Abstract
Neurosensory abnormalities are frequently observed following pediatric mild traumatic brain injury (pmTBI) and may underlie the expression of several common concussion symptoms and delay recovery. Importantly, active evaluation of neurosensory functioning more closely approximates real-world (e.g., physical and academic) environments that provoke symptom worsening. The current study determined whether symptom provocation (i.e., during neurosensory examination) improved classification accuracy relative to pre-examination symptom levels and whether symptoms varied as a function of point of care. Eighty-one pmTBI were recruited from the pediatric emergency department (PED; n = 40) or outpatient concussion clinic (n = 41), along with matched (age, sex, and education) healthy controls (HC; n = 40). All participants completed a brief (∼ 12 min) standardized neurosensory examination and clinical questionnaires. The magnitude of symptom provocation upon neurosensory examination was significantly higher for concussion clinic than for PED patients. Symptom provocation significantly improved diagnostic classification accuracy relative to pre-examination symptom levels, although the magnitude of improvement was modest, and was greater in the concussion clinic. In contrast, PED patients exhibited worse performance on measures of balance, vision, and oculomotor functioning than the concussion clinic patients, with no differences observed between both samples and HC. Despite modest sample sizes, current findings suggest that point of care represents a critical but highly under-studied variable that may influence outcomes following pmTBI. Studies that rely on recruitment from a single point of care may not generalize to the entire pmTBI population in terms of how neurosensory deficits affect recovery.
Keywords: : neurosensory, ocular motor, pmTBI, recovery, vestibular, vision
Introduction
There is increased public concern regarding the incidence and potential long-term effects of pediatric mild traumatic brain injury (pmTBI),1 with recent estimates suggesting 750,000 new cases each year.2 The diagnosis and prognosis of pmTBI is based on symptom self-report,3 with emerging evidence suggesting a prolonged course of recovery in adolescent, relative to adult, patients.4,5 Neurosensory abnormalities (vestibulo-ocular, ocular motor, visual and vestibulospinal) may underlie the expression of several common concussion symptoms and may be associated with persistent symptoms.6 Although previous studies have reported neurosensory deficits in adult7,8 and pediatric9–12 mTBI samples, the prevalence of symptom types and how neurosensory dysfunction varies as a function of healthcare setting (e.g., pediatric emergency department [PED] versus concussion clinic) in pmTBI has not been determined. Point of care may be an important, yet under-studied factor, given the large differences in outcomes reported in the adult literature following mTBI.13,14
There are several benefits for conducting a neurosensory examination in clinical and research settings. First, in contrast to the nonspecific, subjective, self-report nature of concussion symptoms,15,16 certain aspects of neurosensory dysfunction (e.g., near point of convergence [NPC], balance) can be directly quantified, and correspond to a potential underlying biological mechanism.6,17,18 Second, the measurement of symptom exacerbation during the gradual exposure of patients to physical19,20 and/or neurosensory12 tasks (hereafter referred to as “symptom provocation”) is increasingly recognized as an effective strategy for determining recovery.11,21,22 Recent evidence suggests that recovery may vary across different neurosensory and cognitive domains, and likely exceeds the “traditional” 7–10 day recovery window.11,23
pmTBI patients are seen across a variety of clinical and research settings,24,25 with varying outcomes reported as a function of point of care in adult studies.13,14 Data derived from outpatient specialty clinics in the semi-acute phase suggest that 28.6–69% of pmTBI patients have vestibulo-ocular and/or visual symptoms,10,17 with 81% reporting vestibulospinal (i.e., difficulty with balance) problems.26 To our knowledge, the prevalence of vestibulo-ocular, ocular motor, visual, and vestibulospinal deficits has not been investigated in PED patients, nor have the characteristics of these deficits been compared across pmTBI samples derived from different healthcare settings.
The current study, therefore, assessed neurosensory functioning in pmTBI patients recruited from either PED or a concussion clinic and from a community sample of healthy controls (HC) using a standardized battery. Our primary aims were to determine whether symptom provocation would be a more sensitive marker for classification (patient vs. control) and recovery than symptoms at rest, and whether there were differences in symptom provocation as a function of point of care. We hypothesized that: (1) pmTBI patients would exhibit a greater level of concussion symptoms prior to provocative testing than HC, (2) concussion symptom provocation would demonstrate increased sensitivity and specificity relative to pre-examination symptomatology for patient classification, and (3) concussion symptom provocation would be greater in a PED sample because there was a more acute time frame for assessment (i.e., closer to time of injury) relative to a concussion clinic sample.
Methods
Participants
Patients between 12 and 18 years old were consecutively recruited from either the University of New Mexico Hospital Emergency Department (UNMH; n = 42) or The Children's Hospital of Philadelphia Minds Matter Concussion Program (CHOP; n = 42). All patients were seen during the acute to semiacute injury phase of pmTBI (within 28 days of injury). As expected based on the different types of medical care for concussion, patients were seen at longer post-injury intervals in the concussion clinic. Forty-one age-, education-, and sex-matched children served as HC, mostly obtained at the New Mexico site. Two patients (one UNMH and one CHOP) and one HC were extreme outliers on two or more tests (three times the interquartile range) and were excluded from further analyses. One UNMH patient had a high level of pre-examination symptoms with no symptoms following neurosensory testing, and was therefore also excluded. The final cohort consisted of 40 UNMH pmTBI patients (15 females; age, 15.1 ± 2.0; 6.6 ± 2.4 days post-injury), 41 CHOP pmTBI patients (18 females; age, 14.9 ± 1.6; 11.3 ± 7.4 days post-injury), and 40 HC (14 females; age, 14.3 ± 1.9). Informed consent was obtained from participants according to each institution's guidelines.
