Abstract
Excessive reactive oxygen species (ROS) can form an oxidative stress and an associated neuroinflammation. However, the contribution of astrocytes to ROS formation, the cause of the resistance of astrocytes to oxidative stress, and the consequences on neurons remain largely uninvestigated. The transcription factor CCAAT/enhancer-binding protein delta (CEBPD) is highly expressed in astrocytes and has been suggested to contribute to the progress of Alzheimer's disease (AD). In this study, we found that ROS formation and expression of p47phox and p67phox, subunits of NADPH oxidase, were increased in AppTg mice but attenuated in AppTg/Cebpd-/- mice. Cebpd can up-regulate p47phox and p67phox transcription via a direct binding on their promoters, which results in an increase in intracellular oxidative stress. In addition, Cebpd also up-regulated Cu/Zn superoxide dismutase (Sod1) in astrocytes. Inactivation of Sod1 increased the sensitization to oxidative stress, which provides a reason for the resistance of astrocytes in an oxidative stress environment. Taken together, the study first revealed and dissected the involvement of astrocytic Cebpd in the promotion of oxidative stress and the contribution of CEBPD to the resistance of astrocytes in an oxidative stress environment.
Abbreviations: AD, Alzheimer's disease; Aβ, Amyloid-β; AppTg, APPswe/PS1/E9 bigenictransgenic mice; ALS, Amyotrophic lateral sclerosis; Cebpd, CCAAT/enhancer-binding protein delta; GFAP, Glial fibrillary acidic protein; HA/Cd, pcDNA3-HA-Cebpd; NADPH, Nicotinamide adenine dinucleotide phosphate-oxidase; n.s., not significant; ROS, Reactive oxygen species; SOD1, Superoxide dismutase 1; SOD2, Superoxide dismutase 2; SOD3, Superoxide dismutase 3; TETA, Triethylenetetramine
Keywords: ROS, Astrocyte, CEBPD, SOD1 and neuroinflammation
Graphical abstract
Highlights
-
•
Astrocytic Cebpd contributes to ROS formation in neuroinflammation.
-
•
Cebpd regulates p47phox and p67phox transcription in astrocytes.
-
•
Sod1 is significantly activated and protects astrocytes in neuroinflammation.
1. Introduction
Reactive oxygen species (ROS) are the byproducts of respiration, and they play an important role in homeostasis, cell signaling and anti-microorganism capability, increasing dramatically when a cell encounters environmental stress [1], [2]. Previous studies have indicated that excessive ROS can form an oxidative stress and have an effect on neuron survival and, thus, give rise to inflammation-associated neurological disorders, including Alzheimer's disease (AD) and Parkinson's disease (PD) [3], [4]. In AD, β-amyloid accumulation can cause excessive oxidative stress through activation of glia cells and further contributes to the loss of neurons and cognitive deficits [5], [6]. Moreover, microglia, a small portion of glia cells, have been suggested to be a resource contributing to ROS formation following stimuli of proinflammatory factors [7]. However, the knowledge of the details of ROS formation, including the involvement and contribution of astrocytes, the largest set of glia cells, is still limited.
CEBPD is a member of the CCAAT/enhancer binding protein family. As a transcription factor, CEBPD can be regulated by proinflammatory cytokines and growth factors, and the enhanced expression of CEBPD was observed in inflammatory diseases, such as AD and rheumatoid arthritis [8], [9], [10]. In Alzheimer's disease, Aβ and proinflammatory cytokines promote CEBPD expression, and CEBPD can reciprocally induce the generation of proinflammatory cytokines [11]. Majority of CEBPD expression was colocalized with GFAP signals in the cortex of AppTg mice. In astrocytes, activated CEBPD could contribute to the chemoattractant activity and migration of microglia/macrophage via activating monocyte chemoattractant protein-1 (MCP-1) and matrix metalloproteinases (MMPs) [11], reduce macrophage-mediated phagocytosis of damaged neurons via PTX3 [12], and protect astrocytes from cell death through ZNF179-mediated repression of apoptotic genes [8].
Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) is a multimeric ROS-producing complex [13]. p47phox and p67phox are two cytosolic components of NADPH oxidase [13]. Following stimulation, p47phox and p67phox are expressed and can be translocated to the plasma membrane and thereby promote NADPH-oxidase to generate more ROS [14], [15], [16]. However, the regulation of the astrocytic p47phox and p67phox genes in response to inflammatory factor stimulation remains unknown. In addition, stronger superoxide dismutase 1 (SOD1), a detoxifying enzyme that converts superoxide radicals to molecular oxygen and hydrogen peroxide, has been observed in astrocytes in AD [17]. However, a study showed that AppTg/Sod1-/- mice have poor recovery compared with AppTg mice, indicating that clarification of the details of the contribution and regulation of Sod1 function in astrocytes in AD is necessary.
In this study, attenuated ROS formation and low p47phox and p67phox signals were observed in astrocytes of AppTg/Cebpd-/- mice. We found that astrocytic Cebpd can increase extracellular ROS via directly binding to promoter regions of the p47phox and p67phox genes and positively regulating their transcription. Moreover, we also showed new evidence that Cebpd provides an antioxidant effect for astrocytes resistant to intracellular ROS via activation of Sod1 gene expression. The results provided evidence that astrocytic Cebpd contributes to the accumulation of extracellular ROS and the resistance of astrocytes to ROS stress-induced cell death.
2. Materials and methods
2.1. Materials
The CEBPD, p67phox, and nitrotyrosine antibodies and TETA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The GFAP antibody was purchased from Invitrogen (Carlsbad, CA, USA). The p47phox antibody was purchased from MDBio Inc. (Taipei, Taiwan). The SOD1 antibody was purchased from Abcam plc. (Cambridge, MA). The Dulbecco's modified Eagle's medium (DMEM), TRIzol RNA extraction reagent, and SuperScript™ III were purchased from Invitrogen (Carlsbad, CA, USA). Fetal bovine serum (FBS) was purchased from HyClone Laboratories (Logan, UT, USA). All oligonucleotides were synthesized by MDBio Inc. (Taipei, Taiwan).
2.2. Animals
The APPswe/PS1/E9 bigenic (AppTg) mice, bearing chimeric amyloid beta (A4) precursor protein (APPswe) and "DeltaE9" mutation of human presenilin 1, were obtained from the Jackson Laboratory (stock no. 004462, Bar Harbor, ME, USA) and crossed with Cebpd-deficient mice (Cebpd−/−) with a C57BL/6 genetic background obtained from Dr. E. Sterneck. Female AppTg heterozygous mice was intercrossed with Cebpd−/− homozygous mice, and then the offspring (AppTg+/−/Cebpd+/−) were bred to each other to produce the AppTg/Cebpd−/− mice.
2.3. Cell culture and isolation of primary mouse astrocytes
The brain cortex was mechanically dissociated from newborn wild-type or Cebpd−/− mice to isolate the primary mouse brain astrocytes. The isolated cells were filtered through a 70-μm nylon strainer and cultured in DMEM that contained 10% FBS, 100 μg/mL streptomycin, and 100 units/mL penicillin, with an addition of poly-L-lysine (Invitrogen, Carlsbad, CA, USA). The purity of astrocyte cultures was approximately 90–93%, determined by anti-GFAP immunostaining and nuclear staining with DAPI.
