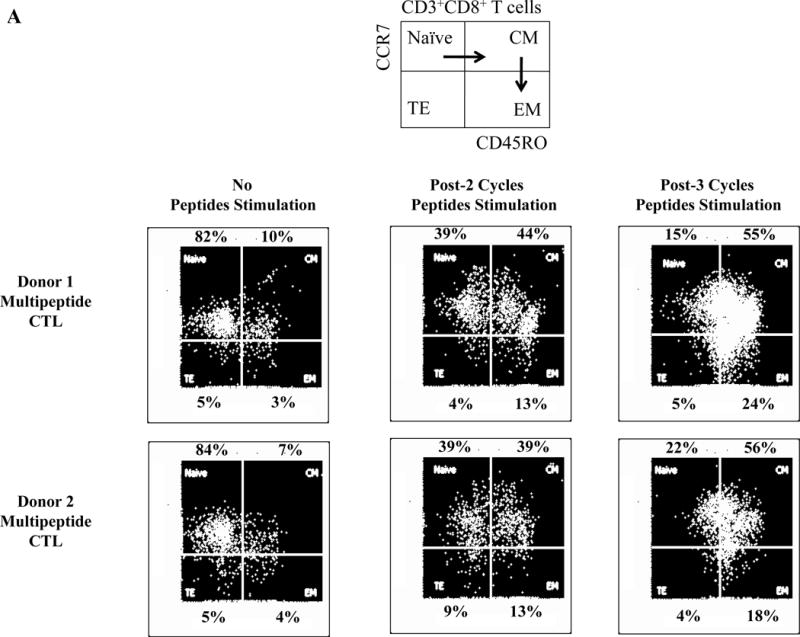
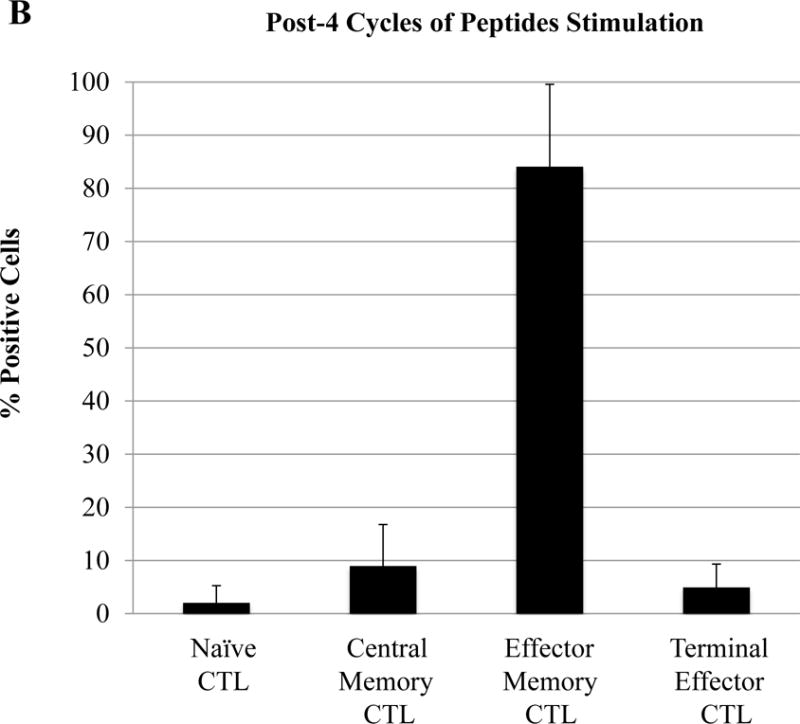
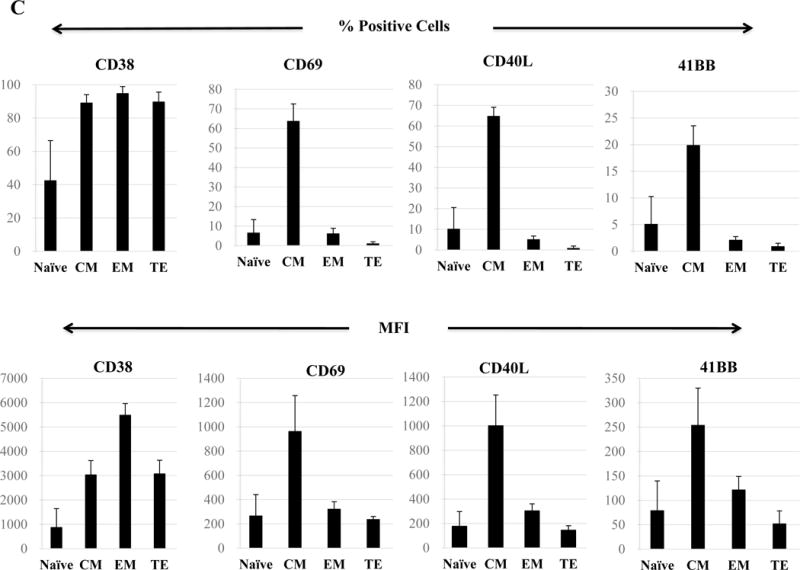
The Naïve:Memory CD3+CD8+ CTL subsets were characterized following each round of stimulation with HLA-A24 multipeptide cocktail. Repeated multipeptide stimulation of CD3+ T cells from HLA-A24+ donors (N=5) induced phenotypic changes from naïve (CD45RO−CCR7+) CD3+CD8+ T cells into central memory (CD45RO+ CCR7+) CD3+CD8+ T cells after 2 cycles of peptides stimulation and then into effector memory (CD45RO+ CCR7−) CD3+CD8+ T cells after 3 cycles of stimulation (Figure 6A), and then into effector memory (CD45RO+ CCR7−) CD3+CD8+ T cells after 4 cycles of stimulation (Figure 6B). Within the multipeptide-specific CTL population, the central memory subset expressed the highest levels of critical activation and co-stimulatory T cell markers (CD38, CD69, CD40L, 41BB; Figure 6C)
Functional anti-tumor activities within the central memory and effector memory cells induced by HLA-A24 XBP1/CD138/CS1 multipeptide
Next, we evaluated anti-MM activities of the antigen-specific CTL subsets generated by HLA-A24-specific XBP1/CD138/CS1 multipeptide. The responses to myeloma was consistently highest within the CM subset, as shown by the production of perforin (71.1%) and