All pmTBI experienced a brain injury associated with the onset of new symptoms. Loss of consciousness (if present) was <30 min, and post-traumatic amnesia (if present) was limited to 24 h. Exclusion criteria for both UNMH and CHOP participants included a positive history of neurological disease, major psychiatric disturbance (i.e., anything other than adjustment disorder), autistic spectrum disorders, prior closed head injuries with >5 min of loss of consciousness, active litigation, substance abuse, or alcohol abuse. HC were excluded for all of the mentioned criteria, as well as for a history of attention-deficit/hyperactivity disorder (ADHD) or learning disorder.
Neurosensory examination
The examination focused on vestibulo-ocular, ocular motor, vestibulospinal, and visual deficits. Selected tests were derived from a number of different sources or newly devised (see Supplementary Text and Fig. S1; see online supplementary material at http://www.liebertpub.com). A measure of symptom validity/effort is highly recommended,27 but does not currently exist for standardized neurosensory tests. All participants, therefore, performed a vigorous dorsiflexion of both feet for ∼20 sec (the double dorsiflexion foot stretch [DDFS]), as this was deemed to be nonspecific to the vestibulo-ocular, ocular motor, visual, or vestibulospinal systems and did not require substantial physical exertion. To avoid bias, the DDFS was always administered first, prior to any other neurosensory examination.
The majority of neurosensory tests (i.e., smooth pursuits, horizontal and vertical saccades, horizontal and vertical vestibulo-ocular reflex, visual motion sensitivity) were derived directly from The Vestibular/Ocular Motor Screening (VOMS),12 with no alterations to the instructions. Per guidelines, a metronome (see Supplementary Materials for rates) was used to assist the participants in guiding the tempo of movements. The VOMS measurement of NPC (measured to 0.5 cm across three trials) was replaced with a more standardized procedure used in clinical trials for convergence insufficiency that uses the Astron Accommodative Rule (Gulden Ophthalmics, Elkins Park, PA) with a standard single 20/30 card as the visual target.28,29 A total of three trials were conducted, and each trial was halted when the participant reported a doubling of the stimuli or when the experimenter noticed binocular loss of convergence. Monocular accommodation was acquired over a single trial for each eye, using the exact same visual stimulus as convergence. The King–Devick test was used to further quantify saccadic eye movements,30,31 with total number of errors and completion time recorded. Finally, a tandem gait task assessed balance, with participants taking 10 steps forwards and backwards with eyes open and closed (five steps each).
All participants were required to rate the degree to which they experienced four symptoms (headache, dizziness, nausea, and fogginess)12 on an 11 point Likert scale prior to the start of the neurosensory examination (i.e., pre-examination symptoms) and immediately following each task listed (i.e., symptom provocation). The primary outcome measures were the total self-reported symptom burden prior to neurosensory testing (i.e., sum of all pre-examination symptoms) and the total burden of symptom provocation reported after performing the neurosensory tests (i.e., sum of positive differences or increased symptoms across each individual test relative to pre-examination levels). The latter variable excluded the score from the nonspecific symptom provocation measure (DDFS) in the calculations. Secondary analyses examined symptom variation (i.e., sum of differences in symptoms for each individual test relative to pre-examination levels).
Statistical analyses
One way group (UNMH vs. CHOP vs. HC) ANOVAs evaluated hypotheses regarding symptom provocation (primary) or total symptom change (secondary) using rank-transformed data caused by non-normality. A Fisher's exact test examined whether the number of participants exhibiting nonspecific symptom provocation (DDFS) differed across the three groups. ANOVAs or multivariate ANOVAs (MANOVAs) were performed on all quantifiable measures (e.g., distances, errors) to assess for group differences using log-based (e.g., skewed data) or rank (error data) transformations as appropriate. Binary logistic regression examined whether symptom provocation was more sensitive and specific for classifying patients from controls relative to pre-examination symptom report. Any simple effects tests were corrected for multiple comparisons (Bonferroni correction) and only significant findings are reported. Statistical tests were conducted on transformed data whereas actual data (i.e., nontransformed) are presented in figures.