2.4. Immunofluorescence analysis
The brains were frozen, sliced, and treated with protein blocker/antibody diluents (Bio SB, Santa Barbara, CA, USA) for 1 h. In the same buffer solution, the brain sections were incubated overnight with primary antibodies at 4 °C. The primary antibodies included nitrotyrosine, GFAP, Aβ, p47phox, p67phox and SOD1. For the staining of cell cultures, primary astrocyte and neuronal cells were post-fixed in 4% paraformaldehyde in PBS for 20 min, followed by 70% methanol in PBS. The fixed primary astrocyte and neuronal cells were further incubated with primary antibodies against target proteins at 4 °C overnight. Pretreated slides of the tissue sections or primary astrocyte and neuronal cells were washed with 0.2% Triton X-100 in PBS. The slides were incubated with Alexa 405-, 488- or 555-conjugated secondary antibodies for 1 h at room temperature and then washed again with 0.2% Triton X-100 in PBS. Next, the glass slides were counter-stained and mounted with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole for immunofluorescence microscopy. ImageJ software was used to analyze the immunofluorescent staining population in the cortex of brain slices from AppTg or AppTg/Cebpd−/− mice.
2.5. Extracellular/intracellular H2O2 production
For the measurement of intracellular H2O2 production, cells were resuspended at 3 × 105 cells/mL in 1× Assay Buffer with 0.5% Triton X-100. The conditional medium for extracellular H2O2 production was centrifuged at 10,000 rpm for 5 min to remove insoluble particles. ADHP/HRP Working Solution [1× Assay Buffer, 100 μM ADHP, .2 U/mL HRP] and 50 µl of each sample were added to a microtiter plate well, and the plate was read with a fluorescence microplate reader equipped for excitation in the 530–570 nm range and for emission in the 590–600 nm range. The concentration of each sample was calculated with the absorbance.
2.6. Reverse transcription-PCR and quantitative PCR
Total RNA was extracted using the TRIzol RNA extraction reagent. SuperScript III was used to complete a reverse transcription (RT) to synthesize the complementary DNA (cDNA). Quantitative PCR (Q-PCR) was conducted with KAPA SYBR FAST qPCR Master Mix (Life Technologies Corporation and Kapa Biosystems Inc.). PCR was conducted using a CFX Connect Real-Time PCR system (Bio-Rad) with the following pairs of specific primers: Cebpd: 5′-CTCCCGCACACAACATACTG-3′ and Cebpd:5′-AGTCATGCTTTCCCGTGTTC-3′; Sod1:5′-AACCATCCACTTCGA
GCAGA-3′; Sod1: 5′-GGTCTCCAACATGCCTCTCT-3′; p47phox:5′-TCAAACCAC
CCCATACCACA-3′; p47phox:5′-AAGTTGACGTAGACCAGCCA-3′; p67phox: 5′-TG
CGCTATACAGACACACCA-3′; and p67phox:5′-TAGCCAGCACACACACAAAC- 3′.
2.7. Western blot
The cells were harvested and lysed with a modified radio-immune precipitation assay (RIPA) buffer [50 mM Tris–HCl (pH 7.4), 150 mM sodium chloride, 1 mM ethylenediamine tetraacetic acid, 1% NP40, 0.25% sodium deoxycholate, 1 mM dithiothreitol, 10 mM NaF, 1 mM PMSF, 1 μg/mL aprotinin, and 1 μg/mL leupeptin]. Lysates were resolved on a sodium dodecyl sulfate-containing 10% polyacrylamide gel and then transferred to a polyvinylidene difluoride nylon membrane and probe with primary antibodies for target proteins at 4 °C overnight. The specific proteins were detected by peroxidase conjugated secondary antibody incubated at room temperature for 1 h. The signals were revealed by an enhanced chemiluminescence Western blot system from Pierce (Rockford, IL, USA).
2.8. Luciferase reporter assay
The 5′ flanking regions of p47phox, p67phox and Sod1 genes were obtained by PCR with primary astrocyte genomic DNA and then individually cloned into a pGL3 basic vector. The primers for the PCR of the genomic DNA were: p47phox(−916):5′-XhoI-CCGCTCGAGCGGCTCTCTCCATCAGTCCCTGCTTTC-3′; p47phox (−671):5′-XhoI-CCGCTCGAGCGGCTCACTTTGTAGACCAGGCTG-3′; p47phox(+39):5′-HindIII-CCCAAGCTTGGGCACTTCTCTCTATAGCCTGGGCTG-3′; p67phox(−592): 5′-MluI-CGACGCGTCGCTTCAGCTTCTCACAGACAGACAG- 3′; p67phox(−164): 5′-MluI-CGACGCGTCGGGTGCCCAGGACATAGAAGAAGCT
C-3′; p67phox(+145): 5′-HindIII-CCCAAGCTTGGGCATGGTTAGGTGCTTACCAG
AAGG-3′; Sod1 (−895):5′-XhoI–CCGCTCGAGCGGCCAATAGATGACTGTGAA
CATCC-3′; and Sod1(+74): 5′-HindIII-CCCAAGCTTGGGGAGAGAGCAAGACG
AGAAGCC-3′. For the reporter assay, cells were transfected with the reporters and expression vectors as indicated using PolyJet (SignaGen, Ijamsville, MD). The lysates of transfected cells were harvested following the manufacturer's instructions for the luciferase assay.
2.9. Chromatin immunoprecipitation assay
Briefly, the primary astrocytes were treated with 1% formaldehyde for 15 min. The cross-linked chromatin was sonicated to 500–1000 bp. The DNA fragments were immunoprecipitated with specific antibodies recognizing CEBPD or control rabbit immunoglobulin G (IgG) at 4 °C for 12–16 h. After reversal of the crosslinking between proteins and genomic DNA, precipitated DNA was amplified by PCR with primers related to the specific regions on the genomic loci of target genes. The primers included p47phox(S):5′-TTATTTATTTATTTTATTAATCTG-3′; p47phox(AS): 5′-CTCTCTACAGTTCTGAAC-3′; p67phox(S):5′-GGTGCCCAGGACATAGAAGA
AGCTC-3′; p67phox(AS): 5′-CAGGAAGTTTCCCCATCGTTCAGG-3′; Sod1(S): 5′-CCAATAGATGACTGTGAACATCC-3′; and Sod1(AS): 5′-GACAGACAAGTG
CTCTGCCA G −3′.
2.10. Caspase3/7 activity assay
Briefly, the same volume of Caspase-Glo® 3/7 reagent (Promega Corporation, Fitchburg, Wisconsin, US) as the conditional medium was prepared and added to the cells. The samples were mixed with a shaker at room temperature for 30 min, and the luciferase activity was measured with a luminometer.
2.11. Statistical analysis
The data were expressed as the means±SEM and analyzed for statistical significance by two-tailed unpaired Student's t-test for experiments with more than two subgroups using Prism 5 software. All experiments were repeated at least two times, each performed in triplicate. A statistically significant difference was defined at * p < 0.05, **p < 0.01, ***p < 0.001.
