Abstract
This review is directed to the redox-modulating properties and anticancer effect of vitamin K. The concept is focused on two aspects: (i) redox-cycle of vitamin K and its effect on the calcium homeostasis, “oncogenic” and “onco-suppressive” reactive oxygen species and the specific induction of oxidative stress in cancer; (ii) vitamin K plus C as a powerful redox-system, which forms a bypass between mitochondrial complexes II and III and thus prevents mitochondrial dysfunction, restores oxidative phosphorylation and aerobic glycolysis, modulates the redox-state of endogenous redox-pairs, eliminates the hypoxic environment of cancer cells and induces cell death. The analyzed data suggest that vitamin C&K can sensitize cancer cells to conventional chemotherapy, which allows achievement of a lower effective dose of the drug and minimizing the harmful side-effects. The review is intended for a wide audience of readers - from students to specialists in the field.
Graphical abstract
1. Chemistry, transport and metabolism of vitamin K
The dietary trace element vitamin K was discovered in the early 1930s by Henrik Dam. During his studies on the cholesterol metabolism in the chicken, Dam has observed that chicks on a sterols-free and low-fat diet have developed large subcutaneous and intramuscular hemorrhages. Henrik Dam has designated this factor as “Koagulation vitamin" (from Danish) or vitamin K in short, because of its vital role for normal haemostasis [1], [2]. Initial experiments with additions of lemon juice, cholesterol, cod-liver oil or ascorbic acid to the diet failed to prevent hemorrhages [1], [2], [3]. Later it has been discovered that vitamin K is an essential cofactor for post-translational modification of hepatic blood-coagulating proteins (as prothrombin, factors II, VII, IX and X) [3], [4]. Vitamin K participates in the converting of their glutamic acid residues into γ-carboxyglutamic acid (Gla) residues. In last years, other functions of vitamin K, unrelated to coagulation, have attracted the attention of researchers, e.g., its role in bone metabolism, vascular calcification, regulation of cell growth and apoptosis [5], [6], [7], [8], [9], [10]. There is also enormous interest in the anticancer activity of vitamin K in combination with vitamin C, which will be specifically addressed below.
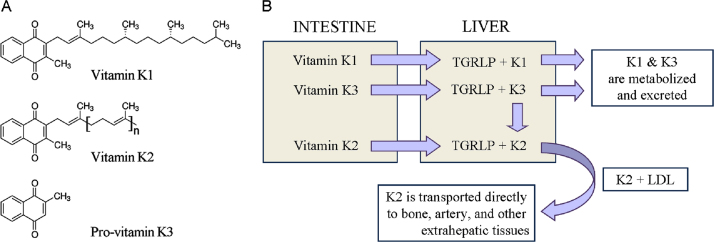
Vitamin K is a group of structurally related molecules that have a 2-methyl-1,4 naphthoquinone ring and a variable aliphatic chain (Fig. 1A). The variable aliphatic chain distinguishes two naturally occurring forms: vitamin K1 (phylloquinone) and vitamin K2 (menaquinone). There is also a synthetic form of vitamin K without aliphatic chain (K3 or menadione), which is classified as a pro-vitamin. Vitamin K1 exists only in one phylloquinone structure, while vitamin K2 exists in multiple structures, which are distinguished by number of unsaturated isoprenyl groups in the aliphatic chain [10].
Fig. 1.
(A) Chemical structures of vitamin K1, vitamin K2, and pro-vitamin K3 (menadione). (B) Transport and metabolism of vitamins K. TGRLP – triglyceride-rich lipoprotein; LDL – low density lipoproteins.
Vitamin K1 is found in green leafy vegetables: broccoli, lettuce, spinach, fermented soy (natto), spring onions, cabbage, etc. The various forms of vitamin K2 (MK-4, MK-7, MK-10) are mainly synthesized by bacteria, especially in nutrient products as natto and yogurt [11], [12]. Vitamin K2 is also found in meat, eggs, curd and cheese [7], [10], [13].
Vitamins K1 and K2 are transported by triglyceride-rich lipoprotein (TGRLP) to the liver, after intestinal absorption [13], [14] (Fig. 1B). Vitamin K1 is metabolized and more than half the amount is excreted by the organism, while vitamin K2 is transported from the low density lipoproteins (LDL) to extra-liver tissues [13], [14]. Vitamin K2 is accumulated preferentially in peripheral tissues. High levels are detected in the brain, aorta, pancreas, fat and low levels are detected in the liver [15], [16], [17].
Pro-vitamin K3 is a synthetic lipid-soluble vitamin precursor, which can be converted to active vitamin K2 (menaquinone) after its alkylation in the liver. In addition, pro-vitamin K3 could be metabolized to glucuronide and sulfate of reduced menadione, which are excreted in the urine [18], [19], [20]. Menadione has a relatively low toxicity and is approved by the Food and Drug Administration for therapeutic purposes. It has been shown that pro-vitamin K3 directly affects the redox status of thiols (including thiol-containing compounds) and calcium homeostasis [20]. The most intriguing property of menadione is its anticancer activity, which will be discussed in details below.
2. Metabolic cycle of vitamin K and its role in calcium homeostasis
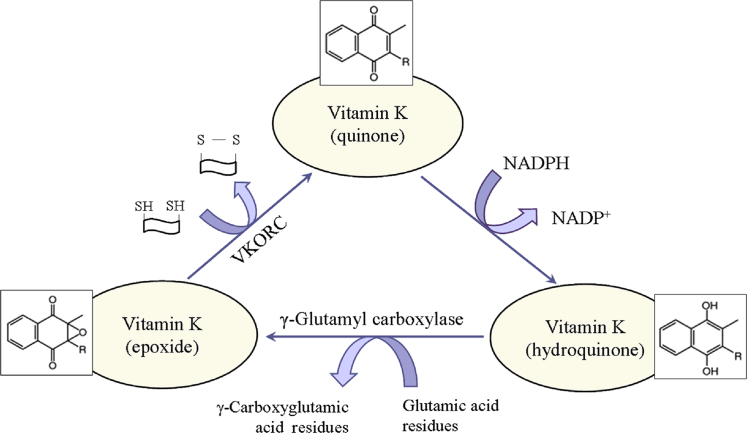
It is generally accepted that the essential role of vitamin K is related to the post-translational γ-carboxylation of glutamate residues of proteins (Fig. 2). This process occurs in the lumen of the endoplasmic reticulum and involves two enzymes: γ-glutamyl carboxylase and vitamin K-dependent epoxide reductase complex (VKORC). Both enzymes together constitute the “vitamin K cycle”, which is a redox-cycle in its biochemical essence [21], [22]. In nature, vitamin K exists in an oxidized form (quinone), but γ-carboxylation of glutamate residues requires its reduced form (hydroquinone). In the organism, quinone is reduced to hydroquinone by NAD(P)H and/or glutathione (GSH). In turn, hydroquinone is converted to epoxide in the process of γ-carboxylation. The epoxide is converted to the initial oxidized quinone state by the VKORC, which is accompanied by a consumption of other reducing equivalents (mainly thiol-groups). Obviously, the activity of vitamin K may affect redox-homeostasis of cells and tissues and can be considered as a regulatory factor in redox-signaling. This function of vitamin K is very important due to variety of glutamic acid-rich proteins in the bones, arteries and soft tissues. For example, vitamin K influences the degree of carboxylation of osteocalcin – a small calcium-binding protein, which is secreted by osteoblasts in bones and serves as an integral protein for the synthesis of bone matrix [23], [24], [25]. The biosynthesis of osteocalcin is regulated by hormones and growth factors, but its post-translational modification is regulated by vitamin K. Osteocalcin contains three Gla residues, which interact with calcium in the hydroxyapatite-crystal lattice of bones [24]. γ-Carboxylation of glutamic acid residues of osteocalcin increases its affinity to calcium and respectively to hydroxyapatite [25]. It was shown, that decarboxylated osteocalcin cannot bind calcium, which emphasizes the essential role of vitamin K in the γ-carboxylation process [26], [27]. In addition, vitamin K inhibits the activity of osteoclasts, thus preventing the breakdown of bones [27], [28].