Th1 type cytokines including IFN-γ (74.0%) and IL-2 (76.3%), as well as upregulation of the co-stimulatory marker 41BB (80.6%) compared to the EM subset (perforin: 8.8%, IFN-γ: 10.4%, IL-2: 16.5%, 41BB: 14.5%) and TE subset (perforin: 0.9%, IFN-γ: 1.3%, IL-2: 2.6%, 41BB: 3.3%) of the multipeptide-specific CTL (Figure 7A). The functional activity of HLA-A24 multipeptide-specific CTL was significantly higher within the CM subset (> 60%, n=3), followed by EM (> 20%) or TE (> 5%) as evidenced by their perforin upregulation, IFN-γ/IL-2 Th1 cytokines production and 41BB upregulation (Figure 7B). Furthermore, we investigated the expression of various immune checkpoint molecules on the Naïve:Memory subsets of multipeptide-specific CTL. Among the different CTL subsets, the CM cell subset showed the highest expression levels (% positive cells and MFI) of the most immune checkpoints evaluated, including CTLA-4, PD-1 and LAG-3 (Figure 7C). Distinctively, TIM3 was detected as the highest in its expression (% positive cells and MFI) in both EM and CM cell subsets.
Figure 7. Anti-tumor activities of Naïve:Memory CD3+CD8+ CTL subsets generated by stimulation with a cocktail of XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) and CS1240-248 (L F V L G L F L W) peptides.