3. Results
3.1. ROS formation was attenuated in astrocytes of AppTg/Cebpd-/- mice
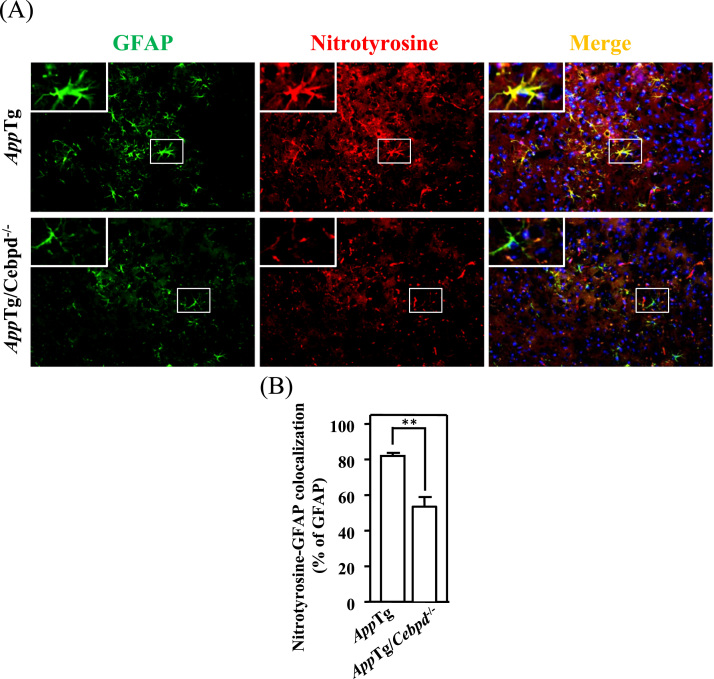
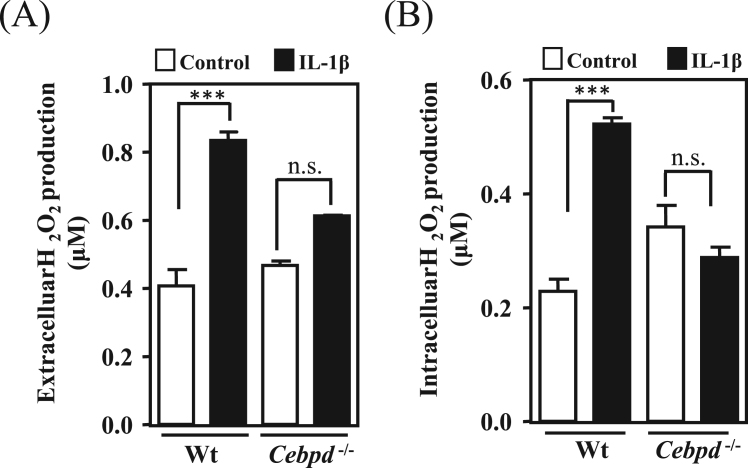
ROS accumulation contributes to the pathological progression of AD. As mentioned above, CEBPD is highly expressed in astrocytes in AppTg mice and human AD patients [9], [11]. To test whether CEBPD contributes to oxidative stress formation, we measured the immunoreactivity of nitrotyrosine (a marker of oxidative stress [18]) in brain sections of AppTg and AppTg/Cebpd-/- mice. As shown in Fig. 1A and 1B, the nitrotyrosine signals were highly co-localized with GFAP, a specific marker of astrocytes, in AppTg mice. However, following the normalization of GFAP signals between AppTg and AppTg/Cebpd-/- mice, the nitrotyrosine signal and the co-localization signal of GFAP and nitrotyrosine were significantly reduced in AppTg/Cebpd-/- mice. These results suggested that the loss of astrocytic Cebpd contributes to the attenuation of ROS formation. Cebpd is an IL-1β-responsive gene in astrocytes [19]. Previous studies showed that IL-1β induces neurotoxicity through the release of free radicals in co-cultures of neurons with astrocytes [7], [20]. We next dissected the relationship between Cebpd and ROS formation in primary astrocytes upon IL-1β treatment. Compared to the primary astrocytes, the extracellular and intracellular H2O2 production was attenuated in Cebpd-/- astrocytes upon IL-1β treatment (Fig. 2A and 2B). The results indicated that astrocytic Cebpd up-regulated ROS formation.
Fig. 1.
Cebpd contributed to ROS formation in astrocytesin vivo. (A) The expression of nitrotyrosine, a ROS marker, was decreased in AppTg/Cebpd-/- mice when compared with AppTg mice. The brain tissue was subjected to immunofluorescence with anti-GFAP and anti-Nitrotyrosine antibodies. The magnification is ×200. (B) Quantitative analysis (n = 3 for each group) of nitrotyrosine-GFAP co-localization in AppTg and AppTg/Cebpd−/− mice using ImageJ software. (**p < 0.01, Student's t-test).
Fig. 2.
Extracellular and intracellular H2O2production inCebpd-/-astrocytes was attenuated. (A) and (B) In primary astrocytes from wild-type and Cebpd-/- mice, IL-1β-induced extracellular and intracellular H2O2 production was attenuated in Cebpd-/- astrocytes. The H2O2 production was detected by a hydrogen peroxide fluorescent detection kit using a fluorescent ELISA reader. Similar results were obtained from two independent experiments, each performed in triplicate, and the data shown here were from one representative assay. n.s. not significant. (***p < 0.001, Student's t-test).