Fig. 2.
Metabolic redox-cycle of vitamin K. VKORC – vitamin K-dependent epoxide reductase complex.
Vitamin K participates in the carboxylation of the matrix Gla-containing protein (MGP) of the arterial walls and plays a crucial role in maintaining their elasticity [7], [8], [29], [30]. MGP belongs to the group of vitamin K2-dependent Gla-containing proteins. MGP is produced in bones and vascular smooth muscle cells and inhibits vascular calcification [30], [31], [32]. Its function is affected by inflammatory factors [32]. The importance of MGP for vascular homeostasis was demonstrated on MGP-deficient animals – all of them died of massive arterial calcification within 6–8 weeks after birth [5], [33]. It was established that non-carboxylated MGP forms calcium depositions in the vascular walls [8], [27], [34]. The calcium phosphate crystals in the arterial wall directly attract macrophages and induce inflammation [27], [35], [36]. Studies have demonstrated that rats with vitamin K-deficiency and chronic kidney disease have an enhanced vascular calcification, which decreases after vitamin K supplementation [31], [37]. Warfarin, an inhibitor of vitamin K reduction, affects γ-glutamyl carboxylation of MGP and induces vascular calcification in experimental animals [4], [31], [34], [38]. The effect is abolished by vitamin K treatment [4], [31], [38]. These findings suggest that vitamin K-deficiency affects calcium homeostasis, which leads to vascular calcification and bone disorders.
3. Redox-modulating effect of vitamin K and anticancer activity
A number of studies have shown a cytotoxicity of vitamin K towards cancer cells [10], [39], [40]. Various mechanisms, responsible for cell growth arrest and suppression of proliferation by vitamin K, have been described. However, almost all of them are focused on modulation of redox-balance and induction of oxidative stress in cancer cells due to quinone structure of vitamin K.
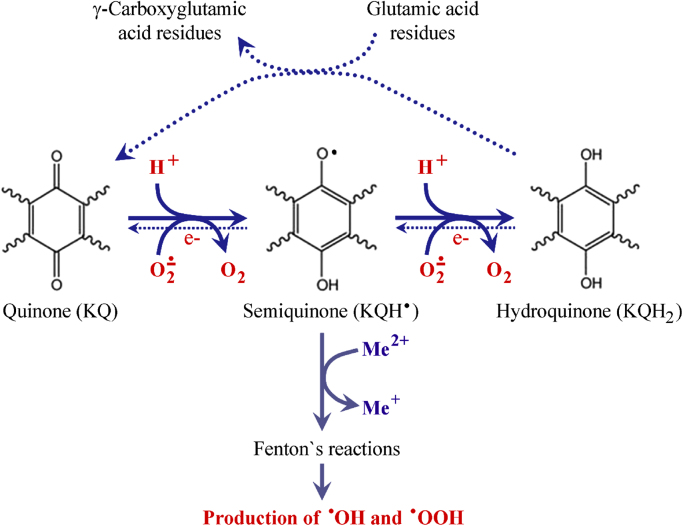
Quinones can undergo one-electron reduction, producing intermediate semiquinone radicals, as well as two-electron reduction with production of hydroquinones (Fig. 3) [41]. Both reactions are accompanied by consumption of superoxide radicals and reducing equivalents (as NADH, NADPH, glutathione), which are essential for cancer cell homeostasis [42], [43]. It is generally accepted that superoxide is “oncogenic ROS”, while hydrogen peroxide is “onco-suppressive ROS” [43], [44], [45], [46]. Numerous studies suggest that cellular state, where the ratio tilts predominantly in favor of superoxide, inhibits apoptosis and promotes cell survival. If the ratio tilts in favor of hydrogen peroxide, this creates an intracellular environment suitable for induction of apoptosis and cell death. This hypothesis is well-grounded by experimental and empirical observations on cells, animals and humans, which are summarized in several excellent review articles [43], [44], [47], [48]. In this context, the decrease of superoxide due to vitamin K redox-cycle may explain, at least partially, its anticancer activity. On the other hand, semiquinone radical of vitamin K can convert transition metal ions, e.g. Fe3+ to Fe2+, thus inducing Fenton's reactions and production of highly reactive and cytotoxic hydroxyl and hydroperoxyl radicals [10], [49].
Fig. 3.
Two-electron reduction of vitamin K and its effect on the levels of reducers and superoxide in biological objects. The γ-carboxylation of glutamic acid residues shifts the equilibrium towards production of hydroquinone, which is accompanied by consumption of reducers and superoxide. Alternative pathway for induction of Fenton's reactions by the intermediate (semiquinone) form of vitamin K is also given in the figure.
It is shown that pro-vitamin K3 induces oxidative stress in cancer cells via production of hydroxyl radicals and DNA strand-breaks [50], [51].
Superoxide dismutase does not influence the anticancer effect of menadione [52], [53]. However, antioxidant enzymes, involved in the depletion of "onco-suppressive" hydrogen peroxide (as catalase and glutathione peroxidase), decrease the anticancer effect of menadione [53], [54]. Similar data have also been reported for transition metal chelators that suppress Fenton's reactions and menadione-mediated cytotoxicity in cancer [55]. The described data suggest that pro-vitamin K3 leads to depletion of “oncogenic” superoxide and probably induces apoptosis via production of “onco-suppressive” hydroperoxides and cytotoxic hydroxyl radicals. This explains, at least partially, the anticancer activity of menadione. However, it should be noted that the induction of Fenton's reactions and the production of hydroxyl radicals suggest for potential side-effects (cytotoxicity) of menadione on normal cells.
Other studies suggest that vitamin K induces apoptosis through different biochemical pathways, including alteration of intracellular calcium homeostasis and activation of the following pro-apoptotic factors: c-Jun N-terminal kinases (JNKs), Fas-dependent and Fas-independent pathways, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [56], [57], [58], [59], [60], [61].