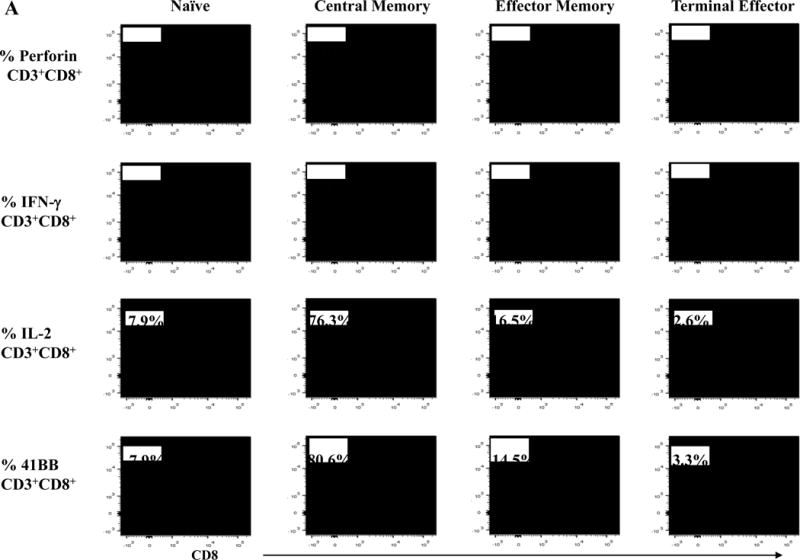
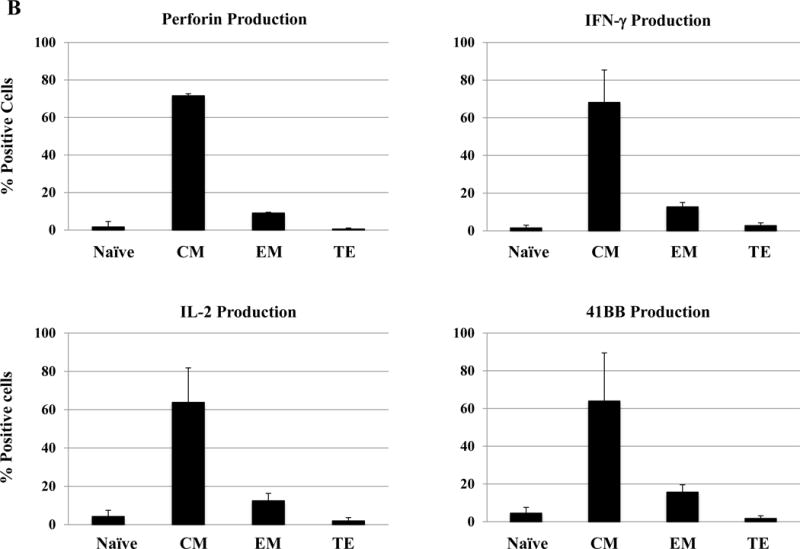
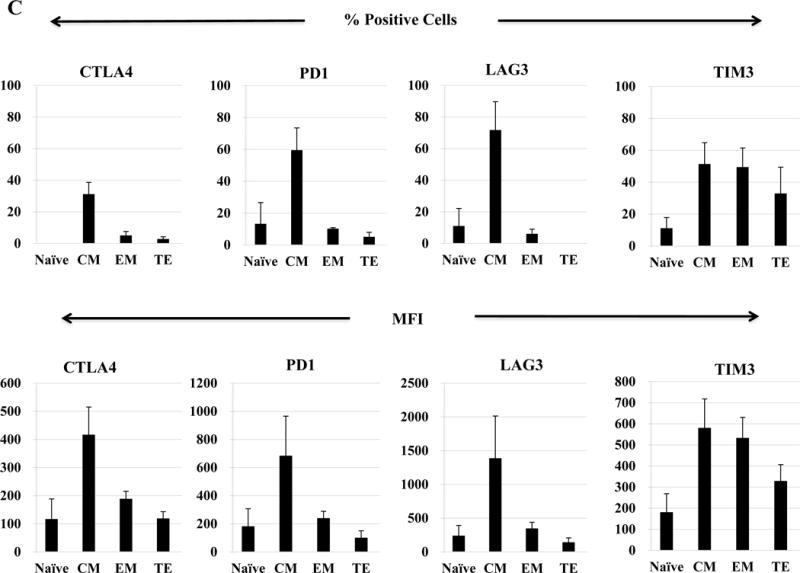
Upon the stimulation of CD3+ T cells from HLA-A24+ donors (N=5) with the HLA-A24-specific multipeptide, the Naïve:Memory CD8+ CTL subsets were evaluated for their anti-tumor activities against multiple myeloma cells. Functionally, the central memory CD8+ CTL subset demonstrated the highest anti-myeloma activities, as evidenced by perforin/IFN-γ/IL-2 production and 41BB upregulation in response to HLA-A24+ KMS11 myeloma cells (Figure 7A). The high levels of anti-tumor activities was observed consistently by the memory CD8+ T cells in the HLA-A24 multipeptide-specific CTL generated (Figure 7B). Within the multipeptide-specific CTL, the central memory cells subset expressed the highest level of immune checkpoints (Figure 7C).
Discussion
Antigen-specific cancer vaccines elicit tumor-suppressive responses by targeting various tumor-associated antigens (TAA) expressed on cancer cells. The discovery of novel immunogenic peptides from selected TAA offers new immunotherapeutic options, either as a vaccine or cell-based immunotherapy. A large number of clinical trials have demonstrated the immunogenicity of vaccines with minimal toxicities and low side effects in cancer patients.13,14 Our group has identified and developed a peptide- based cancer vaccine targeting the highly over-expressed, XBP1, CD138 (Syndecan-1) and CS1 (SLAMF7) antigens, which have been implicated in MM pathogenesis. Specifically, we previously reported on novel immunogenic heteroclitic XBP1 unspliced (US)184-192 (YISPWILAV)10, heteroclitic XBP1 spliced (SP)367-375 (YLFPQLISV) (10), native CD138260-268 (GLVGLIFAV)11, and native CS1239-247 (SLFVLGLFL)12 peptides specific to HLA-A2, which is the most dominant MHC Class I molecule in North America and second most dominant in Asia. The heteroclitic XBP1 peptides YISPWILAV and YLFPQLISV were designed to improve their HLA-A2 binding affinities than their originally identified native XBP1 US184-192 (NISPWILAV) or XBP1 SP367-375 (ELFPQLISV) peptides (10), in order to bypass tolerance and enhance the peptides immunogenicity against cancer cells. These previously identified XBP1/CD138/CS1 peptides, either individually or as a cocktail of four peptides, induced antigens-specific CTL with functional anti-tumor activities against HLA-A2+ MM cells.10-12, 15, 16