3.2. Astrocytic p47phox and p67phox expression was attenuated in the area surrounding β-amyloid plaques in AppTg/Cebpd−/− mice
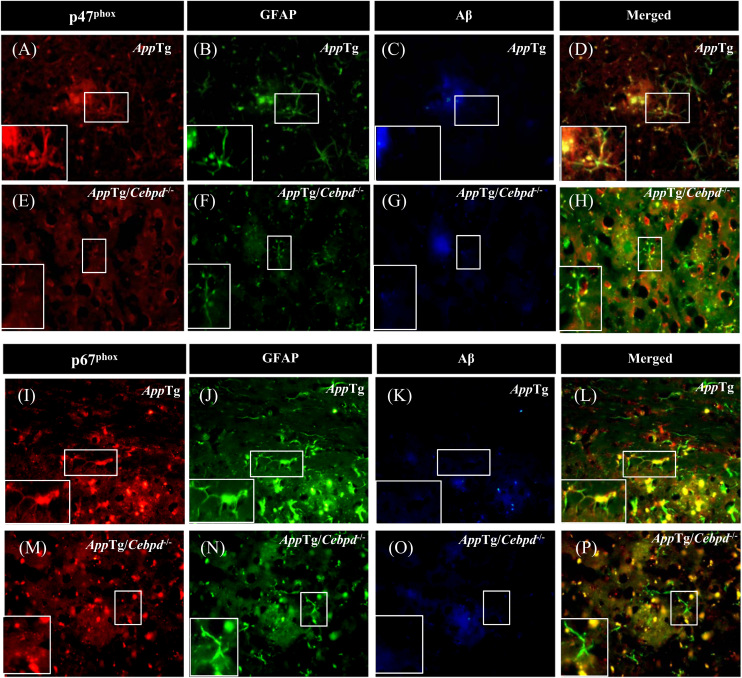
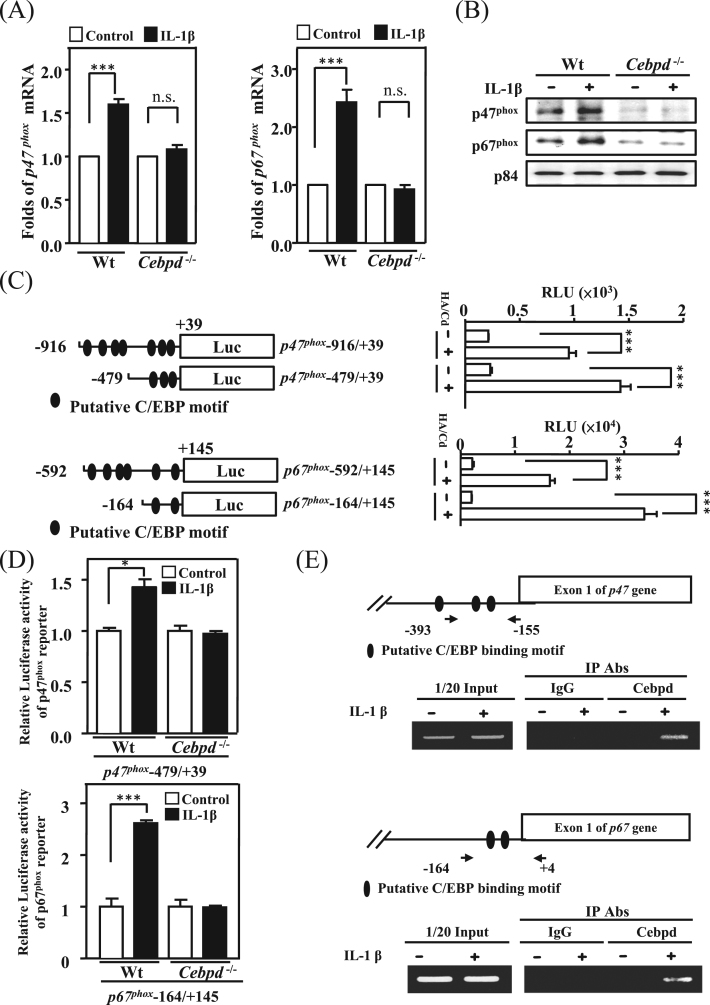
p47phox and p67phox are subunits of NADPH oxidase and play a critical role in ROS production [21], [22]. To examine the effect of Cebpd on p47phox and p67phox expression in astrocytes, we measured the signals of p47phox and p67phox in brain sections of AppTg and AppTg/Cebpd−/− mice. In AppTg mice, both p47phox and p67phox were highly co-localized with GFAP in the area surrounding Aβ plaques (Fig. 3, panel D and L; Supplementary Fig. 1A and 1B). Following the normalization of GFAP signals between AppTg and AppTg/Cebpd−/−mice, the co-localization signals of p47phox and p67phox with GFAP in AppTg/Cebpd−/− mice were significantly attenuated (Fig. 3, panel H and P; Supplementary Fig. 1A and 1B). This result implied that Cebpd is the upstream regulator for p47phox and p67phox expression. We therefore tested whether Cebpd regulated p47phox and p67phox transcription. Compared with primary astrocytes from wild-type mice, the induction effect by IL-1β of both p47phox and p67phox was lost in Cebpd−/− primary astrocytes (Fig. 4A and 4B; Supplementary Fig. 1C and 1D). We further utilized p47phox and p67phox reporters to assess and dissect the Cebpd-responsive regions on their promoter regions. The results of the reporter assay showed that p47phox and p67phox reporter activities were responsive to exogenous transfection of the Cebpd expression vector in primary astrocytes. Meanwhile, the −479/+39 and −164/+145 regions on the p47phox and p67phox genes, respectively, contained Cebpd-responsive motifs (Fig. 4C). We assessed whether the reporters containing Cebpd-responsive regions are also important for the IL-1β response. The result showed that the −479/+39 and −164/+145 regions on the p47phox and p67phox reporters, respectively, were also responsive to IL-1β. The loss of Cebpd significantly attenuated the IL-1β-induced p47phox and p67phox reporter activity in primary mouse astrocytes (Fig. 4D). Moreover, a ChIP assay showed that Cebpd was responsive to IL-1β and bound directly to the promoter regions of the p47phox and p67phox genes (Fig. 4E). These results suggested that Cebpd plays a vital role in IL-1β-induced p47phox and p67phox transcription in primary astrocytes.
Fig. 3.
The expression of p47phoxand p67phoxwere increased in the area surrounding Aβ inAppTg mice. p47phox (A, E) and p67phox (I, M) immunoreactivity co-localized with GFAP (B, F, J, N) and was reduced in the area surrounding Aβ (C, G, K, O) in AppTg/Cebpd-/- mice. The merged photo is shown in D, H, L and P. Coronal sections of the cortex were collected from AppTg and AppTg/Cebpd−/− mice and subjected to immunofluorescence with anti-GFAP, anti-Aβ, anti-p47phox and anti-p67phox antibodies.
Fig. 4.
p47phoxand p67phoxwere directly regulated by Cebpd. (A) IL-1β-induced transcription of p47phox and p67phox were attenuated in Cebpd-/- astrocytes compared with wild-type astrocytes. The total RNA was harvested from IL-1β-treated cells and Q-PCR was conducted with specific primers. (B) Compared with wild-type astrocytes, Cebpd-/- astrocytes had decreased p47phox and p67phox expression after IL-1β treatment. Western blots were performed with total protein lysates harvested from wild-type primary astrocytes (left panel) and Cebpd-/- primary astrocytes (right panel). (C) The identification of Cebpd binding motifs on the p47phox and p67phox promoter region. The luciferase reporter assay was conducted using the luciferase activity of the p47phox and p67phox reporter/Cebpd expression vector co-transfected cell lysates. (D) The IL-1β induced p47phox and p67phox expression was attenuated in Cebpd-/- astrocytes compared with wild-type astrocytes. (E) Cebpd directly binds to the p47phox and p67phox promoter region in vivo. The chromatin immunoprecipitation assay was performed with the immunoprecipitation products with the indicated Abs from wild-type primary astrocytes treated with IL-1β. Similar results were obtained from two independent experiments, each performed in triplicate, and the data shown here were from one representative assay. HA/Cd pcDNA3-HA-Cebpd; n.s. not significant (*p < 0.05, ***p < 0.001, Student's t-test).