Wu et al. have demonstrated that the expression of c-myc and c-fos proto-oncogenes is involved in the mechanism(s) for induction of apoptosis, differentiation and cell cycle arrest by vitamin K [57]. The authors have found that pro-vitamin K3 induces c-fos and c-myc expression in nasopharyngeal carcinoma (NPC) cell lines. Bouzahzah et al. have demonstrated that growth of hepatoma cells could be completely suppressed by addition of vitamin K to the suspension [62]. The authors have found that growth suppression is accompanied by an increased level of transcripts of the following genes: c-myc, c-jun, and prothrombin.
Pro-vitamin K3 is a potent growth inhibitor and an inducer of phosphorylation of the extracellular signal-regulated kinase (ERK) through specific pathway in the stomach cancer cell lines [10], [63], [64], [65], [66]. Osada et al. have shown that non-thiol antioxidants had no effect on pro-vitamin K3-induced tyrosine phosphorylation [63]. The anticancer effect is due to thiol-arylation of critical cysteine residues that mediate the tyrosine phosphorylation. Moreover, pro-vitamin K3 interacts directly with glutathione, which is essential for cancer cells [64]. The cell cycle arrest by pro-vitamin K3 induces also tyrosine phosphorylation of hepatocyte growth factor receptor (c-met) and epidermal growth factor receptors (EGFR), which in turn activate the ras-signaling pathway [63], [64].
The ability of vitamin K to induce cell cycle arrest and cell death may also be explained by the inhibition of cyclin-dependent protein kinases (CDK) [67], [68]. Pro-vitamin K3 inhibits CDK1 and CDK2 by their hyperphosphorylation, modification of the active sites of both proteins and abolishing the dephosphorylating activity of the enzymes. The anticancer effect of vitamin K2 and pro-vitamin K3 is most likely through regulation of p21 gene [69], [70].
Different mechanisms of action of vitamin K on different cell types have been described. However, the potential cross-talk between these mechanisms still remains unclear. Most of the vitamin K-induced pro-apoptotic factors are inflammatory signals, inducing overproduction of ROS (mainly hydroperoxides) and oxidative stress in cancer cells and tissues [43], [48]. The data also suggest that vitamin K decreases the levels of superoxide, NAD(P)H and glutathione, which are essential for hypoxic behavior of cancer cells and their homeostasis.
4. Vitamin C plus K3: A powerful redox-system to sensitize cancer cells towards chemotherapeutics
In vitro studies have reported a strong anticancer effect of the combination of vitamin C and pro-vitamin K3 (C&K3) on cancer cell lines [71], [72], [73], [74], [75], [76], [77], [78]. Most of these studies have demonstrated that anticancer effect of the combination is most well expressed at ratio vitamin C:K3 = 100:1 (mol:mol). Different mechanisms of vitamin C&K3-mediated apoptosis have been discussed: production of free radicals under alkaline conditions, thiol depletion, lipid peroxidation, modulation of ATP level, calcium regulation, activation of NF-kB, etc. Some authors suggest a caspase-independent induction of apoptosis, and others suggest caspase-dependent cell death [71], [73], [74], [76].
Zhang et al. have demonstrated that cancer cells (HSC-2, HSC-3, HL-60) are more sensitive to combined treatment with vitamin C&K3, as compared to normal cells [71]. Vita et al. have reported better cytotoxicity of vitamin C&K3 on glioma cells than on normal brain cells [77]. Our recent study on normal and leukemia lymphocytes, treated with vitamin C&K3, also supports the assumption that cancer cells are more sensitive to vitamin C&K3 than normal cells [78].
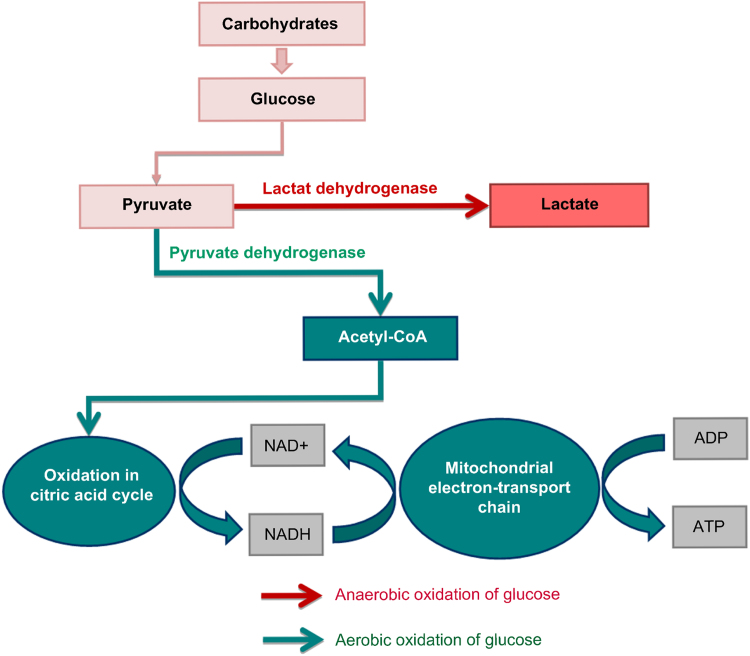
How vitamin C&K3 can activate the specific signaling pathways, involved in the cancer cells death? To answer this question, let's remind briefly the basic biochemical difference between normal and cancer cells, associated with the metabolism of glucose (Fig. 4).
Fig. 4.
Aerobic and anaerobic pathways for degradation of glucose and ATP synthesis.
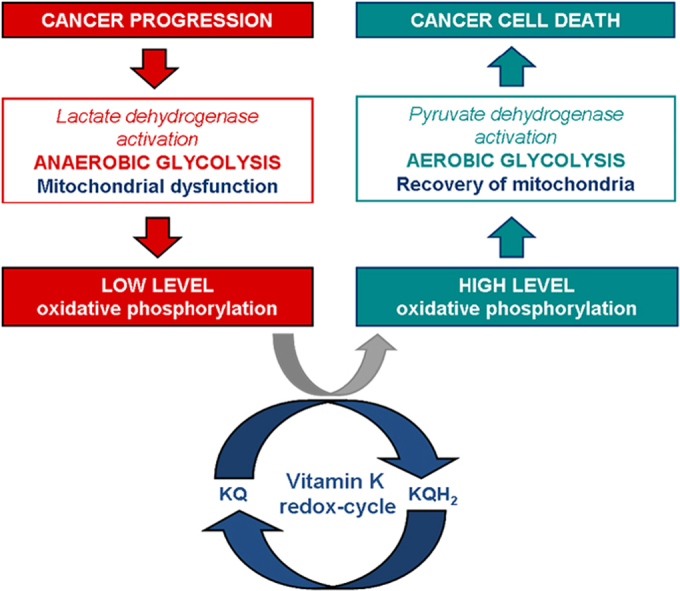
As apposed to degradation of proteins and fats, the glucose metabolism is a unique pathway. It can utilize oxygen if available (aerobic pathway – via conversion of pyruvate to acetyl-CoA) or it can function in the absence of oxygen (anaerobic pathway – via conversion of pyruvate to lactate). Both pathways are necessary for the functioning of cells, tissues and organs, but in norm, the aerobic pathway dominates the anaerobic. In cancer cells, the anaerobic oxidation of glucose is a major source of energy. In many cancers, the major cause of anaerobic glycolysis is mitochondrial dysfunction and inhibition of oxidative phosphorylation. Cancer cells are characterized by high levels of glucose, anaerobic oxidation of glucose (Warburg effect) and accumulation of lactate, compared to normal cells [43], [44], [48], [79]. In last years, lactate dehydrogenase is receiving a great deal of attention as a potential diagnostic marker or a predictive biomarker for many types of cancer and as a therapeutic target for development of new anticancer drugs [80], [81], [82].