A consideration that influences the success of vaccine trials includes selection of the appropriate patient population that can generate an effective immune response. Importantly, MM has the ability to elude immune surveillance through various mechanisms including induction of dendritic cell dysfunction, expansion of myeloid-derived suppressor cells, up-regulation of checkpoint inhibitors like PD-L1 on MM cells, and defects in B-cell immunity.17-21 In addition, defects in T-cell function, including loss of tumor-specific effector T cell activity and induction of Treg cells, as well as cytokines and growth factors including interleukin-6 (IL-6), macrophage inflammatory protein (MIP)-1α, insulin-like growth factor (IGF)-I, VEGF, and hepatocyte growth factor (HGF), contribute to compromised immune function and myeloma pathogenesis.22-24 In ongoing efforts we are therefore vaccinating patients with early stage smoldering multiple myeloma (SMM), who do not have compromised immunity due to disease or therapy, with the goal of delaying or preventing progression to active MM. Importantly, a recently completed Phase I clinical trial using our previously described HLA-A2 multipeptide combination of XBP1, CD138 and CS-1 peptides as a vaccine in SMM patients induced antigen-specific CD3+CD8+ memory CTL with functional Th-1 type immune responses (IFN-γ, IL-2, TNF-α).25 In addition, the first Phase 2a trial using our HLA-A2 multipeptide vaccine in combination with an immune modulatory drug Lenalidomide demonstrated enhanced vaccine efficacy, evidenced by a higher induction of tetramer positive antigen-specific CTL and functional immune responses in HLA-A2 positive SMM patients.25 Beyond myeloma, overexpression of the XBP1 and CD138 antigens are also seen in solid tumor cancers, including breast cancer, colon cancer and pancreatic cancer.26,27 The HLA-A2 multipeptide vaccine is also in clinical trials to treat the patients with triple negative breast cancer.28
In order to expand the therapeutic opportunity in patients beyond HLA-A2 restriction, we report here on novel immunogenic peptides to treat patients expressing HLA-A24, the second most dominant MHC Class I molecule in North America and the most frequent MHC Class I molecule in Asia. HLA-A24 specific peptides derived from unspliced XBP1, spliced XBP1, CD138 or CS1 were identified and characterized as follows: XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F) and CS1240-248 (L F V L G L F L W). Each of the individual HLA-A24 peptides demonstrated their immunogenicity by inducing CD3+CD8+ CTL ex vivo upon repeated stimulation of enriched CD3+ T cells from HLA-A24+ donors. The CTL displayed functional anti-MM immune responses against MM cells in an