3.3. Sod1 was expressed in and protected astrocytes from cell death
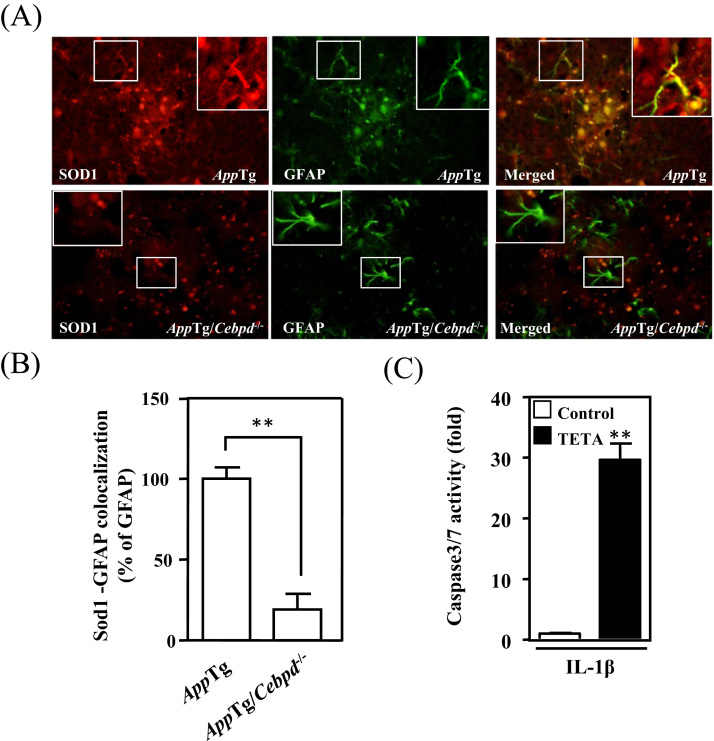
As shown in Fig. 2B and 2C, astrocytic Cebpd contributes to intra- and extra-cellular ROS formation. The transcription of p47phox and p67phox was also shown to be regulated by Cebpd in astrocytes, agreeing with the observation that nitrotyrosine signals were highly co-localized with GFAP signals. The observations led us to investigate why astrocytes are able to resist an oxidative environment. In astrocytes, SOD1, an antioxidant enzyme, can be activated by inflammatory cytokine treatment, is increased in AD patients, and protects cells from ROS damage [23], [24]. Therefore, we tested whether Sod1 was responsive to CEBPD and contributed to the resistance to the oxidative environment. We found that Sod1 is highly co-localized with GFAP signals in AppTg mice. Compared with AppTg mice, the co-localized signals of Sod1 and GFAP were attenuated 80% in AppTg/Cebpd-/- mice (Fig. 5A and 5B). To investigate the antioxidant ability of Sod1 in inflamed astrocytes, triethylenetetramine (TETA), a Sod1 inhibitor, was combined with IL-1β to treat primary mouse astrocytes. As shown in Fig. 5C, caspase-3/7 activity was increased in TETA-pretreated astrocytes upon IL-1β stimulation.
Fig. 5.
Sod1 was abundantly expressed in astrocytes and protected astrocytes from oxidative damage. (A) Sod1 immunoreactivity co-localized with GFAP and was attenuated in AppTg/Cebpd-/- mice compared with that in AppTg mice. The coronal sections of cortex tissue were subjected to immunofluorescence with anti-GFAP and anti-Sod1. (B) Quantitative analysis (n = 3 for each group) of Sod1-GFAP co-localization in AppTg and AppTg/Cebpd−/− mice using ImageJ software. (C) The inhibition of Sod1 by TETA leads to cell death. Cell death was detected by the caspase3/7 activity kit using a fluorescent ELISA reader; data represent the two independent cell cultures analyzed in triplicate wells. n.s. not significant. (**p < 0.01, Student's t-test).
3.4. Cebpd induces Sod1 transcription in astrocytes
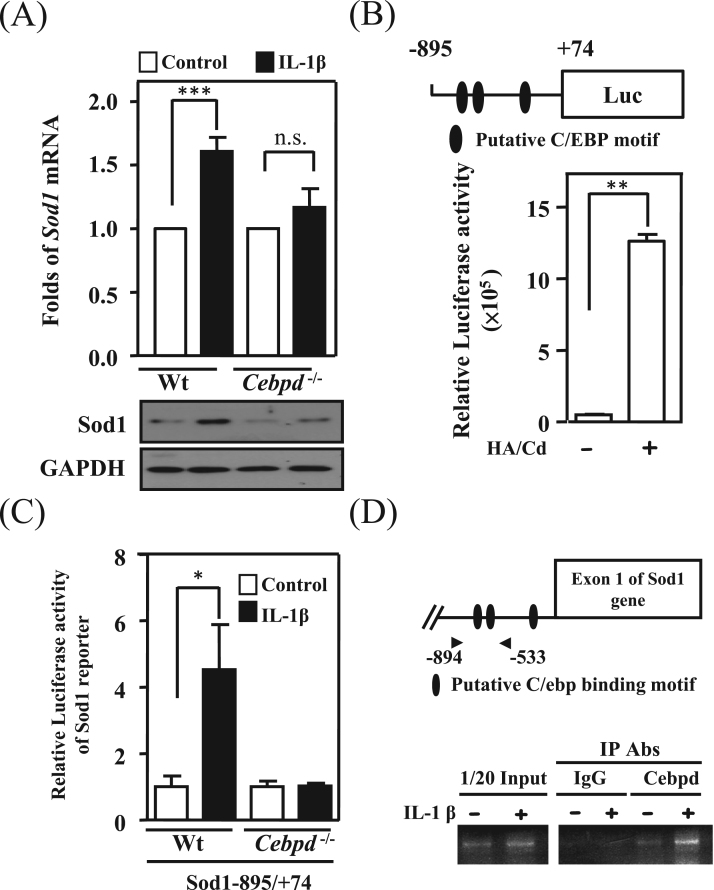
Sod1 signals were significantly associated with GFAP signals and attenuated in the brains of AppTg/Cebpd-/- mice. We further assessed whether Sod1 transcription also occurred in response to IL-1β and was regulated by Cebpd. The results showed that Sod1 transcripts were induced by IL-1β and the loss of Cebpd attenuated the IL-1β-induced Sod1 expression (Fig. 6A). As well as Cebpd- and IL-1β-induced p47phox and p67phox transcription, the results demonstrated that Cebpd could directly bind to the promoter region of the Sod1 gene and was involved in IL-1β-induced Sod1 reporter activity in primary astrocytes (Fig. 6B, 6C and 6D).
Fig. 6.
Sod1 was directly regulated by astrocytic Cebpd. (A) IL-1β-induced Sod1 expression was attenuated in Cebpd-/- astrocytes when compared with that in wild-type astrocytes. Q-PCR and Western blot analyses were conducted with specific primers and the indicated antibodies using total RNA and protein lysates harvested from IL-1β-treated primary astrocytes. (B) The identification of Cebpd binding motifs on the Sod1 promoter region. Sod1 was highly expressed in Cebpd over-expression astrocytes. The luciferase reporter assay was conducted using the luciferase activity of the Sod1 reporter/Cebpd expression vector co-transfected cell lysates. (C) The relative luciferase activity of Sod1 induced by IL-1β was attenuated in Cebpd-/- astrocytes. (D) Cebpd directly binds to the Sod1 promoter region in vivo. The chromatin immunoprecipitation assay was performed with the immunoprecipitation products with the indicated antibodies from wild-type primary astrocytes treated with IL-1β. Similar results were obtained from two independent experiments, each performed in triplicate, and the data shown here were from one representative assay. HA/Cd pcDNA3-HA-Cebpd; n.s. not significant. (*p < 0.05, **p < 0.01, ***p < 0.001, Student's t-test).