Eleff at al. have reported that administration of vitamin C and K3 on the skeletal muscles of a 17-years-old patient with a severe defect in complex III of the mitochondrial electron transport chain (mitochondrial dysfunction) increases the recovery rate, compared to the recovery rate of the young female controls [83]. The defect includes a deficiency of reducible cytochrome b (cyt. b), which is accompanied by an effective prevention of aerobic metabolism and oxidative phosphorylation. The authors bypass the deficient complex III by using pro-vitamin K3 and vitamin C as electron transfer mediators to carry the electrons from coenzyme Q (CoQ) to cyt. c. Thus, they succeeded to increase ATP production rate to about 65% of the normal state in mitochondria, as well as to decrease the lactic acidosis in the patient. The combination of vitamin C and K3 results in a production of more ATP, than in the case of vitamin C applied alone. The authors suggest that both vitamins can reduce directly cyt. c, since the reduction potential of cyt. c is over + 200 mV – more positive than those of menadione or ascorbate [83], [84], [85], [86], [87].
The possibility of improving oxidative phosphorylation by bypass of the cytochrome-deficient site in mitochondria by vitamin C and K3 was also reported by McCord and Fridovich many years ago [84]. Recent in vitro studies provide further evidence that treatment of cells with vitamin C and K3 results in spikes in ATP production due to a shunt around a defective area of complex III of the mitochondrial electron-transport chain (between CoQ and cyt. c) [74], [75], [88], [89], [90], [91]. This reaction causes a shift from anaerobic (glycolytic) to aerobic (oxidative) metabolism, which diminishes hypoxia and lactic acidosis.
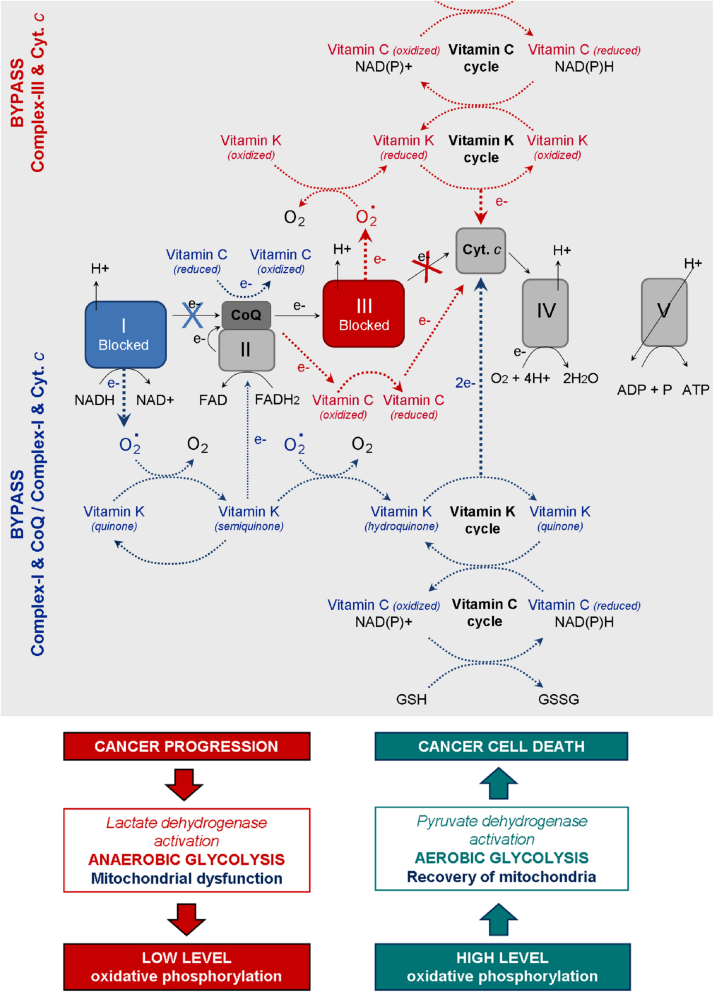
Based on the studies described above, we assume that vitamin C and K can serve as cyt. c reducers either directly or indirectly, but in both cases they can avoid mitochondrial dysfunction and restore the oxidative phosphorylation. The hypothetic mechanism of coupling between “vitamin C redox-cycle” and “vitamin K redox-cycle” and their effect on mitochondrial functionality is shown in Fig. 5. In the case of dysfunction in Complex-III (due to mutations in cyt. b), the electrons can not be transferred from CoQ to cyt. c. In the case of dysfunction in Complex-I (due to mutations in NADH dehydrogenase), the electrons are blocked on the first step of respiratory chain. It is well-known that both types of mutations are accompanied by superoxide production from complex-III and complex-I and suppression of ATP synthesis [43], [48]. The oxidized vitamin K (KQ) can accept electrons from superoxide, converting consequently to semiquinone (KQH•) and hydroquinone (KQH2). Finally, the reduced vitamin K can transfer electrons directly to cyt. c. In turn, the oxidized vitamin C (dehydoroascorbate) can accept electrons directly from Complex-I (NADH) or CoQ, converting consequently to semidehydroascorbate (Asc•) and ascorbate (Asc). Finally, the reduced vitamin C can transfer electrons to cyt. c. The two redox-cycles lead to a bypass between Complex-I (or Complex-III) and cyt. c. This will restore the oxidative phosphorylation and ATP synthesis. ATP, generated due to the vitamin C&K-mediated bypasses, prevents the anaerobic conditions, decreases the levels of “oncogenic” superoxide, decreases the levels of lactate, eliminates the lactic acidosis and hypoxia, and allows the cancer cells to trigger autophagia and “to kill themselves”.
Fig. 5.
Redox-cycles of vitamins C and K and prevention of mitochondrial dysfunction: recovery of mitochondrial oxidative phosphorylation in cancer cells – a possible mechanism for induction of apoptosis and cell death. Blue arrows indicate the possible vitamin K-mediated bypasses between complex-I and CoQ, as well as between complex-I and cyt. c, in the case of dysfunction in complex-I. Direct reduction of CoQ by vitamin C is also possible. Red arrows indicate the possible vitamin K- and vitamin C-mediated bypasses between complex-III and cyt. c, in the case of dysfunction in complex-III. NAD(P)H can reduce both vitamin K (quinone) and dehydroascorbate. Glutathione (GSH) can also reduce dehydroascorbate to ascorbate.
Recent data indicate that the redox cycles of vitamins C and K are cross-linked – ascorbate can reduce pro-vitamin K3, which is accompanied by a production of hydrogen peroxide (“onco-suppressive” and cytotoxic ROS) [92], [93]. The reduction of vitamin K by vitamin C allows also a direct (superoxide-independent) reduction of cyt. c by vitamin K (KQH2). Silvera-Dorta et al. have also reported that vitamin C can reduce CoQ and the process is coupled with reduction of oxygen to hydrogen peroxide [93].