Results
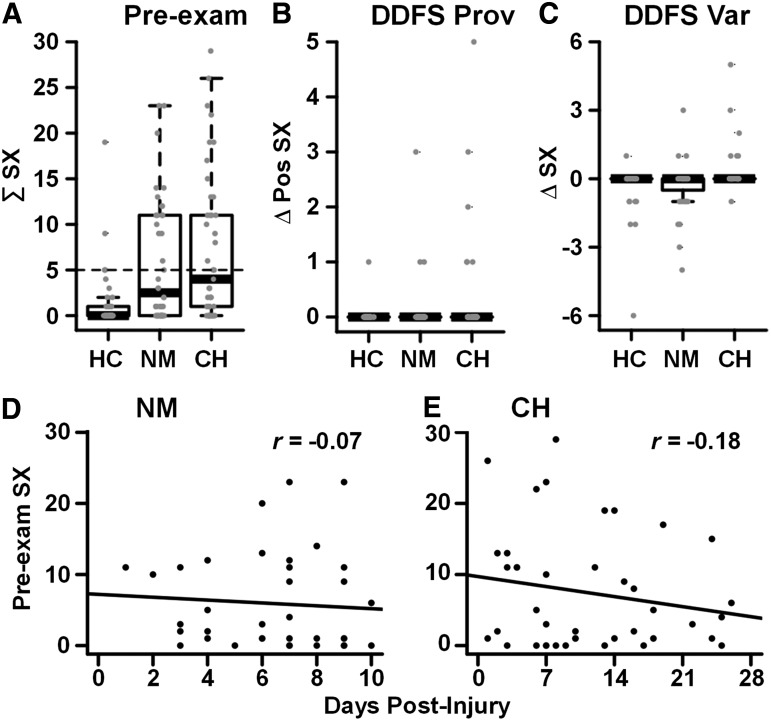
No significant differences in age (p = 0.156) or sex (p = 0.697) were observed (see Table 1). Significant group differences existed in number of prior concussions (F2,118 = 10.00, p < 0.001), with simple effects tests indicating a larger number of previous concussions in both CHOP (t50.5 = 4.60, p < 0.001) and UNMH groups (t54 = 3.08, p = 0.003) relative to HC. As expected based on typical patterns of seeking clinical care, mean days post-injury to assessment was significantly higher (t48.8 = 3.83, p < 0.001) for CHOP than for UNMH. However, days post-injury was not significantly related to pre-examination symptomatology (CHOP r = −0.18, p = 0.255; UNMH r = −0.07, p = 0.655) or symptom provocation (CHOP r = -0.05, p = 0.775; UNMH r = -0.21, p = 0.184) in either sample. Similarly, there was a significantly (X = 4.48; p = 0.034) higher proportion of children with sports-related concussions in the CHOP sample (68.3%) than in the UNMH sample (45.0%).
Table 1.
Demographics and Neurosensory Examination
| Demographics | HC (n = 40) | UNMH (n = 40) | CHOP (n = 41) |
|---|---|---|---|
Age
|
14.3 (1.9) | 15.1 (2.0) | 14.9 (1.6) |
| Gender (% F) | 35.0% | 37.5% | 43.9% |
| n previous mTBIs | 0 (0) | 0 (1) | 0 (2) |
Days post-injury
|
NA | 6.6 (2.4) | 11.3 (7.4) |
| Injury classification (n participants) | |||
| Sports-related concussion | NA | 18 | 28 |
| Motor vehicle crashes | NA | 11 | 3 |
| Falls/assaults | NA | 11 | 10 |
| Clinical conditions (n participants) | |||
| ADHD | 0 | 5 | 3 |
| Learning disability | 0 | 3 | 4 |
| Total clinical conditionsa | 0 | 7 | 5 |
| Neurosensory symptoms | |||
| Baseline | 0 (1.00) | 2.50 (11.00) | 4.00 (10.00) |
| Nonspecific symptom provocation (n participants) | 1 | 3 | 6 |
| Symptom provocation | 0 (1.00) | 4.00 (23.00) | 19.00 (35.00) |
| Total symptom variation | 0 (4.00) | 1.00 (19.00) | 19.00 (36.00) |
| Objective measures | |||
| Near point of convergence (cm) | 5.42 (4.00) | 5.92 (3.00) | 4.50 (2.67) |
| Accommodative amplitude (cm) | |||
| Left eye | 7.50 (2.90) | 7.50 (4.40) | 7.00 (2.00) |
| Right eye | 7.50 (2.40) | 8.50 (5.50) | 6.00 (2.00) |
| Tandem walking errors | 3.50 (6.00) | 4.00 (4.00) | 1.00 (3.00) |
| King–Devick | |||
| Time | 68.30 (17.00) | 66.45 (21.00) | 54.64 (20.00) |
| Errors | 0 (0) | 0 (1) | 0 (0) |
Median (Interquartile ranges) are presented for all variables unless otherwise specified by the symbol  , in which case mean and standard deviation are presented.
, in which case mean and standard deviation are presented.
Individuals could be diagnosed with more than one clinical condition such that the total is not a simple sum. Nonspecific symptom provocation is calculated based on increase in symptom ratings on double dorsiflexion foot stretch. Healthy Control (HC) days post-injury and injury classification is not applicable (NA).
UNMH, University of New Mexico Hospital Emergency Department; CHOP, The Children's Hospital of Philadelphia Minds Matter Concussion Program mTBI, mild traumatic brain injury; ADHD, attention-deficit/hyperactivity disorder.
The ANOVA comparing pre-examination symptomatology was significant (F2,118 = 14.63, p < 0.001), with higher symptom loads in the UNMH (t72.4 = 4.40, p < 0.001) and CHOP (t74.3 = 5.30, p < 0.001) groups than in HC, but with no differences between the two pmTBI samples (t79 = 0.76, p = 0.449; Table 1; Fig. 1A). A total of 95% of HC reported a pre-examination symptom burden score of ≤5, which was then used to operationally define symptomatic versus asymptomatic (i.e., recovered from injury) patients in both samples. Only a few participants in each group reported any symptom provocation (Table 1; Fig. 1B) or symptom variation (Fig. 1C) on the DDFS. A Fisher's exact test was not significant (p = 0.155), although this effect may have been limited by low power.