HLA-A24 restricted manner including proliferation, IFN-γ/IL-2 cytokine production as well as CD107a degranulation (cytotoxicity). In addition, a cocktail of these four immunogenic HLA-A24 peptides elicited multipeptide-specific CTL with a robust immune response against a wide repertoire of the targeted TAA on myeloma cells. This multipeptide approach may therefore overcome the loss of specific CTL activity due to specific antigen mutation or deletion on tumor cells or defects in the T-cell repertoire, which limit single antigen-based vaccine approaches. Our results demonstrate that a cocktail of the four immunogenic HLA-A24 specific unspliced XBP1/spliced XBP1/CD138/CS1 peptides evoke multipeptide-specific CTL with distinct phenotypic profiles against HLA-A24+ myeloma cells including upregulation of co-stimulatory molecules (CD28, CD40L, 41BB), cellular activation markers (CD38, CD69), and immune functional activities (perforin upregulation, Th1-type cytokines production).
Memory CD8+ T cells are a critical component of protective immunity in many different types of diseases including cancer. Optimal cancer vaccine therapies require induction of an antigen-specific “memory” CD8+ T cell population, which can self-renew and respond effectively to tumor antigens, while providing persistent long-term anti-tumor immunity.29-34 Here we provide evidence that repeated stimulation of CD3+ T cells with a cocktail of four HLA-A24 peptides specific to XBP1, CD138 and CS1 antigens evoke memory CD8+ CTL including central memory and effector memory cells. Among the HLA-A24 multipeptide-specific CTL Naïve:Memory cell subsets, the central memory population showed the highest expression of key co-stimulatory and cellular activation markers including CD28, CD38 and 41BB. However, the antigens-specific central memory CTL also had the highest expression of immune checkpoints (CTLA-4, PD-1, LAG-3, TIM-3) as compared to the effector memory and terminal effector CD8+ CTL populations. Despite expression of immune checkpoint antigens, the central memory cells within the HLA-A24+ XBP1/CD138/CS1 peptides-specific CTL retained the highest level of anti-MM activities. These observations suggest the possibility of increasing their functional activities with a blocking antibody against PD-1 or TIM-3, which were the main immune checkpoints induced following the multipeptide stimulation. Future studies will investigate the benefit of combining a checkpoint inhibitor(s) with the immunogenic HLA-A24 peptides to enhance anti-tumor activities by the antigens-specific CTL.