4. Discussion
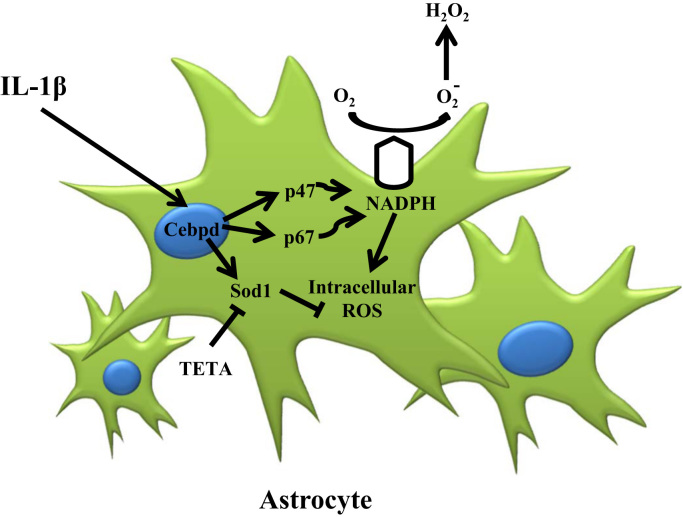
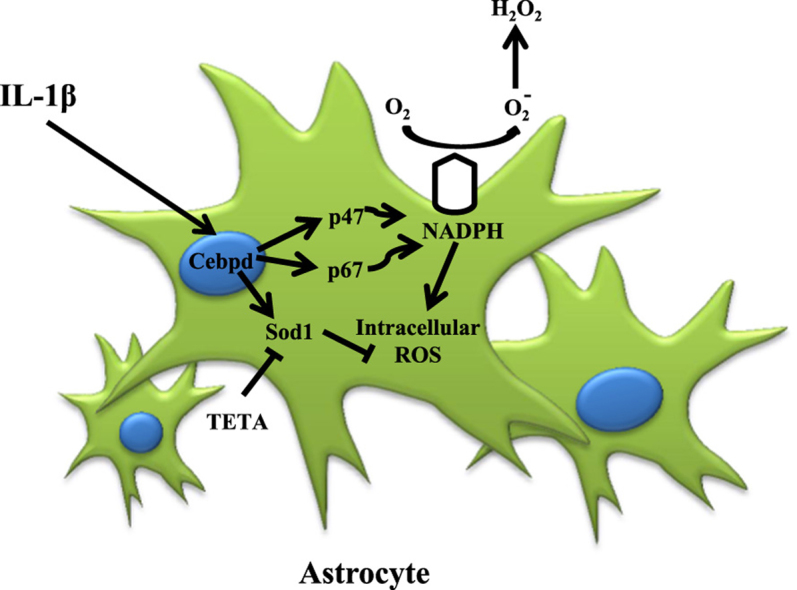
ROS and reactive nitrogen species (RNS), including superoxide anion, hydrogen peroxide, nitric oxide and peroxynitrite, are particularly responsible for oxidative stress and are essential for biological function at low levels of oxidative stress [7]. Excessive ROS production by mitochondria and NADPH oxidase can form an oxidative stress and neurodegenerative disease, such as Parkinson's disease and AD [3], [7], [25]. In AD, β-amyloid forms aggregates that activate microglia and astrocytes and are susceptible to the injurious effects of oxidative stress [6], [7]. Inflammatory cytokine IL-1β can induce neurotoxicity through oxidative stress in co-cultures of neurons with astrocytes [20]. ROS can induce neuron cell death, but astrocytes are more resistant to oxidative stress [26], [27]. However, the underlying mechanism of this phenomenon remains largely unclear. In this study, we found that astrocytic Cebpd activation was responsive to inflammatory factor IL-1β and contributed to intra- and extra-cellular ROS formation. In addition to revealing that Cebpd can directly regulate p47phox and p67phox gene transcription, the antioxidant gene Sod1 was also responsive to Cebpd activation and contributed to antiapoptosis of astrocytes in an inflammatory environment. This study provided a new insight that Cebpd contributes to inter/intracellular ROS formation through activating p47phox and p67phox expression and also enhances Sod1 in astrocytes to resist oxidative stress (Fig. 7).
Fig. 7.
A schematic diagram illustrating the effect of Cebpd on astrocytic ROS formation and cell death resistance in oxidative stress after AD. In AD, IL-1β activates astrocytic expression of Cebpd, which in turn promotes the expression of NADPH oxidase complex subunits p47phox and p67phox and thus increases the ROS formation of the astrocytes located along the surrounding Aβ area. Simultaneously, Cebpd up-regulates astrocytic expression of Sod1. Expression of Sod1 promotes anti-oxidation in astrocytes, particularly those in the Aβ surrounding area.
Astrocytes are the most abundant glial cells in the central nervous system (CNS). Previous studies show that ROS formation is involved in microglia activation in Parkinson's disease [6], [28]. Several recent studies have indicated that astrocytes are also involved in ROS formation [6], [29]. Transcriptional regulation of NADPH oxidase components p47phox and p67phox has been demonstrated in the majority of malignant cells but is rare in normal cells. For example, myeloid cells can activate p47phox and p67phox expression through PU.1 or AP-1 activation [30], [31]. In this study, we demonstrated that CEBPD binds directly to the promoter regions of the p47phox and p67phox genes and activates their expression (Fig. 4C). A previous study showed that CEBPD can interact with Sp1 and contributes to transcriptional activation of IL-10 gene [32]. Furthermore, Sp1 binding sites were found on both the p47phox and p67phox promoters, and a study showed that Sp1 plays a role in the activation of the p47phox and p67phox genes [30], [31]. Therefore, whether CEBPD can interact with Sp1 and cooperatively contribute to the activation of the p47phox and p67phox genes needs further investigation. On the other hand, the master regulator of the antioxidant response, nuclear factor-erythroid 2-related factor-2 (Nrf2), is highly stable in astrocytes and modulates the expression and the coordinated induction of an array of defensive genes encoding phase II detoxifying enzymes and antioxidant proteins explaining their robust antioxidant defense and resistance against oxidative stress [33]. Interestingly, recent studies demonstrated that SOD1 is tightly connected with Nrf2 protein [34]. Our data also showed that overexpression of Cebpd could increase Nrf2 expression in primary astrocytes (Supplementary Fig. 2). Therefore, Nrf2 may play a partial role in CEBPD-mediated ROS protection in astrocytes.