FIG. 1.
(A) Presents scatter box plots representing the total pre-examination symptom (SX) burden for each group (HC, healthy controls; NM, University of New Mexico Hospital Emergency Department patients; CH, The Children's Hospital of Philadelphia Minds Matter Concussion Program patients). The dashed line indicates the 95th percentile for pre-examination symptoms in HC. Symptom provocation (Prov/positive symptom change = Δ Pos SX) and symptom variability (Var/change in symptom = Δ SX; C) following a non-neurosensory task (DDFS, double dorsiflexion foot stretch) are presented in B and C, respectively. D and E present scatter plots depicting the relationship between the number of days post-injury and pre-examination symptoms experienced by the NM and CH groups.
The ANOVA evaluating symptom provocation (Fig. 2) was significant (F2,118 = 28.04, p < 0.001). CHOP patients experienced increased symptom provocation relative to both UNMH (t79 = 2.90, p = 0.005) and HC (t65.4 = 8.20, p < 0.001), with UNMH patients also reporting greater symptom provocation than HC (t62.2 = 4.45, p < 0.001). Results from secondary analyses excluding patients with comorbid disorders from both samples (see Supplementary Materials) were statistically similar for pre-examination and symptom provocation, suggesting that the presence of comorbid disorders did not explain point of care differences observed in principal analyses. Moreover, there were no significant differences in pre-examination symptomatology (p = 0.460) and total symptom provocation (p = 0.520) between pmTBI patients with and without comorbidities when collapsed across groups, although these effects may have been limited by power.
FIG. 2.
Scatter box plots depicting symptom provocation across all tests (sum of the positive change in symptoms following each individual test = ∑ Δ Pos SX; A) and following each individual test (positive symptom change following each of individual test = Δ Pos SX; B–D) for each group (HC, healthy controls; NM, University of New Mexico Hospital Emergency Department patients; CH, The Children's Hospital of Philadelphia Minds Matter Concussion Program patients). Labels for individual tests include: SMP, smooth pursuits; SAH, horizontal saccades; SAV, vertical saccades; VOH, horizontal vestibular-ocular reflex; VOV, vertical vestibular-ocular reflex; VIM, visual motion sensitivity; NPC, near point of convergence; MAC, monocular accommodative amplitude; TG, tandem gait; KD, King–Devick test.
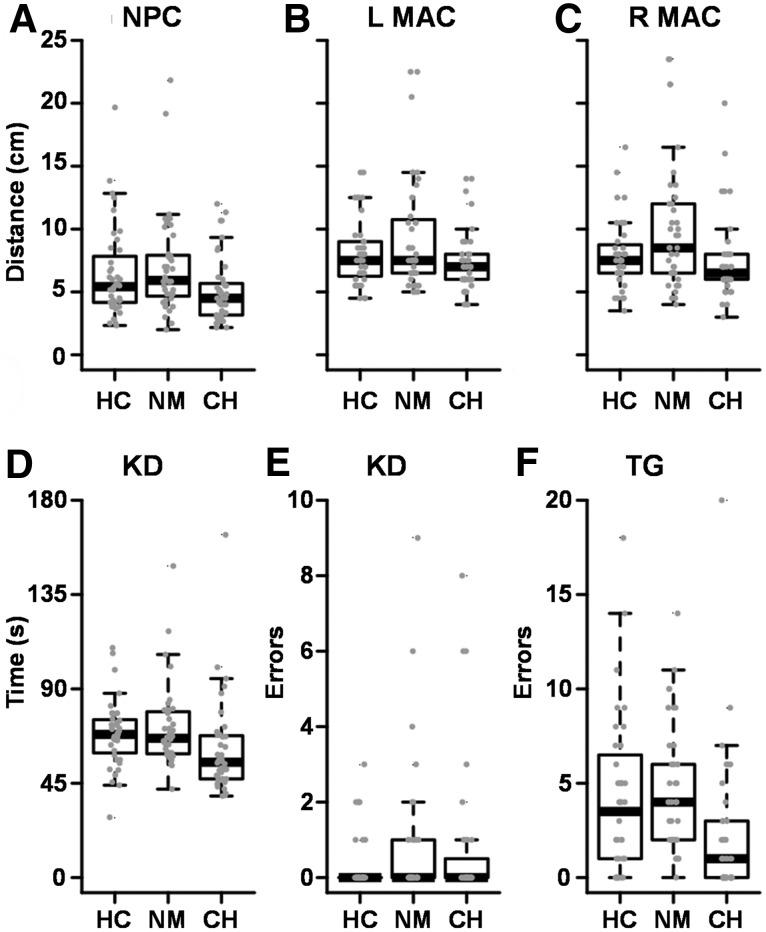
A significant effect of group was observed for NPC distance (F2,118 = 4.18, p = 0.018), with CHOP patients exhibiting significantly lower NPC than UNMH patients (t79 = 2.85, p = 0.006). Completion time for the King–Devick test differed significantly between groups (F2,117 = 5.32, p = 0.006), with CHOP patients completing the test more quickly than UNMH patients (t78 = 3.03, p = 0.003). Error rates on the King–Devick test were nonsignificant between groups (p = 0.459; Fig. 3). ANOVAs comparing monocular accommodation indicated significant differences for group for the left (F2,118 = 4.16, p = 0.018) and right eye (F2,117 = 4.20, p = 0.017), with UNMH patients manifesting significantly greater accommodation distance than CHOP patients for left (t71.8 = 2.75, p = 0.008) and right eye (t78 = 2.65, p = 0.010). The group effect was significant for tandem gait errors (F2,116 = 10.48, p < 0.001), with CHOP patients exhibiting significantly fewer errors than UNMH patients (t77 = −4.80, p < 0.001) and HC (t79 = −2.85 p = 0.006).