In summary, we have identified and validated novel immunogenic HLA-A24 peptides derived from XBP1 unspliced, XBP1 spliced, CD138 and CS1 antigens capable of inducing CD8+ CTL with robust anti-tumor activities against MM cells. These results highlight the potential therapeutic application of a cocktail of HLA-A24 XBP1un, XBP1sp, CD138 and CS1 peptides to evoke the antigens-specific CTL with a broad spectrum of responses against tumor. Combination approach to inhibit immune suppression and enhance the antigens-specific activities will be explored in our future studies.
Supplementary Material
T2-A24 cells were pulsed overnight with a high dose of respective peptide (200 μg/ml) in serum-free AIM-V medium. HLA-A24 specific HIV-1 envelope protein gp41583-591 (RYLKDQQLL) peptide was used as the positive control. Following incubation, the cells were harvested, washed, and stained with HLA-A24-PE mAb for flow cytometric analyses. Among 24 candidate peptides screened, XBP1 UN12-20 (D G T P K V L L L), XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F), CD13821-29 (L P Q I V A T N L), CD13813-21 (L A L S L Q P A L), CS1321-329 (T M P D T P R L F) and CS1240-248 (L F V L G L F L W) had the highest HLA-A24 binding affinities. Peptide affinity to HLA-A24 molecules is shown as an increase in specific HLA-A24-PE mean fluorescence intensity (MFI) in the peptide binding assays (Average ± SE; N=5).
Key Points.
Each HLA-A24 peptide identified induces anti-myeloma specific T lymphocytes targeting XBP1, CD138 and CS1 antigens.
Multipeptide cocktail with HLA-A24 specific XBP1 unspliced, XBP1 spliced, CD138 and CS1 peptides has an immunotherapeutic potential as a vaccine in myeloma patients by evoking the antigens-specific CTL.
Acknowledgments
The authors acknowledge the generous gift of T2-A*2401 cells from Dr. Y. Miyahara at Mie University School of Medicine (Mie, Japan) and Dr. E. Jaffee at the Johns Hopkins University School of Medicine (Baltimore, MD). We also thank Mr. John Daley and Ms. Suzan Lazo-Kallanian from the flow cytometry facility at Dana-Farber Cancer Institute for providing flow cytometry assistance.
This work was supported in part by grants from the National Institutes of Health Grants RO1-124929 to Dr. Nikhil C. Munshi, P50-100007, PO1-78378 and PO1155258 to Drs. Kenneth C. Anderson and Nikhil C. Munshi, and RO1-50947 to Dr. Kenneth C. Anderson. Dr. Kenneth C. Anderson is an American Cancer Society Clinical Research Professor.
Footnotes
Conflict of Interest:
N.C. Munshi is an honorarium of Celgene, Millennium and Novartis; K.C. Anderson is an honorarium of Celgene, Millennium and Onyx; and P.G. Richardson is an honorarium of Celgene, Millennium and Johnson & Johnson. N.C. Munshi, K.C. Anderson and J. Bae have ownership interest and are advisory board members in OncoPep Inc. No relevant conflicts of interest were disclosed by the other authors
References
- 1.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol. 2011;6:249–274. doi: 10.1146/annurev-pathol-011110-130249. [DOI] [PubMed] [Google Scholar]
- 3.Munsh NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, et al. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+ HLA-DRlow myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72:540–547. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 5.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–3949. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 6.Rutella S1, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 7.Rutella S1, Locatelli F. Targeting multiple-myeloma-induced immune dysfunction to improve immunotherapy outcomes. Clin Dev Immunol. 2012;2012:196063. doi: 10.1155/2012/196063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JJ, Choi BH, Kang HK, Park MS, Park JS, Kim SK, et al. Induction of multiple myeloma-specific cytotoxic T lymphocyte stimulation by dendritic cell pulsing with purified and optimized myeloma cell lysates. Leuk Lymphoma. 2007;48:2022–2031. doi: 10.1080/10428190701583975. [DOI] [PubMed] [Google Scholar]