In a neuroinflammatory environment, several inflammatory factors have been suggested to cause oxidative stress and antioxidant imbalance, which induce redox signal-dependent expression of genes for oxidative stress and antioxidants [7]. In contrast with oxidative stress, induction of various antioxidants, such as SOD, catalase or HO-1, could reduce ROS accumulation [7]. As major antioxidant enzymes, superoxide dismutases (SODs) play a crucial role in scavenging the superoxide anion [35]. Superoxide dismutase has three family members, including SOD1 (copper-zinc superoxide dismutase), SOD2 (manganese superoxide dismutase) and SOD3 (extracellular superoxide dismutase) [36]. In the central nervous system, SOD1 and SOD2 are relative highly expressed in astrocytes and neurons, respectively [36], and SOD3 expression is expressed more rarely than other SOD isoform [37]. Moreover, SOD2 is not affected by inflammatory cytokine treatment [38], and our results have shown a marginal increase in SOD1 in response to IL-1β in neuronal cells (Supplementary Fig. 3). A previous study show that transcription activation of SOD1 has been demonstrated that Sp1 is important for basal transcription and inflammatory factor induced transcription through binding SOD1 promoter region [35]. Regarding our current results, SOD1 and NADPH oxidase components, p47phox and p67phox, are activated in astrocytes; therefore, taken together, activation of CEBPD in astrocytes results in the increase in intracellular ROS burden in neurons and glia cells, but SOD1 activation in astrocytes can sustain astrocytic antioxidant capability (Supplementary Fig. 4). On the other hand, previous study demonstrated the relative high production of astrocyte-derived mitochondrial ROS when compared with neurons [39]. However, mitochondrial ROS is majorly produced by intracellular ROS and activate intracellular redox signaling. Our study focuses on extracellular ROS production, which further induces neuronal death. We also checked mitochondrial superoxide production in primary astrocytes with specific mitochondrial probe and showed that IL-1β could increase mitochondrial superoxide production (Supplementary Fig. 5). These results could provide an explanation for the sensitization of neuronal cells and resistance of astrocytes in an oxidative condition in response to inflammation.
The activation of CEBPD is common in various chronic inflammation diseases as well as in ROS formation [40]. We demonstrated that activation of CEBPD and ROS formation was associated in neuroinflammation. This also indicated that CEBPD could be a therapeutic target in ROS-involved chronic inflammation diseases. Accordingly, our findings suggest that the CEBPD cascade can promote ROS formation through inflammatory cytokine treatment. A previous study showed that Rosmanol, a diterpenoid compound, can inhibit CEBPD expression in macrophages [41]. Diterpenoid compounds can inhibit inflammatory [42] and increase antioxidant effects [43]. This suggests that a diterpenoid compound that inhibits CEBPD could be a new potential anti-inflammatory drug.
5. Conclusion
Following the observation of attenuated ROS formation in AppTg/Cebpd-/- mice, we further revealed that Cebpd is responsive to IL-1β in astrocytes and contributes to the activation of p47phox, p67phox and Sod1 genes by directly binding to their promoter regions. The regulation provides the contribution of astrocytic Cebpd in ROS formation and also a new insight for its involvement in increasing intracellular oxidative stress and the resistance of cell death of astrocytes in neuroinflammation.
Acknowledgments
This work was supported by the grants MOST 105–2320-B-038–068-MY2, MOST 105–2321-B-006–029 and MOST 103–2320-B-006–034-MY3 from the Ministry of Science and Technology, Taiwan and 107CM-TMU-13 from Chi Mei Hospital-TMU joint research project.
Acknowledgments
Conflict of interest
None of the authors has a conflict of interest to declare in relation to the present research.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.02.011.
Contributor Information
Chiung-Yuan Ko, Email: ko680108@tmu.edu.tw.
Ju-Ming Wang, Email: yumingw@mail.ncku.edu.tw.
Appendix A. Supplementary material
Supplementary material
References
- 1.Devasagayam T.P., Tilak J.C., Boloor K.K., Sane K.S., Ghaskadbi S.S., Lele R.D. Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 2.Ray R., Shah A.M. NADPH oxidase and endothelial cell function. Clin. Sci. 2005;109(3):217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- 3.Pardillo-Diaz R., Carrascal L., Ayala A., Nunez-Abades P. Oxidative stress induced by cumene hydroperoxide evokes changes in neuronal excitability of rat motor cortex neurons. Neuroscience. 2015;289:85–98. doi: 10.1016/j.neuroscience.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 4.Burguillos M.A., Deierborg T., Kavanagh E., Persson A., Hajji N., Garcia-Quintanilla A., Cano J., Brundin P., Englund E., Venero J.L., Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472(7343):319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 5.Gao H.M., Zhou H., Hong J.S. NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol. Sci. 2012;33(6):295–303. doi: 10.1016/j.tips.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh H.L., Yang C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed. Res. Int. 2013;2013:484613. doi: 10.1155/2013/484613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S.M., Lee Y.C., Ko C.Y., Lai M.D., Lin D.Y., Pao P.C., Chi J.Y., Hsiao Y.W., Liu T.L., Wang J.M. Increase of zinc finger protein 179 in response to CCAAT/enhancer binding protein delta conferring an antiapoptotic effect in astrocytes of Alzheimer's disease. Mol. Neurobiol. 2015;51(1):370–382. doi: 10.1007/s12035-014-8714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R., Strohmeyer R., Liang Z., Lue L.F., Rogers J. CCAAT/enhancer binding protein delta (C/EBPdelta) expression and elevation in Alzheimer's disease. Neurobiol. Aging. 2004;25(8):991–999. doi: 10.1016/j.neurobiolaging.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Cardinaux J.R., Allaman I., Magistretti P.J. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia. 2000;29(1):91–97. [PubMed] [Google Scholar]
- 11.Ko C.Y., Wang W.L., Wang S.M., Chu Y.Y., Chang W.C., Wang J.M. Glycogen synthase kinase-3beta-mediated CCAAT/enhancer-binding protein delta phosphorylation in astrocytes promotes migration and activation of microglia/macrophages. Neurobiol. Aging. 2014;35(1):24–34. doi: 10.1016/j.neurobiolaging.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Ko C.Y., Chang L.H., Lee Y.C., Sterneck E., Cheng C.P., Chen S.H., Huang A.M., Tseng J.T., Wang J.M. CCAAT/enhancer binding protein delta (CEBPD) elevating PTX3 expression inhibits macrophage-mediated phagocytosis of dying neuron cells. Neurobiol. Aging. 2012;33(2):e11–e25. doi: 10.1016/j.neurobiolaging.2010.09.017. (422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babior B.M. NADPH oxidase. Curr. Opin. Immunol. 2004;16(1):42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Heyworth P.G., Curnutte J.T., Nauseef W.M., Volpp B.D., Pearson D.W., Rosen H., Clark R.A. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J. Clin. Investig. 1991;87(1):352–356. doi: 10.1172/JCI114993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagano P.J., Chanock S.J., Siwik D.A., Colucci W.S., Clark J.K. Angiotensin II induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts. Hypertension. 1998;32(2):331–337. doi: 10.1161/01.hyp.32.2.331. [DOI] [PubMed] [Google Scholar]