FIG. 3.
Box plots depicting quantifiable data for near point of convergence distance (NPC; A), left (L) and right (R) monocular accommodative amplitude (MAC; B and C), King–Devick (KD) time (D) and errors (E), and tandem gait errors (TG; F) across groups (HC, healthy controls; NM, University of New Mexico Hospital Emergency Department patients; CH, The Children's Hospital of Philadelphia Minds Matter Concussion Program patients). Per convention, statistical tests were conducted on rank-transformed data, whereas untransformed scores are presented in figures.
Separate binary logistical regression analyses determined whether symptom provocation with neurosensory examination was more sensitive than pre-examination symptomatology for classifying patients and controls. Self-reported pre-examination symptomatology significantly (Wald = 13.83; p < 0.001) classified HC (67.5%) from UNMH patients (72.5%) with a total accuracy of 70.0%. The addition of symptom provocation to the model resulted in significant (Wald = 5.30; p = 0.021), but minimal improvement in total accuracy (73.8%; Δ = 3.8%), which resulted from an improvement in classification of the HC (77.5%; Δ = 10.0%), rather than the UNMH patient group (70.0%; Δ = −2.5%).
Results for pre-examination symptoms were similar for the CHOP sample, with symptoms significantly (Wald = 17.62; p < 0.001; total accuracy = 72.8%) classifying CHOP patients (78.0%) from HC (67.5%). The inclusion of symptom provocation in the model resulted in a significant (Wald = 16.37; p < 0.001) improvement in total accuracy (80.2%; Δ = 7.4%), with classification accuracy increasing slightly in the CHOP patient group (80.5%; Δ = 2.5%) and increasing more for HC (80.0%; Δ = 12.5%). See Supplementary Materials for comparisons between recovered and nonrecovered patients.
Discussion
The current study assessed neurosensory function and symptom provocation in two independent samples of pmTBI patients derived from different points of care (PED vs. concussion clinic). The two patient samples were well matched in terms of biological sex, age, and previous concussion history. Consistent with a priori hypotheses, pmTBI patients exhibited elevated pre-examination concussion symptoms relative to HC, and symptom provocation accurately classified both patient samples versus controls above and beyond pre-examination symptoms. Contrary to predictions, the magnitude of symptom provocation and the improvement in classification accuracy were higher for concussion clinic than for PED patients. In contrast, performance on objectives measure of balance, vision, and oculomotor functioning were generally worse for the PED sample.
Previous research has suggested that recovery times differ based on point of care, with 31–33% of PED patients5 and 73% of specialty clinical patients22 reporting significant impairment 4 weeks post injury. Different recovery patterns in mTBI have also been reported for large-scale studies of adult athletes (i.e., rapid recovery for the majority)14 relative to injured ED/hospitalized (i.e., slow recovery for a substantial minority) patients.13 However, all of these studies confounded point of care with assessment methodology because they were conducted with different measures. Current results suggest that both the amount of pre-test symptomatology and proportion of symptomatic patients (based on cutoff determined from the 95th percentile of the control group) were statistically similar across PED and concussion clinic patients, although the concussion clinic group was assessed later in the recovery course. These results highlight the importance of using a standardized examination and identical criteria for identifying symptomatic patients across points of care, eliminating several methodological concerns associated with symptom-based diagnoses that have hampered previous investigations.3,32
Neurosensory and physical challenges provide a platform for measuring symptom provocation that is similar to a child's real-world experiences (e.g., school and active play) and, therefore, are superior to self-report.12,19,20,33 Consistent with previous results,11,21,22 current findings suggest that: (1) both pmTBI samples exhibited increased symptom provocation relative to HC, (2) symptom provocation increased diagnostic classification accuracy relative to pre-examination symptoms alone, and (3) symptom provocation was a more sensitive marker for identifying patients who were not recovered. To our knowledge, this is the first study to indicate that the magnitude of symptom provocation and the overall impact on classification accuracy were higher for a concussion clinic (classification accuracy Δ = 7.4%) relative to a PED-derived (classification accuracy Δ = 3.8%) sample. Pre-existing learning disorders and ADHD are common in pmTBI,34 and have previously been associated with greater concussion symptoms, as well as with prolonged recovery.22,35 Importantly, symptom provocation significantly differed across points of care even when individuals with comorbid disorders were excluded from analyses. Moreover, secondary analyses indicated that pre-examination symptoms and symptom provocation did not differ between patients with and without comorbid disorders, suggesting that this was not a driving factor in current results.
Considered collectively, symptom-based findings suggest fundamental differences between pmTBI patients recruited from different points of care, with evidence of prolonged recovery and greater symptom burden in a self-referred concussion clinic sample compared with prospective recruiting (i.e., all-comers study) from the PED. Although the specialty clinic patients appears to represent a more symptomatic population seeking additional care based on persistent symptom self-report, the PED sample generally performed worse on objective measures of neurosensory functioning than the concussion clinic sample (tandem gait errors), with significant differences in other visual measures as well (accommodation and NPC).