The redox-system vitamin C&K3 could also influence the ratio between the oxidized and reduced forms of the main endogenous redox-pairs: NAD+/NADH, NADP+/NADPH, GSH/GSSH, etc. (Fig. 5) [10], [72], [88]. These redox-pairs are enzymatic cofactors, involved in various biochemical reactions and serve as key regulators of cellular metabolism, including lactate dehydrogenase, pyruvate dehydrogenase, glutathione-dependent enzymes, etc. The balance between oxidized and reduced forms is crucial for the cell behavior, as well as for cell survival or death. It is widely accepted that the increased mitochondrial oxidative stress and high levels of NAD(P)H and glutathione are distinctive features of the metabolic phenotype of cancer cells [43], [44], [47], [48]. Since the reduction potentials of NAD(P)H, FADH2 and GSH are very low (below −200 mV), all substances can directly reduce vitamins C and K.
The analyzed data suggest that the combination of vitamins C and K (in particular K3) is not just an antioxidant system. Vitamin C&K3 is a powerful redox-couple that can prevent mitochondrial dysfunction, restore oxidative phosphorylation, modulate redox-homeostasis, eliminate hypoxia, and induce apoptosis and cell death.
5. Conclusion
The redox-cycles of vitamins C and K are cross-linked and both vitamins can serve as associated redox-modulators (a redox-shuttle). The combination vitamin C&K can serve as a bypass between complex I (or complex-III) and cyt. c of mitochondrial respiratory chain and to donate electrons (directly or indirectly) to cyt. c, passing from reduced to oxidized form. Both vitamins can be also converted into reduced forms, consuming NAD(P)H and/or GSH. These processes are accompanied by an increase in ATP synthesis and accumulation of high levels of NAD(P)+ and GSSH, which restores oxidative phosphorylation and aerobic glycolysis and eliminates the hypoxic environment. Тhe specific behavior of the cancer cells is disrupted and apoptosis is initiated. It is assumed that changing the ratio NAD(P)+/NAD(P)H and/or GSH/GSSH in cancer cells could be the “switch mechanism” from survival to cell death. Targeting defective mitochondria in cancer cells and preventing their dysfunction, as well as modulating redox-state of endogenous redox-pairs can be a successful strategy for anticancer therapy. This strategy allows target cytotoxicity, which is not directed to normal cells and tissues [43], [48].
There is one more interesting aspect of vitamin C&K-dependent modulation of the cellular redox-status. If the redox-system vitamin C&K can prevent mitochondrial dysfunction and change energy metabolism of cancer cells, we can expect a synergistic cytotoxicity after combining with conventional chemotherapeutics, targeting different oncogenes. Recently, we established that vitamin C&K3 sensitize leukemia lymphocytes (Jurkat) to variety of anticancer drugs [78]. Fifteen anticancer drugs were covered in this study. Almost all combinations showed synergistic cytotoxicity to leukemia cells, whereas the effect on normal lymphocytes was significantly less expressed. Thus, the combined treatment with anticancer drug and vitamin C&K3 can be considered as a new therapeutic approach – to sensitize cancer cells and to achieve a lower effective dose of the drug, minimizing the harmful side-effects of conventional chemotherapy. However, considering the fact that a significant number of people use anticoagulants due to concomitant diseases, the use of а procoagulant such as vitamin K should be done very carefully under the supervision of the physician.
Acknowledgements
This work was partially supported by the Project for Cancer Research And Therapeutic Evolution (P-CREATE) (Project No.16 cm0106202h0001) from AMED and the Center of Innovation Stream from JST and the National Science Fund of Bulgaria (Grant DN11/2 to B.N.).
References
- 1.Dam H., Schonheyder F. A deficiency disease in chicks resembling scurvy. Biochem. J. 1934;28:1355–1359. doi: 10.1042/bj0281355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dam H. The antihaemorrhagic vitamin of the chick. Biochem. J. 1935;29:1273–1285. doi: 10.1042/bj0291273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rannels S.R., Gallaher K.J., Wallin R., Rannels D.E. Vitamin K-dependent carboxylation of pulmonary surfactant-associated proteins. Proc. Natl. Acad. Sci. USA. 1987;84:5952–5956. doi: 10.1073/pnas.84.16.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furie B., Furie B.C. Molecular basis of vitamin K-dependent gamma-carboxylation. Blood. 1990;75:1753–1762. [PubMed] [Google Scholar]
- 5.Cranenburg E.C.M., Schurgers L.J., Vermeer C. Vitamin K: the coagulation vitamin that became omnipotent. Thromb. Haemost. 2007;98:120–125. [PubMed] [Google Scholar]
- 6.Kaneki M., Hosoi T., Ouchi Y., Orimo H. Pleiotropic actions of vitamin K: protector of bone health and beyond? Nutrition. 2006;22:845–852. doi: 10.1016/j.nut.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Berkner K.L., Runge K.W. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J. Thromb. Haemost. 2004;2:2118–2132. doi: 10.1111/j.1538-7836.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 8.Sweatt A., Sane D.C., Hutson S.M., Wallin R. Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats. J. Thromb. Haemost. 2003;1:178–185. doi: 10.1046/j.1538-7836.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama T., Miyazawa K., Naito M., Toyotake J., Tauchi T., Itoh M., Yuo A., Hayashi Y., Georgescu M.M., Kondo Y., Kondo S., Ohyashiki K. Vitamin K2 induces autophagy and apoptosis simultaneously in leukemia cells. Autophagy. 2008;4:629–640. doi: 10.4161/auto.5941. [DOI] [PubMed] [Google Scholar]
- 10.Lamson D.W., Plaza S.M. The anticancer effects of vitamin K. Altern. Med. Rev. 2003;8:303–318. [PubMed] [Google Scholar]
- 11.Knapen M.H., Braam L.A., Teunissen K.J., Zwijsen R.M., Theuwissen E., Vermeer C. Yogurt drink fortified with menaquinone-7 improves vitamin K status in a healthy population. J. Nutr. Sci. 2015;4:e35. doi: 10.1017/jns.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahdinia E., Demirci A., Berenjian A. Optimization of Bacillus subtilis natto growth parameters in glycerol-based medium for vitamin K (menaquinone-7) production in biofilm reactors. Bioprocess. Biosyst. Eng. 2018;41:195–204. doi: 10.1007/s00449-017-1857-0. [DOI] [PubMed] [Google Scholar]