- 16.Wu F., Schuster D.P., Tyml K., Wilson J.X. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic. Biol. Med. 2007;42(1):124–131. doi: 10.1016/j.freeradbiomed.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 17.Choi J., Rees H.D., Weintraub S.T., Levey A.I., Chin L.S., Li L. Oxidative modifications and aggregation of Cu,Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J. Biol. Chem. 2005;280(12):11648–11655. doi: 10.1074/jbc.M414327200. [DOI] [PubMed] [Google Scholar]
- 18.Darwish R.S., Amiridze N., Aarabi B. Nitrotyrosine as an oxidative stress marker: evidence for involvement in neurologic outcome in human traumatic brain injury. J. Trauma. 2007;63(2):439–442. doi: 10.1097/TA.0b013e318069178a. [DOI] [PubMed] [Google Scholar]
- 19.Wang S.M., Hsu J.C., Ko C.Y., Chiu N.E., Kan W.M., Lai M.D., Wang J.M. Astrocytic CCAAT/enhancer-binding protein delta contributes to glial scar formation and impairs functional recovery after spinal cord injury. Mol. Neurobiol. 2016;53(9):5912–5927. doi: 10.1007/s12035-015-9486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornton P., Pinteaux E., Gibson R.M., Allan S.M., Rothwell N.J. Interleukin-1-induced neurotoxicity is mediated by glia and requires caspase activation and free radical release. J. Neurochem. 2006;98(1):258–266. doi: 10.1111/j.1471-4159.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Perez A.I., Borrajo A., Rodriguez-Pallares J., Guerra M.J., Labandeira-Garcia J.L. Interaction between NADPH-oxidase and Rho-kinase in angiotensin II-induced microglial activation. Glia. 2015;63(3):466–482. doi: 10.1002/glia.22765. [DOI] [PubMed] [Google Scholar]
- 22.Noh K.M., Koh J.Y. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J. Neurosci.: Off. J. Soc. Neurosci. 2000;20(23):RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunton-Stasyshyn R.K., Saccon R.A., Fratta P., Fisher E.M. SOD1 function and its implications for amyotrophic lateral sclerosis pathology: new and renascent themes. Neurosci.: Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2015;21(5):519–529. doi: 10.1177/1073858414561795. [DOI] [PubMed] [Google Scholar]
- 24.Sharma V., Mishra M., Ghosh S., Tewari R., Basu A., Seth P., Sen E. Modulation of interleukin-1beta mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain Res. Bull. 2007;73(1–3):55–63. doi: 10.1016/j.brainresbull.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Blesa J., Trigo-Damas I., Quiroga-Varela A., Jackson-Lewis V.R. Oxidative stress and Parkinson's disease. Front. Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita T., Tozaki-Saitoh H., Inoue K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia. 2009;57(3):244–257. doi: 10.1002/glia.20749. [DOI] [PubMed] [Google Scholar]
- 27.Furuta T., Ohshima C., Matsumura M., Takebayashi N., Hirota E., Mawaribuchi T., Nishida K., Nagasawa K. Oxidative stress upregulates zinc uptake activity via Zrt/Irt-like protein 1 (ZIP1) in cultured mouse astrocytes. Life Sci. 2016;151:305–312. doi: 10.1016/j.lfs.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W., Wang T., Pei Z., Miller D.S., Wu X., Block M.L., Wilson B., Zhang W., Zhou Y., Hong J.S., Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 29.Sheng W.S., Hu S., Feng A., Rock R.B. Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem. Res. 2013;38(10):2148–2159. doi: 10.1007/s11064-013-1123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S.L., Valente A.J., Zhao S.J., Clark R.A. PU.1 is essential for p47(phox) promoter activity in myeloid cells. J. Biol. Chem. 1997;272(28):17802–17809. doi: 10.1074/jbc.272.28.17802. [DOI] [PubMed] [Google Scholar]
- 31.Li S.L., Valente A.J., Wang L., Gamez M.J., Clark R.A. Transcriptional regulation of the p67phox gene: role of AP-1 in concert with myeloid-specific transcription factors. J. Biol. Chem. 2001;276(42):39368–39378. doi: 10.1074/jbc.M106111200. [DOI] [PubMed] [Google Scholar]
- 32.Chiang B.T., Liu Y.W., Chen B.K., Wang J.M., Chang W.C. Direct interaction of C/EBPdelta and Sp1 at the GC-enriched promoter region synergizes the IL-10 gene transcription in mouse macrophage. J. Biomed. Sci. 2006;13(5):621–635. doi: 10.1007/s11373-006-9101-y. [DOI] [PubMed] [Google Scholar]
- 33.Jimenez-Blasco D., Santofimia-Castano P., Gonzalez A., Almeida A., Bolanos J.P. Astrocyte NMDA receptors' activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 2015;22(11):1877–1889. doi: 10.1038/cdd.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milani P., Ambrosi G., Gammoh O., Blandini F., Cereda C. SOD1 and DJ-1 converge at Nrf2 pathway: a clue for antioxidant therapeutic potential in neurodegeneration. Oxid. Med. Cell. Longev. 2013;2013:836760. doi: 10.1155/2013/836760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao L., St Clair D.K. Regulation of superoxide dismutase genes: implications in disease. Free Radic. Biol. Med. 2009;47(4):344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller A.F. Superoxide dismutases: active sites that save, but a protein that kills. Curr. Opin. Chem. Biol. 2004;8(2):162–168. doi: 10.1016/j.cbpa.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Ljubisavljevic S. Oxidative Stress and Neurobiology of Demyelination. Mol. Neurobiol. 2016;53(1):744–758. doi: 10.1007/s12035-014-9041-x. [DOI] [PubMed] [Google Scholar]
- 38.Ishihara Y., Takemoto T., Itoh K., Ishida A., Yamazaki T. Dual role of superoxide dismutase 2 induced in activated microglia: oxidative stress tolerance and convergence of inflammatory responses. J. Biol. Chem. 2015;290(37):22805–22817. doi: 10.1074/jbc.M115.659151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Fabuel I., Le Douce J., Logan A., James A.M., Bonvento G., Murphy M.P., Almeida A., Bolanos J.P. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc. Natl. Acad. Sci. USA. 2016;113(46):13063–13068. doi: 10.1073/pnas.1613701113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko C.Y., Chang W.C., Wang J.M. Biological roles of CCAAT/Enhancer-binding protein delta during inflammation. J. Biomed. Sci. 2015;22:6. doi: 10.1186/s12929-014-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang L.H., Huang H.S., Wu P.T., Jou I.M., Pan M.H., Chang W.C., Wang D.D., Wang J.M. Role of macrophage CCAAT/enhancer binding protein delta in the pathogenesis of rheumatoid arthritis in collagen-induced arthritic mice. PLoS One. 2012;7(9):e45378. doi: 10.1371/journal.pone.0045378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paiva L.A., Gurgel L.A., Silva R.M., Tome A.R., Gramosa N.V., Silveira E.R., Santos F.A., Rao V.S. Anti-inflammatory effect of kaurenoic acid, a diterpene from Copaifera langsdorffi on acetic acid-induced colitis in rats. Vasc. Pharmacol. 2002;39(6):303–307. doi: 10.1016/s1537-1891(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 43.Bajpai V.K., Sharma A., Kang S.C., Baek K.H. Antioxidant, lipid peroxidation inhibition and free radical scavenging efficacy of a diterpenoid compound sugiol isolated from Metasequoia glyptostroboides. Asian Pac. J. Trop. Med. 2014;7(1):9–15. doi: 10.1016/S1995-7645(13)60183-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material