Previous data suggest that vestibulospinal impairments (i.e., balance) predicted return to school, represent important treatment targets in pmTBI, and are typically more abnormal during the 1st week of injury.36,37 The differences in accommodation and NPC may be related to abnormal parasympathetic autonomic function, which may be more pronounced earlier in the course of injury (worse performance observed when presenting earlier rather than later post-injury). Previous results also suggest that the King–Devick test is most sensitive in the very acute (i.e., within first 48 h) injury phase.38 Collectively, these findings may be partially reflective of the fact that PED patients were tested at time points more proximal to their injury. Importantly, neither group differed from the HC sample. Therefore, the current stratification by point of care may also reflect differences in socioeconomic status, state laws for patient referrals, injury characteristics (e.g., sport-related concussion vs. motor vehicle crash, time post-injury), and/or other associated factors. Therefore, current findings will require replication in larger samples to ensure that the findings generalize.
Several limitations of the current study should be noted. Foremost, sample sizes may have limited power to detect smaller effect sizes or to explore relationships between nonspecific symptom exacerbation, concussion-specific symptom provocation, objective findings, and the effect of comorbid conditions. Second, examiners were not blinded to patient status. Third, additional studies are required to (1) reliably establish cutoffs for use of neurosensory testing in clinical samples across a variety of healthcare settings and (2) determine if neurosensory testing can increase the 70% predictive accuracy for prolonged recovery obtained with nine demographic and symptom factors in a recent PED study.5 Finally, the examination did not include a test of auditory functioning, which has been shown to be impaired in both military and civilian samples of adult mTBI.39,40 This precludes the assessment of certain forms of common pathology following TBI (e.g., tinnitus, effortful comprehension) or impairments that span multiple sensory modalities.41
In summary, previous work suggests that disturbances in neurosensory function have good diagnostic (i.e., differentiating pmTBI patients from controls)9,12 as well as prognostic utility for academic problems,42 disrupted neurocognitive function,43delayed return to school,26 and prolonged recovery.11 The current study confirms these results, but further emphasizes the complexities involved in symptom-based diagnosis. Specifically, current findings highlight the important but frequently overlooked role of point of care and associated factors (e.g., time-post injury) which may prove to be important for determining outcomes in pmTBI research.24,25 Several more recent studies have further quantified the effects of concussion on vestibular (e.g., with gyroscopes) and visual functioning (e.g., with eye tracking and/or pupillometry) using more precise equipment than our clinically based examination,44,45 providing new diagnostic and prognostic opportunities for investigating how neurosensory dysfunction impacts recovery.
Supplementary Material
Acknowledgments
This study was funded by National Institutes of Health grant number 1 R01 NS098494-01A1 to A.R.M.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Marin J.R., Weaver M.D., Yealy D.M., and Mannix R.C. (2014). Trends in visits for traumatic brain injury to emergency departments in the United States. JAMA 311, 1917–1919 [DOI] [PubMed] [Google Scholar]
- 2.Zemek R.L., Grool A.M., Duque D.R., DeMatteo C., Rothman L., Benchimol E.I., Guttmann A., and Macpherson A.K. (2016). Annual and seasonal trends in ambulatory visits for pediatric concussion in Ontario between 2003 and 2013. J. Pediatr. 181, 222–228 [DOI] [PubMed] [Google Scholar]
- 3.Mayer A.R., Quinn D.K., and Master C.L. (2017). The spectrum of mild traumatic brain injury: a review. Neurology 89, 623–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeates K.O., Kaizar E., Rusin J., Bangert B., Dietrich A., Nuss K., Wright M., and Taylor H.G. (2012). Reliable change in postconcussive symptoms and its functional consequences among children with mild traumatic brain injury. Arch. Pediatr. Adolesc. Med. 166, 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zemek R., Barrowman N., Freedman S.B., Gravel J., Gagnon I., McGahern C., Aglipay M., Sangha G., Boutis K., Beer D., Craig W., Burns E., Farion K.J., Mikrogianakis A., Barlow K., Dubrovsky A.S., Meeuwisse W., Gioia G., Meehan W.P., III, Beauchamp M.H., Kamil Y., Grool A.M., Hoshizaki B., Anderson P., Brooks B.L., Yeates K.O., Vassilyadi M., Klassen T., Keightley M., Richer L., DeMatteo C., and Osmond M.H. (2016). Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 315, 1014–1025 [DOI] [PubMed] [Google Scholar]
- 6.Hoffer M.E. (2015). Mild traumatic brain injury: neurosensory effects. Curr. Opin. Neurol. 28, 74–77 [DOI] [PubMed] [Google Scholar]