- 13.Shearer M.J., Bach A., Kohlmeier M. Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. J. Nutr. 1996;126(Suppl 4):1182S–1186S. doi: 10.1093/jn/126.suppl_4.1181S. [DOI] [PubMed] [Google Scholar]
- 14.Schurgers L.J., Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochem. Biophys. Acta. 2002;1570:27–32. doi: 10.1016/s0304-4165(02)00147-2. [DOI] [PubMed] [Google Scholar]
- 15.Thijssen H.H., Drittij-Reijnders M.J. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br. J. Nutr. 1996;75:121–127. doi: 10.1079/bjn19960115. [DOI] [PubMed] [Google Scholar]
- 16.Okano T., Shimomura Y., Yamane M., Suhara Y., Kamao M., Sugiura M., Nakagawa K. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 2008;283:11270–11279. doi: 10.1074/jbc.M702971200. [DOI] [PubMed] [Google Scholar]
- 17.Sato T., Ohtani Y., Yamada Y., Saitoh S., Harada H. Difference in the metabolism of vitamin K between liver and bone in vitamin K-deficient rats. Br. J. Nutr. 2002;87:307–314. doi: 10.1079/BJNBJN2001519. [DOI] [PubMed] [Google Scholar]
- 18.Losito R., Owen C.A., Jr, Flock E.V. Metabolism of [14C]Menadione. Biochemistry. 1967;6:62–68. doi: 10.1021/bi00853a012. [DOI] [PubMed] [Google Scholar]
- 19.Thor H., Smith M.T., Hartzell P., Bellomo G., Jwell S.A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J. Biol. Chem. 1982;257:12419–12425. [PubMed] [Google Scholar]
- 20.Chung J.-H., Seo D.-C., Chung S.-H., Lee J.-Y., Seung S.-A. Metabolism and cytotoxicity of menadione and its metabolite in rat platelets. Toxicol. Appl. Pharmacol. 1997;142:378–385. doi: 10.1006/taap.1996.8048. [DOI] [PubMed] [Google Scholar]
- 21.Stafford D.W. The vitamin K cycle. J. Thromb. Haemost. 2005;3:1873–1878. doi: 10.1111/j.1538-7836.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 22.Tie J.K., Stafford D.W. Functional study of the vitamin K cycle enzymes in live cells. Methods Enzymol. 2017;584:349–394. doi: 10.1016/bs.mie.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkins G.J., Welldon K.J., Wijenayaka A.R., Bonewald L.F., Findlay D.M. Vitamin K promotes mineralization, _osteoblast-to-ostecyte transition, and an anticatabolic phenotype by γ-carboxylation-dependent and -independent mechanisms. Am. J. Physiol. Cell Physiol. 2009;297:C1358–C1367. doi: 10.1152/ajpcell.00216.2009. [DOI] [PubMed] [Google Scholar]
- 24.Gundberd C.M., Lian J.B., Booth S.L. Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv. Nutr. 2012;3:149–157. doi: 10.3945/an.112.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferron M., Lacombe J. Regulation of energy metabolism by skeleton: osteocalcin and beyond. Arch. Biochem. Biophys. 2014;561:137–146. doi: 10.1016/j.abb.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Mizuguchi M., Fujisawa R., Nara M., Nitta K., Kawano K. Fourier-transform infrared spectroscopic study of Ca2+-binding to osteocalcin. Calcif. Tissue Int. 2001;69:337–342. doi: 10.1007/s002230010042. [DOI] [PubMed] [Google Scholar]
- 27.Falcone T.D., Kim S.W., Cortazzo M.H. Vitamin K: fracture prevention and beyond. PM&R. 2011;3:S82–S87. doi: 10.1016/j.pmrj.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Koshihara Y., Hoshi K., Okawara R., Ishibashi H., Yamamoto S. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J. Endocrinol. 2003;176:339–348. doi: 10.1677/joe.0.1760339. [DOI] [PubMed] [Google Scholar]
- 29.Theuwissen E., Smit E., Vermeer C. The role of vitamin K in soft-tissue calcification. Adv. Nutr. 2012;3:166–173. doi: 10.3945/an.111.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danzieger J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin. J. Am. Soc. Nephrol. 2008;3:1504–1510. doi: 10.2215/CJN.00770208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demer L.L., Tintut Y. Inflammatory, metabolic and genetic mechanisms of vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2014;34:715–723. doi: 10.1161/ATVBAHA.113.302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueland T., Dahl C.P., Gullestad L., Aakhus S., Broch K., Skardal R., Vermeer C., Aukrust P., Schurgers L.J. Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clin. Sci. (Lond.) 2011;121:119–127. doi: 10.1042/CS20100589. [DOI] [PubMed] [Google Scholar]
- 33.Luo G., Ducy P., McKee M.D., Pinero G.J., Behringer R.R., Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 34.Becker R.C. Warfarin-induced vasculopathy. J. Thromb. Thrombolysis. 2007;23:79–81. doi: 10.1007/s11239-006-9021-8. [DOI] [PubMed] [Google Scholar]
- 35.Nadra I., Mason J.C., Philippidis P., Florey O., Smythe C.D.W., McCarthy G.M., Landis R.C., Haskard D.O. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ. Res. 2005;96:1248–1256. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 36.Tintut Y., Patel J., Territo M., Saini T., Parhami F., Demer L.L. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105:650–655. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 37.McCabe K.M., Booth S.L., Fu X., Shobeiri N., Pang J.J., Adams M.A., Holden R.M. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int. 2013;83:835–844. doi: 10.1038/ki.2012.477. [DOI] [PubMed] [Google Scholar]
- 38.Kruger T., Oelenberg S., Kaesler N., Schurgers L.J., Van De Sandt A.M., Boor P., Schlieper G., Branderburg V.M., Fekete B.C., Veulemans V., Ketteler M., Vermeer C., Jahnen-Dechent W., Floege J., Westenfeld R. Warfarin induces cardiovascular damage in mice. Arterioscler. Thromb. Vasc. Biol. 2013;33:2618–2624. doi: 10.1161/ATVBAHA.113.302244. [DOI] [PubMed] [Google Scholar]
- 39.Dasari S., Ali S.M., Zheng G., Chen A., Dontaraju V.S., Bosland M.C., Kajdacsy-Balla A., Munirathinam G. Vitamin K and its analogs: potential avenues for prostate cancer management. Oncotarget. 2017;8:57782–57799. doi: 10.18632/oncotarget.17997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis-Yadley A.H., Malafa M.P. Vitamins in pancreatic cancer: a review of underlying mechanisms and future applications. Adv. Nutr. 2015;6:774–802. doi: 10.3945/an.115.009456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gant T.W., Rao D.N., Mason R.P., Cohen G.M. Redox cycling and sulfhydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem. Biol. Interact. 1988;65:157–173. doi: 10.1016/0009-2797(88)90052-x. [DOI] [PubMed] [Google Scholar]