- 9.Yi Q, Szmania S, Freeman J, Qian J, Rosen NA, Viswamitra S, et al. Optimizing dendritic cell-based immunotherapy in multiple myeloma: intranodal injections of idiotype-pulsed CD40 ligand-matured vaccines led to induction of type-1 and cytotoxic T-cell immune responses in patients. Br J Haematol. 2010;150:554–564. doi: 10.1111/j.1365-2141.2010.08286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae J, Carrasco R, Lee AH, Prabhala R, Tai YT, Anderson KC, et al. Identification of novel myeloma-specific XBP1 peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma. Leukemia. 2011;25:1610–1619. doi: 10.1038/leu.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae J, Tai YT, Anderson KC, Munshi NC. Novel epitope evoking CD138 antigen-specific cytotoxic T lymphocytes targeting multiple myeloma and other plasma cell disorders. Br J Haematol. 2011;155:349–361. doi: 10.1111/j.1365-2141.2011.08850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae J, Song W, Smith R, Daley J, Tai YT, Anderson KC, Munshi NC. A novel immunogenic CS1-specific peptide inducing antigen-specific cytotoxic T lymphocytes targeting multiple myeloma. Br J Haematol. 2012;157:687–701. doi: 10.1111/j.1365-2141.2012.09111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabaker K, Shell K, Kaufman HL. Vaccines for colorectal cancer and renal cell carcinoma. Cancer J. 2011;17:283–293. doi: 10.1097/PPO.0b013e318232ff44. [DOI] [PubMed] [Google Scholar]
- 14.Rezvani K, de Lavallade H. Vaccination strategies in lymphomas and leukaemias: recent progress. Drugs. 2011;71:1659–1674. doi: 10.2165/11593270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Bae J, Prabhala R, Voskertchian A, Brown A, Maguire C, Richardson P, et al. A multiepitope of XBP1, CD138 and CS1 peptides induces myeloma-specific cytotoxic T lymphocytes in T cells of smoldering myeloma patients. Leukemia. 2015;29:218–229. doi: 10.1038/leu.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae J, Smith R, Daley J, Mimura N, Tai YT, Anderson KC, et al. Myeloma-specific multiple peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma and other plasma cell disorders. Clin Cancer Res. 2012;18:4850–4860. doi: 10.1158/1078-0432.CCR-11-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuranda K, Berthon C, Dupont C, Wolowiec D, Leleu X, Polakowska R, et al. A subpopulation of malignant CD34+CD138+B7-H1+ plasma cells is present in multiple myeloma patients. Exp Hematol. 2010;38:124–131. doi: 10.1016/j.exphem.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 19.Romano A, Conticello C, Cavalli M, Vetro C, La Fauci A, Parrinello NL, et al. Immunological dysregulation in multiple myeloma microenvironment. Biomed Res Int. 2014;2014:198539. doi: 10.1155/2014/198539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshua D, Suen H, Brown R, Bryant C, Ho PJ, Hart D, et al. The T cell in myeloma. Clin Lymphoma Myeloma Leuk. 2016;16:537–542. doi: 10.1016/j.clml.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Tucci M, Stucci S, Savonarola A, Ciavarella S, Cafforio P, Dammacco F, et al. Immature dendritic cells in multiple myeloma are prone to osteoclast-like differentiation through interleukin-17A stimulation. Br J Haematol. 2013;161:821–831. doi: 10.1111/bjh.12333. [DOI] [PubMed] [Google Scholar]