- 7.Mayer A.R., Bellgowan P.S., and Hanlon F.M. (2015). Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci. Biobehav. Rev. 49, 8–18 [DOI] [PubMed] [Google Scholar]
- 8.Lew H.L., Pogoda T.K., Baker E., Stolzmann K.L., Meterko M., Cifu D.X., Amara J., and Hendricks A.M. (2011). Prevalence of dual sensory impairment and its association with traumatic brain injury and blast exposure in OEF/OIF veterans. J. Head Trauma Rehabil. 26, 489–496 [DOI] [PubMed] [Google Scholar]
- 9.Storey E.P., Master S.R., Lockyer J.E., Podolak O.E., Grady M.F., and Master C.L. (2017). Near point of convergence after concussion in children. Optom. Vis. Sci. 94, 96–100 [DOI] [PubMed] [Google Scholar]
- 10.Ellis M.J., Cordingley D., Vis S., Reimer K., Leiter J., and Russell K. (2015). Vestibulo-ocular dysfunction in pediatric sports-related concussion. J. Neurosurg. Pediatr. 16, 248–255 [DOI] [PubMed] [Google Scholar]
- 11.Sufrinko A.M., Marchetti G.F., Cohen P.E., Elbin R.J., Re V., and Kontos A.P. (2017). Using acute performance on a comprehensive neurocognitive, vestibular, and ocular motor assessment battery to predict recovery duration after sport-related concussions. Am. J. Sports Med. 45, 1187–1194 [DOI] [PubMed] [Google Scholar]
- 12.Mucha A., Collins M.W., Elbin R.J., Furman J.M., Troutman-Enseki C., DeWolf R.M., Marchetti G., and Kontos A.P. (2014). A Brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am. J. Sports Med. 42, 2479–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lingsma H.F., Yue J.K., Maas A.I., Steyerberg E.W., and Manley G.T. (2015). Outcome prediction after mild and complicated mild traumatic brain injury: external validation of existing models and identification of new predictors using the TRACK-TBI pilot study. J. Neurotrauma 32, 83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCrea M., Guskiewicz K.M., Marshall S.W., Barr W., Randolph C., Cantu R.C., Onate J.A., Yang J., and Kelly J.P. (2003). Acute effects and recovery time following concussion in collegiate football players: The NCAA Concussion Study. JAMA 290, 2556–2563 [DOI] [PubMed] [Google Scholar]
- 15.Iverson G.L., and Lange R.T. (2003). Examination of “postconcussion-like” symptoms in a healthy sample. Appl. Neuropsychol. 10, 137–144 [DOI] [PubMed] [Google Scholar]
- 16.Losoi H., Silverberg N., Waljas M., Turunen S., Rosti-Otajarvi E., Helminen M., Luoto T.M., Julkunen J., Ohman J., and Iverson G.L. (2016). Recovery from mild traumatic brain injury in previously healthy adults. J. Neurotrauma 33, 766–776 [DOI] [PubMed] [Google Scholar]
- 17.Master C.L., Scheiman M., Gallaway M., Goodman A., Robinson R.L., Master S.R., and Grady M.F. (2016). Vision diagnoses are common after concussion in adolescents. Clin. Pediatr. (Phila) 55, 260–267 [DOI] [PubMed] [Google Scholar]
- 18.DuPrey K.M., Webner D., Lyons A., Kucuk C.H., Ellis J.T., and Cronholm P.F. (2017). Convergence insufficiency identifies athletes at risk of prolonged recovery from sport-related concussion. Am. J. Sports Med. 45, 2388–2393 [DOI] [PubMed] [Google Scholar]
- 19.Leddy J. (2018). Safety and prognostic utility of provocative exercise testing in acutely concussed adolescents: a randomized trial. Clin. J. Sport Med. 28, 13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCrory P., Meeuwisse W.H., Aubry M., Cantu B., Dvorak J., Echemendia R.J., Engebretsen L., Johnston K., Kutcher J.S., Raftery M., Sills A., Benson B.W., Davis G.A., Ellenbogen R.G., Guskiewicz K., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., and Turner M. (2013). Consensus statement on concussion in sport the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br. J. Sports Med. 47, 250–258 [DOI] [PubMed] [Google Scholar]
- 21.Anzalone A.J., Blueitt D., Case T., McGuffin T., Pollard K., Garrison J.C., Jones M.T., Pavur R., Turner S., and Oliver J.M. (2016). A positive Vestibular/Ocular Motor Screening (VOMS) is associated with increased recovery time after sports-related concussion in youth and adolescent athletes. Am. J. Sports Med. 45, 474–479 [DOI] [PubMed] [Google Scholar]
- 22.Corwin D.J., Zonfrillo M.R., Master C.L., Arbogast K.B., Grady M.F., Robinson R.L., Goodman A.M., and Wiebe D.J. (2014). Characteristics of prolonged concussion recovery in a pediatric subspecialty referral population. J. Pediatr. 165, 1207–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry L.C., Elbin R.J., Collins M.W., Marchetti G., and Kontos A.P. (2016). Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery 78, 232–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbogast K.B., Curry A.E., Pfeiffer M.R., Zonfrillo M.R., Haarbauer-Krupa J., Breiding M.J., Coronado V.G., and Master C.L. (2016). Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 170, e160294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutis K., Weerdenburg K., Koo E., Schneeweiss S., and Zemek R. (2015). The diagnosis of concussion in a pediatric emergency department. J. Pediatr. 166, 1214–1220 [DOI] [PubMed] [Google Scholar]