- 42.Chen G., Wang F., Trachootham D., Huang P. Preferential killing of cancer cells with mitochondrial dysfunction by natural compounds. Mitochondrion. 2010;10:614–625. doi: 10.1016/j.mito.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediating mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 44.Pervaiz S., Clement M.V. Superoxide anion: oncogenic reaction oxygen species? Int. J. Biochem. Cell Biol. 2007;39:1297–12304. doi: 10.1016/j.biocel.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Lennicke C., Rahn J., Lichtenfels R., Wessjohann L.A., Seliger B. Hydrogen peroxide – production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015;13:39. doi: 10.1186/s12964-015-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivanova D., Bakalova R., Lazarova D., Gadjeva V., Zhelev Z. The impact of reactive oxygen species on anticancer therapeutic strategies. Adv. Clin. Exp. Med. 2013;22:899–908. [PubMed] [Google Scholar]
- 47.Fulda S., Galluzzi L., Kroemer G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 48.Trachootham D., Lu W., Ogasawara M.A., Velle N.R., Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen R., Pignatello J.J. Role of quinine intermediates as electron shuttles in Fenton and photoassisted Fenton oxidations of aromatic compounds. Environ. Sci. Technol. 1997;31:2399–2406. [Google Scholar]
- 50.Nutter L.M., Ngo E.O., Fisher G.R., Gutierrez P.L. DNA strand scission and free radical production in menadione-treated cells. Correlation with cytotoxicity and role of NADPH quinone acceptor oxidoreductase. J. Biol. Chem. 1992;267:2474–2479. [PubMed] [Google Scholar]
- 51.D`Odorico C., Novotny L., Vachalkova A.: Quinone-induced DNA single strand breaks in a human colon carcinoma cell line. Carcinogenesis. 1997;18:43–46. doi: 10.1093/carcin/18.1.43. [DOI] [PubMed] [Google Scholar]
- 52.Sun J.S., Tsuang Y.H., Huang W.C., Chen T.L., Hang Y.S., Lu E.J. Menadione-induced cytotoxicity to rat osteoblasts. Cell Mol. Life Sci. 1997;53:967–976. doi: 10.1007/s000180050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiou T.J., Tzeng W.F. The roles of glutathione and antioxidant enzymes in menadione-induced oxidative stress. Toxicology. 2000;154:75–84. doi: 10.1016/s0300-483x(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 54.Loop G., Kondapalli J., Schriewer J.M., Chandel N.S., Vanden Hoek T.L., Schumacker P.T. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 2010;49:1925–1936. doi: 10.1016/j.freeradbiomed.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calderaro M., Martins E.A.L. Oxidative stress by menadione affects cellular copper and iron homeostasis. Mol. Cell Biochem. 1993;126:17–23. doi: 10.1007/BF01772204. [DOI] [PubMed] [Google Scholar]
- 56.Di Monte D., Bellomo G., Thor H., Nicotera P., Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch. Biochem. Biophys. 1984;235:343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- 57.Wu F.Y., Chang N.T., Chen W.J., Juan C.C. Vitamin K3-induced cell cycle arrest and apoptotic cell death are accompanied by altered expression of c-fos and c-myc in nasopharyngeal carcinoma cells. Oncogene. 1993;8:2237–2244. [PubMed] [Google Scholar]
- 58.Caricchio R., Kovalenko D., Kaufmann W.K., Cohen P.L. Apoptosis provoked by the oxidative stress inducer menadione (vitamin K3) is mediated by the Fas/Fas ligand system. Clin. Immunol. 1999;93:65–74. doi: 10.1006/clim.1999.4757. [DOI] [PubMed] [Google Scholar]
- 59.Jones B.E., Lo C.R., Liu H., Pradhan Z., Garcia L., Srinivasan A., Valentino K.L., Czaja M.J. Role of caspases and NF-kB signaling in hydrogen peroxide- and superoxide-induced hepatocyte apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G693–G699. doi: 10.1152/ajpgi.2000.278.5.G693. [DOI] [PubMed] [Google Scholar]
- 60.Laux I., Nel A. Evidence that oxidative stress-induced apoptosis by menadione involves Fas-dependent and Fas-independent pathways. Clin. Immunol. 2001;101:335–344. doi: 10.1006/clim.2001.5129. [DOI] [PubMed] [Google Scholar]
- 61.Ma X., Du J., Nakashima I., Nagase F. Menadione biphasically controls JNK-linked cell death in leukemia Jurkat T cells. Antioxid. Redox Signal. 2002;4:371–378. doi: 10.1089/15230860260196173. [DOI] [PubMed] [Google Scholar]
- 62.Bouzahzah B., Nishikawa Y., Simon D., Carr B.I. Growth control and gene expression in a new hepatocellular carcinoma cell line, Hep40: inhibitory actions of vitamin K. J. Cell Physiol. 1995;165:459–467. doi: 10.1002/jcp.1041650303. [DOI] [PubMed] [Google Scholar]
- 63.Osada S., Saji S., Osada K. Critical role of extracellular signal-regulated kinase phosphorylation on menadione (vitamin K3) induced growth inhibition. Cancer. 2001;91:1156–1165. [PubMed] [Google Scholar]
- 64.Checker R., Sharma D., Sandur S.K., Khan N.M., Patwardhan R.S., Kohli V., Sainis K.B. Vitamin K3 suppressed inflammatory and immune responses in a redox-dependent manner. Free Radic. Res. 2011;45:975–985. doi: 10.3109/10715762.2011.585647. [DOI] [PubMed] [Google Scholar]
- 65.Wu J., Chien C.C., Yang L.Y., Huang G.C., Cheng M.C., Lin C.T., Shen S.C., Chen Y.C. Vitamin K3-2,3-epoxide induction of apoptosis with activation of ROS-dependent ERK and JNK protein phosphorylation in human glioma cells. Chem. Biol. Interact. 2011;193:3–11. doi: 10.1016/j.cbi.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Perez-Soler R., Zou Y., Li T., Ling Y.H. The phosphatases inhibitor menadione (vitamin K3) protects cells from EGFR inhibition by erlotinib and cetuximab. Clin. Cancer Res. 2011;17:6766–6777. doi: 10.1158/1078-0432.CCR-11-0545. [DOI] [PubMed] [Google Scholar]
- 67.Hitomi M., Yokoyama F., Kita Y., Nonomura T., Masaki T., Yoshiji H., Inoue H., Kinekawa F., Kurokohchi K., Uchida N., Watanabe S., Kuriyama S. Antitumor effects of vitamins K1, K2 and K3 on hepatocellular carcinoma in vitro and in vivo. Int. J. Oncol. 2005;26:713–720. [PubMed] [Google Scholar]
- 68.Lee M.H., Cho Y., Kim D.H., Woo H.J., Yang J.Y., Kwon H.J., Yeon M.J., Park M., Kim S.H., Moon C., Tharmalingam N., Kim T.U., Kim J.B. Menadione induces G2/M arrest in gastric cancer cells by down-regulation of CDC25C and proteasome mediated degradation of CDK1 and cyclin B1. Am. J. Transl. Res. 2016;8:5246–5255. [PMC free article] [PubMed] [Google Scholar]
- 69.Zenmyo M., Komiya S., Hamada T., Hiraoka K., Kato S., Fujii T., Yano H., Irie K., Nagata K. Transcriptional activation of p21 by vitamin D3 or vitamin K2 leads to differentiation of p53-deficient MG-63 osteosarcoma cells. Hum. Pathol. 2001;32:410–416. doi: 10.1053/hupa.2001.23524. [DOI] [PubMed] [Google Scholar]