- 22.Podar K, Richardson PG, Hideshima T, Chauhan D, Anderson KC. The malignant clone and the bone-marrow environment. Best Pract Res Clin Haematol. 2007;20:597–612. doi: 10.1016/j.beha.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Dhodapkar MV1, Krasovsky J, Osman K, Geller MD. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med. 2003;198:1753–1757. doi: 10.1084/jem.20031030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutella S, Locatelli F. Targeting multiple-myeloma-induced immune dysfunction to improve immunotherapy outcomes. Clin Dev Immunol. 2012;2012:196063. doi: 10.1155/2012/196063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nooka AJ, Wang M, Yee AJ, Thomas SK, O’Donnell EK, Shah JJ, et al. Final Results of a Phase 1/2a, Dose Escalation Study of Pvx-410 Multi-Peptide Cancer Vaccine in Patients with Smoldering Multiple Myeloma (SMM) American Society of Hematology. 2016;128:2124. [Google Scholar]
- 26.Bae J, Samur M, Munshi A, Hideshima T, Keskin D, Kimmelman A, et al. Heteroclitic XBP1 Peptides evoke tumor-specific memory cytotoxic T lymphocytes against breast cancer, colon cancer and pancreatic cancer cells. OncoImmunology. 2014;3:e970914. doi: 10.4161/21624011.2014.970914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae J, Keskin D, Cowens K, Lee AH, Dranoff G, Munshi NC, et al. Lenalidomide polarizes Th1-specific anti-rumor immune response and expands XBP1 antigen-specific central memory CD3+CD8+ T cells against various solid tumors. J Leuk. 2015;3:178–190. doi: 10.4172/2329-6917.1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isakoff S, Tolaney S, Tung N, Adams S, Hussein H, Brachtel E, et al. A Phase 1b study of safety and immune response to PVX-410 vaccine alone and in combination with Durvalumab in HLA-A2+ subjects following standard treatment of stage II or III triple negative breast cancer. American Society of Clinical Oncology. 2017;35:15. [Google Scholar]
- 29.Olson JA, Jameson SC. Keeping STATs on memory CD8+ T cells. Immunity. 2011;35:663–665. doi: 10.1016/j.immuni.2011.11.006. 2011. [DOI] [PubMed] [Google Scholar]
- 30.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 31.Junker N, Kvistborg P, Køllgaard T, Straten Pt, Andersen MH, Svane IM. Tumor associated antigen specific T-cell populations identified in ex vivo expanded TIL cultures. Cell Immunol. 2012;273:1–9. doi: 10.1016/j.cellimm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Dobrzanski MJ, Reome JB, Hylind JC, Rewers-Felkins KA, Abdulsamad K, Adams SL. Ag-specific type 1 CD8 effector cells enhance methotrexate-mediated antitumor responses by modulating endogenous CD49b-expressing CD4 and CD8 T effector cell subpopulations producing IL-10. Immunol Invest. 2008;37:315–338. doi: 10.1080/08820130802083762. [DOI] [PubMed] [Google Scholar]
- 33.Ye SW, Wang Y, Valmori D, Ayyoub M, Han Y, Xu XL, et al. Ex-vivo analysis of CD8+ T cells infiltrating colorectal tumors identifies a major effector-memory subset with low perforin content. J Clin Immunol. 2006;26:447–456. doi: 10.1007/s10875-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 34.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
T2-A24 cells were pulsed overnight with a high dose of respective peptide (200 μg/ml) in serum-free AIM-V medium. HLA-A24 specific HIV-1 envelope protein gp41583-591 (RYLKDQQLL) peptide was used as the positive control. Following incubation, the cells were harvested, washed, and stained with HLA-A24-PE mAb for flow cytometric analyses. Among 24 candidate peptides screened, XBP1 UN12-20 (D G T P K V L L L), XBP1 UN185-193 (I S P W I L A V L), XBP1 SP223-231 (V Y P E G P S S L), CD138265-273 (I F A V C L V G F), CD13821-29 (L P Q I V A T N L), CD13813-21 (L A L S L Q P A L), CS1321-329 (T M P D T P R L F) and CS1240-248 (L F V L G L F L W) had the highest HLA-A24 binding affinities. Peptide affinity to HLA-A24 molecules is shown as an increase in specific HLA-A24-PE mean fluorescence intensity (MFI) in the peptide binding assays (Average ± SE; N=5).