- 26.Corwin D.J., Wiebe D.J., Zonfrillo M.R., Grady M.F., Robinson R.L., Goodman A.M., and Master C.L. (2015). Vestibular deficits following youth concussion. J. Pediatr. 166, 1221–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkwood M.W., and Kirk J.W. (2010). The base rate of suboptimal effort in a pediatric mild TBI sample: performance on the Medical Symptom Validity Test. Clin. Neuropsychol. 24, 860–872 [DOI] [PubMed] [Google Scholar]
- 28.Scheiman M., Gallaway M., Frantz K.A., Peters R.J., Hatch S., Cuff M., and Mitchell G.L. (2003). Nearpoint of convergence: test procedure, target selection, and normative data. Optom. Vis. Sci. 80, 214–225 [DOI] [PubMed] [Google Scholar]
- 29.Scheiman M., Mitchell G.L., Cotter S., Cooper J., Kulp M., Rouse M., Borsting E., London R., and Wensveen J. (2005). A randomized clinical trial of treatments for convergence insufficiency in children. Arch. Ophthalmol. 123, 14–24 [DOI] [PubMed] [Google Scholar]
- 30.Oride M.K., Marutani J.K., Rouse M.W., and DeLand P.N. (1986). Reliability study of the Pierce and King-Devick saccade tests. Am. J. Optom. Physiol. Opt. 63, 419–424 [DOI] [PubMed] [Google Scholar]
- 31.Weise K.K., Swanson M.W., Penix K., Hale M.H., and Ferguson D.. (2017). King-Devick and pre-season visual function in adolescent athletes. Optom. Vis. Sci. 94, 89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iverson G.L., Silverberg N.D., Mannix R., Maxwell B.A., Atkins J.E., Zafonte R., and Berkner P.D. (2015). Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 169, 1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leddy J., Baker J.G., Haider M.N., Hinds A., and Willer B. (2017). A physiological approach to prolonged recovery from sport-related concussion. J. Athl. Train. 52, 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Max J.E., Smith W.L., Jr., Sato Y., Mattheis P.J., Castillo C.S., Lindgren S.D., Robin D.A., and Stierwalt J.A. (1997). Traumatic brain injury in children and adolescents: psychiatric disorders in the first three months. J. Am. Acad. Child Adolesc. Psychiatry 36, 94–102 [DOI] [PubMed] [Google Scholar]
- 35.Bloom D.R., Levin H.S., Ewing-Cobbs L., Saunders A.E., Song J., Fletcher J.M., and Kowatch R.A. (2001). Lifetime and novel psychiatric disorders after pediatric traumatic brain injury. J. Am. Acad. Child Adolesc. Psychiatry 40, 572–579 [DOI] [PubMed] [Google Scholar]
- 36.Aligene K., and Lin E. (2013). Vestibular and balance treatment of the concussed athlete. NeuroRehabilitation 32, 543–553 [DOI] [PubMed] [Google Scholar]
- 37.Gurley J.M., Hujsak B.D., and Kelly J.L. (2013). Vestibular rehabilitation following mild traumatic brain injury. NeuroRehabilitation 32, 519–528 [DOI] [PubMed] [Google Scholar]
- 38.Silverberg N.D., Luoto T.M., Ohman J., and Iverson G.L. (2014). Assessment of mild traumatic brain injury with the King-Devick Test in an emergency department sample. Brain Inj. 28, 1590–1593 [DOI] [PubMed] [Google Scholar]
- 39.Gallun F.J., Lewis M.S., Folmer R.L., Diedesch A.C., Kubli L.R., McDermott D.J., Walden T.C., Fausti S.A., Lew H.L., and Leek M.R. (2012). Implications of blast exposure for central auditory function: a review. J. Rehabil. Res. Dev. 49, 1059–1074 [DOI] [PubMed] [Google Scholar]
- 40.Oleksiak M., Smith B.M., St Andre J.R., Caughlan C.M., and Steiner M. (2012). Audiological issues and hearing loss among Veterans with mild traumatic brain injury. J. Rehabil. Res. Dev. 49, 995–1004 [DOI] [PubMed] [Google Scholar]
- 41.Lew H.L., Weihing J., Myers P.J., Pogoda T.K., and Goodrich G.L. (2010). Dual sensory impairment (DSI) in traumatic brain injury (TBI)—An emerging interdisciplinary challenge. NeuroRehabilitation 26, 213–222 [DOI] [PubMed] [Google Scholar]
- 42.Swanson M.W., Weise K.K., Dreer L.E., Johnston J., Davis R.D., Ferguson D., Hale M.H., Gould S.J., Christy J.B., Busettini C., Lee S.D., and Swanson E. (2017). Academic difficulty and vision symptoms in children with concussion. Optom. Vis. Sci. 94, 60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearce K.L., Sufrinko A., Lau B.C., Henry L., Collins M.W., and Kontos A.P. (2015). Near point of convergence after a sport-related concussion: measurement reliability and relationship to neurocognitive impairment and symptoms. Am. J. Sports Med. 43, 3055–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King L.A., Horak F.B., Mancini M., Pierce D., Priest K.C., Chesnutt J., Sullivan P., and Chapman J.C. (2014). Instrumenting the balance error scoring system for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch. Phys. Med. Rehabil. 95, 353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samadani U., Ritlop R., Reyes M., Nehrbass E., Li M., Lamm E., Schneider J., Shimunov D., Sava M., Kolecki R., Burris P., Altomare L., Mehmood T., Smith T., Huang J.H., McStay C., Todd S.R., Qian M., Kondziolka D., Wall S., and Huang P. (2015). Eye tracking detects disconjugate eye movements associated with structural traumatic brain injury and concussion. J, Neurotrauma 32, 548–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.