- 70.Degen M., Alexander B., Choudhury M., Eshghi M., Konno S. Alternative therapeutic approach to renal-cell carcinoma: induction of apoptosis with combination of vitamin K3 and D-fraction. J. Endourol. 2013;27:1499–1503. doi: 10.1089/end.2013.0207. [DOI] [PubMed] [Google Scholar]
- 71.Zhang W., Negoro T., Satoh K., Jiang Y., Hashimoto K., Kikuchi H., Nishikawa H., Miyata T., Yamamoto Y., Nakano K., Yasumoto E., Nakayachi T., Mineno K., Satoh T., Sakagami H. Synergistic cytotoxic action of vitamin C and vitamin K3. Anticancer Res. 2001;21:3439–3444. [PubMed] [Google Scholar]
- 72.Calderon P., Cadrobbi J., Marques C., Hong-Ngoc N., Jamison J.M., Gilloteaux J., Summers J.L., Taper H.S. Potential therapeutic application of the association of vitamins C and K3 in cancer treatment. Curr. Med. Chem. 2002;9:2271–2285. doi: 10.2174/0929867023368674. [DOI] [PubMed] [Google Scholar]
- 73.Verrax J., Cadrobbi J., Marques C., Taper H., Habraken Y., Piette J., Calderon P.B. Ascorbate potentiates the cytotoxicity of menadione leading to an oxidative stress that kills cancer cells by a non-apoptotic caspase-3 independent form of cell death. Apoptosis. 2004;9:223–233. doi: 10.1023/B:APPT.0000018804.26026.1a. [DOI] [PubMed] [Google Scholar]
- 74.Bonilla-Porras A.R., Del-Rio M.J., Velez-Pardo C. Vitamin K3 and vitamin C alone or in combination induced apoptosis in leukemia cells by a similar oxidative stress signaling mechanism. Cancer Cell Intern. 2011;1011:1–11. doi: 10.1186/1475-2867-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGuire K., Jamison J., Gilloteaux J., Summers J.L. Vitamin C and K3 combination causes enhanced anticancer activity against RT-4 bladder cancer cells. J. Cancer Sci. Ther. 2013;5:325–333. [Google Scholar]
- 76.Tomasetti M., Nocchi L., Neuzil J., Goodwin J., Nguyen M., Dong L., Manzella N., Staffolani S., Milanese C., Garrone B., Aleva R., Borghi B., Santarelli L., Guerrieri R. Alpha-tocopheryl succinate inhibits autophagic survival of prostate cancer cells induced by vitamin K3 and ascorbate to trigger cell death. PLoS One. 2012;7:e52263. doi: 10.1371/journal.pone.0052263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vita M.F., Nagachar N., Avramidis D., Delwar Z.M., Cruz M.H., Siden A., Paulsson K.M., Yakisich J.S. Painkiller effect of prolonged exposure to menadione on glioma cells: potentiation by vitamin C. Investig. New Drugs. 2011;29:1314–1320. doi: 10.1007/s10637-010-9489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ivanova D., Zhelev Z., Lazarova D., Getsov P., Bakalova R., Aoki I. Vitamins C and K3: a powerful redox system for sensitizing leukemia lymphocytes to everolimus and barasertib. Anticancer Res. 2018;38:1407–1414. doi: 10.21873/anticanres.12364. [DOI] [PubMed] [Google Scholar]
- 79.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rani R., Kumar V. Recent update on human lactate dehydrogenase enzyme 5 (hLDH5) inhibitors: a promising approach for cancer chemotherapy. J. Med. Chem. 2016;59:487–496. doi: 10.1021/acs.jmedchem.5b00168. [DOI] [PubMed] [Google Scholar]
- 81.Di Stefano G., Manerba M., Di Ianni L., Fiume L. Lactate dehydrogenase inhibition: exploring possible applications beyond cancer treatment. Future Med. Chem. 2016;8:713–725. doi: 10.4155/fmc.16.10. [DOI] [PubMed] [Google Scholar]
- 82.Manerba M., Di Ianni L., Govoni M., Roberti M., Recanatini M., Di Stefano G. Lactate dehydrogenase inhibitors can reverse inflammation induced changes in colon cancer cells. Eur. J. Pharm. Sci. 2017;96:37–44. doi: 10.1016/j.ejps.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Eleff S., Kennaway N.G., Buist N.R., Darley-Usmar V.M., Capaldi R.A., Bank W.J., Chance B. 31P NMR study of improvement in oxidative phosphorylation by vitamins K3 and C in a patient with a defect in electron transport at complex III in skeletal muscle. Proc. Natl. Acad. Sci. USA. 1984;81:3529–3533. doi: 10.1073/pnas.81.11.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCord J.M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J. Biol. Chem. 1970;245:1374–1377. [PubMed] [Google Scholar]
- 85.Matsui T., Kitagawa Y., Okumura M., Shigeta Y. Accurate standard hydrogen electrode potential and application to the redox potentials of vitamin C and NAD/NADH. J. Phys. Chem. A. 2015;119:369–376. doi: 10.1021/jp508308y. [DOI] [PubMed] [Google Scholar]
- 86.Tur`yan Y.I., Kohen R. Formal redox potentials of the dehydro-L-ascorbic acid/L-ascorbic acid system. J. Electroanal. Chem. 1995;380:273–277. [Google Scholar]
- 87.Wagner G.C., Kassner R.J., Kamen M.D. Redox potentials of certain vitamin K: implications for a role in sulfite reduction by obligatively anaerobic bacteria. Proc. Natl. Acad. Sci. USA. 1974;71:253–256. doi: 10.1073/pnas.71.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.May J.M., Qu Z.-C., Li X. Ascorbic acid blunts oxidant stress due to menadione in endothelial cells. Arch. Biochem. Biophys. 2003;411:136–144. doi: 10.1016/s0003-9861(02)00715-4. [DOI] [PubMed] [Google Scholar]
- 89.KC S., Cárcamo J.M., Golde D.W.: Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005;19:1657–1667. doi: 10.1096/fj.05-4107com. [DOI] [PubMed] [Google Scholar]
- 90.McGuire K., Jamison J.M., Gilloteaux J., Summers J.L. Synergistic antitumor activity of vitamins C and K3 on human bladder cancer cell lines. J. Cancer Ther. 2013;4:7–19. [Google Scholar]
- 91.Bonuccelli G., De Francesco E.M., de Boer R., Tanowitz H.B., Lisanti M.P. NADH autofluorescence, a new metabolic biomarker for cancer stem cells: identification of vitamin C and CAPE as natural products targeting “stemness”. Oncotarget. 2017;8:20667–20678. doi: 10.18632/oncotarget.15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beck R., Verrax J., Dejeans N., Taper H., Calderon P.B. Menadione reduction by pharmacological doses of ascorbate indices an oxidative stress that kills breast cancer cells. Int. J. Toxicol. 2009;28:33–42. doi: 10.1177/1091581809333139. [DOI] [PubMed] [Google Scholar]
- 93.Silvera-Dorta G., Monzon D.M., Crisostomo F.P., Martin T., Martin V.S., Carrillo R. Oxidation with air by ascorbate-driven quinone redox cycling. Chem. Commun. (Camb.) 2015;51:7027–7030. doi: 10.1039/c5cc01519g. [DOI] [PubMed] [Google Scholar]